Case-Based Self-Assessment Questions
Clinical Scenario
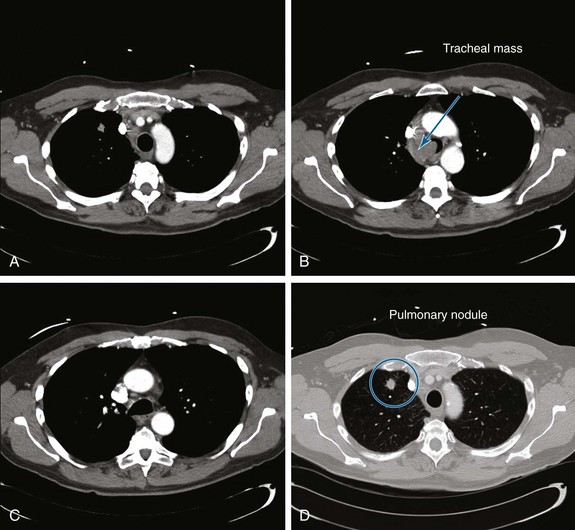
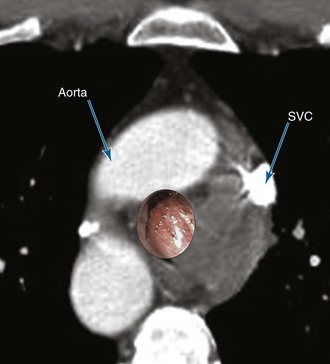
A 55-year-old white male with a 20–pack-year history of smoking presented with syncope, a 2 month history of increasing exertional dyspnea, and a cough that had been refractory to bronchodilators and antibiotics. He was married and lived with his wife. The patient’s father and one brother had died of lung cancer. The emergency department physician believed that the patient was disabled, required special care and assistance, and warranted immediate hospitalization. The physical examination, however, was normal except for stridor heard over the trachea during forced expiration. The workup for syncope included an electrocardiogram, two-dimensional echocardiogram, bilateral carotid duplex ultrasonography, and computed tomography (CT) of the head, all of which were normal. A pulmonary consultant ordered a CT scan of the chest, which revealed a 1.5 cm right upper lung (RUL) nodule and a 4 cm right paratracheal mass invading the trachea (Figure 1).
Estimated survival is an important factor for decision making in all disease processes. If this patient has primary lung cancer, he would be clinically staged IIIB because of tracheal involvement (T4 tumor). In such cases of advanced cancer, prognostic considerations are especially important because treatment goals nearing the end of life may change from efforts to prolong life at all costs, to those of palliating symptoms, preserving quality of life, and maintaining dignity. Estimating survival based on objective data is warranted because physicians’ subjective assessments of predicted survival are often incorrect, with the direction of error being usually optimistic.*1 Patients, in most circumstances, want their doctors to be realistic when it comes to prognosis. Although exact numbers may not be requested by patients or their families, having knowledge of relatively accurate prognostic indicators helps physicians conduct meaningful and honest discussions.
A clinical symptom such as stridor, although a sign of severe laryngeal or tracheal obstruction,2 does not delineate a benign or malignant disease process. In general, stridor and other symptoms of upper or central airway obstruction (CAO), including exertional dyspnea, wheezing, or even syncope, are nonspecific.3 In fact, results from analysis of 100 variables from several studies showed that only dyspnea, dysphagia, weight loss, xerostomia, anorexia, and cognitive impairment were strongly and independently associated with cancer patient survival. These signs and symptoms were outranked, however, by assessment of performance status,1 which deservedly has become a “sixth vital sign” in clinical oncology.† Because performance status is the strongest prognostic indicator of survival in patients with cancer, it is frequently used as an entry criterion and adjustment factor in clinical trials of anticancer treatment.1 One commonly used measure of performance is the Karnofsky Performance Status score (range, 0 to 100 in 10 point increments, where 0 is death and 100 is perfect health), a general measure of functional impairment in which the lower the score, the worse is survival for most serious illnesses.1
This patient was assigned a Karnofsky score of only 40. In the setting of lung cancer, known central airway obstruction, and Karnofsky scores below 50, a combination of interventional bronchoscopy and external beam radiation therapy (EBRT) to relieve the airway obstruction has been shown to be the therapeutic option of choice, often resulting in rapid restoration of airway patency, improved symptoms, improved performance status, and increased survival.4
Question 2: The next appropriate step is to:
A Postpone the procedure until bronchodilator treatments result in decreased peak airway pressure
B Change the endotracheal tube to a larger diameter (e.g., 8 or 8.5 mm), which will accommodate the 6 mm outer diameter flexible bronchoscope
C Cancel the procedure because peak airway pressures are too high
Ideally, a No. 8 or larger ETT is preferred for bronchoscopy because it allows proper ventilation during the procedure and potentially prevents a further increase in peak airway pressure or development of auto-PEEP. These tubes provide at least a 2 mm difference between the scope and the ETT diameter, preventing critical alterations in ventilatory parameters.5 Changing the ETT, however, by extubation and repeat laryngoscopy or by use of a tube exchanger is hazardous in a patient with critical tracheal obstruction. Reintubation may be difficult, and should the airway be lost, ventilation cannot be ensured. A safer approach may be to perform inspection flexible bronchoscopy through the existing ETT while monitoring peak airway pressures, tidal volumes, heart rate, and oxygenation. In case of tachycardia, a rise in blood pressure, or oxygen desaturation during the procedure, the bronchoscope can be immediately withdrawn until stable vital signs return.
Flexible bronchoscopy was performed after a bite block was inserted into the mouth and was secured around the existing ETT. A swivel adapter with a fitted rubber cap was attached, allowing bronchoscopy with minimal loss of tidal volume. The FiO2 was increased to 1.0, starting 5 minutes before the procedure, and continued until after the procedure, then was titrated down to prebronchoscopy levels. PEEP was removed during bronchoscopy to avoid raising peak airway pressures by as much as 25 mm Hg.6 If discontinuation of PEEP had not been feasible, it would have been reduced by 50%. Volume control ventilatory mode was preferred so that the increasing airway resistance secondary to the bronchoscopy would not result in reduced tidal volume. If pressure-controlled ventilation had been used, the peak pressure setting would have been increased to compensate for the loss of tidal volume consequent to the increased resistance. Moderate sedation was achieved with 2 mg intravenous midazolam. Had the patient been unable to cooperate or otherwise tolerate the procedure comfortably, supplemental doses may have been needed in 1- to 2-minute increments and according to moderate sedation guidelines used in our institution. To remove airway secretions, bronchoscopic suction was applied using short, 3 second or less bursts, because as much as 200 to 300 cm3 of the patient’s tidal volume can be removed during each suction period.6
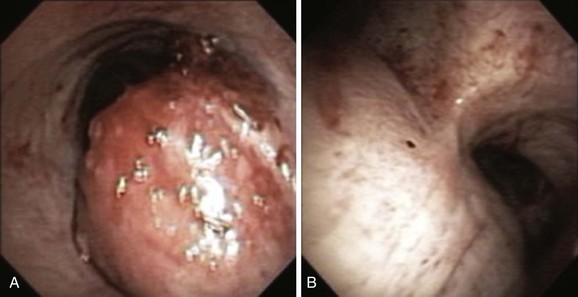
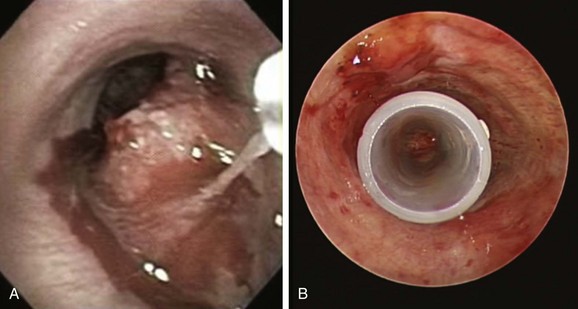
A mass was seen protruding from the right lateral and posterior walls of the lower trachea. The tracheal lumen was narrowed by more than 70%. A severe mixed extrinsic compression and endoluminal obstruction pattern was noted. The remaining airways were normal except for an enlarged carina (Figure 2). The procedure lasted only 1 minute, during which the peak airway pressure increased to 80 cm H2O but without hypoxemia or hemodynamic instability.
A Flattening of the inspiratory curve
B Flattening of the expiratory curve
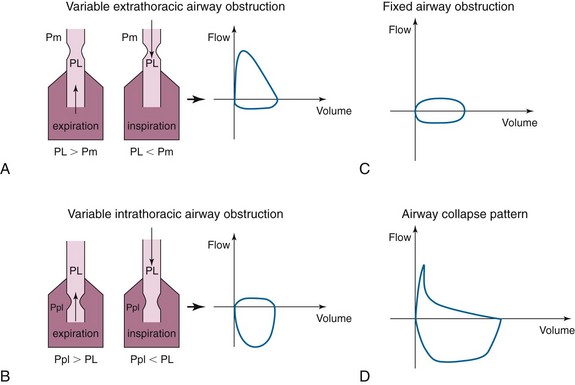
In cases of extrathoracic tracheomalacia, laryngomalacia, or vocal cord processes, the flow-volume loop pattern may be that of flattening of the inspiratory curve (Figure 3, A). This is due to the fact that intraluminal pressure during inspiration (PL) is lower than extraluminal pressure (Pm = atmospheric pressure) for the extrathoracic airway. Therefore, any obstruction in this airway segment will be made worse during inspiration and will cause limitation of the inspiratory flow. During expiration, however, to have an expiratory flow, the intraluminal pressure (PL) is higher than the atmospheric pressure (Pm), the extrathoracic airway will dilate, and the flow will be normal.
The opposite is true in cases of variable intrathoracic obstruction such as intrathoracic tracheomalacia. In this case, the obstruction is worsened during expiration when the pleural pressures (Ppl) exceed the intraluminal pressure (PL) (Figure 3, B), and there will be flattening of the expiratory curve of the flow-volume loop.
Our patient had a tracheal mass limiting airflow both during inspiration and during exhalation. The pattern on the flow-volume loop would likely have been that of blunting of both inspiratory and expiratory curves (Figure 3, C), the so-called square pattern. This pattern may be seen before spirometry yields abnormal results but may not be appreciated until the airway diameter is already narrowed to about 8 to 10 mm and is often masked in severe COPD.
The airway collapse pattern (Figure 3, D) is usually seen in severe COPD and may suggest excessive dynamic airway collapse. This flow-volume loop pattern suggests compression of the mainstem bronchi and trachea. The sudden drop in flow velocity occurs early during expiration when intrathoracic pressure is still increasing, as documented by esophageal pressure measurements. Because there is no reduction of force expelling air from the lungs, the sudden fall in velocity can be explained only by a sudden increase in resistance to airflow, which occurs because of collapse of the central airways. The small, relatively constant flow (i.e., plateau) that occurs after the sudden drop in velocity is consistent with air being forced through a narrow airway.
A Rigid bronchoscopy and restoration of airway patency might allow subsequent systemic therapy in a more stable and spontaneously breathing patient.
B Rigid bronchoscopy and restoration of airway patency will improve exercise capacity and dyspnea.
C Rigid bronchoscopy and restoration of airway patency might allow removal from mechanical ventilatory support and discharge from the intensive care unit.
Patients with respiratory failure and malignant CAO treated by bronchoscopic intervention, who subsequently underwent definitive systemic therapy, were shown to have improved dyspnea and longer survival (median, 38.2 months; range, 1.7 to 57.0 months) compared with those who did not undergo bronchoscopic intervention (median, 6.2 months; range, 0.1 to 33.7 months; P < .001).7 Restoring airway patency also improves exercise capacity and dyspnea.7 In this study, a significant improvement in symptoms was noted in 94% of patients undergoing airway stent insertion, although ultimately 40% of patients required multiple bronchoscopic procedures. This raises concerns about the cost per quality of life-years gained in patients with advanced disease.8 The expense of palliative interventional procedures such as bronchoscopic laser resection and stent insertion, however, is probably small compared with the costs of repeated external beam radiation therapy or prolonged hospitalization in the intensive care unit. Rapid tissue diagnosis, restoration of airway patency, subsequent extubation and removal from mechanical ventilation, and discharge from the intensive care unit to a ward or even outpatient setting because of this single procedure likely result in considerable savings that positively impact health care costs and resource utilization.
Evidence that removal from mechanical ventilation in critically ill patients with airway obstruction and respiratory failure is possible because of interventional bronchoscopic procedures is limited but reproducible. Lo et al. successfully extubated 7 of 7 patients who were on mechanical ventilation for respiratory failure from malignant obstruction.9 Colt and Harrell successfully removed ventilatory support in 10 of 19 patients (52.6%) with malignant and benign obstruction,10 suggesting that when procedures were unsuccessful, immediate referral to hospice and comfort care was justified. Schaffer and Allen inserted metal stents in 6 patients with malignant CAO and in 2 with benign CAO, successfully extubating 6 of them 2 to 11 days later.11 Furthermore, successful removal from mechanical ventilation has been associated with prolonged survival when systemic treatment is also administered (98 vs. 8.5 days).12
During our dialogue with the patient’s wife, it was believed that the risks of further airway compromise and clinical deterioration were outweighed by the benefit of restoring airway patency with an ability to immediately diagnose the abnormality, wean the patient from mechanical ventilation, and offer systemic therapy. Although we planned to make the diagnosis on frozen section of the tumor removed from the airway, we could have also chosen to perform endobronchial needle aspiration (EBNA), brushing, or endobronchial forceps biopsy. In our experience, EBNA has a lower risk of bleeding than brushing or biopsy, and it provides immediate diagnosis when on-site cytologic examination is available. On-site cytology is not yet the standard of practice in many institutions; however, it may increase procedure-related costs, and, contrary to endobronchial biopsy, tissue architecture is not seen.13
A CT-guided percutaneous needle aspiration of the right upper lobe nodule
B Endobronchial ultrasound (EBUS) radial probe-guided transbronchial biopsy of the nodule
C Electromagnetic navigation (EMN) for guiding transbronchial biopsy of the nodule
D Surgical consultation for video-assisted thoracic surgery (VATS) or thoracotomy
E Flexible bronchoscopy with EBNA at the bedside in the intensive care unit (ICU)
Rather than approaching the tracheal mass, some might address the pulmonary nodule for diagnosis. CT-guided percutaneous needle aspiration of the right upper lobe nodule has a high diagnostic rate (91%) but would not provide information on staging, and it carries an increased risk for pneumothorax, especially in patients on positive-pressure ventilation (5% to 60%).14 Endobronchial ultrasound (EBUS) radial probe-guided transbronchial biopsy has a diagnostic yield of 70%15 but requires special training for image acquisition and interpretation and is not universally available. Electromagnetic navigation (EMN) in addition to EBUS radial probe biopsy increases the yield to 88%. Pneumothorax occurs in about 5%16 of patients, but this rate might be higher (14%) in mechanically ventilated patients.17
In patients with visible endobronchial lesions, endobronchial needle aspiration (EBNA) with on-site cytology examination has a diagnostic yield of 96% when combined with biopsy, washings, and brushing, compared with about 76% using any of these procedures alone.13 Therefore EBNA at the bedside in the ICU is a reasonable alternative, particularly if diagnosis is desired before consideration for rigid or flexible bronchoscopic resection.18
Systemic therapies such as EBRT and systemic chemotherapy might be appropriate treatment strategies once a tissue diagnosis has been obtained. In case of non–small cell lung carcinoma (NSCLC), this tumor would be a clinical stage IIIB with an estimated median survival of 10 months and a 5 year survival of 7%.19 Current evidence shows that for individuals with unresectable disease, good performance score, and minimal weight loss, treatment with combined chemoradiotherapy results in better survival than radiotherapy alone. Concurrent chemoradiotherapy seems to be associated with improved survival compared with sequential chemoradiotherapy.20 Restoration of airway patency should improve performance score and accelerate appropriate initiation of systemic therapy. Therefore even in the absence of rigid bronchoscopy, restoration of airway patency is warranted.
Brachytherapy is an endobronchial therapy with proven efficacy in patients with endoluminal tumor and a substantial extrabronchial component. A Cochrane meta-analysis concluded, however, that for palliation of NSCLC symptoms, endobronchial brachytherapy (EBB) alone was less effective than EBRT. For patients previously treated by EBRT who are symptomatic from recurrent endobronchial central obstruction, EBB may be considered.21 Tissue effects are delayed and necrotic debris after radiation can worsen airway obstruction.
Photodynamic therapy (PDT) has resulted in successful weaning from mechanical ventilation of a small number of patients with malignant CAO and respiratory failure.22 The therapeutic effect of PDT is delayed, however, for at least 48 hours, and this might actually worsen airway obstruction during the initial post-treatment period because of sloughing of airway mucosa and retained tumor debris. PDT usually is not indicated in patients with critical tracheal narrowing but appears to be most effective when there is more than 50% narrowing from lobar or segmental bronchial mucosal disease.23
Cryotherapy results in thrombosis and necrosis of tumor tissues and would address only the exophytic, endoluminal component of the obstructing airway lesion. Cryotherapy has been shown to be most effective when performed in combination with EBRT.24 Similar to PDT and EBB, the effect is delayed and initially might worsen airway obstruction.
Electrocautery and argon plasma coagulation (APC) remove the exophytic component and allow superficial cauterization (3 to 6 mm), which may not suffice to stop large airway bleeding in cases of obstructing tracheal tumor. As gas is forced into the airway wall, it can collect in a blood vessel, causing embolism or perforation. Argon gas is heavy, inert, and 17 times less soluble in the body than carbon dioxide. Thus, it may also pass into the systemic circulation.25 Although the tracheal wall is fairly dense, erosion from tumor presents a risk to major vessels. Systemic, life-threatening gas embolism occurring as a complication of bronchoscopic application of APC has been reported26 and might be of particular risk in view of the highly vascular pattern of this patient’s particular tracheal lesion.
Fully or partially covered metal stent insertion is a plausible alternative in cases of malignant CAO. This can be performed through the ETT in the ICU.27 It is also a suitable alternative in patients with significant comorbidities precluding general anesthesia. These stents are more costly than silicone stents and can be difficult to remove. Because the bulk of the obstruction resulted from malignant extrinsic compression, the need for excessive debulking was unlikely,28 and stent insertion alone could probably restore airway patency. These stents can be placed with or without fluoroscopic guidance.27,29
Question 7: Which type of anesthesia method would you suggest for this patient’s rigid bronchoscopy?
A Spontaneous-assisted ventilation with total intravenous anesthesia
B Spontaneous-assisted ventilation with inhalational anesthetics
Concern for loss of airway during induction exists because of critical tracheal narrowing.30 Induction should be cautious and preferably should be conducted without neuromuscular blockers; use of these might result in complete loss of muscular tone of the posterior membrane, possibly worsening an already critically narrowed airway.31 Spontaneous-assisted ventilation with intravenous anesthetics has been shown to have a good cardiac safety profile in patients undergoing rigid bronchoscopy under general anesthesia. Spontaneous-assisted ventilation is provided through an open circuit during the rigid bronchoscopic procedure.32 This usually is done without the use of muscle relaxants and requires an Ambu bag with a high-flow oxygen source connected to the side port of the rigid bronchoscope. In case of an air leak resulting in an inability to ventilate and oxygenate satisfactorily, the mouth and nose can be packed with gauze and Vaseline nasal packing.
The delivery of volatile anesthetics may be problematic when rigid bronchoscopy is performed using an open system with spontaneous-assisted ventilation because the quantity of anesthetic gas reaching the patient is uncertain, and operating room personnel and the operator are at risk for pollution from escaped anesthetics.33
Positive-pressure ventilation will increase flow velocity and turbulent flow past the region of stenosis. Subsequently, a laminar flow pattern cannot be resumed, potentially resulting in ineffective ventilation of the distal airways and loss of effective gas exchange.34
Jet ventilation can be considered for severe tracheal obstruction.35 High-frequency jet ventilation (HFJV) or intermittent low-frequency jet ventilation can be delivered through a rigid or flexible bronchoscope. HFJV through catheters with internal diameters as small as 2.0 mm placed beyond the tracheal obstruction allows safe levels of oxygenation, provided an adequate expiratory passage is present.36 Jet ventilation poses a risk for barotrauma and pneumothorax and therefore was not our method of choice for this patient.
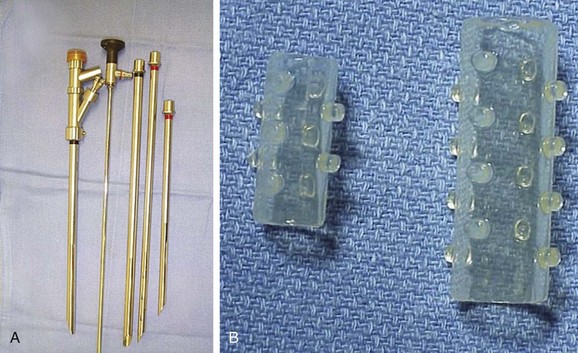
Rigid bronchoscopy with spontaneous ventilation was performed. The patient was extubated and under direct visualization was intubated with a 12 mm Efer-Dumon rigid nonventilating bronchoscope (Bryan Corp., Woburn, Mass). Neodymium-doped yttrium aluminum garnet (Nd : YAG) laser was available on standby in case of bleeding or for tumor coagulation. Various types and sizes of silicone stents were also readily available (Figure 4).
Potential dangers during bronchoscopic resection of a lesion in this location involve the superior vena cava and azygos vein located adjacent to the anterior and lateral wall of the lower trachea. The aorta is anterior (Figure 5).
Moderate bleeding occurred when the tumor was probed with the suction catheter (Figure 6). This stopped after instillation of 60 mL of saline. The tumor was debulked using the beveled edge of the rigid bronchoscope. Laser was not necessary. A 14 × 50 mm straight studded silicone stent was inserted into the lower trachea so that the distal aspect of the stent was located 1 cm above the main carina (see Figure 6). Preliminary tissue diagnosis was non–small cell carcinoma.
Question 8: What is the most appropriate next step?
A Compete staging, including whole body PET/CT and brain MRI
B Consult palliative care medicine
Complete staging should be performed to better assess prognosis and treatment strategy. This includes a whole body positron emission tomography (PET)/computed tomography (CT) scan and brain magnetic resonance imaging (MRI), which in this patient showed no extrathoracic disease. Because this patient had stage IIIB NSCLC (T4 tumor due to tracheal involvement), he was not a surgical candidate. Optimal treatment for stage IIIB NSCLC depends on several variables, including extent of disease, age, comorbidities, performance scores, and weight loss.20 This patient’s Karnofsky performance status score post procedure had increased to 90; therefore referral for palliative or hospice care was considered premature. Concurrent chemoradiotherapy, which prolongs survival and is at the present time considered to be the standard of care for inoperable locally advanced NSCLC,37 is usually restricted to healthy cancer patients who maintain a good performance status. Because this patient’s status had significantly improved,38 concurrent chemoradiotherapy was offered following discussion in our multidisciplinary chest conference (with representatives from cardiothoracic surgery, oncology, critical care medicine, interventional pulmonology, and radiation oncology).
Question 9: The most appropriate next step is to:
A Perform flexible bronchoscopy
B Obtain a three-dimensional computed tomography
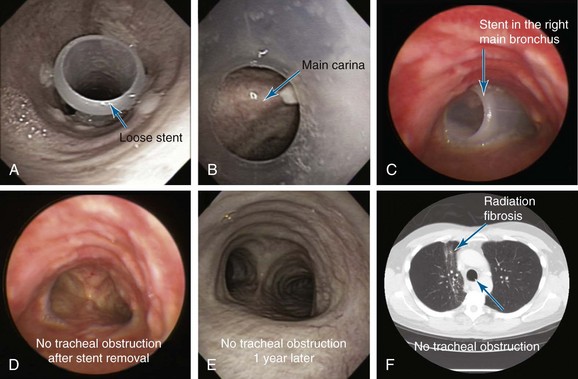
Acute onset of shortness of breath and difficulty raising secretions are likely due to a stent-related complication, rather than to progression of disease or pulmonary embolism. Stent migration should be expected after radiation therapy, especially in cases of tumor shrinkage, causing loss of intimate contact between the stent and the airway wall. In one report, indwelling airway stents could be removed in 50% of patients with malignant CAO at a median of 31.7 days after successful systemic therapy.39 The risk of stent migration has an estimated incidence of 1% to 5%.28 Other stent-related complications include kinking, obstruction by tumor or granulation tissue overgrowth, bacterial colonization, and impaired mucociliary clearance causing retained secretions and stent obstruction. Repeat flexible bronchoscopies are reportedly necessary to remove secretions in up to 40% of patients with indwelling stents.8 Additional rigid bronchoscopic procedures are seldom required in patients with malignant CAO, in part because of their limited survival.10,28
In fact, outpatient flexible bronchoscopy showed that the stent had migrated distally to the main carina (Figure 7). Rigid bronchoscopy with stent revision was promptly scheduled for the same morning. The patient had begun to cough violently. By the time of rigid bronchoscopy, the stent had partially migrated into the right main bronchus, crossing over the carina and nearly occluding the entrance of the left main bronchus. The stent was removed with rigid forceps. The airway was patent, and no evidence of recurrent endoluminal disease was found (see Figure 7). One year later, the patient continued to do well, had not required stent reinsertion or repeat bronchoscopic interventions, and had no respiratory complaints. Chest CT showed only chronic radiation pneumonitis changes with fibrosis (see Figure 7).
1. Lamont EB, Christakis NA. Survival estimates in advanced cancer. www.UpToDate.com, 2010. Accessed March 16
2. Hollingsworth HM. Wheezing and stridor. Clin Chest Med. 1987;8:231-240.
3. Fries MR, Galindo RL, Flint PW, et al. Giant fibrovascular polyp of the esophagus: a lesion causing upper airway obstruction and syncope. Arch Pathol Lab Med. 2003;127:485-487.
4. Canak V, Zarić B, Milovancev A, et al. Combination of interventional pulmonology techniques (Nd:YAG laser resection and brachytherapy) with external beam radiotherapy in the treatment of lung cancer patients with Karnofsky Index < or = 50. J BUON. 2006;11:447-456.
5. Lawson RW, Peters JI, Shelledy DC. Effects of fiberoptic bronchoscopy during mechanical ventilation in a lung model. Chest. 2000;118:824-831.
6. Tai DY. Bronchoscopy in the intensive care unit (ICU). Ann Acad Med Singapore. 1998;27:552-559.
7. Jeon K, Kim H, Yu CM, et al. Rigid bronchoscopic intervention in patients with respiratory failure caused by malignant central airway obstruction. J Thorac Oncol. 2006;1:319-323.
8. Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg. 2003;76:167-174.
9. Lo CP, Hsu AA, Eng P. Endobronchial stenting in patients requiring mechanical ventilation for major airway obstruction. Ann Acad Med Singapore. 2000;29:66-70.
10. Colt HC, Harrell J. Therapeutic rigid bronchoscopy allows level of care changes in patients with acute respiratory failure from central airways obstruction. Chest. 1997;112:202-206.
11. Shaffer JP, Allen JN. The use of expandable metal stents to facilitate extubation in patients with large airway obstruction. Chest. 1998;114:1378-1382.
12. Stanopoulos IT, Beamis JFJr, Martinez FJ, et al. Laser bronchoscopy in respiratory failure from malignant airway obstruction. Crit Care Med. 1993;21:386-391.
13. Mazzone P, Jain P, Arroliga AC, et al. Bronchoscopy and needle biopsy techniques for diagnosis and staging of lung cancer. Clin Chest Med. 2002;23:137-158.
14. Toloza EM, Harpole L, Detterbeck F, et al. Invasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:157S-166S.
15. Herth FJ, Eberhardt R, Becker HD, et al. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest. 2006;129:147-150.
16. Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36-41.
17. O’Brien JD, Ettinger NA, Shevlin D, et al. Safety and yield of transbronchial biopsy in mechanically ventilated patients. Crit Care Med. 1997;25:440-446.
18. Colt H, Prakash U, Offord K. Bronchoscopy in North America: survey by the American Association for Bronchology, 1999. J Bronchol. 2000;7:8-25.
19. Goldstraw P, Crowley J, Chanksy K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:704-706.
20. Jett JR, Schild SE, Keith RL, et al. American College of Chest Physicians. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:266S-276S.
21. Cardona AF, Reveiz L, Ospina EG, et al. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2, 2008. CD004284
22. Shah SK, Ost D. Photodynamic therapy: a case series demonstrating its role in patients receiving mechanical ventilation. Chest. 2000;118:1419-1423.
23. Edell ES, Cortese DA. Photodynamic therapy: its use in the management of bronchogenic carcinoma. Clin Chest Med. 1995;16:455-463.
24. Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer: preliminary results. Chest. 1992;102:1436-1440.
25. Kono M, Yahagi N, Kitahara M, et al. Cardiac arrest associated with use of an argon beam coagulator during laparoscopic cholecystectomy. Br J Anaesth. 2001;87:644-646.
26. Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest. 2008;134:1066-1069.
27. Chung FT, Lin SM, Chen HC, et al. Factors leading to tracheo-bronchial self-expandable metallic stent fracture. J Thorac Cardiovasc Surg. 2008;136:1328-1335.
28. Lemaire A, Burfeind WR, Toloza E, et al. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann Thorac Surg. 2005;80:434-438.
29. Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124:1993-1999.
30. McMahon CC, Rainey L, Fulton B, et al. Central airway compression: anaesthetic and intensive care consequences. Anaesthesia. 1997;52:158-162.
31. Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth. 1989;36:681-688.
32. Perrin G, Colt HG, Martin C, et al. Safety of interventional rigid bronchoscopy using intravenous anesthesia and spontaneous assisted ventilation: a prospective study. Chest. 1992;102:1526-1530.
33. Purugganan RV. Intravenous anesthesia for thoracic procedures. Curr Opin Anaesthesiol. 2008;21:1-7.
34. Sibert KS, Biondi JW, Hirsch NP. Spontaneous respiration during thoracotomy in a patient with a mediastinal mass. Anesth Analg. 1987;66:904-907.
35. Baraka AS, Siddik SS, Taha SK, et al. Low frequency jet ventilation for stent insertion in a patient with tracheal stenosis. Can J Anaesth. 2001;48:701-704.
36. Brodsky JB. Bronchoscopic procedures for central airway obstruction. J Cardiothorac Vasc Anesth. 2003;17:638-646.
37. Trodella L, D’Angelillo RM, Ramella S, et al. Multimodality treatment in locally advanced non-small cell lung cancer. Ann Oncol. 2006;17:ii32-ii33.
38. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379-392.
39. Witt C, Dinges S, Schmidt B, et al. Temporary tracheobronchial stenting in malignant stenoses. Eur J Cancer. 1997;33:204-208.