Case 9
HISTORY AND PHYSICAL EXAMINATION
Electrodiagnostic (EDX) examination was performed.
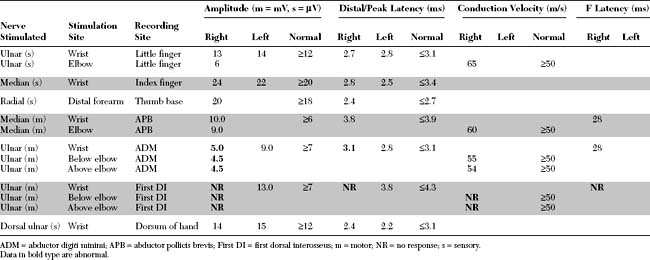
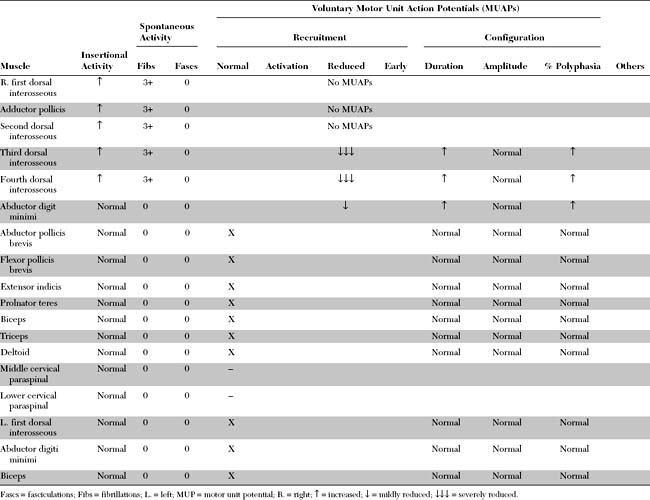
Please now review the Nerve Conduction Studies and Needle EMG tables.
QUESTIONS
EDX FINDINGS AND INTERPRETATION OF DATA
Pertinent EDX findings include:
This is consistent with ulnar mononeuropathy at the wrist, affecting the motor branch exclusively, distal to the main branch to the hypothenar muscles, but proximal to the branch to the fourth dorsal interosseus (i.e., at the pisohamate hiatus [PHH]). The normal ulnar sensory study rules out a proximal ulnar nerve, or a lower brachial plexus lesion. This case is not due to C8/T1 radiculopathy is because the median CMAP is preserved, and there is no denervation seen in other C8/T1-innervated muscles.
DISCUSSION
Applied Anatomy
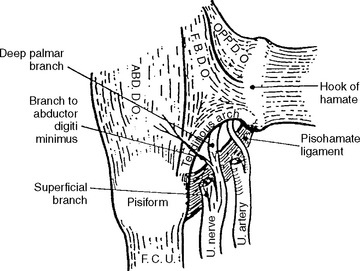
Guyon canal is formed proximally by the pisiform bone and distally by the hook of the hamate. Its floor is formed by the triquetrum and hamate bones along with the thick transverse carpal ligament, while its roof is composed of a loose connective tissue (Figure C9-1). In the distal portion of Guyon canal lies the pisohamate hiatus (PHH). This aperture is bounded anteriorly by a fibrous arch formed by the two musculotendinous attachments of the flexor brevis digiti minimi (or quinti), a hypothenar muscle, to the hook of hamate and the pisiform bone (Figure C9-2). The posterior boundary of the PHH, is formed by a thick pisohamate ligament which extends from the pisiform bone to the hook of the hamate. The origin of the major motor branch to the ADM is proximal to this hiatus in the majority of hands.

Figure C9-1 Anatomy of the ulnar nerve within Guyon canal at the wrist. 1 = ulnar artery, 2 = superficial branch of the ulnar nerve, 3 = hamulus, 4 = fibrous arch of the hypothenar muscles (see also Figure C9-2), 5 = pisiform, 6 = transverse carpal ligament, 7 = palmaris brevis, 8 = palmar carpal ligament.
(From Gross MS, Gelberman RH. The anatomy of the distal ulnar tunnel. Clin Orthop 1985;196:238–247, with permission.)
Clinical Features
Patients with ulnar neuropathy at the wrist often presents with painless unilateral hand atrophy. These ulnar nerve lesions pose a diagnostic challenge, particularly when the weakness is not associated with sensory loss. It is useful in sorting out the cause of hand weakness or atrophy to distinguish between atrophy of all intrinsic hand muscles from atrophy that is restricted to the thenar or hypothenar muscles. Table C9-1 lists the various causes of wasting and weakness of the hand.
Table C9-1 Causes of Unilateral Atrophy and Weakness of Intrinsic Hand Muscles
Several classifications of ulnar mononeuropathy at the wrist have been proposed; most separate the lesions into four types. Table C9-2 lists the common types of ulnar mononeuropathy at the wrist and hand, with their corresponding sites of lesion and clinical presentation. In ulnar neuropathy at the wrist, selective compression of the deep palmar motor branch, with normal superficial sensory branch functions, is the most common type accounting for 39 to 75+ of distal ulnar nerve lesions depending on published series.
Table C9-2 Various Types of Distal Ulnar Mononeuropathy
| Lesion Site | Nerve Affected | Clinical Presentation |
|---|---|---|
| Proximal Guyon canal | Main trunk of ulnar nerve | Ulnar palmar sensory loss and weakness of all ulnar intrinsic hand muscles |
| or | or | |
| Ulnar cutaneous branch | Ulnar palmar sensory loss only | |
| Distal Guyon canal | Deep palmar branch (proximal to branch to the abductor digiti minimi) | Weakness of all ulnar intrinsic hand muscles (interossei, ulnar lumbricals, and hypothenars) without sensory loss |
| Pisohamate hiatus | Deep palmar branch (distal to branch to the abductor digiti minimi) | Weakness of ulnar intrinsic hand muscles with sparing of the hypothenar muscles and without sensory loss |
| Palm (rare) | Deep palmar branch (distal to hypothenars) | Weakness of adductor pollicis, 1st, 2nd, and possibly the 3rd interossei only, usually sparing 4th interossei and without sensory loss |
Table C9-3 lists the common causes of ulnar mononeuropathy at the wrist. A ganglion is the most common cause accounting for 28 to 45+ of cases depending on reported series. The second most common cause is entrapment of the motor branch at the pisohamate hiatus, which may be spontaneous or as an occupational or recreational hazard (such as after prolonged bicycling, use of hand tools, etc.). Compression at this anatomic hiatus explains the selective involvement of the deep motor branch at the wrist with complete or relative sparing of the hypothenar muscles and the normal superficial sensory functions.
Table C9-3 Common Causes of Ulnar Mononeuropathy at the Wrist
Decisions regarding the management of these lesions are made on the basis of their etiologies, presentations, and clinical courses. In patients with fractures, ganglia, or mass lesions, surgical intervention is necessary. However, in patients with ulnar neuropathy at the wrist who do not harbor an obvious mass or fracture and have a predominantly demyelinating lesion, careful review of the history for any occupational or recreational trauma should be initiated; if found, sources of the trauma should be eliminated. Then, the patient should be followed clinically and by serial EDX studies. If recovery is not evident, surgical exploration of Guyon canal extending into the PHH should be done. The prognosis for patients with this disorder is usually good after surgical decompression because the lesion is distal and reinnervation to the target hand muscles is efficient.
Electrodiagnosis
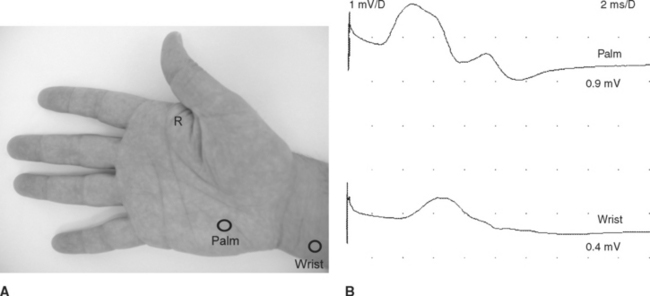
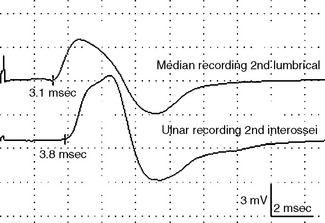
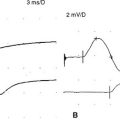
Figure C9-3 Palm and wrist stimulations recording first dorsal interosseous muscle. This 37-year-old woman developed subacute painless weakness of left hand. Neurological examination reveals severe weakness of all ulnar-innervated muscles in the hand with a positive Froment sign. There was no sensory loss. Tinel signs were negative at the wrist and elbow. Routine EDX studies revealed findings compatible with an axon loss ulnar mononeuropathy at the distal portion of Guyon canal (see Table C9-4). (A) The stimulation points at the palm and wrist (circles) while recording first DI (R). (B) Note that both wrist and palm stimulations result in low ulnar CMAPs, but there is a localizing partial conduction across both stimulation points (>50+ drop in amplitude).
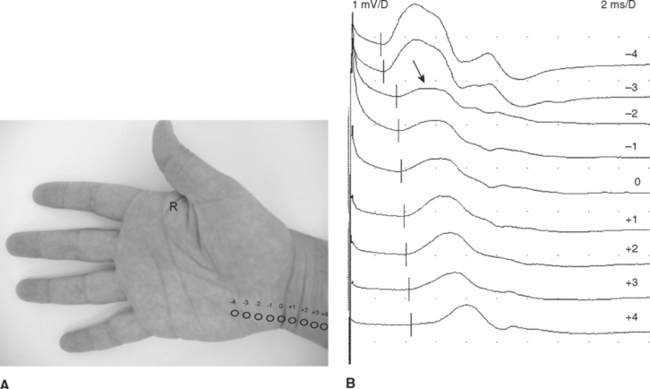
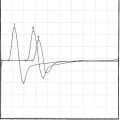
Figure C9-4 Ulnar short segment stimulations across the wrist (“inching”) recording first dorsal interosseous muscle. (A) Nine sites of stimulation (circles) in 1 cm increments along the length of the ulnar nerve while recording first DI (R): 0 level is at the distal crease of the wrist, while negative sites are progressively distal points and positive sites are proximal. (B) Same patient as Figure C9-3. Note that the most distal response, stimulating the palm at point –4, is low (0.9 mV), consistent with axon loss. However, there is also a localizing partial conduction block between points –3 and –2 (arrow), consistent with additional segmental demyelination. This is evidenced by a drop in CMAP amplitude from 0.7 mV at point –3 to 0.2 mV at point –2 (70+ amplitude decay). There is also focal slowing at the same segment (between points –3 and –2) as evidenced by a larger increase in latency between the same two points resulting in a latency difference of 0.9 ms. This is contrasted to 0.1 to 0.2 ms latency differences at all other sites.
The EDX findings in ulnar mononeuropathy at the wrist parallel the clinical manifestations and vary with the site of the lesion (Table C9-4). In addition to try to localize the lesions accurately within Guyon canal, at the PHH or in the palm, the EDX study play a pivotal role in excluding an ulnar neuropathy at the elbow, a common entrapment neuropathy. Several features on the EDX examination are not consistent with an ulnar neuropathy at the wrist:
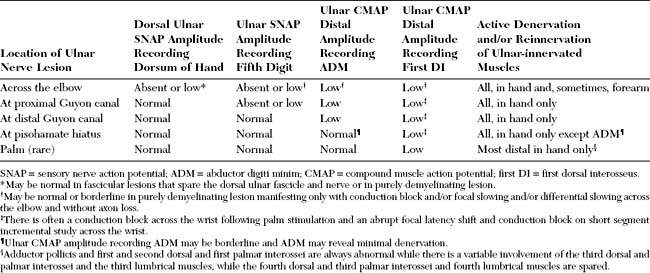
Among all ulnar mononeuropathies at the wrist, selective lesion of the deep motor branch at the PHH, sparing the hypothenar muscles completely or partially, is the most common. This entrapment is suspected when a patient presents with wasting and weakness of all intrinsic muscles of the hand except the thenar and hypothenar muscles, and without sensory manifestations. Table C9-5 lists the pathognomonic EDX features of such a lesion.
Table C9-5 Pathognomonic Electrodiagnostic Features of Deep Palmar Ulnar Neuropathy at the Pisohamate Hiatus
ADM = abductor digiti minimi; CMAP = compound motor action potential; DI = dorsal interosseus; SNAPs = sensory nerve action potentials.
* Following palm stimulation and short segment stimulations.
FOLLOW-UP
On further questioning, the patient recalled that a month before the onset of symptoms, he had spent an entire weekend vigorously chopping wood. Because the patient demonstrated no improvement, a surgical exploration of the proximal portion of Guyon canal was performed, which revealed normal structures. Despite this, there was no evidence of clinical or electrophysiologic improvement over the ensuing 6 months. A second operation was performed that achieved more distal exploration of the deep palmar branch into the PHH. This revealed a fibrous band constricting the deep motor branch at the PHH, which was resected. The patient had significant and gradual improvement of strength and experienced reversal of atrophy over the next year. Twelve months after the second decompression, the ulnar CMAP amplitude, which recorded the first DI and the ADM, showed significant improvement (Table C9-6). Needle EMG of the first and fourth DI showed significant reinnervation and a decline in fibrillation potentials.
Cowdery SR, Preston DC, Herrmann DN, et al. Electrodiagnosis of ulnar neuropathy at the wrist: conduction block versus traditional tests. Neurology. 2002;59:420-427.
Ebling P, Gilliatt RW, Thomas PK. A clinical and electrical study of ulnar nerve lesions in the hand. J Neurol Neurosurg Psychiatry. 1960;23:1-9.
Gross MS, Gelberman RH. The anatomy of the distal ulnar tunnel. Clin Orthop. 1985;196:238-247.
Katirji B, Dokko Y. Electrodiagnosis of deep palmar ulnar neuropathy at the pisohamate hiatus. Eur J Neurol. 1996;3:389-394.
Kothari MJ, Preston DC, Logigian EL. Lumbrical and interossei recordings localize ulnar neuropathy at the wrist. Muscle Nerve. 1996;19:170-174.
Jabley ME, Wallace WH, Heckler FR. Internal topography of the major nerves of the forearm and hand. J Hand Surg. 1980;5:1-21.
McIntosh KA, Preston DC, Logigian EL. Short segment incremental studies to localize ulnar entrapments at the wrist. Neurology. 1998;50:303-306.
Olney RK, Wilbourn AJ. Ulnar nerve conduction study of the first dorsal interosseus muscle. Arch Phys Med Rehabil. 1985;66:16-18.
Peterson AR, et al. Variations in dorsomedial hand innervation. Electrodiagnostic implications. Arch Neurol. 1992;49:870-873.
Shea JD, McClain EJ. Ulnar nerve compression syndromes at or below the wrist. J Bone Joint Surg. 1969;51A:1095-1103.
Stewart JD. The variable clinical manifestations of ulnar neuropathies at the elbow. J Neurol Neurosurg Psychiatry. 1987;3:429-432.
Streib EW, et al. Distal ulnar neuropathy: clinical and electrophysiologic aspects. Surg Neurol. 1985;23:281.
Uriburu IJF, Morchio FJ, Marin JC. Compression syndrome of the deep motor branch of the ulnar nerve (pisohamate-hamate hiatus syndrome). J Bone Joint Surg. 1976;58A:145-147.
Ventakesh S, Kothari MJ, Preston DC. The limitations of the dorsal ulnar cutaneous sensory response in patients with ulnar neuropathy at the elbow. Muscle Nerve. 1995;18:345-347.
Wu JS, Morris JD, Hogan GR. Ulnar neuropathy at the wrist: case report and review of the literature. Arch Phys Med Rehabil. 1985;66:19-21.