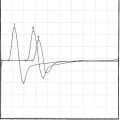
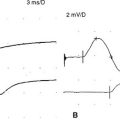
Case 2
HISTORY AND PHYSICAL EXAMINATION
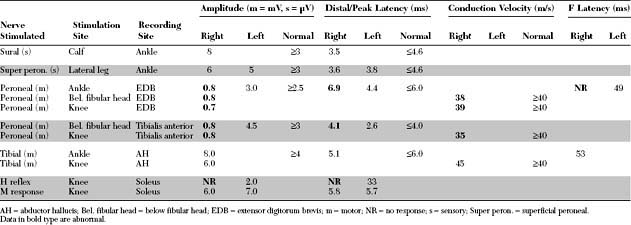
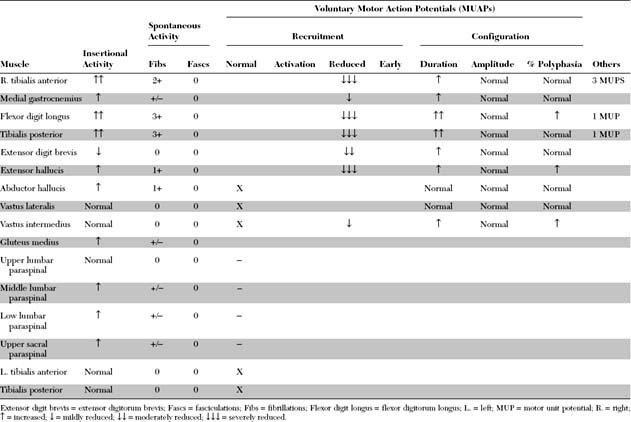
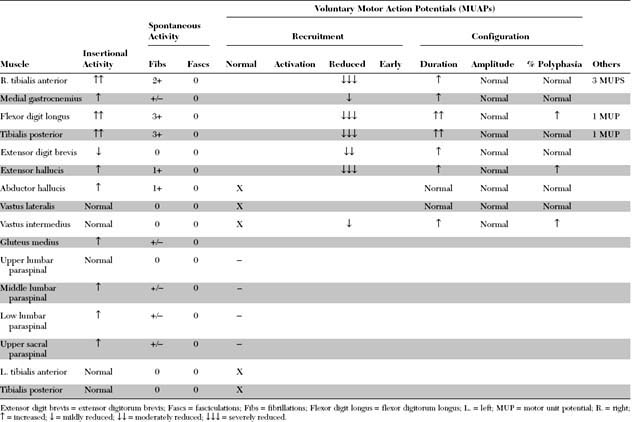
Please now review the Nerve Conduction Studies and Needle EMG tables.
QUESTIONS
EDX FINDINGS AND INTERPRETATION OF DATA
Relevant EDX findings in this patient are:
DISCUSSION
Applied Anatomy
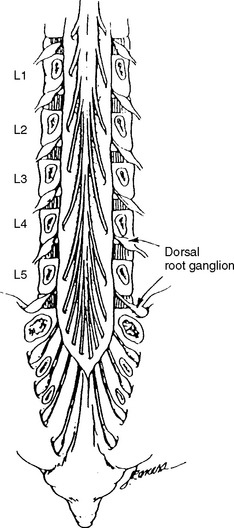
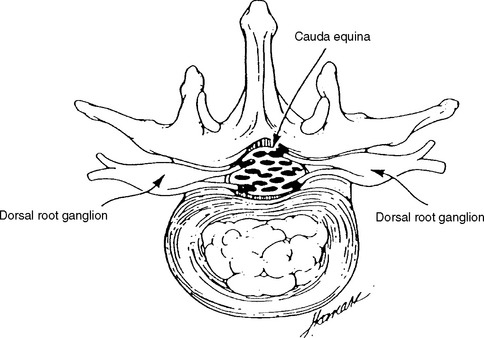
The dorsal root axons originate from the sensory neurons of the dorsal root ganglia (DRG), which lie outside the spinal canal within the intervertebral foramen (Figure C2-1), and immediately before the dorsal and ventral roots are joined. These sensory neurons are unique because they are unipolar. They have proximal projections through the dorsal root, called the preganglionic sensory fibers, which extend to the dorsal horn and column of the spinal cord. The distal projections of these neurons, called the postganglionic sensory fibers, join the motor fibers in the ventral root to form the spinal nerve, and then pass through the corresponding peripheral nerve to reach their respective sensory end-organs. The ventral root axons are mainly motor, and originate from the anterior horn cells within the spinal cord. Passing through the spinal nerves and the peripheral nerve, these motor fibers terminate in the corresponding muscles. At each intervertebral foramen, a mixed spinal nerve is formed by the fusion of the dorsal (afferent, sensory) and ventral (efferent, motor, and sympathetic) roots. Nerve roots have no epineurium and less collagen than peripheral nerves, which result in increased susceptibility to compression, stretch, and infiltration.
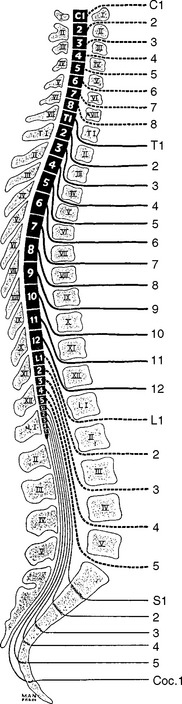
In humans, there are 31 pairs of spinal nerve roots: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal. In the cervical spine, each cervical root exits above the corresponding vertebra that shares the same numeric designation. For example, the C5 root exits above the C5 vertebra (i.e., between the C4 and C5 vertebrae). Because there are seven cervical vertebrae but eight cervical roots, the C8 root exits between the C7 and T1 vertebrae; subsequently, all thoracic, lumbar, and sacral roots exit below their corresponding vertebrae (Figure C2-2). For example, the L3 root exits below the L3 vertebra (i.e., between the L3 and L4 vertebrae).
In adults, the spinal cord ends at the L1 vertebra, resulting in a disparity between the lengths of the vertebral column and the spinal cord. Hence, spinal cord segments, mostly the thoracic and lumbar, are higher than the corresponding vertebras. This disparity is most pronounced in the lumbar region where there is a difference of approximately three segments, while there is usually a two-segment disparity in the thoracic region (see Figure C2-2). Since the cord terminates at L1 vertebra, the lumbosacral roots traverse relatively long intraspinal courses before exiting through their respective intervertebral foramina, thus forming the cauda equina. Due to the intricate anatomic relationships between the cauda equina and lumbar spinal column, a disc herniation at one level may injure different nerve roots and the vertebral level of a lumbosacral root compression does not always correlate with its exit level. For example, the S1 root is most often compressed by a posterolateral L5–S1 disc herniation (Figure C2-3). Also, the L5 root may be compressed by any disc herniation up to the level of conus medullaris at or rostral to L5–S1 (see Figure C2-2), including a lateral L5–S1 disc herniation and a posterolateral L4–L5 disc herniation. Less often, the L4–L5 disc may protrudes laterally into the foramen at that level and compress the exiting L4 root. If large, the lateral disc may injure both the L4 and L5 roots. Finally, if the L4–L5 disc herniation is central, it may compress several roots on one or both sides of the cauda equina, often asymmetrically.
Clinical Features
Low back pain is an extremely common symptom, but only a relatively small number of patients with low back pain have root compression in the lumbar region. Lumbosacral radiculopathy may be due to a variety of causes (Table C2-1), but is often caused by disc herniation, spondylitic changes (especially at the facetal joints leading to foraminal stenosis), or calcification of the ligamentum flavum. When combined, these changes can result in acquired lumbar canal stenosis. Disc herniation is more common in patients younger than 50 years while degenerative and spondylotic changes are more common in patients older than 50 years.
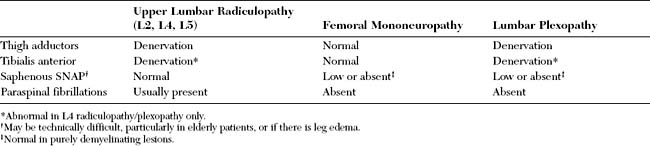
Table C2-2 lists common findings in patients with the various lumbosacral radiculopathy. Straight leg raise test is a maneuver that causes stretching of the sciatic nerve, sacral plexus, and the L5 and S1 nerve roots. While the patient is supine, the pain is reproduced with passive straight leg raising or when the examiner flexes the leg at the hip and then extends it at the knee. The test is most reliable when it is positive between 30° and 70°. Reverse straight leg raise testing is performed by passive hip extension while the patient is prone and causes stretching of the femoral nerve, the lumbar plexus, and upper lumbar roots (L2, L3, or L4). Pain in the groin or anterior thigh is considered a positive test.
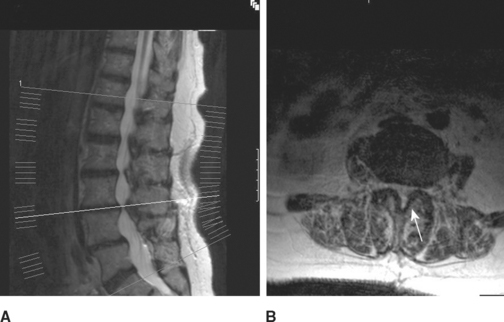
Lumbosacral root compression may involve a single root (monoradiculopathy) or multiple roots that are contiguous and may be bilateral (polyradiculopathy). Cauda equina lesions should be considered when more than two contiguous nerve roots are involved. Midline cauda equina syndrome results in early compression of sacral nerve roots, which lie medially within the cauda equina, leading to low back pain, sphincteric and sexual dysfunction, and paresthesias and sensory loss in sacral dermatomes (“saddle anesthesia”). When the lesion is large (such as with large midline L4–5 disc herniation), lumbosacral nerve roots may be involved resulting in leg weakness and sensory loss that may develop either early or later in the course, and sometimes result in paraplegia when multiple bilateral nerve roots are involved. Another clinically distinct cauda equina syndrome is the one caused by lumbar canal stenosis (Figure C2-4). This often presents with intermittent neurogenic claudication which is characterized by low back and leg pain, sometimes with paresthesias and weakness, brought on by standing and often worsened by walking. Typically, the symptoms are completely relieved several minutes after the patient sits down and are occasionally improved by bending at the waist. The symptoms are often bilateral but may be unilateral. The neurological examination in patients with lumbar canal stenosis may be entirely normal or show evidence of a single lumbosacral monoradiculopathy or patchy lumbosacral polyradiculopathy often involving the L4, L5, or S1 roots.
Electrodiagnosis
General Concepts
Goals of the Electrodiagnostic Study
Exclude a More Distal Nerve Lesion
Distinguishing a mononeuropathy from radiculopathy is relatively easy when the focal peripheral nerve lesion is associated with conduction block or focal slowing, such as in peroneal mononeuropathy at the fibular neck. Also, fibrillation potentials and MUAP denervation and reinnervation changes are limited to the muscles of the affected peripheral nerve in axon-loss mononeuropathy, while these abnormalities are widespread in radiculopathy and involve muscles that share a segmental innervation, irrespective of their peripheral nerves.
Confirm Evidence of Root Compression
Two criteria are necessary to establish the diagnosis of radiculopathy:
Table C2-3 Lower Extremity Sensory Nerve Action Potentials (SNAPs) and their Segmental Representation
| Root | SNAP |
|---|---|
| S1 | Sural |
| L5 | Superficial peroneal |
| L4 | Saphenous* |
* Saphenous SNAP is not always technically reliable especially in the elderly.
Localize the Compression to One or Multiple Roots
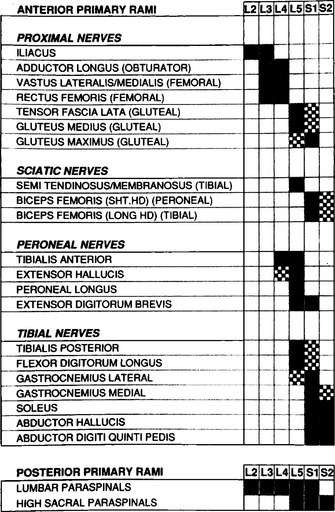
This requires meticulous knowledge of the segmental innervation of limb muscles (myotomes). Many myotomal charts have been devised, with significant variability; this may lead to confusion and disagreement between the EMG and the level of root compression as seen by imaging techniques or during surgery. EMG-derived charts also are very helpful and have had anatomic verification (see Tsao et al.). Figure C2-6 shows a common and most useful EMG-extracted myotomal chart.
A minimal “root search” should be performed in all patients with suspected lumbosacral radiculopathy to ensure that a radiculopathy either is confirmed or excluded. In other words, certain muscles of strategic value in EMG, because of their segmental innervation, should be sampled in these patients (Table C2-4). When abnormalities are found or when the clinical manifestations suggest a specific root compression, more muscles must be sampled, after being selected based on their innervation (see Figure C2-6), to verify the diagnosis and to establish the exact root(s) compressed. In contrast to limb muscles, fibrillation potentials in the paraspinal muscles are not useful in the diagnosis of the specific compressed root, though they often confirm that the lesion is intraspinal. This is due to observations that each primary posterior ramus has a highly variable segmental innervation that may extend up to 6 segments beyond the vertebral level of its root exit.
Table C2-4 Suggested Muscles to be Sampled in Suspected Lumbosacral Radiculopathy
| Muscle | Root Innervation* |
|---|---|
| Tibialis anterior | L4, L5 |
| Medial gastrocnemius | S1, S2 |
| Flexor digitorum longus and tibialis posterior | L5, S1 |
| Extensor digitorum brevis | L5, S1 |
| Vastus lateralis and medialis | L2, L3, L4 |
| Biceps femoris (short or long head) | L5, S1 |
| Gluteus medius and tensor fascia lata | L5, S1 |
| Mid: lumbar paraspinal | |
| Low: lumbar paraspinal |
Define the Age and Activity of the Radiculopathy
As time elapses, collateral sprouting from intact axons results in MUAPs with polyphasia and satellite potentials. These MUAPs, usually seen after 2 to 3 months from acute injury, are often unstable by showing moment-to-moment variation in morphology. With further time, MUAPs with high amplitude and long duration dominate, reflecting a more complete reinnervation and the chronicity of the root compression.
Define the severity of the radiculopathy
Electrodiagnostic Findings in Lumbosacral Radiculopathies
In patients with unilateral lumbosacral radiculopathy on needle EMG, signs of denervation are frequently present in a contralateral root, usually of the same myotome, and despite the lack of clinical manifestations. This is caused by the unique anatomy of the cauda equina (not present in the cervical and thoracic regions), in which more than one root can be compressed by a single disc herniation. Thus, it is essential to sample a few contralateral muscles, at least of the same affected myotome, in patients with severe lumbosacral radiculopathy.
L2, L3, L4 Radiculopathies
L5 Radiculopathy
It is not unusual for patients with severe L5 radiculopathy, in whom significant motor axon loss has occurred, to present with footdrop. In cases associated with motor axon loss, motor nerve conduction studies reveal low-amplitude peroneal CMAPs, recording extensor digitorum brevis and/or tibialis anterior. This mimics a peroneal mononeuropathy, especially of the deep branch. In these situations, denervation in the tibialis posterior and/or the flexor digitorum longus excludes a selective peroneal lesion because these are tibial innervated muscles (Table C2-6). Thus, needle EMG of the tibialis posterior and/or the flexor digitorum longus is essential in all patients presenting with footdrop.
Table C2-6 Electrophysiological Differentiation Between L5 Radiculopathy and Peroneal Mononeuropathy
| L5 Radiculopathy | Peroneal Mononeuropathy | |
|---|---|---|
| Nerve Conduction Studies | ||
| Peroneal CMAP recording extensor digitorum brevis | Normal or low amplitude | Conduction block at fibular head or low amplitude or both |
| Peroneal CMAP recording tibialis anterior | Normal or low amplitude | Conduction block at fibular head or low amplitude or both |
| Superficial peroneal SNAP | Frequently normal | Low/absent; normal in deep peroneal or purely demyelinating lesions |
| Needle EMG | ||
| Tibialis anterior | Abnormal | Abnormal |
| Extensor digitorum brevis | Abnormal | Abnormal |
| Extensor hallucis | Abnormal | Abnormal |
| Peroneus longus | Abnormal | Abnormal; normal in selective deep peroneal lesions |
| Tibialis posterior | Abnormal | Normal |
| Flexor digitorum longus | Abnormal | Normal |
| Gluteus medius | May be normal | Normal |
| Tensor fascia lata | May be normal | Normal |
| Lumbar paraspinals | May be normal | Normal |
S1, S2 Radiculopathy
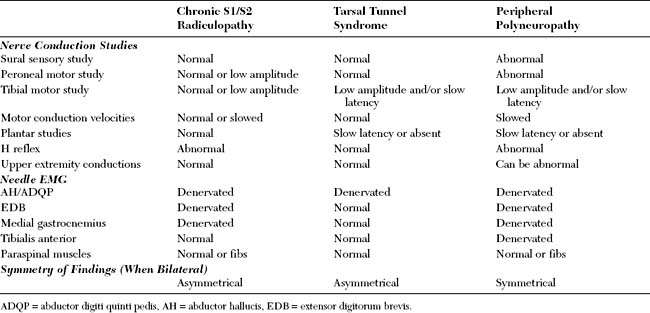
S1 radiculopathy is common and often due to posterolateral disc herniation (see Figure C2-3). It is difficult to distinguish S1 from S2 radiculopathy (though the latter is rare), because their myotomal representations overlap almost completely. As with the L5 root, the segmental distribution of the S1 root is diffuse, with both proximal and distal muscle representation. Again, here distal muscles (below the knee), such as the medial and lateral heads of the gastrocnemius, soleus, or abductor hallucis, are more likely to reveal fibrillation potentials. Unfortunately, these S1/S2 muscles are all of tibial innervation, and fibrillation potentials must be found in other nerve distributions. The extensor digitorum brevis is the only distal peroneal muscle with substantial S1 innervation, but this muscle is subject to atrophy and chronic denervation, probably from local trauma. Proximal muscles such as the biceps femoris or gluteus maximus can be useful, but these are more subject to sprouting, which abolishes fibrillation potentials.
It is generally accepted that the H reflex is helpful in the diagnosis of S1 radiculopathy. The tibial H reflex is the clinical counterpart of the ankle jerk; it tests the integrity of the entire S1 reflex arc, including the Ia afferent fibers, the spinal cord S1 segment, and the alpha motor efferent fibers (see Chapter 3). It is the only test available within the routine EDX test that includes the preganglionic segment of the sensory fibers of the S1 root. The amplitude of the tibial H wave correlates well with the magnitude of the ankle jerk. Controversy continues regarding whether the amplitude or the latency asymmetry is more valuable in S1 radiculopathy. Although a unilaterally absent or abnormally low amplitude or slow latency is common in S1 radiculopathy, certain limitations exist:
As with other lumbosacral radiculopathies, bilateral S1/S2 radiculopathies are relatively common, and most are chronic. Because the symptoms usually are bilateral and involve the feet predominantly, these cases may imitate a peripheral polyneuropathy or bilateral tarsal syndromes. Differentiating these three entities requires meticulous EDX examination and is often difficult, especially in elderly patients (Table C2-7). In elderly patients, the sural SNAPs and H reflexes are frequently absent bilaterally, and the foot muscles may show denervational changes of unclear etiology.
Lumbar Canal Stenosis
The EDX findings in lumbar canal stenosis are extremely variable due to the variable level and degree of root(s) compression (see Figure C2-4). The abnormalities seen on EDX testing often mirror the variable clinical presentations in these patients which may vary from neurogenic claudication with normal neurologic examination, to severe disability with weakness, reflex changes, and sensory loss. The EDX findings in lumbar canal stenosis may manifest as one of the following scenarios:
Could an EMG Be Normal in a Patient With Definite Lumbosacral Radiculopathy?
FOLLOW-UP
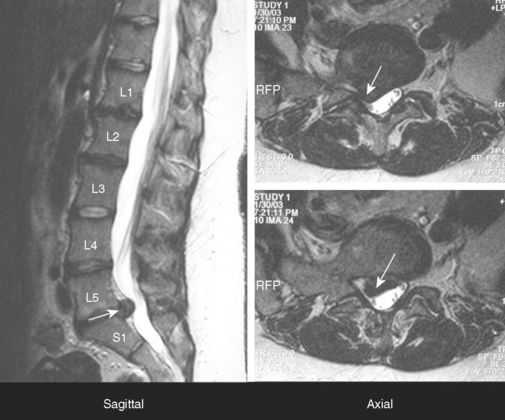
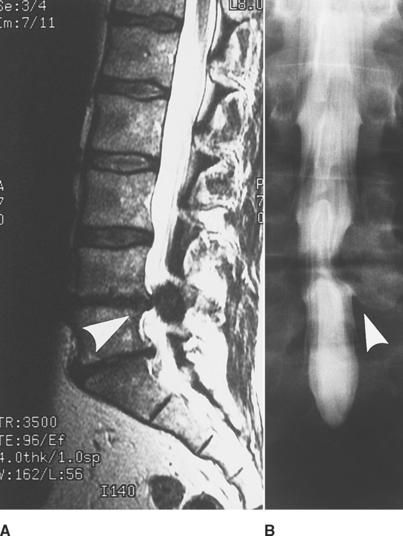
The patient underwent magnetic resonance imaging of the lumbar spine and a computed tomography/myelography (Figure C2-7). This revealed a large L4–L5 posterolateral disc herniation, with compression and displacement of the right L5 and S1 roots, and secondary canal stenosis at that level. The patient underwent an L4–L5 laminectomy and diskectomy. Four months later, he demonstrated significant improvement in the footdrop and did not require an ankle brace anymore. When seen 6 years later, he was completely asymptomatic.
Aminoff MJ, et al. Electrophysiological evaluation of lumbosacral radiculopathies: electromyography, late responses and somatosensory evoked potentials. Neurology. 1985;35:1514-1518.
Chu J. Lumbosacral radicular symptoms: Importance of bilateral electrodiagnostic studies. Arch Phys Med Rehabil. 1981;62:522.
Hardy RW, editor. Lumbar disc disease, 2nd ed., New York: Raven Press, 1992.
Katirji B, Weissman JD. The ankle jerk and the tibial H-reflex: a clinical and electrophysiological correlation. Electromyogr Clin Neurophysiol. 1994;34:331-334.
Lajoie WV. Nerve root compression: correlation of electromyographic, myelographic and surgical findings. Arch Phys Med Rehabil. 1972;53:390-392.
Levin KH. L5 radiculopathy with reduced superficial peroneal sensory responses: intraspinal and extraspinal causes. Muscle Nerve. 1998;21:3-7.
Levin KH. Electrodiagnostic approach to the patient with suspected radiculopathy. Neurol Clin N Am. 2002;20:397-421.
Nishada T, et al. H reflex in S-1 radiculopathy: latency versus amplitude controversy revisited. Muscle Nerve. 1996;19:915-917.
Phillips LH, Parks TS. Electrophysiologic mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve. 1991;14:1213-1218.
Sunderland S. Nerves and nerve injuries, 2nd ed. New York: Churchill Livingstone, 1978.
Tonzola RF, et al. Usefulness of electrophysiological studies in the diagnosis of lumbosacral root disease. Ann Neurol. 1981;9:305-308.
Tsao B, Levin KH, Bodner R. Comparison of surgical and electrodiagnostic findings in single root lumbosacral radiculopathies. Muscle Nerve. 2003;27:60-64.
Wilbourn AJ, Aminoff MJ. The electrophysiologic examination in patients with radiculopathies. Muscle Nerve. 1988;11:1099-1114.