Chapter 1 Cartilage Morphology
Hyaline cartilage provides the diarthrodial joint with a low-friction surface, resilience, and compressive stiffness, and this unique tissue is, under normal conditions, wear resistant.
Loss of cartilage function may lead to a painful joint with a decreased mobility. Many factors (epidemiological, biochemical, and morphological) are associated with cartilage destruction. However, only trauma is known directly to cause osteoarthritis.1 It is well known that once the cartilaginous tissue has been destroyed, the intrinsic reparative ability is poor. Therefore, it is of uttermost importance to increase knowledge about the cartilage, the tissue reaction to trauma, and the intrinsic attempts to repair the defects as well as extrinsic methods.
Cartilage Biochemistry and Morphology
The collagen fibers are responsible for the structure of cartilage and consist mainly of collagen type II. They are highly cross-linked via collagen type IX fibers.2
Chondrocytes are the producers of the surrounding ground substance: matrix.
The cells have different appearances depending on where in the cartilage they are situated. The cells in the top layer appear flattened, whereas the cells in the deeper layer are more rounded and aligned along vertically orientated type II collagen.3
Proteoglycans are large protein-polysaccharide molecules making up 5% to 10% of the wet weight of the cartilage.4 They are composed by chains of the glycosaminoglycans keratan sulfate and chondroitin sulfate covalently bound to a central protein core molecule. Large aggregates are formed with several proteoglycan monomers via a link protein connecting the central protein cores to a chain of hyaluronic acid.
All the components of the proteoglycan aggregates are synthesized by the chondrocytes.
The proteoglycans are unevenly distributed throughout the cartilage layers with the highest concentration in the middle part and the lowest concentration in the superficial layers.5 The proteoglycans give the cartilage its elasticity and resilience.
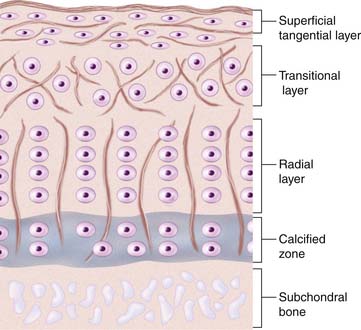
There is a difference in cartilage composition between the cartilage surface and the subchondral bone plate. These structural differences give rise to four separate layers or zones (see Fig. 1-1).
In the top zone, the superficial zone, there is first a cell-free fibril-layer, called the lamina splendens.6 Beneath this thin layer, chondrocytes are dispersed in an elongated manner parallel to the surface, reflecting as well the tangential orientation of the collagen fibers. This is the tangential layer.
The calcified zone provides an important transition to the less resilient subchondral bone. For a long time this was regarded more or less as an inactive zone, until Hunziker (1992)7 noted that also the chondrocytes here could take up and incorporate (35S) sulfate into the pericellular and territorial matrix. Hunziker speculated that, following trauma, the metabolic activity here becomes temporarily impaired.7
Regarding experimental animals, it is important to know that it is only in adult animals that the division into zone I to zone III is possible.8 In the immature animal, the cells are more randomly distributed with a gradient in cell size from the top to the calcified zone, with the cells in the deeper parts being largest. Thus, the articular cartilage organization during prepubertal growth imitates the structure of the growth plate, and during that time the biomechanical properties of the cartilage change with an increase in stiffness and in shearing and compressive resistance.7,9
Metabolic Events in the Cartilage
The majority of the proteoglycans have a life span of about 600 days, but a small proportion of the proteoglycans in adult cartilage act as a fast fraction with a half-life of about 8 days. The proteoglycans are thus also more vulnerable to enzymatic degradation.10,11
This is a sophisticated and well-balanced process regulated by the chondrocytes, and a disturbance of any of these events could lead to destruction of the cartilaginous matrix. This happens in osteoarthritis where an early sign is an imbalance in synthesis and degradation of the matrix.
Pathology Evaluation
It is necessary to know the exact location from where the biopsy is taken and how it was taken. The International Cartilage Society (ICRS) has developed a mapping system, which is useful to utilize when describing the location.12 A biopsy should be taken in the following manner:
Biopsies can be either wax embedded for sections or frozen for cryo sections.
As recommended by the ICRS Histology Endpoint Committee,13 cartilage morphology can be evaluated by hematoxylin and eosin staining. Normal and polarized light are used.
Molecular Biology
In situ hybridization studies the genes that the chondrocyte expresses and indicates what the cells are capable to synthesize.14
Histological Repair Scores
Various histological scoring systems exists for analysis of osteoarthritic or normal, in vivo repaired, or tissue-engineered cartilage, but only a few have been validated:15
Imaging Evaluation of Cartilage Repair
To get a better total idea of the repair area, imaging techniques need to be used.
Arthrography combined with computed tomography (CT) provides information about the contour and surface characteristics of the cartilage and cartilage repair.23 Combined with three-dimensional reconstruction of the bone structure, useful information is yielded.
The main advantages of MRI as a method for cartilage imaging are as follows:
Fat-suppressed three-dimensional gradient echo (3D-GRE)24 allows the exact description of the thickness and surface of cartilage.
T2-weighted (dual) fast spin echo (FSE) techniques with or without fat-suppression24 give the information about the normal and abnormal internal structure of hyaline cartilage.
dGEMRIC25 relies on intravenous injection of a negatively charged MR contrast agent and the acquisition of a T1 map after equilibration of the contrast agent in the cartilage to estimate the glycosaminoglycan distribution within cartilage.
Quantitative T2 MR26 mapping of articular cartilage is a noninvasive imaging technique that has the potential to characterize hyaline articular cartilage and repair tissue.24 Normal articular cartilage demonstrates an increase in T2 values from the subchondral bone to the articular surface that has been correlated with type II collagen fiber matrix organization (anisotropy) in these zones.
Qualitative and quantitative T2 mapping helped differentiate hyaline cartilage from reparative fibrocartilage after cartilage repair at 1.5-T MR imaging.24
Cartilage T2 mapping at 1.5-T MR imaging shows promise as a noninvasive tool to study and differentiate cartilage composition after surgical cartilage repair procedures.26
One MRI technique, magnetization transfer (MT) imaging,27 is known to generate a useful image contrast in cartilage in vitro, which is sensitive to the macromolecular content of the cartilage. Palmieri and coworkers27 have studied cartilage repair with microfracture, comparing the repair with autologous chondrocyte implantation (ACI) repair. The differences between damaged and repaired cartilage magnetization transfer ratio (MTR) were too small to enable MT imaging to be a useful tool for postoperative follow-up of cartilage repair procedures. However, there was an evolution toward normal MTR values in the cartilage repair tissue (especially after ACI repair), while the MTR of microfracture repaired cartilage still showed a significant difference from normal cartilage at a 24-month follow-up.27
Furthermore, another technique called T1rho can potentially be used to noninvasively to quantitatively assess the biochemical and biomechanical characteristics of articular cartilage in humans during the progression of osteoarthritis.28
1. O’Connor B.L., Brandt K.D. Neurogenic factors in the ethiopathogenesis of osteoarthritis. Rheum Dis North Am. 1993;19:581-605.
2. Mow V.C., Ratcliffe A., Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67-97.
3. Jeffrey A.K., Blunn G.W., Archer C.W., Bentley G. Three-dimensional collagen architecture in bovine articular cartilage. J Bone Joint Surg Br. 1991;73(5):795-801.
4. Muir I.H.M., Sokoloff L., editor., the joints and synovial fluid. The chemistry of the ground substance of joint cartilage, Academic Press, New York, 27-94, 1980.
5. Ratcliff A., Fryer P.R., Hardingham T.E. The distribution of aggregating proteoglycans in articular cartilage. Comparisons of quantitative immunoelectron microscopy with radio-immuno assay and biochemical analysis. J Histochem Cytochem. 1984;32:193-201.
6. MacConaill M.A. The movement of bone and joints: the mechanical structure of articulating cartilage. J Bone Joint Surg Br. 1951;33B:251-257.
7. Hunziker E. Articular cartilage structure in humans and experimental animals. In: Kuettner K.E., Schleyerbach R., Peyron J.G., Hascall V.C., editors. Articular cartilage and osteoarthritis. New York: Raven Press; 1992:183-199.
8. Schenk R.K., Eggli P.S., Hunziker E.B. Articular cartilage morphology. In: Kuettner K.E., Schleyerbach R., Hascall V.C., editors. Articular cartilage biochemistry. New York: Raven Press; 1986:3-22.
9. Mow V.C., Rosenwasser M.P. Articular cartilage biomechanics. In: Woo S.L.Y., Buckwalter J.A., editors. Injury and repair of musculoskeletal soft tissues. Park Ridge II, Am Acad Orthop Surg; 1987:427-463.
10. Mankin H.J., Lippiello L. The turnover of adult rabbit articular cartilage. J Bone Joint Surg Am. 1969;51((8)A):1591-1600.
11. Hall D., Mankin H.J., Lippiello L. Turnover of proteoglycan of adult rabbit articular cartilage. Trans Orthop Res Soc. 1977;2:10.
12. Brittberg M., Winalski C.S. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(suppl 2):58-69.
13. Mainil-Varlet P., Aigner T., Brittberg M., Bullough P., Hollander A., Hunziker E., Kandel R., Nehrer S., Pritzker K., Roberts S., Stauffer E. International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A(suppl 2):45-57.
14. Robers S., Evans H. Microscopic assessment of cartilage and cartilage repair. In: Zanasi S., Brittberg M., Marcacci M., editors. Basic science, Clinical repair and reconstruction of articular cartilage defects; current status and prospects. Bologna: Timeo Editore; 2006:123-131.
15. Rutgers M., van Pelt M.J., Dhert W.J., Creemers L.B., Saris D.B. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010 Jan;18(1):12-23. Epub 2009 Sep 2
16. Mankin H.J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523-537.
17. Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A., et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13-29.
18. O’Driscoll S.W., Keeley F.W., Salter R.B. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68(10):1017. 35
19. Pineda S., Pollack A., Stevenson S., Goldberg V., Caplan A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat (Basel). 1992;143:335. e40
20. Wakitani S., Goto T., Pineda S.J., Young R.G., Mansour J.M., Caplan A.I., et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579-592.
21. Mainil-Varlet P., Van Damme B., Nesic D., Kuntsen G., Kandel R., Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010 May;38(5):880-890. Epub 2010 Mar 4
22. Mainil-Varlet P., Rieser F., Grogan S., Mueller W., Saager C., Jakob R.P. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthritis Cartilage. 9(Suppl A), 2001. S6-15
23. Lecouvet F.E., Dorzée B., Dubuc J.E., Vande Berg B.C., Jamart J., Malghem J. Cartilage lesions of the glenohumeral joint: diagnostic effectiveness of multidetector spiral CT arthrography and comparison with arthroscopy. Eur Radiol. 2007 Jul;17(7):1763-1771. Epub 2006 Dec 21
24. Trattnig S., Domayer S., Welsch G.W., Mosher T., Eckstein F. MR imaging of cartilage and its repair in the knee–a review. Eur Radiol. 2009 Jul;19(7):1582-1594. Epub 2009 Mar 13. Review
25. Potter H.G., Chong le R. Magnetic resonance imaging assessment of chondral lesions and repair. J Bone Joint Surg Am. 2009 Feb;91(suppl 1):126-131.
26. White L.M., Sussman M.S., Hurtig M., Probyn L., Tomlinson G., Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006 Nov;241(2):407-414.
27. Palmieri F., De Keyzer F., Maes F., Van Breuseghem I. Magnetization transfer analysis of cartilage repair tissue: a preliminary study. Skeletal Radiol. 2006 Dec;35(12):903-908.
28. Wheaton A.J., Dodge G.R., Elliott D.M., Nicoll S.B., Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005 Nov;54(5):1087-1093.