10
Cartilage Healing
1. Discuss the composition and function of articular cartilage.
2. Identify common causes of injury to articular cartilage.
3. Describe the sequence of healing and the extent of intrinsic repair of articular cartilage.
4. Define invasive and noninvasive techniques of stimulating articular cartilage repair.
5. Define and describe the composition and function of fibrocartilage.
6. Identify and discuss common mechanisms of injury to fibrocartilage.
7. Describe the mechanisms of intrinsic healing of the meniscus.
ARTICULAR CARTILAGE
Composition
Articular cartilage covers the ends of bones of synovial joints. It is composed primarily of water (approximately 65% to 80%),11,13 which provides for load deformation of the cartilage surface.16 The tensile strength of articular cartilage depends on type II collagen, which is approximately 20% of the total composition of articular cartilage.19 Proteoglycans contribute 10% to 15% of the structure of articular cartilage. These proteoglycans are made up of glycosaminoglycans, which are in part responsible for bearing the compressive strength of articular cartilage. Finally, chondrocytes (mature cartilage cells) make up 5% of the articular cartilage.16,19
Articular Cartilage Zones
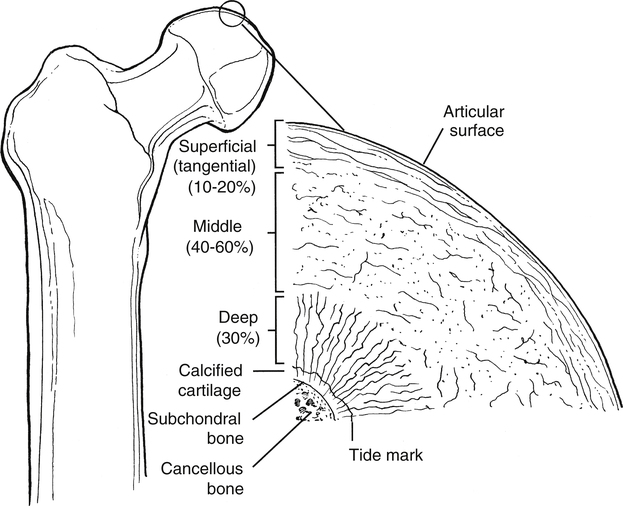
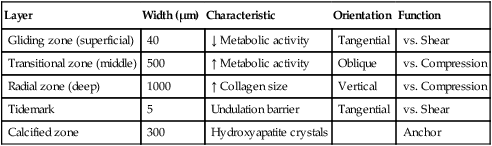
Articular cartilage is not homogeneous. The composition of articular cartilage varies considerably among four distinct zones. The superficial zone of articular cartilage is composed of water and parallel, highly organized collagen fibrils with very limited concentration of proteoglycans.14 The middle or transitional zone of articular cartilage demonstrates randomly arranged, large diameter collagen and rounded chondrocytes.14 The deep zone is rich in proteoglycans and lowest in water concentration. In this zone, collagen is large with a more organized structure that is arranged vertically.14 The zone of calcified cartilage is the deepest zone that separates cartilage tissue from subchondral bone. This small distinct layer is composed mainly of cells, cartilage, matrix, and inorganic salts (Fig. 10-1 and Table 10-1).
Table 10-1
| Layer | Width (μm) | Characteristic | Orientation | Function |
| Gliding zone (superficial) | 40 | ↓ Metabolic activity | Tangential | vs. Shear |
| Transitional zone (middle) | 500 | ↑ Metabolic activity | Oblique | vs. Compression |
| Radial zone (deep) | 1000 | ↑ Collagen size | Vertical | vs. Compression |
| Tidemark | 5 | Undulation barrier | Tangential | vs. Shear |
| Calcified zone | 300 | Hydroxyapatite crystals | Anchor |
From Brinker MR, Miller MD: Fundamentals of orthopaedics, Philadelphia, 1999, Saunders.
Collagen in Articular Cartilage
Articular cartilage is an extremely unique biological tissue with both durable and permeable characteristics. Collagen represents more than 50% of the entire dry weight of articular cartilage.14 The vast majority of collagen in articular cartilage is type II; however, the extracellular matrix also contains types IV, V, VI, X, and XL.
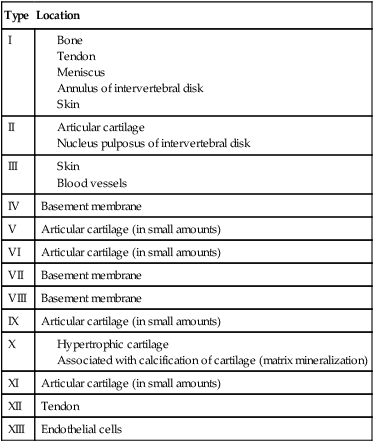
Clinicians must recognize the composition of various collagen types to fully appreciate the remarkable resilience of articular cartilage tissue. Collagen in general contains various amounts of proline, hydroxyproline, and hydroxylysine. The proline content of collagen provides a structure that is highly resistant to tensile forces. Conversely, hydroxyproline composition of collagen provides for the compressive stability of articular cartilage. Articular cartilage is composed of collagen types that are distinct in organization, structure, and fiber arrangement that are consistent with the zones of cartilage and their unique physiologic requirements of compression, tension, stiffness, strength, and durability (Table 10-2).14
Table 10-2
| Type | Location |
| I |
From Brinker MR, Miller MD: Fundamentals of orthopaedics, Philadelphia, 1999, Saunders.
Vascular Supply of Articular Cartilage
Vascularized tissues heal in an organized, predictable fashion. Trauma to vascular tissue incites an intense cascade of events characterized by hemorrhage, inflammation, and fibrin clot formation. Angiogenesis, or neovascularization, is the hallmark of intrinsic repair through mobilization of repair molecules and undifferentiated cells capable of synthesizing matrix and new tissue.14
Conversely, articular cartilage is a nonhomogeneous and avascular structure that lacks the ability to stimulate, regulate, or organize intrinsic repair.1 Without an intense vascular response to injury, articular cartilage cannot form a fibrin scaffold or mobilize cells to repair the defect. Chondrocytes are essentially trapped within the dense extracellular matrix and are therefore incapable of traveling to the damaged site via a vascular access channel.14
Function
The viscoelastic structure of articular cartilage, by virtue of its component parts of collagen, water, and proteoglycans, makes articular cartilage incredibly durable.16,19 Generally, articular cartilage is only 2 to 4 mm thick, yet it is capable of bearing compressive loads many times greater than body weight.24 Articular cartilage is resistant to wear; has an extremely low coefficient of friction; and is responsible for influencing and dissipating compression, shear, and tension forces within synovial joints.1,16,19,24
Articular cartilage is also permeable. The chondrocytes within the cartilage must receive nutrition to remain viable. The synovial fluid surrounding the articular cartilage provides the necessary nutrients through diffusion, convection, or both.19 Diffusion and convection are achieved through joint motion and normal physiologic weight bearing. Therefore normal joint motion is needed to maintain the cartilage integrity, fluid movement (lubrication between articulating surfaces), and nutrition of hyaline cartilage.19
Immobilization and Response to Healing
Articular cartilage requires physiologic stress (e.g., cyclical compression) to maintain its unique environment as a strong, tough, fatigue-resistant, permeable, and low friction tissue.14,15 The biochemical components of proteoglycans or glycosaminoglycans (GAGs), chondrocytes, matrix-molecules, and collagen significantly contribute to its structure, composition, and mechanical properties.14,15
Just as collagen is varied and distinct among the zones of articular cartilage, so are proteoglycans distributed in different concentrations between zones. Because these proteoglycan molecules—including chondroitin sulfate, keratan sulfate, and dermatan sulfate—bind and attract water (hydrophilic), their concentration and distribution among zones influence the various mechanical wear characteristics of articular cartilage.14,15 The removal of normal physiologic loading, unloading, and joint motion have profoundly negative effects on the biochemical and mechanical characteristics of articular cartilage.14,15
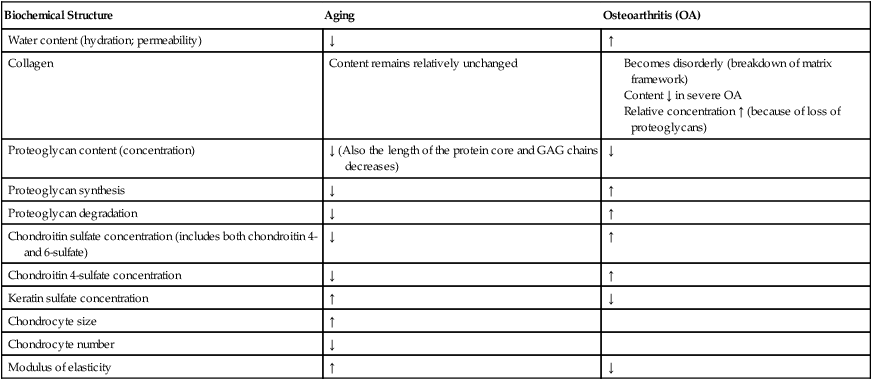
The significance of articular cartilage atrophy and degeneration is related to the magnitude and duration of immobilization. Joint contact surfaces suffer greater degenerative changes than noncontact areas of articular cartilage (Table 10-3).14,15
Table 10-3
Biochemical Changes of Articular Cartilage
| Biochemical Structure | Aging | Osteoarthritis (OA) |
| Water content (hydration; permeability) | ↓ | ↑ |
| Collagen | Content remains relatively unchanged |
GAG, Glycosaminoglycan; ↑, increased; ↓, decreased.
From Brinker MR, Miller MD: Fundamentals of orthopaedics, Philadelphia, 1999, Saunders.
Chondrocyte necrosis and subchondral bone degenerative lesions occur with prolonged rigid immobilization. Generally, immobilization and lack of physiologic stress cause a reduction in the synthesis and concentration of proteoglycans, which ultimately leads to surface fibrillation, fissures, and ulceration of the various zones of articular cartilage.14,15
Injury
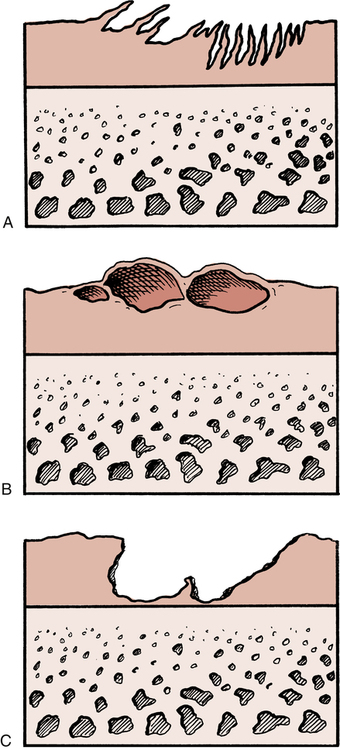
Articular cartilage can be damaged in many ways.1,19,24 Erosion and degeneration of the articular surface can be seen clinically in patients ranging from young athletes to the elderly. Causes of degenerative joint disease include related joint instability, blunt trauma, repetitive overloading, and immobilization.24 Articular cartilage degeneration is generally characterized by three progressively overlapping degenerative events (Fig. 10-2).24 Initially the hyaline cartilage begins to fray or fibrillate. Progressive destruction leads to blistering of the articular surface. Further joint deterioration leads to splitting or clefting (fissuring) of the surface. This affects the deeper layers of cartilage and eventually progresses to denuded bone.24 Although blunt trauma, progressive friction abrasion, and a focal concentration of weight-bearing forces mechanically erode articular cartilage, joint immobilization does not cause these mechanical changes. However, joint immobilization may lead to loss of the load-bearing structural compression-resistant component GAGs.19 Such loss is related to decreased normal joint loading and motion, which is needed for cartilage nutrition.
Healing and Repair
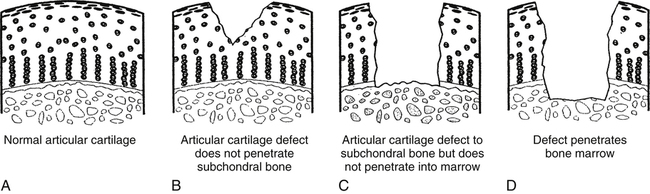
Articular cartilage defects heal differently, depending on the extent or depth of the injury.1,16,19,24 Less serious, more superficial lesions of articular cartilage do not spontaneously remodel or heal as well as deeper or full-thickness injuries due to the lack of vascularization. Healing of these superficial layers occurs through proteoglycans and limited chondrocyte proliferation, but the strength, composition, and durability of this healing tissue are inferior to those of normal articular cartilage.19
Superficial articular cartilage defects do not heal as well as deeper injuries because these injuries do not stimulate an inflammatory reaction.19 The thickness of the articular cartilage (2 to 4 mm) forms a barrier between the superficial layers of the cartilage and subchondral blood vessels, effectively eliminating any contact among fibrin, fibroblasts, and inflammatory response cells (neutrophils, macrophages). In effect, the chondrocytes do not adhere to the defect and do not fill the injury site with new tissue.
Deeper wounds or full-thickness injuries expose subchondral bone blood vessels to the defect site, which stimulates the acute inflammatory response. Full-thickness cartilage injuries heal spontaneously with large amounts of Type I collagen. In fact, 1 year after injury, approximately 20% of the repaired defect remains type I collagen.19 Although full-thickness injuries heal considerably better than superficial wounds, the quality of the scar formed within the defect remains inferior to normal articular cartilage because of the lack of type II collagen. The healed scar does not maintain its integrity over time.19
Nonoperative Management for Articular Cartilage Pathology
Continuous passive motion (CPM) is sometimes used in the care of articular cartilage injury. The benefits from the use of CPM appear to be limited to full-thickness hyaline cartilage defects.21,22 Salter and colleagues21,22 demonstrated that CPM used on full-thickness cartilage injury in rabbits showed healing of the defect with tissue resembling hyaline cartilage. Salter and associates,20–22 who believe that CPM can help stimulate chondrocyte formation, also found that using CPM with full-thickness hyaline cartilage injuries improves articular cartilage nutrition by enhancing fluid mechanics, inhibiting adhesions, and clearing the joint of noxious material.
Oral administration of glucosamine and chondroitin sulfate
Generally, glucosamine is an amino saccharide that participates in the synthesis of GAGs and proteoglycans by chondrocytes.6 Glucosamine is also an active substrate for the production of chondroitin sulfate, hyaluronic acid, and other proteoglycans in the articular cartilage matrix.6
Clinical trials and commercial products recommend 500 mg three times daily as an effective regimen.6 Glucosamine sulfate generally appears to have a more rapid onset of effects (4 to 8 weeks), as compared with chondroitin sulfate (3 to 6 months).6 Results in the literature are mixed with positive trends noted in patients with moderate to severe symptoms.7 Because glucosamine is considered a supplement, it is not regulated by the US Food and Drug Administration (FDA). This means that the purity and concentration of the product can vary despite claims made by the manufacturer.
Viscosupplementation through intraarticular hyaluronic acid injection
Hyaluronic acid is a proteoglycan constituent of articular cartilage matrix and synovial fluid. Both fibroblasts and synoviocytes synthesize hyaluronic acid into the joint space of the knee.26
The normal, nonpathologic human adult knee contains approximately 2 mL of synovial fluid with a hyaluronic acid concentration of 2.5 to 4.0 mg per mL.26 Osteoarthritis creates a deficit in volume and molecular mass of hyaluronic acid.26 This reduction in hyaluronic acid causes lower viscosity, high stress concentration, and lowered elastic properties of the synovium and articular cartilage matrix.26
Injecting hyaluronic acid is thought to benefit the osteoarthritic knee by reducing pain and inflammation, replenishing decreased volume of hyaluronic acid, and stimulating intrinsic synovial synthesis of hyaluronic acid.26 Hyaluronic acid injections are shown to reduce inflammation by limiting prostaglandins, reducing circulating proinflammatory cytokine, and decreasing the release of arachidonic acid from synovial fibroblasts.26
Typically, commercially available hyaluronic acid preparations are injected into the affected knee joint once a week for a total of 3 weeks. At present, there is no consensus about the clinical efficacy of hyaluronic acid intraarticular injections. Several clinical trials have demonstrated no increased joint function or pain relief when compared with traditional nonsteroidal antiinflammatory agents or intraarticular steroidal preparations. However, clinical trials of hyaluronic acid injections versus placebo favor its use. The rate of side effects is approximately 1% per injection. Local site reaction of pain, warmth, and swelling are most common and last 1 to 2 days.26
Operative Management for Articular Cartilage Pathology
Various surgical interventions are at the disposal of the surgeon that are designed to stimulate an intense inflammatory response or to débride and irrigate (lavage) cartilage debris from within the joint (Fig. 10-3).
Débridement
Short-term temporary relief of symptomatic articular cartilage lesions can be achieved with arthroscopic lavage or débridement of cartilaginous debris. Essentially, arthroscopic débridement removes particles of cartilage, degradative enzymes such as MMPs, and proinflammatory cytokines, all of which contribute to the painful disability of articular cartilage osteoarthritic lesions. The benefit of arthroscopic débridement is often temporary because of the lack of a true inflammatory response or cellular proliferation. Joint lavage (irrigation) with saline inflow provides a substantial isolated benefit by removing tissue debris and inflammatory cells even without surgical intervention of articular shavers with suction.10
Microfracture
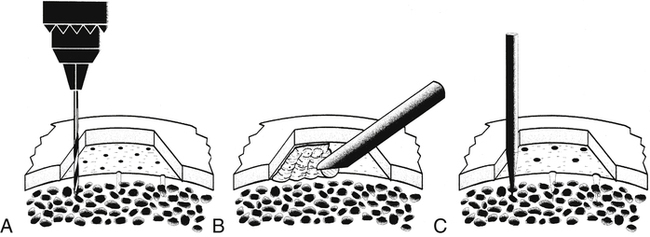
Articular cartilage repair is promoted when the injury site can go through the inflammatory process and when factors lead to chondrocyte proliferation. With some cartilage defects, surgically abrading or fracturing multiple small holes through the cartilage layers down to bone stimulates bleeding and initiates the healing process (Fig. 10-4).2
Initially, the bleeding response produces a “superclot” over the chondral defect. The desired outcome of this penetrating technique is the development of fibrocartilage consisting of type I collagen, which attempts to fill the defect. Being different from the type II collagen of normal articular cartilage, the repair tissue that forms from microfracture is histochemically and biochemically fragile and weak when compared with structurally intact articular cartilage. The repair also lacks the anatomically distinct layers of differing collagen types characteristic of normal articular cartilage, which provide additional protection from compression, shear, and tensile loads.2
Microfracture has been shown to provide short-term functional improvement, but long-term efficacy is still unknown.17 Outcomes are highest when performed on younger patients with cartilage defects that are surrounded by normal articular cartilage.2
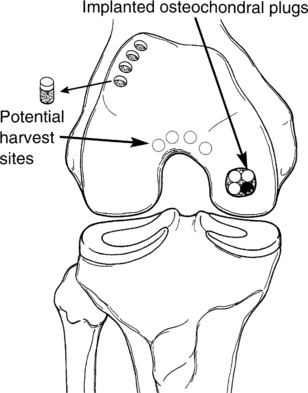
Arthroscopic osteochondral autografts
Full-thickness articular lesions (osteochondral) can be surgically filled with transplanted plugs of intact bone and articular cartilage using the technique of mosaicplasty or osteochondral autograft transplantation system (OATS) (Fig. 10-5).
The surgeon prepares the lesion by removing all nonviable surrounding tissue from the crater, thereby creating precise borders of the lesion. Multiple full-thickness bone plugs are surgically harvested from a relatively non–load-bearing surface of the femur, usually the superior lateral femoral condyle or, less often, the inferior condylar notch. Harvest site morbidity is a concern because full-knee ROM provides significant contact pressure of the mentioned harvest sites.10 These cylindrical osteochondral plugs become revascularized (incorporated) into the lesion. These “press-fit” plugs contain viable hyaline cartilage, which tends to survive over time and remains structurally stable.2,10
Several key factors may compromise the outcome of the OATS technique. The convex geometry of the femoral condyles contributes to a relative joint incongruence because the shape of the plugs may not match the surrounding surface with anatomic precision. In addition, the depth placement of the chondral plugs is critical because the donor plugs may collapse or settle over time or may be inserted with too much “pride” (the surface of the plugs may sit too high above the horizon of the chondral plate).10
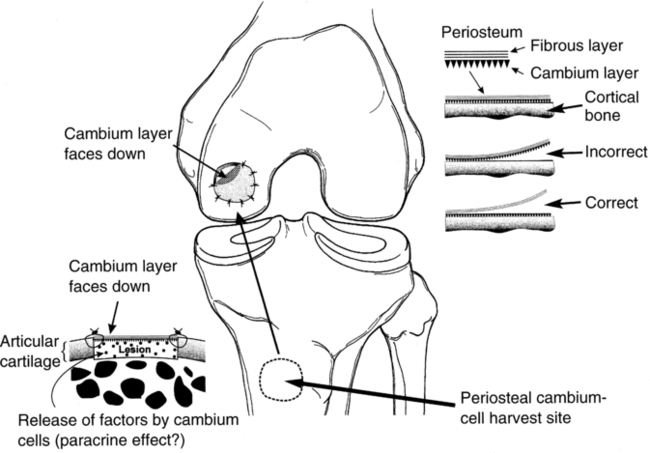
Autologous chondrocyte implantation
Because of substantial variations in collagen types, orientation, proteoglycan content, extracellular matrix composition, and avascularity of the four anatomically distinct zones of articular cartilage, a tissue implantation procedure is available to generate a biologic substitute tissue.2,10
The autologous chondrocyte implantation (ACI) procedure calls for two separate surgical interventions. First the surgeon harvests articular cartilage from the superomedial edge of the trochlea or the lateral edge of the intercondylar notch. This harvested tissue is sent to a specific laboratory, where the chondrocytes are enzymatically separated from the extracellular matrix, cultured, and multiplied. The second procedure typically occurs 6 to 18 weeks after harvesting. At this time, a periosteal patch may be harvested from the proximal medial tibia.2 However, concern regarding hypertrophy of the periosteum has lead to the development of second generation patches such as porcine derived collagen.5,8 This patch is then secured with fibrin glue and sutures over the defect. The cultured chondrocytes are injected under the patch to fill the defect with biologically active viable chondrocytes (Fig. 10-6).
FIBROCARTILAGE
Function
The menisci of the knee are semilunar (C-shaped) fibrocartilage tissues that have several functions. Generally the meniscus dissipates extreme compressive (vertical) loads. By virtue of its anatomic position within the knee and its collagen makeup, the meniscus acts as a mechanical buffer between the load-bearing surfaces of the tibia and the femur. The meniscus of the knee also functions as a shock absorber.3 Studies have shown that a knee without a meniscus has 20% less shock-absorbing capacity than normal knees.25
The meniscus may also function as a secondary restraint in joint stability. Several factors influence its effect, including ligament stability, joint surface congruency, and joint compression loads.3 Significant joint instability does not occur with an isolated, total meniscectomy. However, a meniscectomy combined with anterior cruciate ligament injuries produces profound joint instability.23
The meniscus also limits knee hyperextension as a passive restraint and functions in joint lubrication and nutrition.12 Normal physiologic joint motion promotes the lubricating effects of a thin layer of fluid between the joint surfaces. The meniscus may spread this lubrication medium during motion.18
Healing
The vascular anatomy of fibrocartilage profoundly influences the type of healing that occurs and the degree of remodeling, such as with articular cartilage. The peripheral borders of the medial and lateral meniscus of the knee are vascularized between 10% and 30% of the width of the tissue.3 If an injury occurs within a nonvascular region of the meniscus, spontaneous, intrinsic repair is not possible because no vascular supply communicates with the injury site. However, if the injury extends to the periphery where the cartilage is vascularized, healing is possible through the inflammatory–response mechanism.
Decisions regarding surgical repair or excision of meniscal tissue are based on the extent of the injury and location. If the tear is within the vascularized peripheral border (only 15% to 20% of all meniscal injuries occur within the vascularized bed of the meniscus), arthroscopic repair can be done by placing sutures in the meniscus to approximate the torn tissue.3 Surgeons refer to the following zone system of evaluating meniscal injuries9:
 Zone I: Both portions of the meniscus are torn within the vascularized periphery; “red-on-red”
Zone I: Both portions of the meniscus are torn within the vascularized periphery; “red-on-red”
 Zone II: One portion of the meniscus is torn within the vascularized periphery, whereas the other portion is in the avascular region; “red-on-white”
Zone II: One portion of the meniscus is torn within the vascularized periphery, whereas the other portion is in the avascular region; “red-on-white”
 Zone III: There is no blood supply on either side of the injury; “white-on-white”
Zone III: There is no blood supply on either side of the injury; “white-on-white”
Both red-on-red and red-on-white zones are considered reparable. When injuries occur in an avascular portion of the meniscus, the surgeon must perform either a partial or total meniscectomy. However, in animal studies,4 when an injury was present within the white-on-white nonvascularized Zone III area of the meniscus, researchers surgically created a “vascular access channel” to connect the blood supply of the periphery to the area of injury without circulation.3,4 These changes allowed blood vessels to migrate to the injury site and provided an avenue for repair.
GLOSSARY
Chondrogenesis Intrinsic cartilage repair.
Chondromalacia Softening of hyaline cartilage.
Hyaline cartilage A synonym for articular cartilage.
Hydrophilic A quality of a molecule that causes it to bind to and attract water.