Chapter 11 Cartilage Fragment Implantation
Introduction
Similar to autologous chondrocyte implantation (ACI) techniques, CAIS results in chondrocyte-based tissue repair but does not require ex vivo isolation and culture expansion of the cartilage tissue biopsy. Rather, donor cartilage is morselized in situ into small fragments using a customized harvesting device. The resulting increase in surface area of the cartilage fragments promotes cell outgrowth and expansion of the chondrocytes incarcerated within the dense cartilaginous matrix. Fragments are then incorporated on a chondro-conductive scaffold and affixed in the lesion site using bioresorbable staples in a single-stage procedure.1,2
Technical Overview (Knee Joint)
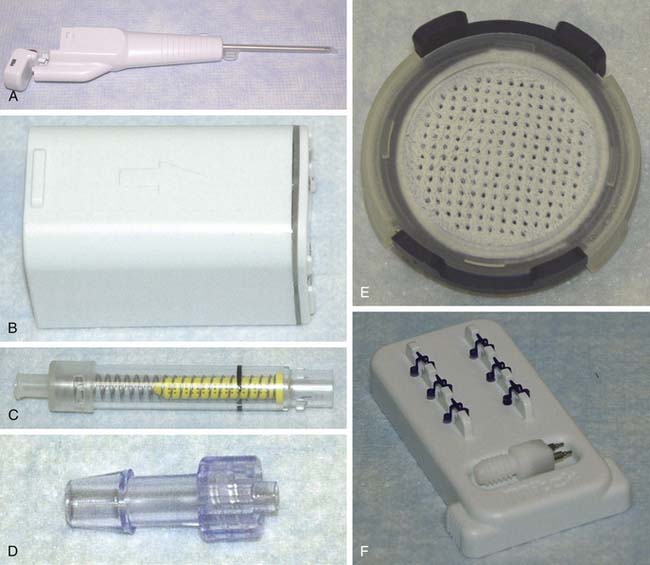
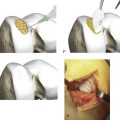
The CAIS procedure requires the utilization of two single-use items (a CAIS harvester and a disperser) and two implantable devices (a CAIS scaffold implant and staples) (Fig. 11-1, A-H).
CAIS Harvester
The CAIS harvester is a single-use device with a battery-operated motor. The harvester tip, with the aid of surgical vacuum, directs the morselized cartilage mixed with irrigation fluids into a tissue collector.
Harvest and Collection of Cartilage Fragments
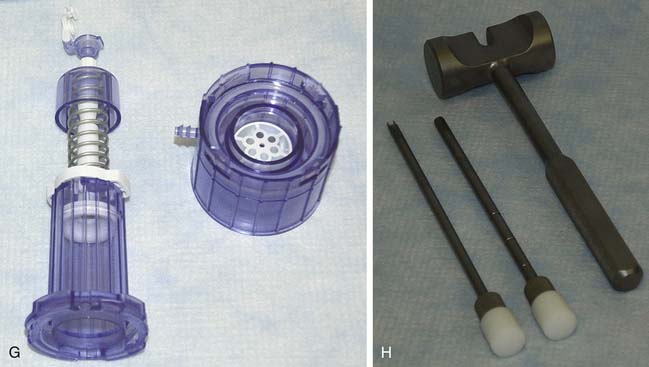
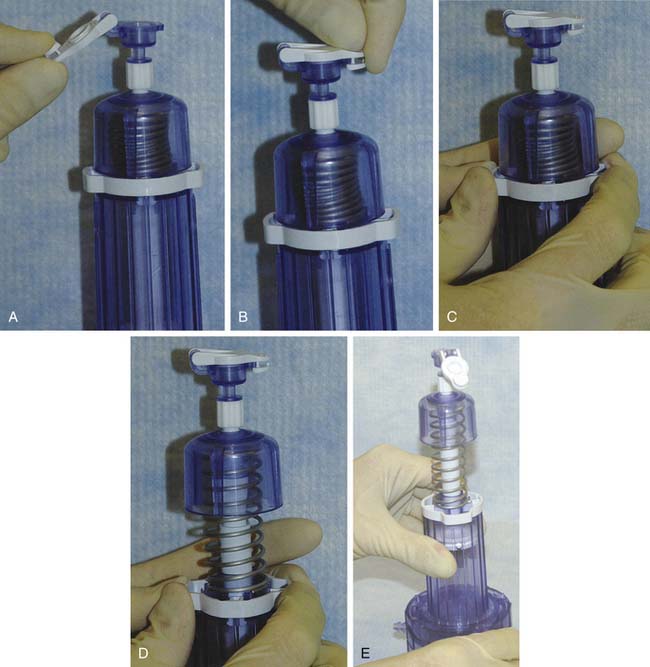
The CAIS disperser is prepared by placing the CAIS scaffold into the bottom of the disperser with the clear side of the cartridge ring facing up and the blue side facing down. The top tube is then assembled to the disperser base using alignment arrows and rotating clockwise to a hard stop. The disperser spring is then compressed by depressing the disperser cap until an audible click is heard (Fig. 11-2, A-E).
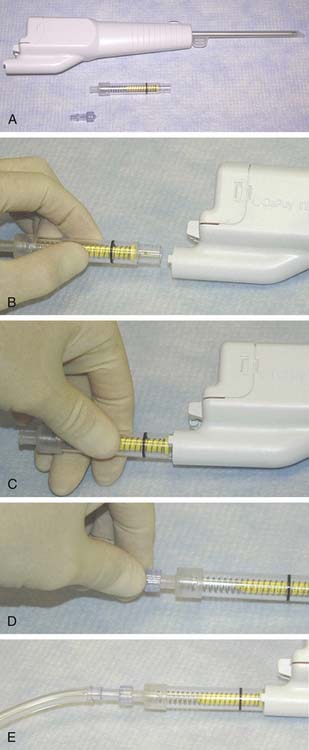
The CAIS harvester is prepared by loading the battery pack into the device. The fragment collector tube is inserted into the harvester and rotated clockwise to a hard stop (Fig. 11-3, A-E). Following attachment of surgical vacuum, the harvester is then inserted through the portal and placed with its tip near the cartilage harvest site (Fig. 11-4).
Full thickness cartilage is harvested down to the calcified layer. Cartilage is harvested until the flange of the yellow basket in the collector aligns with the black demarcation line on the tube, indicating a minimum harvest quantity of 200 mg has been collected.
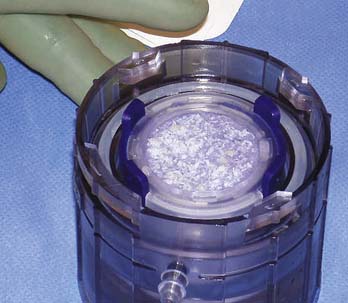
The collector is then removed and the disperser connection is closed. The disperser is activated by compressing the plunger retaining ring. The cap springs upward to suspend the cartilage fragments in the saline solution. The fragments then settle because of gravity and disperse uniformly across the surface of the scaffold. The dispersion step is performed two times.
The scaffold is examined to confirm a uniform tissue fragment dispersion (Fig. 11-5, A-E, and Fig. 11-6). Provided the distribution of fragments is acceptable, the scaffold is removed from base of the disperser.
Defect Preparation
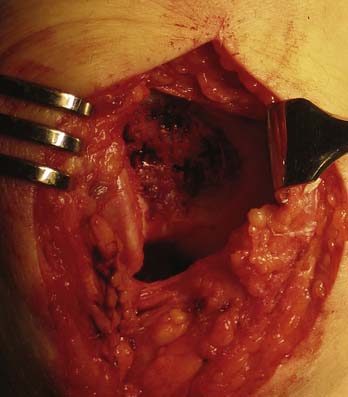
The joint is accessed through a miniarthrotomy. The lesion is debrided to healthy, orthogonal margins and templated as described in Chapter 3.
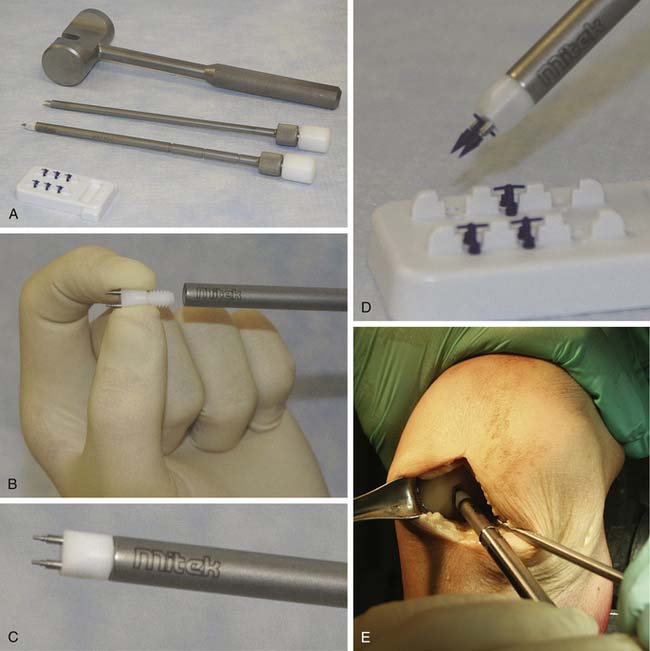
The template is used to trim the scaffold to the appropriate size and shape (Fig. 11-7). The implant should be slightly oversized to ensure it will completely cover the defect once staples have been placed. Finally, the implant is placed onto the debrided lesion with the fragment side facing the subchondral bone. The implant is held in a stable position using forceps, and pilot holes for the staples are made using the CAIS punch and mallet (Fig. 11-8a-e). With constant pressure on the scaffold, the punch is carefully removed.
Staples are loaded onto the CAIS inserter instrument by inserting the prongs of the inserter tip into a staple. The staples are then placed into the pilot holes using the CAIS inserter and mallet (approximately one staple per 0.75 cm2 of implant) (Fig. 11-8, A-E, and Fig. 11-9).
The scaffold implant is trimmed peripherally to ensure the implant is not proud, and the joint is exercised through a range of motion of flexion and extension to ensure no impingement from graft or staples.
1. Lu Y., Dhanaraj S., Wang Z., et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261-1270.
2. Frisbie D.D., LU Y., Kawcak C.E., DiCarlo E.F., Binette F., Mcllwraith C.W. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009 Nov;37(Suppl 1):71S-80S.