19 Carotid Endarterectomy
Introduction
The first description of a carotid endarterectomy (CEA) for stroke was published in the Lancet in 1954.5 Since that time, more clinical trials have been completed for CEA than for any other surgical procedure, first addressing the question of surgery versus best medical practice (clearly answered in favor of surgery in most cases) and, most recently, addressing the question of surgery versus endovascular carotid intervention. (These latest trials currently also favor open surgery versus endovascular intervention.)
We can review current indications for surgical intervention for carotid stenosis as follows. The first large cooperative trials randomized surgical reconstruction versus best medical therapy. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) established that symptomatic patients with internal carotid artery stenoses of 50% or more benefit from surgical resection of the offending lesion.1,3 The Asymptomatic Carotid Atherosclerosis Study (ACAS) and the European Asymptomatic Carotid Surgery Trial (ACST) trial6,7 provided compelling evidence that asymptomatic patients with 60% or more stenosis of the cervical internal carotid artery have a more favorable outcome with endarterectomy, provided that perioperative morbidity and mortality remain low (<3%),2 and that the patient otherwise has a 5-year life expectancy (the time needed to achieve a surgical benefit).
Surgical reconstruction (CEA) has now been randomized against carotid stenting (CAS) in several trials. To date, none has shown an advantage of CAS in either safety or efficacy. The latest trials, EVA-3S and SPACE, continue to demonstrate that open surgery is the safest and best treatment in most cases.28,29 The SAPPHIRE trial randomized “high-risk” asymptomatic and symptomatic carotid stenosis patients to CAS or CEA.32 Both techniques were effective, with statistically equal risk, in the treatment of symptomatic patients. For asymptomatic patients, there was a trend (p = NS) to lower risk with CAS. Questions have been raised, however, whether high-risk asymptomatic patients should be treated at all, since American Heart Association (AHA) guidelines have specified that surgical treatment is inappropriate for these patients.4 The only true answer to this question would be a study of CAS versus medical therapy in this group. No such study exists at present, although one large but non-randomized three-arm trial, the Japan Carotid Atherosclerosis Study (JCAS), will be forthcoming from Japan.
The National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Health (NIH)–funded Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) completed enrollment of 2522 patients (53% symptomatic, 47% asymptomatic) on July 18, 2008 using the same criteria as NASCET and ACAS.10,30 Final results of this trial have yet to be published. CREST and other similar trials in Europe (The International Carotid Stenting Study–Carotid and Vertebral Artery Transluminal Angioplasty Study [ICSS–CAVATAS 2]) and in Japan will hopefully provide level 1 evidence for therapeutic decision making. Curiously, the lead-in CREST data show that the risk of CAS, 30-day rates of stroke and death = 11.1%, is unacceptably high in elderly patients, aged >75, a group that was thought to represent excellent candidates for the procedure because of medical comorbidities.8,10
Indications for surgery
Most patients with symptomatic carotid artery stenoses of ≥50%, as measured by the NASCET method, are offered surgery at our institution. We offer endovascular counseling to these patients particularly if they have major medical comorbidities but we educate them that there is no level 1 evidence that stenting is equivalent to surgery in any but the highest-risk cases. The patients are counseled pre-operatively about surgical risks and benefits. There is good evidence that nondiabetic men with hemispheric symptoms have the greatest benefit from surgery; diabetes certainly increases the surgical risk, and amaurosis fugax appears to be a marker for lower stroke risk carotid lesions.26
Surgical technique
Principles
We have several cardinal principles of carotid reconstruction: complete knowledge of the patientElsevier Masson SASapos;s vascular anatomy, complete vascular control at all times, anatomic knowledge of the surgical field to prevent harm to surrounding structures, and finally, that a widely patent carotid repair free of technical errors must be achieved. Detailed descriptions of our carotid surgical technique with Hemashield patch graft can be found in multiple texts.9,11,13,14,16–20,22–24,26,27,31
Positioning
The patient is placed in the supine position with the head extended and turned away from the side of the operation (Figure 19–1). Folded sheets or pillowcases are placed beneath the shoulder blades to aid in extension of the neck; the preoperative arteriogram is reviewed to visualize the relationship between the internal and external carotid arteries (ICA and ECA) to choose the appropriate degree of head turning. Normally, the carotid vessels are superimposed in the anteroposterior plane, and rotating the head away from the operative side improves intraoperative visualization of the ICA by forcing it into a more lateral position. When the ICA is medial to the ECA (perhaps 10% of cases), no amount of head rotation will bring the ICA lateral enough, and the surgeon should recognize this and be prepared to dissect posterior and inferior to the ECA to find the “hidden” ICA and mobilize it laterally into the surgical position.
Operative technique
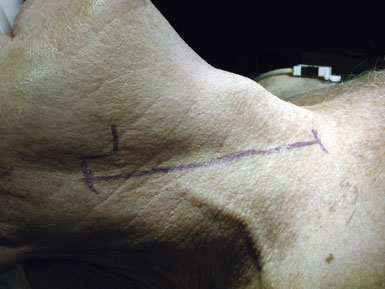
The cerebral angiogram is used to locate the height of carotid bifurcation, and the incision is planned accordingly. We prefer a linear incision along the medial border of the sternocleidomastoid muscle (see Figure 19–1), which may extend as high as the retroaural region and as low as the suprasternal notch, as needed. Following sterile prep (never a vigorous scrub) and drape, the skin and subcutaneous tissues are dissected sharply down through the platysma, unavoidably transecting the transverse cervical nerve. (For this reason, patients should be instructed preoperatively to expect numbness anterior to the incision, which usually resolves after 6 months.) Meticulous hemostasis is obtained using monopolar and bipolar cautery superficially, and bipolar alone once the dissection is deep to the sternomastoid muscle. The medial edge of the sternocleidomastoid muscle is located and kept in view by placing a superficial blunt-bladed self-retaining retractor and dissecting through the overlying fat (Figure 19–2). The medial retractor blade (of this and all retractors) remains superficial to prevent injury to the recurrent laryngeal nerve in the tracheo-esophageal groove, while the lateral blade may safely be placed more deeply, on or under the sternocleidomastoid muscle.
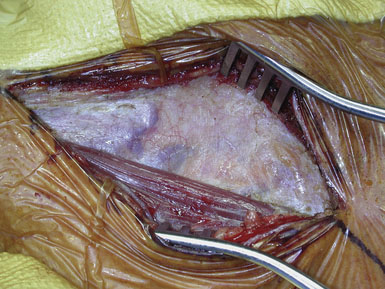
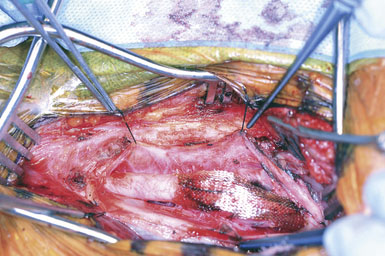
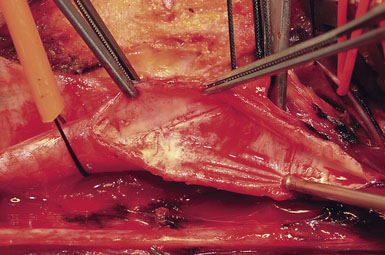
Attention is next focused on the middle of the incision where the dissection proceeds down the sternocleidomastoid muscle until the internal jugular vein is reached. When dissection is carried under the muscle, the surgeon must be careful not to injure the laterally-placed spinal accessory nerve by inadvertent transection or over-retraction. The jugular vein is a key landmark in the dissection, and it typically lies lateral, parallel, and slightly anterior to the ICA and common carotid artery (CCA). The medial jugular border is fully exposed, and the vein can be retracted as needed using blunt blades over a cottonoid pattie; it is crucial that the retractor blades are blunt to prevent vascular injury. In this portion of the dissection, the rather large common facial vein, and occasionally several smaller veins, are doubly ligated and divided. The common facial vein is a key landmark as it generally crosses the field at the level of the carotid bifurcation and carotid bulb (Figure 19–3).
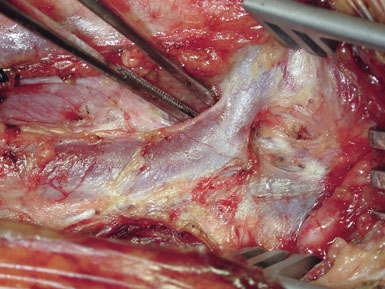
The CCA is typically encountered first, and at first visualization we instruct the anesthesiologist to administer 5000 units of intravenous heparin, which we do not reverse with protamine sulfate at the end of the procedure. The carotid is lifted and separated from surrounding structures upon opening of the carotid sheath with the aid of four sutures (4-0 silk) tied to more superficial tissue (Figure 19–4). The individual vessels (CCA, ICA, and ECA) are then dissected and encircled with 0 silk ties passed with a right-angle clamp. The carotid sinus is injected with 2 to 3 cc of 1% Xylocaine using a 25-gauge needle only if the anesthesiologist notes significant vital sign changes during vessel dissection in the region of the bifurcation. (We find this is very rarely necessary.) The CCA and ECA are not dissected free of the underlying tissue, in order to prevent postoperative kinking of these vessels; but they must, of course, be dissected circumferentially in the area where the silk ties or clamps are placed around them. The ICA is freed more extensively posteriorly, to facilitate full control of the vessel, and also where tacking sutures may later be placed.
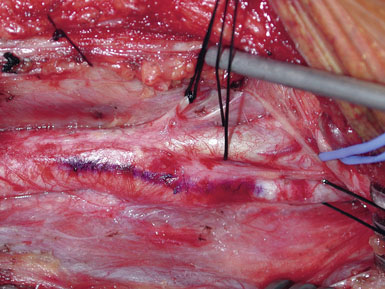
We like to use a sterile marking pen to draw out the proposed arteriotomy (Figure 19–5), lessening the risk of a jagged or curving suture line. We then ensure that the small spring-loaded Loftus shunt clamp will fit snugly around the ICA (Figure 19–6). Next, we notify the electroencephalograph (EEG) and SSEP technicians of the impending cross-clamp.12–21 For the past several years we have been monitoring with both modalities simultaneously and are currently acquiring data to determine which modality is most sensitive in detecting ischemia. Following baseline EEG/SSEP acquisition, the ICA is occluded first with a small low closing force bulldog clamp. (Occluding the ICA first protects the brain from any debris released by clamp application elsewhere.) The CCA is then occluded with a large soft-shoe Fogarty vascular clamp and the ECA is closed with a second, larger and stronger, straight bulldog clamp. The arteriotomy is begun in the CCA with a no. 11 scalpel blade, and Potts angled scissors are used to extend the incision once the lumen is fully visualized. One needs to take care not to insert the no. 11 scalpel blade too deeply to avoid injury to the back wall of the carotid (Figures 19–7 and 19–8). The blue-marked arteriotomy line is followed from the CCA up to the bifurcation, and then up the ICA until normal vessel is encountered. In severely stenotic vessels, identification of the lumen is not always straightforward, and great care must be taken not to cut the back wall of the carotid. The possibility of a false lumen should be excluded prior to shunt placement.
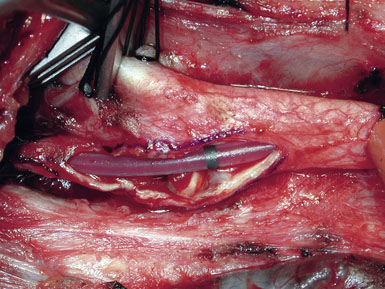
In our practice, EEG/SSEP changes in any form lead to placement of an intraluminal shunt (Figure 19–9). Our preference is the Loftus Carotid Endarterectomy Shunt (Integra LifeSciences, Plainsboro, NJ), which is a 15-cm straight silicone tube with a black marker band in the center, allowing for easy identification and correction of shunt migration; the shunt is tapered at the ends for easy insertion, and the proximal end has a bulb that facilitates anchoring by the Rummel tourniquet.15,24,25
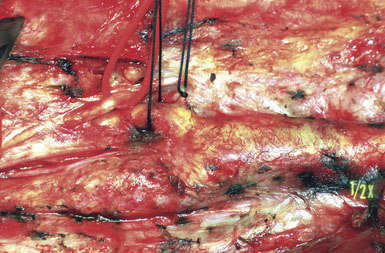
The CCA is cannulated first, and the shunt is secured by pulling up on the silk ties; a mosquito clamp is used to hold the rubber sleeve in place. The shunt tubing is held shut by a pair of heavy vascular forceps grasping the region of the black marker band. The tubing is cleared of air and debris by briefly releasing the vascular forceps, filling the shunt with fresh blood, and allowing confirmation of blood flow as well. The lumen of the ICA is visualized, and the distal end of the shunt is placed at the ICA orifice; the shunt is bled into the ICA to blow apart the vessel walls. The bulldog clamp on the ICA is then released and the shunt is advanced up the ICA until the black marker band lies in the center of the arteriotomy (see Figure 19–9). The shunt should slide up and down the ICA easily; no unnecessary force should be used, to avoid intimal damage and possible dissection. Our small Scanlan-Loftus custom clamp (Figure 19–6), or a Javid clamp, is then placed to secure the shunt in the distal ICA. A hand-held Doppler probe is used to confirm blood flow in the shunt.
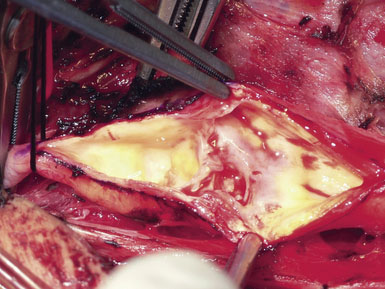
A Scanlan plaque dissector, or a Freer elevator, is used to dissect the plaque away from the arterial wall (Figure 19–10). The wall of the vessel is held with a fine vascular pickup while the instrument is moved from side to side, first developing a plane in the lateral wall of the arteriotomy. We go approximately halfway around the wall from the lateral side when the plaque comes easily, and then repeat the dissection on the medial side of the CCA. The plaque is then transected proximally with Potts scissors, leaving a smooth transition zone, since a clean feathering away of the plaque is almost never possible in the CCA. The proximal end may create a flap despite the direction of blood flow; hence, care should be taken to ensure that the CCA endpoint is not free-floating.
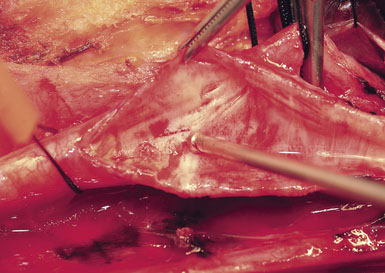
Next, the plaque is dissected away from the ICA in the same manner. However, the plaque usually feathers down much more smoothly at the ICA endpoint (Figure 19–11). Occasionally, a shelf of normal tissue protrudes and must be tacked down with suture.
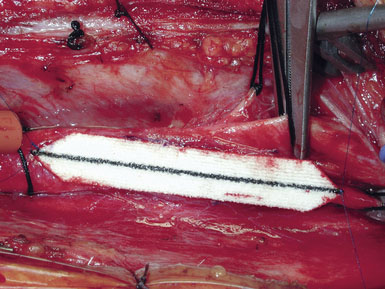
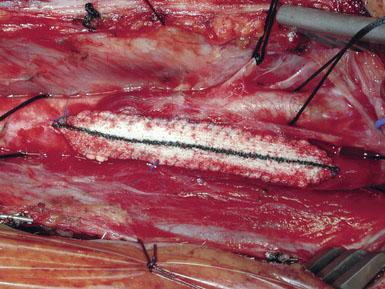
Occasionally, removal of a stony hard plaque, the most difficult type, results in areas of thinning of the posterior arterial wall where only the adventitial layer remains. One or two double-armed, interrupted stitches of 6-0 prolene are used to primarily plicate the thin spot, similar to a tacking suture (from the inside out, with the knot extraluminal). Following declamping, if the arterial wall has a transparent or overly thin appearance, an encircling piece of vascular Hemashield can be used to reinforce the vessel (this is rare). The Hemashield surrounds the artery and is sewn together at the top (Figure 19–12), taking care not to pull it too snug.
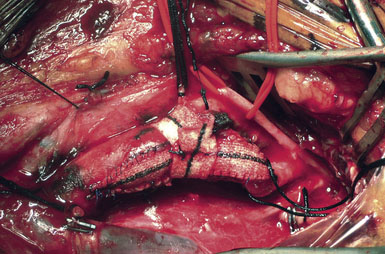
While some surgeons prefer to use the microscope to perform the arteriotomy closure, we prefer to continue working under 3.5x loupe magnification. If tacking sutures in the distal ICA are required, two such sutures may be placed, at the four and eight oElsevier Masson SASapos;clock positions. The patch material is then fashioned by placing it over the arteriotomy, cutting it to the length of the opening, and tapering the patch ends. Double-armed 6-0 prolene suture is then used to attach each of the patch ends to the arteriotomy, and rubber-shod clamps are used to secure the needles (Figure 19–13). A running, non-locking stitch is used to close the medial wall suture line from the ICA anchor to the CCA anchor; the anchors are tied together. The suture line is then inspected, and the lateral wall is closed to the level of the carotid bulb with the remaining limb of the ICA anchor stitch. The CCA anchor stitch is then used to close the lateral CCA wall up to the ICA limb. Care must be taken to include all arterial layers and to advance the stitch in small enough intervals so that leaking will not be problematic; no stray adventitial tags or suture ends are sewn into the lumen (Figure 19–14).
Special situations
Recurrent Stenosis
Recurrent stenosis following primary CEA does occur. The only risk factor repeatedly shown to play a role is continued smoking, but other factors such as hypertension and diabetes mellitus may also contribute. The operation for recurrent stenosis is much more technically demanding and, even as performed by experienced surgeons, is clearly associated with higher risks (Figure 19–15). Our approach is mostly conservative for moderate-grade asymptomatic recurrent stenosis, where we offer the patient either observation for progression or a primary endovascular strategy. For symptomatic recurrent stenosis, or for rapidly progressing (to high-grade) asymptomatic recurrent disease, we are more aggressive, especially with medical therapy failure patients, and we do not hesitate to operate in such cases if the patient clearly understands the attendant risks.
1 Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445-453.
2 Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421-1428.
3 Barnett H.J., Taylor D.W., Eliasziw M., et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
4 Biller J., Feinberg W.M., Castaldo J.E., et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1998;29:554-562.
5 Eastcott H.H., Pickering G.W., Rob C.G. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954;267:994-996.
6 Halliday A., Mansfield A., Marro J., et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491-1502.
7 Halliday A.W., Thomas D., Mansfield A. The Asymptomatic Carotid Surgery Trial (ACST). Rationale and design. Steering Committee. Eur J Vasc Surg. 1994;8:703-710.
8 Hobson R.W.II, Howard V.J., Roubin G.S., et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40:1106-1111.
9 Honeycutt J., Loftus C. Carotid endarterectomy: general principles and surgical technique. Neurosurg Clin North Am. 2000;11:279-297.
10 Lal B.K., Brott T.G. The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: lessons learned and anticipated results. J Vasc Surg. 2009;50:1224-1231.
11 Loftus C. Technical aspects of carotid endarterectomy with hemashield patch graft. Neurol Med Chir (Tokyo). 1997;37:805-818.
12 Loftus C. Anesthesia for carotid endarterectomy: general vs. local. In: Bederson J., Tuhrim S., editors. Treatment of Carotid Disease: A Practitioner’s Manual. Park Ridge, IL: AANS; 1998:181-190.
13 Loftus C. Surgical anatomy, technique, and indications for carotid endarterectomy. In Matsuno H.: Proceedings of the 12th Microsurgical Anatomy Seminar Program. Tokyo: SciMed Publications, 1998.
14 Loftus C. Surgical management of extracranial carotid artery disease. Surg Cerebral Stroke (Japan). 1999;27:7-13.
15 Loftus C. Design characteristics and clinical application of a newly designed carotid artery shunt. Neurol Res. 2000;22:443-448.
16 Loftus C. Surgical management of extracranial carotid stenosis. In: Schmidek H., Sweet W., editors. Operative Neurosurgical Techniques. Philadelphia: WB Saunders; 2000:1067-1079.
17 Loftus C., Biller J. Modern management of carotid atheromatosis. Neurocirugia. 1989;3:62-72.
18 Loftus C., Kresowik T. Carotid Artery Surgery. New York: Thieme, 2000.
19 Loftus C., Stanfield M. Carotid endarterectomy. In: Batjer H., Loftus C., editors. Textbook of Neurological Surgery. Philadelphia: Lippincott, Williams and Wilkins; 2003:2259-2260.
20 Loftus C.M. Technique of carotid endarterectomy. Contemp Neurosurg. 1988;10:1-6.
21 Loftus C.M. Monitoring during extracranial carotid reconstruction. In: Loftus C.M., Traynelis V.C., editors. Intraoperative Monitoring Techniques in Neurosurgery. New York: McGraw-Hill; 1994:3-8.
22 Loftus C.M. Carotid Endarterectomy: Principles and Technique. St. Louis: Quality Medical Publishing, 1995.
23 Loftus C.M. Carotid endarterectomy: how the operation is done. Clin Neurosurg. 1997;44:243-265.
24 Loftus C.M. Technical fundamentals, monitoring, and shunt use during carotid endarterectomy. Tech Neurosurg. 1997;3:16-24.
25 Loftus C.M. Overview of shunt controversy. In: Loftus C., Kresowik T., editors. Carotid Artery Surgery. New York: Thieme Medical Publishers; 2000:409-420.
26 Loftus C.M. Carotid Endarterectomy: Principles and Technique. New York: Informa Healthcare, 2007.
27 Loftus C.M., Kresowik T.F. Anatomical basis and technique of carotid endarterectomy. In: Loftus C., Kresowik T., editors. Carotid Artery Surgery. New York: Thieme Medical Publishers; 2000:245-256.
28 Mas J.L., Chatellier G., Beyssen B., et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660-1671.
29 Ringleb P.A., Allenberg J., Bruckmann H., et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239-1247.
30 Sheffet A.J., Roubin G., Howard G., et al. Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST). Int J Stroke. 2010;5:40-46.
31 Snell B., Wienecke R., Loftus C.: Surgical management of extracranial vascular occlusive disease. In Lawton M, editor: Controversies in Cerebrovascular Disease, New York, Thieme/AANS (in press)
32 Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.