15 Carotid Artery Stenting
Diagnosis of Carotid Artery Disease: Anatomical Imaging
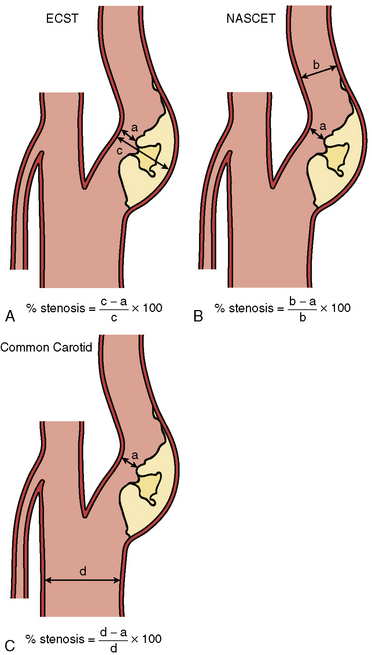
Digital subtraction angiography (DSA) is the gold standard for defining carotid anatomy with the NASCET method of stenosis measurement the most widely used (Fig. 15-1). Cerebral catheter-based angiography carries a risk of cerebral infarction of 0.5% to 1.2%; therefore, noninvasive imaging should be the initial strategy for evaluation.
Surgical Therapy
• Carotid artery stenosis after prior radical neck surgery
• High cervical carotid artery lesions that are surgically inaccessible
• Sequential lesions of the proximal to distal ICA
• Lesions of the common carotid artery and associated ICA lesions
• Recurrent stenosis of the carotid artery after CEA
• Non-atherosclerotic cause of carotid artery stenosis (e.g., fibromuscular dysplasia, Takayasu’s arteritis)
• Ipsilateral stenosis due to prior radiation therapy to the neck
• Increased operative risk due to concomitant illnesses such as CAD requiring coronary artery bypass surgery
• Contralateral occlusion and concurrent high-grade ipsilateral stenosis
Procedural Techniques
Baseline Aortography and Cerebral Angiography
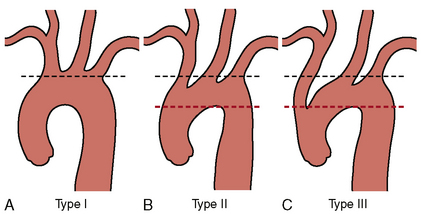
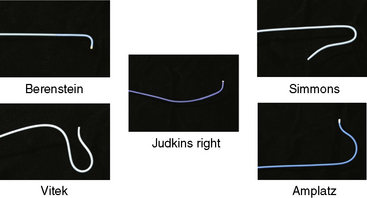
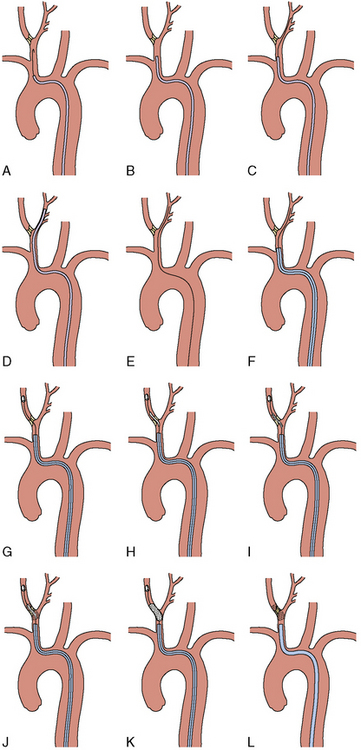
Once the morphology of the aortic arch is determined (Fig. 15-2), catheters are chosen for selective angiography of the cervical arteries supplying the brain (right and left carotid and vertebral arteries) and the cerebral vasculature. In a type I arch, Berenstein or Judkins right (JR) catheters are often used. In type II or III arch morphologies, shepherd’s crook–shaped catheters (i.e., Simmons or Vitek catheters) may be best (Fig. 15-3).
Internal Carotid Intervention
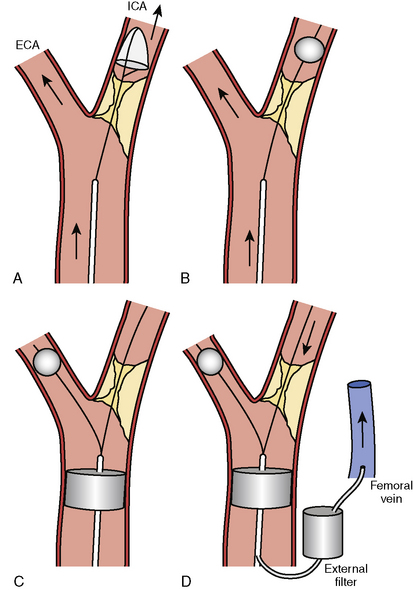
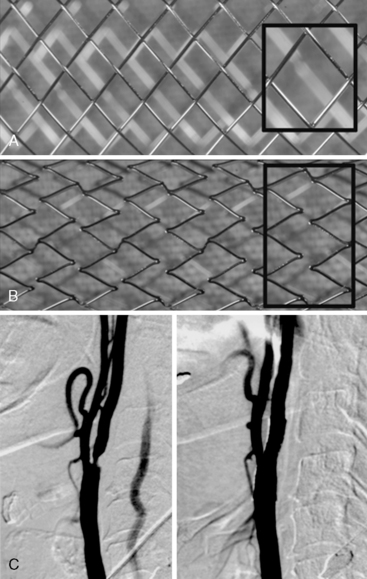
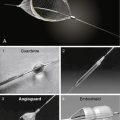
For procedures performed with a filter-type distal embolic protection device (EPD), the target lesion is crossed with the EPD. Although there are no randomized trials comparing stenting with EPDs to stenting alone, one study found that 57% of EPDs contained debris upon retrieval. EPDs are standard of care in the United States, and several types exist (Fig. 15-4). If the distal EPD will not cross the lesion, the stenosis may be crossed with a conventional 0.014-inch guidewire and subsequently predilated with a small (2.5 mm) balloon. Then the EPD should be placed. After distal EPD deployment, the lesion is often predilated with an undersized coronary balloon, typically 3 to 4 mm in diameter. A self-expanding stent is then placed across the lesion. The stent is sized to fit the CCA, and as a general rule, self-expanding stents are typically sized at least 1 mm larger than the reference diameter. There is no demonstrated benefit for using tapered stents. It is common practice, when treating an internal carotid bifurcation lesion, to place the stent across the ostium of the external carotid artery (Fig. 15-5).
An alternative to distal embolic protection is proximal protection. Two devices are available: the Gore flow reversal system (WL Gore & Associates, Flagstaff, AZ) and the Mo.Ma system (Medtronic, Minneapolis, MN). Both are positioned in a similar fashion. With the Gore device, the external carotid artery is accessed as described earlier and a balloon-tipped sheath is advanced over the 0.035-inch Amplatz wire into the CCA. This sheath has a port for an occlusion balloon to be placed in the external carotid artery. The external and common carotid balloons are inflated, arresting antegrade flow. The Mo.Ma system is similar but consists of a single sheath with two balloons: a proximal balloon in the CCA and a distal balloon in the external carotid artery. When the balloons are inflated, blood flow is arrested. In either system, once patient tolerance of balloon occlusion is confirmed, the internal carotid lesion is crossed with a 0.014-inch wire, dilated, and stented as described earlier. With the Mo.Ma system, blood is manually aspirated after the stenting procedure to clear the debris distal to the common carotid balloon. The Gore system, however, provides continuous flow reversal by having the arterial sheath connected to a venous sheath (Fig. 15-4). Although experience with these devices is limited, data indicate that they can provide excellent results.
Stents
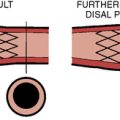
There are two types of self-expanding stents: closed-cell and open-cell (Fig. 15-6). Open-cell stents are more flexible and may better navigate tortuous vessels. Closed-cell stents are more rigid but may better “cover” atherosclerotic plaque. Whereas some evidence suggests that the frequency of embolic complications in symptomatic patients is lower with closed-cell stents, others have found no significant correlation between stent design and outcomes. Typical stent sizes are 6 to 10 mm in diameter and 2 to 4 cm in length. Gentle postdilation with a ≤ 5-mm balloon is often performed to improve stent apposition with the vessel wall. There is no benefit to aggressive postdilation since the rates of restenosis and late loss are very low in the carotid artery. Balloons are conservatively sized (≤ 1:1) to minimize vessel trauma/dissection, plaque embolization, and stimulation of the carotid sinus. A poststent carotid diameter stenosis of ≤ 50% is an acceptable result. Figure 15-6C shows a carotid bifurcation lesion after self-expanding stent placement.
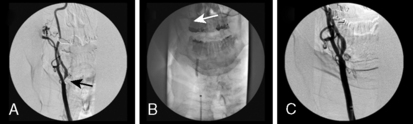
Following the procedure, if a filter-type EPD is used, the EPD is retrieved and final carotid and cerebral angiography is performed (Fig. 15-7). If a proximal protection device is used, the balloons are deflated and final angiography is performed. It is important to confirm that the carotid artery is free of dissection and that the cerebral vasculature is intact. Prior to removal of equipment, a neurologic exam assessing speech, movement, and mental status should be performed. If a neurologic deficit is found, a culprit lesion is sought and neurovascular rescue attempted.
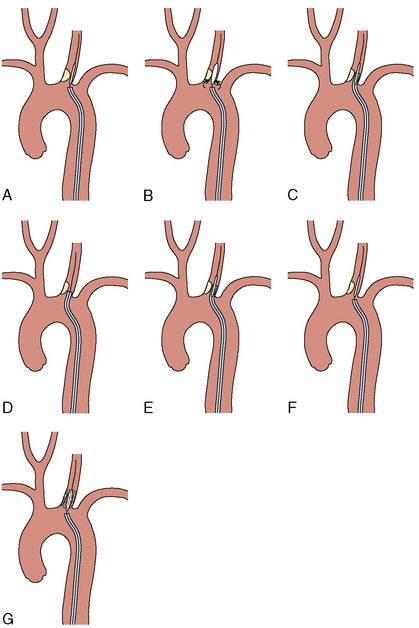
Aorto-Ostial Interventions
The lesion is predilated with a balloon sized 1:1 with the CCA. As the balloon deflates, the guide is gently advanced or “telescoped” over the balloon and across the lesion. This will protect the stent as it is delivered to the lesion. The predilation balloon is removed, and a balloon-expandable stent is placed (in arteries protected by the axial skeleton, balloon-expandable stents are more often used). After positioning the stent at the target lesion, the guide catheter is withdrawn, uncovering the stent and placing it in contact with the target lesion. The proximal stent should protrude slightly into the aorta (≤ 1 mm) to ensure adequate lesion coverage. After verifying adequate placement with contrast injections through the guide catheter, the stent is deployed at nominal pressure (Fig. 15-8). As the balloon deflates, the guide is again gently telescoped over the balloon to allow further stents to be delivered distally if needed. Final angiography and neurologic assessment are performed. The access site is managed similarly to other interventional procedures. Sheath removal is performed when the ACT is ≤ 170 seconds if a closure device is not used.
Complications and Troubleshooting
Carotid Stenting: Clinical Outcomes
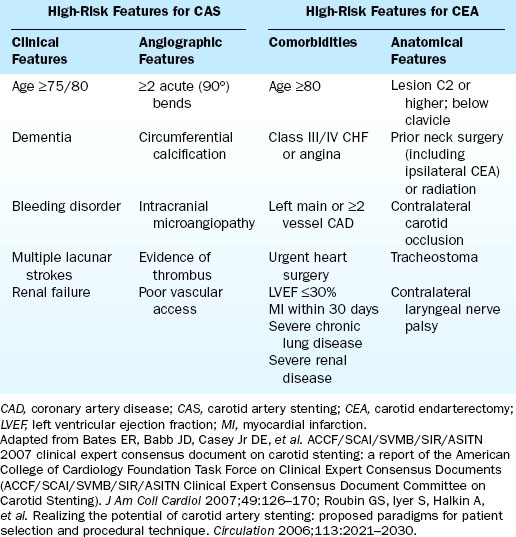
When interpreting data on carotid stenting, it is important to realize that a patient who is high risk for surgery is not necessarily high risk for stenting (and vice versa). Features that place a patient at increased risk for complications from CEA and CAS are summarized in Table 15-1.
High Surgical Risk Patients
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial is the only randomized trial comparing high surgical risk (HSR) patients treated with CEA to those treated with CAS. Patients (N = 334) with a symptomatic stenosis of ≥ 50% or an asymptomatic stenosis ≥ 80% (~30% were symptomatic) were randomized to either CEA or CAS. The primary end point of death, stroke, or MI at 30 days plus ipsilateral stroke or death from neurologic cause between day 31 and 1 year occurred in 12.2% of patients in the stenting group and 20.1% in the CEA group (P = 0.004 for noninferiority; Fig. 15-9). The 30-day stroke and death rate among the asymptomatic patients was 4.6% for the CAS group and 5.4% for the CEA group. At 3 years, there were no differences between the groups.
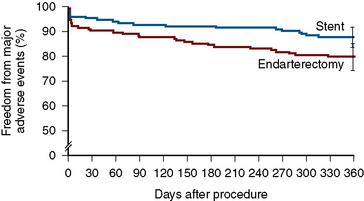
Figure 15-9 Freedom from major adverse events at 1 year in the SAPPHIRE trial.
(From Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–1501. Reprinted with permission.)
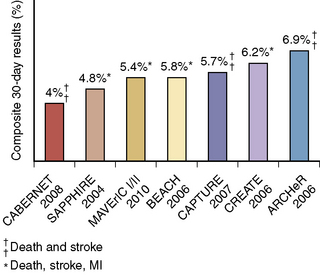
Most of the contemporary registry data focuses on HSR patients, and data from more than 10,000 HSR patients have been published. These registries generally include symptomatic patients with ≥ 50% stenosis and asymptomatic patients with ≥ 70–80% stenosis. Data from some of these studies are summarized in Figure 15-10.
Usual Surgical Risk Patients
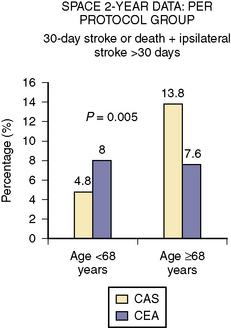
Four large randomized studies in average or usual surgical risk patients compared CAS to CEA. The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial found that the 30-day incidence of stroke or death was 9.6% in the CAS group and 3.9% in the CEA group. The Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial noted that the 30-day rate of ipsilateral stroke or death was not different between the two groups (6.8% in the CAS group and 6.3% in the CEA group, P = 0.09 for noninferiority). However, the 2-year outcomes for this trial demonstrated a statistically significant benefit for CAS over CEA in patients <68 years of age (Fig. 15-11). The International Carotid Stenting Study (ICSS) published only their interim safety analysis, which demonstrated that the 30-day rate of death, stroke, or MI was 7.4% in the CAS group and 4% in the CEA group (P = 0.003). The rate of death and stroke alone also favored CEA (7.4% vs. 3.4%, P = 0.0004).
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) is the largest randomized trial published comparing CAS with EPD to CEA (Fig. 15-12). The primary outcome of periprocedural stroke, death, or MI or follow-up ipsilateral stroke was not significantly different between the two groups (7.2% for CAS and 6.8% for CEA). The 30-day risk of stroke was higher for CAS (4.1% vs. 2.3%, P = 0.01), whereas CEA was associated with a higher 30-day risk of MI (2.3% vs. 1.1%, P = 0.03). CAS appeared safer than CEA for patients ≤ 69 years of age while CEA yielded better outcomes in those >70 years of age.
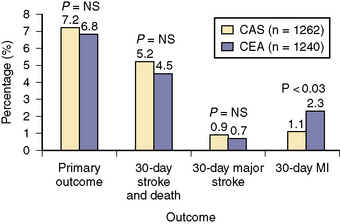
Figure 15-12 Results from CREST. CAS, carotid artery stenting; CEA, carotid endarterectomy; MI, myocardial infarction.
CREST differed from the previous three trials in three significant ways. Most importantly, the European trials, EVA-3S, SPACE, and ICSS, allowed inexperienced operators to treat patients. All allowed stent operators, but not surgery operators, to be “tutored” during the randomized trial. CREST requirements were more stringent. In fact, many of the “experienced” CAS operators in the first three trials were not very experienced. The fact that so many neurologic events involve the nonculprit carotid circulation is testament to the importance of catheter skills, and the value of experience cannot be overstated. Second, CREST mandated the use of EPDs, whereas the other trials did not. Lastly, just over 50% of the patients in CREST were symptomatic, whereas the other trials were specifically for symptomatic patients. When taken together, the message from these four trials is that CAS with embolic protection is a legitimate alternative to CEA for average surgical risk patients but only when performed by experienced operators. The Asymptomatic Carotid Trial (ACT-1) will further help evaluate CEA vs. CAS in asymptomatic, usual surgical risk patients. Preliminary data have been reported (Table 15-2).
| Event | 30 Days, N = 135 |
|---|---|
| Death, stroke, and MI* | 1.4% |
| All stroke and death* | 1.4% |
| Major stroke/death* | 0% |
| Death | 0% |
| All stroke | 1.4% |
| Major stroke | 0% |
| Minor stroke | 1.4% |
| MI | 0% |
| Ipsilateral stroke, days 31–365 | 0% |
ACT-1, Asymptomatic Carotid Trial; MI, myocardial infarction.
Abou-Chebl A., Reginelli J., Bajzer C.T., et al. Intensive treatment of hypertension decreases the risk of hyperperfusion and intracerebral hemorrhage following carotid artery stenting. Catheter Cardiovasc Interv. 2007;69:690–696.
Adams R.J., Albers G., Alberts M.J., et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647–1652.
Amarenco P., Bogousslavsky J., Callahan A.3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559.
Bates E.R., Babb J.D., Casey D.E.Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting). J Am Coll Cardiol. 2007;49:126–170.
Brott T.G., Halperin J.L., Abbara S., et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. 2011;57:e16–e94.
Brott T.G., Hobson R.W.Jr, Howard G., et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23.
Diener H.C., Bogousslavsky J., Brass L.M., et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337.
Easton J.D., Saver J.L., Albers G.W., et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293.
Eckstein H.H., Ringleb P., Allenberg J.R., et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902.
Everett B.M., Glynn R.J., MacFadyen J.G., et al. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Circulation. 2010;121:143–150.
Fayed A.M., White C.J., Ramee S.R., et al. Carotid and cerebral angiography performed by cardiologists: cerebrovascular complications. Catheter Cardiovasc Interv. 2002;55:277–280.
Goldstein L.B., Adams R., Alberts M.J., et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633.
Gray W.A., Hopkins L.N., Yadav S., et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258–268.
Gray W.A., Yadav J.S., Verta P., et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69:341–348.
Gurm H.S., Yadav J.S., Fayad P., et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579.
Halliday A., Mansfield A., Marro J., et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502.
Hart J.P., Peeters P., Verbist J., et al. Do device characteristics impact outcome in carotid artery stenting? J Vasc Surg. 2006;44:725–730. discussion 30–31
Higashida R.T., Popma J.J., Apruzzese P., et al. Evaluation of the Medtronic exponent self-expanding carotid stent system with the Medtronic guardwire temporary occlusion and aspiration system in the treatment of carotid stenosis: combined from the MAVErIC (Medtronic AVE Self-expanding CaRotid Stent System with distal protection In the treatment of Carotid stenosis) I and MAVErIC II trials. Stroke. 2010;41:e102–e109.
Hopkins L.N., Myla S., Grube E., et al. Carotid artery revascularization in high surgical risk patients with the NexStent and the Filterwire EX/EZ: 1-year results in the CABERNET trial. Catheter Cardiovasc Interv. 2008;71:950–960.
International Carotid Stenting Study Investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomized controlled trial. Lancet. 2010;375:985–997.
Jackson B.M., English S.J., Fairman R.M., et al. Carotid artery stenting: identification of risk factors for poor outcomes. J Vasc Surg. 2008;48:74–79.
Kelso R., Clair D.G. Flow reversal for cerebral protection in carotid artery stenting: a review. Perspect Vasc Surg Endovasc Ther. 2008;20:282–290.
Kleindorfer D., Panagos P., Pancioli A., et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–723.
Lloyd-Jones D., Adams R.J., Brown T.M., et al. Heart disease and stroke statistics—2010 update. A report from the American Heart Association. Circulation. 2009;121:e1–e171.
Mas J.L., Chatellier G., Beyssen B., et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671.
Moulakakis K.G., Mylonas S.N., Sfyroeras G.S., et al. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg. 2009;49:1060–1068.
Rothwell P.M., Eliasziw M., Gutnikov S.A., et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116.
Rothwell P.M., Gutnikov S.A., Warlow C.P. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003;34:514–523.
Roubin G.S., Iyer S., Halkin A., et al. Realizing the potential of carotid artery stenting: proposed paradigms for patient selection and procedural technique. Circulation. 2006;113:2021–2030.
Sacco R.L., Adams R., Albers G., et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617.
Sacco R.L., Diener H.C., Yusuf S., et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251.
Safian R.D., Bresnahan J.F., Jaff M.R., et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol. 2006;47:2384–2389.
Schillinger M., Gschwendtner M., Reimers B., et al. Does carotid stent cell design matter? Stroke. 2008;39:905–909.
Stabile E., Salemme L., Sorrropago G., et al. Proximal endovascular occlusion for carotid artery stenting: results from a prospective registry of 1300 patients. J Am Coll Cardiol. 2010;3:298–304.
Touze E., Trinquart L., Chatellier G., et al. Systematic review of the perioperative risks of stroke or death after carotid angioplasty and stenting. Stroke. 2009;40:e683–e693.
White C.J., Iyer S.S., Hopkins L.N., et al. Carotid stenting with distal protection in high surgical risk patients: the BEACH trial 30 day results. Catheter Cardiovasc Interv. 2006;67:503–512.
Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501.