CHAPTER 89 Carotid Artery Disease
The ancient Greeks believed that compression of the carotid artery caused sudden sleep (karoo, “to stupefy”). Ambroise Paré later named these arteries “the right and left carotides or sleepy vessels” in the 16th century.1 In 1914, J. Ramsay Hunt noted that partial or complete occlusion of the carotid artery could be responsible for “cerebral intermittent claudication.”1 In 1935, Aring clarified the differences between brain hemorrhages and infarctions in autopsy cases. The notion that carotid artery occlusive disease can produce a dementia state was proposed soon thereafter.1 It was postulated that restoration of blood supply could reverse the condition. This inspired the first carotid reconstruction (1951) by Carrea, Molins, and Murphy in Argentina.1 They performed an anastomosis of the distal internal carotid artery to the external carotid artery after partial resection of a stenosed internal carotid artery. Although DeBakey is credited with performing the first carotid endarterectomy (CEA), the report in 1954 by Eastcott, Pickering, and Rob of a successful carotid reconstruction in a woman after acute stroke provided a major impetus for operative intervention in carotid occlusive disease and introduced CEA as an important procedure in the management of stroke.1 In the ensuing decades, atherosclerotic carotid occlusive disease has been recognized as, by far, the most common disorder affecting the extracranial carotid artery and has become one of the most intensively studied of vascular diseases. Treatment decisions are appropriately guided by several randomized trials and additional well-designed large cohort studies.
CAROTID ARTERY STENOSIS
Prevalence and Epidemiology
Stroke is the third leading cause of death in the United States. Each year, 600,000 patients suffer a new stroke, and 170,000 deaths per year are attributable to cerebrovascular disease.2 Stroke is also the leading cause of disability among elderly Americans. Although there has been a 50% reduction in mortality during the last two decades, 21% of survivors will still have a second stroke and 7% a third stroke. Forty percent will require special nursing home care; 10% will require institutionalization involving an expenditure of billions of dollars of the health care budget.2
About one third of strokes are thromboembolic in etiology, and atherosclerotic carotid occlusive disease is the most common single cause. The extent to which carotid stenosis or occlusion can be attributed to stroke has been difficult to determine from population studies. Between 1993 and 1997, the Northern Manhattan Study identified cerebral infarction attributable to carotid stenosis in 17 (95% CI, 8 to 26) per 100,000 blacks, 9 (5 to 13) per 100,000 Hispanics, and 5 per 100,000 (2 to 8) whites.3 Approximately 7% of all first ischemic strokes occurred in the presence of extracranial carotid artery stenosis of 60% or more. Between 1985 and 1989, in the Mayo Clinic study conducted among the population of Rochester, Minnesota, 18% of all first ischemic strokes occurred in the presence of significant extracranial or intracranial carotid artery stenosis.4
A variety of risk factors contribute to an increased risk of stroke. The Framingham study5 confirmed that there was no difference in incidence related to gender. However, increasing age, systolic and diastolic blood pressure, diabetes, smoking, and cardiovascular diseases were associated with an increased risk for stroke. The age-adjusted relative risk for stroke among hypertensive patients (>160/95 mm Hg) in contrast to normotensive persons (<140/90 mm Hg) is 3.0 in men and 2.9 in women. Smokers may have a 50% increase in risk of atheroembolic stroke compared with nonsmokers.
Etiology and Pathophysiology
The unique hemodynamics at the carotid bifurcation predisposes this area to atherosclerosis. Along the inner wall of the carotid bulb, blood flow remains laminar, with high velocity and high shear stress. Conversely, along the outer wall, there are areas of flow separation, stasis, turbulent flow, and a complex oscillating shear stress pattern that predisposes to atherosclerotic plaque deposition.6 Although neurologic events have been attributed to progressive stenosis and decreased blood flow from enlarging atherosclerotic plaques, most such events are usually secondary to plaque disruption and atheroembolization from the lesion. Loss of the fibrous cap with exposure of atherosclerotic debris to the flow lumen appears to be responsible for these embolic complications. Additional factors, such as adequacy of collateralization, plaque ulceration or hemorrhage, hypotension, and low cardiac output, also play a contributory role.
Histologic evaluation of atherosclerotic plaques has demonstrated that they originate from fatty streaks that, over time, accumulate into a lipid core.7 The fatty streak becomes a fibroatheroma as fibrous tissue accumulates over the core and forms a fibrous cap (Fig. 89-1). Through unknown mechanisms, some plaques become unstable, resulting in an enlarging lipid core, intraplaque hemorrhage, plaque enlargement, fibrous cap rupture, ulceration, and luminal thrombosis. These histomorphologic features have been associated with the production of atheroemboli and neurologic symptoms. Specifically, large lipid cores located close to the flow lumen, intraplaque hemorrhage, and fibrous cap disruption with ulceration have frequently been observed in CEA specimens obtained from patients with symptomatic plaques (Fig. 89-2A).8 Conversely, small lipid cores located deep within the plaque with a thick fibrous cap have been observed in CEA specimens from patients with asymptomatic carotid stenosis (see Fig. 89-2B).8
Manifestations of Disease
Clinical Presentation
Because one of the major goals in the management of carotid stenosis is to prevent stroke, the identification of individuals with asymptomatic stenosis becomes an important objective. The incidence of asymptomatic carotid stenosis in the general population is difficult to estimate. In one study of 2000 asymptomatic patients screened by duplex ultrasonography, significant carotid stenosis was found in 32.8% of patients with peripheral vascular disease, 6.8% of patients with coronary artery disease, and 5.9% of patients with significant risk factors.9 Perhaps the most frequent reason for suspecting a carotid stenosis is a cervical bruit in a high-risk patient. However, bruit is nonspecific and is associated with significant stenosis in only 47% of patients. Conversely, only 60% of significant carotid stenoses will be manifested as a bruit.10 Although auscultation of a bruit is not a sensitive or specific predictor of carotid stenosis, it remains an important clinical sign in an otherwise asymptomatic patient and must prompt a duplex ultrasound evaluation. Of additional interest is the association of cervical bruit with coronary artery disease. Its presence should also prompt a clinical evaluation for coronary artery disease because the bruit may actually be a more accurate predictor of myocardial infarction than stroke.10
Alternatively, patients may first present with a variety of symptoms related to atheroembolic ischemic events. Eye symptoms can result from embolization to the retinal, ophthalmic, or cerebral circulation and include one or more of the following: amaurosis fugax (blurring or loss of vision in the ipsilateral eye, typically described as a curtain or shade falling over the eye), homonymous hemianopia (resulting from emboli to optic radiation), intermittent retinal blindness (loss of vision on exposure to bright light), neovascularization of iris (resulting from ophthalmic artery ischemia), and, rarely, complete blindness (resulting from ischemic optic neuropathy). More commonly, atheroemboli travel to the intracranial circulation and result in lateralizing deficits that may follow several patterns. A transient ischemic attack tends to be a stereotypic and temporary loss of sensory, motor, or visual function that usually lasts for less than 15 minutes. Per definition, patients always return to baseline within 24 hours. Alternatively, patients may have recurring attacks of deficits similar to a transient ischemic attack without an interval allowing complete recovery. The deficit is often the same with each attack, and there is no deterioration in function. These symptoms are associated with a worse outcome and are referred to as crescendo transient ischemic attacks. Patients may also present with recurring attacks of focal neurologic deficits with progressive deterioration in neurologic function. This is termed a stroke in evolution and generally lasts longer than 24 hours. Finally, a completed stroke occurs when a deficit has stabilized beyond 24 hours. The definitions of transient ischemic attack and stroke are evolving. Studies of patients with diffusion-weighted MRI demonstrate that many patients with transient ischemic attacks lasting only hours will actually be shown to have sustained cerebral infarction.11
All patients with suspected or confirmed carotid stenosis must undergo a complete neurologic examination to identify potential neurologic deficits and to establish a clinical baseline with which to compare outcomes of future therapeutics. No single outcome measure can describe or predict all dimensions of recovery and disability after acute stroke. Several scales have proved reliability and validity in stroke trials, including the National Institutes of Health Stroke Scale (NIHSS), the modified Rankin Scale, the Barthel Index, the Glasgow Outcome Scale, and the Stroke Impact Scale. The NIHSS comprises 15 items that enable standardized application and measurement of a simple neurologic examination.12 The scale assesses the level of consciousness, extraocular movements, visual fields, facial muscle function, extremity strength, sensory function, coordination, language, speech, and hemi-inattention. It has undergone extensive validation and is the preferred method of quantification of neurologic deficits in a clinical trial setting.13–16
Imaging Indications and Algorithm
A clinically detected carotid bruit, or neurologic symptoms attributable to the carotid artery territory as outlined before, should prompt a noninvasive assessment of the carotid arteries. The most commonly performed study is an ultrasonographic evaluation. Alternative approaches include evaluation with a CT scan or MRI, which have the advantage of being able to incorporate intracranial views to assess for infarctions. However, these studies are more expensive, and the yield for intracranial disease is low. Noninvasive studies typically yield information about the lesion that is reliable enough to plan further management.17 In cases in which the stenosis is determined to be borderline significant, a second noninvasive study provides complementary information. Exceptional circumstances, such as the inability to differentiate between an occluded and a very tight stenosis, warrant an invasive study such as angiography.
Imaging Techniques and Findings
Angiography
The degree of carotid stenosis generally correlates with risk of stroke in symptomatic extracranial carotid occlusive disease. Cerebral angiography (Fig. 89-3) was the first definitive investigative tool used to visualize and to quantify carotid stenosis. Multiplanar angiography still remains the gold standard for preoperative delineation of extracranial carotid occlusive disease. Complete details of diagnostic procedures have been included in other chapters in this book. The definition of a significant carotid stenosis has been conventionally accepted as a 50% decrease in the luminal diameter, which translates into a 75% reduction in cross-sectional area. On cerebral angiography, the maximal stenosis is determined on the anteroposterior, lateral, or oblique view. Two methods to estimate the percentage of diameter reduction are in common use (Fig. 89-4). The first (North American) method compares the least transverse diameter at the stenosis with the luminal diameter of the distal internal carotid artery once its walls become parallel. This technique has been used by the majority of carotid atherosclerosis trials.13–15 The second method compares it with the presumed normal luminal diameter at the stenosis and has been used in Europe.18 The percentage stenosis measured by the North American method will be lower than the European measurement; for example, North American measurements of 30%, 40%, 50%, 60%, 70%, 80%, and 90% stenosis will correspond to 65%, 70%, 75%, 80%, 85%, 91%, and 97% stenosis, respectively, in Europe. It is therefore important to specify the method used for estimation of percentage stenosis for purposes of comparison with published data.
Ultrasonography
Duplex ultrasonography (Fig. 89-5) emerged about 2 decades ago as a reliable, inexpensive, and safe method of assessing carotid occlusive disease. It is ideal for screening, for observing disease progression or postoperative status, and for intraoperative assessment of CEA. It is being used increasingly as the sole preoperative test before CEA.17 On a scan, the percentage stenosis is estimated on the basis of peak systolic and end-diastolic velocities. Although the modified University of Washington criteria define this relationship,19 the Intersocietal Commission for the Accreditation of Vascular Laboratories has recommended that each vascular laboratory generate its own estimates based on the performance of individual devices and technologists.
Plaque Morphology
A subset analysis of patients randomized in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) showed that for symptomatic patients with no carotid plaque ulceration, the risk of stroke remained constant for all degrees of stenosis of 75% or more.20 Therefore, it is possible that other factors may add to the risk of stroke, such as the histologic composition of the plaque, the plaque surface characteristics (ulceration, hemorrhage), the presence of prior silent but CT-confirmed cerebral infarction, and the status of collateral cerebral circulation. Currently, the most commonly employed clinical predictor for risk of stroke is the severity of stenosis of the carotid arterial plaque. However, the presence of ulceration on carotid plaques is also a marker for stroke risk.20 Plaques that appear hypoechoic or heterogeneous on duplex ultrasound B-mode imaging may have a high content of lipid or intraplaque hemorrhage that may produce plaque ulceration and a greater embolic potential.8 Duplex ultrasound examination of the plaque followed by computer-assisted image analysis has recently been suggested as an effective method of objectively characterizing plaque morphology (Fig. 89-6).21 These morphologic features may also influence the neurologic complication rate associated with revascularization procedures such as carotid artery stenting. MRI also provides excellent soft tissue contrast, offering delineation of important plaque composition features such as the fibrous cap, necrotic core, intraplaque hemorrhage, and calcification (Fig. 89-7). MRI methods that use black blood are effective in delineating the flow lumen and the inner and outer walls of the plaque while eliminating the flow signal. In this protocol, signal heterogeneity has been shown to reflect the complexity of the plaque, consisting of admixtures of necrotic tissue, calcific shards, and intraplaque hematoma. These plaques are probably a greater source of microemboli than is homogeneous fibrous tissue. Significant effort continues to be focused on the identification of noninvasive tissue signatures that may portend future stroke, but additional work is required to identify a definitive marker.
Synopsis of Treatment Options
The first randomized controlled trial comparing pharmacologic with surgical treatment of carotid occlusive disease appeared in 1970.22 The study reported that the patients undergoing CEA had a lower incidence of stroke and death during long-term follow-up. However, the procedure resulted in a prohibitively high incidence of perioperative stroke rate in that study. With improved surgical technique and selection of patients for operation, a significant decrease in the complication rate of this procedure was noted during the ensuing decade. The ensuing controversy about the appropriate management of carotid stenosis prompted several randomized controlled multi-institutional trials that have provided statistically reliable results that form the basis for current treatment guidelines.
Symptomatic Carotid Stenosis
Several centers in North America and internationally participated in NASCET,13 which studied patients with recent hemispheric and retinal transient ischemic attacks or nondisabling strokes and ipsilateral high-grade stenosis (70% to 99%) of the internal carotid artery. The results, published in 1991, showed that the risk of an ipsilateral stroke at 2 years was 26% in medically treated patients and 9% in the surgical patients—an absolute risk reduction of 17%. The European Carotid Surgery Trial18 published a confirmatory report demonstrating significant benefit of CEA and optimal medical therapy versus best medical care alone. Finally, the Veterans Affairs trial of symptomatic carotid stenosis23 also confirmed benefit of CEA in a small number of patients; the trial was closed after initial announcement of the NASCET data. Subsequently, the NASCET trialists15 reported benefit from CEA in patients with 50% to 69% stenosis. The 5-year rate of ipsilateral stroke was 15.7% among patients treated surgically and 22.2% in those treated medically. On the basis of these clinical trials, the Society for Vascular Surgery24 and the American Heart Association25 have recommended that symptomatic patients with 50% or more carotid artery stenosis are best treated with CEA and medical therapy.
Asymptomatic Carotid Stenosis
The Veterans Affairs Cooperative Study Group16 enrolled men with asymptomatic carotid stenosis with diameter reduction of 50% or more. Whereas the combined rate of transient neurologic events and stroke was reduced after CEA, the Asymptomatic Carotid Atherosclerosis Study investigators14 were the first to report reduction in stroke alone after CEA. In patients with 60% or more diameter-reducing stenosis, the projected 5-year ipsilateral stroke rate for surgically treated patients was 5.1%, whereas it was 11.0% for the medically treated group. These results were mirrored in a larger European trial, in which the benefit of CEA was proved to extend to women also.26 These trials established the beneficial role of carotid revascularization in reducing the risk of neurologic sequelae in patients with asymptomatic carotid stenosis. On the basis of these results, the Society for Vascular Surgery24 and the American Heart Association25 have recommended that asymptomatic patients with 60% or more carotid artery stenosis are best treated with CEA and medical therapy, provided periprocedural morbidity and mortality are less than 3%.
Medical
The major randomized trials addressing management of carotid stenosis have demonstrated the benefit of pharmacologic (antiplatelet) therapy over surgery in a selected subgroup of patients. Furthermore, low periprocedural morbidity in the surgical arm of these studies was achieved in the presence of simultaneous pharmacologic therapy. The Society for Vascular Surgery24 and the American Heart Association25 have therefore recommended optimal medical management for all patients with carotid stenosis. Medical therapy should be targeted to achieve a blood pressure below 120/80 mm Hg by lifestyle interventions and antihypertensive treatment, glucose control to hemoglobin A1c below 7% by dietary modifications and hypoglycemic agents, and low-density lipoprotein cholesterol level to 100 mg/dL or even lower for high-risk patients by lifestyle modification and statin therapy. Patients who have smoked in the last year should be counseled to quit. Antiplatelet agents are recommended for patients with ischemic stroke or transient ischemic attack and include aspirin (50 to 325 mg/day), the combination of aspirin, or clopidogrel (75 mg/day).
Surgical
Carotid Endarterectomy
We routinely use general anesthesia for the procedure, although some surgeons perform CEA under cervical block anesthesia. Along with routine monitoring, invasive arterial blood pressure monitoring is recommended during and immediately after the procedure. The operation is accomplished through a longitudinal incision along the anterior border of the sternocleidomastoid muscle. The dissection to expose the internal, external, and common carotid arteries must avoid damage to the superior laryngeal, hypoglossal, glossopharyngeal, marginal mandibular, and vagus nerves. Manipulation of the carotid body must be minimized to prevent bradycardia or hypotension. Systemic heparin is given, and vascular clamps are applied on the proximal uninvolved common carotid artery and the distal nondiseased internal carotid artery and external carotid artery. Many surgeons recommend placement of a temporary shunt from the common carotid artery to the distal internal carotid artery to maintain prograde flow to the brain during the endarterectomy. Others use shunting selectively, based on variables such as internal carotid artery backpressure measurements, operative electroencephalography, sensory evoked potentials, or observation for neurologic events during regional anesthesia. The artery is then incised longitudinally, and the plaque is separated from the common carotid artery and the internal carotid artery. Great care is taken to leave behind a smooth luminal surface with no debris or intimal flaps. The arteriotomy is usually closed with a synthetic or vein patch to prevent restenosis and to reduce perioperative stroke risk. A closed suction drain may also be placed (Fig. 89-8).
Emerging Therapy: Carotid Artery Stenting
During the recent decade, carotid artery stenting (CAS) has been advocated as an alternative treatment to CEA for patients who have carotid stenosis. Several case series, single institutional trials, or registries have reported support for this approach.24 One randomized trial reported potential equivalence of outcomes after CAS versus CEA in high-risk patients.27 In response to this early enthusiasm, the U. S. Food and Drug Administration approved the use of CAS in selected high-risk patients with symptomatic carotid stenosis in 2004. However, two subsequent randomized trials have shown equivocal results.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial28 randomized symptomatic patients with at least 60% carotid stenosis into CAS and CEA. The primary outcome measured was the composite of stroke or death within 30 days of the procedure. As a prerequisite to participation, surgeons had to have successfully performed at least 25 CEA procedures in the previous year, and CAS operators had to have performed at least 12 CAS procedures or 35 stenting procedures in any other artery. Importantly, stent operators with no previous CAS experience could still participate if they were proctored by another qualified operator. The use of an embolic protection device was not mandatory. Enrollment to EVA-3S was stopped in 2005 after 520 patients had been randomized because of a higher stroke and other adverse event rate in patients randomized to CAS. The 30-day stroke and death rate was 9.6% for CAS compared with 3.9% for CEA. Whereas the results provided important information, this study had some limitations that precluded a definitive answer. The experience of the surgeons and CAS operators participating in the trial was unequal, the use of an embolic protection device during CAS was not uniform, the study did not complete enrollment, and more patients assigned to CAS had occlusions of the contralateral carotid artery (5.0% vs. 1.2%), a high-risk anatomic feature limiting the success of CEA.
The Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial29 was designed to test whether CAS would be noninferior to CEA in symptomatic patients with carotid artery stenosis of 70% or more. Between 2001 and 2006, 1200 patients were randomized to CEA (n = 595) or CAS (n = 605). The primary outcome was composite 30-day stroke or death. The prerequisites for participation in the trial were that surgeons should have performed at least 25 CEAs and physicians performing stenting should have carried out at least 25 endovascular interventions, not necessarily involving the carotid arteries. Shunting during CEA and embolic protection during CAS were optional. The study had aimed to randomize 1900 patients but had to be terminated because of inadequate enrollment. Of the patients enrolled, demographics and lesion characteristics were similar in the two treatment groups. At 30 days, there was no significant difference in outcomes between CAS and CEA (30-day stroke and death, 6.8% vs. 6.3%; absolute difference, 0.51% [95% CI, −1.89%-2.91%]. The SPACE study suffered from limitations similar to those observed in the EVA-3S trial, namely, an uneven experience of surgeons versus CAS operators, nonuniform use of embolic protection during CAS, and reduced power due to the inability to complete enrollment.
The safety and efficacy of any new therapy must be clearly established in a controlled, appropriately powered, adequately monitored clinical trial, with appropriately credentialed operators, under Institutional Review Board and U.S. Food and Drug Administration guidelines. In the tradition of the two previous National Institutes of Health–sponsored carotid trials,13,14 the Carotid Revascularization Endarterectomy versus Stent Trial (CREST)30 was funded by the National Institute of Neurological Disorders and Stroke. It completed enrollment of more than 2500 asymptomatic and symptomatic patients who were randomized to undergo CAS or CEA in July 2008, and results are anticipated in late 2009. Until the safety and efficacy of CAS can be proved, the widespread clinical application of this technology is not justified. Such procedures must be performed only by properly credentialed physicians in a monitored trial setting.
Procedure
Routinely, two preprocedural antiplatelet agents (aspirin and clopidogrel) are used. Access is generally gained by a transfemoral 6F introducer sheath. Transbrachial or direct carotid exploration and cannulation can be undertaken in selected patients with severe aortoiliac disease. The patient is heparinized to an activated clotting time of 250 to 300 seconds. A 6F long sheath is positioned within the common carotid artery proximal to the lesion. A 0.014-inch guidewire is used to cross the carotid stenosis, over which an embolic protection device (filter or balloon) is passed and deployed distal to the lesion. The lesion is then dilated with a 4-mm low-profile balloon. The same wire is used to deploy a stent across the lesion. Post-stent dilation is accomplished with 5- or 6-mm balloons. Intermittent hand-injection angiography is performed during the entire procedure to confirm appropriate balloon and stent placements; bone landmarks are used for the same purpose. Clopidogrel is discontinued 4 weeks after CAS; aspirin is continued indefinitely (Fig. 89-9).
Reporting: Information for the Referring Physician
KEY POINTS
 Randomized trial data confirm that carotid artery revascularization in patients with carotid stenosis reduces the incidences of stroke and death beyond that which can be achieved by optimal medical therapy.
Randomized trial data confirm that carotid artery revascularization in patients with carotid stenosis reduces the incidences of stroke and death beyond that which can be achieved by optimal medical therapy. Because patients must survive several years to derive a benefit from revascularization, the risks of offering the procedure to high-risk individuals must be carefully considered.
Because patients must survive several years to derive a benefit from revascularization, the risks of offering the procedure to high-risk individuals must be carefully considered. There is Level 1 evidence that revascularization with CEA in carefully selected patients offers an advantage over medical therapy.
There is Level 1 evidence that revascularization with CEA in carefully selected patients offers an advantage over medical therapy. Current information also indicates that revascularization with CAS is technically feasible and can be performed with low periprocedural morbidity, thereby offering a reasonable alternative to CEA.
Current information also indicates that revascularization with CAS is technically feasible and can be performed with low periprocedural morbidity, thereby offering a reasonable alternative to CEA.Barrett KM, Brott TG. Carotid artery stenting versus carotid endarterectomy: current status. Neurosurg Clin North Am. 2008;19:447-458.
Hobson RW2nd. Randomized clinical trials: how will results influence clinical practice in the management of symptomatic and asymptomatic extracranial carotid occlusive disease? J Vasc Surg. 2007;45(Suppl A):A158-A163.
Hobson RW2nd, Mackey WC, Ascher E, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48:480-486.
Lal BK, Hobson RW2nd. Treatment of carotid artery disease: stenting or surgery. Curr Neurol Neurosci Rep. 2007;7:49-53.
1 Thompson J. Carotid artery surgery—historical review. In: Greenhalgh R, Hollier L, editors. Surgery for Stroke. Philadelphia: WB Saunders; 1993:3-10.
2 Elkins JS, Johnston SC. Thirty-year projections for deaths from ischemic stroke in the United States. Stroke. 2003;34:2109-2112.
3 White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327-1331.
4 Petty GW, Brown RDJr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513-2516.
5 Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke. The Framingham study. Stroke. 1982;13:290-295.
6 Zarins CK, Giddens DP, Bharadvaj BK, et al. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502-514.
7 Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177-1178.
8 Lal BK, Hobson RW2nd, Hameed M, et al. Noninvasive identification of the unstable carotid plaque. Ann Vasc Surg. 2006;20:167-174.
9 Hobson RW. Carotid artery occlusive disease. In: Dean RH, Yao JST, Brewster DC, editors. Current Diagnosis and Treatment in Vascular Surgery. New York: Appleton & Lange; 1995:88-104.
10 Wolf PA, Kannel WB, Sorlie P, McNamara P. Asymptomatic carotid bruit and risk of stroke. The Framingham study. JAMA. 1981;245:1442-1445.
11 Mlynash M, Olivot JM, Tong DC, et al. Yield of combined perfusion and diffusion MR imaging in hemispheric TIA. Neurology. 2009;72:1127-1133.
12 Brott T, Adams HPJr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864-870.
13 Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445-453.
14 Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421-1428.
15 Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
16 Hobson RW2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221-227.
17 Kuntz KM, Skillman JJ, Whittemore AD, Kent KC. Carotid endarterectomy in asymptomatic patients—is contrast angiography necessary? A morbidity analysis. J Vasc Surg. 1995;22:706-714.
18 Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
19 Faught WE, Mattos MA, van Bemmelen PS, et al. Color-flow duplex scanning of carotid arteries: new velocity criteria based on receiver operator characteristic analysis for threshold stenoses used in the symptomatic and asymptomatic carotid trials. J Vasc Surg. 1994;19:818-827.
20 Eliasziw M, Streifler JY, Fox AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1994;25:304-308.
21 Lal BK, Hobson RW. Pixel distribution analysis to improve patient selection for CEA and CAS. Endovascular Today. 2006;5:23-26.
22 Fields WS, Maslenikov V, Meyer JS, et al. Joint study of extracranial arterial occlusion. V. Progress report of prognosis following surgery or nonsurgical treatment for transient cerebral ischemic attacks and cervical carotid artery lesions. JAMA. 1970;211:1993-2003.
23 Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA. 1991;266:3289-3294.
24 Hobson RW2nd, Mackey WC, Ascher E, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48:480-486.
25 Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation. 1995;91:566-579.
26 Halliday A, Mansfield A, Marro J, et al. MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491-1502.
27 Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.
28 Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660-1671.
29 Eckstein H-H, Ringleb P, Allenberg J-R, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893-902.
30 Hobson RW2nd. CREST (Carotid Revascularization Endarterectomy versus Stent Trial): background, design, and current status. Semin Vasc Surg. 2000;13:139-143.

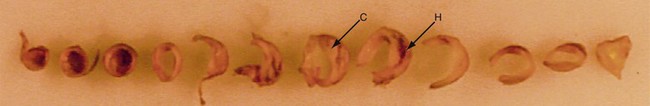
 FIGURE 89-1
FIGURE 89-1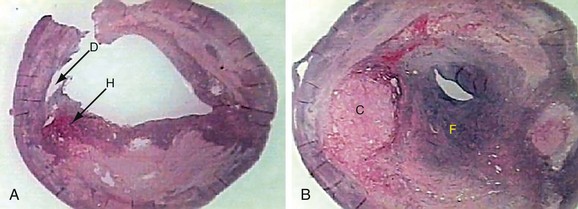
 FIGURE 89-2
FIGURE 89-2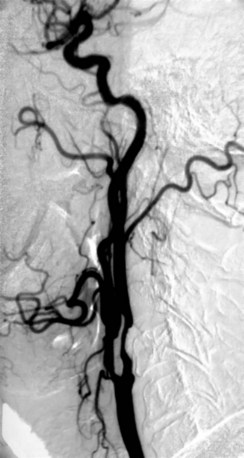
 FIGURE 89-3
FIGURE 89-3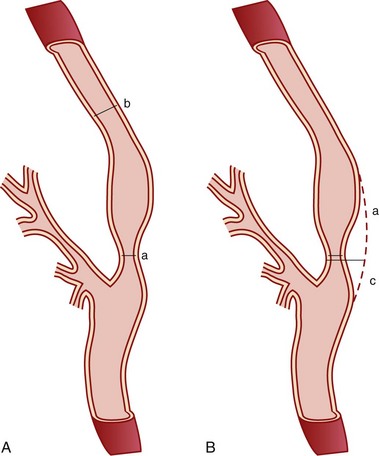
 FIGURE 89-4
FIGURE 89-4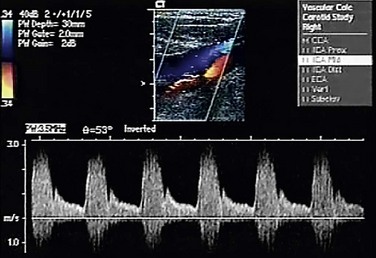
 FIGURE 89-5
FIGURE 89-5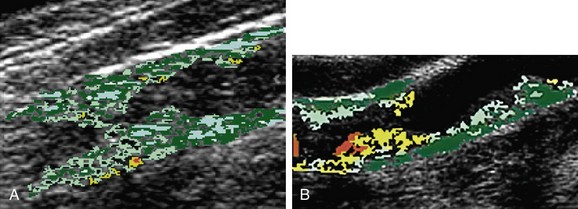
 FIGURE 89-6
FIGURE 89-6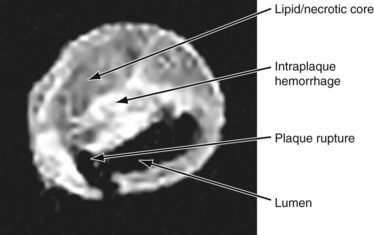
 FIGURE 89-7
FIGURE 89-7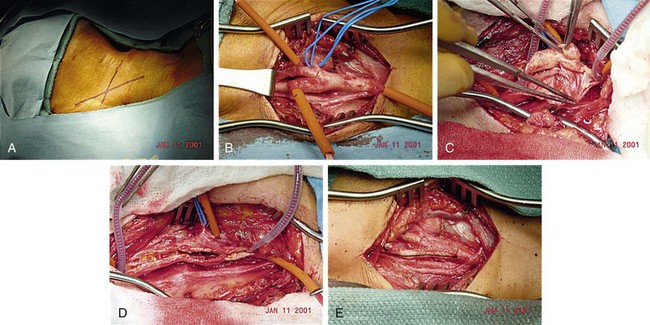
 FIGURE 89-8
FIGURE 89-8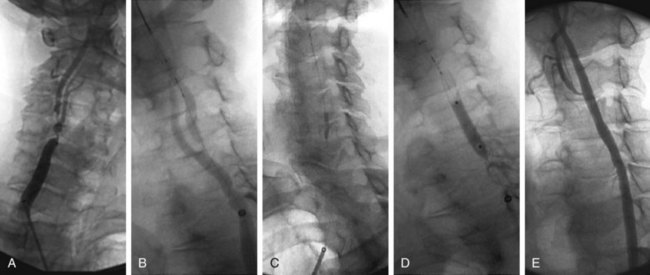
 FIGURE 89-9
FIGURE 89-9

