13 Caring for the patient undergoing radiotherapy
Introduction
After surgery, radiotherapy is the most effective curative treatment for cancer (Burnet et al 2000). Approximately 50% of patients in the UK will receive radiotherapy. Unlike the treatments discussed in previous chapters that are given systemically, the value of radiotherapy is in the local management of cancer. The success of radiotherapy is dependent on how bulky the tumour is, how sensitive the tissues are to radiation and the location of the tumour.
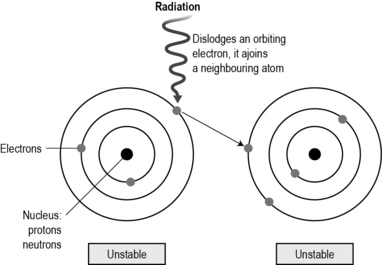
Radiation is a natural part of the electromagnetic spectrum. At one end of the electromagnetic spectrum are radio waves which have low energy and bounce around us; at the other end of the spectrum, X-rays, beta and gamma rays are highly energised and can penetrate into the body, causing ionisation. Ionisation is where the atoms within the cell are altered by the radiation. Atoms are electrical in nature. When they come into contact with radiation, the highly energised waves from the radiation cause an orbiting electron to be dislodged and join an adjacent atom which is said to be ionised (Fig. 13.1).
There are four principles (‘four Rs’) that support our understanding of how radiotherapy works:
• Repair: radiotherapy damages cells both directly and indirectly. Directly, if the damage to DNA is great, the repair genes will not be able to fix the problem and the cell will commit suicide or undergo apoptosis. Indirectly, the radiotherapy interacts with oxygen and water molecules in the cell to damage DNA as it is synthesised. Very few cells are killed straight away – it takes several cell divisions before the cell dies. Cells that die immediately are very radiosensitive, such as lymphocytes and germ cells. These are very vulnerable and responsive to radiotherapy.
• Reoxygenation: the success of radiotherapy depends on the presence of oxygen. Many cancers have a poor blood supply and are often hypoxic. This reduces the indirect action of radiotherapy which requires oxygen to damage the synthesis of DNA. To maximise the amount of oxygen, external radiotherapy is generally given in fractions. These are individual doses of radiotherapy which allow well-oxygenated cells to be killed, making way for hypoxic cells to have access to more oxygen, making them more sensitive to the next fraction of radiotherapy. Fractionisation also allows normal cells to repair and repopulate which minimises side effects.
• Redistribution: those cells in G0 will not be killed initially as they are not actively in the cell cycle, but as cells that are actively cycling die, cells in G0 are recruited into the cell cycle to replace damaged cells. Giving fractions over a period of days/weeks means that cells are more likely to be in the cell cycle and be killed. Cells are most sensitive in the M and G2 phase.
• Repopulation: cancer cells that are not actively dividing when the radiotherapy is given will start to divide after the dose, in order to replace those cells destroyed. If another dose of radiation is given, these cells will also be damaged. If the cancer repopulation happens more quickly than the radiotherapy is given, the success of the treatment will be reduced. For example, when radiotherapy is given, a number of cancer cells are killed off and other cancer cells repopulate to replace those lost. If there is a delay in the treatment schedule, these cancer cells will continue replicating, reducing the chance of success. Some radiotherapy schedules, such as accelerated hyperfractionated (CHART) radiotherapy, are give twice a day to try to deal with the problem of cancer cells repopulating more quickly than normal cells.
Read Rosenfield and Stahl (2006) (see References). Discuss the role of radiotherapy and the use of other strategies used to manage bone pain, and the nursing implications for each, with your mentor
Where radiotherapy is used to palliatiate symptoms rather than as a definitive treatment, the effects will only last until the cancer grows. This condition is explained more fully in Chapter 15.
Other radiation treatments
An example of a sealed radioactive treatment is iridium wires which are inserted into the skin and tissues under a general anaesthetic. These wires are used to treat anal, vaginal and urethral cancers. The risk to healthcare professionals is much higher with this treatment as the radiation cannot be temporarily removed and the patient will be radioactive for the duration of the treatment. Similarly, iodine 131 is an unsealed source which is given as an oral capsule or liquid. When consumed it is absorbed into the thyroid gland and cancer cells in the thyroid will be killed. This is used in conjunction with, or instead of, a thyroidectomy. Because the iodine 131 is given orally, these patients will stay radioactive until the radiation starts to degrade – this may be several weeks. Patients who have had iodine 131 are required to stay in a lead-lined room for between 4 and 7 days (depending on the time taken to degrade the radioactivity). During their admission, patients are highly radioactive, therefore they should require minimal assistance with their activities of daily living and self-administer their medications, minimising contact with healthcare professionals. As a student, you should not enter a patient’s room if they have received either iodine 131 or iridium wires. This is discussed in Chapter 7 under health and safety issues.
Read Stajduhar et al (2000) (see References). Think about the preparation, care and safety issues that are required in delivering this treatment.
The impact of undergoing radiotherapy
Patients often are frightened or misunderstand what radiotherapy treatment actually is. This is often based on catastrophic events such as the World War Two atomic bombings of the Japanese cities of Nagasaki and Hiroshima and the nuclear power disasters in Chernobyl in the Ukraine in 1986 and Japan in 2011. Because of the technical nature of the treatment, patients and healthcare professionals often lack knowledge and understanding of how radiotherapy works. It is an abstract concept compared to surgical treatment where the cancer is physically removed or cytotoxic drugs are seen to enter the body. Radiotherapy is invisible. Peck and Boland (1977) demonstrated that approximately 60% of patients feel unprepared for treatment. Although this research is old, the figure is still relevant today. Information is required to outline what is involved in radiotherapy planning; how the treatment works; what to expect while in the radiotherapy department; the practicalities, such as parking; the duration of each treatment; what the likely side effects are and how these can be prevented; who to tell if a toxicity develops, etc. (Halkett et al 2010).
Like cytotoxic therapy, radiotherapy kills cells that are frequently dividing, so any normal cell that is active in the cell cycle and comes in contact with the radiation will potentially be damaged or killed. As all external radiotherapy beams have to enter the body through the skin, adverse skin reactions are common. The severity of the damage will depend on the dose; the number of fractions; and site of treatment (skin folds and areas of friction are more likely to increase the risk of a skin reaction). Exposure to sunlight will increase the risk of damage as will mechanical irritation such as clothes rubbing, shaving, etc. (Porock et al 2004). Other factors will influence how quickly and easily the body repairs the damage to the skin, such as age (the older we get, the slower the body repairs damage) and increased body weight (reduces healing, reduces blood supply, increased skin folds, friction and moisture).
To minimise radiation skin reaction, patients should be advised to wash their skin gently with mild soap and warm water; shaving the area of treatment should be avoided and clothing should be made from natural fibres that do not rub. When assisting a patient undergoing radiotherapy with their hygiene needs in an inpatient setting, you should also follow this advice. Patients should have their skin assessed throughout and after treatment to identify reaction and for prompt intervention (NHS Quality Improvement Scotland 2004). Eighty per cent of patients undergoing radiotherapy experience erythema, a redness and slight inflammation of the site, which occurs approximately 10–14 days after the first fraction. This coincides with the damaged basal layers migrating to the upper layers of the skin. The skin compensates by increasing cell division to replace the damaged cells. The new cells are immature and are more easily damaged. If the new cells are produced quicker than the old ones are shed, then dry desquamation develops; this looks like dry, flaky, itchy skin. If the old cells are shed before the new ones can reach the top, then the thin epidermal layer is easily eroded; this is known as moist desquamation. Moist desquamation is more likely to develop in skin folds like under the breast, groin and axilla. When the skin breaks down, there is a high infection risk and the wound must be managed carefully to avoid further damage so that healing is promoted (MacBride et al 2008).
Read Hollinworth and Mann (2010) (see References) and find out how radiation skin reactions are treated in your area. Which dressings are used? How are dressings held in place? What can be done to promote wound healing in a radiotherapy-related wound?
Another commonly reported general side effect is extreme fatigue. This tends to increase over the period of treatment. It is estimated that between 30% and 80% of patients undergoing radiotherapy will experience fatigue. This range is so wide, reflecting the subjective nature of the symptom and the difficulty in measuring fatigue (Jereczek-Fossa et al 2002). It is not clear why radiotherapy causes fatigue. It may be caused by travelling to and from the hospital daily and other side effects like loss of sleep, and the effort of the continual renewal of damaged cells.
As well as the general side effects, there are usually a number of site-specific toxicities depending on the site of the radiotherapy (Table 13.1).
| Site of radiotherapy | Specific toxicities |
|---|---|
| Chest | Cough, oesophagitis, pain, pneumonitis, lung fibrosis, spinal cord damage, dyspnoea, nausea, indigestion |
| Breast (may include the axilla) | Mild breast oedema, lymphoedema in arm, brachial plexopathy, pain, breast shrinkage, lung fibrosis, bone necrosis (ribs), cardiac damage |
| Brain | Cerebral oedema (may cause raised intercranial pressure), alopecia, exacerbation of existing symptoms, somnolence (sleeping), white matter changes, cognitive changes, reduced hormone production |
| Abdomen/pelvis | Abdominal cramps, diarrhoea, proctitis (inflammation of the anus and rectum), tenesmus (feeling of incomplete defecation) discharge, cystitis, frequency and urgency, incontinence, dysuria, infection, bowel and/or vaginal stenosis, fistula, impotence, infertility |
| Head and neck | Mucositis, infection, dysphagia, taste alteration, weight loss, increased salivary production (late effects might be reduced saliva production), dental caries, trismus (lock jaw), bone necrosis |
Burnet N., Benson R., Williams M. Improving cancer outcomes through radiotherapy. British Medical Journal. 2000;320:198–199.
Halkett G., Kristjanson L., Lobb E. Meeting breast cancer patients’ information needs during radiotherapy: what can we do to improve the information and support that is currently provided? European Journal of Cancer Care (Engl.). 2010;19(4):538–547.
Hollinworth H., Mann L. Managing acute skin reactions to radiotherapy treatment. Nursing Standard. 2010;24(24):53–54.
Jereczek-Fossa B.A., Marsiglia H.R., Orecchia R. Radiotherapy-related fatigue. Critical Reviews in Oncology/Hematology. 2002;41:317–325.
MacBride S., Wells M., Hornsby C., et al. A case study to evaluate a new soft silicone dressing, Mepilex Lite, for patients with radiation skin reactions. Cancer Nursing. 2008;31(1):E8–E14.
NHS Quality Improvement Scotland. Skincare of patients receiving radiotherapy: best practice statement. Edinburgh: NHS Quality Improvement Scotland; 2004.
Peck A., Boland J. Emotional reactions to radiation treatment. Cancer. 1977;40:180–184.
Porock D., Nikoletti S., Cameron F. The relationship between factors that impair wound healing and the severity of acute radiation skin and mucosal toxicities in head and neck. Cancer Nursing. 2004;27(1):1–78.
Rosenfield R., Stahl D. Pain management of bone metastases in breast cancer. Journal of Hospice and Palliative Nursing. 2006;8(4):233.
Stajduhar K., Neithercut J., Chu E. Thyroid cancer: patients’ experiences of receiving iodine-131 therapy. Oncology Nursing Forum. 2000;27(8):1213.
Bolderston A. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support. Care Cancer. 2006;14(8):802–817.
Faithfull S. Radiotherapy. In: Kearney N., Richardson A. Nursing patients with cancer: principles and practice. Edinburgh: Churchill Livingstone, 2006.
Faithfull S.. Radiotherapy. Corner J., Bailey C. Cancer nursing: care in context, 2nd ed, Oxford: Blackwell, 2008.
Faithfull S., Wells M. Supportive care in radiotherapy. Edinburgh: Churchill Livingstone; 2003.
Porock D. Factors influencing the severity of radiation skin and oral mucosal reactions: development of a conceptual framework. European Journal of Cancer Care (Engl.). 2002;11:33–43.
Souhami R., Tobias J. Cancer and its management, 5th ed. Oxford: Blackwell; 2005.
Yarbro C.H., Hansen Frogge M., Godman M. Cancer symptom management. Boston: Jones and Bartlett; 2004.
Yarbro C.H., Hansen Frogge M., Godman. Cancer nursing: principles and practice, 6th ed. Boston: Jones and Bartlett; 2005.