12 Caring for the patient undergoing biological therapy
Introduction
– attack or control the growth of cancer cells
– restore blood cells following an episode of cytotoxic-related neutropenia
– interfere with the way cells interact and signal to each other.
Read Waugh and Grant (2010) (see References) or a similar textbook to refresh your knowledge of the different parts and cells of the immune system. How does the immune system work to get rid of non-self cells?
There are numerous types of biological therapies. Table 12.1 identifies some of the main groups and provides examples of drugs/agents. Each group is discussed in turn.
| Groups of biological therapies | Examples of agents |
|---|---|
| Cytokines | Interferon, interleukins, tumour necrosis factor, colony-stimulating factors (G-CSF: pegfilgrastim/filgrastim and epoetin alfa) |
| Monoclonal antibodies (MoAbs) | Trastuzumab (Herceptin), rituximab, bevacizumab, cetuximab |
| Cancer growth blockers | Tyrosine kinase inhibitors: erotinib (Tarceva), imatinib (Glivec), getitinib (Iressa), sunitinib, dasatinib, lapatinib Proteasome inhibitors: bortezomib (Velcade) |
| Anti-angiogenic agents | Thalidomide |
| Cancer vaccines | Bacillus Calmette–Guérin (BCG) |
| Gene therapies | In development |
Cytokines
Another group of cytokines are the interleukins which are given intravenously (Batchelor 2006).
Yet another group is the haemopoietic growth factors. These stimulate production of white blood cells and help them mature. Rather than being used to eliminate cancer, these factors can be made synthetically, and boost recovery of the immune system when it has been damaged by cytotoxic therapy. Generally, granulocyte colony-stimulating factor (G-CSF) is used subcutaneously to stimulate neutrophil production and maturation, to prevent or minimise the severity and length of neutropenia and to lower the risk of infection when a patient is undergoing cytotoxic therapy and is likely to become immunocompromised. G-CSF is also used in haemopoietic transplant to increase the number of stems cells in the blood, before harvesting (discussed in Ch. 11).
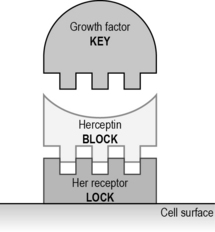
Monoclonal antibodies
Not all patients (even with cancer cells with extra receptors) will respond. Cancer cells can learn to avoid binding to the antibodies, by downregulating (reducing) the number of receptors available. In addition, MoAbs are big molecules and cannot reach all the cells in a large cancer which has narrow blood vessels (Fig. 12.1).
Battiato L.A.. Biological and targeted therapy. Yarbro C.H., Hansen Frogge M., Godman M. Cancer nursing: principles and practice, sixth ed, Boston: Jones and Bartlett, 2005.
Pecorino L. Molecular biology of cancer: mechanisms, targets and therapeutics, second ed. Oxford: Oxford University Press; 2008.
Souhami R., Tobias J. Cancer and its management, fifth ed. Oxford: Blackwell; 2005.
Yarbro C.H., Hansen Frogge M., Godman M. Cancer symptom management. Boston: Jones and Bartlett; 2004.