49 Care of the pediatric patient
Anesthetic: An agent used to produce anesthesia.
Anesthesia: Partial or complete loss of sensation, with or without loss of consciousness; referred to in this chapter as the administration of an anesthetic agent via injection or inhalation.
Antagonist: To counteract the action of something else, such as a drug that binds to a receptor site and prevents receptor stimulation.
Anticholinergic: A parasympatholytic; blocks the parasympathetic nerve fibers and parasympathetic nerve impulse conduction.
Anxiolytic: Medication used to reduce, relieve, or counteract anxiety.
Apnea/Apneic: Suspension of breathing.
Aspiration: The general use of this term is to draw in or out via suction; however, specifically referred to in this chapter are situations in which an individual is at risk for entry of gastric secretions, oropharyngeal secretions, or exogenous food or fluids into tracheobronchial passages, because of loss of the normal protective mechanisms as occurs with induction of general anesthesia.
Atelectasis: A collapse, lack of expansion, or airless condition of the lungs.
Barotrauma: An injury caused by a change in atmospheric pressure relative to a potentially closed space within a surrounding area.
Child: Younger than 13 years of age; before puberty.
Conception: Onset of pregnancy with implantation of a fertilized ovum in the uterine wall; fertilization.
Delirium: An acute and reversible condition characterized by agitation, confusion, disorientation, hallucinations or delusions, difficulty focusing attention, and inability to rest.
Desaturation: When oxygen is dissociated from hemoglobin.
Dissociative: A type of anesthesia with marked catalepsy, amnesia, and analgesia.
Dysphoria: A mood disorder of restlessness without apparent cause, anxiety, dissatisfaction, and discomfort.
Emergence: To evolve or rise out of anesthesia to a level of consciousness and status of protective reflexes, motor activity, and orientation.
Emergence Delirium: Occurs during initial cessation from general anesthesia to an awake state and initial transfer into the postanesthesia care unit.
Erythropoiesis: Forming red blood cells.
Gestation: Period of intrauterine fetal development from conception to birth.
Hemostasis: To stop bleeding; stasis refers to standing still.
Hypercarbia, Hypercapnia: Elevated above normal levels of carbon dioxide in the blood (>45 mm Hg).
Hyperflexion: Increased flexion of a joint; in this text, refers to the neck.
Hyperoxia: Increased levels of oxygen in the blood.
Hyperthermia, Hyperpyrexia: An elevated body temperature greater than the normal range.
Hypervolemia: An abnormal increase in circulating blood volume.
Hypothermia: A lower body temperature below normal range.
Hypovolemia: An abnormal decrease in circulating blood volume.
Hypoxemia: Decreased levels of oxygen in the blood.
Induction: Anesthetization, onset of general anesthesia.
Infant: Includes the neonatal period and extends through 12 months of age.
Inhalation: To draw a breath, vapor, or gas into the lungs.
Inspiratory Pressure: An active positive pressure ventilatory maneuver in which a delivered volume of gas is given to a set peak level of pressure before passive expiration.
Isotonic: In this chapter, pertains to an intravenous solution with the same osmotic pressure as normal body fluid.
Laryngospasm: A spasm of the laryngeal muscles.
Larynx: The musculocartilaginous organ at the upper end of the trachea, below the root of the tongue, and part of the airway and vocal apparatus.
Macroglossia: An abnormally small tongue.
Maintenance: Stage of anesthesia in which relaxation of muscles and loss of sensation and consciousness are adequate for the performance of surgery.
Micrognathia: Refers to the jaw; abnormal smallness, particularly of the lower jaw.
Neonatal Period: The first 28 days of life.
Newborn (“Newly Born”): Younger than 72 hours.
Occiput: The back part of the skull.
Parenteral: Any route of administration for a medication other than alimentary; such as intravenous, subcutaneous, intramuscular, or mucosal.
Pediatrics: The medical science specific to the care of children and treatment of diseases that occur in childhood.
Pharynx: Refers to the passageway from the nasal and oral cavity to the larynx and esophagus.
Postconceptual Age: Postgestational age (number of weeks since birth) plus conceptual age (number of weeks at delivery).
Premature Newborn: Birth before 37 weeks’ gestation.
Rebreathing: Inhalation of a gas or gases previously exhaled.
Retrognathia: When the mandible lies behind the frontal plane of the maxilla.
Thermogenesis: Heat production. Nonshivering thermogenesis is a physiologic response of the newborn infant during periods of hypothermia with stimulation of the sympathetic catabolism of brown fat with release of energy in the form of heat. Brown fat is primarily located in the neck and chest of the infant.
Anatomic and physiologic considerations
Respiratory system
Understanding the differences between the adult and pediatric respiratory systems is essential to properly manage the pediatric airway. There are several distinctions of the pediatric airway that make these patients more susceptible to airway obstruction and hypoxemia.1,2–5 Respiratory distress will occur quickly in the pediatric patient if respiratory complications are not managed quickly and properly.2
The newborn has small nares, a large tongue, a small mandible, a short neck, and a large amount of upper airway lymphoid tissue.2–6 Newborns are considered obligate or preferential nose breathers4–6; therefore anything that partially or fully blocks the nares can result in respiratory compromise. In the neonate, the epiglottis is at the level of the first cervical vertebra (C1); however, the epiglottis usually moves down to the level of C3 by 6 months of age (this makes oral breathing more feasible).3 The epiglottis of the newborn is U shaped versus being flatter in the adult. A straight laryngoscope blade may be more maneuverable in the pediatric airway and is most commonly used for intubation in pediatric patients. The tracheal length is relatively short in children, which makes proper placement and securing of the endotracheal tube critical to avoid bronchial intubation (or accidental extubation).7 When the endotracheal tube is secured (and anytime the patient is repositioned), the presence of bilateral breath sounds and end-tidal carbon dioxide (ETCO2) should be reconfirmed.
The vocal cords of the newborn are more anterior (C4) than in adults (C6)3–5; this makes it more difficult to properly align the airway for ventilation and intubation.5,6 Historically, the shape of the child’s larynx has been thought to resemble that of an inverted cone with the narrowest portion of the trachea residing at the cricoid cartilage.1,3,4,6 Recent research suggests that the narrowest portion of the pediatric trachea may actually be the glottis.5 No matter where the smallest diameter lies, the diameter of the endotracheal tube that can be used is limited. In addition, because the diameter of the pediatric airway is small, airway edema may lead to significant narrowing (and potential occlusion) of the airway.
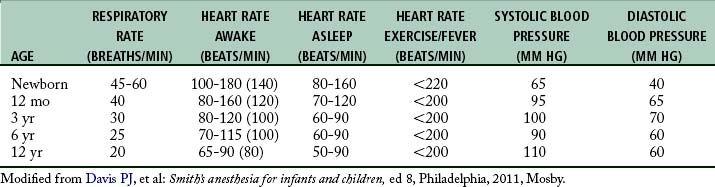
Newborns are diaphragmatic breathers.5,6 The ribs of newborns are situated horizontally in a cylindrical thorax, which limits thorax expansion. Consequently, ventilatory efforts are the result almost entirely of the movement of the diaphragm. Newborns are susceptible to ventilator problems when excursion of the diaphragm is impeded. As a result, gastric distention caused by faulty bag and mask ventilation, improper positioning, or bowel obstruction can produce inadequate ventilation.5 In the pediatric patient, the sternum and anterior rib cage are compliant, and the intercostal and accessory muscles of respiration are poorly developed. The respiratory rates of infants and young children (Table 49-1) are faster than those of adults.5 This faster rate is a result of (1) the lung volumes in infants being extremely small in relation to their body size and (2) the higher metabolic rates in infants (oxygen consumption per unit body weight is double that of adults). This is the main reason that pediatric patients rapidly desaturate during short periods of hypoventilation or apnea.
The control of breathing in infants during the first several weeks of life differs significantly from that of the adult patient. As in the adult, the newborn’s primary drive to ventilation is carbon dioxide; however, hypoxemia depresses rather than stimulates respiration in the newborn.5 This secondary response is potentiated further by hypothermia, a condition that can occur at any point in the perioperative period.
The respiratory control center in both full-term and premature infants can fatigue easily; therefore ventilatory reaction to high carbon dioxide tensions or to low percentage of oxygen is not as rapid in the newborn.5 As a result, the newborn might not be able to compensate for rapid changes in arterial blood gas levels. By 3 weeks of age, hypoxemia induces sustained hyperventilation, as in older children and adults.5 In addition, newborns and infants may breathe irregularly because of the lack of a mature respiratory center. Periodic breathing is often seen in this age group.5
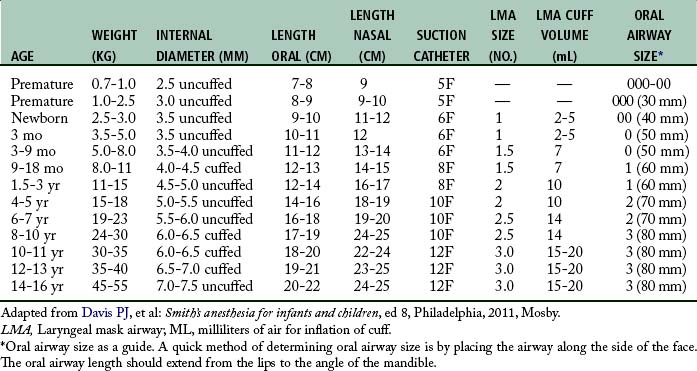
Endotracheal intubation is more widely used in pediatric anesthesia today and is considered the preferable airway management technique for general anesthesia in premature infants and most neonates. The reason for this change is that the premature infant and neonate can prove difficult to ventilate by mask, which increases the risk of filling the stomach with air during mask ventilation.1 Both endotracheal tube and laryngeal mask airways have been used safely in children of all ages (Table 49-2). The advantages of endotracheal intubation include decreased dead space, avoidance of laryngospasm and gastric distention, and prevention of aspiration; however, the incidence rate of post intubation edema from trauma and infection may be increased.
Cardiovascular system
As the pediatric patient matures, the cardiovascular system undergoes substantial changes. Normally the respiratory rate and heart rate decreases with increasing age.5 Advancing age and increasing body size result in increases in the systolic and diastolic blood pressure. The cardiovascular age-related changes for newborns, infants, and children are summarized in Table 49-1. The newborn heart functions near its peak ventricular function and therefore has little cardiac reserve. Thus, in the newborn, heart rate plays a major role in determination of cardiac function.5 The newborn is relatively unable to compensate for suboptimal conditions such as hypoxemia, acidosis, or myocardial depression.5 With the advent of more sophisticated blood pressure monitoring devices, measurements in infants can be taken with greater accuracy. The pediatric patient ordinarily has the usual signs of impending shock or airway obstruction, but physiologic status deteriorates rapidly if the problem is not rectified quickly.1–3,5,6 The perianesthesia nurse should closely observe children for subtle changes in cardiovascular status. If abnormalities arise, prompt intervention is essential.
At birth, fetal hemoglobin levels are high compared with those in the adult patient; however, the fetal hemoglobin does not readily release the oxygen it carries to tissues. Hemoglobin values decrease progressively and reach their lowest values by 2 to 3 months of age.5 By 4 to 6 months of age, the amount of oxygen available to tissues begins to increase and reaches the highest value usually by 10 months of age. This increase remains steady during the first decade of life. Research regarding the physiologic anemia of childhood suggests that, although children’s hemoglobin levels are lower than adults, oxygen unloading at the tissue level is increased in children.5 This allows a lower level of hemoglobin in infants and children to be as efficient in tissue oxygenation as a higher hemoglobin in adult patients (Table 49-3).
Composition and regulation of body fluids
Maturation of the kidneys in newborns occurs rapidly. In the neonate, renal function is characterized with obligate salt loss, slow clearance of fluid overload, and an inability to conserve fluid.8 Consequently, newborns are intolerant of both dehydration and fluid overload. The newborn can conserve sodium to some degree despite a low glomerular filtration rate and limited tubular function5; however, premature infants are prone to hyponatremia and water overloading. Dehydration in the neonate of any gestational age has harmful effects on renal function.5 Moreover, decreased renal function can delay the excretion of drugs primarily eliminated by renal clearance. At 20 weeks after birth, maturation of glomerular filtration and tubular function is nearly complete.4,5
The blood volume of the newborn younger than 1 month of age is approximately 80 to 90 mL/kg3; however, the blood volume of the premature newborn is as high as 100 mL/kg. The estimated blood volume of an infant from 3 months until 3 years of age is 75 to 80 mL/kg. In children older than 6 years, the estimated blood volume approximates that of an adult (65 mL/kg in the adult female; 70 mL/kg in the adult male).3
Water distribution in the various body compartments is markedly different among the premature newborn, the full-term newborn, the child, and the adult. Water distribution is significant because body water composition affects the volume of distribution of drugs. Premature infants have the highest percentage of fluid in the extracellular fluid compartment. A progressive decrease in total body water and distribution to the extracellular fluid compartment is seen during the first year of life. Complete maturation of renal function occurs when the child reaches 2 to 3 years of age. The fluid requirements for infants and children are reviewed in Table 49-4.
Table 49-4 Formula for Hourly Maintenance Fluid Requirements in Infants and Children
| BODY WEIGHT (KG) | HOURLY FLUID REQUIREMENT* |
|---|---|
| 0-10 | 4 mL/kg/h for each 1 kg body weight |
| 10-20 | 40 mL + 2 mL/kg/h for each 1 kg >10 kg |
| >20 | 60 mL + 1 mL/kg/h for each 1 kg >20 kg |
* Based on 1 mL of fluid per 1 kcal of caloric expenditure.
From Davis PJ, et al: Smith’s anesthesia for infants and children, ed 8, Philadelphia, 2011, Mosby.
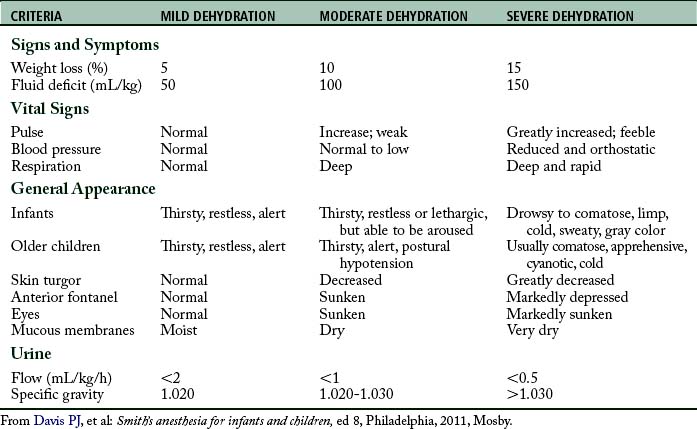
It is important to assess the hydration status of the pediatric patient to formulate an appropriate therapeutic strategy. Guidelines for assessing dehydration in children are provided in Table 49-5. Laboratory data, history and physical, and assessment of fluid input and output should be used to aid in the diagnosis of dehydration and guide therapy.
Thermal regulation
Newborns and infants are sensitive to heat loss because they have a relatively large body surface area, a relatively small amount of subcutaneous fat, poor vasomotor control, and a decreased ability to produce heat.9 The primary mechanism of heat production in a neonate is nonshivering thermogenesis mediated by brown fat.1,5,10 Shivering is of little significance to thermal regulation. When ambient temperature falls (<33° C), epinephrine is released by the sympathetic nervous system to activate thermogenesis. The preterm newborn needs a higher ambient temperature (at least 35° C) to minimize oxygen consumption.5 Ordinarily, to maintain a body temperature within normal limits, infants metabolize brown fat, cry, and move about vigorously. Newborns and infants respond to a cold environment by increasing their metabolism, which ultimately leads to an increase in oxygen consumption and the production of organic acids.
Prematurity
A premature newborn is defined as birth before 37 weeks’ gestation.3,5,6,8 The often labile condition of a premature neonate demands meticulous and vigilant perianesthesia care. Careful attention must be given to airway maintenance, medication dosage, fluid management, and temperature regulation. Premature infants and infants younger than 6 months are prone to airway obstruction and apneic episodes.3,5 Most infants in whom postanesthesia apnea develops are less than 46 weeks of postconceptual age; however, apnea has been reported in infants up to 60 weeks of postconceptual age.2 In addition to apneic spells, pulmonary complications include hyaline membrane disease and bronchopulmonary dysplasia. In addition, the premature neonate is immunocompromised and at greater risk for postoperative infection. In the sick premature neonate, the likelihood of blood transfusions, artificial ventilation, and the need for parenteral nutrition is greater.2 The risk of apnea in the postanesthesia care unit (PACU) may be decreased with intravenous (IV) administration of caffeine (10 mg/kg).5 In neonates, the half-life of caffeine is 37 to 231 hours.5 By 4 months of age, the half-life of caffeine decreases dramatically to approximately 6 hours and is similar to that in an adult. In addition, several authors cite the initial discovery of xanthine derivatives, such as theophylline or aminophylline, as a respiratory stimulant that can be used to decrease the frequency of apneic episodes in the newborn.2,5,8
Retinopathy of prematurity
Retrolental fibroplasia is a fibrovascularization and scarring of the retina. Although this disease is associated with hyperoxia (too much oxygen), a multitude of other risk factors may be involved, and the role of oxygen therapy is controversial.5,8 The risk of this retinal disorder is to newborns, especially premature infants who are born before 36 weeks’ gestation and weigh less than 1000 to 1500 g.5 Vascularization of the retina is complete at approximately 44 weeks’ gestation.8 The extreme prematurity may be the single most important factor in the development of retinopathy of prematurity. The normal PaO2 in neonates is between 60 and 80 mm Hg. Oxygenation is recommended to be continuously monitored with pulse oximetry, and hyperoxia should be avoided; therefore a saturation of 90% to 95% results in a PaO2 in the range of 60 to 80 mm Hg.5,6,8 In addition, the pulse oximeter probe must be placed on the right upper extremity or ear lobe, in case of a patent ductus arteriosus. Placement of two pulse oximeter probes on the premature infant may be helpful. Moreover, when an arterial catheter is indicated, it also should be placed in the right upper extremity.
In susceptible patients who are exposed to a hyperoxic environment, blood gas tension should be measured and an oxygen analyzer used to confirm the oxygen concentration. It is not known what level of oxygenation, or what exact length of exposure time, might lead to the development of retinopathy of prematurity.8 One must consider that attempts to prevent arterial hyperoxia and visual impairment must be tempered with the realization that unrecognized arterial hypoxemia can result in irreversible brain damage.
Infant respiratory distress syndrome
Infant respiratory distress syndrome (IRDS), once called hyaline membrane disease, is a severe disorder of the lungs of the newborn.11 The incidence rate of IRDS increases in premature infants. The basis of the pathogenesis of IRDS is insufficient surfactant levels.8 Surfactant is beneficial for the following two functions: (1) reduction of surface tension so that less pressure is required to hold the alveoli open and (2) maintenance of alveolar stability with adjustment of surface tension to changes in alveolar size. Insufficient surfactant levels increase surface tension at the alveolar air-liquid interface, resulting in alveolar collapse, an inordinate increase in the work of breathing, and impaired gas exchange. This impaired gas exchange results in hypoxemia and hypercarbia. In addition, the pulmonary vascular resistance is increased and leads to hypoperfusion of pulmonary and systemic circulation. This hypoperfusion, along with hypoxemia, causes tissue hypoxia and metabolic acidosis. An increase in survival with a decrease in serious complications is associated with administration of surfactant into the lungs at birth.5 As lung compliance improves, a progressive decrease in tidal volume and positive inspiratory pressure helps to prevent further lung injury.
Treatment for neonates with severe IRDS includes oxygen therapy, maintenance of intravenous fluids and nutritional support, temperature regulation, arterial blood gas monitoring, and laboratory sampling.8,11,12 Extremely preterm infants and those with severe disease often need intubation during delivery room resuscitation or shortly after birth.8 In addition, intermittent positive-pressure mechanical ventilation with positive end-expiratory pressure may be necessary to ventilate the exceptionally stiff lungs of these neonates. Chronic air trapping in preterm infants can occur, and excessive inflation pressures must be avoided.5
Pediatric perianesthesia considerations and techniques
Preoperative period
The preoperative meeting with the pediatric patient and his or her guardians begins the foundation of the perioperative care for this child. A thorough preoperative examination of the pediatric patient and the child’s medical record enable the nurse to assess the patient’s general state of health and identify chronic or acute disease processes. The preoperative evaluation includes (1) reviewing the patient’s chart; (2) reviewing current and past medical history of the patient with the patient and guardian(s); (3) determining medication, latex, and food allergies; (4) determining the fasting (nothing by mouth [NPO]) status of the patient; (5) formulating a list of the patient’s current medications (including herbal medications); and (6) reviewing any lab tests. Most importantly, the preoperative interview gives the nurse the opportunity to gain the confidence of the patient and caregiver which can help to alleviate anxiety. Depending on the age of the child, after the interview the nurse may be responsible for starting the intravenous catheter for the pediatric patient. If the child is healthy, this task may be postponed until the operating room, after mask induction with general anesthesia has been initiated.
Before initiating any preoperative interventions, the NPO status of the patient must be confirmed. NPO guidelines have been formulated to help prevent the aspiration of stomach contents into the lungs during surgery. The most current guidelines suggest the following: (1) solids are prohibited within 6 to 8 hours of surgery, (2) formula is prohibited within 6 hours of surgery, (3) breast milk is prohibited within 4 hours of surgery, and (4) clear liquids are prohibited within 2 hours of surgery.1
Even if the nurse gains the trust of the child and guardians, this may not be enough to alleviate all of the pediatric patient’s anxiety. Premedication with various anxiolytic medications might aid in these situations. It must be remembered, however, that the most common fear for children concerning hospitalization is the pain from needles or venopuncture; therefore the route of administration should be considered carefully. Children 1 year of age and older benefit from anxiolytic premedication to decrease preoperative anxiety and modify behavioral changes after discharge.13 Many anesthesia practitioners use oral midazolam premedication in pediatric patients 1 year of age and older, in whom a greater likelihood of uncontrollable separation anxiety exists. In addition, midazolam can be given intramuscularly, intravenously, rectally, or intranasally as an alternative route of administration for pediatric premedicant.13 However, the oral route is generally preferred unless preoperative intravenous access is available. The effect of premedication in children varies from sedation to extreme excitation. Flumazenil is a competitive antagonist at the benzodiazepine receptor and is used as a reversal agent for midazolam.5,8,14 An initial dose has been recommended of 0.2 mg of flumazenil IV (8 to 15 mcg/kg IV) given over 15 seconds14; this usually reverses the central nervous system effects of midazolam within 2 minutes. If need, additional doses of 0.1 mg IV to a total of 1 mg IV can be administered at 60-second intervals.14
An anticholinergic drug, such as glycopyrrolate or atropine, may be given preoperatively to protect against bradycardia, which can occur with the induction of general anesthesia.5,8,14 The most popular inhalation anesthetic agents used for pediatric anesthesia are halothane, sevoflurane, isoflurane, and desflurane. Halothane and sevoflurane are the most common induction inhalation agents, although halothane is no longer available for use in the United States. Sevoflurane may be superior to halothane for mask induction of general anesthesia because of (1) a quicker induction of anesthesia, (2) less incidence of cardiac arrhythmias, (3) more rapid psychomotor recovery postoperatively, and (4) less nausea and vomiting.15 After induction of general anesthesia with either sevoflurane or halothane, and when the child reaches the maintenance phase of general anesthesia, the anesthesia provider may switch to either isoflurane or desflurane. To help prevent emergence delirium, an analgesic should be administered before emergence from general anesthesia and transfer to the PACU.
Ketamine, a dissociative anesthetic, is sometimes used in pediatrics as an induction agent or for short procedures, such as painful dressing changes that do not require muscle relaxation. Emergence time depends on route of administration and whether the drug was repeated during the operation. The most serious disadvantage to ketamine is a high incidence rate of emergence delirium, hallucinations, and possible psychosis.14 Nystagmus can also occur. After the use of ketamine, the postanesthesia recovery area should be quiet and conducive to a slow peaceful emergence and recovery. A premedicant with a sedative, such as midazolam, can significantly reduce these side effects.8 It is suggested that, if possible, ketamine should always be administered with a drug such as midazolam1,8,14; this helps to reduce the emergence delirium. Ketamine has the advantage of providing analgesia and supporting spontaneous respirations.14 Caution should be used when additional opioids are given in the PACU. For uncooperative children or patients with Down syndrome or mental retardation, an intramuscular injection of midazolam combined with ketamine may be helpful.2
Source: Dahmani S, et al: Ketamine for perioperative pain management in children: a meta-analysis of published studies, Paediatr Anaesth 21(6):636, 2011.
The opioid fentanyl can be given as a premedicant either IV or in the form of a lollipop called an Oralet (5 to 15 mcg/kg).4 When using the Oralet, when the child becomes sedate, the Oralet naturally falls away from the child’s mouth. With the Oralet, fentanyl levels continue to rise during surgery and contribute to postoperative analgesia. The fentanyl Oralet has fallen out of favor because of the potential for children to associate the medication with candy.
The introduction of the intravenous agent propofol has had a dramatic effect on pediatric anesthesia. Propofol can be used for sedation or general anesthesia and has minimal recovery time with few side effects.8,14,16 Propofol can be used when administering anesthesia in remote locations and provides deep sedation for painful or frightening procedures.1–3,5,8,14,17 This drug has advanced anesthesia care for children in remote locations such as magnetic resonance imaging or during procedures or therapy for hematology and oncology.17,18 Propofol provides rapid onset of action and quick emergence from anesthesia with minimal residual effects.19 Earlier discharge and decreased recovery time are particularly notable when propofol is the only anesthetic agent used.20 Maintenance of anesthesia can be accomplished through repeated dosing or continuous infusion.18 In addition, propofol has an antiemetic effect which has demonstrated usefulness in preventing postoperative nausea and vomiting in susceptible patients.1,8,14
Postoperative care of the pediatric patient
A large percentage of children become hypoxic during the transfer from the operating room to the PACU. In addition, the primary organ dysfunction seen in children involves the respiratory system.8 For these reasons, it is prudent to always transport the pediatric patient with supplemental oxygen. Furthermore, upon the patient’s arrival to the PACU, the first task of the nurse is to administer humidified oxygen and then connect the pulse oximeter. While sufficient oxygenation is being confirmed, the blood pressure cuff and electrocardiogram (ECG) leads can be applied. Always remember airway, breathing, and circulation (ABCs). The initial assessment should include identification of a patent airway and confirmation of stable vital signs, including temperature. A humidified air-oxygen mixture (high-flow) should be administered, and a safe environment secured before report from the anesthetist is given. In a restless child or in one with a history of seizure disorder, side rail pads may be used. The pediatric patient is usually placed in the lateral position on the stretcher in the operating room for transport to PACU (Fig. 49-1). However, after an intraoral surgical procedure, the patient may be placed in the three-fourths prone position, more commonly known as the tonsillar position, to facilitate adequate drainage of secretions and blood.
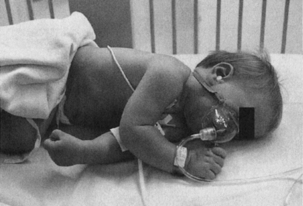
FIG. 49-1 An infant is transported to the postanesthesia care unit in lateral position.
(From Motoyama E, Davis P: Smith’s anesthesia for infants and children, ed 7, St. Louis, 2006, Mosby.)
Over the years, anesthesia techniques have improved. With combined rapid-acting anesthetic agents, the pediatric patient usually arrives in the PACU awake and responsive, with good abdominal muscle tone. These patients are usually quickly responsive to verbal and tactile stimulation. However, it has been reported that as many as 30% of pediatric patients experience some type of agitation or delirium during their PACU stay.8 Pediatric patients, especially those experiencing agitation, need constant nursing care to prevent injury to themselves. These patients can be extremely restless, vocal, and difficult to manage. It must always be remembered that this agitation or excitement could be related to hypoxemia, the pharmacologic side effects of certain drugs, pain, or awakening in strange surroundings. Therefore, if a pediatric patient has hyperactive behavior, the perianesthesia nurse should assess the patient in the aforementioned areas to provide the appropriate interventions.
Psychosocial considerations
The nurse must address issues of parental and child anxiety, which helps to lessen the stress on the child.21 The parents should be allowed to be with the child as soon as is practical and preferably before the child awakens.5,21 Remember that all surgical procedures and hospitalizations are extremely stressful for a family. When the pediatric patient emerges from anesthesia, the perianesthesia nurse should meet certain emotional needs of all children to facilitate positive outcomes of the perioperative experience. Explanations must be provided to the child in terms that can be understood. In addition, the nurse should always be completely honest with the child. The developmental age of the child determines which areas are of greatest concern (Table 49-6). For example, infants can become distressed when physical needs are not met. With the type of surgery and the degree of emergence from anesthesia taken into consideration, the primary caregiver should hold or rock the patient, if it is age-appropriate, or do both. This action usually relaxes the patient, and infants especially enjoy being swaddled in a warm blanket, cuddling, rocking, having the head or back rubbed, and hearing the voice of the nurse. In the infant younger than 6 months, the nurse is “mom.”7 Because the infant mimics facial expressions, the nurse should smile and use facial expressions of happiness when caring for all children. Another consideration is that the PACU is a strange environment for the child. Often, dolls, teddy bears, and other familiar objects from home are taken to surgery and arrive with the patient in the PACU. These special toys should remain with the child, especially during emergence, to help the child cope with the environmental change.
In the older infant and preschool child, stranger anxiety remains the greatest fear. This stage is a particularly vulnerable one in childhood development. Children at 2 to 3 years of age are at the stage of autonomy versus self doubt. They may exhibit independence that alternates with sudden dependence and the need for periodic cuddling and reassurance. Their greatest fears include separation from parents, pain, physical harm, strange environments, and the unknown.5 Often the only mechanism of self expression is crying. Negativism may be the child’s means of demonstrating control; thus, “no” may actually mean “yes.” Children of this age are prone to temper tantrums, ritualistic behavior, and breath-holding spells. The perianesthesia nurse should be sure to differentiate apnea from breath-holding spells when assessing the respiratory status of the patient. Three-year-old children are too young to use their own reason and can become impatient at times; therefore the perianesthesia nurse must avoid criticism and provide acceptable behavior alternatives to the patient.
Between 3 and 6 years of age, the child begins to become independent; however, in the PACU, dependency can occur because of pain, course of disease, or immobilization. Guilt can occur when the child desires to remain dependent.8 Consequently, the perianesthesia nurse should provide as much opportunity for independence as possible. The nurse can foster independence by allowing the child to select alternatives in care.
The ages of 6 to 12 years coincide with entering school. These children are striving for approval when tasks are completed and usually do not tolerate failure because it promotes their sense of inferiority and inadequacy. The hospital environment is new to them, and the child may be unprepared to handle the situation and thus have difficulty with impulse control. The nurse should allow children as much individualization and self-care as possible, because children lose control when they are immobilized or ill. The nurse should also encourage self expression and compliment the child on accomplishments during recovery from anesthesia. Remember that a child of this age has a vivid imagination and could easily distort reality.8
Monitoring
Initially, monitoring in the PACU includes respiratory rate, blood pressure, pulse oximetry, ECG, fluid balance, and temperature control.1,5,8 When the vital signs are obtained, the PACU values should be first compared with the preoperative and intraoperative recordings of vital signs. After the initial blood pressure is ascertained, particularly for outpatients, pulse oximetry monitoring may be all that is needed. The pulse oximeter is superior to clinical judgment in providing the earliest warning of a desaturation event.5,8 The frequency in measurement of other physiologic parameters depends on the status of the patient and the surgical procedure.
The most likely causes of respiratory failure in children with surgical disease include extrathoracic airway swelling and injury, thoracic dystrophy consistent with congenital disease or intraabdominal swelling, respiratory control abnormality such as occurs with congenital anomalies or drug induced, and loss of functional residual capacity that results in atelectasis.8 The most common etiology involves the extrathoracic airway with swelling of the pharynx, larynx, or trachea. Infants and small children usually have a low incidence rate of postoperative atelectasis, because crying from pain or awakening in an unfamiliar environment stimulates ventilation. Older children tend to remain in one position and not move. They must be encouraged to cough and to perform the sustained maximal inspiration (SMI) maneuver to prevent atelectasis. If the pediatric patient is unable to perform the SMI maneuver, deep breathing should be encouraged.5,8
A change in heart rate of the pediatric patient is one of the first clues of impending physiologic dysfunction.5 The PACU provider should initially consider hypoxia as the most likely cause of bradycardia. In the PACU, the heart rate of infants and children is influenced by physical activity, fluid volume replacement, and the administration of atropine, glycopyrrolate, and anesthetic agents. Glycopyrrolate (Robinul), an anticholinergic atropine-like drug, may elevate the heart rate mildly, and not to the same degree as atropine.14 Crying, struggling, or pain can also increase the heart rate.
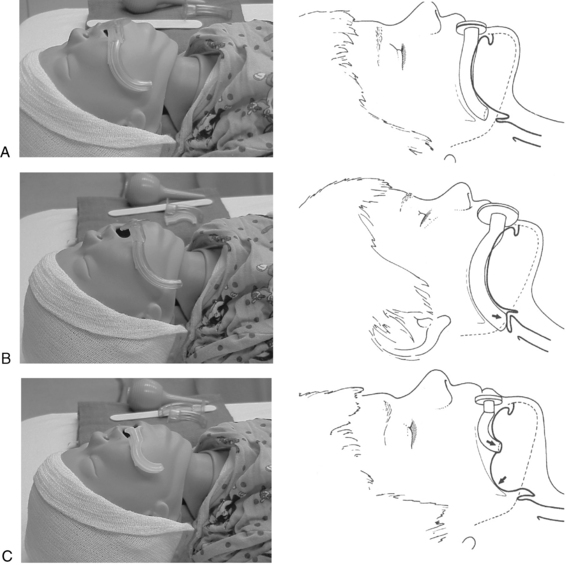
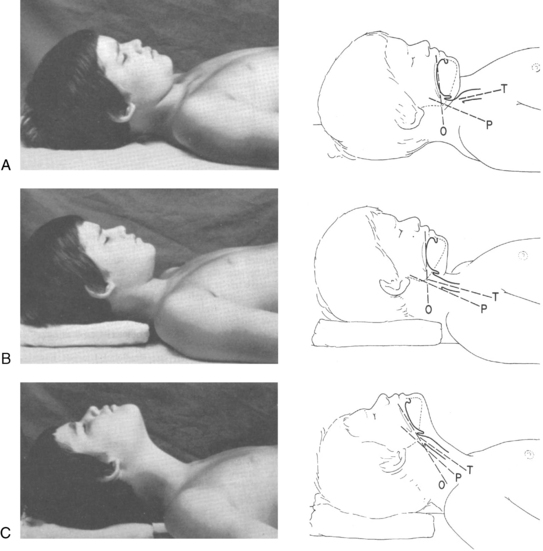
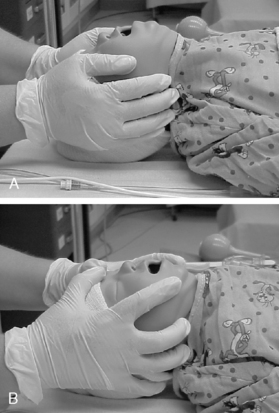
In the event of respiratory dysfunction, the designated PACU attending anesthesia provider should be notified immediately. An ongoing airway assessment should be performed, and emergency airway management may be necessary. An oral airway must be of proper size and correct placement to relieve airway obstruction (Fig. 49-2). In addition, correct positioning of the patient assists with ventilation and intubation if necessary; however, correct positioning varies depending on the age of the child. For example, children 6 years of age and older benefit from a folded towel or small pillow placed under the occiput in combination with extension of the head (Fig. 49-3). This position has often been referred to as the sniffing position. In infants and younger children, usually the size of the head is large relative to the trunk and hyperflexion of the neck occurs with lying flat on a bed. Further elevation of the occiput with a folded towel most likely hinders airway management. Mild flexion of the neck, with slight extension of the head, can be accomplished with the placement of a shoulder roll. Optimal positioning of the head and neck should assist in maintaining a patent airway and helping to ensure successful bag-mask ventilation when indicated. Excessive elevation of the occiput or exaggerated extension of the head and neck should be avoided.
Reversal agents such as flumazenil (0.1 mg/kg IV) and naloxone (1 to 10 mcg/kg IV) should be readily available in the event of hypoventilation that is unresponsive to stimulation and to improve or reverse respiratory depression secondary to benzodiazepine (midazolam) or narcotic overdose respectively.2,8,14 In addition, the anesthesia care team providers should be notified to offer additional assistance and follow-up care. Pressure-cycled ventilators are used in the neonatal intensive care unit to prevent barotrauma.3 With a pressure-cycled ventilator, the risk of pressure trauma to the lungs is reduced by allowing the peak airway pressure to be varied to support optimum ventilation. However, a peak airway pressure alarm and limit must be maintained to decrease the risk of trauma to the lungs. Therefore, in the event of necessary postoperative mechanical ventilation of an infant, the setting is most likely an intermittent mandatory ventilation rate of 20 to 40 breaths/min, with a peak positive inspiratory airway pressure set at approximately 20 to 24 cm H2O.3
The most likely indications for a central venous catheter include the need to monitor central venous pressure, cardiac surgery, inotropic drug administration, neurosurgery (with the potential risk of air embolism), major orthopedic procedures (such as spinal fusion), and abdominal surgical procedures when massive fluid shifting or blood loss is expected.5 The central venous pressure line can be inserted via the internal jugular, external jugular, subclavian, femoral, basilic, or axillary veins.5,8 This line provides for information on blood volume and serves as an avenue for fluid replacement. Remember that all rapid infusions of blood products or fluids should be warmed before administration. A fluid or blood warming device should have a visible thermometer and an audible warning that indicates excessive heating greater than 42° C.3 The arterial line, which is inserted through the umbilical (neonate) or radial artery, can measure blood pressure and heart rate and provide for instantaneous blood gas or laboratory sampling. The ECG results provide information on cardiac rate and rhythm. In the event of circulatory abnormalities, blood pressure and pulse should be ascertained and recorded frequently. Any deviation in the cardiac or pulmonary physiologic parameters should be reported immediately to the attending physician.
Newborns and infants expend a great amount of energy maintaining alveolar ventilation, cardiac output, muscular activity, and an appropriate temperature. Because of these high-energy metabolic processes, glycogen and fat stores may be mobilized and depleted rapidly.5,8 Consequently, cold, stress, pain, and increased muscle activity compound the need for adequate caloric intake in the PACU. In high-risk situations, plasma glucose concentrations should be monitored.12 The patient’s clinical condition dictates the final fluid and electrolyte requirements incurred as a result of trauma or complications from surgery. A glucose-containing infusion can be used for postoperative maintenance fluid replacement for children in high-risk circumstances2,3,5,6,8; however, rapid infusion of glucose-containing solutions should be avoided. During the administration of fluids, the perianesthesia nurse should ensure that the indwelling catheter is patent and not infiltrated and should use a constant infusion pump to facilitate the proper administration of the correct volume and rate of fluids. To prevent inadvertent overhydration, not more than one third of the day’s maintenance intravenous fluid volume should be measured into the intravenous bag at any time.
Important considerations for fluid management of the pediatric patient include monitoring for hypervolemia or hypovolemia. Proper administration of fluids must be ensured. When attending to issues related to hydration, additional areas of assessment and monitoring can be helpful and include the following: (1) urine output, specific gravity, and osmolality; (2) body temperature; (3) ECG status, pulse, and blood pressure; (4) hydration of the mucosa; (5) assessment of the fontanel, in the newborn, for bulging or depression; and (6) blood loss.5 An accurate assessment of blood loss must be made during and after surgery, because a small miscalculation of a few milliliters can have a serious effect on the total blood volume of an infant.
Permissible blood loss should be defined individually for each patient and is based on the patient’s current medical condition, surgical procedure, and cardiovascular and respiratory function.3,5,8 In healthy children with normal cardiovascular function, a lower hematocrit should be tolerated by increasing cardiac output when ventilation is not compromised. A higher inspired oxygen concentration should be provided. In the neonate or child with significant cardiac or pulmonary disease, initiation of blood loss replacement occurs earlier because of the patient’s inability to compensate and because of a greater association with increased morbidity. Initially, ongoing blood loss is replaced with isotonic crystalloid at a ratio of 1:3; for every 1 mL of blood lost, 3 mL of isotonic fluid, such as lactated Ringer solution, is given. When losses are high, the usual blood replacement is such that for each 1 mL lost, 1 mL of packed red blood cells is administered (1:1) to the patient. Alternatively, if a colloid solution is used, it is replaced at the same ratio (1:1) as that for blood products, 1 mL of 5% albumin per 1 mL of blood lost, until the patient’s hematocrit level reaches a predetermined critical level that requires packed red blood cells.5
Thermal regulation in the postanesthesia care unit
Hypothermia can delay emergence from anesthesia and prolong the stay in the postoperative care unit (see Chapter 53). Hypothermia can have a detrimental affect on termination of neuromuscular blockade, metabolic balance, coagulation, and ventilatory control.1–3,5,8 If an infant or small child arrives in the PACU with inadvertent hypothermia, the nurse should assess the patient for (1) vital signs (core temperature, pulse, and respiratory rate), (2) pulse oximetry waveform and saturation, and (3) the degree of emergence from anesthesia. If the patient has a delayed emergence from anesthesia, the nurse should protect the patient from aspiration of gastric contents and from hypoventilation with positioning (see Fig. 49-1) and stimulation. Dysrhythmias and cardiovascular depression are associated with profound hypothermia. Hypothermia can also lead to a detrimental effect on coagulation, ventilatory control, and metabolism.1,3,5,8 Children with profound hypothermia should remain intubated and sedated. Continuous ECG monitoring is necessary until the core temperature reaches at least 35° C.5 Finally, to avoid excess oxygen demand and acidosis associated with hypothermia, newborns and infants should be maintained in a neutral thermal environment in the PACU with the use of incubators, warm blankets, infrared heating lamps, warm-air heating blankets, or elevated room temperature when possible. Care should be taken to minimize environmental exposure during physical assessment, and the head should remain covered.
Postoperative pain management and regional anesthesia
Analgesia is a right of all patients, including pediatric patients.22 Inadequate management of a pediatric patient’s pain can have severe negative consequences both acutely and chronically.23 Therefore the management of postoperative pain in the pediatric patient must be a priority for the perioperative nurse. Numerous patient and situational factors must be considered when selecting and administering pain medications to children. If the postoperative plan of care includes hospital admission, then the analgesic regimen can be more flexible because the patient will have skilled nursing care available. Conversely, an alternate regimen may need to be selected for the outpatient.
The issues related to pain management for children have received increased attention in recent years.5,23–28 Depending on the age and maturity of the child, various choices for pain management are available. Choices for pain management include parenteral or oral opioids, nonsteroidal antiinflammatory drugs (NSAIDs), regional anesthesia, and even patient-controlled analgesia (PCA). Medications such as NSAIDs, and oral or rectal acetaminophen in combination with parenteral opioids have been shown to be effective for pain control in the postoperative period. The use of this type of multimodal technique also has the advantage of decreasing opioid requirements, which may be beneficial in the outpatient setting. The overall opioid dose and incidence rate of opioid side effects is decreased when combined with NSAIDs.22,25
Acetaminophen and NSAIDs can treat minor to moderate pain and have been shown to be effective at reducing postoperative pain in pediatric patients.22–30 Ketorolac is approved by the U.S. Food and Drug Administration for parenteral use (0.5 mg/kg, IV or intramuscular [IM], q6h).25 The recommended dosing of ibuprofen is 8 to 10 mg/kg, orally, every 6 hours. Acetaminophen is a nonopioid analgesic and antipyretic that can be given via the oral (10 to 15 mg/kg q6h) or rectal (20 mg/kg q4h) route.14 OFIRMEV (acetaminophen), the first IV formulation of acetaminophen in the United States, is indicated for the management of mild to moderate pain, management of moderate to severe pain with adjunctive opioid analgesics, and reduction of fever. It can be used in children who are 2 years old or older.29
Opioids are administered for moderate to severe pain. Routes of administration for opioids include oral, rectal, oral transmucosal (under the tongue), intramuscular, intravenous, transdermal, epidural, subarachnoid, and subcutaneous.1–3,5,8,10,28–30 The most common postoperative parenteral opioids used in the pediatric population include fentanyl (0.5 to 1.0 mcg/kg, IV/IM), meperidine (0.5 to 1 mg/kg, IV/IM,) and morphine (0.05 to 0.1 mg/kg, IV/IM).5 However, meperidine use is limited because its metabolite normeperidine can cause dysphoria, agitation, and seizures.5,8 In low doses, meperidine can be used for the treatment of rigors and shivering in the PACU. The most common side effects that occur with opioid administration are sedation, pruritus, nausea, vomiting, constipation, and respiratory depression.1–3,5,8 These side effects are dose dependent. Respiratory depression is of great concern in the pediatric patient; therefore vigilant monitoring of the patient’s respiratory status after administration of opioids is imperative. Supplemental oxygen may be required when administering these medications. Fentanyl and its analogs can produce chest wall rigidity when administered as a bolus, which makes mask ventilation extremely difficult.
Ketamine is a sedative, hypnotic medication that produces dissociative anesthesia.14 Ketamine is one of the only sedative, hypnotic drugs that also has analgesic properties. The preoperative or intraoperative use of ketamine has been shown to lower postoperative opioid requirements.1,8,31 A benefit of ketamine is that it promotes spontaneous respirations.1–3,8,14 This drug has been shown to be a highly effective adjunct to the management of postoperative pain in pediatric patients.31 When used in small (subanesthetic) doses (0.1 to 0.5 mg/kg), the common side effects (i.e., tachycardia, delirium, hallucinations, nightmares) are minimal.2,3 It has been suggested that ketamine always be administered with a benzodiazepine (midazolam) to help reduce the incidence of delirium, hallucinations, and nightmares.1,8 For more information regarding ketamine, refer to the Evidence-Based Practice box.
PCA has been used successfully in children as young as 6 years of age, but requires the understanding and cooperation of the patient.6,7,10,20 The inherent safety of PCA is based on the idea that a child who becomes too sleepy is not able to push the button to self-administer another dose of pain medication. Family members and nurses must not push the button for the child or medication overdose may result. By pushing a button, the child is able to deliver a precise opioid dose preprogrammed into the infusion pump. A minimum interval between dosing (a lock-out mechanism) and a maximum dose delivered over a set period of time are also preprogrammed to prevent overdose. A basal metabolic rate can be preprogrammed into the infusion pump to prevent severe breakthrough pain. In some cases, a basal infusion rate is not suggested for fear of additive sedation and for prevention of respiratory depression. Research has shown that the overall total drug consumption with PCA use is less.5,22,30 The potential complications associated with PCA include overdose from incorrect programming of parameters or mechanical malfunction of the device. The perianesthesia care provider should assess the child for side effects associated with opioids (nausea, vomiting, itching, and ileus). An order should be written to discontinue all previous pain medications. Children should be instructed on the use of a pain score method, such as the visual analogue scale, and a record for assessment of pain should be included on the vital sign sheet (Table 49-7). Morphine is most often the opioid of choice for postoperative PCA use in children.5 During orientation, all nurses should be trained in PCA use.
Regional anesthesia in children can dramatically improve pain management and lower general anesthesia requirements.5 The most common regional blocks in children include a penile block for circumcision, ilioinguinal block for hernia repair, spinal anesthesia (most often in the neonate who is very ill), and caudal epidural. Caudal blocks have been used for a variety of surgical procedures, including circumcision, inguinal herniorrhaphy, hypospadias repair, clubfoot repair, anal surgery, and other procedures below the umbilicus and of the lower extremities.8 A caudal catheter can be threaded to the thoracic epidural space and can provide a thoracic-level block for pain relief in small children; this is particularly useful for control of pain after open heart surgery.
Selected postoperative concerns in pediatrics
The most common reasons for postdischarge readmission to the hospital are protracted vomiting and surgery-related complications.5,8 PACU nursing care of children must include constant assessment of airway patency, ventilation, and circulatory stability.5 In addition, common postoperative concerns in children include the potential for a postanesthetic excitement phase commonly referred to as emergence delirium, and pain management.1–3,5,8,30 Because the pediatric patient does not have the physiologic reserves of the adult patient, when complications occur, serious untoward sequelae take place. The perianesthesia nurse must monitor for and react to any complication in a timely fashion.
Laryngospasm
The larynx is the musculocartilaginous organ located at the upper end of the trachea. It is part of the airway and vocal apparatus.1–3,8 Laryngospasm (closure of the larynx) is caused by sensory stimulation of the superior laryngeal nerve.5,8 When this nerve is stimulated, a forceful involuntary spasm of the laryngeal musculature occurs. In the PACU, laryngospasm can occur as the child awakens. Laryngospasm is usually caused by blood or pharyngeal secretions draining toward the vocal cords.1,5,8,31 For this reason, children are placed in the three-fourths prone position to promote drainage of oral secretions away from the vocal cords. Posterior oral pharyngeal suctioning can cause additional trauma and should be avoided (or performed very carefully) after the child has been extubated.
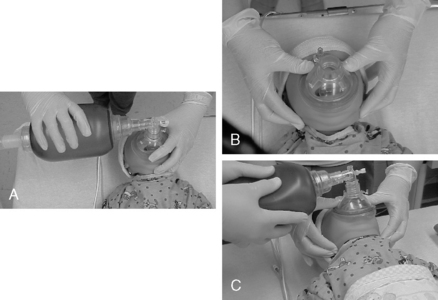
Initial treatment of laryngospasm includes positive-pressure ventilation with a bag-mask device (Fig. 49-4). The two-person bag-mask ventilation technique provides superior ventilation in the event of significant airway obstruction or poor lung compliance. Intravenous lidocaine (1 to 1.5 mg/kg) also can be helpful.1–3,8 If hypoxia develops and the laryngospasm is not relieved from positive-pressure ventilation via mask, then succinylcholine (0.25 to 1 mg/kg) should be given to allow control of ventilation with paralysis of the laryngeal muscles.5,31 Succinylcholine is a rapid-acting and ultra-short–duration depolarizing muscle relaxant and is useful in situations that necessitate rapid endotracheal intubation and securing of the airway.1,14 A word of caution is needed because succinylcholine use is associated with many profound side effects such as cardiovascular complications, severe hyperkalemia, increased intraocular pressure, increased intracranial pressure, prolonged apnea, and injured muscle membranes with associated hyperkalemia and can be a trigger for myotonia, masseter spasm, and malignant hyperthermia. The U.S. Food and Drug Administration issued a “box” warning against the elective use of succinylcholine outside of the operating room. Because of the combination of a box warning and an increased availability of alternative agents, succinylcholine use is limited outside the operating room, except when clearly indicated such as in emergency airway situations.
A perianesthesia care provider should have airway equipment and drugs readily available to facilitate re-intubation if necessary. When laryngospasm develops, large intrathoracic pressures are generated. A negative-pressure pulmonary edema can result even in healthy children, and close attention should be given to further respiratory compromise after the laryngospasm has resolved.1–3,5,8 Positive-pressure ventilation is used to treat pulmonary edema after a laryngospasm. If the pulmonary edema is severe and the patient begins to show signs and symptoms of inadequate respiration (labored breathing, decreasing oxygen saturation even with supplemental oxygenation), then more aggressive measures should be used. These measures include the use of diuretics and possibly intubation of the patient.
Airway obstruction
In the PACU, every pediatric patient, particularly children who have been intubated during anesthesia, should be monitored for signs of airway obstruction. Postintubation croup is caused by glottic or tracheal edema. When laryngeal swelling occurs, the diameter of the airway of the infant or small child can become significantly reduced; in fact, 1 mm of edema in the infant’s trachea at the cricoid level decreases the diameter of the airway by 75%. The symptoms of laryngeal obstruction, in order of appearance, are croupy cough, hoarseness, inspiratory stridor, and aphonia. These symptoms are accompanied by increasing restlessness, tachypnea, use of accessory muscles of respiration, retraction of the suprasternal notch and intercostal spaces, and drawing in of the upper abdomen.5 If these symptoms appear, the perianesthesia nurse should act immediately to relieve the obstruction, administer supplemental oxygen, and send someone to notify the PACU anesthesia provider. The progression of these symptoms can be rapid.
Treatment of postintubation croup involves use of a high-humidity atmosphere that is oxygen-enriched. Nebulized racemic epinephrine (0.5 mL of a 2.25% solution in 2.5 mL of normal saline solution) has been useful in the treatment of postintubation croup. In addition, corticosteroids such as dexamethasone (Decadron, 0.2 mg/kg, IV) have been useful to decrease the laryngeal inflammation associated with other causes of croup5,8,14; however, corticosteroid use for postintubation croup remains controversial.6 If laryngeal edema is allowed to progress, the patient may need reintubation, although this rarely occurs.
Nausea and vomiting
Nausea and vomiting (see Chapter 29) is a leading cause of delayed discharge from the PACU. Children who are undergoing tonsillectomy, strabismus, or orchiopexy surgery are at greater risk.5 The incidence rate of postoperative nausea and vomiting (PONV) varies; anything that can be done to minimize PONV wins the approval of the patient, the parents, and the staff members. Early or forced administration of liquids before the child is ready can cause vomiting. If a greater likelihood of PONV exists, administration of prophylactic antiemetics is best whenever possible.5 In most cases, vomiting can be treated successfully with the use of phenothiazines (rectal promethazine, 0.5 mg/kg), metoclopramide (0.15 mg/kg, IV), or the serotonin-3 antagonist ondansetron (0.05 to 0.15 mg/kg, IV; maximum dose, 4 mg). Phenothiazines and metoclopramide can cause dystonic reactions that can be treated with diphenhydramine (0.5 to 1 mg/kg, IV).14 Ondansetron (Zofran) is safe to use without significant side effects, is approved for use in patients 1 month of age and older, and has been shown to be the most effective drug for treating PONV.5 In the absence of an IV catheter, ondansetron can be given under the tongue for quick absorption without the need for swallowing. Propofol used alone for sedation has antiemetic properties, and it may decrease PONV when it is combined with other drugs for maintaining anesthesia.1
Malignant hyperthermia
Although a detailed discussion on malignant hyperthermia (MH) is presented in an alternate chapter of this text (Chapter 53), a brief description of the condition is given here. The incidence rate of MH is approximately 1:3000 to 1:15,000 in children, compared with 1:40,000 to 1:100,000 in adults.1–3,5,8 Halogenated inhalation anesthetic agents and the depolarizing muscle relaxant succinylcholine trigger this genetically determined condition. The pathophysiology of this condition centers on the enhanced release and diminished reuptake of calcium in the skeletal muscle. This decreased uptake of calcium causes sustained skeletal muscle contraction which results in profound hyperthermia. The muscle cells convert to anaerobic metabolism, and lactic acidosis ensues. Ultimately, muscle cell breakdown occurs. The drug dantrolene sodium effectively treats MH with inhibition of further release of calcium in the skeletal muscle. In most instances, MH occurs in the operating room; however, a patient may first be seen with the disorder in the PACU, or the patient with successfully treated MH may have an exacerbation of MH symptoms later in the recovery process.
To facilitate a reversal of this condition, the perianesthesia nurse must understand the pathophysiology of MH and should know exactly where the MH emergency cart is located. If a patient seems to be exhibiting signs and symptoms of MH, the nurse should send for help immediately. The nurse should start to assist ventilation of the patient with high-flow 100% oxygen and ensure that the IV catheter is patent. When the appropriate personnel arrive, more than one person should mix the dantrolene sodium (20 mg per 60 mL of sterile water). A note of warning is needed to ensure that the sterile water does not contain preservatives and that much sterile water will be used. The usual starting dose of dantrolene sodium is 2.5 mg/kg IV. This dose can be repeated up to 10 mg/kg over 45 minutes or until the patient’s condition stabilizes and temperature is reduced.
When the protocol is initiated, the need for endotracheal intubation and active cooling with frequent temperature monitoring begins. Recognition and treatment of arrhythmias along with correction of the associated acidosis and electrolyte imbalance (hyperkalemia) should be anticipated. Most likely an arterial line, nasogastric tube, and three-way urinary catheter is placed. The most successful outcome occurs when the syndrome is identified and treated early.6,26 The child is transferred to a pediatric intensive care unit usually for at least 24 hours for close monitoring and continued therapy.
Special considerations
Otolaryngologic surgery
The most common surgical procedures performed in pediatrics involve the ear, nose, and throat. The leading cause of obstructive sleep apnea (OSA) and hypoventilation in children is adenotonsillar hypertrophy.5 Other anatomic factors that lead to OSA include micrognathia, retrognathia, or macroglossia. In addition, morbid obesity in children or a congenitally small airway narrows the nasopharynx. Chronic OSA can disrupt sleep and breathing patterns and lead to impaired daytime performance and more serious complications such as polycythemia, growth failure, heart failure, pulmonary hypertension, and arrhythmias.5,8
Trauma victim
Special consideration should be considered with airway management for the child with head or cervical spine injury. When assisted airway support is needed to relieve airway obstruction in the PACU, the jaw-thrust maneuver is indicated to open the airway (see Figs. 49-3 to 49-5).5,8,30 An anesthesia provider should be notified at once to offer assistance and airway management. If a second care provider is available, assistance should be placed with emphasis on immobilization of the cervical spine with maintenance of a neutral alignment. The head tilt–chin lift is contraindicated in the presence of cervical spine injury.1,5,8 When the airway is controlled, a semirigid cervical collar, spine board, linen rolls, and tape can be used to immobilize the child. To support oxygenation and ventilation, intubation may be indicated. Inline traction and spine immobilization are necessary during mask ventilation, laryngoscopy, intubation, and transport.
Discharge from the postanesthesia care unit
With the advancement in pharmacologic drugs and inhalational agents for general anesthesia, rapid recovery with decreased side effects has led to earlier discharge from the PACU for children. Certain criteria must be met for safe transition from the PACU to a short-stay recovery unit or hospital ward; however, the goals of recovery vary depending on the discharge location planned for the patient.5 In the evaluation of a child for possible discharge from the PACU, the perianesthesia nurse should observe for each of the following: (1) an alert and easily aroused child; (2) protective airway reflexes; (3) strong muscle strength; (4) oxygen saturation maintained above 95% on room air or at the baseline preoperative level; (5) normothermia; (6) pain under control; (7) absence of vomiting; (8) no sign of active bleeding; and (9) stable vital signs. Children continue to recover in an ambulatory or short-stay recovery unit after outpatient procedures.
Factors that delay postoperative recovery in children include residual anesthetic or neuromuscular blockade, hypothermia, hypoxemia, acid-base imbalance, hypocarbia, hypercarbia, hypovolemia, and elevated intracranial pressure.5 Forcing fluids by mouth to facilitate discharge is never advisable, and one should wait until the child vocalizes a desire to decrease the likelihood of vomiting. A delay of discharge until the child has voided is not necessary. The anesthesia provider should be notified to assess the child; write the discharge notes, including any findings or recommendations for postoperative care; and sign for discharge from the PACU. The parents or guardian must be instructed concerning discharge care. A phone number should be included with written information on what to do in case of an emergency for further clarification of post discharge questions or concerns.
Summary
Successful transition of the pediatric patient through the perioperative period requires careful coordination by skilled perioperative nurses. The pediatric patient’s journey begins with a thorough assessment and proper preparation by the preoperative nurse. The care is then transitioned to the intraoperative nurse and anesthetist. In the PACU, the nurse must be constantly vigilant and aware of any minor changes in the pediatric patient that may be early signs and symptoms of problems. The perioperative nurse must be skilled in airway assessment and the provision of basic airway support and management, in the use of oral and nasal airways, bag-mask ventilation, and assistance with intubation and extubation. The nurse must be prepared to manage emergence delirium and postoperative pain and potentially assist with basic and advanced life support measures. All these tasks must be performed while providing an age-appropriate level of comfort and reassurance to a frightened child.
1. Barash PG, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
2. Morgan GE, et al. Clinical anesthesiology, ed 4. New York: Lange Medical Books/McGraw Hill Publishing Division; 2006.
3. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
4. Moore KL, et al. Clinically oriented anatomy, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2010.
5. Davis PJ, et al. Smith’s anesthesia for infants and children. ed 7. St. Louis: Mosby; 2011.
6. Holzman RS, et al. A practical approach to pediatric anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2008.
7. Galbusera V, et al. The assistance of the ventilated infant: role of the nurse in the management of the endotracheal tube. Minerva Pediatrica.2010;62(3 Suppl 1):169.
8. Miller R. Anesthesia, ed 7. New York: Churchill Livingstone; 2009.
9. Hines RL, Marschall KL. Stoelting’s anesthesia and coexisting disease. ed 5. Philadelphia: Saunders; 2008.
10. Cousins MJ, et al. Cousins & Bridenbaugh’s neural blockade in clinical anesthesia and pain medicine, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2009.
11. Townsend GM, et al. Sabiston textbook of surgery. ed 18. New York: Saunders; 2008.
12. Mason RJ, et al. Murray and Nadel’s textbook of respiratory medicine, ed 5. New York: Saunders; 2010.
13. Eckhenhoff J. Relationship of anesthesia to postoperative personality changes in children. Am J Dis Childhood. 1953;86:587–591.
14. Stoelting RK. Pharmacology and physiology in anesthetic practice, ed 3. Philadelphia: Lippincott Williams & Wilkins; 1999.
15. Redhu S, et al. A comparative study of induction, maintenance and recovery characteristics of sevoflurane and halothane anaesthesia in pediatric patients (6 months to 6 years). Journal of Anaesthesiology-Clinical Pharmacology. 2010;26(4):484.
16. Cravero J, et al. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the pediatric sedation research consortium,. Anesthesia and Analgesia.2009;108(3):795.
17. Weiss M, et al. Deep propofol sedation for vacuum-assisted bite-block immobilization in children undergoing proton radiation therapy of cranial tumors. Paediatric Anaesthesia. 2007;17(9):867.
18. Martin L, et al. Total intravenous anesthesia with propofol in pediatric patients outside the operating room. Anesth Analg. 1992;74:609–612.
19. Westrin P. The induction dose of propofol in infants 1–6 months and children 10–16 years of age. Anesthesiology. 1991;74(3):455–459.
20. Hannallah R. Propofol: effective dose and induction characteristics in unpremedicated children. Anesthesiology.1991;74(2):217–219.
21. Bevin J, et al. Preoperative parental anxiety predicts behavioral and emotional responses to induction of anaesthesia in children. Can J Anaesth. 1990;37:177–182.
22. Savoia G, et al. Postoperative pain treatment SIAARTI recommendations 2010, (short version). Minerva Anestesiologica. 2010;76(8):657.
23. Ali S, et al. Pain management of musculoskeletal injuries in children: current state and future directions. Pediatric Emergency Care.2010;26(7):518.
24. Mubroy J. Safety and efficacy of alfentanil and halothane in paediatric surgical patients. Can J Anaesth. 1991;38(4):445–449.
25. Sutters K, et al. Comparison of morphine patient-controlled analgesia with and without ketorolac for postoperative analgesia in pediatric orthopedic surgery. Am J Orthop. 1999;28(6):351–358.
26. Vesely C. Pediatric-patient-controlled analgesia: enhancing the self-care construct. Pediatr Nurs Rev.1995;21(2):124–128.
27. Migita R, et al. Sedation and analgesia for pediatric fracture reduction in the emergency department: a systematic review,. Archives of Pediatrics Adolescent Medicine. 2006;160(1):46.
28. Neuhuser, et al. Analgesia and sedation for painful interventions in children and adolescents. Deutsches International. 2010;107(14):241.
29. Groudine S, Fossum S. Use of intravenous acetaminophen in the treatment of postoperative pain. J Perianesth Nurs. 2011;26(2):74–80.
30. Macintyre PE, et al. Acute pain management: scientific evidence. ed 3. Melbourne, Australia: Australian and New Zealand College of Anaesthetist and Faculty of Pain Medicine; 2010.
31. Dahmani S, et al. Ketamine for perioperative pain management in children: a meta-analysis of published studies. Paediatric Anaesthesia.2011;21(6):636.