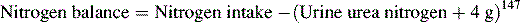20 Care of the Child with Burns
Pearls
• Intentional injury (child abuse) should be considered and ruled out as a cause of burns and scalds in children.
• Burns are painful; therefore analgesia should be provided and supplemented as needed before burn care.
• Formulas developed to estimate burn size in adults should not be used to calculate the burn surface area and fluid resuscitation requirements for children.
• Children with large burns should receive nursing and medical care in units with nurses and physicians who have pediatric expertise. Strong consideration should be given to transfer of a child to a pediatric critical care unit when large burns or complicating factors, such as smoke inhalation, are present.
Introduction
The incidence of pediatric burn injuries has declined as a result of preventive measures and legislation. However, more than 1 million burn injuries still occur each year in the United States. Although most of these burn injuries are minor, each year in the United States approximately 45,000 patients suffer moderate to severe burns that require hospitalization. Of these cases, 67% are young males, and 40% are children younger than 15 years.31a Burns are the second leading cause of unintentional death in children younger than 5 years. It is estimated that the number of serious disabilities from burns is triple the number of deaths. Three fourths of these burns are thought to be preventable.22
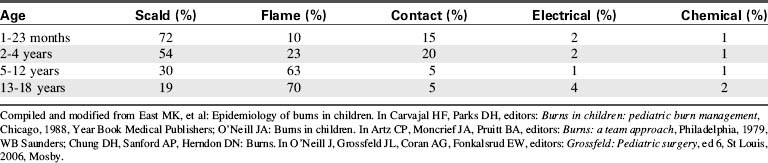
Eighty-five percent of thermal injuries in children occur at home, usually in the kitchen or bathroom. Infants and toddlers are injured most frequently by scald burns (Table 20-1),1 whereas contact burns become more common once the infant is crawling or walking. Flame burns are seen in children 2 to 4 years of age and older and are the most common cause of burn injury in children 5 to 18 years of age. Electrical and chemical burns are uncommon in children and can be lethal if they are severe.128
Essential anatomy and physiology
The skin is composed of three layers: epidermis, dermis, and subcutaneous tissues (Fig. 20-1). The epidermis is a superficial layer of stratified epithelial tissue that is composed of five microscopic levels of maturing cells. The epidermis is thinner in infants than in older children, and its thickness also varies over parts of the body. This layer is constantly shed to the environment, so that it regenerates continually. After a superficial burn, the epidermis will regenerate because portions of the epidermal appendages are present.
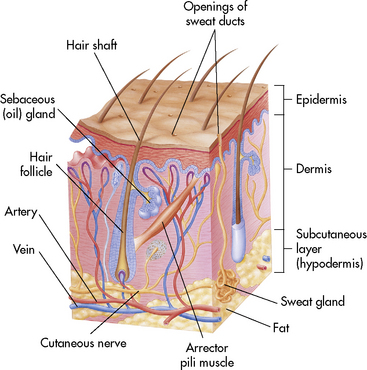
Fig. 20-1 Anatomy of the skin.
(From Thibodeau GA, Patton KT: Anatomy and physiology, ed 5, St Louis, 2003, Mosby.)
Severity and Classification of Injury
Depth of Burn
The severity of the burn injury is determined by estimating the depth and extent of the injury. The degree of tissue destruction is affected by the burning agent, its temperature, and the duration of exposure to the heat source. Healthy skin can tolerate brief exposure to temperatures up to 40° C (104° F) without injury, but higher temperatures will produce burns. Severity of the injury increases as the temperature and duration of contact increase.180
Classically, description of burn injury refers to the three concentric zones of tissue damage.143,180 The central area of the burn wound, called the zone of coagulation, is injured most severely and is characterized by coagulation necrosis. The zone of stasis is an area of direct but milder injury, which can be damaged further if ischemia develops.232 The zone of hyperemia is the area of tissue most peripheral to the initial burn and is injured only minimally.
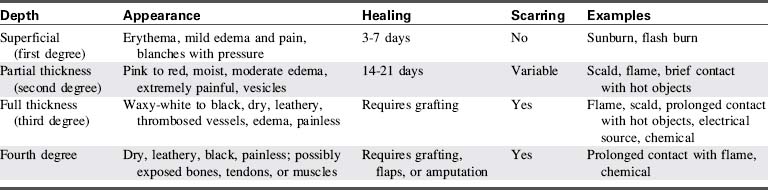
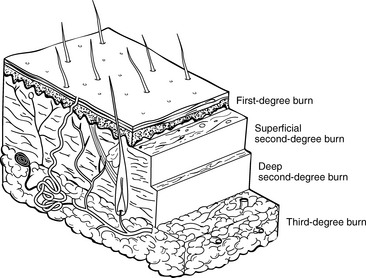
A second method of burn classification describes the specific depth of injury (Table 20-2). A first-degree burn involves the top portion of the epidermis and does not extend into the dermis layer (Fig. 20-2). The burn area is characterized by erythema, mild edema, pain, and blanching with pressure. There is no vesicle formation. First-degree burns (e.g., sunburn) heal spontaneously without scarring in 7 to 10 days.
Extent of Injury
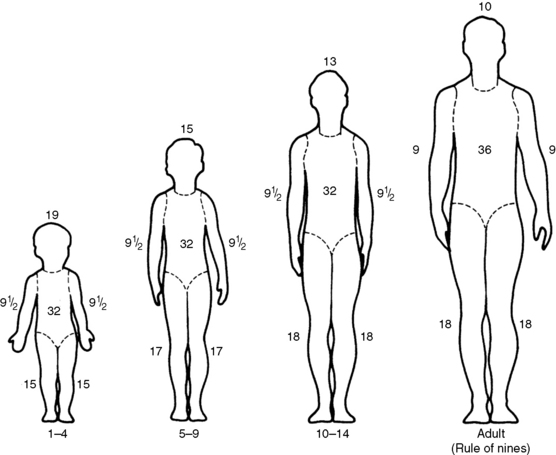
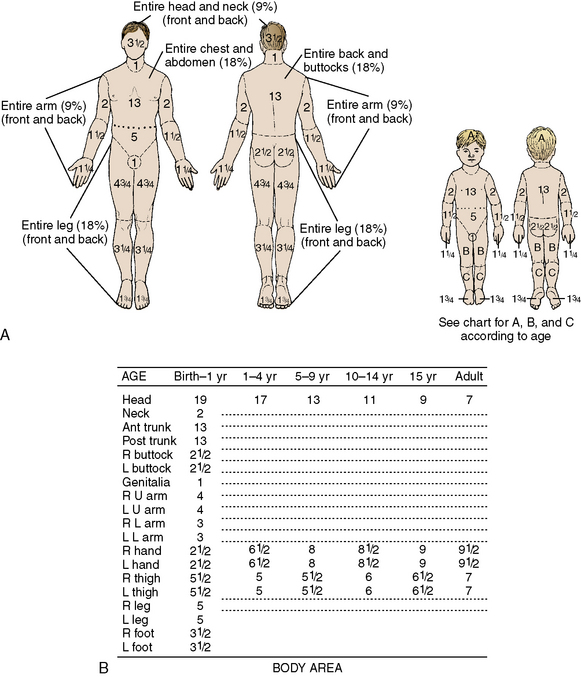
A rapid method of calculating burn area in adolescents and adults, developed in the 1940s by Pulaski and Tennison,174a is called the rule of nines (Fig. 20-3).1 In the rule of nines, each upper extremity and the head constitute 9% of the TBSA, and the lower extremities and the anterior and posterior trunks are each 18% of TBSA. The perineum, genitalia, and neck comprise the remaining 1% of the TBSA. A quick estimate of burn size can also be obtained by using the patient’s palm to represent 1% of TBSA and transposing that measurement to estimate the wound size.
Use of the rule of nines can be misleading in children because the child’s body proportions differ from those in adolescents and adults. In children, the head and neck constitute a relatively larger portion of the TBSA, and the lower extremities constitute a smaller portion. For example, an infant’s head constitutes 19% of TBSA, compared with 9% in an adult. Thus, a modified rule of nines, based on the anthropomorphic differences of infancy and childhood, is generally used to assess pediatric burn size (see Fig. 20-3). Clinical criteria can also be used to estimate the percentage of TBSA burned, based on the patient’s age and the body part burned (see Classification of Burns).
Another widely used method of determining the extent of pediatric burn injury is the Lund and Browder method (Fig. 20-4). This method allows for changes in body surface area as the average-sized child grows.119
Classification of Burns
After providing first aid and initiating appropriate fluid resuscitation, providers should consider transferring the patient to a tertiary burn center. Burn units with experienced multidisciplinary teams are best prepared to treat patients with major burns. In addition to physicians and nurses, respiratory and rehabilitation therapists play a critical role in managing acute burns. Any patient who sustains a major burn injury, as defined by the American Burn Association (Box 20-1), should be transferred to a nearby burn center for further care.
Box 20-1 American Burn Association Criteria for Major Burn Injury
• Second- and third-degree burns over more than 10% TBSA in patients less than 10 or greater than 50 years
• Second- and third-degree burns over more than 20% TBSA in other age groups (i.e., between 10 and 50 years)
• Third-degree burns over more than 5% TBSA in any age group
• Burns involving the face, hands, feet, genitalia, perineum, and skin overlying major joints
• Significant electrical burns, including lightning injury
• Burns with significant concomitant trauma
• Burns with significant preexisting medical disorders
• Burns in patients requiring special social, emotional, and rehabilitative support (including suspected inflicted injury and neglect)
Pathophysiology of a Burn
Local Circulatory Destruction
The vessels supplying the burned skin are occluded, and there is reduction in or cessation of blood flow through the arteries and veins. Release of vasoactive substances (especially histamine) from injured cells will produce vasoconstriction,143 and peripheral vessel thrombosis ultimately may occur. The reduction in skin perfusion can produce tissue necrosis and increase the depth of the burn.
Capillary Permeability (Third-Spacing) Period
As protein rich fluids, electrolytes, and plasma escape into the interstitial space, peripheral edema develops. Movement of proteins into the interstitial space will increase tissue colloid osmotic pressure, enhancing the intravascular-to-interstitial fluid shift.136
Fluid lost from the vascular space is relatively isotonic; therefore if it is replaced with isotonic or hypertonic fluids, electrolyte balance should be maintained. Dilutional hyponatremia, hypocalcemia, and hypomagnesemia are seen occasionally,213 particularly if antidiuretic hormone secretion is significant (antidiuretic hormone secretion causes water retention in excess of sodium—see Chapter 12). It is rarely necessary to replace these electrolytes if isotonic fluids are administered; however, electrolyte balance should be monitored closely. Hypotonic fluids (e.g., 5% dextrose and water or 5% dextrose and 0.45% sodium chloride) should not be administered during this period.
The concentration of base bicarbonate in the extracellular fluid decreases after a burn, and fixed acids are released from the injured tissues into the extracellular fluid, including the plasma. These acids normally are excreted by the kidney and buffered by respiratory compensation. If fluid resuscitation is inadequate, or respiratory function is compromised, the patient may develop metabolic acidosis. Young infants are less able to compensate for significant metabolic acidosis, because the infant kidneys are unable to excrete large quantities of acids or absorb large quantities of bicarbonate.179
Cardiovascular Dysfunction
Cardiac output falls after a burn as the result of decreased intravascular volume and the development of myocardial dysfunction.123 Myocardial dysfunction after a burn is not explained entirely by intravascular fluid loss. Within 30 minutes after a large burn (i.e., 50% or more of TBSA), cardiac output may decrease to 30% of preburn levels and may remain depressed for 18 to 36 hours. Cardiac output returns to normal levels long before plasma volume has been restored completely.47
The fall in cardiac output after a burn has been attributed to the presence of circulating myocardial depressant factor or the development of a catecholamine (stress induced) increase in systemic and pulmonary vascular resistances and increased ventricular afterload.229 Treatment of low cardiac output requires supportive care; the efficacy of vasoactive (inotropic) drug therapy in the treatment of this cause of myocardial dysfunction has not been determined.
In general, treatment of inadequate cardiovascular function requires support of maximal oxygen delivery (including support of oxygenation, ventilation, and cardiac output) with titration of intravenous volume administration. The effectiveness of vasoactive agents for children with significant burns has not been studied (refer to discussion of shock in Chapter 6).
Pulmonary Injuries
Respiratory insufficiency can result from the inhalation of superheated air, steam, toxic fumes, or smoke, and it is a major cause of morbidity and mortality in burned children.94,97,126,146,197 This respiratory failure may result from airway edema or obstruction or from microcirculatory changes and increased capillary permeability. Pulmonary edema can result from inhalation injuries, excessive volume administration during resuscitation, or sepsis.
Inhalation of smoke, steam or other irritants will produce upper airway edema, erythema, and blistering. Progressive edema can cause upper airway obstruction. Ciliated epithelial cells may be damaged during inhalation, so that foreign particles can enter the bronchi. The damaged mucosal layer may slough 48 to 72 hours after a burn, producing acute airway obstruction.30,94
Damage to the pulmonary parenchyma can result from an inhalation injury and can complicate shock and fluid resuscitation (see Respiratory Failure, later in this chapter and Acute Respiratory Distress Syndrome in Chapter 9). Increased alveolar capillary membrane permeability will produce pulmonary edema with resultant intrapulmonary shunting and hypoxemia, decreased lung compliance, and increased work of breathing.118
Carbon Monoxide Poisoning
Carbon monoxide (CO) is produced in almost every fire, and CO poisoning can cause immediate death during the fire or death during the first 12 hours after a burn. CO has much higher affinity for hemoglobin than does oxygen; as a result, it will bind tightly with hemoglobin, forming carboxyhemoglobin (COHb). Each gram of hemoglobin bound to CO is unable to carry oxygen, so impaired oxygen transport, decreased oxygen delivery, tissue hypoxia, and metabolic acidosis will result if CO levels are high. COHb levels greater than 40% usually produce significant tissue and organ ischemia and dysfunction, and levels greater than 60% are usually fatal.78,216
Gastrointestinal Dysfunction
Gastrointestinal ischemia can increase the permeability of gastrointestinal mucosa to gram-negative bacteria and endotoxins. As a result, translocation of gram-negative bacteria or endotoxin can occur and may precipitate gram-negative sepsis (see Septic Shock in Chapter 6, and Septic Shock: Mediators of the Septic Cascade in the Chapter 6 Supplement on the Evolve Website).
The incidence of Curling’s ulcer is unknown, because it typically is diagnosed at autopsy. Superficial gastric and duodenal mucosal changes are common in children with major burns,67 but ulcer prophylaxis has ensured that clinically significant bleeding and ulceration are still relatively uncommon.
Gastrointestinal ulceration may produce pain, hemorrhage, or perforation. Gastric suction and stool samples should be tested for the presence of blood (heme protein), and the use of antacids or sucralfate (a hydrogen ion diffusion barrier) should be considered.131 Administration of histamine receptor antagonists (e.g., cimetidine or ranitidine) is controversial, because the morbidity of these drugs may be higher than the risk of stress ulceration. Severe pneumonias may result from aspiration of gastric bacteria that can flourish after these drugs are administered. The gastric pH should be maintained at 3.5 to 5.0 (see Chapter 14).
Metabolic Changes
The patient with a burn is in a hypermetabolic state, with high oxygen consumption and caloric requirements. Metabolic rate reaches its peak at double (or more) normal values approximately 4 to 12 days after a burn.5b Catecholamine secretion activates the stress response, and heat production and substrate mobilization will result in protein and fat catabolism, increased urinary nitrogen losses, and rapid utilization of glucose and calories.70 An increased metabolic rate continues until after the burn is healed or covered by graft.
Central thermoregulation is altered at this time, and the hypermetabolic condition often produces a low-grade fever.205 In contrast, heat loss and a fall in body temperature may be observed in the very young child with an extensive burn.
Because a burn is a major body stress, muscle protein catabolism increases to provide amino acids for gluconeogenesis and fuel sources for local tissue needs.69 Insufficient protein administration and nutrition will result in a marked catabolic state (negative nitrogen balance) and major muscle loss. Large amounts of urea in the urine indicate increased nitrogen loss.218
Thermal injury and hypermetabolism result in increased serum free fatty acids. Hydrolysis of stored triglycerides is accelerated, and catecholamine secretion stimulates mobilization of fat stores. Hypoalbuminemia results from increased protein loss at the burn surface and can, in turn, reduce fatty acid transport.75
Compromise in Immune Function
After a burn, several circulating immunosuppressive substances are present. Nonspecific suppressor T cells compromise lymphocyte response for approximately 48 hours.154 Leukocyte phagocytosis is reduced, and the reticuloendothelial system is often depressed.220 Burn toxin, a high-molecular-weight protein, is thought to contribute to postburn immunosuppression. The patient’s immune function may be compromised further by the application of topical antimicrobial agents and the insertion and contamination of intravascular catheters.
Infection or injury can activate the complement system, resulting in a normal inflammatory response.88 Extensive burns result in a decrease in serum complement levels and a potential reduction in the inflammatory response during infection (see Septic Shock in Chapter 6, and Septic Shock: Mediators of the Septic Cascade in the Chapter 6 Supplement on the Evolve Website).
Common clinical conditions
Care of the child with burns requires support of cardiorespiratory function, prevention of infection, and preparation of the burn surface for healing or grafting. In addition, potential complications of the burn and its treatment must be prevented. An overview of this nursing care is provided in the nursing care plan (Box 20-2), and the major potential patient problems are reviewed in the following discussion.
Box 20-2 Nursing Care of the Child with Thermal Injuries
Inadequate Cardiac Output and Tissue Perfusion (Alteration in Tissue Perfusion) Related to: Extravascular Fluid Shift and Relative Hypovolemia, Inadequate or Delayed Fluid Resuscitation, Constriction of Eschar
Expected Patient Outcome
Patient will demonstrate adequate tissue perfusion as evidenced by:
Nursing Activities
• Constantly assess fluid balance, systemic perfusion. Monitor for signs of hypovolemic shock, including: tachycardia, oliguria, cool skin and cool extremities, weak peripheral pulses, altered level of consciousness, negative fluid balance, low central venous or pulmonary artery wedge pressure, development of lactic acidosis, and absence of hepatomegaly (by clinical examination) and of cardiomegaly (on chest radiograph).
• Calculate fluid replacement requirements and discuss inadequate fluid administration or excessive fluid losses with the on-call provider immediately.
• Administer fluid boluses and maintenance fluids as needed to restore adequate intravascular volume and systemic perfusion and to replace ongoing fluid losses.
• Assess perfusion and appearance of and movement and sensation in extremities and digits.
 Report decreased or absent peripheral pulses, delayed capillary refill, edema, cyanosis, or cool extremities or digits to the on-call provider immediately.
Report decreased or absent peripheral pulses, delayed capillary refill, edema, cyanosis, or cool extremities or digits to the on-call provider immediately. Assess blood flow to extremities using Doppler; if Doppler indicates low flow to extremities, monitoring of compartment pressures may be ordered (see Chapter 19).
Assess blood flow to extremities using Doppler; if Doppler indicates low flow to extremities, monitoring of compartment pressures may be ordered (see Chapter 19).• Assist with escharotomies or fasciotomies on arms, legs, and chest as needed.
• Perform passive and active range-of-motion exercises to extremities as ordered.
• Position patient carefully to prevent compromised blood flow to extremities.
Potential Hypovolemia or Inadequate Fluid Volume Related to: Fluid Loss Through Evaporation from Burn Surface, Increased Capillary Permeability and Extravascular Fluid Shift, Inadequate Fluid Administration, Excessive Fluid Losses Through Fever, Diarrhea
Expected Patient Outcomes
• Patient will demonstrate adequate intravascular volume as evidenced by: effective systemic perfusion, balanced fluid intake and output (with consideration of fluid loss from surface of burn), urine volume of 1 mL/kg per hour, electrolyte balance, central venous pressure or pulmonary artery wedge pressure of approximately 2 to 5 mm Hg (or higher as needed to maintain adequate systemic perfusion), normal arterial pH, and normal serum lactate.
• Patient will demonstrate no signs of hemoconcentration or dehydration, such as dry mucous membranes, poor skin turgor, increase in serum electrolyte concentration and hematocrit, sunken fontanelle (in infants), and oliguria with increased urine specific gravity.
Nursing Activities
• Monitor patient systemic perfusion: signs of hypovolemia include signs of poor systemic perfusion associated with a low central venous pressure or pulmonary artery wedge pressure, small heart size on chest radiograph, and absence of hepatomegaly. Report these findings to the on-call provider immediately.
• Monitor for signs of dehydration, including: sunken fontanelle in infants, dry mucous membranes, poor skin turgor, evidence of weight loss, rise in serum electrolyte concentration, and increase in hematocrit. Report findings to the on-call provider.
• Monitor urine output and fluid balance every hour; report oliguria or negative fluid balance to the on-call provider (consider fluid loss through burn).
• Titrate fluid administration as needed (and with physician or other provider order) to maintain systemic perfusion.
• Monitor heart size and evidence of pulmonary edema on chest radiograph; discuss these findings with on-call provider.
• Monitor electrolyte balance, serum albumin, and hematocrit.
• Obtain daily weights, report them to the on-call provider, and discuss changes in fluid requirements.
Potential Hypervolemia or Fluid Volume Excess Related to Excessive Fluid Administration and Renal Failure
Nursing Activities
• Monitor systemic perfusion and fluid balance; report signs of congestive heart failure (tachycardia, tachypnea, periorbital edema, hepatomegaly, increased respiratory effort, cardiomegaly, and oliguria), excessive weight gain, or positive fluid balance to the on-call provider (note that most patients will demonstrate weight gain following fluid resuscitation after a burn).
• Assess for evidence of pulmonary edema, including crackles and increased respiratory effort; be prepared to support oxygenation and ventilation as needed.
• Palpate liver margin; report the presence or progression of hepatomegaly to the on-call provider.
• Monitor electrolyte balance; report a fall in electrolyte concentration or hematocrit to the on-call provider.
Potential Airway Obstruction Related to: Airway Inflammation, Pulmonary Interstitial Edema, Reduced Ciliary Function Following Inhalation Injury, Altered Level of Consciousness
Expected Patient Outcomes
• Patient will demonstrate patent airway as demonstrated by: normal spontaneous respiratory rate and effort, adequate depth of respirations and air movement, effective oxygenation and carbon dioxide removal (per arterial blood gases, pulse oximetry and exhaled carbon dioxide tension [PETCO2]), and absence of crackles and stridor
• If patient develops airway obstruction, intubation and appropriate support will be provided immediately.
Nursing Activities
• Monitor patient respiratory rate, effort, and air movement. Notify on-call provider of signs of airway obstruction, including tachypnea, retractions, nasal flaring, stridor, or weak cry. Be prepared to assist with emergency intubation as needed. Resuscitation bag and mask with oxygen source should be available at the bedside.
• Note that the diagnosis of respiratory failure from airway obstruction is a clinical diagnosis and can be present despite normal arterial blood gases and pulse oximetry. Hypoxemia and hypercarbia will only be late signs of airway obstruction, and intubation should be accomplished before these develop.
• Monitor for evidence of inhalation injury, including singed nasal hairs, excessive secretions, progressive respiratory distress; report these findings to the on-call provider immediately.
• Provide oxygen therapy as needed and monitor the effect on systemic oxygenation, including pulse oximetry and arterial blood gases.
• Perform tracheal suctioning as needed to maintain a clear upper airway.
• Encourage the alert patient (as age-appropriate) to take deep breaths and cough as needed to clear the airway.
• Insert oral or nasal airway as needed (and ordered by on-call provider).
• Position child to maintain airway patency (particularly important if level of consciousness is impaired).
• Assess patient responsiveness; discuss elective intubation if the patient is obtunded or demonstrates decreased response to stimulation
Hypoxemia, Hypoxia and Impaired Gas Exchange Related to: Airway Obstruction, Inhalation Injury, Pulmonary Edema, Acute Respiratory Distress Syndrome, Carbon Monoxide Poisoning, Impaired Level of Consciousness
Expected Patient Outcomes
• Patient will demonstrate adequate respiratory function as evidenced by: appropriate respiratory rate, depth and effort; patent airway; normal arterial blood gases; COHb level less than 10%; absence of crackles or pulmonary edema on chest radiograph.
• If patient demonstrates hypoxemia despite oxygen therapy, or ineffective ventilation, support of airway, oxygenation, and ventilation will be provided,
• Patient will remain alert and oriented with adequate respiratory rate and effort.
Nursing Activities
• Monitor respiratory rate and effort, and notify a provider of development of respiratory distress.
• Monitor pulse oximetry, exhaled carbon dioxide (PETCO2), and arterial blood gases; notify a provider of any development of hypoxemia or hypercarbia.
• Monitor for evidence of inhalation injury, including headache, dizziness, confusion, flushed appearance (late finding), visual disturbance, seizures, and metabolic acidosis. Report these findings to the on-call provider immediately.
 If inhalation injury and carbon monoxide poisoning are suspected, obtained blood gas specimen for qualification of COHb level; notify on-call provider of result (COHb levels greater than 10% can be associated with significant compromise of oxygen delivery).
If inhalation injury and carbon monoxide poisoning are suspected, obtained blood gas specimen for qualification of COHb level; notify on-call provider of result (COHb levels greater than 10% can be associated with significant compromise of oxygen delivery). Severe carbon monoxide poisoning may be present despite a normal oxyhemoglobin saturation as measured by pulse oximetry, because the pulse oximeter does not recognize COHb. To determine actual hemoglobin saturation, the hemoglobin saturation must be measured using a cooximeter in the blood gas laboratory.
Severe carbon monoxide poisoning may be present despite a normal oxyhemoglobin saturation as measured by pulse oximetry, because the pulse oximeter does not recognize COHb. To determine actual hemoglobin saturation, the hemoglobin saturation must be measured using a cooximeter in the blood gas laboratory.• Administer oxygen as ordered, and monitor the patient’s response.
• Be prepared to assist with non-invasive positive-pressure ventilation, hyperbaric oxygen therapy or intubation and mechanical ventilation as needed. Ensure that emergency equipment is readily available.
Pain Related to Burn, Multiple Invasive or Painful Catheters, and Painful Dressing Changes and Procedures
Expected Patient Outcomes
• Patient will demonstrate no pain, as evidenced by verbal reports of pain (as age-appropriate), facial expression, crying or whining, and increased heart rate and blood pressure.
• Patient will demonstrate relaxed body position, relaxed facial expression, ability to sleep when undisturbed, and appropriate heart rate and blood pressure for age and clinical condition.
• Patient will indicate reduction or elimination of pain as indicated and quantified using consistent pain assessment tools (see Chapter 5).
Nursing Activities
• Assess nature, location, quality, and intensity of pain every hour and as needed using consistent pain assessment tool (see Chapter 5).
• Monitor patient closely for nonverbal evidence of pain, including: restlessness, guarding of burned areas, wrinkled brow, clenched fingers, reluctance to move, facial pallor or flushing, diaphoresis, tachycardia or hypertension, pupil dilation.
• Assess analgesic administration and discuss modification of analgesic dose, schedule, or type with a physician or other on-call provider if pain persists.
• Administer analgesics as ordered and evaluate effectiveness; if continuous infusion medications are ordered, ensure that bolus administration of the drug is used to initiate infusion therapy and achieve therapeutic drug levels. Bolus therapy may also be required if dose of drug is increased (see Chapter 5). Ensure adequate analgesia before painful procedures.
• Identify factors that aggravate or alleviate pain and modify patient support accordingly.
Potential Burn Wound Infection or Septic Shock Related to: Open Wound, Presence of Multiple Invasive Catheters, Compromise in Immune Function
Expected Patient Outcomes
• Patient will demonstrate no signs of infection, including fever or hypothermia, leukocytosis or leukopenia, wound erythema, wound drainage, or positive blood cultures.
• If infection develops, it will be promptly treated with antibiotics and the patient will be closely monitored for evidence of sepsis.
• Burn wound and graft sites will heal within appropriate interval.
Nursing Activities
• Assess wound appearance for evidence of local signs of infection, including: erythema; change in color, appearance, odor, or amount of wound drainage; change in color of wound to black or dark brown, progression of burn to full thickness injury; sloughing of graft or wound breakdown after closure.
• Monitor patient temperature and white blood cell count and differential; notify the on-call provider of changes.
• Obtain blood cultures as ordered.
• Administer antibiotics as ordered (precisely on time) and monitor for side effects.
• Ensure strict aseptic technique during all invasive procedures; ensure clean technique during all noninvasive procedures including wound care.
• Maintain closed delivery system for all intravenous lines.
• Ensure good hand-washing technique by all members of the healthcare team before and after patient contact.
• Monitor the patient closely for signs of sepsis, including fever, hypothermia, tachycardia, tachypnea, alteration in responsiveness, leukocytosis, leukopenia, metabolic acidosis, evidence of organ system dysfunction (e.g., oliguria, diarrhea or vomiting, abdominal distension, elevation in liver transaminases), evidence of relative hypovolemia (and increased intravascular fluid requirements to maintain perfusion), and signs of septic shock (sepsis plus cardiovascular and one other organ system failure). See Boxes 6-2 and 6-3.
Potential Temperature Instability Related to Heat Loss Through Burn Surface
Nursing Activities
• Monitor body temperature hourly initially, then every 2 to 4 hours when the patient’s condition is stable.
• Monitor for signs of cold stress, including shivering or chills, or tachycardia, tachypnea and hypoxemia in young infant.
• Use a heat shield, over bed warmer, or warming blanket as needed to maintain the patient’s body temperature.
• Keep ambient air warm, and prevent any room draft.
• Use warmed (not hot) solution during burn care.
• Perform burn care under a heat shield or overbed warmer for infants or young children and as needed for older children.
• Minimize patient exposure during treatments or wound care (e.g., perform burn care on one extremity at a time, and redress that extremity before the next extremity is unwrapped).
• Consider warming of intravenous fluids and blood products; warming should be performed according to hospital policy for infants and young children.
Potential for Inadequate Nutrition Related to: Excessive Caloric Requirements, Inadequate Caloric Intake, Altered Metabolism
Nursing Activities
• Calculate the patient’s caloric and protein needs and notify the on-call provider if these are not being met. Monitor patient tolerance of tube, oral, or parenteral feedings. Ensure appropriate distribution of calories within protein, fat, and carbohydrates.
• Begin enteral feedings as ordered; notify a provider of feeding intolerance (including abdominal distension, vomiting, diarrhea, increased residual volume after gastric feeding, reflux). For additional information, see Chapter 14.
• Implement measures to reduce nausea and vomiting as needed, including position changes and administering an antiemetic.
• Ensure adequate oral intake when tolerated; calculate caloric intake and ensure provision of multiple opportunities to eat a wide variety of appetizing foods.
• Obtain daily weight; notify a provider of weight loss or failure to gain weight.
• Obtain consultation with dietician as needed. Evaluate oxygen and caloric expenditure using calorimetry as needed.
Inadequate Intravascular Volume and Cardiac Output: Third-Spacing Phase
Etiology and Pathophysiology
The magnitude of the intravascular volume deficit will depend on the depth and surface area of the injury and the time elapsed before adequate fluid resuscitation.28 In general, deeper and larger (15% or more of TBSA) burns are associated with more significant fluid shifts and circulatory complications.
Clinical Signs and Symptoms
After a significant burn, intravascular volume loss will eventually produce signs of hypovolemia (Box 20-3). Children often do not exhibit significant signs of hypovolemia, including hypotension until more than 25% of the circulating volume is depleted and complete cardiovascular collapse is imminent.
Significant hypovolemia will compromise systemic perfusion and may produce shock. Such hypovolemia will produce tachycardia, prolonged capillary refill time, and cold extremities. Anuria is often present. The development of a metabolic acidosis (i.e., fall in arterial pH, rise in serum lactate) indicates critical compromise in tissue perfusion. The young infant in shock often will demonstrate temperature instability and hypoglycemia. Hypotension may develop only as a late sign of shock.47
Management
Intravenous Access and Monitoring
Whenever a major burn is present or systemic perfusion is compromised significantly, insertion of a central venous catheter (into the subclavian, internal jugular, or femoral vein) should be considered to provide reliable, large-bore venous access. Use of a multilumen central venous catheter will provide two or more ports to facilitate fluid administration and central venous pressure (CVP) monitoring. Any central venous catheter must be inserted under strict sterile conditions and cared for using strict aseptic technique (see Chapter 21 and Box 22-6).
Determination of Fluid Requirements
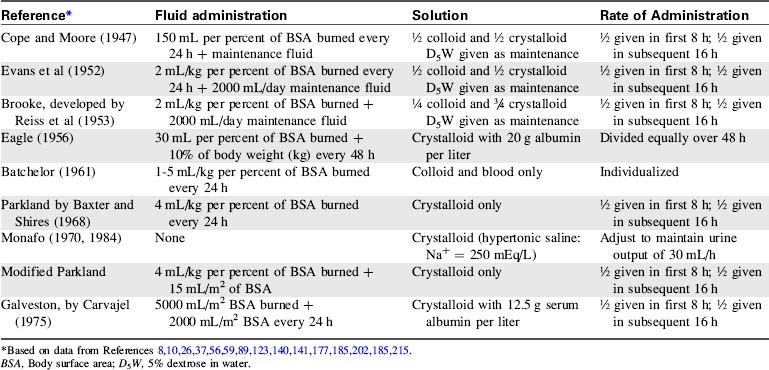
A variety of formulas have been developed to assist in determining fluid losses and requirements in patients with burns (Table 20-3). Many formulas, however, have been designed for use in adult patients and are based solely on body weight and percentage of TBSA burned. Use of these adult formulas will result in inadequate pediatric fluid resuscitation.62,135
The most popular formula for use in adolescent and adult patients with burns is the Parkland (by Baxter), formula.10 Modification of the Parkland formula for children provides for crystalloid administration during the first 24 hours of therapy. The volume administered during this time is based on the burn surface area (4 mL/kg per percent of TBSA burned) plus maintenance fluid requirements (1500 mL/m2 BSA).215 Half of this calculated fluid is administered during the first 8 hours of therapy, and the remaining half is administered during the next 16 hours of therapy.
The child’s fluid resuscitation requirements should be based on body surface area rather than weight. Because children have a greater body surface area in relation to weight, weight-based formulas can underestimate the fluid requirements of children with minor burns and may grossly overestimate the fluid requirements of those with extensive burns.79 TBSA can be rapidly estimated from height and weight using standard nomograms (see inside back cover of this text).
The Galveston formula26 (developed by Carvajal at the Shriners Hospital for Children in Galveston, Texas) provides 5000 mL/m2 BSA burned plus 2000 mL/m2 BSA of lactated Ringer’s solution given over the first 24 hours after the injury, with half the volume administered during the first 8 hours and the remaining half over the next 16 hours. The Carvajal formula26 recommends crystalloid and colloid administration based on the absolute surface area of the child’s burn, plus generous maintenance fluid administration.
Selection of Fluid Content
There is continued debate regarding the relative benefits of crystalloid versus colloid administration during burn resuscitation.27,42,51,176 Proponents of crystalloids advocate the use of isotonic or hypertonic crystalloids because they are physiologic, inexpensive, and readily available.
Critics of crystalloid administration note that immediately after administration, isotonic crystalloids will equilibrate between the intravascular and interstitial spaces, and only a fraction of administered intravenous crystalloids will remain in the vascular space.178 Therefore, large quantities of crystalloids generally are required to restore intravascular volume. In addition, the fluid that moves into the interstitial space may contribute to worsening systemic edema. Pulmonary interstitial water usually does not increase substantially during this time, because pulmonary capillary permeability remains normal unless significant inhalation injury occurs. In addition, lymph flow is usually proportional to the amount of pulmonary interstitial water movement.
Colloid resuscitation may restore intravascular volume and pressure more efficiently than will crystalloid administration. If capillary permeability is normal, administered colloids will remain in the vascular space for several hours, exerting oncotic pressure. This oncotic pressure will increase intravascular volume and maintain intravascular osmolality, so that continued fluid shift from the vascular space is less likely. Because colloids are thought to diffuse more slowly into the interstitial space, colloid resuscitated patients may develop less edema than crystalloid-resuscitated patients.163 Adequate fluid resuscitation should be possible with relatively small volumes of colloids,86,201 so that the patient receives a small volume and salt load.
Critics of colloid administration note that membrane permeability is not normal in patients immediately after burns, and proteins may move from the vascular to the interstitial space during the first 24 hours after a burn.9 Movement of administered colloids into the interstitial space can increase interstitial oncotic pressure, enhancing the fluid shift from the intravascular space into the interstitial space.
Colloid administration during the first day after a burn was avoided in the past, based on the fear that it would increase the severity of third-spacing of fluid.182 However, the validity of this criticism has been challenged during the last decade. Although albumin may leave the vascular space, an equal amount of albumin may be returned to the vascular space by lymphatics approximately 8 hours or more after a burn. Therefore many institutions have successfully added small amounts of colloids to their early burn resuscitation protocols.
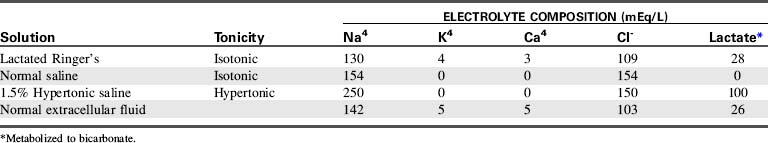
In general, adequate resuscitation can be provided if isotonic crystalloids are administered in sufficient quantity.48 Lactated Ringer’s (LR) solution, an isotonic crystalloid, is the most widely used solution for burn resuscitation. The composition of LR’s solution closely mimics extracellular (including intravascular) fluid composition (Table 20-4); therefore LR’s solution is ideal for replenishing intravascular water and electrolytes. In addition, LR’s solution contains lactate, which is metabolized to bicarbonate, so it will buffer mild acidosis. Lactated Ringer’s solution is inexpensive, readily available, and effective in the treatment of nonhemorrhagic hypovolemia.
During fluid resuscitation, the child’s systemic perfusion and urine output must be monitored closely. These parameters should improve if fluid administration is adequate (Table 20-5). The serum hemoglobin concentration, electrolyte balance, and acid-base status (including serum lactate) must also be monitored closely.
Table 20-5 Clinical Responses to Fluid Resuscitation in Burned Patients
| Parameter | Desirable Response (Fluid Resuscitation Adequate) | Undesirable Response (Fluid Administration Inadequate) |
| Urine output | 1 mL/kg per hour (up to 30 kg, then 25-30 mL/h) | <1 mL/kg per hour (for children above 30 kg, less than 25 mL/h) |
| Specific gravity | 1.010-1.025 | >1.025 |
| Weight | Preburn level | 10% less than preburn level |
| Blood pressure | Normal for age or high* | Low for age* |
| Pulse | Normal for age* | Normal or high* |
| Level of consciousness | Alert, clear, and lucid | Lethargic and stuporous |
| Hematocrit | 35%-45% | 48%-55% |
| Serum sodium | 135-145 mEq/L | >150 mEq/L |
| Blood urea nitrogen | 5-20 mg/dL | >25 mg/dL |
| Creatinine | 0.8-1.4 mg/dL | >2.0 mg/dL |
| Osmolality (serum) | 275-295 mOsm/L | >300 mOsm/L |
| Urine sodium | 60-100 mEq/L | ≤40 mEq/L |
| Blood pH | 7.20-7.50 | <7.20 |
| Serum lactate | Venous: 0.5-2.2 mmol/L Arterial: 0.5-1.6 mmol/L |
>4 mmol/L |
| Peripheral circulation | Brisk capillary refill; normal color in unburned areas | Cyanosis; prolonged capillary refill |
| Central venous pressure (CVP) | 4-8 mmHg | <2-4 mmHg |
| Pulmonary artery pressure (PAP) | Systolic, 20-30 mmHg | Systolic, <20 mmHg |
| Diastolic, 5-15 mmHg | Diastolic, <5 mmHg | |
| Cardiac index | 3.0-4.5 L/min per m2 BSA | <3.0 L/min per m2 BSA |
BSA, Body surface area.
* See normal blood pressure and heart rate ranges for age in Tables 1-1 and 1-3 (and on pages inside front cover).
Hypertonic saline resuscitation can be beneficial in treating burn-induced shock.16,17,64,84 This process maintains intravascular volume more effectively because it induces movement of free water from the interstitial to the intravascular space, thus decreasing generalized tissue edema. However, hypertonic saline is not widely used because of the potential risk of hypernatremia, hyperosmolarity, renal failure, and alkalosis.98,173,222 Some favor the use of a modified hypertonic solution—adding an ampule of sodium bicarbonate to each liter of lactated Ringer’s solution during the first 24 hours of resuscitation.19
Routine Care
Regardless of the type of resuscitation fluid being used, the nurse must closely monitor the patient’s response to volume resuscitation (see Table 20-5). Adequate systemic perfusion, demonstrated by warm extremities, brisk capillary refill, strong peripheral pulses, and adequate (1-2 mL/kg body weight per hour) urine volume should be observed.
The child’s level of consciousness should be appropriate for clinical condition. Irritability may be an early sign of cardiovascular or neurologic deterioration,150 and lethargy or decreased response to painful stimulation is abnormal and requires investigation.
During fluid resuscitation, the nurse should be alert for the development of pulmonary edema, and the team should have a plan for a sequence of appropriate respiratory support. Elective intubation should be performed before decompensation occurs (see Respiratory Failure in this chapter and Chapter 9).
The urine sodium may also be monitored. Normal urinary excretion of sodium is approximately 60 to 100 mEq/L. A low urine sodium (less than 40 mEq/L) usually results from aldosterone secretion in the presence of inadequate intravascular volume123 and indicates the need for further volume administration.
Evaluation of Therapy
Increased fluid administration is probably necessary if inadequate systemic perfusion and continuing acidosis are associated with a low CVP. Vasoactive drug therapy will not improve systemic perfusion produced by hypovolemia. Poor systemic perfusion and extreme acidosis despite adequate fluid administration indicate severe shock and are associated with a high mortality (see Shock in Chapter 6).
Hypervolemia: Fluid Mobilization Phase
Clinical Signs and Symptoms
As fluid returns to the vascular space, the patient’s peripheral edema should subside, and evidence of pulmonary edema (on chest radiograph and by pulmonary function) should disappear. Reduction in pulmonary interstitial fluid may be difficult to appreciate if the patient has sustained significant inhalation injury or if acute respiratory distress syndrome (ARDS) has developed. Urine volume should increase significantly, and the child’s weight should begin to decrease toward the baseline (preburn) weight (Box 20-4).
Box 20-4 Clinical Signs Observed During Fluid Mobilization Period
Hyponatremia may persist as the result of increased renal sodium excretion. Potassium will shift back into the cells, and urinary potassium loss is increased, so hypokalemia is usually noted.28 Finally the hematocrit falls as the result of hemodilution and increased destruction of RBCs.
Management
Fluid and Electrolyte Therapy
Fluid loss during this period will consist of continued evaporative water losses from the burn surface and basal metabolic (insensible) water losses (Box 20-5). Evaporative water losses become significant approximately 24 hours after the burn, and they may be as high as 2000 mL/day in the child. Because fluid lost by evaporation is predominantly water, replacement with 5% dextrose and 0.45% sodium chloride solution is provided.
Box 20-5 Fluid Requirements 24-48 h after Burn Injury
Maintenance fluid* = Basal fluid requirements + Evaporative water loss
• Basal fluid per hour = (1500 mL × m2 BSA) ÷ 24
• Evaporative water loss per hour = (35 + % burn) m2 BSA
Colloid = 20% of circulating blood volume
• mL/hour = 0.20 × (70† mL/kg × kg body weight)/24
BSA, Body surface area; kg, body weight in kilograms; % burn is the estimated percent of body surface area that is burned (see Fig. 20-3 and 20-4).
Blood administration may be necessary if significant anemia develops. The hematocrit should be maintained at approximately 25% to 30%, using whole blood or packed RBCs. Administration of 10 mL/kg of packed RBCs will increase the hematocrit by approximately 10 percentage points (e.g., from 25% to 35%). This volume should be administered over 3 to 4 hours, and patient tolerance of the volume should be assessed constantly during the transfusion. A diuretic may be administered immediately before the transfusion to prevent hypervolemia (for further information regarding transfusion therapy, see Chapter 15).
Evaluation of Therapy
If cardiorespiratory function remains adequate, systemic perfusion should continue to improve. If hypervolemia is present, the child will demonstrate high CVP, and hepatomegaly and pulmonary edema may develop or persist (Table 20-6).
Table 20-6 Clinical Parameters Indicating Hypervolemia During Fluid Resuscitation 24-48 h after Burn Injury
| Parameter* | Signs of Hypervolemia |
| Urine output | >2 mL/kg per hour |
| Specific gravity | <1.010 |
| Weight | ≥20% above preburn level |
| Blood pressure | Elevated† |
| Heart rate | Normal or high† |
| Level of consciousness | Can be alert or lethargic |
| Hematocrit | 25-30% |
| Serum sodium | <130 mEq/L |
| Blood urea nitrogen | <5 mg/dL |
| Creatinine | <0.5 mg/dL |
| Osmolality | <250 mOsm/L |
| Urine sodium | ≥100-120 mEq/L |
| Blood pH | >7.50 |
| Peripheral circulation | Bounding peripheral pulses |
| Central venous pressure | >10 mmHg |
| Pulmonary artery pressure PAP | Systolic, >30 mmHg Diastolic, >15 mmHg |
| Cardiac index | >8.0 L/min per m2 BSA |
BSA, Body surface area.
* See Box 20-4 for desirable responses.
† See normal blood pressure and heart rate values for age in Tables 1-1 and 1-3 and on pages inside front cover.
If cardiovascular dysfunction is associated with the hypervolemia, poor systemic perfusion will be noted in addition to the signs of pulmonary and systemic edema, and oliguria may be present. Ventricular dilation and reduced contractility will be apparent on echocardiography. In these patients, diuretic therapy and support of cardiovascular function should be intensified, and vasoactive drug therapy probably is necessary. See Chapters 6 and 8 for further information about management of shock and congestive heart failure.
If urine volume does not improve and the serum BUN and creatinine rise during this time, renal failure may be present. See Chapter 13 for information regarding management of renal failure.198,211
Respiratory Failure
Etiology
Respiratory failure in the burn patient may be the result of inhalation of toxic substances, airway edema, or increased capillary permeability pulmonary edema. These pulmonary insults will produce the problems of airway obstruction or of acute respiratory failure with permeability pulmonary edema (i.e., ARDS). CO poisoning will compromise oxygen delivery. These problems will be discussed briefly here; see Chapter 9 for a more detailed discussion of respiratory failure and ARDS.
Pathophysiology
Inhalation of smoke, hot gas, and combustion products can produce oropharyngeal edema and injury to the ciliated mucosal epithelial layer of the trachea. Edema and airway obstruction usually are evident during the first 24 hours after the burn. If the mucosal layer is injured severely, it may slough 48 to 72 hours after the burn, causing acute airway obstruction.94
Permeability pulmonary edema and ARDS result from damage to the pulmonary alveolar capillary membrane.183 This damage allows both proteins and fluids to move from the vascular space into the interstitium of the lung, causing pulmonary edema. This edema produces intrapulmonary shunting, hypoxemia, decreased pulmonary compliance, and increased work of breathing.118
The insult of the burn, inhalation injury, resulting shock, and fluid resuscitation all can contribute to the development of ARDS. Other pathologic mechanisms may contribute to the progression of pulmonary injury, including production of arachidonic acid metabolites and release of vasoactive substances. However, the most common cause of postburn pulmonary capillary injury, pulmonary edema, and respiratory failure is the development of sepsis (see Septic Shock in Chapters 6 and 16, and Septic Shock, Mediators of the Septic Cascade, in the Chapter 6 Supplement on the Evolve Website).
CO injury or chemical injury may result from inhalation of smoke or other products of combustion. CO binds readily with hemoglobin, so the hemoglobin it binds is not available to carry oxygen.82 Small amounts of inhaled CO can substantially reduce hemoglobin oxygen-carrying capacity and tissue oxygen delivery. Progressive tissue hypoxia and acidosis will result in tissue and organ (including neurologic) dysfunction.
Inhalation of other products of combustion can produce pharyngeal or tracheobronchial edema or ulceration, with a resultant risk of airway obstruction. In addition, the inhaled substances can decrease ciliary action or produce bronchorrhea, bronchospasm, airway ulceration, or pulmonary edema (Table 20-7).
Table 20-7 Toxic Products and Clinical Symptoms Produced from Burning Substances
| Substances | Toxic Products | Clinical Symptoms |
| Polyvinylchloride | Hydrogen chloride, phosgene | Dyspnea, burning mucous membranes, lightheadedness, laryngeal and pulmonary edema |
| Wood, cotton, paper | Acetaldehyde, formaldehyde, acrolein, acetic acid, methane | Decrease in ciliary action, decrease in macrophage activity, pulmonary edema |
| Polyurethane foam | Isocyanate, hydrogen cyanide | Dyspnea, lightheadedness, confusion, dizziness, unconsciousness |
| Wool, silk | Ammonia, sulfur dioxide, hydrogen sulfide | Bronchorrhea, bronchospasm, ulceration, pulmonary edema, hoarseness, stridor, dyspnea |
| Nylon | Ammonia, hydrogen cyanide | Dyspnea, dizziness, bronchospasm, pulmonary edema, unconsciousness |
| Teflon | Octafluoroisobutylene | Dyspnea, wheezing, pulmonary edema |
Clinical Signs and Symptoms
Respiratory symptoms will be determined by the location of the burn and the quantity and type of gas inhaled. Inhalation injuries can produce respiratory failure over time (Table 20-8).
| Stage | Onset (Hours After Burn) | Characteristics |
| Ventilatory insufficiency | 0-8 | Bronchospasm and alveolar damage |
| Pulmonary edema | 8-48 | Edema of upper or lower airways and pulmonary interstitial edema, hypoxemia and decreased lung compliance |
| Bronchopneumonia | ≥72 | Bronchorrhea, pneumonia, decrease in ciliary and mucosal activity |
Acute Respiratory Distress Syndrome
Within 24 to 96 hours after the pulmonary insult, the child with ARDS will demonstrate clinical evidence of significant pulmonary edema and decreased pulmonary compliance (tachypnea with increased respiratory effort). Crackles often are noted on clinical examination. Arterial blood gases or pulse oximetry will demonstrate hypoxemia, which is not relieved by supplementary oxygen administration.184
Carbon Monoxide Poisoning
CO poisoning will produce a fall in measured hemoglobin saturation if a cooximeter (e.g., Corning IL282 or Corning 2500; Corning, N.Y.) is used for blood-gas analysis. The hemoglobin saturation obtained by pulse oximetry can be normal despite significant CO poisoning, because the COHb is not recognized as functioning hemoglobin by the oximeter. If the hemoglobin saturation from a blood gas sample is calculated based on the child’s arterial oxygen tension and pH, a normal saturation may be derived despite progressive hypoxemia.43 Dissolved oxygen in the arterial blood may produce a normal arterial oxygen tension, even in the presence of significant compromise in arterial oxygen content and tissue oxygenation. For these reasons the arterial oxyhemoglobin saturation should be measured with a cooximeter.
Mild CO poisoning will produce headache and shortness of breath, but CO toxicity will result in cardiorespiratory distress, coma, severe metabolic acidosis, and multisystem organ failure (Table 20-9). Significant CO toxicity produces vasodilation and a characteristic cherry red color in the mucous membranes and cheeks. Late neurologic dysfunction following CO inhalation has been reported in children and includes headache, personality and behavioral changes, memory loss, and poor school performance; this dysfunction is thought to result from hypoxic injury to the cerebral cortex.112
Table 20-9 Relationship Between Blood Carboxyhemoglobin Levels and Clinical Signs and Symptoms
| CoHb Level (%) | Symptoms |
| <10 | None |
| 10-20 | Mild headache, dyspnea, visual changes, confusion |
| 20-40 | Dizziness, shortness of breath, nausea and vomiting, irritability, weakness, ringing in the ears, hypotension, tachycardia |
| 40-60 | Hallucinations, confusion, coma, cardiopulmonary instability, arrhythmias |
| >60 | Usually fatal |
COHb, Carboxyhemoglobin.
Management
Inhalation injury should be suspected in any burn victim with evidence of oral burns, singed nasal hairs, pharyngeal ulceration, carbonaceous material in the nose or mouth, congestion, or a high-pitched cough.188 Tachypnea, dyspnea, stridor, wheezing, cough, and increased respiratory secretions indicate development of significant airway obstruction. Resultant hypoxia may produce changes in color and responsiveness.
Airway Obstruction
Occasionally, severe upper airway edema makes intubation difficult, and a tracheostomy may be necessary. However, early elective intubation, rather than late emergency intubation may preclude the need for tracheostomy and its potential complications.132
Maintenance of proper tube placement is essential for the child with critical airway obstruction. If severe facial burns are present, facial tape cannot be used to secure the endotracheal tube. Twill tape can be wrapped around the neck and the endotracheal tube to secure the tube. The twill tape should be placed above one ear and under the other ear to minimize nasal distortion.2 Commercial tube holders may also be used.
Acute Respiratory Distress Syndrome
If the child develops permeability pulmonary edema and ARDS, then intubation and mechanical ventilation will be required.184 Mechanical ventilation support must be skilled, and high inspired oxygen concentration and titrated positive end-expiratory pressure (PEEP) may be required to achieve optimal oxygen delivery.
PEEP effectively reduces intrapulmonary shunting because it expands atelectatic areas of the lung and improves functional residual capacity.188 In addition, PEEP probably moves edema fluid to harmless areas of the lung.
Titration of PEEP is designed to ensure maximal oxygen delivery—optimal arterial oxygen content without significant depression of cardiac output. Ideally, titration of PEEP will enable reduction of potentially harmful levels of inspired oxygen and will improve arterial oxygen content and lung compliance. However, it is important to note that high levels of PEEP may produce barotrauma, which can be as harmful to the lung as high levels of inspired oxygen. High PEEP can also reduce systemic venous return and cardiac output, with resultant reduction in oxygen delivery. Therefore the levels of inspired oxygen and PEEP should be the lowest levels consistent with satisfactory oxygen delivery and end organ function. For further information about the management of ARDS, see Acute Respiratory Distress Syndrome in Chapter 9.
Carbon Monoxide Poisoning
Unfortunately, the development of increased intracranial pressure following cerebral hypoxia is usually a sign of devastating neurologic insult, which is typically unresponsive to conventional therapy for increased intracranial pressure. For further information regarding assessment and management of increased intracranial pressure, see Chapter 11.
Use of hyperbaric oxygen (HBO) has been advocated as a treatment for CO poisoning,78,234 although its efficacy has not been studied in controlled clinical trials. Brief (30-90-minute) periods in HBO (at 2.5-3.0 times atmospheric pressure) will approximately double the amount of dissolved oxygen present in the blood, so oxygen delivery will improve. In addition, HBO therapy displaces CO from hemoglobin, myoglobin, and cells; this reduces the half-life of carboxyhemoglobin to approximately 20 to 30 minutes, compared with approximately 5 to 6 hours in room air and 80 to 90 minutes in 100% oxygen. HBO therapy also results in rapid removal of CO from intracellular cytochromes, so effects of cellular hypoxia may be halted.216
HBO therapy is performed in designated HBO units. If the child’s condition is unstable, a multiplace chamber must be used to allow constant attendance by physicians and nurses. HBO is used most frequently for treating patients with isolated CO poisoning, particularly if COHb levels are high (exceeding 25%-30%). HBO therapy may be effective even after COHb levels have returned to normal. Several pediatric case reports have noted promising results of this therapy.78
Evaluation of Therapy
If burns are extensive, respiratory function is likely to deteriorate during the first days of hospitalization. As a result, continuous assessment of respiratory function and evaluation of the effectiveness of mechanical ventilation support is mandatory. If high concentrations of inspired oxygen and PEEP are required to maintain oxygenation, severe parenchymal injury is probably present, and prolonged mechanical ventilation support may be required (refer to Chapter 9 for treatment of ARDS).
Pain
Clinical Signs and Symptoms
If the child is awake and responsive, assessment tools such as the Eland Color Tool, the Hester Poker Chips, or the Beyers “Oucher” may be used to quantify and perhaps localize pain. These assessment tools are discussed in Chapter 5 (see also Evolve Fig. 5-1 in the Chapter 5 Supplement on the Evolve Website).
Management
A variety of narcotic and psychotropic drugs are currently available to provide analgesia and amnesia. The potential complications of the drugs should not preclude their use. Instead, the nurse and physician should be familiar with the drugs used, anticipate the complications, and monitor the patient accordingly. The goal of therapy is to provide effective analgesia with minimal side effects (see Chapter 5).
Antihistamines frequently are prescribed to relieve pruritus. These drugs may produce tachycardia and drowsiness. Nonpharmacologic methods of pain relief, including imagery, relaxation techniques, transcutaneous nerve stimulation, and hypnosis also may provide effective pain relief under appropriate conditions. These methods are discussed further in Chapter 5.
Potential Infection, Sepsis, and Septic Shock
Etiology
After a burn, the child is at risk for infection from the burn wound itself, from postburn immunosuppression, and through invasive monitoring and therapy equipment. Despite the recent advances in the care of burned children, infection remains the leading cause of post burn morbidity and mortality.45
Pathophysiology
Normal Inflammatory Response
The burn wound is the most common site of infection in patients with burns who develop sepsis. For infection to occur, a microorganism must colonize the wound and survive local conditions at the site of entry,153 then the organism or its toxins must disseminate into the surrounding tissue.
Once the organism enters the body, it triggers a local inflammatory response that includes vasodilation and increased capillary permeability (for further information, see Septic Shock in Chapters 6 and 16, and Septic Shock: Mediators of the Septic Cascade in the Chapter 6 Supplement on the Evolve Website). The inflammatory response is designed to deliver white blood cells (WBCs; particularly the neutrophils) to the area of infection. The organism also may be ingested by macrophages or eliminated by circulating neutrophils.
The complement system is a network of serum proteins that normally are present in the inactive form; activation of any of the complement proteins will result in activation of a series of proteins in a cascading fashion. The complement proteins contribute to the inflammatory process and immunity when they bind with invading organisms, facilitating phagocytosis in a process called opsonization.88 In addition, activation of the complement system results in stimulation of the clotting cascade and may result in changes in vascular tone and alteration in platelet function.
Enzymes and granules released by macrophages and WBCs will also contribute to the inflammatory response and destruction of the organism.45,221 A specific immune response may be initiated by the lymphocytes to enable the development of immunity.
If the organism is not destroyed at the tissue level, it may enter the blood stream; at this point, bacteremia is present (if the organism is a fungus, fungemia is present; if the organism is a virus, viremia is present). As blood passes through lymph tissue, specific antibodies and lymphocytes may combat the infection.203 The success of the response to an invading organism will depend on the virulence of the organism itself and the strength of the body’s lymphocyte and immune response.223
Effects of Thermal Injury
Thermal injury activates the body’s inflammatory response and creates changes in immune function. The ability of the body to fight infection is compromised by decreased neutrophil phagocytosis, alteration in complement function, circulation of burn generated toxins, suppression of lymphocyte function, and administration of antimicrobial agents (especially tetracycline).157,159 The extent of postburn immunosuppression depends on a variety of factors, including the severity of the burn, the patient’s nutritional status, and the patient’s hormonal balance.
Neutrophil phagocytic function is typically normal immediately after a burn. However, approximately 5 or more days after the burn, phagocytic function may be normal, depressed, or increased.46,80 Because neutrophils provide the first-line response to infection, neutrophil depression can significantly increase the patient’s risk of infection.
Circulating immunosuppressive substances are present in patients with burns. These substances appear within 24 hours of injury and may persist until the wound is closed.152 The origin of these suppressors is not known, although substances secreted from WBC granules or membranes have been implicated.4,5a,36,53,87,154,160,209 Burn toxin, a high-molecular-weight protein, is known to contribute to postburn immunosuppression.108,109,158
The complement system may be activated after a burn; this can produce blood pressure instability, fever, peripheral vasodilation with increased capillary permeability, changes in leukocyte function, coagulopathies, and microcirculatory obstruction.220 The complement system also may be dysfunctional after a thermal injury.
Immediately after a burn, lymphocyte response to antigen usually is depressed.156 This depression lasts approximately 48 hours and may compromise the patient’s immune response.
Clinical Signs and Symptoms
General Findings
Burn wound sepsis is the most serious complication of burn injury and is defined as a bacterial count of greater than 105 organisms per gram of tissue associated with invasion of viable tissue beneath the eschar.194 Infections and sepsis also may be caused by other organisms, including Candida species, other fungi,49,208 or viruses.
Signs of possible local burn wound infection are listed in Box 20-6; they include a change in wound appearance or drainage, vesicular or coloration changes in the skin surrounding the burn, and the presence of a distinctive odor. If any of these changes are noted, burn wound infection should be suspected, and a wound biopsy should be performed.189
Box 20-6 Signs of Possible Burn Wound Infection
• Conversion of partial-thickness to full-thickness injury
• Hemorrhagic discoloration or ulceration of healthy skin at the burn margins
• Erythematous, modular lesions in unburned skin and vesicular lesions in healed skin
• Edema of healthy skin surrounding the burn wound
• Pale, boggy, dry, or crusted granulation tissue
Signs of sepsis in the burned child are listed in Box 20-7 and include alteration in neurologic, gastrointestinal, and skin perfusion, subtle changes in vital signs (including unexplained tachycardia and early tachypnea or the need for increased oxygen or mechanical ventilation support), alteration in temperature (fever or hypothermia) and alteration in WBCs.165 Clinical and laboratory evidence of end organ dysfunction (e.g., lactic acidosis, oliguria, disseminated intravascular coagulation) will be observed when septic shock develops (see, also, Boxes 6-2 and 6-3).
Box 20-7 Signs of Sepsis in the Child with Burns: Clinical Findings*
Signs of systemic inflammatory response (two or more of the following four)
Initially the child with sepsis may demonstrate peripheral vasodilation with increased capillary permeability similar to that seen during the third-spacing phase following a burn. Increased fluid administration suddenly may be necessary to maintain systemic perfusion, and systemic and pulmonary edema may develop. In addition, laboratory findings may indicate nonspecific signs of stress, including hyperglycemia (or hypoglycemia in infants), early disseminated intravascular coagulation (particularly thrombocytopenia), and metabolic acidosis. Leukocytosis or leukopenia may develop. When septic shock develops, cardiovascular dysfunction (e.g., hypotension and signs of poor perfusion, such as lactic acidosis) and other organ failure will develop (see Septic Shock in Chapter 6 and Box 6-3).
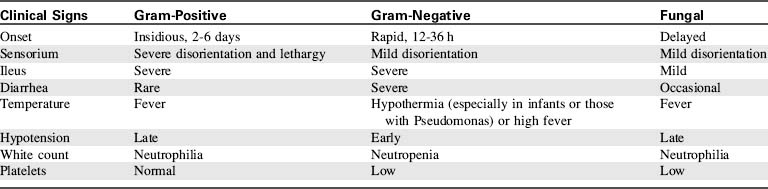
There is no single laboratory or clinical finding that confirms the presence of sepsis.2a A wound biopsy will aid in identifying an infecting organism and its sensitivities, and histologic examination will determine whether bacterial invasion of healthy tissue has occurred.189 In addition, sepsis caused by gram-positive, gram-negative, and fungal infections can produce characteristic clinical findings. The characteristics of potential infections and resulting sepsis are summarized briefly here (Table 20-10).
Gram-Positive Infections and Sepsis
This infection is characterized by wound erythema, pain, induration, and swelling. The erythema may extend from the margin of the burn wound, indicating streptococcus invasion of normal tissue.124
Staphylococcus aureus and Staphylococcus epidermidis are also gram-positive organisms; they are easily transmitted by contact or airborne routes. These infections usually have an insidious course, and 2 to 5 days may elapse between the onset of signs of infection and the development of sepsis. Staph wound infections are characterized by microabscesses, tissue necrosis, and increased exudate,29 and they may cause graft loss. Staph sepsis will produce high fever and leukocytosis, and a gastrointestinal ileus is often present.
Gram-Negative Infections and Sepsis
Other gram-negative organisms, including Escherichia coli, Klebsiella, Proteus, Enterobacter, and Providencia species have been observed with increasing frequency in burn units. These infections usually colonize the wound from the patient’s own flora and produce infection when other organisms are eliminated by antibiotic therapy.3 Translocation of gram-negative bacteria or endotoxin from the patient’s own gastrointestinal tract can also contribute to the development of gram negative sepsis.
Management
Prevention
Some physicians advocate excising the burn eschar within 72 hours of injury to reduce the possibility of burn wound sepsis.61,137,196 Early excision is thought to be effective, because it enables early closure of the wound (with biologic dressings or grafting), eliminates the potential source of immunosuppressive factors, stops the consumption of immune defense factors, reduces the length of hospital stay (see Management of Escharotomy), and provides better functional and cosmetic results.
Treatment of Sepsis
Initially, broad-spectrum antibiotics are prescribed until results of blood cultures and sensitivity studies are available; more specific antibiotics are then used. If aminoglycoside antibiotics are administered, peak and trough levels are monitored to ensure effective blood concentrations (see Chapter 4).
Occasionally, antibiotics may be injected or infused into the wound itself in an attempt to eliminate the infection at its site.142,174 This therapy is controversial, however, because it may increase the formation of resistant organisms.
Detailed discussion of the treatment of septic shock is included in Chapter 6 (see Fig. 6-8). Aggressive fluid resuscitation (with administration of bolus therapy) is indicated; typically three or four boluses of 20 mL/kg are administered in the first hour after the development of signs of septic shock, unless signs of heart failure (e.g., hepatomegaly, pulmonary edema, and respiratory distress) suggestive of myocarditis develop. Beta-adrenergic and vasoactive drug support should be initiated if shock persists, and support of the airway, oxygenation, and ventilation are required.
Evaluation of Therapy
Throughout therapy the burn wounds should be inspected to determine progress in healing.150 When sepsis is present, intravenous catheters should be changed every 72 hours, and more often if sites appear inflamed. Routine culture of catheter tips was shown to have no demonstrable benefit,190 because these tips often are contaminated during removal. However, if catheter infection is suspected, tip culture may aid in confirmation of the diagnosis. Meticulous catheter care should be performed at least every 24 to 48 hours, using aseptic technique per unit policy (See Box 22-6).
Nutritional Compromise
Pathophysiology
Metabolic Rate and Oxygen Consumption
After a burn, catecholamine secretion in response to stress will stimulate metabolic rate, oxygen consumption, heat production, and substrate mobilization.70 When a major burn is present, the basal metabolic rate may be twofold higher than normal. The actual metabolic rate can be determined by measuring the exchange of respiratory gases and calculating heat production from oxygen consumption and carbon dioxide production (see Indirect Calorimetry, later in this section).206
Oxygen consumption increases when the metabolic rate increases. However, this increase in oxygen consumption varies in different tissue beds after a burn. Despite the fact that blood flow to the burn wound is enhanced,73 the burn uses little or no oxygen for its metabolic processes. As a result, anaerobic burn metabolism can produce localized metabolic acidosis. Visceral oxygen consumption increases markedly with a burn injury, whereas peripheral oxygen uptake remains a fixed percentage of total aerobic metabolism.206
Glucose and Fat Metabolism
Hyperglycemia is observed after a burn, resulting from accelerated gluconeogenesis, reduced insulin levels, and abnormal glucose utilization. Hepatic gluconeogenesis is stimulated by catecholamine release, and the quantity of glucose made is directly related to the extent of the injury.207
Glucose utilization is not uniform throughout the body after a burn. The net glucose flux across healthy tissues and skeletal muscles is low, whereas glucose uptake by burned tissue is extremely high. In addition, injured tissues release large quantities of bacteria, which consume most of the available glucose.206 Renal glucose consumption is also elevated, whereas central nervous system glucose consumption remains normal.
Exogenous glucose from intravenous fluids is not utilized appropriately at this time, so serum glucose concentration often remains elevated long after glucose administration. Hepatic gluconeogenesis will continue despite exogenous glucose administration.226
Major thermal injury and hypermetabolism produce an increase in serum free fatty acids. Hydrolysis of stored triglycerides is accelerated, and mobilization of fat stores is stimulated by catecholamine secretion and elevated glucagon levels.25 Postburn hypoalbuminemia also contributes to the elevation in free fatty acids,75 because the serum albumin is not available to transport free fatty acids across cell membranes. Albumin administration at this time may help reduce serum free fatty acids.
Negative Nitrogen Balance
A thermal injury results in the breakdown of protein from skeletal muscle in burned and unburned areas.218 This muscle breakdown provides amino acids for gluconeogenesis and fuel sources for local tissue needs.69 If protein intake and synthesis do not increase and protein breakdown from skeletal muscle continues, a marked negative nitrogen balance ensues and nitrogen is excreted with urea in the urine. Urinary nitrogen loss is related primarily to the metabolic rate of the child, but is also affected by the child’s nutritional status and muscle mass.
Clinical Signs and Symptoms
Physical Assessment of Nutritional Status
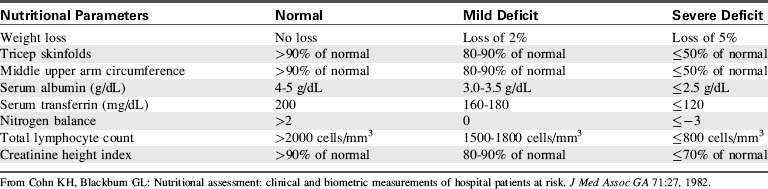
A variety of parameters must be examined to determine the child’s nutritional status (Table 20-11). However, these standard parameters do not allow for the effects of a large burn and its therapy on metabolic rate, so the child’s nutritional support must be evaluated constantly.
Anthropometric measurements include daily weight, triceps skin fold, and middle upper arm circumference.33 The most useful of these measurements is the daily weight.
Daily weights should be recorded on a weight chart. A weight change of 10% or more is significant and requires evaluation of caloric and fluid intake. Weight loss of 5% or more of baseline body weight usually indicates inadequate nutritional support.206 A weight gain can indicate fluid retention, early sepsis, or muscle or fat accumulation. Changes in weight will most accurately reflect nutritional status late after a burn, once edema has disappeared.
Laboratory Evaluation of Nutritional Status
Protein Levels
Visceral proteins (e.g., transferrin, albumin, retinol binding protein, thyroxin binding prealbumin) are essential for wound healing, host defense, substrate transport, and many enzyme functions in the body.104,206 The serum concentrations of these proteins can fall abruptly after a burn as the result of depletion of body fat reserves and skeletal muscle protein.13
Serum transferrin is one of the most reliable indicators of visceral protein status and malnutrition because it has a short half-life.33 A serum transferrin concentration less than 120 mg/dL is consistent with protein depletion230 and is associated with an increased risk of bacteremia in burn patients.105,145,161
Serum albumin concentration is measured frequently, but this protein has a long half-life. In addition, it can be increased by exogenous albumin administration and so may fail to reflect acute changes in nutritional status. The serum albumin level usually does fall within a few days of a burn injury, as a result of albumin movement from the vascular to the interstitial space.144,155
Nitrogen Balance
If the child has a large burn, nonurinary nitrogen losses also must be estimated; this requires the use of a multiple regression equation based on measured urinary urea nitrogen, the age of the child, and the percentage of TBSA burned. A complete 24-hour urine collection for urine urea is fundamental to each of these calculations.11
Creatinine Height Index
The creatinine height index is calculated from a 24-hour urine collection. Urinary creatinine excretion and the creatinine height index will decrease when the lean body mass decreases during periods of malnutrition.144 Results may be inaccurate if the child is receiving tobramycin sulfate, narcotics, ascorbic acid, or dietary creatinine, because these substances will alter urinary creatinine.
Determining Nutritional Requirements
The child’s nutritional requirements are determined by the amount of calories, nitrogen, and protein needed for normal homeostasis, plus those needed during burn-induced catabolism and healing of the burn wound.218 Initial estimate of nutritional requirements is made at the time of admission. A variety of equations are available to determine nutritional requirements; all pediatric equations use the child’s age, body weight or body surface area (see BSA nomogram on inside back cover of this text), and the percent of TBSA burned. Nutritional requirement equations developed for use in adult patients are not suitable for use in pediatric patients.40,85,117
The most popular pediatric formula for determining nutritional needs of children with burns is the Polk formula (Box 20-8); it provides for basal metabolic requirements plus additional calories based on the percent of the child’s body surface area burned.74 Alternative formulas calculate basal metabolic requirements based on the child’s body surface area with additional fluid requirements based on burn surface area.96 Use of body surface area provides a more accurate estimation of caloric requirements than those based on weight alone.
Additional Nutritional Requirements
High protein intake will be required after a burn to replace protein lost as a result of the increased metabolic rate, through the burn wound itself, and from tissue breakdown and infection. Protein intake can be calculated based on urinary nitrogen excretion, because 1 g of urinary nitrogen represents the loss of 30 g of lean body tissue, or 6.25 g of protein.70 An estimate of protein requirements also can be made from the child’s weight (3 g protein required per kilogram body weight) plus percent of TBSA burned (1 g protein required per 1% of TBSA burned). Serum protein measurements are unreliable as parameters to guide protein replacement.5
Approximately 20% to 30% of the child’s total caloric intake should be in the form of proteins, and approximately 50% to 60% of total calories should be administered as carbohydrates. Fats should constitute approximately 5% to 15% of nonprotein caloric intake.138 It is now thought that W-3 fatty acids, such as those derived from fish oil, are the most desirable form of fat supplement.76 Excessive carbohydrate intake can result in hyperglycemia,12 and excessive fat intake can result in immunosuppression, hyperlipidemia, and hepatic dysfunction.207,209
Vitamin and mineral supplementation is necessary, although specific requirements after a burn injury have not been determined. Fat-soluble vitamins (A, D, E, and K) are stored in fat deposits and are depleted during prolonged feeding without supplementation. Water-soluble vitamins (B and C) are not stored in large quantities, so they also are depleted rapidly. Although vitamin and mineral deficiency can impair healing,99 excessive vitamin administration also may be toxic.52,225 National Academy of Science34 and American Medical Association6 recommendations should be followed until more specific information is known about vitamin and mineral requirements after a burn.
Indirect Calorimetry
Indirect calorimetry has only recently been used to determine caloric requirements in children.102,193 This technique determines kilocalories of energy expenditure based on the measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2).200 An accurate weight measurement is also required.
The amount of oxygen consumed and carbon dioxide produced is determined by the difference in concentration of these gases between inspiration and exhalation.117 Energy expenditure is calculated by means of standard equations.23 Anything that interferes with gas exchange in the lungs (e.g., pneumothorax) or gas conduction to the calorimetry circuit will produce inaccurate results.
Measurements obtained during calorimetry also can be used to calculate the respiratory quotient (RQ). RQ is the ratio of oxygen consumption to carbon dioxide production, and it is useful in assessing energy expenditure. The RQ will vary with the adequacy of feeding and the type of fuel used as energy. An RQ of 0.70 is seen in starvation, and an RQ greater than 1.0 suggests that overfeeding has occurred, with resultant pure carbohydrate metabolism and fat synthesis.192
The use of indirect calorimetry still requires refinement. Because oxygen consumption can vary significantly throughout the day as the result of activity, pain, and change in temperature, all measurements must be performed under identical conditions. In addition, calculated allowances (available from the manufacturer) for activity are not accurate for pediatric patients.200 Calorimetry does not measure nitrogen balance, so it is usually necessary to continue to monitor urinary nitrogen excretion.
Management
Nutritional therapy requires identification of nutritional needs, reduction of net nitrogen losses, promotion of protein repletion, provision of adequate nutrients, and assessment of the effectiveness of therapy. When burns are extensive, it may be difficult to provide high caloric intake on a daily basis. Some form of feeding supplementation almost certainly will be necessary after large burns, because caloric requirements are high, and the child may develop loss of appetite.127
Oral Feeding
Although oral feeding is the preferred route of nutrition, only children with uncomplicated burns totaling 15% or less of TBSA can be expected to ingest sufficient calories by this route. To maximize effectiveness of oral feeding, every attempt must be made to maximize the quantity and content of the child’s caloric intake. The child’s likes and dislikes must be noted, and favorite foods must be available at all times on the unit.224 When the child is thirsty, high-calorie liquids, including fruit juices, fortified milk drinks, and commercial oral feeding preparations, should be offered instead of water. Commercial nitrogen and caloric supplements should be added to food and beverages to optimize intake. Mealtimes should be made special, and strenuous activity and therapy should not be scheduled immediately before or after eating.
Tube or Enteral Feeding
Whenever possible, the child’s gut should be used for feeding. Oral and gastric feeding preserve gut mucosal mass and maintain digestive enzyme control.218 Tube feeding may be used to supplement oral intake, if the child is unable to ingest at least 75% of caloric requirements.164 Tube feedings should be planned for any child with burns in excess of 15% of TBSA. Feeding should begin as soon as possible after the burn, because delayed feedings are associated with loss of mucosal mass, elevated catabolic hormones, increased metabolic rate, and decreased feeding tolerance.54,139 Enteral feeding may prevent or minimize translocation of gram-negative bacteria and endotoxin across the gastrointestinal mucosa.63
Continuous tube feedings usually are required to provide maximal caloric intake. Nasogastric feeding may be provided as long as active bowel sounds are present, usually within 48 to 72 hours after a burn injury. Nasoduodenal feeding can begin immediately after a burn, even if bowel sounds are absent, so this method of feeding has recently become popular.76,77,107 Intravenous albumin administration may help to maintain serum albumin soon after a burn until the child demonstrates ability to tolerate tube feeding.
Tube feeding should be started with a small volume of formula, and the hourly feeding volume is increased gradually as tolerated every 4 to 8 hours. The head of the child’s bed should be elevated to reduce the risk of regurgitation. Infusion pumps should be used to ensure consistent feeding volume and rate. Intermittent bolus feedings should be avoided, because they are associated with a higher incidence of gastric cramping, diarrhea, gastric distension, regurgitation, and aspiration.95,99
Commercial tube feedings designed for adults are inappropriate for use in young infants, because they contain amounts of protein that are excessive for immature kidneys.76 Infant formulas such as Similac (Abbott, Abbott Park, IL) or Enfamil (Mead Johnson Nutrition, Glenview, IL) are preferred. Caloric content of these formulas can be increased gradually from 20 calories/ounce to 24-27 calories/ounce as tolerated, using commercial feeding supplements if needed.
Isotonic commercial tube feeding preparations (such as Isocal [Mead Johnson Nutrition, Glenview, IL] and Osmolite [Abbott, Abbott Park, IL]) can be used for children older than 1 year; they may be enriched with additional protein (e.g., whey or Pro-mix) to provide additional protein caloric intake.172 High-nitrogen content formulas, including Isocal HN, have been developed to meet the high nitrogen needs of burn and trauma patients.
Daily multivitamin and mineral supplements including ascorbic acid and zinc sulfate should be administered. Administration of intravenous or enteral glutamine may reduce translocation of gram-negative bacteria across gastrointestinal mucosa during parenteral nutrition. The efficacy of this therapy is under evaluation.63
Parenteral Feeding
Because hypertonic glucose is an excellent growth medium, strict aseptic technique must be maintained when changing alimentation tubing and catheter entrance site dressings. Ideally, parenteral alimentation catheters should be used only for alimentation and not for drug administration or blood sampling. Potential complications of parenteral alimentation include metabolic imbalance and sepsis.134 For further information regarding parenteral alimentation, see Chapter 14.
Temperature Instability
Management
Blood should be warmed in a water bath heated to 37.5 to 40.5° C prior to administration. A microwave oven should not be used to warm fluids, because it will result in uneven heating and may lyse proteins contained in the fluids. Solutions used for wound care also are typically warmed in baths heated to 37.5 to 40.5° C.186 Follow unit protocols.
Potential Skin and Joint Contractures
Etiology and Pathophysiology
Collagen gradually develops on the surface of the burn wound and continues to develop after healing of the open areas and after grafting is complete. In this collagen layer, many fibroblasts gradually proliferate and attach to surrounding tissue. The fibroblasts progressively contract, causing the collagen fibers to disrupt the normal parallel layers and to form a wave pattern.199 Eventually, the collagen bundles take on a supercoiled appearance, and collagen nodules develop115; this can lead to joint and skin contractures.
Management
Rehabilitation must begin during the acute phase of burn care.71 The child in pain will assume a position of comfort, which is generally a position of flexion of the extremities. If appropriate positioning and exercises are not provided, contractures will develop. Therapeutic positioning of extremities is needed, and splinting of extremities should be accomplished when the child’s condition stabilizes.
Positioning
Hands should be splinted in the position of function, with the wrist dorsiflexed at 45 degrees or neutral, according to the physical therapist’s preference. Burns in the area of the axillae frequently result in severe contractures. When axillary burns are present, the arms should be positioned at 90-degree angles from the body.60
The foot should be positioned at a 90-degree angle from the leg, avoiding both dorsiflexion and plantar flexion. If the feet are not burned, the use of high-topped athletic shoes will facilitate proper positioning. Splints or footboards also may be used. Knees should remain extended, and hips should not be flexed.199
Evaluation of Therapy
Constant and thorough assessment of joint function and range of motion is needed to identify any signs of contraction and prevent contracture development. Interruption of the program may be necessary during grafting procedures, but it is imperative that it be resumed as soon as possible after graft stabilization.38,39,55,83,116,133,166,195
Psychosocial Challenges
Etiology and Pathophysiology
A major burn is viewed as a crisis in the life of the child and the family—it is a sudden, traumatic event that alters their lives. The child’s response to injury is influenced by the child’s age, culture, socioeconomic background, coping skills, body image, family support systems, and previous experience with injury and pain.187,210 Every child will respond differently and must be treated as an individual while coping strategies are assessed and supported.
The effect of the burn injury begins immediately.106 The acute stress of pain, hospital procedures, disruption of body chemistry, strange people, and separation from family begins to mold the child’s perception and response.1
Clinical Signs and Symptoms
The child’s behavioral response to the burn must be interpreted in light of the emotional impact of the burn trauma, rather than in terms of normal behavior for age. The child may demonstrate withdrawal, manipulation, anger, depression, or sleep disturbances. The child will be frightened by the hospital environment, procedures, and strangers. Regression frequently is observed in school-aged children and adolescents.111
Management
Parents should be allowed to visit continuously and to assist with some comforting aspects of care; this will reassure the child and family by allowing the parents to continue to nurture the child in a special way.111 By minimizing or eliminating separation from the parents, the child will be better able to focus on interaction with the environment and coping with the burn injury and its treatment.
The child should be allowed to participate in care whenever possible. The child’s feeling of powerlessness may be reduced if the child is allowed to make age-appropriate choices about some aspects of care (e.g., which arm dressing will be changed first or sequence of bath and meals).103
A burn injury may have permanent psychosocial consequences for the child and family. The nurse will play a pivotal role in shaping the response of the child and family to the burn. For more comprehensive references regarding psychosocial care, see Chapters 2 and 3.14,15,18,31,32,66,120,110,127,129,149,204,214
Burn care
Initial Burn Care
Wound care should begin after the child has received adequate fluid resuscitation and systemic perfusion is acceptable (see Shock in Chapter 6). Wound care is designed to: (1) protect the patient from infection, (2) remove nonviable tissue, (3) clean the wound surface, (4) prepare the area for healing and grafting, and (5) provide patient comfort. Burn care can be performed in a treatment room or at the bedside, but bedside care is most practical if the child is seriously ill and requires mechanical ventilation. Appropriate analgesia must be provided (see Chapter 5).
The management of intact blisters depends on their size, location, and appearance.181 Blisters located on the palms of the hands or soles of the feet should be left intact; the blisters serve as a protective barrier that assists with wound healing and reepithelialization. The blister fluid will be absorbed in 5 or 6 days, and attenuated epidermis and keratinized skin will remain, leaving a bright pink, healed epidermis underneath.58 The blister promotes rapid healing with minimal scarring or pain.
Clean washcloths or gauze sponges are recommended for burn cleansing. Gowns or aprons, head coverings, and masks should be worn for all dressing changes. Nonsterile gloves can be used without increased risk of infection.191 Boxes of nonsterile gloves should not, however, be kept for use with different patients, because contamination may occur.
Management of Escharotomy
If arterial compromise to the involved extremity is suspected, tissue pressure measurements are performed. These measurements will provide more information than Doppler assessment of pulses. A wick catheter is inserted under sterile conditions into a muscular compartment beneath the eschar, and the catheter then is connected to a fluid-filled monitoring system, including a pressure transducer and monitor. Measurements are performed in both an anterior and a posterior compartment of the extremity. If tissue or compartment pressure exceeds 30 mm Hg, blood flow to the tissues will be compromised,114 and an escharotomy should be performed.
An escharotomy is an incision into the burn eschar (with electrocautery, scalpel, or enzyme) to relieve pressure and improve circulation. The incision is extended into the subcutaneous tissue, breaking the tourniquet effect of the eschar and allowing edematous tissue to bulge through the incision.100 Incisions are made carefully to avoid nerves and blood vessels. The procedure may be performed without anesthesia, because nerve endings to the eschar have been destroyed. If the child is awake and frightened, sedation, local anesthesia, or intravenous hypnotics (see Chapter 5) may be required. If tissue pressure measurements remain high after the escharotomy, a fasciotomy is performed. A fasciotomy is an incision extending through the subcutaneous tissue and the fascia.
Topical Antibiotic Agents
Topical antibiotics are applied to burn wounds to prevent bacterial colonization of the wounds.125 These agents restrict the bacterial population of the wound until the child’s immune system recovers sufficiently to destroy the bacteria or until the wound is closed surgically. No topical agent will sterilize the wound; bacterial growth can only be diminished. Furthermore, if the burn wound is extensive (60% or more of TBSA), infection often will develop despite these agents. For this reason, major burns usually are treated with early excision and grafting.
Topical agents can mask signs of infection; therefore in unusual cases, burn wound biopsies may be necessary to detect invasive infections at an early stage.162 Bacterial counts of 10,000 or more organisms per gram of tissue indicate impending burn wound infection; counts exceeding 100,000 organisms per gram of tissue indicate bacterial invasion.
The ideal topical agent should be bactericidal or bacteriostatic against the most common burn infections, should penetrate burn eschar actively, should lack local or systemic toxicity and significant side effects, should be painless and easy to apply, should prevent desiccation and allow reepithelialization, should not injure viable tissue, and should be inexpensive.151 No one topical agent meets all these requirements. As a result, several topical agents commonly are used, and the most popular are presented in Table 20-12. The agent selected for unit use will depend on specific wound care policies and typical unit pathogens and their sensitivities. Effectiveness of the agent used will be demonstrated by a low or decreased incidence of burn wound infections and sepsis. Silver sulfadiazine is used most frequently.
Silver Sulfadiazine
Silver sulfadiazine is the most popular topical antimicrobial agent available.219 It is effective against gram-negative and gram-positive organisms as well as yeast. The silver ion produces ultrastructural changes in bacterial cell membranes and cell walls and also binds to bacterial DNA to kill bacteria and prevent its replication.162,202
Silver sulfadiazine is applied liberally to a wound after it has been washed and debrided. A layer  to
to 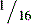 inch in thickness is applied (“buttered”) with a clean, gloved hand.186 Although the area can be left open, pediatric burn wounds generally are covered with gauze, so that the medication is not transferred onto linens. Because this drug may produce eye or nasal irritation,81 it should be applied to the face with caution.
inch in thickness is applied (“buttered”) with a clean, gloved hand.186 Although the area can be left open, pediatric burn wounds generally are covered with gauze, so that the medication is not transferred onto linens. Because this drug may produce eye or nasal irritation,81 it should be applied to the face with caution.
Silver sulfadiazine does not penetrate eschar as well as other topical agents. In addition, it may produce rash and itching if it comes in contact with unburned areas. Temporary, mild leukopenia has been reported,167 but this may be the result of the burn rather than the ointment.24,212,217,231 The child’s WBC count should be monitored daily; it generally returns to normal within 72 to 96 hours, even if the drug is continued.
Other Wound Care Modalities
A variety of wound care techniques and materials are currently available. The type of material and modality used will depend on unit protocols, efficacy of the material, product availability, and physician preference. Biologic dressings, including homograft, artificial skin, autologous cultured epithelium, and synthetic dressings are discussed in the following sections. Refer to Table 20-13 for a more comprehensive list of wound care modalities.
Biological Dressings
The term biological dressings refers to any natural or synthetic material that can be applied to an open burn wound to facilitate healing or prepare the wound for grafting. The most effective biologic dressings will adhere quickly to the burn surface and hasten healing; they will also provide a water and thermal barrier while remaining permeable to vapor and gas.148 The dressing should control bacterial growth and facilitate debridement of the wound; it should be painless, readily available, and inexpensive. As with topical antibiotics, no single biologic dressing possesses all these characteristics. As a result, the selection of dressings will be determined by the balance of desirable and undesirable characteristics, availability, and surgeon preference.
Biobrane (UDL Laboratories Inc., Mylan, Rockford, IL) is a thin, synthetic material composed of an inner layer of nylon coated with porcine collagen and an outer layer of rubberized silicone. It is pervious to air but not fluids and is available in simple sheets or preshaped gloves.113 After placement on clean, fresh, superficial second-degree burn wounds using Steri-strips and bandages, the Biobrane dressing dries, becoming adherent to burn wounds within 24 to 48 hours. Once the dressing is adherent, the covered areas are kept open to air and examined closely for the first few days to detect any signs and symptoms of infection. As epithelialization occurs beneath the Biobrane, the sheet is easily peeled off the wound. If serous fluid accumulates beneath the Biobrane, sterile needle aspiration can preserve its use. However, if foul-smelling exudate is detected, the Biobrane should be removed and topical antimicrobial dressings applied.
Synthetic and biologic dressings are also available to provide coverage for full-thickness burn wounds. Integra (Integra LifeSciences Corporation, Plainsboro, NJ), made of a collagen matrix with an outer silicone sheet, is a synthetic dermal substitute for the treatment of full-thickness burn wounds. After the collagen matrix engrafts into the wound in approximately 2 weeks, the outer silicone layer is replaced with epidermal autografts.72 Epidermal donor sites heal rapidly without significant morbidity, and Integra-covered wounds scar less; however, they are susceptible to wound infection and must be monitored carefully.7
Autologous Cultured Epithelium
Epithelial sheets now can be produced from cultures of epidermal cells obtained from small skin biopsies. These sheets can then be grafted to generate a permanent epidermal surface.35,64,84
Epithelial sheets are especially useful for the child with extensive burns when available donor sites are inadequate to provide wound coverage. Within 3 to 4 weeks, a biopsy specimen of 2 cm2 can be expanded to provide an epithelial sheet sufficient to cover the entire body surface. Currently, the 2- to 3-week growth period is too long to enable timely coverage of the burn, and the sheets are highly fragile. However, further development of the culture technique should enable improved use of this dressing.19,68
Epidermal Growth Factor
Initial clinical studies of partial-thickness donor sites in adult patients demonstrated accelerated epidermal regeneration when epidermal growth factor was added to conventional antimicrobial cream.20 Further clinical studies in children will be required to determine the effects and potential complications of epidermal growth factor on burn surfaces, donor sites, and other wounds, but initial results are promising.101
Surgical Intervention
Excision and Grafting
Early excision with skin grafting has been shown to decrease operative blood loss and length of hospital stay and ultimately improve the overall survival of burn patients.61,90–93 Typically, tangential excision of a full-thickness burn wound is performed within 3 days of injury, after relative hemodynamic stability has been achieved.137
Eschar is sequentially shaved using a powered dermatome (e.g., made by Zimmer, Wiltshire, UK) or knife blades (e.g., Watson or Weck surgical blades) until a viable tissue plane is achieved. Early excision of eschar (usually less than 24 hours after the burn injury) generally decreases operative blood loss, because vasoconstrictive substances, such as thromboxane and catecholamines are active. Once the burn wound becomes hyperemic, approximately 48 hours after injury, bleeding during excision of the eschar can be excessive. Tourniquets and subcutaneous injections of epinephrine-containing solution can lessen the blood loss, but these techniques may hinder the surgeon’s ability to differentiate viable from nonviable tissue.130 A topical hemostatic agent such as thrombin can also be used, but it is expensive and not very effective against excessive bleeding from open wounds. In patients with deep full-thickness burns, electrocautery is used to rapidly excise eschar with minimal blood loss. More importantly, the earlier the excision, the less is the expected blood loss in burns greater than 30% of TBSA.50 With scald burns, it is more difficult to assess the burn depth initially; therefore such burns require a more conservative approach, with delayed excision.
With massive burns, the closure of burn wounds is achieved by a combination of widely meshed autografts (4:1 to 6:1) with allograft (2:1) overlay. Repeated grafting is required for large burns, with sequential harvesting of split-thickness autograft from limited donor sites until the entire burn wound is closed. As the meshed autografts heal, allografts slough off, but the formation of significant scar is a major disadvantage of this technique.171 Therefore the use of widely meshed graft is avoided for the face and hands. A full-thickness graft that includes both dermal and epidermal components provides the best outcome in wound coverage, with diminished contracture and better pigment match.227 However, its use is generally limited to small areas, because there is a lack of abundant full-thickness donor skin available.
Donor Sites
Patient donor sites should resemble the area to receive the graft, so that hair growth and skin texture will conform to that surrounding the wound.44 Ideally, the donor sites should be covered by clothing or regrowth of hair, so that any scarring will not be visible. The scalp is an excellent donor site for children, because the head has a large available surface area and hair growth will cover the donor site. Small burns can also be covered with skin removed from the anterior or lateral surfaces of the upper thighs and lower abdomen.
Postoperatively, the split-thickness donor site should be treated as a partial thickness burn injury.57 A wide variety of care modalities have been used with comparable results. The site may be covered with dry or antimicrobial-permeated gauze, polyurethane, or a silver sulfadiazine dressing. Gauze is left in place for 2 to 3 weeks, until it naturally separates from the site as the area heals. This technique is relatively simple for the nurse, but is usually uncomfortable for the child, because the gauze tends to pull as it dries and mobility at the donor site is compromised.
Postoperative Care
The grafted skin must remain in constant contact with the tissue bed to receive nutrients and oxygen supply. Once the graft is applied, a fibrin layer forms between the granulating bed and the graft. Capillary action allows absorption of serum from the bed into the graft during the first days after grafting. Capillary buds then form a fine network of vascular channels to the fibrin layer,228 and blood flow to the graft is present within 3 days. Complete capillary ingrowth will be established approximately 7 to 10 days after grafting. It is imperative that the graft be secure (e.g., by wrapped dressings), without stress or shear forces applied during this time, to ensure delivery of nutrients to the graft and to prevent the disruption of the fragile capillary network.
Sheet grafts are usually left open to the air, without dressings, for the first days after surgery. Serum and exudate that accumulate under the graft must be evacuated, or the graft may fail.168 The serum may be allowed to seep from under the surface of the graft via small slices in the graft (similar to the small slices on a pie crust). Alternatively, the serum may be aspirated or expressed. Serum aspiration is accomplished with a needle and syringe. To express the serum, a sterile cotton applicator is rolled gently over the sheet graft from the center of the graft toward the nearest incision. The rolling technique should only be performed over a small amount of tissue, without application of pressure, because extensive or firm rolling might interrupt capillary development and blood flow to the graft.
1 Aatoon A., Remensnyder J.P. Burns in children. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
2 Adolfson L., Halebian P., Shires G.T.: Fixation of nasotracheal tubes based on blood supply to the nasal alae (abstract). American Burn Association Abstracts of 16th Annual Meeting, 1984:73 San Francisco
2a Alexander J.W. The body’s response to infection. In: Artz C.P., Moncrief J.A., Pmitt B.A., editors. Burns: a team approach. Philadelphia: WB Saunders Co, 1979.
3 Alexander J.W. The role of infection in the burn patient. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
4 Alexander J.W., Stinnett J.D., Ogle C.K. Alterations in neutrophil function. In: Ninnemann J.L., editor. The immune consequences of thermal injury. Baltimore: Williams and Wilkins, 1981.
5 Alexander J.W., et al. Beneficial effects of aggressive protein feeding in severely burned children. Ann Surg. 1980;192:505.
5a Arturson M.G. Arachidonic acid metabolism and prostaglandin activity following burn injury. In: Ninnemann J.L., editor. Traumatic injury: infection and other immunologic sequelae. Baltimore: University Park Press, 1983.
5b Aulick LH, Wilmore D.W. Hypermetabolism in trauma. In: Girardier L., Stock M.S., editors. Mammalian thermogenesis. London: Chapman & Hall, 1983.
6 American Medical Association, Department of Foods and Nutrition. Guidelines for essential trace element preparations for parenteral use. J Am Med Assoc. 1979;241:2051.
7 Barret J.P., et al. Cost-efficacy of cultured epidermal autografts in massive pediatric burns. Ann Surg. 2000;231:869.
8 Batchelor A.D.R., Kirk J., Sutherland A.B. Treatment of shock in the burned child. Lancet. 1961;1:123.
9 Baxter C.R. Controversies in the resuscitation of burn shock. Curr Concepts Thermal Care. 1982;5:5.
10 Baxter C.R., Shires G.T. Physiological response to crystalloid resuscitation of severe burns. Ann NY Acad Sci. 1968;150:874.
11 Bell S.J., et al. Prediction of total urinary nitrogen from urea nitrogen in burned patients. J Am Diet Assoc. 1985;85:1100.
12 Bessey P.Q., Wilmore D.W. Metabolic and nutrition support for trauma and burn patients, Nutrition Symposium. West Virginia: Mead Johnson Nutritional Division; 1982.
13 Blackburn G.L., Harvey K.B. Nutritional assessment as a routine in clinical medicine. Postgrad Med. 1982;71:46.
14 Blakeney P., et al. Long-term psychosocial adjustment following burn injury. J Burn Care Rehabil. 1988;9:661.
15 Boswick J.A. Emotional problems in burn patients. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
16 Bowser-Wallace B.H., Caldwell F.T. Fluid requirements of severely burned children up to3 years old: hypertonic lactated saline versus Ringer’s lactate-colloid. Burns Incl Therm Inj. 1986;12(8):549-555.
17 Bowser-Wallace B.H., Caldwell F.T. The effect of resuscitation with hypertonic versus hypotonic versus colloid on the wound and urine fluid and electrolyte losses in severely burned children. J Trauma. 1983;23:916.
18 Brill N., et al. Caring for chronically ill children: an innovative approach for care. Child Health Care. 1987;16:105.
19 Brown A., Barot L. Biological dressings and skin substitutes. Clin Plast Surg. 1986;13:69.
20 Brown G.L., et al. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989;321:76.
21 Burke J.F., et al. Successful use of physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;199:413.
22 Safe Kids Campaign. Burn injury fact sheet. Washington, DC: National Safe Kids Campaign; 2004.
23 Burszstein S., et al. Utilization of protein, carbohydrate and fat in fasting and post absorptive subjects. Am J Clin Nutr. 1980;33:998.
24 Caffee H.H., Bingham H.G. Leukopenia and silver sulfadiazine. J Trauma. 1982;22:586.
25 Carpentiera Y.A., et al. Effects of hypercaloric glucose infusion on lipid metabolism in injury and sepsis. J Trauma. 1979;19:649.
26 Carvajal H.F. Acute management of burns in children. South Med J. 1975;68:129.
27 Carvajal H.F. Management of severely burned patients: sorting out the controversies. Emer Med Rep. 1985;6:89.
28 Carvajal H.F. Resuscitation of the burned child. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
29 Carvajal H.F. Septicemia and septic shock. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
30 Charnock E.L., Meehan J.J. Postburn respiratory injuries in children. Pediatr Clin North Am. 1980;27:666.
31 Chedekel D.S. The psychologist’s role in comprehensive burn care. In: Bernstein N.R., Robson M.C., editors. Comprehensive approaches to the burned patient. New York: Medical Examination Publishing, 1983.
31a Chung D.H., Sanford A.P., Herndon D.N. Burns. In O’Neill J., Grossfeld J.L., Coran A.G., Fonkalsrud E.W., editors: Grossfeld: pediatric surgery, ed 6, St Louis: Mosby, 2006.
32 Clarke A.M. Thermal injuries: the case of the whole child. J Trauma. 1980;20:823.
33 Cohn K.H., Blackburn G.L. Nutritional assessment: clinical and biometric measurements of hospital patients at risk. J Med Assoc Ga. 1982;71:27.
34 Committee on Dietary Allowances. Recommended dietary allowances. Washington, DC: National Academy of Science; 1980.
35 Compton C.C., et al. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. Lab Invest. 1989;60:600.
36 Constantian M.B. Association of sepsis with an immunosuppressive polypeptide in the serum of burn patients. Ann Surg. 1978;188:209.
37 Cope O., Moore F.D. The redistribution of body water and the fluid therapy of the burn patient. Ann Surg. 1947;126:1010.
38 Covey M.H. Occupational therapy. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
39 Covey M.H., et al. Efficacy of continuous passive motion (CPM) devices with hand burns. J Burn Care Rehabil. 1988;9:397.
40 Curreri P.W., et al. Dietary requirements of patients with major burns. J Am Diet Assoc. 1974;65:415.
41 Cuzzell J.Z. Wound care forum: artful solutions to chronic problems. Am J Nurs. 1985;85:162.
42 Dahn M.S., et al. Negative inotropic effect of albumin resuscitation for shock. Surgery. 1979;86:235.
43 Dailey M.A. Carbon monoxide poisoning. J Emerg Nurs. 1989;15:120.
44 David J.A. Wound management: a comprehensive guide to dressing and healing. London: Martin Dunitz; 1986.
45 Deitch E.A. Immunologic considerations in the burned child. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
46 Deitch E.A., Gelder F., McDonald J.C. Sequential prospective analysis of the nonspecific host-defense system after thermal injury. Arch Surg. 1984;119:83.
47 Demling R.H. Fluid replacement in burned patients. Surg Clin North Am. 1987;67:15.
48 Demling R.H. Fluid resuscitation. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
49 Desai M.H., Herdon D.N., Abston S. Candida infection in massively burned patients. J Trauma. 1987;27:1186.
50 Desai M.H., et al. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211:753.
51 Dingeldein G.P. Fluid and electrolyte therapy in the burn patient. In: Salisbury R.E., Newmann N.M., Dingeldein G.P., editors. Manual of burn therapeutics: an interdisciplinary approach. Boston: Little Brown, 1983.
52 DiPalma J.R., Ritchie D.M. Vitamin toxicity. Ann Rev Pharmacol Toxicol. 1977;17:133.
53 Dobke M., et al. Autoimmune effects of thermal injury. In: Ninnemann J.L., editor. The immune consequences of thermal injury. Baltimore: Williams and Wilkins, 1981.
54 Dominioni L., et al. Prevention of severe post-burn hypermetabolism and catabolism by immediate intragastric feedings. J Burn Care Rehabil. 1984;5:106.
55 Duncan C.E., Cathcart M.E. A multi-disciplinary model for burn rehabilitation. J Burn Care Rehabil. 1988;9:191.
56 Eagle J.F. Parenteral fluid therapy of burns during the first 48 hours. NY J Med. 1956;56:1613.
57 Engeman S.A. The burned patient: perioperative nursing care. AORN J. 1984;10:36.
58 Ersek R.A., et al. A report of the simple two-step system for scalds. Bull Burn Inj. 1988;5:46.
59 Evans El, et al. Fluid and electrolyte requirements in severe burns. Ann Surg. 1952;135:804.
60 Fader P. Preserving function and minimizing deformities: the role of the occupational therapist. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
61 Fidler J.P. Debridement and grafting of full-thickness burns. In: Hummel R.P., editor. Clinical burn therapy: a management and prevention guide. Boston: John Wright Publishing, 1982.
62 Fimberg L. Interrelationships between electrolyte physiology, growth and development. In: Fimberg L., Kravath R.E., Fleischman A.R., editors. Water and electrolytes in pediatrics. Philadelphia: WB Saunders, 1982.
63 Fink M.P. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med. 1991;19:627.
64 Flores J.B., et al. Use of cultured human epidermal keratinocytes for allografting burns and conditions for temporary banking of the cultured allografts. Burns. 1990;16:3.
65 Frank D.H., et al. Comparison of biobrane, porcine, and human allograft as biologic dressings for burn wounds. J Burn Care Rehabil. 1983;4:186.
66 Friedman J.K., Shapiro J., Plon L. Psychosocial treatment and pain control. In: Archauer B.M., editor. Management of the burn patient. Los Altos, Calif: Appleton and Lange, 1987.
67 Fuchs G.J., Gleason W.A. Gastrointestinal complications in burned children. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
68 Gallico G., et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448.
69 Gamelli R.L. Nutritional problems of the acute and chronic burn patient. Arch Dermatol. 1988;124:756.
70 Giel L.C. Nutrition. In: Archauer B.M., editor. Management of the burned patient. Los Altos, CA: Appleton and Lange, 1987.
71 Gillespie R.W., Halpern M. Rehabilitation services. In: Smith D.J., editor. Symposium: reconstruction and rehabilitation. Seattle: American Burn Association, 1988.
72 Goodenough R.D., Molnar J.A., Burke J.F. Changes in burn wound closure. In: Ninnemann J.L., editor. Traumatic injury, infection and other immunologic sequelae. Baltimore: University Park Press, 1983.
73 Goodwin C.W. Metabolism and nutrition in the thermally injured patient. Crit Care Clin. 1985;1:97.
74 Gordon M.D. Nursing care of the burned child. In: Artz C.P., Moncrief J.A., Pruitt B.A., editors. Burns: a team approach. Philadelphia: WB Saunders, 1979.
75 Gottschlich M.M., Alexander J.W. Fat kinetics and recommended dietary intake in burns. J Parenter Enter Nutr. 1987;11:80.
76 Gottschlich M.M., Warden G.D., Alexander J.W. Dietary regimens for the burned pediatric patient. In: Herdon D.N., editor. Nutrition and metabolism symposium. Chicago: American Burn Association, 1986.
77 Gottschlich M.M., et al. Therapeutic effects of modular tube feeding recipe in pediatric burn patients. Proc Am Burn Assoc. 1986;18:84.
78 Gozal D., et al. Accidental carbon monoxide poisoning; emphasis on hyperbaric oxygen treatment. Clin Pediatr. 1985;24:132.
79 Graves T.A., et al. Fluid resuscitation of infants and children with massive thermal injury. J Trauma. 1988;28:1656.
80 Grogan J.B. Altered neutrophil phagocytic function in burn patients. J Trauma. 1976;16:734.
81 Guzzett P.C., Holihan J.A. Burns. In: Eichelberger M.R., Pratsch G.L., editors. Pediatric trauma care. Rockville, MD: Aspen Publishers, 1985.
82 Halpern J. Chronic occult carbon monoxide poisoning. J Emerg Nurs. 1989;15:107.
83 Hanson N.N. Practice and planning in physical therapy. In: Berstein N.R., Robson M.C., editors. Comprehensive approaches to the burned patient. New York: Medical Examination Publishing, 1983.
84 Harmel R.P., Vane D.W., King D.R. Burn care in children: special considerations. Clin Plast Surg. 1986;13:95.
85 Harris J.A., Benedict F.G. A biometric study of basal metabolism in man. Washington: Carnegie Institute of Washington; 1919. publication 279
86 Hauser C.J., et al. Oxygen transport response to colloids and crystalloids in critically ill surgical patients. Surg Gynecol Obstet. 1980;150:811.
87 Heggers J.P., Robson M.C. Prostaglandins and thromboxanes. In: Ninnemann J.L., editor. Traumatic injury, infection and other immunologic sequelae. Baltimore: University Park Press, 1983.
88 Heideman M. Complement activation by thermal injury and its possible consequences for immune defense. In: Ninnemann J.L., editor. The immune consequences of thermal injury. Baltimore: Williams & Wilkins, 1981.
89 Heimbach D.M. American Burn Association 1988 presidential address “we can see so far because…”. J Burn Care Rehabil. 1988;9:340.
90 Heimbach D.M., Engrav L.H. Burn wound excision. Curr Concepts Trauma Care. 1981;4:14.
91 Heimbach D.M., Engrav L.H. Surgical management of the burn wound. New York: Raven Press; 1984.
92 Herndon D.N., et al. Determinants of mortality in pediatric patients with greater than 70% full-thickness total body surface area thermal injury treated by early total excision and grafting. J Trauma. 1987;27:208.
93 Herndon D.N., Parks D.H. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma. 1986;26:149.
94 Herndon D.N., et al. Incidence, mortality, pathogens and treatment of pulmonary injury. J Burn Care Rehabil. 1986;7:185.
95 Hiebert J.M., et al. Comparison of continuous versus intermittent tube feedings in adult burn patients. J Parenter Enter Nutr. 1981;5:73.
96 Hildreth M., Carvajal H.F. Caloric requirements in burned children: a simple formula to estimate daily caloric requirements. J Burn Care Rehabil. 1982;3:7880.
97 Horovitx J.H. Heat and smoke injuries of the airway. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
98 Huang P.P., et al. Hypertonic sodium resuscitation is associated with renal failure and death. Ann Surg. 1995;221:543.
99 Huggins B.M., Dingeldein G.P. Nutritional support in thermal injuries. In: Salisbury R.E., Newman N.M., Dingeldein G.P., editors. Manual of burn therapeutics: an interdisciplinary approach. Boston: Little Brown, 1983.
100 Hummel R.P. Curling’s ulcer and other complications. In: Hummel R.P., editor. Clinical burn therapy: a management and prevention guide. Boston: John Wright Publishing, 1982.
101 Hunt T.K., La Van F.B. Enhancement of wound healing by growth factors. N Engl J Med. 1989;321:111.
102 Ireton C.S., et al. Do changes in burn size affect measured energy expenditure? J Burn Care Rehabil. 1985;6:419.
103 Jansen M.T., et al. Meeting psychosocial and developmental needs of children during prolonged intensive care unit hospitalization. Child Health Care. 1989;18:91.
104 Jeejeebhoy K.N. Protein nutrition in clinical practice. Brit Med Bul. 1981;37:11.
105 Jensen J.G., et al. Nutritional assessment indications of post-burn complications. J Am Diet Assoc. 1985;85:68.
106 Kibbee E. Life after severe burns in children. J Burn Care Rehabil. 1981;2:44.
107 Kravitz M. Nutritional needs of burn patients. In: Herdon D.N., editor. Nutrition and metabolism symposium. Chicago: American Burn Association, 1986.
108 Kremer B., et al. Burn toxin. In: Ninnemann J.L., editor. The immune consequences of thermal injury. Baltimore: Williams and Wilkins, 1981.
109 Kremer B., et al. The present status of research in burn toxins. Int Care Med. 1981;7:77.
110 Knudson-Cooper M.S. Adjustment to visible stigma: the case of the severely burned. Soc Sci Med. 1981;15-B:31.
111 Knudson-Cooper M.S., Thomas C.M. Psychosocial care for severely burned children. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
112 Lacey D.J. Neurologic sequelae of acute carbon monoxide intoxication. Am J Dis Child. 1981;135:145.
113 Lal S., et al. Biobrane improves wound healing in burned children without increased risk of infection. Shock. 2000;14:314.
114 Larson M., Leigh J., Wilson L.R. Detecting compartmental syndrome using continuous pressure monitoring. Focus Crit Care. 1986;13:51.
115 Law E.J. Minimizing burn scar and contracture. In: Hummel R.P., editor. Clinical burn therapy: a management and prevention guide. Boston: John Wright Publishing, 1982.
116 Linares H.A. Hypertrophic healing: controversies and etiopathogenic review. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
117 Long C.L., et al. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. J Parenter Enter Nutr. 1979;3:452.
118 Lough M., Doershuk C., Stern R. Pediatric respiratory therapy. Chicago: Year Book Medical Publishers, Inc; 1985.
119 Lund C.C., Browder N.C. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79:352.
120 Luther S., Price J.H. Burns and their psychologic effect on children. J School Health. 1981;51:419.
121 Luterman A. Artificial skin. In: Warden G.D., editor. Wound healing symposium. Washington, DC: American Burn Association, 1987.
122 MacMillan B.G. Infections following burn injury. Surg Clin North Am. 1980;60:185.
123 MacMillan B.G. Initial replacement therapy. In: Hummel R.P., editor. Clinical burn therapy: a management and prevention guide. Boston: John Wright Publishing, 1982.
124 MacMillan B.G. The problem of infection in burns. In: Hummel R.P., editor. Clinical burn therapy: a management and prevention guide. Boston: John Wright Publishing Co, Inc, 1982.
125 MacMillan B.G. Wound management. In: Wagner M.M., editor. Care of the burn-injured patient: multidisciplinary involvement. Boston: PSG Publishing, 1981.
126 Madden M.R., Finkelstein J.L., Goodwin C.W. Respiratory care of the burn patient. Clin Plast Surg. 1986;13:29.
127 Magrath H.L. Nursing pediatric burns from a growth and development perspective. In: Wagner M.M., editor. Care of the burn-injured patient: multidisciplinary involvement. Boston: PSG Publishing, 1981.
128 Mancusi-Ungaro H.R. Chemical burns in children. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
129 Mannon J.M. Caring for the burned. Springfield, Ill: Charles C Thomas; 1985.
130 Marano M.A., et al. Tourniquet technique for reduced blood loss and wound assessment during excisions of burn wounds of the extremity. Surg Gynecol Obstet. 1990;171:249.
131 Martyn J.A. Cimetidine and/or antacid for the control of gastric acidity in pediatric burn patients. Crit Care Med. 1985;13:1.
132 Maschinot N., et al. Laryngotracheal stenosis as a complication of upper-airway thermal injury in children: two cases. Resp Care. 1987;32:785.
133 McDonald K., Johnson B., Prasad J.K. Collaborative physical therapy for a 4-month old infant. J Burn Care Rehabil. 1988;9:193.
134 Mechanic H.F., Dunn L.T. Nutritional support for the burn patient. Dim Crit Care Nurs. 1986;5:20.
135 Merrell S.W., et al. Fluid resuscitation in thermally injured children. AM J Surg. 1986;152:664-669.
136 Metheny N.M. Fluid and electrolyte balance, nursing considerations. Philadelphia: JB Lippincott Co; 1987.
137 Moberg A.W., et al. The comparative advantages of early tangential excision and grafting (TEG) in burn wound management. Plast Surg Forum. 1982;109:197.
138 Mochizuki H., et al. Optimal lipid content for enteral diets following thermal injury. J Parenter Enter Nutr. 1984;8:638.
139 Mochizuki H., et al. Mechanism of prevention of post burn hypermetabolism and catabolism by early enteral feedings. Ann Surg. 1984;200:297.
140 Monafo W.W. The treatment of burn shock by the intravenous and oral administration of hypertonic lactated saline solution. J Trauma. 1970;10:575.
141 Monafo W.W., Chuntrasakul C., Ayvazian V.H. Hypertonic sodium solutions in the treatment of burn shock. Am J Surg. 1973;126:778.
142 Monafo W.W., Salisbury R.E., Dimick A.R. The perils of sepsis. Emerg Med. 1983;15:47.
143 Moncrief J.A. The body’s response to heat. In: Artz C.P., Moncrief J.A., Pruitt B.A., editors. Burns: a team approach. Philadelphia: WB Saunders, 1979.
144 Morath M.A., et al. Interpretation of nutritional parameters in burn patients. J Burn Care Rehabil. 1983;4:361.
145 Morath M.A., Miller S.F., Finley R.K. Nutritional indicators of post burn bacteremic sepsis. J Parenter Enter Nutr. 1981;5:488.
146 Mosley S. Inhalation injury: a review of the literature. Heart Lung. 1988;17:3.
147 Murphy M., Bell S.J. Assessment of nutritional status in burn patients. J Burn Care Rehabil. 1988;9:432.
148 Nahas L.F., Swartz B.L. Use of semipermeable polyurethane membrane for skin graft dressing. Plast Reconstr Surg. 1981;67:791.
149 Nelson M. Identifying the emotional needs of the hospitalized child. MCN Am J Matern Child Nurs. 1981;6:181.
150 Newman N.M. Monitoring the burn patient. In: Salisbury R.E., Newman N.M., Dingeldein G.P., editors. Manual of burn therapeutics: an interdisciplinary approach. Boston: Little Brown, 1983.
151 Newman N.M. Nursing procedures. In: Salisbury R.E., Newman N.M., Dingeldein G.P., editors. Manual of burn therapeutics: an interdisciplinary approach. Boston: Little Brown, 1983.
152 Ninnemann J.L. Immune depression in burn and trauma patients: the role of circulating suppressors. In: Ninnemann J.L., editor. Traumatic injury, infection and other immunologic sequelae. Baltimore: University Park Press, 1983.
153 Ninnemann J.L. Immunologic defenses against infection: alterations following thermal injuries. J Burn Care Rehabil. 1982;3:355.
154 Ninnemann J.L., Condie J.T., Stein M.D. Lymphocyte response following thermal injury: the effect of circulating immunosuppressive substances. J Burn Care Rehabil. 1981;2:196.
155 Ninnemann J.L., Fischer J.C., Wachtel T.L. Effect of thermal injury and subsequent therapy on serum protein concentrations. Burns. 1980;6:165.
156 Ninnemann J.L., Fischer J.C., Wachtel T.L. Thermal injury associated immunosuppression: occurrence and in vitro blocking effect of post recovery serum. J Immunol. 1979;122:1736.
157 Ninnemann J.L., Stein M.D. Induction of suppressor cells by burn treatment with povidone-iodine. J Burn Care Rehabil. 1980;1:12.
158 Ninnemann J.L., Stein M.D. Suppression of in vitro lymphocyte response by “burn toxin” isolates from thermally injured skin. Immunol Lett. 1981;2:339.
159 Ninnemann J.L., Stein M.D. Suppressor cell induction by povidone-iodine: in vitro demonstration of a consequence of clinical burn treatment with Betadine. J Immunol. 1981;126:1905.
160 Ninnemann J.L., Stockland A.E., Condie J.T. Induction of prostaglandin synthesis dependent suppressor cells by endotoxin: occurrence in patients with thermal injuries. J Clin Immunol. 1983;3:142.
161 Ogle C.K., Alexander J.W., MacMillan B.G. The relationship of bacteremia to levels of transferrin, albumin and total serum protein in burn patient. Burns. 1981;8:32.
162 O’Neill J.A. Burns in children. In: Artz C.P., Moncrief J.A., Pruitt B.A., editors. Burns: a team approach. Philadelphia: WB Saunders, 1979.
163 O’Neill J.A. Fluid resuscitation in the burned child: a reappraisal. J Pediatr Surg. 1982;17:604.
164 O’Neill J.A., Roeber J. Burn care protocals: nutritional support. J Burn Care Rehabil. 1986;7:351.
165 Parish R.A., et al. Fever as a predictor of infection in burned children. J Trauma. 1987;27:69.
166 Parrott M., et al. Structured exercise circuit program for burn patients. J Burn Care Rehabil. 1988;9:666.
167 Pegg S.P. The role of drugs in management of burns. Drugs. 1982;24:256.
168 Pensler J.M., Mulliker J.B. Skin grafts: to mesh or not to mesh. Contemp Surg. 1988;32:45.
169 Perry A.W., et al. Mafenide-induced pseudochondritis. J Burn Care Rehabil. 1988;9:145.
170 Peterson H.D. Topical antibacterials. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, Inc, 1987.
171 Petry J.J., Wortham K.A. Contraction of wounds covered by meshed and non-meshed split thickness graft. Brit J Plast Surg. 1986;39:478.
172 Prokop-Oliet M., et al. Whey protein supplementation of complete tube feeding in the nutritional support of thermally injured patients. Proc Am Burn Assoc. 1983;15:37.
173 Prough D.S., et al. Effects on intracranial pressure of resuscitation from hemorrhagic shock with hypertonic saline versus lactated Ringer’s solution. Crit Care Med. 1985;13:407.
174 Pruitt B.A. Burn infection prophylaxis today: an overview. In: Alexander J.W., Munster A.M., Pruitt B.A., editors. Applied immunology: severe burns and trauma. Berkeley, CA: Cutter Biological, 1983.
174a Pulaski G.R., Tennison A.C. Estimation of the amount of burned surface area. J Am Med Assoc. 1948;103:34-38.
175 Quinby W.C., et al. Clinical trials of amniotic membranes in burn wound care. Plast Reconstr Surg. 1982;70:711.
176 Rackow E.C. Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch and saline solutions in patients with hypovolemic and septic shock. Crit Care Med. 1983;11:839.
177 Reiss E., et al. Fluid and electrolyte balance in burns. J Am Med Assoc. 1953;152:1309.
178 Rice V. Shock management: fluid volume replacement. Crit Care Nurse. 1984;4:69.
179 Robillard J.E., et al. Renal hemodynamics and functional adjustments to postnatal life. Semin Perinatol. 1988;12:143.
180 Robson M.C., Heggers J.A. Pathophysiology of the burn wound. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, Inc, 1988.
181 Rockwell W.B., Ehlich P. Should burn blister fluid be evacuated? J Burn Care Rehabil. 1990;11:93-95.
182 Ross A.D., Angarar D.M. Colloids versus crystalloids: a continuing controversy. Drug Intell Clin Pharm. 1984;18:202.
183 Royall J.A., Levin D.L. Adult respiratory distress syndrome in pediatric patients. I. Clinical aspects, pathophysiology, pathology and mechanisms of lung injury. J Pediatr. 1988;112:169.
184 Royall J.A., Levin D.L. Adult respiratory distress syndrome in pediatric patients. II. Management. J Pediatr. 1988;112:335.
185 Rubin W.D., Mani M.M., Hiebert J.M. Fluid resuscitation of the thermally injured patient. Clin Plast Surg. 1986;13:9.
186 Sadowski D.A. Burn wound care: silver sulfadiazine application. J Burn Care Rehabil. 1987;8:429.
187 Sadowski D.A. Fears expressed by burned children during the first eighteen months after injury. University of Cincinnati; 1984. master’s thesis
188 Sadowski D.A. Smoke inhalation/carbon monoxide poisoning. In: Sommers M.S., editor. Difficult diagnosis in critical care nursing. Rockville, MD: Aspen Publishers, 1988.
189 Sadowski D.A., Kishman M. Monitoring burn wounds for infection. J Burn Care Rehabil. 1987;8:568.
190 Sadowski D.A., et al. The value of culturing central line catheter tips in thermally injured patients. J Burn Care Rehabil. 1988;9:66.
191 Sadowski D.A., et al. Use of nonsterile gloves for routine noninvasive procedures in thermally injured patients. J Burn Care Rehabil. 1988;9:613.
192 Saffle J.R., Young E. Energy requirements in thermal injury. In: Herdon D.N., editor. Nutrition and metabolism symposium. Chicago: American Burn Association, 1986.
193 Saffle J.R., et al. Use of indirect calorimetry in the nutritional management of burned patients. J Trauma. 1985;25:32.
194 Salisbury R.E. Wound care. In: Salisbury R.E., Newman N.M., Dingeldein G.P., editors. Manual of burn therapeutics: an interdisciplinary approach. Boston: Little Brown, 1983.
195 Salisbury R.E., Petro J.A. Rehabilitation of burn patients. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
196 Sandove A.M., et al. Early excision: a financial assessment. J Burn Care Rehabil. 1985;6:442.
197 Sataloff D., Sataloff R. Tracheostomy and inhalation injury. Head Neck Surg. 1984;6:1024.
198 Schnares R.H., et al. Plasma exchange for failure of early resuscitation in thermal injuries. J Burn Care Rehabil. 1986;7:230.
199 Schneider R.M., Simonton-Thorne S. Treatment of joints and scars. In: Achauer B.M., editor. Management of the burn patient. Los Altos, CA: Appleton and Lange, 1987.
200 Sehune J., Goede M., Silverstein P. Comparison of energy expenditure measurement techniques in severely burned patients. J Burn Care Rehabil. 1987;8:366.
201 Shoemaker W.C., et al. Fluid therapy in emergency resuscitation: clinical evaluation of colloid and crystalloid. Crit Care Med. 1981;9:367.
202 Silvadene Cream, Product monograph. Kansas City: Marion Laboratories, Inc; 1983.
203 Smith S.L. Physiology of the immune system. Crit Care Q. 1986;9:7.
204 Soloman J.R. Care and needs in a children’s burn unit. In: Rickham P.P., Hecker W.C., Preirot J., editors. Progress in pediatric surgery. Baltimore: Urban and Schwarzenberg, 1981.
205 Souba W.W., Bessey P.Q. Nutritional support of the trauma patient. Infect Surg. 1984;3:727.
206 Souba W.W., Schindler B.A., Carvajal H.F. Nutrition and metabolism. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
207 Souba W.W., Wilmore D.W. Gut-liver interaction during accelerated gluconeogenesis. Arch Surg. 1985;120:66.
208 Spevor M.J., Pruitt B.A. Candidiasis in the burned patient. J Trauma. 1981;21:237.
209 Stein M.D., Ninnemann J.L. Interferon production in patients with thermal injuries. Immunol Lett. 1981;2:207.
210 Stoddard F.J. Body image development in the burned child. J Am Acad Child Psychol. 1982;21:502.
211 Stratta R.J., et al. Plasma exchange therapy during burn shock. Curr Surg. 1983;40:429.
212 Thomson P.D., et al. Leukopenia in acute thermal injuries: evidence against topical silver sulfadiazine as the caustic agent. J Burn Care Rehabil. 1989;10:418.
213 Thorton J.W. Resuscitation. In: Archauer B.M., editor. Management of the burn patient. Los Altos, CA: Appleton and Lange, 1987.
214 Tse A.M., Perez-Woods C., Opie N.D. Children’s admissions to the intensive care unit: parent’s attitudes and expectations of outcomes. Child Health Care. 1987;16:68.
215 Uchiyama N., German J. Pediatric considerations. In: Archauer B.M., editor. Management of the burned patient. Los Altos, CA: Appleton and Lange, 1987.
216 VanHoesen K.B., et al. Should hyperbaric oxygen be used to treat the pregnant patient for acute carbon monoxide poisoning? A case report and literature review. J Am Med Assoc. 1989;261:1039.
217 Vigness R.M., Frey C.S., Long J.M. Acute leukopenia in burn patients treated with silver sulfadiazine and cimetidine. Proc Am Burn Assoc. 1981:13.
218 Wachtel T.L. Nutritional support of the burn patient. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
219 Wachtel T.L. Topical antimicrobials. In: Carvajal H.F., Parks D.H., editors. Burns in children: pediatric burn management. Chicago: Year Book Medical Publishers, 1988.
220 Warden G.D. Immunologic response to burn injury. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
221 Warden G.D. Immunology. In: Achauer B.M., editor. Management of the burned patient. Los Altos, CA: Appleton and Lange, 1987.
222 Warden G.D. Fluid resuscitation and early management. In: Herndon D.N., editor. Total burn care. Philadelphia: WB Saunders; 1996:53.
223 Warden G.D., Ninnemann J.L. The immune consequences of thermal injury: an overview. In: Ninnemann J.L., editor. The immune consequences of thermal injury. Baltimore: Williams and Wilkins, 1981.
224 White S., Kampler G. Dietary noncompliance in pediatric patients in the burn unit. J Burn Care Rehabil. 1990;11:167.
225 Winter S.L., Boyer J.L. Hepatic toxicity from large doses of vitamin B3 (nicotinamide). N Engl J Med. 1973;289:1180.
226 Wolfe R.R., et al. Response of protein and urea-kinetics in burn patients to different levels of protein intake. Ann Surg. 1983;197:163.
227 Wooldridge M., Surveyer J.A. Skin grafting for full-thickness burn injury. AJN. 1980;80:2000.
228 Yannas I.V., Burke J.F. Design of artificial skin; basic design principles. J Biomed Mater Res. 1980;14:65.
229 Yarbrough D.R. Pathophysiology of the burn wound. In: Wagner M.M., editor. Care of the burn-injured patient. Boston: PSG Publishing, 1981.
230 Young M.E. Malnutrition and wound healing. Heart Lung. 1988;17:60.
231 Zamierowski D.A., et al. Leukopenia in acute thermal injury: Is silver sulfadiazine responsible? Proc Am Burn Assoc. 1977;9:118.
232 Zawacki B.E. The local effects of burn injury. In: Boswick J.A., editor. The art and science of burn care. Rockville, MD: Aspen Publishers, 1987.
233 Zawacki B.E. Topical antimicrobial therapy using 0.5% aqueous silver nitrate solution. In: Warden G.D., editor. Wound healing symposium. American Burn Association, 1987. 19
234 Zeller W.P., et al. Accidental carbon monoxide poisoning. Clin Pediatr. 1984;23:694.