35 Care of the cardiac surgical patient
Allograft (Homograft): Tissue from another human, commonly procured from cadaver aortic valves with a portion of the attached aorta. The aortic allograft is obtained in a sterile manner; tested to rule out communicable disease, malignant disease, diabetes, and other pathologic conditions; treated with antibiotics; measured; placed in a sterile bag; and cryopreserved in a special freezer. When needed for surgery to replace the aortic valve or a portion of the ascending aorta and valve, the correct size of allograft is thawed and implanted.
Annuloplasty: The surgical repair of a dilated valve annulus, which causes valvular regurgitation. A cloth-covered prosthetic annuloplasty ring is sewn to the valve annulus (usually mitral or tricuspid) to reduce the overall circumference of the annular tissue, thereby reducing the amount of regurgitant blood flow.
Aortic Aneurysm: A localized or diffuse dilation of the arterial wall. Weakening and degeneration of the medial layer of the aortic wall leads to progressive enlargement of all layers of the aorta. Thoracic aortic aneurysms can occur in the ascending aorta, the aortic arch, or the descending aorta and extend into the abdominal aorta and branch vessels.
Aortic Dissection: A unique condition that affects the aortic media in which repeated stress (often from hypertension) and a congenital predisposition produce a tear in the intimal layer. Blood enters the tear, creating a dissecting hematoma within the tunica media and forming a false lumen. As the false lumen enlarges, with more blood entering the tear, the true lumen compresses and eventually compresses branches of the aorta. The intimal tear usually forms in the ascending aorta, although it can originate in any portion of the thoracic aorta. Surgery (excision of the aorta that contains the tear and replacement with a graft) is indicated for ascending aortic and transverse aortic arch dissections.
Aortic Insufficiency (AI): Also called aortic regurgitation; a condition that occurs when the aortic valve leaflets do not close properly (or have tears) and the valve becomes incompetent, thereby allowing regurgitation of blood from the aorta back into the left ventricle during diastole. AI can be the result of a primary valve disorder or aortic root disease. Valve disorders may be the result of congenital or rheumatic heart disease, infective endocarditis, torn leaflets, or trauma; aortic root disease may include aneurysmal dilatation, aortic dissection, Marfan syndrome, or some other condition that predisposes the root to dilatation (e.g., ectasia).
Aortic Stenosis (AS): The development of stiff and fibrotic valve leaflets or a narrowing of the orifice of the aortic valve itself. Valvular AS is often characterized by a fusion of the commissures of the valve leaflets that leaves only a small opening. A calculated aortic valve orifice of less than 1 cm2 is considered critical AS. Cardinal symptoms are syncope, angina pectoris, and dyspnea; these symptoms are related to insufficient cerebral blood flow, myocardial blood flow, and impaired left ventricle function, respectively. AS can occur as a congenital process, as in the bicuspid valve (a common congenital anomaly first diagnosed in adulthood), as an acquired disease process related to a history of rheumatic heart disease, or as calcific AS.
Aortocoronary Bypass Grafts: See Myocardial Revascularization.
Atrial Septal Defect (ASD): An opening in the atrial septum, ASDs are among the frequently seen congenital anomalies in adulthood. The most common form in the adult is the ostium secundum defect. Depending on the size of the defect (commonly the size of a quarter coin), there is a shunting of blood from the left atrium to the right atrium and subsequent increased pulmonary flow.
Balloon Valvotomy or Valvuloplasty: A catheter-based interventional (i.e., nonsurgical) procedure to enlarge a (stenotic) valve opening. For aortic valvuloplasty, the balloon tip is positioned across the aortic valve, and the balloon is inflated to dilate the aortic valve orifice. Balloon valvotomy for the mitral valve is performed with a catheter percutaneously inserted into a large vein and threaded to the right atrium, where a septal puncture is made to allow access to the mitral valve.
Bicuspid Aortic Valve: See Aortic Stenosis.
Cardioplegia: Literally, “paralysis of the heart”; pharmacologically induced cardiac arrest during surgery on the heart. Potassium is the most commonly used arresting agent, but the solutions may also contain electrolytes, buffers to maintain appropriate pH, glucose, metabolic substrates, calcium antagonists, tromethamine, heparin, and antiarrhythmic agents; the carrying solution may be crystalloid or blood (preferable for its oxygen carrying capacity). The purposes of cardioplegia are protection of the myocardium against irreversible ischemic injury during the aortic cross-clamp period when the heart is arrested and conservation of energy resources that can be used after removal of the cross clamp.
Cardiopulmonary Bypass (CPB): A temporary substitution for the heart and lungs with a mechanical device that drains systemic venous return (thereby decompressing the heart) into an oxygenator that removes excess carbon dioxide and adds oxygen (simulating the lungs) and pumps arterialized blood into the systemic circulation (simulating the heart). The development of CPB enabled surgeons to isolate the heart by cross clamping the aorta and to pharmacologically induce cardiac arrest to create a dry, quiet operative field in which to perform the surgical repairs. During the period of cardiac arrest, the rest of the body can be adequately perfused via CPB.
Coarctation of the Aorta: A congenital narrowing of the thoracic aorta, usually in the area of the ligamentum arteriosum (formerly the fetal patent ductus arteriosus, which shunts blood from the pulmonary artery to the aorta).
Commissurotomy: The opening or separation of fused valve leaflet commissures (in the adult, generally the mitral or pulmonary valve).
Congenital Heart Disease: Cardiac anomalies apparent at birth or later in life. In this section, some of the congenital anomalies that may be seen in adulthood are discussed.
Dysrhythmia Surgery for Atrial Fibrillation (AF): Surgery designed to block or redirect aberrant atrial fibrillatory impulses toward the atrioventricular node to achieve normal sinus rhythm.
Hybrid Suite: Procedure or operating room that integrates interventional and traditional surgical equipment and supplies, and imaging and monitoring capability.
Hypothermia: Cooling of the patient during cardiac surgery. Hypothermia can be induced (elective) or inadvertent. Induced systemic hypothermia is achieved with a heat exchanger incorporated into the cardiopulmonary bypass circuit; induced cardiac hypothermia is achieved with infusion of cooled cardioplegia solution into the coronary circulation. Topical cardiac cooling is achieved by pouring cold solutions onto the heart.
Implantable Cardioverter-Defibrillator (ICD): A device that provides a shock to the heart for tachycardia above a predetermined rate or ventricular fibrillation and also has the capability to provide bradycardia therapy. The device is usually inserted transvenously in the electrophysiology or cardiac catheterization laboratory. Patients with ICDs who undergo surgery have a magnet placed over the generator (usually in the right or left upper chest) to turn off the device and avoid frequent shocks during induced cardiac arrest. After surgery, the magnet is removed and the ICD is again activated.
Minimally Invasive Cardiac Surgery: Operations that use smaller incisions, percutaneous and or endoscopic access, endovascular techniques, or off-pump (i.e., no cardiopulmonary bypass) procedures. A common procedure in minimally invasive surgery is the endoscopic video-assisted excision of saphenous vein coronary bypass grafts. In other cases, smaller sternal or thoracic chest incisions or ports may be used for target areas of the heart that can be accessed through these minimal incisions (e.g., certain coronary bypass operations for single vessel disease and some valve procedures).
Mitral Regurgitation (MR): A condition that occurs when one or more components of the mitral valve apparatus prevents competent closure of the valve, with subsequent regurgitation of blood into the left atrium.
Mitral Stenosis (MS): A narrowing of the mitral valve orifice that creates an impedance to flow across the valve.
Myocardial Protection Techniques: Techniques or procedures designed to conserve myocardial energy resources and limit the amount of ischemic tissue injury that can occur. The use of cardioplegia and induced hypothermia (see previous definition) to arrest and preserve the heart form the foundation of myocardial protection. Additional myocardial protective measures can include minimization of the risk of an air embolus from ambient room air trapped inside the heart with insufflation of CO2 gas through a catheter into the pericardial well. The CO2 gas not only displaces ambient air but also dissolves approximately 25 times faster in the blood than room air and is less harmful to cardiac tissue.
Myocardial Revascularization: Also called coronary artery bypass grafting. Surgical procedure performed for coronary artery disease in which conduits of the patient’s own tissue (autograft), most commonly the internal mammary artery or other arteries (e.g., radial artery, gastroepiploic artery) and the greater saphenous vein, are attached directly to the affected coronary artery at a site distal to the narrowed atherosclerotic lesion. Generally, coronary narrowing of 70% or greater in arteries that are 1 mm or larger is an indication for bypass grafting; narrowing of less than 70% can create competitive flow between the conduit and the native artery and reduce the amount of blood that perfuses the bypass graft (and the anastomosed coronary artery).
Patent Ductus Arteriosus (PDA): A duct between the pulmonary artery and the aorta, present in fetal life. The duct usually closes within 1 or 2 weeks after birth and becomes the ligamentum arteriosum.
Percutaneous Transluminal Coronary Angioplasty (PTCA) with Stent Insertion: A catheter-based interventional technique performed with fluoroscopy in the cardiac catheterization (see previous definition) laboratory for acute or chronically obstructed coronary arteries. A percutaneously inserted balloon catheter is threaded to the right or left coronary os, where the catheter is positioned across the coronary artery at the site of the narrowing. The balloon is inflated to compress the atherosclerotic lesion and enlarge the coronary lumen. A stent (bare metal or coated) is commonly inserted in conjunction with the PTCA to maintain coronary artery patency.
Pericardiectomy, Pericardial Window: Pericardiectomy is the partial excision of an adhered, thickened fibrotic pericardium to relieve constriction of the heart and great blood vessels. In patients with chronic cardiac effusions, the creation of a pericardial window (excision of a portion of the pericardial or pleural wall) between the pericardial sac and the pleural space drains the fluid. In patients with chronic effusions, the window to the pleural space generally allows future fluid accumulation to be reabsorbed.
Pulmonary Stenosis: Fusion of the pulmonary valve cusps at the commissures, which creates an obstruction to the right-ventricular outflow tract. Another form of pulmonary stenosis occurs in the infundibular portion where fibromuscular obstruction occurs proximal to the valve, creating right ventricular outflow tract obstruction.
Tetralogy of Fallot (TOF): A congenital entity with four distinctive features: (1) a high ventricular septal defect (VSD); (2) pulmonary stenosis that affects the valve or the infundibular region; (3) overriding of the ventricular septal defect by the aorta; and (4) hypertrophy of the right ventricle. In the adult and commonly in the child, correction includes closure of the VSD with a patch and enlargement of the pulmonary valve (or stenosed infundibular region) with dilators, via commissurotomy, valve replacement, infundibular resection, or insertion of an allograft, depending on the underlying pathology.
Transplantation: Replacement of the patient’s heart with a human donor heart; replacement of one or both lungs with lungs from a donor, or one lung from a living donor). Heart donors are persons with irreversible catastrophic brain injury who do not have atherosclerotic heart disease; age limitations have become less restrictive, and donors and recipients may be 60 years or older in certain cases. Severe pulmonary disease is a contraindication for lung donation.
Tricuspid Regurgitation: A condition that occurs when the tricuspid valve does not totally close because the leaflets do not completely approximate in diastole.
Tricuspid Stenosis: A narrowing of the orifice of the tricuspid valve.
Valve Replacement: A surgical procedure in which the native valve is replaced with a mechanical or biologic prosthesis; allograft valve replacement also may be performed.
Valvuloplasty: The repair of one or more components of the right (tricuspid) or left (mitral) atrioventricular valve complex that consists of the valve annulus (see Annuloplasty), the valve leaflets, the chordae tendineae, the papillary muscles, and the endoventricular wall.
Ventricular Assist Device (VAD): Mechanical devices to support the left ventricle (LVAD), the right ventricle (RVAD), or both ventricles (BiVAD) can be used long term as a bridge to transplant or destination therapy, depending on the underlying pathology. VADs decrease the workload of the heart by diverting blood from the ventricle to an artificial pump that maintains systemic (LVAD) or pulmonary (RVAD) perfusion. Currently, patients can be ambulatory with implantable VADs powered by batteries, which has substantially improved the quality of life for many VAD patients.
Ventricular Septal Defect (VSD): Consists of a hole through the ventricular septum. VSDs may be congenital or acquired (i.e., postinfarction VSD).
The first suture repair of a right ventricular stab wound in 1896 demonstrated that the heart could be manipulated and sutured without causing ventricular fibrillation and without entering the pleural cavity, thereby creating a pneumothorax. The introduction of positive-pressure endotracheal anesthesia obviated the dangers of a pneumothorax because clinicians could access the heart and enter the pleural cavities without causing the lungs to collapse. Gibbon’s introduction in 1953 of extracorporeal circulation (i.e., cardiopulmonary bypass) enabled surgeons to isolate the heart and lungs from the circulation without producing irreversible tissue anoxia.1 The development of electric defibrillation and induced chemical cardiac arrest made stopping and restarting the heart possible with consistent predictability; myocardial protection techniques protected and conserved myocardial energy resources during the period of induced cardiac arrest. Newer diagnostic imaging and monitoring devices have allowed clinicians to visualize the heart, identify specific pathoanatomic changes, record the heart’s electric activity, measure blood pressure and flow, and assess ventricular function. These imaging and monitoring improvements, along with the refinement of mechanical and bioprosthetic materials, improved anesthetic agents, percutaneous and newer minimally invasive surgical techniques, and additional technologic advances have made development of more effective procedures possible to revascularize the myocardium, repair and replace valves, treat aneurysms, alter conduction disturbances, and assist and replace a failing heart.1 The reader will find related information in Chapters 11, 34, and 36.
Preoperative considerations
Before surgery, documentation of the history and physical examination, surgical consents, the results of diagnostic and laboratory tests, and additional pertinent information are reviewed by the nurse. Among the significant factors are estimates of left ventricle (LV) function (e.g., ejection fraction), a history of medications that may affect perioperative bleeding (e.g., aspirin, anticoagulants), oxygen-carrying capacity (e.g., hematocrit, hemoglobin), renal function (creatinine), pulmonary function (spirometry), cardiac anatomy (e.g., of the valves, coronary arteries), and the presence of concomitant carotid artery disease.2 Many patients are admitted the morning of surgery, which shortens the period of time for teaching. Often preoperative laboratory testing and requests for packed red blood cells are performed a few days before the scheduled surgery; patient teaching can be accomplished at this time (Table 35-1).
Table 35-1 Patient Teaching for Coronary Artery Bypass Graft Surgery and Valve Surgery
| TOPIC | CABG SURGERY | VALVE SURGERY |
|---|---|---|
| Perioperative Pointers | ||
| Medical diagnosis | Coronary artery occlusive disease | Valve regurgitation, stenosis, or mixed lesion |
| Diagnostic tests | ECG, chest radiograph, nuclear imaging, cardiac catheterization, carotid duplex studies | Same, plus echocardiogram |
| Routine preoperative tests | CMP, ECG, T&C, pulmonary function, PT, PTT, INR, urinanalysis | Same |
| Incision site | Midsternal or anterior thoracotomy; multiple leg incisions for vein harvest, arm incision for radial artery harvest | Mini-sternotomy, full sternotomy, or mini-thoracotomy |
| Resumption of eating | After removal of ET and NG tubes (postoperative day 1) clear liquids, then advance to high calorie/high protein diet | Same |
| Pain control | IM, PO, PCA | Same |
| Estimated length of procedure | 3-6 hours | Same |
| Estimated length of hospital stay | 4-6 days | 5-7 days |
| Long-term effects of surgery | Possible loss of saphenous vein or internal mammary or radial arteries; possible intermittent lower leg ischemia | Possible chronic anticoagulation therapy; differences between biologic and mechanical prostheses, valve repair |
| Drains or tubes | 2 days: mediastinal chest tube, pleural tube; 2-3 days: leg drains, urinary drainage catheter; remove tubes when drainage >100 mL for 8 hours | 2 days: mediastinal and pleural tubes, urinary catheter |
| Postoperative Pointers and Home Instructions | ||
| Food | Cardiac diet | Same |
| Wound care | Wounds covered if draining; redress after shower or bath; contact clinician if signs of infection | Same |
| Bathing | Daily | Same |
| Driving | 4-6 weeks (automatic shift only) | Same |
| Sex | Restricted by limits of ability to bear weight on upper arms and chest | Same |
| Return to work | 8-12 weeks | Same |
| Medications | Aspirin anticoagulant, cardiac drugs | Warfarin (Coumadin), cardiac drugs |
| Follow-up | 7-14 days | Same, plus laboratory tests for determination of bleeding times |
| Special restrictions | Upper body movement restricted for 6 weeks for sternal healing | Same |
| Lifestyle changes | Reduction of coronary risk factors; cardiac rehabilitation | Risk factor reduction; cardiac rehabilitation |
| Worrisome but normal | Fatigue, swelling in leg; leg discomfort 4-6 weeks; weakness, emotional let down | Fatigue; sound of mechanical valve; weakness; emotional let down |
ECG, Electrocardiogram; CMP, comprehensive metabolic panel (includes glucose, blood urea nitrogen, sodium, potassium, chloride, creatinine, albumin, bilirubin, calcium, alkaline phosphatase, total protein); T&C, type and cross match (blood); PT, prothrombin time; PTT, partial thromboplastin time; INR, International Normalized Ratio; ET, endotracheal; NG, nasogastric; IM, intramuscular; PO, by mouth; PCA, patient-controlled analgesia.
Adapted from Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Anesthesia
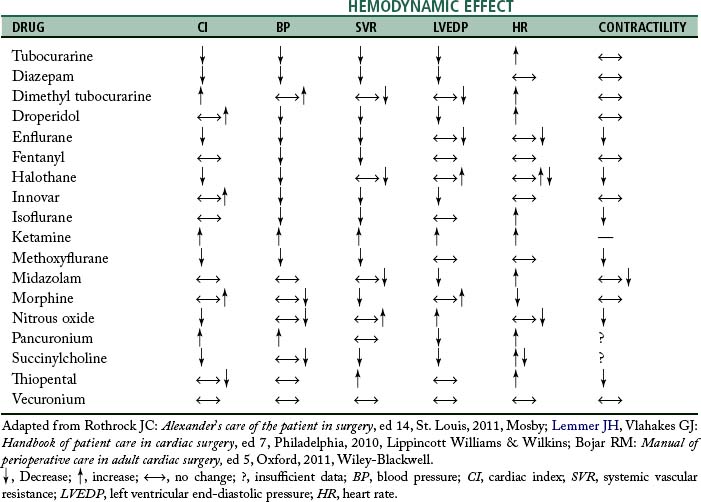
Anesthesia for cardiac surgery varies by hospital and can also vary by the type of cardiac repair. Ideally, anesthetic management includes drugs that have rapid onset and termination, minimize ischemia, and are nontoxic to myocardial and other tissue.3 For example, in patients with significant pulmonary involvement, agents that increase pulmonary vascular resistance should be avoided.2 Hemodynamic effects of some anesthetic drugs are shown in Table 35-2.
Procedures
Monitoring
Monitoring (Table 35-3) of the patient’s electrocardiogram (ECG) and direct blood pressure (commonly via the radial artery in the nondominant wrist) results is initiated when the patient arrives in the preoperative area. Central lines are usually inserted after transport into the operating room (OR), but occasionally are inserted in another designated location, such as the preoperative area. Whether the patient first undergoes intubation before insertion of central lines (i.e., central venous pressure, pulmonary artery catheter) or after line insertion is at the discretion of the anesthesia provider; the sequence varies among institutions. Increasingly, an anesthesia provider inserts a transesophageal echocardiography (TEE) probe to illustrate cardiac function. Use of TEE for valve surgery and for patients with compromised LV function is routine.2,3
| MONITORING DEVICE | LOCATION | ASSESSES/MEASURES |
|---|---|---|
| Cardiovascular System | ||
| Electrocardiogram (ECG) | Electrodes placed on shoulders, hips, and left axillary line | Electrical activity of heart: lead II useful to monitor cardiac rhythm (good visualization of P wave and QRS) and myocardial ischemia (inferior surface); lead V5 useful to detect myocardial ischemia (anterior surface) |
| Intraarterial catheter | Radial artery (also femoral artery) aorta, bypass circuit; in children, may use superficial temporal or dorsalis pedis arteries; in neonates, may use umbilical artery | Direct arterial BP; blood gases; blood chemistries |
| Blood pressure cuff | Right or left arm | Indirect BP |
| CVP line | RA | RA pressure (CVP); RV filling pressure; RV preload |
| PA catheter (addition of fiberoptics provides additional information about mixed venous oxygen saturation [SvO2]) | PA (proximal and distal) | PA pressures: systolic, diastolic, mean, wedge; pulmonary vascular resistance; LV filling pressure; LV preload; CO; assessment of stroke volume, stroke work, systemic vascular resistance; mixed venous saturation (continuous indirect assessment of CO and reflection of tissue oxygenation); RV function |
| LA catheter (when used) | LA | LA pressure (direct); LV filling pressure; LV preload |
| TEE | Esophagus | Valve function before and after repair; LV wall motion, failure; intracardiac air bubbles |
| Urinary drainage catheter | Urinary bladder | Urinary output, renal perfusion; indirect measure of CO |
| Respiratory System | ||
| Mass spectrometry | Anesthesia circuit | Inspired or expired O2, CO2, and anesthetic gases; used to avoid hypoxia, hypercarbia, anesthetic overdose |
| Pulse oximeter | Finger or toe cot; earlobe, nose | Oxygen saturation of arterial hemoglobin; tissue oxygenation |
| Capnography | Anesthesia circuit | End-tidal CO2; used to detect integrity of anesthesia circuit; avoid disconnections of monitor, endotracheal tube; detect spontaneous ventilation, rebreathing, obstructive pulmonary disease |
| Central Nervous System | ||
| Temperature | Esophagus, nasopharynx, urinary bladder, rectum, ventricular septum, bypass circuit, PA catheter | Core, and peripheral temperature of heart, brain, and other organs |
| Electroencephalogram | Scalp electrodes | Detect cerebral ischemia, embolus; indication of depth of anesthesia |
| Renal System | ||
| Urinary discharge catheter | Bladder | Urinary output; indirect measure of cardiac output |
BP, Blood pressure; CVP, central venous pressure; RA, right atrial; RV, right ventricular; PA, pulmonary artery; LA, left atrial; CO, cardiac output; TEE, transesophageal echocardiography.
Adapted from Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Incisions
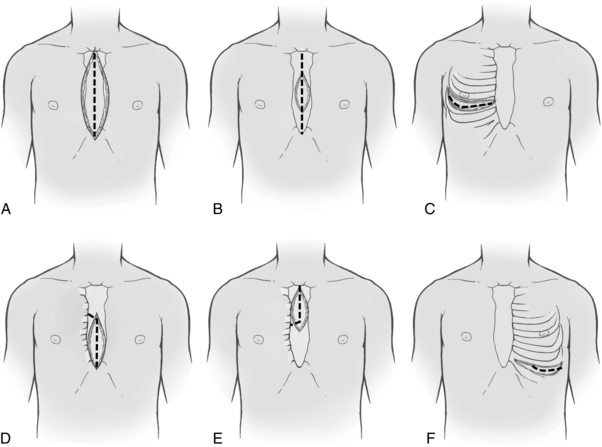
After the patient has been anesthetized, positioned, and washed (prepared), the chest is opened. The most commonly used chest incision in cardiac surgery is the median sternotomy; this is the preferred incision when a thorough assessment of, and complete access to, the heart (as with global coronary artery disease) is needed. However, ministernotomy, anterolateral or posterolateral thoracotomy incisions, or transverse sternotomy also may be used (Fig. 35-1). The surgeon splits the sternum with a saw from the sternal notch to the xiphoid process. After the sternum is opened, the exposed pericardial sac is incised anteriorly, and the pericardial edges are tacked up along the chest wall incision to allow complete access to the entire pericardium without the necessity of entering the pleural cavities. Minimally invasive surgery uses smaller incisions and can speed patient recovery, enable the patient to return faster to normal activities, and reduce costs.
Cardiopulmonary bypass
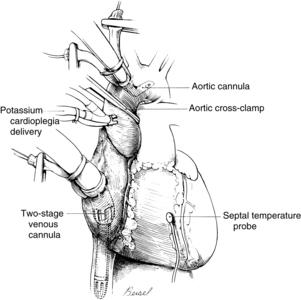
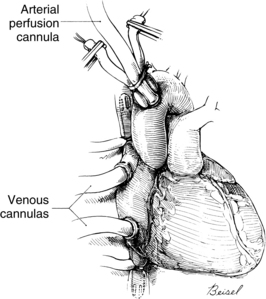
Systemic venous return to the heart flows by gravity drainage (the level of the patient needs to be above that of the bypass machine to facilitate drainage) into one or two large-bore (e.g., 32F to 36F) cannulas inserted into the right atrium. With the single two-stage cannula (Fig. 35-2), openings in the distal tip drain blood returning from the inferior vena cava (IVC) and openings in the middle portion of the cannula drain venous blood from the superior vena cava (SVC) and the coronary circulation exiting from the coronary sinus. Some blood enters the right atrium (RA) and the pulmonary circulation. With double cannulation (Fig. 35-3), individual cannulas are inserted into the IVC and the SVC, thereby forcing all venous return into the cannulas. Generally the two-stage cannula is used for coronary artery bypass grafting and aortic valve surgery when total right side decompression is not generally needed and blood entering the right side of the heart does not obscure the surgical field. Individual (double) venous cannulation can be used for procedures in the right side (e.g., tricuspid valve repair) to keep the right heart free of blood. Occasionally, two cannulas can be used when greater decompression of the RA is necessary (e.g., mitral valve surgery).
After the blood is oxygenated, it is pumped into the systemic circulation via an arterial cannula, commonly located in the aorta (see Fig. 35-3). When aortic pathology (e.g., aortic aneurysm) prevents cannulation, the femoral artery can be cannulated (retrograde flow) for arterial return. The axillary artery may be needed when the aorta or both of the femoral arteries are unavailable.
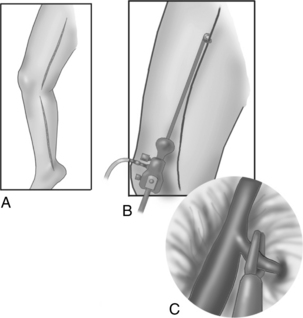
In addition to the standard cannulation techniques for cardiopulmonary bypass (CPB), minimally invasive systems use endovascular catheters (Fig. 35-4). A venous catheter is inserted into the femoral vein, and the arterial cannula is placed into the femoral artery. The ipsilateral femoral artery is also used for insertion of a multilumen catheter. One lumen serves as an endovascular cross clamp that occludes the aorta with inflation of an intraaortic balloon at the tip of the catheter, and the other lumen can be used for infusion of antegrade cardioplegia. Pressure lines, venting catheters, and a coronary sinus retrograde cardioplegia catheter can be inserted via the jugular vein. This system does not require median sternotomy and is an important adjunct for minimally invasive surgery.4
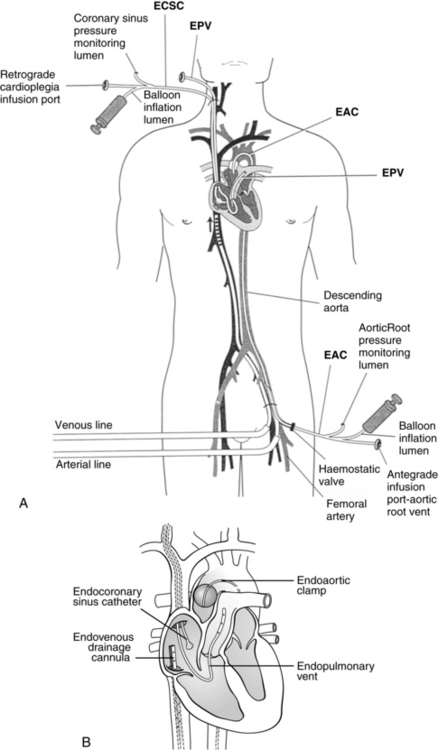
FIG. 35-4 Minimally invasive cardiopulmonary bypass technique. A, Positioning of endovascular catheters). B, Correct positions of endocoronary sinus catheter, endoaortic clamp, endovenous drainage cannula, and endopulmonary vent for port-access cardiopulmonary bypass. EAC, Endoaortic clamp; ECSC, endocoronary sinus catheter; EPV, cardiopulmonary vent.
(A, From Toomasian M, et al: Extracorporeal circulation for port-access cardiac surgery, Perfusion 12:83, 1997. B, From Kaplan J, editor: Kaplan’s cardiac anesthesia, ed 5, Philadelphia, 2006, Saunders.)
A number of risks are associated with the use of CPB (e.g., particulate or air embolus), and some form of checklist is often used before CPB is instituted (Box 35-1) and before the patient is weaned from CPB (Box 35-2). Such checklists help to enhance the safety of CPB. In addition, the clinical sequelae of CPB can affect all body systems. Caregivers in the postoperative period should be aware of the possible effects of CPB (Table 35-4).
BOX 35-1 Checklist for Initiation of Pump
• Activated coagulation time checked and at least 350 to 400 seconds
• Muscle relaxant adequate (or infusion turned on for maintenance)
• Inotropic infusions turned off
• Pupil symmetry assessed for later comparison (unilateral dilation may indicate unilateral carotid perfusion on cardiopulmonary bypass)
• Pulmonary artery catheter (if used) pulled back 5 cm
• Urinary output emptied before bypass (start anew with initiation of bypass)
From Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
BOX 35-2 Checklist for Weaning from the Pump
• Patient is warm (to at least 36° C)
• All monitors are functioning; electrocardiogram, pulmonary artery tracing, arterial line tracing, central venous pressure reflect heart filling
• Heart rate is 70 to 100 beats/min (<60 beats/min is reduced cardiac output; ≥120 beats/min is detrimental to left ventricular filling)
• Infusions are prepared as necessary
• Lungs are inflated, but kept out of the surgeon’s field
• Pressure becomes pulsatile as ventricles fill
• Blood is drawn for measurement of electrolytes, hematocrit, arterial blood gases, and activated clotting time
• The surgeon clamps venous drainage cannula and removes the cannula
From Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
| EFFECTS | CONTRIBUTING FACTORS |
|---|---|
| Cardiovascular System | |
| Perioperative myocardial infarction | Inadequate myocardial protection and emboli |
| Low cardiac output syndrome after surgery | Preexisting heart disease, inadequate myocardial protection, alteration in colloidal osmotic pressure, left ventricular dysfunction, hypoperfusion injury, hypothermia, long pump run |
| Increased afterload | Catecholamine release |
| Hypertension | Elevated rennin, angiotensin, and aldosterone levels |
| Hypotension | Postoperative diuresis, sudden vasodilation (rewarming), third spacing |
| Pulmonary System | |
| Respiratory insufficiency | Alterations in colloidal osmotic pressure, interstitial pulmonary edema, decreased perfusion, alterations in ventilatory patterns, decreased surfactant production, pulmonary microemboli |
| Atelectasis | Complement activation and inflammatory response, emboli, alveolar-capillary membrane damage |
| Neurologic System | |
| Cerebrovascular accident | Cerebral emboli |
| Transient motor defects | Decreased cerebral blood flow |
| Cerebral hemorrhage | Systematic heparin administration |
| Neuropsychological deficits | Microemboli, ischemia, altered perfusion flow of bypass |
| Gastrointestinal System | |
| Gastrointestinal bleeding | Hormonal stress and coagulation diatheses |
| Intestinal ischemia or infarction | Emboli and decreased perfusion |
| Acute pancreatitis | Pancreatic vasculature emboli |
| Renal System | |
| Acute renal failure | Decreased renal blood flow, microemboli, and myohemoglobin release |
| Hemoglobinuria | Red blood cell hemolysis |
| Fluid and Electrolyte Balance | |
| Interstitial edema, weight gain | Increased extravascular fluid and organ dysfunction, fluid shifts, decreased plasma protein concentration, increased capillary permeability |
| Intravascular hypovolemia | Decreased intravascular volume, bleeding, and interstitial edema |
| Hypokalemia | Dilution, polyuria, intracellular shifts of potassium ions |
| Hyperkalemia | Potassium cardioplegia and increased intracellular exchange of glucose and potassium, cellular destruction |
| Hyponatremia, hypocalcemia, and hypomagnesemia | Dilution, fluid shifts, diureses |
| Endocrine System | |
| Water and sodium retention | Increase in antidiuretic hormone |
| Hypothyroidism | Increased levels of thyroxine (T4) and decreased levels of triiodothyronine (T3) and thyroid-stimulating hormone |
| Hyperglycemia | Depressed insulin response, stimulation of glycogenesis |
| Immune System | |
| Infection | Exposure to multiple pathogens, decreased immunoglobin levels, and hypothermia |
| Postperfusion syndrome | Release of anaphylactic toxins, complement activation |
| Hematologic Factors | |
| Bleeding | Blood cell hemolysis, heparin rebound, reduction in platelet count and coagulation factors, coagulopathy, systemic heparin administration, depressed liver function from hypothermia |
Adapted from Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Myocardial protection
The goal of myocardial protection is to conserve cardiac energy resources; the goal is achieved with induced hypothermia and rapid diastolic arrest. Hypothermia reduces the metabolic rate (and therefore the energy demands) of the tissue being cooled; effective intraoperative myocardial protection positively affects the patient’s postoperative recovery. For most cardiac procedures of less than 1 hour of induced cardiac arrest (e.g., coronary artery bypass grafting), the perfusionist cools the systemic circulation from a normal temperature of 37° C (98.6° F) to approximately 32° C (89.6° F). The cardioplegia solution is cooled to a lower temperature (near freezing) for intracardiac cooling as a method of myocardial protection. For procedures with a longer cross-clamp time, the systemic temperature may be further reduced to protect the brain, kidneys, and other organs while the heart is arrested by reducing energy demands and limiting ischemic injury. Topical cooling of the heart with cold lavage or frozen “slush” placed on the heart and in the pericardial well helps to achieve transmural cooling. Although induced hypothermia plays an important role in minimization of tissue ischemia during selected portions of cardiac procedures, inadvertant hypothermia has become an important consideration for cardiac patients because of associated risks for perioperative complications. Before surgery, shivering increases myocardial oxygen demands and further taxes hearts that have diminished myocardial energy supplies. During surgery, before and after CPB, inadvertent cooling of the patient is associated with increased surgical bleeding and a diminished immune response. After surgery, hypothermia contributes to patient discomfort, impaired wound healing, prolonged bleeding, longer lengths of stay, and cardiac events such as ischemia and tachyarrhythmias.2,3 Active measures, such as the application of warm blankets before surgery and during the intraoperative period (before and after induced hypothermia), the postoperative use of forced warm air devices and intravenous fluid warmers, and an increased ambient room temperature, can help to maintain normothermia.
Rapid diastolic arrest conserves existing cellular energy resources (e.g., adenosine triphosphate) by avoiding the energy-expensive state of ventricular fibrillation before the heart achieves an arrested state. Quick arrest of a beating heart (e.g., 10 to 20 seconds) enhances myocardial energy conservation. Potassium is the most commonly used arresting agent, but the solutions may also contain electrolytes, buffers to maintain appropriate pH, glucose, metabolic substrates, calcium antagonists, tromethamine, heparin, and antiarrhythmic agents. The carrying solution can be crystalloid or blood (preferable for its oxygen carrying capacity).4
Methods of infusing cardioplegia include the antegrade and retrograde routes and the direct coronary ostial route of infusion. With the antegrade method, a needle catheter is inserted into the anterior aorta proximal to the aortic cross clamp (see Fig. 35-2). The cardioplegia solution is infused with sufficient pressure to close the aortic valve. With the cross clamp applied distally and a competent aortic valve proximally, the only paths for the solution to travel are the right and left coronary ostia lying between the cross-clamped aorta and the closed aortic valve. In patients with aortic valve insufficiency, cardioplegia flow into the coronary ostia is significantly reduced because the incompetent aortic valve provides a lower pressure pathway into the inner ventricular chamber, thereby distending the heart and increasing myocardial wall tension. Retrograde delivery is indicated in the presence of coronary artery lesions that impair the transmural distribution of the cardioplegia when delivered solely by the antegrade route. For the retrograde route, the surgeon inserts a catheter through a stab wound into the right atrial wall and threads the catheter into the coronary sinus. The cardioplegic solution infused into the coronary sinus flows retrograde through the cardiac veins and arteries. The solution exits via the coronary ostia, where it is removed by suction.
Surgery for coronary artery disease
Coronary artery disease remains the leading cause of death among men and women.5 Left heart cardiac catheterization is commonly performed for identification of areas affected by obstructive atherosclerotic coronary artery disease. Selective coronary angiography with an injection of contrast medium into the right and left coronary ostia illustrates coronary anatomy, distal coronary perfusion, the location of atherosclerotic lesions, the status of coronary collateral circulation, and the percentage of narrowing of coronary arteries affected by coronary artery disease (CAD). Dye is injected into the LV (ventriculography) to determine wall motion (e.g., hypokinesia, dyskinesia, paradoxic motion), and valvular function (e.g., mitral regurgitation, prosthetic valve function). Generally, a right-heart catheterization yields data concerning the inferior and superior vena cava, the right atrium and ventricle, tricuspid and pulmonary valves, and the pulmonary artery.
Percutaneous coronary interventions, specifically percutaneous coronary interventions angioplasty (PTCA) with stent insertion, can be performed for discrete lesions. Equalized pressure measurements above and below the lesion indicate a successful angioplasty. The restenosis rate for angioplasty at 6 months has been approximately 30%, necessitating additional angioplasties or surgery. Complications from the interventional procedure include prolonged chest pain, myocardial infarction, coronary spasm, or coronary artery dissection that can necessitate emergency coronary artery bypass grafting (CABG). Evidence supports surgical revascularization (i.e., CABG) over percutaneous coronary interventions in patients with left main coronary artery disease.6,7
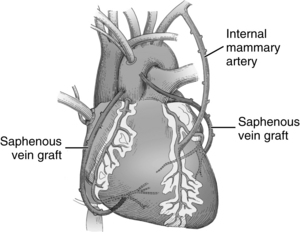
When CABG is recommended for patients, the number and type of bypass grafts (e.g., saphenous vein, internal mammary artery) are assessed. Fig. 35-5 illustrates possible arterial and venous autologous conduits. Grafts from cadaver human saphenous vein, human and bovine umbilical vein, and synthetic grafts have been used when other conduits were unavailable. CABG does not cure CAD; its purpose is to increase blood flow to the myocardium beyond the obstructive lesions. CAD is a progressive disease, and retardation of the atherosclerotic process requires life style changes and pharmacologic support (e.g., statins). Gender-based differences in CABG outcomes are under scrutiny, and guidelines have been published to provide evidence for the selection of techniques.5
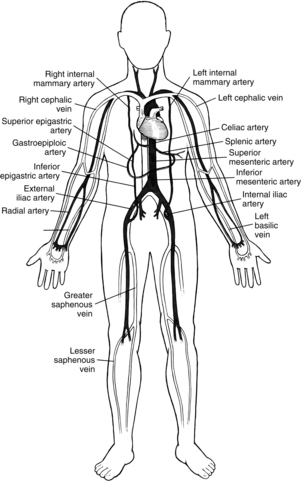
FIG. 35-5 Arterial and venous conduits for coronary bypass surgery.
(From Seifert PC: Cardiac surgery, St. Louis, 2002, Mosby.)
Implications for practice
1. Use of the internal mammary artery (IMA)
2. Management of hyperglycemia
3. Intraoperative management of anemia
4. Use of off-pump CABG (OPCAB)
5. Optimization of thyroxine treatment for women with hypothyroidism
6. Consideration of preoperative hormone replacement therapy (HRT)
Source: Edwards FH, et al: Gender-specific practice guidelines for coronary artery bypass surgery: perioperative management, Ann Thorac Surg 79:2189-2194, 2005; Puskas JD, et al: Off-pump techniques benefit men and women and narrow the disparity in mortality after coronary bypass grafting, Ann Thorac Surg 84:1447-1456, 2007; Toumpoulis IK, et al: Assessment of independent predictors for long-term mortality between women and men after coronary artery bypass grafting: are women different from men? J Thorac Cardiovasc Surg 131:343-351, 2006; Mosca L, et al: Effectiveness-based guidelines for the prevention of cardiovascular disease in women, Circulation 123:1243-1262, 2011; Coulter SA: Heart disease and hormones, Texas Heart Inst J 38(2):137-141, 2011.
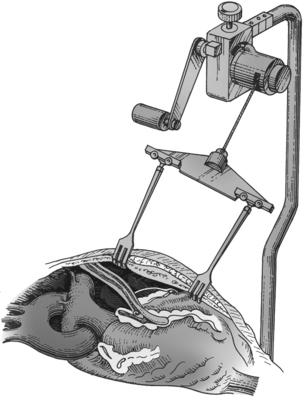
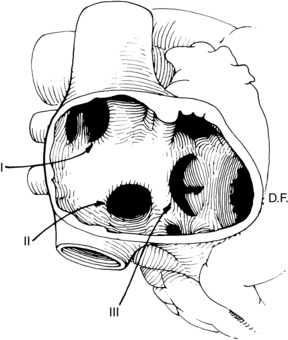
Once the sternum is opened, the surgeon dissects the required length of the internal mammary artery (Fig. 35-6). The internal mammary artery (IMA) is dissected from its retrosternal bed to the required length, leaving intact the proximal attachment to the left subclavian artery. The assistant harvests additional bypass conduits, such as the saphenous vein (Fig. 35-7) or the radial artery.8 Greater saphenous vein is commonly harvested with video-assisted endoscopic techniques.9 Because leg veins have valves that facilitate flow toward the right heart, reversal of the vein is necessary so that flow as a bypass graft is not impeded. After conduit harvesting is completed, or if another procedure is planned (e.g., valve repair), the surgeon proceeds to cannulate for CPB. The body is cooled to the desired temperature, and the heart is arrested with cardioplegia. The surgeon then proceeds to attach the grafts to the heart. The left IMA is commonly used in bypass of the left anterior descending coronary artery. The distal anastomosis is created with attaching the end of the graft conduit to the side of the coronary artery; proximally, the IMA graft remains attached to the subclavian artery. For vein grafts, the surgeon attaches the proximal end of the vein to an opening created in the side of the proximal aorta. Veins grafts may be attached to the diagonal, obtuse marginal, posterior descending, or right coronary arteries, as indicated (Fig. 35-8).
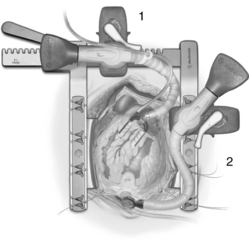
In operations that necessitate entry into the heart (valve repair or replacement), CPB is required for perfusion of the body while the heart is arrested and opened. During CABG, the procedure is performed on the epicardial coronary arteries that lie on the surface of the heart, not inside the heart. Coronary bypass surgery can be performed without the use of CPB (off-pump CABG), thereby avoiding the deleterious effects associated with extracorporeal circulation (see Table 35-4). Off-pump surgery may be feasible when pulmonary function is poor but LV function is not severely compromised, and access to the target coronary arteries does not require excessive manipulation of the heart.2,3 During off-pump procedures, the heart beats to perfuse the systemic, pulmonary, and coronary circulations. This beating heart surgery has been facilitated with the use of stabilizing devices that isolate and reduce the motion of the portion of the artery to be anastomosed (Fig. 35-9, 1). An attachment applies suction to the LV apex and allows the heart to be retracted for access to the lateral (i.e., obtuse marginal branches) and posterior (i.e., posterior descending or distal right coronary artery) portions of the heart (see Fig. 35-9, 2). Patient teaching considerations related to on-pump or off-pump surgery are listed in Table 35-5.
Table 35-5 Patient Teaching Considerations for Minimally Invasive Surgery (On/Off Pump)
| BEATING HEART/OFF-PUMP | ARRESTED HEART | |
|---|---|---|
| Definition | CABG without CPB or induced cardiac arrest; HR and contractile force may be pharmacologically reduced; stabilizer used at anastomotic site; apical retractor used to expose lateral and posterior coronary arteries | CABG with CPB and endovascular technique for CPB and induced cardiac arrest |
| Indications | Multiple-vessel disease, angioplasty contraindicated, medical problems, poor anatomy, accessible target arteries, previous CABG with blocked grafts | Multiple-vessel disease; angioplasty contraindicated, need to stop heart to enhance technical precision, accessible target arteries, mitral valve disease |
| Contraindications | Intramyocardial lesions, hemodynamic instability | High complex lesions, posterior targets |
| Incisions | Sternotomy or ministernotomy (cephalad or caudad); 1-3 small right or left, rib or submammary incisions | 1-4 small rib incisions, 1 or 2 groin incisions |
| CPB | No, available on standby | Yes |
| Cardioplegia | No | Yes |
| Procedure time | 2 hours or more | 2 hours or more |
| Hospital LOS | 3-5 days (versus 4-6 days for sternotomy) | 3-5 days (versus 4-6 days for sternotomy) |
| Advantages | Avoids CPB, ischemic arrest, and hypothermia; may enable more complete revascularization with postoperative insertion of intracoronary stents into posterolateral coronary arteries in cardiac catheterization laboratory (hybrid procedure) | Allows repair of more complex lesions without technical challenge of moving heart; better ability to produce more complete revascularization |
| Potential complications and disadvantages | Learning curve technically more challenging; may cause VF; may have to revert to standard sternotomy with CPB and induced arrest | Learning curve technically more challenging; may have to revert to standard sternotomy; potential for endovascular injury to cannulated blood vessels |
| Discharge planning | Anticipated faster recovery of 1-2 weeks (versus 4-12 weeks for sternotomy), earlier ambulation, need to identify reportable signs and symptoms (angina, difficulty breathing, infection) | Anticipated faster recovery of 1-2 weeks (versus 4-12 weeks for sternotomy), earlier ambulation, need to identify reportable signs and symptoms (angina, difficulty breathing, infection) |
CABG, Coronary artery bypass grafting; CPB, cardiopulmonary bypass; HR, Heart rate; LOS, length of stay; VF, ventricular fibrillation.
Adapted from Rothrock JC: Alexander’s care of the patient in surgery, ed 14, St. Louis, 2011, Mosby; Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Other technical advances have brought together interventional procedures and traditional surgical treatments. This combination is seen in the hybrid cardiovascular suite, which enables patients to receive both percutaneous coronary stent placement and traditional surgical revascularization.10
Left ventricular aneurysm repair
Another form of myocardial revascularization is repair of an LV aneurysm. An LV aneurysm most often results from a large myocardial infarction or numerous smaller adjacent infarctions that cause a portion of the myocardial wall to become scarred, necrotic, thin, and weak. Scar tissue does not contract during systole but instead bulges outward (paradoxic motion or dyskinesia), which decreases the patient’s cardiac output. Thrombus can form within the trabeculations of the LV, and pieces of thrombus can break off and embolize to the systemic circulation. In addition, the perimeter around the scarred fibrotic aneurysmal area can alter conduction pathways and create reentrant ventricular dysrhythmias. Surgical repair consists of excision of the aneurysmal tissue, removal of existing thrombus, and insertion of a patch into the LV in a manner that restores a more normal (i.e., elliptic) ventricular geometry. If the patient continues to have recurrent ventricular tachycardia, electrophysiologic mapping and possible insertion of an implantable cardioverter-defibrillator (ICD) may be performed after surgery in the electrophysiologic laboratory.3
Valvular heart surgery
Valve surgery is performed more commonly on valves in the left side of the heart (i.e., aortic and mitral valves), because the higher pressures on the left side aggravate traumatic, infectious, or other preexisting injury to the valves and valve components. Repair of the native valve is preferred to replacement when possible. A left heart catheterization yields information concerning the left atrium and ventricle, the proximal aorta, aortic and mitral valves, and the coronary arteries. Echocardiography increasingly is the gold standard for the diagnosis of valvular heart disease. Other imaging processes include aortography that displays the size and function of the aorta and can be used to identify the location of the intimal tear in a dissection. Computed tomographic (CAT) scan and magnetic resonance arteriography have largely replaced aortography for imaging of aortic disease.11
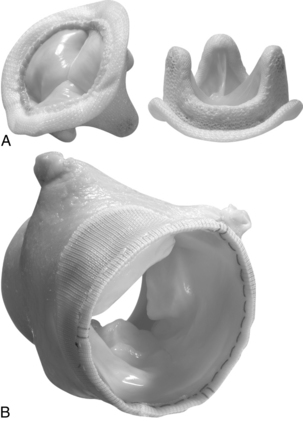
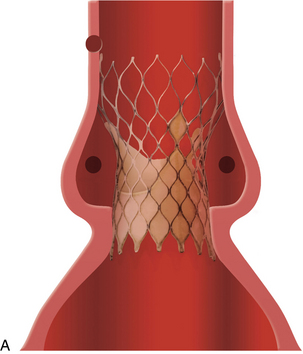
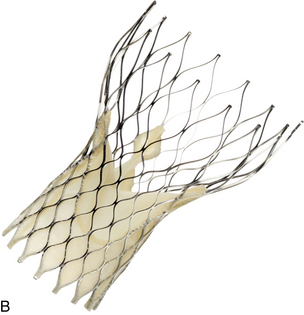
When surgery for valve replacement or repair is indicated, the surgeon selects and inserts the appropriate prosthesis. Valve replacement can be accomplished with biologic (Fig. 35-10) or mechanical (Fig. 35-11) prostheses. Newer, percutaneous aortic valve prostheses (Fig. 35-12) are increasingly available, mainly to patients who may be unable to withstand surgical replacemtnt.12 Allografts (Fig. 35-13) can serve as biologic valve replacements. Allografts are advantageous because they do not require warfarin anticoagulation, are resistant to infection, and have excellent hemodynamics. They are also useful in patients with small aortic roots, because no sewing ring exists to decrease the size of the aortic valve orifice, unlike prosthetic biologic or mechanical valves. Disadvantages include less availability and technical difficulties associated with insertion. Bioprostheses have the advantage of not requiring chronic anticoagulation postoperatively unless there are other indications, such as chronic atrial fibrillation; their disadvantage is that they do not last as long as mechanical prostheses. Bioprostheses are stored in glutaraldehyde before implantation; the solution must be rinsed off the prosthesis, usually in three baths of normal saline solution.
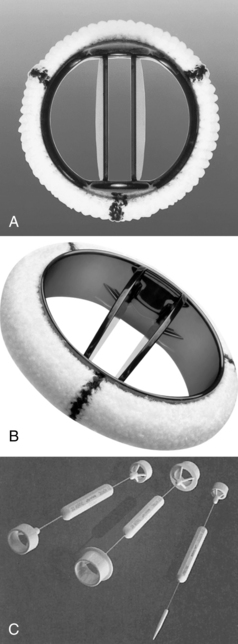
(A, Courtesy St. Jude Medical, St. Paul, Minn. B and C, Courtesy Medtronic Inc., Minneapolis, Minn.)
Minimally invasive techniques are increasingly popular. For example, a catheter-based interventional (i.e., nonsurgical) procedure can be performed in a hybrid suite for percutaneous insertion of a balloon-tipped catheter (commonly inserted into the femoral artery or femoral vein) that is threaded through the aorta or IVC to the intended location to enlarge a (stenotic) valve opening. For aortic valvuloplasty, the balloon tip is positioned across the aortic valve, and the balloon is inflated to dilate the aortic valve orifice. Complications of valvotomy include development of aortic or mitral regurgitation (depending on the affected valve), cardiac perforation, pulmonary edema, and cerebral embolus. Percutaneous aortic valve implantation also can be performed along with traditional aortic valve replacement.12
Aortic valve surgery
Aortic insufficiency
The regurgitating blood in AI creates an increased volume load on the LV. Mild chronic AI can be tolerated by the LV for almost 20 years with more forceful dilation and contraction to eject the additional regurgitant volume. Eventually the LV is stimulated to hypertrophy and decompensates if corrective therapy is not implemented. Symptoms include dyspnea from pulmonary edema and fatigue and other symptoms associated with LV failure. Sudden acute AI (e.g., from bacterial endocarditis that causes tearing of the leaflets [Fig. 35-14] or acute aortic dissection that dilates the aortic annulus) is poorly tolerated, because the normal-sized LV cannot accommodate the sudden increased volume and the lowered diastolic pressure that results from a portion of the cardiac output (CO) being regurgitated into the LV impairs coronary filling. Emergency aortic valve replacement is indicated for acute AI. Aortic valve replacement (see the definition of valve replacement) is generally indicated for AI; some reparative techniques are available for the aortic valve, but they tend to have less successful outcomes.
Aortic stenosis
Aortic stenosis (AS) can occur as a congenital process, as in the bicuspid valve (a common congenital anomaly first diagnosed in adulthood),13 as an acquired disease process related to a history of rheumatic heart disease, or as calcific AS (Fig. 35-15). An inflammatory process has been associated also with the development of aortic stenosis.2
Other forms of AS include narrowing of the outflow tract below or above the valve. Subvalvular stenosis occurs when the intraventricular septum becomes hypertrophied and creates an obstruction to LV outflow. This lesion is known as idiopathic hypertrophic subaortic stenosis or hypertrophic obstructive cardiomyopathy; surgical treatment for subaortic stenosis with a hypertrophied septum involves excision of a portion of the hypertrophied septum; cardiomyopathy may require heart transplantation. Supraaortic valvular stenosis occurs when the aorta above the valve is narrowed from a congenital anomaly. Repair involves opening the affected portion of the aorta and inserting a patch graft to enlarge the narrowed vessel.
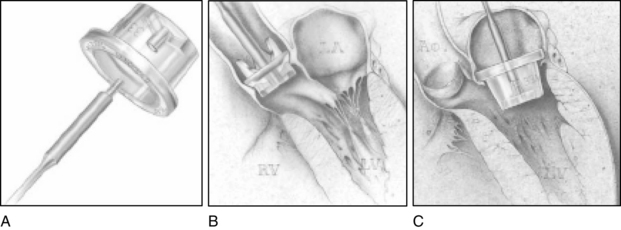
Procedure
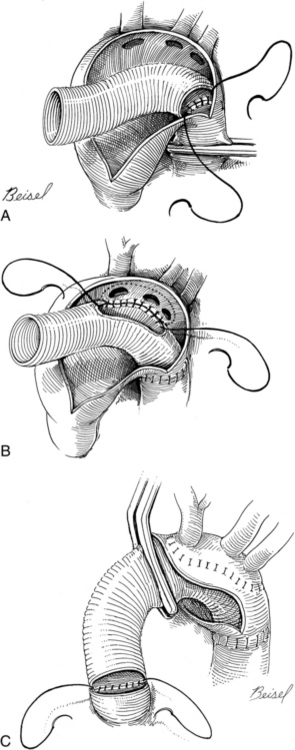
For aortic valve replacement (Fig. 35-16), the leaflets are excised, the annulus is sized with obturators (i.e., sizers) specific to the prosthesis desired (Fig. 35-17), and the selected prosthesis is implanted. After surgery, anticoagulation therapy is usually initiated 3 to 5 days after surgery, when the patient’s intravascular lines and chest tubes are discontinued and immediate postoperative hemorrhage is no longer a concern. Oral warfarin therapy is then started and titrated appropriately to achieve an international normalized ratio of 2.0 to 3.0 times normal (target, 2.5 for mechanical valves); a target international normalized ratio of 2.5 to 3.5 may be sought in the first 3 months following mechanical valve replacement.3,14
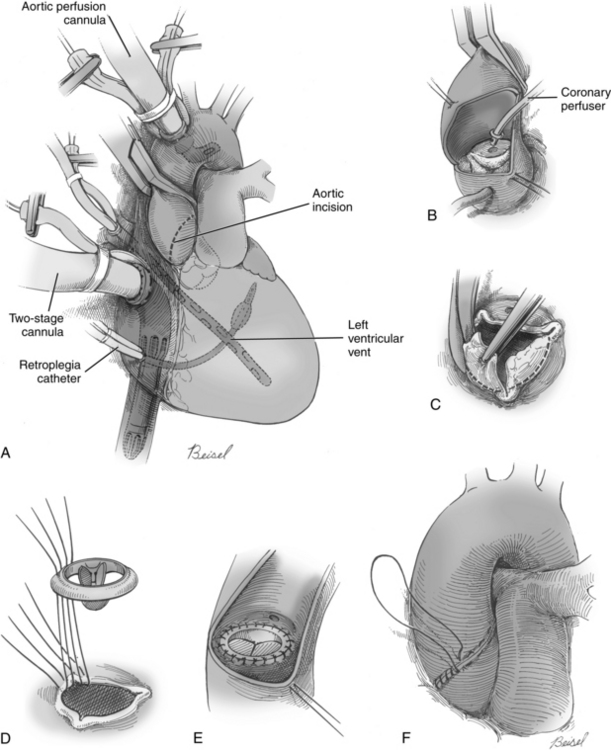
FIG. 35-16 A, Aortic valve replacement. Cardiopulmonary bypass cannulation, retrograde cardioplegia, and left ventricular vent (inserted via right superior pulmonary vein) are shown. Note incision site on aorta (dotted line). B, If retrograde cardioplegia is not used, handheld ostial catheters may be used for cardioplegia infusion. C, Valve leaflets are completely excised. D, Sutures are placed in aortic annulus and prosthetic sewing ring. E, Stitches are tied and cut. F, Aorta is closed.
(From Waldhausen JA, et al: Surgery of the chest, ed 6, St. Louis, 1996, Mosby.)
Mitral valve surgery
Mitral regurgitation (MR)
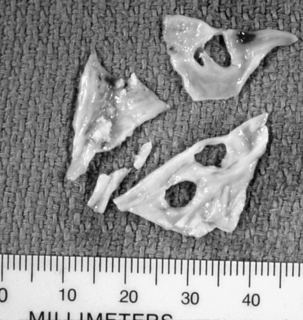
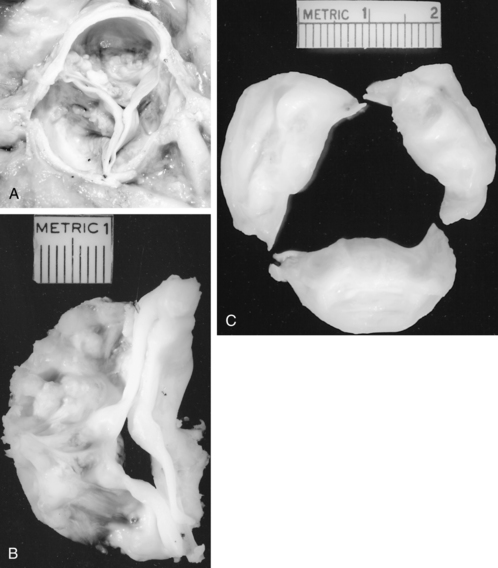
Any of the mitral valve components may be affected. An enlarged annulus is a common cause of mitral regurgitation (MR). The valve leaflets may be normal, but they are unable to coapt and create a competent valve because the dilated annulus pulls the leaflet edges away from one another. The causes of MR may be inflammatory, degenerative, infective, ischemic, structural, or congenital.15–17 The myxomatous mitral valve (Fig. 35-18) reflects leaflet degeneration from fibroelastic changes. MR also may be related to fibrosis, calcification, or tears in a previously implanted valve prosthesis (Fig. 35-19). Chordae tendineae may be ruptured or excessively elongated or shortened and fused from bacterial endocarditis, one or more papillary muscle heads may have ruptured as a result of ischemic heart disease, or the ventricular endocardium may be dilated.
Mitral stenosis (MS)
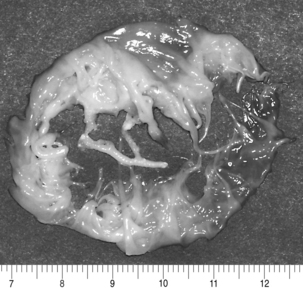
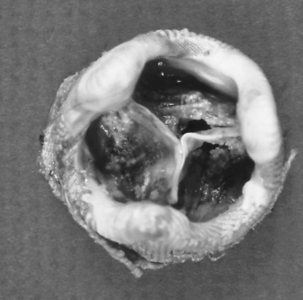
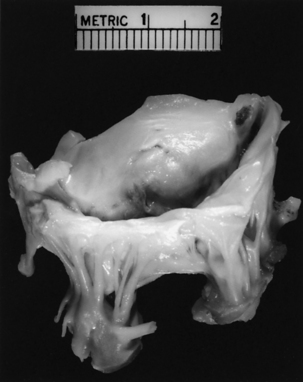
Unlike AS, AI, or MR, stenosis of the mitral valve is the only lesion that does not place either a volume or a pressure load on the LV. However, chronic mitral stenosis (MS; like MR) produces an enlarged atria and stasis of blood (with the risk of thromboembolism) and frequently leads to atrial fibrillation. Functional tricuspid regurgitation may develop. MS is the result of changes in one or more of the components of the mitral apparatus. The causes of MS may be inflammation, calcification, tumors (i.e., left atrial myxoma), and infection. Bacterial vegetations and the sequelae of rheumatic fever continue to be significant causes of MS.15 With MS the leaflets become progressively more fibrotic and stenotic. Fig. 35-20 illustrates the classic “fish mouth” appearance of a rheumatic mitral valve: smooth fibrotic thickened leaflets, commissural fusion, and shortened thickened chordae tendineae.
Procedure
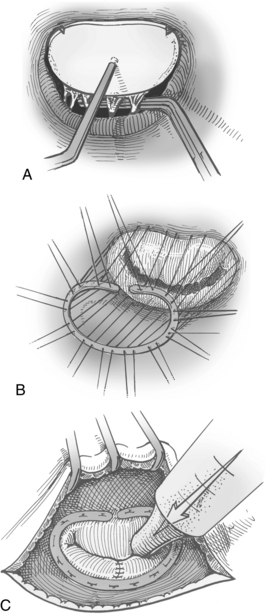
Annuloplasty and valvuloplasty may be performed on the affected component. An annuloplasty procedure commonly is performed on the mitral or tricuspid valve or both. A cloth-covered prosthetic annuloplasty ring (Fig. 35-21) in a size smaller than the dilated native annulus is sutured to the valve annulus (Fig. 35-22). When the stitches are tied, excess annular tissue is pulled up against the ring, thereby reducing the overall circumference of the valve orifice and allowing the valve leaflets to coapt properly. Annuloplasty rings have different configurations (e.g., circular, almond shaped, C shaped) and are flexible, rigid, or semirigid. The surgeon’s selection of the prosthesis is based on factors such as the underlying pathology, anatomy, annular dynamics, remodeling of the annular geometry, and preservation of valve function. Prosthetic valvular annuloplasty itself does not require postoperative long-term anticoagulation therapy.
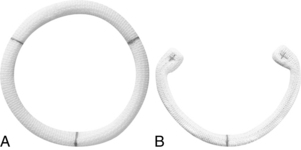
FIG. 35-21 Annuloplasty rings. A, Duran AnCore® ring. B, CG Future® ring.
(Courtesy Medtronic Inc., Minneapolis, Minn.)
In addition to insertion of a prosthetic annuloplasty ring for annular dilation (see Figs. 35-21 and 35-22), valvuloplasty can be performed. Excess portions of the posterior leaflet of the mitral valve are excised, and the remaining edges are sewn together to promote leaflet coaptation and to reduce the annular circumference. Torn leaflets can be repaired with a patch of pericardium. Ruptured chordae tendineae can be replaced with suture or reattached to the valve leaflet; shortened thickened chordae can be incised to increase length and improve flexibility; and excessively lengthened chordae can be shortened by implanting a portion of the chord into a papillary muscle head. Debridement of calcific nodules can be done, although valve replacement may be needed with severe calcification.
Balloon valvotomy for the mitral valve is performed with a catheter percutaneously inserted into a large vein and threaded to the RA, where a septal puncture is made to allow access into the left atrium and to the mitral valve. This technique is useful for children, young adults, and patients who are too elderly or medically compromised to withstand an operation. Occasionally the catheter is threaded up the aorta and through the aortic valve into the LV and then placed across the mitral valve where the balloon is inflated. Percutaneous mitral valve repair can be performed, but the procedure is less effective than surgery for reducing mitral regurgitation.18
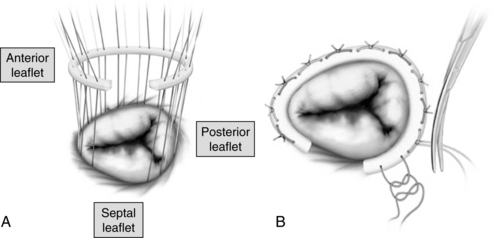
When valvular deformation injury is severe, prosthetic replacement may be required. For mitral valve replacement (Fig. 35-23), the anterior leaflet is usually excised, but often all or part of the posterior leaflet and its attached chordae are retained to preserve the LV geometry and enhance LV function. Although valve replacement improves cardiac function by removing the stresses associated with volume or pressure overloading, transient symptoms of ventricular failure can exist after mitral valve replacement for chronic MR because the LV no longer has the release valve represented by the incompetent valve. With the insertion of a competent mitral prosthetic valve, the LV must develop sufficient pressure to push all the LV end-diastolic volume forward through the aortic valve. Afterload reduction (e.g., with sodium nitroprusside) assists LV ejection. Potential complications associated with prosthetic valve replacement are thromboembolism, anticoagulation-related hemorrhage, prosthetic valve endocarditis, perivalvular leak, and prosthetic failure.2,3
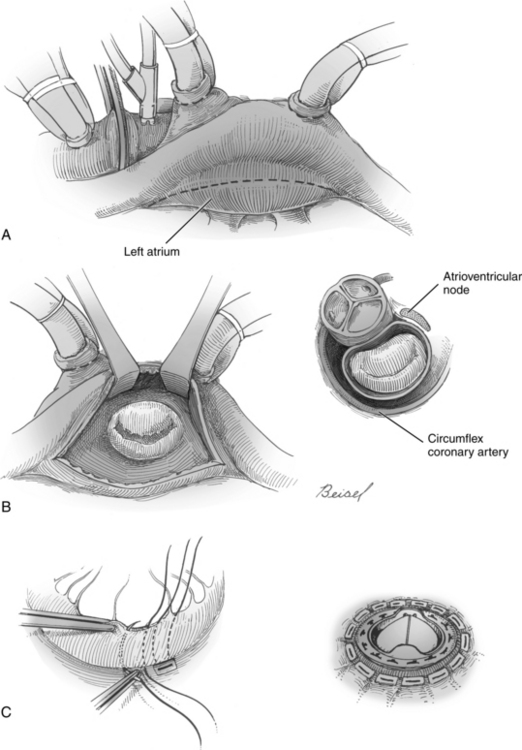
FIG. 35-23 Mitral valve replacement. A, Incision (dotted) line in left atrium, exposure of mitral valve, and illustration of relationship between mitral and aortic valves and atrioventricular node. Double venous cannulas shown for cardiopulmonary bypass; antegrade cardioplegia catheter inserted into aorta below the cross clamp and aortic cannula are shown. B, Sutures are placed in native valve annulus and then prosthetic. C, Completed valve replacement.
(From Waldhausen JA, et al: Surgery of the chest, ed 6, St. Louis, 1996, Mosby.)
Tricuspid valve repair
Annuloplasty repair is generally performed, although tricuspid valve replacement is performed if a repair to the native valve cannot be accomplished. During ring insertion for tricuspid annulus repair (Fig. 35-24), the surgeon is especially cautious when inserting stitches near the atrioventricular node and the bundle of His (in the area of the tricuspid septal leaflet) to avoid creating complete heart block or other injury to the conduction system. A suture annuloplasty (i.e., without the insertion of a prosthetic ring) can be used also for some tricuspid valve repairs (see also the definition of valvuloplasty).
Surgery for thoracic aortic aneurysms and dissections
Localized or diffuse dilation of the aortic wall leads to progressive enlargement of all layers of the aorta. Surrounding tissue may be compressed by the enlarging vessel, and eventual rupture with exsanguination can occur without treatment. Diagnosis is made with CT scan and magnetic resonance arteriography, which display the size and shape of the aorta and can be used to identify the location of the intimal tear in a dissection.19 Surgical treatment consists of excision of the aneurysmal tissue with insertion of a prosthetic graft (e.g., Dacron; Fig. 35-25). An endovascular stented graft made from expanded polytetrafluoroethylene (PTFE) can be used to repair f descending (and abdominal) aortic aneurysms. The prosthesis is initially compressed, inserted percutaneously into the femoral artery, and guided into the aorta where it is expanded and deployed in the desired position.
Aortic dissection is a unique condition affecting the aortic media. Surgery is indicated for ascending aortic and transverse aortic arch dissections and involves excising the portion of the aorta that contains the tear and replacing excised tissue with a graft. Uncomplicated descending thoracic aortic dissections are more commonly managed medically with antihypertensive medications and analgesics. Surgery, with a traditional incision or an endovascular prosthesis, is indicated with impending rupture.20,21
Blunt trauma to the thoracic aorta can lead to aortic rupture. Newer imaging techniques have enabled clinicians to intervene more promptly in these cases, thereby improving survival.11 Endovascular graft insertion or traditional repair may be performed.22
Ventricular assistance
Ventricular assist devices (VADs) decrease the workload of the heart by diverting blood from the ventricle to an artificial pump that maintains systemic (left VAD [LVAD]) or pulmonary (right VAD [RVAD]) perfusion. A BiVAD supports both right and left ventricles. Placement of a cardiac transplant candidate on a VAD enhances anabolism, ambulation, and improved organ function; these physiologic improvements enable the patient to become a stronger transplant candidate. In this case, the VAD is used as a bridge to transplantation. Improvements in VAD therapy have significantly reduced the incidence rate of infection and thromboembolism. VADs have been especially valuable in the treatment of heart failure.23,24 A vented electric LVAD for destination therapy or permanent replacement for the LV enables patients to be relatively independent by reducing the reliance on in-line power sources.25
Short-term assist devices include the intraaortic balloon pump (IABP), a sausage-shaped balloon mounted on a catheter percutaneously inserted into the aorta distal to the left subclavian artery via the femoral artery. The balloon inflates during diastole, propelling blood proximally to the aortic root and into the coronary ostia to enhance coronary perfusion. Distally, blood is propelled into the peripheral vascular bed to enhance organ blood flow. When the balloon deflates during LV systole, LV afterload is reduced by the movement of blood during balloon inflation. With the increase in coronary blood flow and the decrease in afterload, the total work of the heart is reduced, thereby providing an environment that supports the recovery of a failing myocardium and improves peripheral vascular perfusion.24
Transplantation
With the improvement in pharmacologic therapy, comorbidity management and infection prophylaxis, cardiac transplantation 1-year survival rates approach 90%.26 Potential candidates are patients who are considered to be in the New York Heart Association Functional Classification System’s class IV, which denotes the most compromised cardiac status, and who have less than a 10% chance of survival for 6 months. Heart donors are persons with irreversible catastrophic brain injury who do not have atherosclerotic heart disease. Age limitations have become less restrictive, and donors and recipients may be 60 years or older in certain cases.
Two new cardiac transplantation subpopulations include patients with previously repaired congenital heart disease and transplantation recipients requiring retransplantation. Ten-year survival after transplantation in the former group is excellent.26 Retransplantation patient survival improves in the absence of primary allograft failure and refractory rejection within the first 6 months after retransplantation. Guidelines for this subpopulation remain vague and are undergoing refinement.26,27
Lung transplantation involves replacement of one or both lungs with lungs from a donor or a single lung from a living donor. Newer allograft organ protective strategies have contributed to improved outcomes in lung transplantation candidates. Contraindications for transplantation include significant pulmonary hypertension, the presence of systemic disease, a recent pulmonary infarction, or active systemic infection.28,29
Surgery for adult congenital heart disease
A number of congenital anomalies might not be treated until adulthood. These congenital lesions tend to be less severe and therefore asymptomatic (or mildly symptomatic) in childhood. Some of the most common are listed in the following sections.30
Atrial septal defect
A frequently seen congenital anomaly in adulthood is the atrial septal defect (Fig. 35-26).31 The most common form of atrial septal defect in the adult is the ostium secundum defect, located in the midportion of the septum in the vicinity of the fossa ovalis (formerly the intrauterine shunt known as the foramen ovale). Less common is the sinus venosus defect located near the entrance of the SVC in the high RA. Sinus venosus defects are associated with partial anomalous pulmonary venous return (commonly of the right superior pulmonary vein), whereby the anomalous pulmonary vein enters the RA (rather than the left atrium) and returns oxygenated blood from the lungs into the SVC or the upper RA. Closure of the secundum and sinus venosus septal defects is accomplished with suturing or patching the defect with a patch of pericardium or prosthetic material. During repair of a sinus venosus defect, the patch is placed in such a way that anomalous pulmonary venous drainage is diverted into the left atrium.
Dysrhythmia surgery
Atrial fibrillation (AF) is associated with significant morbidity and mortality rates. In 1991, the Cox Maze procedure32 showed excellent results in redirecting the impulses of AF with incisions made and the sutured closed. Electric impulses cannot cross sutured (or ablated) tissue. Because electric impulses can travel only through normal tissue, the surgeon locates the various incisions to create a pathway for impulses that direct those impulses toward the atrioventricular node, thereby returning the heart to normal sinus rhythm.32 More recent research has shown that the source of 90% of the aberrant impulses comes from the area that surrounds the pulmonary veins.33 Ablation of the targeted tissue (with cold energy, radiofrequency, or other energy sources) has been successful, with a more than 90% success rate in achieving sinus rhythm.34 Further improvement in outcomes has been seen in patients who are followed with a protocol that coordinates treatments by cardiologists, surgeons, and nurse coordinators.35
Completion of surgery
After the cardiac repair is completed, temporary pacing wires are attached to the right atrial and ventricular surface, brought out through the chest below the sternal incision, and connected to an external pacemaker generator. One or more chest tubes are placed anteriorly, laterally, or posteriorly in the pericardium to facilitate drainage. The tubes exit via stab wounds below the sternal incision. If either of the pleurae were opened during the procedure, the tip of one of the pericardial chest tubes is inserted into the affected pleura. The pericardial sac is rarely closed so that any remaining blood or fluid can drain freely into the chest tubes, avoiding the creation of a cardiac tamponade. Occasionally, when a repeated operation is anticipated (e.g., after a biologic valve replacement in a young person), the upper (cephalad) portion of the pericardium may be closed so that less scar tissue forms over the cannulation sites for CPB (e.g., the aorta). The lower portion of the sac remains open to drain fluid. The sternum is closed with stainless steel wires, and the fascia, subcutaneous tissue, and skin are closed with suture. Before transfer from the OR to the postanesthesia care unit (or intensive care unit [ICU]), portable monitoring lines for heart rate and rhythm, arterial blood pressure, and oxygen saturation are connected for transport to the postoperative unit. Patients can have acute circulatory instability develop, because of either the normal recovering physiologic state or inadvertent movement or displacement of the numerous invasive lines and tubes needed. A portable defibrillator is often placed on the transport bed for use if ventricular tachycardia or ventricular fibrillation develops during transport.
Postanesthesia care
Cardiac surgery patients commonly go to a specialized cardiovascular surgery ICU or postanesthesia care unit (PACU). Whether a critical care nurse or a postanesthesia nurse, a caregiver should be aware of the level of knowledge, understanding, and anxieties of both the patient and the family before surgery. When possible, and depending on the individual institution’s policies and procedures, the postoperative caregiver nurse can provide preoperative instruction for the patient and family or make an introductory visit before surgery. Teaching content for coronary or valve surgery (see Table 35-1) can be initiated in the preoperative period and reinforced after surgery. Discharge from the cardiovascular surgery ICU or open heart PACU to a step-down unit increasingly occurs within hours. A reduction has also been seen in the overall length of stay (LOS) from 1 or longer to a few days. The movement toward rapid postoperative endotracheal extubation and shortened LOS has been spurred not just by costs but also by improved pain control that facilitates earlier extubation and by fundamental shifts in anesthetic management from a high-dose opioid technique to a more balanced approach with moderate-dose opioids, shorter acting muscle relaxants, and volatile anesthetics. The so-called fast-track approach (i.e., extubation immediately after or within a few hours of surgery)2 is becoming a standard of care in a number of centers.3 The longer LOS sometimes seen in the rapidly growing number of patients 80 years and older may be the result of comorbid conditions such as peripheral vascular disease, renal pathology, diabetes mellitus, hyperlipidemia, and hypertension, but advances in anesthetic techniques and myocardial protection during surgery have improved morbidity and mortality rates in older and younger patients. This chapter is designed to familiarize the PACU nurse with the perioperative care for the adult cardiac patient. Understanding what the patient has experienced before and during surgery assists the nurse to tailor care after surgery to the patient’s needs and to strengthen the continuity of that care.
Invasive monitoring lines are attached to transducers or manometers. Patency is ascertained, and values and wave tracings are continuously displayed and recorded. Commonly measured intravascular parameters include mean arterial, right atrial, mean pulmonary artery, pulmonary artery systolic, pulmonary artery diastolic, pulmonary capillary wedge, and left atrial pressures. In addition to these directly measured parameters, these values assist in calculation of indirect or derived hemodynamic parameters, such as cardiac output and cardiac index, systemic vascular resistance, and pulmonary vascular resistance. These parameters assist in the assessment of both LV and RV status and are used in determination of pharmacologic, fluid, and mechanical therapies for the postoperative patient. Once these invasive lines are connected, the nurse reviews and assesses with the anesthesia provider the intravascular lines and solutions in regard to type, drugs infused, flow rates, patency, and expiration times. Intake and output recordings with running totals are made and documented hourly or more often as clinically indicated. Volume administration and replacement treatments are largely determined by the individual patient’s hemodynamic parameters, and responses can vary greatly from hour to hour.
The patient’s neurologic status is assessed on admission and every 30 to 60 minutes and thereafter until arousal from anesthesia. Neurologic complications can affect the central or the peripheral nervous system and include cerebral or peripheral thromboembolism, encephalopathy, coma, impaired intellectual function. Use of off-pump (versus on-pump) techniques for patients undergoing CABG have not shown improvements in neurocognitive outcomes.3,36 Once the patient has been aroused, neurologic assessments are decreased to every 2 hours.
An admission temperature is obtained and active rewarming therapies are instituted. Temperatures are recorded hourly during this period, and rewarming devices are discontinued when the patient’s condition reaches normothermia. One temperature-related condition is hypothermia-related hypocarbia. The body temperature usually remains lower than normal during the early postoperative hours. The body temperature returns to normal slowly; rebound hyperthermia occurs occasionally. As rewarming takes place, carbon dioxide is produced. Consequently, hypercarbia can develop, and ventilator settings should be adjusted to produce a minute volume necessary to facilitate normocarbia. In addition, if rebound hyperthermia occurs or if rewarming devices are not regulated and monitored appropriately, a hyperthermic overshoot can occur, which increases myocardial oxygen demand and consumption.3
An abdominal assessment is performed on admission and every 2 hours thereafter until bowel sounds return. A nasogastric or orogastric tube inserted after induction of anesthesia relieves gastric distention and facilitates removal of gastric contents.2 It is usually attached to low intermittent suction or gravity drainage and removed at the time of extubation. Analysis of pH and tests for the presence of occult blood can be performed on gastric secretions if they begin to resemble coffee grounds. After the nasogastric tube is removed, the patient is given ice chips and resumes a clear liquid diet within the first 24 hours.
Urinary drainage from the catheter inserted in the OR should be recorded on admission to the PACU and then hourly. The appearance of the urine is also monitored closely. During the first few hours after surgery, massive diuresis of 2 to 3 L of pale dilute urine is usually common from diuretics that are generally administered during the discontinuation of CPB to facilitate the removal of fluid that has sequestered during surgery in the interstitial space. When this initial diuresis resolves, urine color and consistency return to normal. Urinary output should be more than 0.5 mL/kg/h; a lower urine output is commonly the result of decreased renal perfusion.3
Cardiopulmonary bypass affects every body system, and a brief review of some of the effects on the patient seen in the postoperative period is useful to the nurse. For example, epinephrine and norepinephrine levels are markedly elevated during CPB. Hyperglycemia and impaired insulin responses are present and result in use of fat stores for energy until carbohydrate metabolism returns to normal levels. Secretion of antidiuretic hormone and aldosterone is also increased, stimulated by different mechanisms associated with the physiologic components of stress. Serum complement that is activated when blood contacts the inner surfaces of the bypass circuit cause an inflammatory response with a marked increase in total body water and interstitial edema. Postoperative fluid requirements are affected by rewarming because rewarming itself causes fluid shifts. This excess fluid usually redistributes itself by the second or third day after surgery and is excreted spontaneously or with diuretic therapy. CPB and hypothermia also alter coagulation factors, platelet formation, and the immune system.2
A written assessment is performed after admission routines. The frequency of assessment and documentation of the hemodynamic parameters and routines is dictated by the patient’s response to and recovery from surgery. Recovery time varies from among patients but generally occurs within 12 to 24 hours, except with impaired cardiac, pulmonary, renal, or other organ function. During that time numerous expected postoperative physiologic alterations occur, largely because of the techniques of CPB, hypothermia, anesthesia, and surgical manipulation of the myocardium. With correct interventions, these physiologic responses are short lived and reversible; however, two problems exist. First, although these alterations are reversible, they can lead to complications if not identified early and treated quickly. Such is the case with uncontrolled hypertension, which can develop into hemorrhage if fresh suture lines are disrupted. Second, these alterations often resemble complications and thus may be missed in the early stages. For example, the initial absence of a pedal pulse may be attributed to hypothermia and vasoconstriction only to be traced later to a vascular embolism. For these reasons, the PACU nurse must be knowledgeable about the causes, assessment factors, and interventions for both expected physiologic alterations and complications that can occur after cardiac surgery. These alterations and potential complications are reviewed briefly in the following section.
Complications
Cardiovascular system
These complications also contribute to increased costs. One multiyear, statewide study of isolated CABG patients demonstrated the greatest additive costs for prolonged ventilation ($40,704), renal failure ($49,128), and mediastinitis ($62,773).37 Noncardiac complications include hemorrhage, wound dehiscence and infection, and neurologic, renal, pulmonary, and gastrointestinal problems. Each complication is addressed individually.
Cardiac complications of coronary artery bypass grafting and valvular surgery
Myocardial infarction
Another contributing factor may be reperfusion injury from sudden reperfusion of an ischemic area causing necrosis as a result of a rapid influx of calcium ions, oxygen-free radicals, and other metabolic waste products into the ischemic myocardial cells. The appearance of new Q waves after surgery also has been shown to adversely affect early and late survival. Other predictors of perioperative MI include left main coronary artery stenosis, multivessel disease, minimal collateral circulation, and the duration of CPB.2,3
Congestive heart failure (alterations in myocardial contractility)
After surgery, treatment can include a combination of pharmacologic and mechanical circulatory support throughout the period of recuperation. Depending on the hemodynamic status of the patient, different combinations of inotropic and vasopressor agents are used as initial therapy, with the addition of mechanical support (i.e., IABP) if cardiac output remains low despite inotropic stimulation and optimal filling pressures, as indicated by a pulmonary capillary wedge pressure greater than 22 to 25 mm Hg.2,3
Cardiac tamponade
Cardiac tamponade develops when the amount of blood or fluid accumulated in the pericardial cavity is sufficient to compress the heart. Cardiac compression produces ineffective filling and ejection of an adequate CO. Signs of tamponade consist of low CO and cardiac index, hypotension, tachycardia, equalization of the RA and LA pressures, development of pulsus paradoxus, narrowed pulse pressure, muffled heart sounds, widening of the mediastinum on chest radiograph, and alteration in neurologic status. Observation of the quality of chest tube drainage is critical, especially in the first 6 hours when tamponade is more likely to occur. Generally, chest tube drainage in cardiac surgical patients is thin, red, or serosanguineous and not clotted, because blood is exposed to the mechanical effects of the contracting heart and the motion of the lungs. Blood in the pericardium that normally begins to clot is defibrinated by this mechanical trauma and becomes thin, nonclotted chest tube drainage.3 When clots begin to appear in the chest tubes, relatively fresh bleeding may be indicated. In this situation, the incidence rate of tamponade is higher because clot can occlude the chest tube lumen, preventing chest drainage. Sudden cessation of previously and heavily clotted drainage should be promptly investigated for signs of tamponade. The specific cause can be either rapid, active bleeding from a suture line or continuous, slow oozing from a coagulopathy. Mediastinal exploration in the OR, or in the postoperative unit if the patient’s condition is unstable, may be necessary to control surgical bleeding. Treatment for coagulopathy is determined with assessment of the underlying cause of the bleeding (e.g., insufficient number of functional platelets) and treatment of the specific problem.
Dysrhythmias
Rhythm disturbances of the atria or ventricles are common after surgery and may be caused by ischemia, hypoxia, hypotension, or pharmacologic or metabolic factors. Inotropic drugs (with their contractile and chronotropic effects), acid-base imbalances, and electrolyte abnormalities can also cause dysrhythmias. The high incidence rate of dysrhythmias necessitates continuous monitoring for the first 48 hours in the critical unit and the stepdown unit. Correction of electrolyte imbalances and hypoxia are basic requirements. Massive diuresis can precipitate hypokalemia, promoting the development of dysrhythmias; potassium replacement should be aggressive to maintain serum potassium concentrations at levels higher than 4 mEq/L. An intravenous infusion of magnesium may reduce premature atrial contractions and promote atrial contraction to enhance preload and CO.2,3
AF is common after cardiac surgery 2,3 Amiodarone may be infused to promote new onset AF conversion to sinus rhythm. For patients with persistent or permanent AF, the goal is rate control of the ventricular response to the atrial ectopic impulses. If cardioversion is considered, a TEE first should be performed to identify the presence of left atrial thrombus, which that could break off and embolize.3 If thrombus is present, there is an increased risk for thromboembolism after cardioversion.
Peripheral vasoconstriction (postoperative hypertension)
Factors that contribute to the development of peripheral vasoconstriction include the patient’s own sympathetic drive triggered by anxiety, surgical manipulation of the heart and the great vessels with the attached pressor receptors, vasoactive drugs, and systemic hypothermia. Patients may appear pale, with cool extremities and body temperature less than 37° C (98.6° F). Patients may also display increased systemic vascular resistance, weak or impalpable pulses, tachycardia, and varying degrees of hypertension. Hypertension needs to be controlled so that the elevated pressure does not disrupt the surgical anastomoses. Increased systemic vascular resistance increases ventricular afterload and stresses the myocardium. Treatment approaches include active rewarming and administration of vasodilating agents (e.g., intravenous sodium nitroprusside, nitroglycerin, nicardipine, clevidipine).3,38 These agents have relatively immediate effects and can be easily reversed when use is discontinued. Dosages should be titrated to achieve a mean arterial blood pressure of 70 to 80 mm Hg.
Noncardiac complications of coronary artery bypass grafting and valvular surgery
Hemorrhage
Coagulation disorders and hemorrhage pose a potential threat to the cardiac surgical patient. Coagulation dysfunction is often the result of CPB,2,3 from direct trauma to the blood components from solid synthetic surfaces and inline tubing connectors in the CPB circuit, inadequate surgical hemostasis, coagulation disorders, preoperative use of antiplatelets or anticoagulants, inadvertent hypothermia, or insufficient heparin reversal with protamine sulfate. Preoperative evaluation can be used to detect some coagulation disturbances that can be treated before surgery (e.g., discontinuation of anticoagulant medications or switching from warfarin to heparin). Previous health problems such as uremia and hepatic disease also should be considered as possible causes of coagulation disturbances. Adequate heparin reversal with confirmation with a whole-blood activated coagulation time (ACT) or activated partial thromboplastin time (APTT) should be performed. Even if the results of these studies are normal, heparin may still be released from body stores (e.g., adipose tissue) and cause a heparin rebound phenomenon. An initially normal ACT or APTT result does not guarantee that subsequent bleeding is unrelated to the effects of heparin.
Indications for transfusion based on the hematocrit and other clinical factors have undergone reassessment. One study has demonstrated that using a hematocrit value of 24% (versus 30%) as a trigger for transfusion did not result in increased morbidity and mortality.39 Another study demonstrated that red blood cell transfusion was associated with more adverse outcomes (compared to patients not receiving red blood cell transfusion.40
Mediastinal exploration is indicated when signs of cardiac tamponade develop with bleeding of more than 500 mL/h or less or more than 300 mL/h for 6 hours or longer. The decision to operate again should be made before the patient’s condition becomes hemodynamically unstable. On reexploration for bleeding, oozing from the mediastinal wound or active bleeding may be found. During exploration, clots and hematoma are removed, the heart and pericardium are explored, and active bleeding is controlled. The pericardium is irrigated, and the chest is closed. Occasionally, no discrete source of bleeding can be found, even after extensive exploration of the surgical site.2,3
Sternal wound dehiscence and infection
Superficial wound infection, sternal osteitis, and mediastinitis can occur after surgery, although superficial wound problems are the most common. Predisposing factors for impaired sternal healing include advanced age, obesity, diabetes mellitus, long-term steroid use, and chronic obstructive pulmonary disease. Postoperative bleeding that necessitates reexploration also contributes to sternal wound infection. Infection can occur at any time, but is usually diagnosed 6 to 12 days after surgery. Treatment consists of intravenous antibiotics, opening of the wound, removal of clot, debridement, and irrigation. After the sternal wound has been debrided, primary closure may be performed. In more severe cases, sternal reconstruction (occasionally with muscle flaps)2 may be needed and be performed about 1 or 2 weeks after debridement. Intravenous antibiotics are continued for at least 1 week after wound closure.
Inadequate volume status
Hypervolemia develops as interstitial fluid moves back into the intravascular space or if overaggressive volume replacement occurs. Assessment of these states requires extensive hemodynamic monitoring and understanding of the numerous processes involved. Signs and symptoms specific to hypovolemia or hypervolemia should be investigated. Interventions for hypovolemia initially consist of replacement with crystalloid fluids. Colloidal solutions may be more appropriate in patients with significant peripheral edema because colloidal solutions can pull fluid more aggressively into the vascular bed where excess fluid can be excreted through the kidneys. If persistent hemorrhage from coagulopathies exists, transfusion with fresh-frozen plasma, platelet concentrates, or other factors may be indicated. If hemorrhage is related to technical (i.e., surgical) factors, reoperation is necessary. Replacement solutions in the interim can consist of blood, whole blood, or packed cells; however, blood transfusion is generally avoided unless oxygen-carrying capacity is significantly compromised.39,40
Respiratory system
The effects of anesthesia, sedation, and the deflation of the lungs during CPB commonly create moderate episodes of impaired gas exchange with concurrent alterations in the arterial blood gas values. These episodes, largely atelectatic in nature, are usually self limiting or easily resolved with sustained maximal inspiration, chest physiotherapy, and administration of supplemental oxygen. If a hemothorax or a pneumothorax develops, more negative pressure may be added to the drainage systems, or additional chest tubes may be inserted. A volume overload from overaggressive replacement or mobilization of fluid from the third spaces may exist and can hamper gas exchange; diuretic therapy is indicated.2,3
Nervous system
Temporary and permanent sensory, motor, perceptual, and cognitive deficits can occur during the perioperative period. Permanent deficits can usually be attributed to a low cerebral perfusion state from inadequate cardiac output or to an embolic phenomenon from intracardiac thrombi, calcified valve fragments, aortic plaque dislodgement and subsequent embolization from application of the aortic crossclamp, or air embolization from intracardiac chambers of invasive lines. The magnitude of the deficit is determined by the degree of neurologic involvement. Deficits are usually identified early in the postoperative period when the effects of anesthesia have resolved. Some of these deficits may not be identified until after extubation. Transient deficits, lasting from hours to days, can range in degree from slowness to arousal to confusion and delirium. Deficits can be caused by microemboli from tissue debris, air bubbles, or platelet aggregation and are associated with CPB.3
Renal system
Prerenal and acute renal failure states can develop after cardiac surgery. Inadequate CO from myocardial depression or inadequate volume replacement can lead to prerenal oliguria. Blood urea nitrogen and serum sodium levels increase, and serum creatinine levels remain the same. Low sodium content is seen in the urine as the body attempts to save sodium and thus increase its intravascular volume. If these states continue for prolonged periods, acute renal failure can ensue. Treatment focuses on maintenance of adequate volume replacement and increase in CO, with an inotropic agent if necessary. In addition, renal emboli from intracardiac thrombi or hemolysis from blood transfusions or prolonged CPB runs (from trauma to the formed elements of the blood producing hematuria) can also lead to the development of acute renal failure. In acute renal failure, serum creatinine and urea levels elevate and remain in a 10:1 ratio, urine sodium levels increase, and the plasma urine osmolality ratio falls to 1:1. Transient hematuria is usually short lived and may resolve after infusion of an osmotic diuretic (e.g., mannitol).2,3
Gastrointestinal system
Gastrointestinal complications are similar to those for general surgery.41 After extubation, the patient may remain on nothing by mouth (i.e., NPO) status for a few hours. Small amounts of ice or water are then allowed. Gastric distention can occur if air enters the stomach and can cause cardiac problems and pulmonary complications. A nasogastric tube can be inserted to decompress the stomach. Rarely, mesenteric or splenic ischemia or infarction from intracardiac thrombi or air emboli may occur; surgical revascularization can be performed.
Peripheral vascular system
Vascular complications can include both venous and arterial thrombus formation and embolism development. Venous thrombus can develop as a result of blood stasis from immobilization and inactivity in the immediate postoperative period. Arterial complications are largely associated with various intravascular devices such as intraarterial lines and intraaortic balloon catheters, when present. Assessment of pulses should be ongoing, and a Doppler pencil may be used to confirm the patency of the radial artery after removal of the intraarterial pressure monitoring line. The status of lower extremity pulses, skin color and temperature, and motor activity should be monitored closely, particularly in the presence of an intraaortic balloon catheter and particularly during insertion and removal. Passive and active range-of-motion exercises and early ambulation are advocated and encouraged in these patients to prevent complications. Patients are instructed and assisted in performing active dorsiflexion and extension of the feet and ankles. These maneuvers facilitate venous return and decrease stasis.2,3
Summary
This chapter is intended to familiarize the PACU nurse with the perioperative care for the adult cardiac patient. The cardiac surgery patient has complex needs and may have resulting complications. Knowledge of the patient’s experiences before and during surgery assists the nurse after surgery to tailor the care to the patient’s needs and to strengthen the continuity of that care.
1. Stoney WS. Pioneers of cardiac surgery. Nashville: Vanderbilt University Press; 2008.
2. Lemmer JH, Vlahakes GJ. Handbook of patient care in cardiac surgery, ed 7. Philadelphia: Lippincott Williams & Wilkins; 2010.
3. Bojar RM. Manual of perioperative care in adult cardiac surgery, ed 5. Oxford: Wiley-Blackwell; 2011.
4. Seifert PC. Cardiac surgery. Rothrock JC, ed. Alexander’s care of the patient in surgery, ed 14, St. Louis: Mosby, 2011.
5. Mosca L, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women. Circulation. 123, 2011.
6. Wijns W, et al. Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J.2010;31:2501–2555.
7. Park SJ, et al. Randomized trial of stent versus bypass surgery for left main coronary artery disease. New Eng J Med. 2011;364:1718–1727.
8. Goldman S, et al. Radial artery grafts vs. saphenous vein grafts in coronary artery bypass surgery. JAMA. 2011;305(2):167–174.
9. Dacey LJ, et al. Long-term outcomes of endoscopic vein harvesting after coronary artery bypass grafting. Circulation. 2011;123:147–153.
10. Kpodonu J. Hybrid cardiovascular suite: The operating room of the future. J Card Surg.2010;25(6):704–709.
11. Zamorano JL, et al. The ESC textbook of cardiovascular imaging. New York: Springer; 2010.
12. Coeytaux RR, et al. Percutaneous heart valve replacement for aortic stenosis: State of the evidence. Ann Intern Med.2010;153:314–324.
13. Aboulhosn J, Child JS. Left ventricular outflow obstruction. Circulation. 2006;114:2412–2422.
14. Salem DN, et al. Valvular and structural heart disease: American College of Chest Physicians evidence-based practice guidelines ed 8. Chest.2008;133(6 Suppl):593S–629S.
15. Foster E. Mitral regurgitation due to degenerative mitral-valve disease. N Eng J Med. 2010;363:156–165.
16. Yosefy C, et al. Mitral regurgitation after anteroapical myocardial infarction: New mechanistic insights. Circulation.2011;123:1529–1536.
17. Darke M, et al. Rheumatic mitral and aortic stenosis: To replace or not to replace – That is the question – Part 1. J Cardiothorac Vasc Anes.2010;24(1):191–192.
18. Feldman T, et al. Percutaneous repair or surgery for mitral regurgitation. N Eng J Med. 2011;364:1395–1406.
19. Hiratzka L, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e266–e369. circ.ahajournals.org.
20. Braverman AC. Acute aortic dissection. Circulation. 2010;122:184–188.
21. Cozijnsen L, et al. What is new in dilatation of the ascending aorta? Review of current literature and practical advice for the cardiologist. Circulation. 2011;123:924–928.
22. Propper BW, Clouse D. Thoracic aortic endografting for trauma: A current appraisal. Archives of Surgery.2010;145(10):1006–1011.
23. Birks EJ, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: A prospective study. Circulation.2011;123:381–390.
24. Naidu SS. Novel percutaneous cardiac assist devices: The science of and indications for hemodynamic support. Circulation.2011;123:533–543.
25. Patel-Raman SM, Chen EA. Past, present, and future regulatory aspects of ventricular assist devices. J of Cardiovasc Trans Res. 2010;3:600–603.
26. Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010;122:173–183.
27. Davies RR, et al. Listing and transplanting adults with congenital heart disease. Circulation.2011;123:759–767.
28. Thabut G, et al. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304(1):53–60.
29. Mascia L, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation. JAMA. 2010;304(23):2620–2627.
30. Sable C, et al. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issuesa scientific statement from the American Heart Association. Circulation.2011;123:1454–1485.
31. Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation.2006;114:1645–1653.
32. Cox JL, et al. The surgical treatment of atrial fibrillation; development of a definitive surgical procedure. J Thoracic Cardiovasc Surg. 1991;101:569–583.
33. Haisaguerre M, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666.
34. Seifert PC, et al. Surgery for atrial fibrillation. AORN J. 2007;86(1):23–40.
35. Ad N, et al. The implementation of a comprehensive clinical protocol improves ling-term success after surgical treatment of atrial fibrillation. J Thorac Cardiovasc Surg. 2010;139:1146–1152.
36. Marasco SF, et al. No improvement in neurocognitive outcomes after off-pump versus on-pump coronary revascularization: a meta-analysis. Eur J Cardiotorac Surg.2008;33:961–970.
37. Speir AM, et al. Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in Virginia. Ann Thorac Surg. 2009;88:40–46.
38. Singla N, et al. Treatment of acute postoperative hypertension in cardiac surgery patients: an efficacy study of clevidipine assessing its postoperative antihypertensive effect in cardiac surgery-2 (ESCAPE-2), a randomized, double-blind, placebo controlled trial. Anesth Analg.2008;107:59–67.
39. Hajjar LA, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA.2010;304(14):1559–1567.
40. Murphy GJ, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552.
41. Khan JH, et al. Abdominal complications after heart surgery. Ann Thorac Surg. 2006;82:1796–1801.
Coulter SA. Epidemiology of cardiovascular disease in women: risk, advances, and alarms. Texas Heart Inst J.2011;38(2):145–147.
Coulter SA. Heart disease and hormones. Texas Heart Inst J. 2011;38(2):137–141.
Ferraris VA, et al. Special report: STS workforce on evidence based surgery 2011, update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. 10.1016/j.athoracsur.2010.11.078. available at
Kolh P. Guidelines on myocardial revascularization. Eur J Cardio-thorac Surg. 2010;38:S1–S52.
Moraca RJ, et al. Strategies and outcomes of cardiac surgery in Jehovah’s Witnesses. J Card Surg. 2011;26:135–143.
Rahimtoola SH. Choice of prosthetic heart valve in adults: an update. J Amer Coll Cardiol.2010;55(22):2413–2426.
Sable C, et al. on behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease: Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation.2011;123:1454–1485.