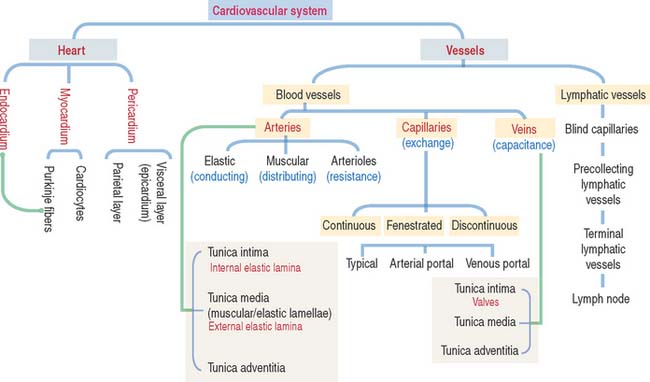
12 CARDIOVASCULAR SYSTEM
General characteristics of the cardiovascular system
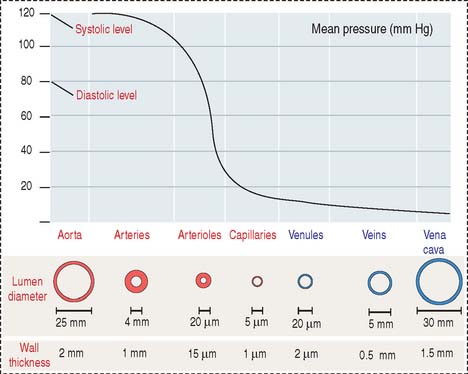
Arteries transport blood under high pressure and their muscular walls are thick (Figure 12-1). The veins are conduits for transport of the blood from tissues back to the heart. The pressure in the venous system is very low and the walls of the veins are thin.
There are variations in blood pressure in various parts of the cardiovascular system (see Figure 12-1). Because the heart pumps blood continuously in a pulsatile fashion into the aorta, the pressure in the aorta is high (about 100 mm Hg) and the arterial pressure fluctuates between a systolic level of 120 mm Hg and a diastolic level of 80 mm Hg.
HEART
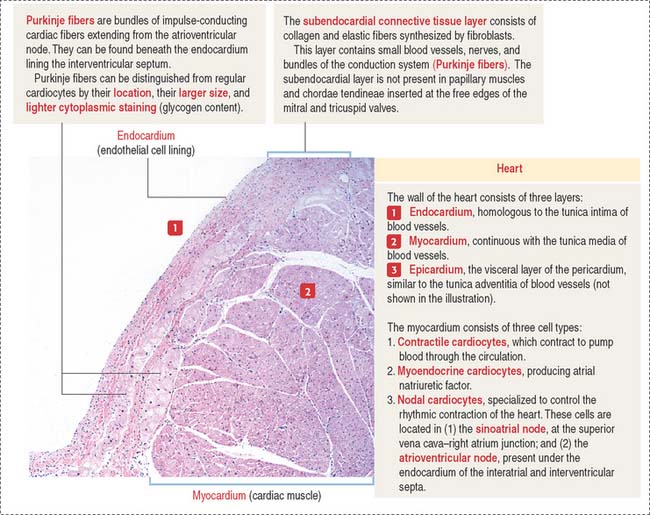
The heart is a folded endothelial tube whose wall is thickened to act as a regulated pump. The heart is the major determinant of systemic blood pressure.
The cardiac wall consists of three layers:
Conductive system of the heart
The heart has two specialized conductive systems:
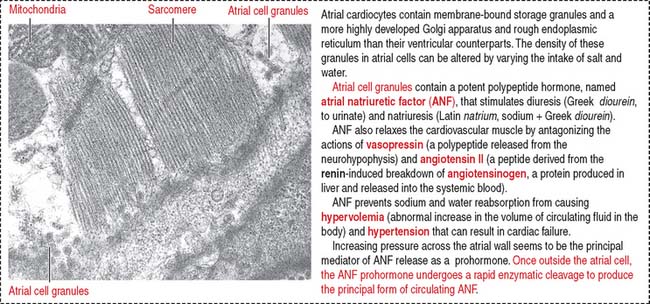
When stretched, cardiac muscle cells of the atrium (atrial cardiocytes) secrete a peptide called atrial natriuretic factor (ANF) (Figure 12-3) that stimulates both diuresis and excretion of sodium in urine (natriuresis) by increasing the glomerular filtration rate. By this mechanism, the blood volume is reduced.
Histologically (see Figure 7-18 in Chapter 7, Muscle Tissue), individual cardiac muscle cells have a central nucleus and are linked to each other by intercalated disks. The presence of gap junctions in the longitudinal segment of the intercalated disks between connected cardiac muscle cells allows free diffusion of ions and the rapid spread of the action potential from cell to cell. The electrical resistance is low because gap junctions bypass the transverse components of the intercalated disk (fasciae adherentes and desmosomes).
Differences between cardiac muscle fibers and Purkinje fibers
The Purkinje fibers lie beneath the endocardium lining the two sides of the interventricular septum (see Figure 12-2). They can be distinguished from cardiac muscle fibers because they contain a reduced number of myofibrils located at the periphery of the fiber and the diameter of the fiber is larger. In addition, they give a positive reaction for acetylcholinesterase, and they contain abundant glycogen. Purkinje fibers lose these specific characteristics when they merge with cardiac muscle fibers. Like cardiac muscle fibers, Purkinje fibers are striated and are linked to each other by atypical intercalated disks.
ARTERIES
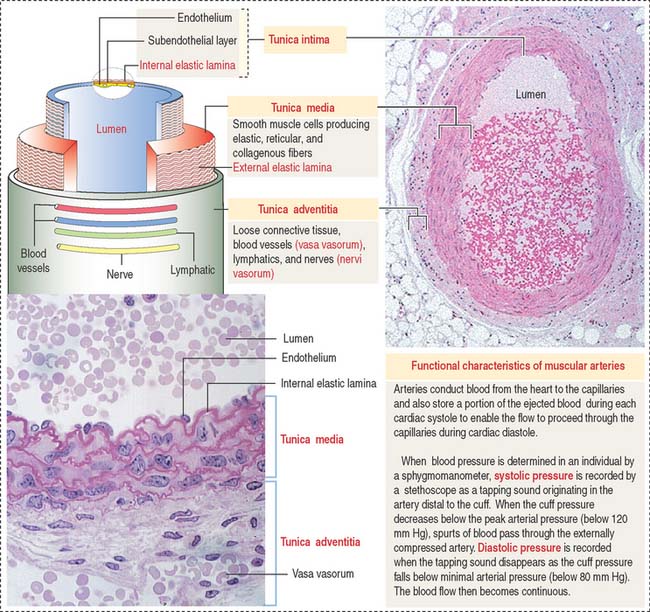
Arteries are organized in three major tunics or layers (Figure 12-4):
From the heart to the capillaries, arteries can be classified into three major groups: (1) large elastic arteries, (2) medium-sized muscular arteries (see Figure 12-4), and (3) small arteries and arterioles.
Large elastic arteries are conducting vessels
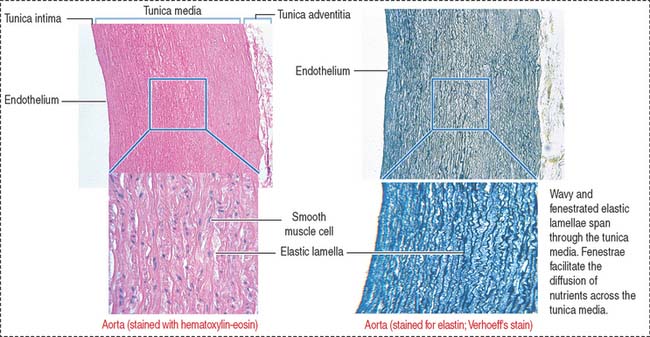
The aorta and its largest branches (the brachiocephalic, common carotid, subclavian, and common iliac arteries) are elastic arteries (Figure 12-5). They are conducting arteries because they conduct blood from the heart to the medium-sized distributing arteries.
Large amounts of fenestrated elastic sheaths are found in the tunica media, with bundles of smooth muscle cells permeating the narrow gaps between the elastic lamellae. Collagen fibers are present in all tunics, but especially in the adventitia. We have seen in Chapter 4, Connective Tissue, that smooth muscle cells can synthesize both elastic and collagen fibers. Blood vessels (vasa vasorum), nerves (nervi vasorum), and lymphatics can be recognized in the tunica adventitia of large elastic arteries.
Clinical significance: Aortic aneurysms
The two major types of aortic aneurysms are the syphilitic aneurysm (relatively rare because syphilis is no longer common) and the abdominal aneurysm. The latter is caused by a weakening of the aortic wall produced by atherosclerosis (see Figure 12-14). Aortic aneurysms generate murmurs caused by blood turbulence in the dilated aortic segment. A severe complication is rupture of the aneurysm followed by immediate death.
Marfan syndrome (see Chapter 4, Connective Tissue) is an autosomal dominant defect associated with aortic dissecting aneurysm and skeletal and ocular abnormalities due to mutations in the fibrillin 1 gene. Fibrillins are major components of the elastic fibers found in the aorta, periosteum, and suspensory ligament of the lens.
Medium-sized muscular arteries are distributing vessels
There is a gradual transition from large arteries, to medium-sized arteries, to small arteries and arterioles. Medium-sized arteries are distributing vessels, allowing a selective distribution of blood to different organs in response to functional needs. Examples of medium-sized arteries include the radial, tibial, popliteal, axillary, splenic, mesenteric, and intercostal arteries. The diameter of medium-sized muscular arteries is about 3 mm or greater.
The tunica intima consists of three layers: (1) the endothelium, (2) the sub-endothelium, and (3) the internal elastic lamina (see Figure 12-4).
Arterioles are resistance vessels
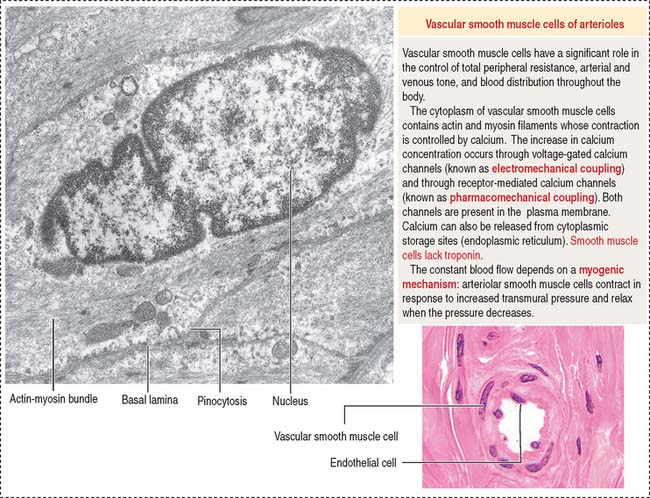
Arterioles are the final branches of the arterial system. Arterioles regulate the distribution of blood to different capillary beds by vasoconstriction and vasodilation in localized regions. Partial contraction (known as tone) of the vascular smooth muscle exists in arterioles. Arterioles are structurally adapted for vasoconstriction and vasodilation because their walls contain circularly arranged smooth muscle fibers. Arterioles are regarded as resistance vessels and are the major determinants of systemic blood pressure (Figure 12-6).
The diameter of arterioles and small arteries ranges from 20 to 130 μm. Because the lumen is small, these blood vessels can be closed down to generate high resistance to blood flow. The tunica intima has an endothelium, subendothelium, and internal elastic lamina. The tunica media consists of two to five concentric layers of smooth muscle cells. The tunica adventitia, or tunica externa, contains slight collagenous tissue, binding the vessel to its surroundings.
Capillaries are exchange vessels
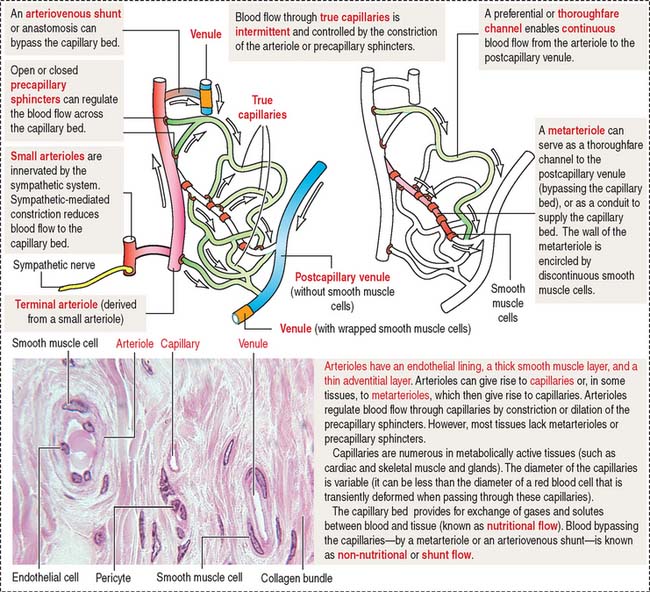
The microvascular bed, the site of the microcirculation (Figure 12-7), is composed of the terminal arteriole (and metarteriole), the capillary bed, and the postcapillary venules. The capillary bed consists of slightly large capillaries (called preferential or thoroughfare channels), where blood flow is continuous, and small capillaries, called the true capillaries, where blood flow is intermittent.
When functional demands decrease, most precapillary sphincters are closed, forcing the flow of blood into thoroughfare channels. Arteriovenous shunts, or anastomoses, are direct connections between arterioles and postcapillary venules and bypass the microvascular bed.
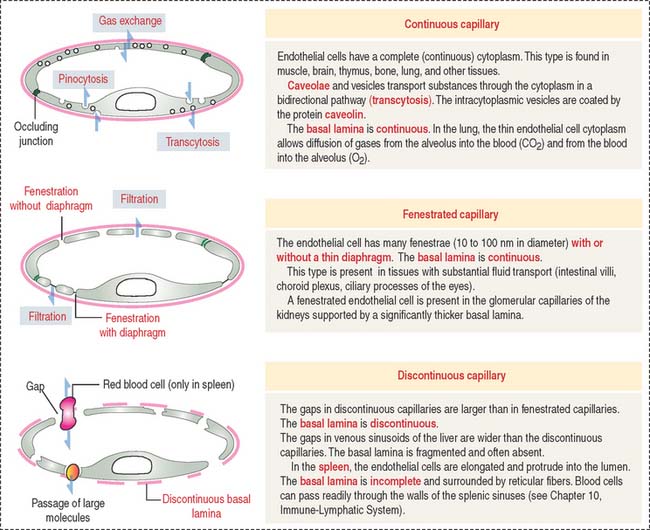
Three types of capillaries: Continuous, fenestrated, and discontinuous
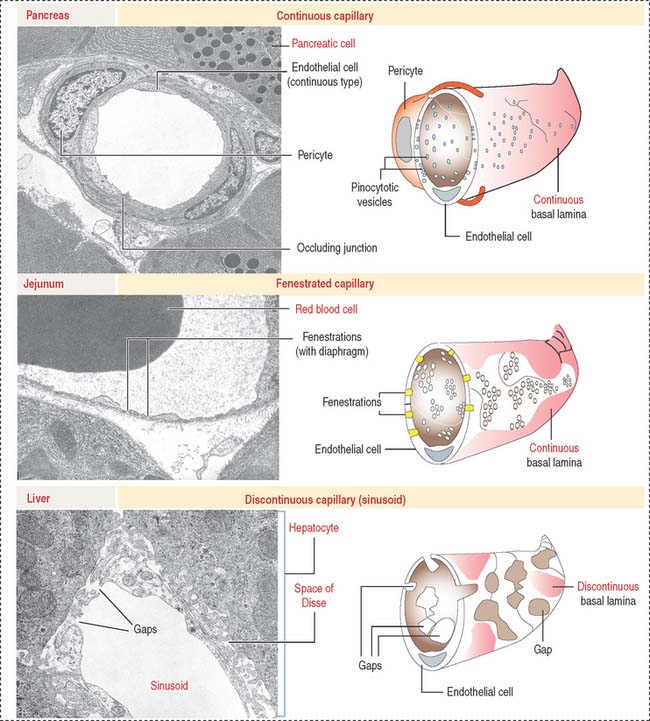
Three morphologic types of capillaries are recognized (Figures 12-8 and 12-9): continuous, fenestrated, and discontinuous (sinusoids).
Fenestrated capillaries have pores, or fenestrae, with or without diaphragms. Fenestrated capillaries with a diaphragm are found in intestines, endocrine glands, and around kidney tubules. Fenestrated capillaries without a diaphragm are characteristic of the renal glomerulus. In this particular case, the basal lamina constitutes an important permeability barrier, as we will analyze in Chapter 14, Urinary System.
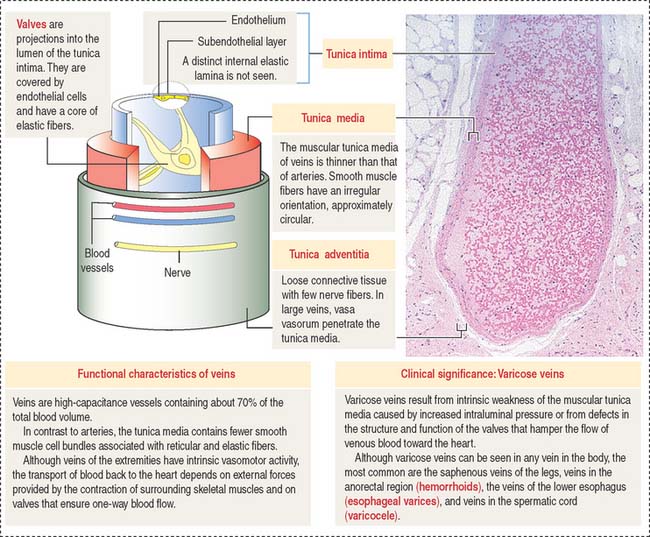
Veins are capacitance, or reservoir, vessels
In lymphatic tissues, the endothelial cells are taller. High endothelial venules are associated with the mechanism of homing of lymphocytes in lymphoid organs (see Chapter 10, Immune-Lymphatic System).
Veins have a relatively thin wall in comparison with arteries of the same size (Figure 12-10). The high capacitance of veins is attributable to the distensibility of their wall (compliance vessels) and, therefore, the content of blood is large relative to the volume of the veins. A small increase in the intraluminal pressure results in a large increase in the volume of contained blood.
The tunica adventitia consists of collagen fibers and fibroblasts with few nerve fibers. In large veins, the vasa vasorum penetrate the wall.
Lymphatic vessels
The functions of the lymphatic vascular system are to (1) conduct immune cells and lymph to lymph nodes, (2) remove excess fluid accumulated in interstitial spaces, and (3) transport chylomicrons, lipid-containing particles, through lacteal lymphatic vessels inside the intestinal villi (see Chapter 16, Lower Digestive Segment). The flow of lymph is under low pressure and unidirectional.
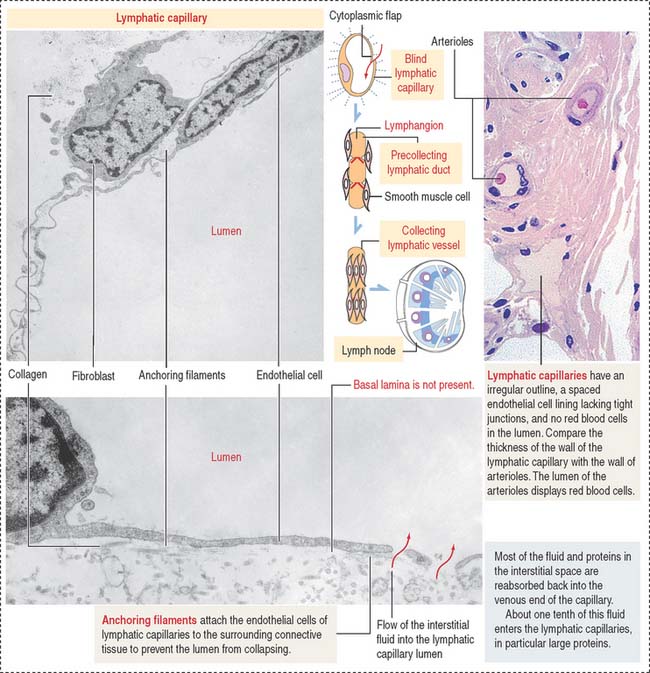
Lymphatic capillaries form networks in tissue spaces and begin as dilated tubes with closed ends (blind tubes) in proximity to blood capillaries. Lymphatic capillaries collect tissue fluid, the lymph. The wall of a lymphatic capillary consists of a single layer of endothelial cells lacking a complete basal lamina (Figure 12-11). Bundles of anchoring filaments associated to the endothelium prevent the lymphatic capillaries from collapsing during changes in interstitial pressure and enable the uptake of soluble tissue components. Lymphatic capillaries can be found in most tissues. Exceptions are cartilage, bone, epithelia, the central nervous system, bone marrow, and placenta.
The collecting vessels consist of bulblike segments separated by luminal valves. The sequential contraction of each segment, called lymphangions, propels the unidirectional flow of lymph (see Box 12-A). A collecting lymphatic vessel gives rise to terminal lymphatic vessels in the proximity of a lymph node. These terminal lymphatic vessels branch and become lymphatic afferent vessels, which penetrate the lymph node capsule and release lymph and its contents into the subcapsular sinus. Lymph nodes are distributed along the pathway of the lymph vessels to filter the lymph before reaching the thoracic and right lymphatic ducts. A total of 2 to 3 L of lymph is produced each day.
The tunica intima consists of an endothelium and a thin subendothelial layer of connective tissue.
The tunica adventitia is connective tissue with fibroelastic fibers.
Clinical significance: Edema
Edema occurs when the volume of interstitial fluid increases and exceeds the drainage capacity of the lymphatics, or lymphatic vessels become blocked. Subcutaneous tissue has the capacity to accumulate interstitial fluid and gives rise to clinical edema (see Box 12-B).
Box 12-B Lymphatic vascular disorders
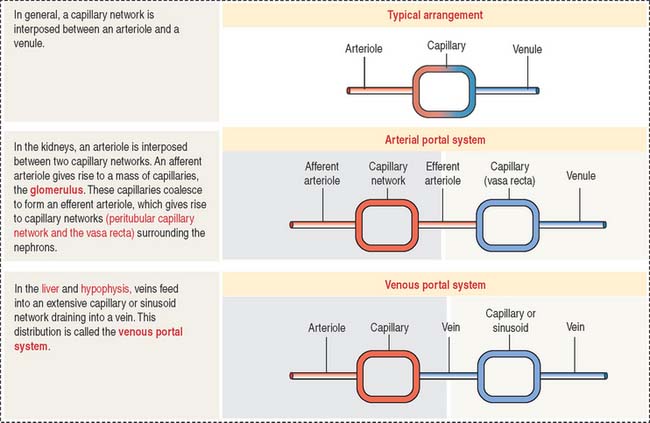
Special capillary arrangements: Glomerulus and portal systems
In general, blood from an arteriole flows into a capillary network and is drained by a venule. There are two specialized capillary systems that depart from this standard arrangement (Figure 12-12): (1) the glomerulus and (2) the portal system.
In the kidneys, an afferent arteriole drains into a capillary network called the glomerulus. The glomerular capillaries coalesce to form an efferent arteriole, which branches into another capillary network called the vasa recta. The vasa recta surround the limbs of the loop of Henle and play a significant role in the formation of urine. The glomerulus system is essential for blood filtration in the renal corpuscle (see Chapter 14, Urinary System).
Endothelial cell-mediated regulation of blood flow
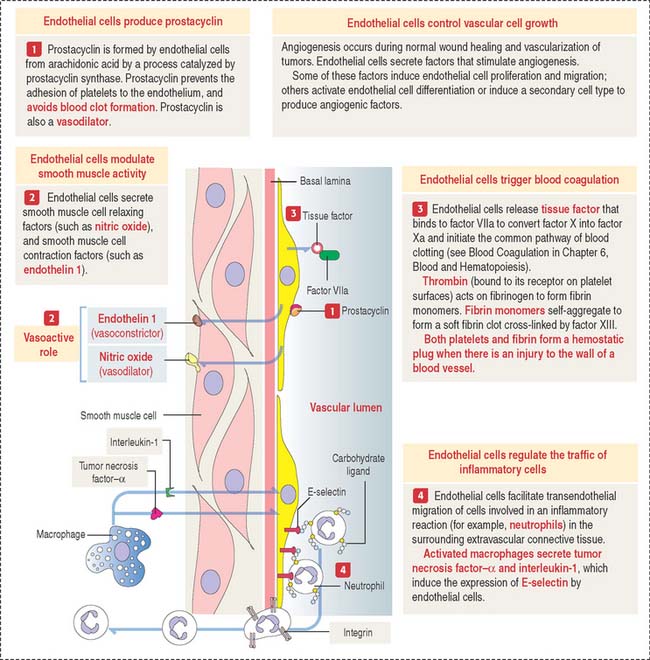
The general assumption that the endothelium is just an inert simple squamous epithelium lining blood vessels is no longer correct. In addition to enabling the passage of molecules and gases and retaining blood cells and large molecules, endothelial cells produce vasoactive substances that can induce contraction and relaxation of the smooth muscle vascular wall (Figure 12-13).
Prostacyclin, synthesized from arachidonic acid by the action of cyclooxygenase and prostacyclin synthase in endothelial cells, determines the relaxation of vascular smooth muscle cells by the action of cyclic adenosine monophosphate (cAMP). Synthetic prostacyclin is used to produce vasodilation in severe Raynaud’s phenomenon (pain and discoloration of the fingers and toes produced by vasospasm), ischemia, and in the treatment of pulmonary hypertension. Prostacyclin also prevents platelet adhesion and clumping leading to blood clotting.
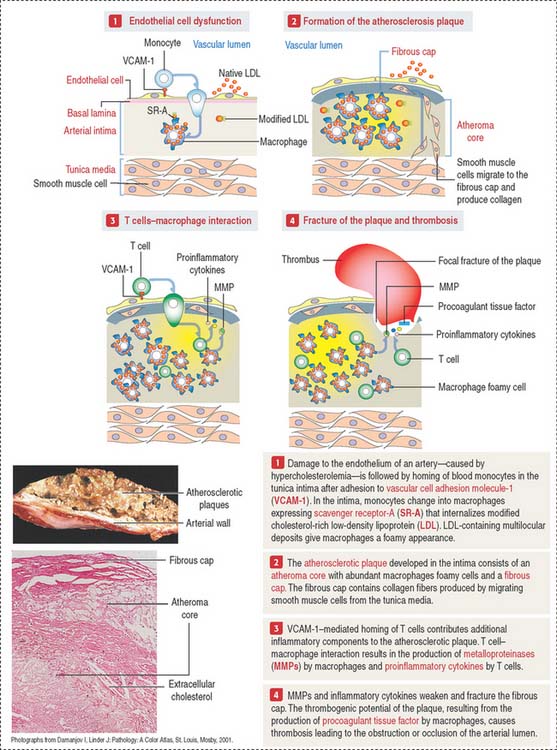
Clinical significance: Atherosclerosis
Atherosclerosis is now recognized as a chronic inflammatory disease, characterized by features of inflammation at all stages of its development (Figure 12-14). The atherosclerotic process is initiated when cholesterol-containing low-density lipoproteins (LDLs) accumulate in the intima as a consequence of endothelial cell dysfunction. A dysfunctional endothelium expresses vascular cell adhesion molecule-1 (VCAM-1) that enables monocytes to attach to the endothelial cell surface, cross the endothelium and penetrate the intima of the blood vessel. Monocytes then differentiate into macrophages expressing on their surface scavenger receptor-A (SR-A). SR-A uptakes a modified form of LDL (oxidized LDL) and the massive accumulation transforms macrophages into cholesterol-laden foam cells. Macrophage foam cells constitute the atheroma core of the atherosclerotic plaque.
Atherosclerosis correlates with the serum levels of cholesterol or low-density lipoprotein (LDL). A genetic defect in lipoprotein metabolism (familial hypercholesterolemia) is associated with atherosclerosis and myocardial infarction before patients reach 20 years of age. We discussed in Chapter 2, Epithelial Glands, that familial hypercholesterolemia is caused by defects in the LDL receptor, resulting in increasing LDL circulating levels in blood. In contrast to LDL, high-density lipoprotein (HDL) transports cholesterol to the liver for excretion in the bile (see in the gallbladder section of Chapter 17, Digestive Glands).
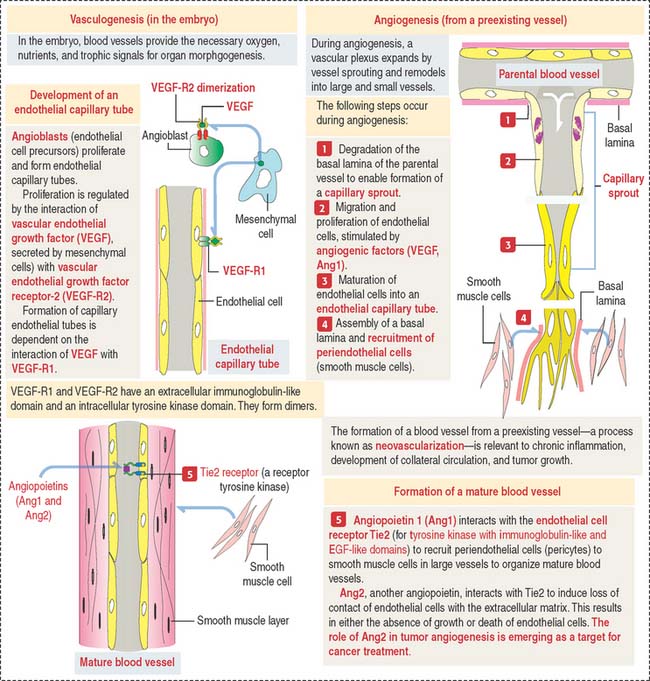
Vasculogenesis and angiogenesis
After birth, angiogenesis contributes to organ growth. In the adult, most blood vessels remain stable and angiogenesis occurs in the endometrium and ovaries during the menstrual cycle, and in the placenta during pregnancy. Under pathologic conditions, angiogenesis is excessive during malignant (see Box 12-C), ocular (age-related macular degeneration), and inflammatory conditions.
Box 12-C Kaposi sarcoma
The vascular system is formed by two processes (Figure 12-15):
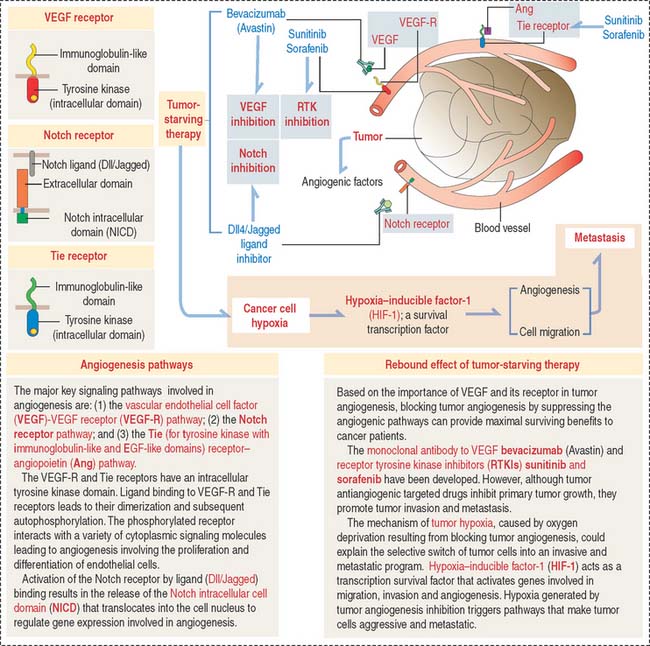
The following molecules are central to vascular morphogenesis: (1) Vascular endothelial cell factors (VEGFs), with binding affinity to two different receptors, VEGF-R1 and VEGF-R2, present on endothelial cells; (2) Tie2, a receptor tyrosine kinase that modulates a signaling cascade required for the induction or inhibition of endothelial cell proliferation. Angiopoietins 1 and 2 (Ang1 and Ang2) bind to the Tie2 receptor (for tyrosine kinase with immunoglobulin-like and EGF-like domains). Ang1 binding to Tie2 has a stabilizing effect on blood vessels (proangiogenic), whereas Ang2 has a destabilizing effect (anti-angiogenic).
The Notch receptor is a third pathway (Figure 12-16). Notch receptor signaling facilitates endothelial cell survival by activating the expression of a VEGF-R that protects endothelial cells from apoptosis. Notch receptor Delta-like ligands (Dll1, Dll3 and Dll4) and Jagged (Jagged 1 and Jagged 2) play significant roles in normal and tumoral angiogenesis by regulating the actions of VEGF.
Clinical significance: Tumor angiogenesis and tumor-starving therapy
In Chapter 4, Connective Tissue, we discussed the molecular biology of tumor invasion. We briefly mentioned that tumors secrete angiogenic factors that increase the vascularization and nutrition of an invading tumor. These angiogenic factors are similar to those produced during normal wound healing. In addition, we indicated that newly formed blood vessels facilitate the dissemination of tumor cells to distant tissues (metastasis).
Given the belief that blocking blood supply starves tumors and the importance of VEGF and its receptor and receptor tyrosine kinase inhibitors (RTKIs) in angiogenesis, tumor antiangiogenic therapeutic approaches have been developed to provide cancer patients maximal survival time. Therapy with angiogenesis inhibitors reduce tumor growth but promote tumor invasiveness and metastasis (see Figure 12-16).
How can tumor invasiveness and metastasis be explained following VEGF-targeted therapy? A possible mechanism is tumor hypoxia. Following antiangiogenic tumor treatment, a lack of oxygen supply to the tumor selects for metastasis the less sensitive cells to treatment. These cells escape the hypoxic environment leading to increasing metastasis by expressing hypoxia-inducible factor-1 (HIF-1). HIF-1 is a survival factor of cancer cells by activating transcription of genes involved in angiogenesis. We discussed in Chapter 6, Blood and Hematopiesis, the role of hypoxia-inducible factor-1α in the production of erytropoietin, a regulator of erythropoiesis, under conditions of low oxygen tension.
Cardiovascular System
Fenestrated capillaries have pores, or fenestrae, with or without diaphragms.