Cardiovascular Involvement by Systemic Diseases
These entities and the spectrum of their cardiac effects are outlined in Table 82-1. We have included both common diseases that have cardiovascular features and uncommon lesions in which cardiovascular manifestations are prominent. A small number of selected entities are discussed in the following sections. Many of the lesions outlined in Table 82-1 overlap with other chapters and thus are not specifically discussed here. Some well-known syndromes, such as Marfan syndrome, have both cardiac and other organ manifestations, but because they are covered in other chapters, further discussion is omitted here (see Chapter 79).
Toxins/Drugs
Fetal alcohol syndrome is a common disorder affecting 0.5 to 2.0 per 1000 live births. Affected infants have moderate to severe growth retardation both in the prenatal and postnatal period, along with a characteristic facies. Most children with fetal alcohol syndrome have associated neurologic problems including mental retardation and learning disabilities, as well as altered behavior.1,2
Cardiac malformations are common with fetal alcohol exposure; the most frequent is ventricular septal defect. Other cardiac anomalies include pulmonary artery hypoplasia, coarctation or interruption of the aortic arch, atrial septal defect, patent ductus arteriosus, and tetralogy of Fallot (Fig. 82-1).3
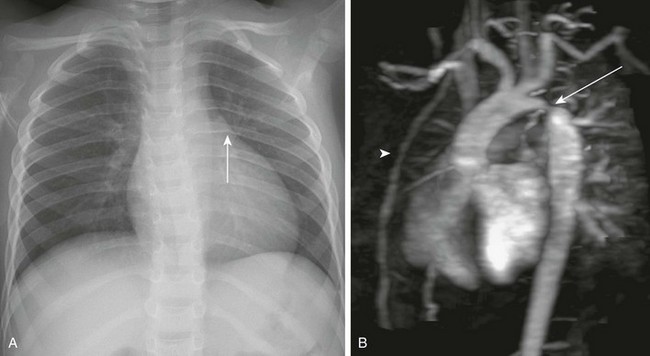
Figure 82-1 Fetal alcohol syndrome and aortic coarctation in a 4-year-old boy.
A, A chest radiograph shows a prominent aortic arch and descending aorta. Notching of the posterior left sixth rib (arrow) from an intercostal aortic collateral vessel is present. B, Oblique sagittal three-dimensional magnetic resonance angiography image demonstrates marked coarctation of the aorta (arrow) distal to the left subclavian artery. Multiple large intercostal collateral vessels are present, as well as enlarged internal mammary collaterals (arrowhead).
Infectious, Inflammatory, and Autoimmune Disorders
Overview: Acute infectious aortitis in children often is caused by bacterial septicemia originating from infected lines and intravascular devices and from valvular endocarditis or occasionally by direct spread from an adjacent infection or abscess (Fig. 82-2).4 Staphylococci and streptococci are the organisms most frequently responsible for acute infectious aortitis.4,5 Predisposing conditions include congenital heart disease and an immunocompromised state. Once they are in the bloodstream, virulent organisms may adhere to and invade the aortic wall. The resulting inflammation leads to suppurative necrosis that weakens the aortic wall and forms an aneurysm (see Fig. 82-2, A). A contained leak may lead to pseudoaneurysm formation (see Fig. 82-2, B). Staphylococcal aortitis is particularly prone to overt rupture of the aneurysm or pseudoaneurysm and is the most serious complication of infectious aortitis. Fungal agents, especially Aspergillus or Candida, also may be the causative agent of infectious aortitis, especially in immune-compromised individuals.6 Syphilitic and tuberculous aortic aneurysms are rare complications of chronic infection by those organisms and are very uncommon in children.
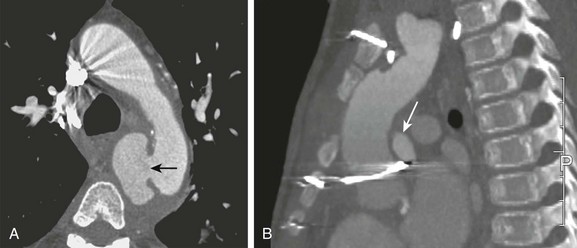
Figure 82-2 Infectious aortitis.
A, Axial image from a computed tomography angiogram (CTA) shows a thick-walled saccular aneurysm communicating with the aortic arch through a narrow opening (arrow). B, An 8-year-old child after repair of aortic coarctation and aortic stenosis with prosthetic aortic valve replacement whose postoperative course was complicated by Staphylococcus aureus mediastinitis. A sagittal maximal intensity projection image from a gated CTA demonstrates a small posterior aortic pseudoneurysm (arrow) adjacent to the aortic valve. Note diffuse anterior soft tissue edema/inflammation.
Diagnosis of infectious aortitis is difficult because many children with infected aneurysms are asymptomatic or they present with nonspecific complaints, such as fever and abdominal or back pain. Commonly used laboratory markers of infection can be normal. One adult study showed that blood cultures were negative in 28% of cases and white blood cell counts were normal in 42% of cases; however, an elevated erythrocyte sedimentation rate, a nonspecific finding of inflammation, was found in 92% of patients.5
Imaging: Few clinical studies have evaluated the imaging appearance and distribution of infected aortic aneurysms in children. Experience from adult patients suggests that these aneurysms are more often saccular (93%) than fusiform (7%) and can be distributed throughout the course of the aorta: 6% in the ascending aorta, 23% in the descending thoracic, 19% in the thoracoabdominal aorta, 10% in the juxtarenal aorta, and 32% in the infrarenal aorta.7 Periaortic fluid, stranding, or a soft tissue mass was present in 48% of patients with infectious aortitis. Periaortic gas, a specific sign, was present in only 7%. Rapid progression of aneurysm size was found in infected aneurysms in both adults and children.7 Computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) imaging have largely supplanted conventional angiography in the diagnosis of aortic aneurysms and their complications (see Fig. 82-2). Ultrasound may be an initial screening examination but usually is not definitive enough to support management decisions.
Treatment: Antibiotic treatment with the goal of eradicating the offending organism is the first step in the treatment of infectious aortitis.5 At the same time, imaging to document stability of the aortic lumen is necessary. If an aneurysm has formed, it should be surgically repaired after an adequate period of antibiotic treatment.4,5 Deployment of endovascular stent grafts in infected aortic aneurysms has been attempted.8 Although this deployment is not considered a treatment of choice, it may be useful to act as a bridge to open surgical repair, especially in the presence of low-virulence organisms or rapidly expanding aneurysms.
Takayasu Arteritis
Overview: Takayasu arteritis, also known as pulseless arteritis, is a chronic inflammatory arteritis of large vessels.9 The aorta is the artery that is most commonly involved, with the abdominal aorta involved in 59% to 75% of cases and the thoracic aorta involved in 40% to 56% of cases. Takayasu arteritis involvement is more common in the systemic arteries than in the pulmonary arteries.10 Takayasu arteritis is a rare disease, occurring in 2.6 per 1 million people in North America.11 It is more common in patients of Asian descent, and females make up 80% to 90% of patients.
Diagnosis of Takayasu arteritis is based on patient symptoms, physical findings, clinical laboratory values, serologic markers, and vascular findings.9 The American College of Rheumatology criteria include arm or leg claudication, age younger than 40 years, a blood pressure difference between extremities of greater than 10 mm Hg, subclavian or aortic bruit, decreased brachial artery pulse, and aortic or branch narrowing.12 Three of these criteria provide a diagnosis of Takayasu arteritis with a sensitivity of 90.5% and a specificity of 97.8%.11 Other clinical manifestations of Takayasu arteritis that are not involved in diagnosis include fever, headache, neurologic symptoms including stroke, postural dizziness, arthralgias, weight loss, myalgias, and systemic or pulmonary hypertension.11,13
Takayasu arteritis has a triphasic pattern: a systemic nonvascular phase, a vascular inflammatory phase, and a quiescent “burnt out” phase, although the inflammatory and fibrotic changes often overlap.10,11 In children, a long delay often occurs before Takayasu arteritis is diagnosed, especially when systemic symptoms predominate.11,13,14
The lesions of Takayasu arteritis are segmental with a patchy distribution. The vasculitis can lead to stenosis, occlusion, and aneurysm formation.10 Severe stenosis or occlusive thrombosis of the pulmonary vasculature may lead to pulmonary infarction and pulmonary hypertension.15 Cardiac symptoms include aortic regurgitation, dilated cardiomyopathy, myocarditis, pericarditis, congestive heart failure, and myocardial ischemia.
Etiology and Classification: The specific cause of Takayasu arteritis is unknown, but it is probably a T-cell–mediated autoimmune process. Infection, particularly tuberculosis, has been linked to the development of Takayasu arteritis, especially in children.13 The diseased vessel wall is thickened and shows granulomatous changes from the adventitia to the media. Giant cell (or temporal arteritis) has an identical pathologic appearance to Takayasu arteritis but affects an older population and typically involves the temporal artery.16
Takayasu arteritis currently is divided into six types depending on the location of aortic involvement. Coronary (C+) or pulmonary (P+) involvement may occur in all types (Fig. 82-3).10
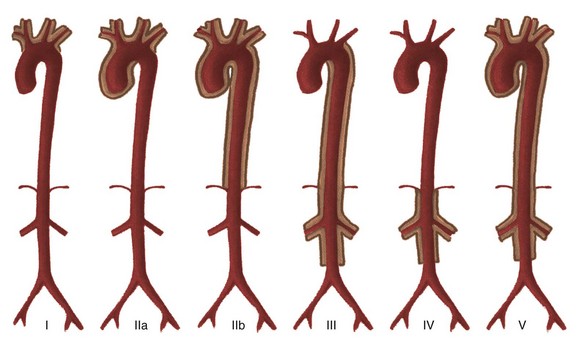
Figure 82-3 The classification schema of Takayasu arteritis.
Coronary (C+) and/or pulmonary (P+) involvement can occur in all types. Type I involves aortic arch branches only; type IIa involves the ascending aorta, arch, and branches; type IIb involves the descending thoracic aorta, with or without involvement of the ascending aorta, arch, and branches; type III involves the descending thoracic aorta, abdominal aorta, and the renal arteries; type IV involves the abdominal vessels only; and type V is generalized. (From Nastri MV, Baptista LPS, Baroni RH, et al. Gadolinium-enhanced three-dimensional MR angiography of Takayasu arteritis. Radiographics. 2004;24:773-786.)
Imaging: Vascular stenosis and aneurysm are the primary causes of mortality and morbidity and therefore should be evaluated with angiographic techniques. Conventional angiography has been the gold standard for diagnosis, but it is being supplanted by MRA (e-Fig. 82-4 and Fig. 82-5).10,11 MRA has several advantages, including its lack of ionizing radiation, its noninvasive nature, and its ability to show abnormal vascular wall signal and thickening before luminal narrowing becomes apparent.9 It also does not require iodinated contrast agents. Active inflammation is suggested when a high T2 signal is noted within the vessel wall (see Fig. 82-5). Vessel wall enhancement can be seen with the administration of gadolinium and also is used to gauge the activity of the inflammatory process (see e-Fig. 82-4).10,11,14 Inversion recovery delayed enhancement of the vessel wall also may be seen; the significance of this phenomenon is uncertain.11
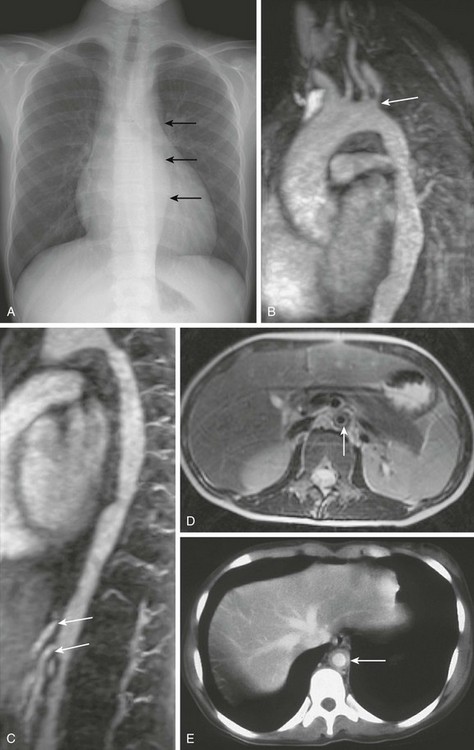
Figure 82-5 An 11-year-old girl with type V Takayasu arteritis.
A, A chest radiograph shows a heart that is top-normal in size and has an abnormal wavy contour to the descending thoracic aorta (arrows). B, An oblique sagittal thin maximal intensity projection (MIP) from a magnetic resonance angiogram (MRA) image shows four vessels arising from the aortic arch: the right brachiocephalic artery, left common carotid artery, left vertebral artery, and left subclavian artery. Stenosis is present at the origin of the left subclavian artery (arrow) along with areas of stenosis and aneurysmal dilation of the visible portions of the descending aorta. C, Thin sagittal MIP image reconstruction of the MRA shows an irregular contour and narrowing of the descending aorta, as well as at the origins of the celiac and the superior mesenteric arteries (arrows). D, A T2-weighted axial magnetic resonance image shows increased signal and thickening of the wall with a narrowed lumen (arrow) of the inframesenteric abdominal aorta. E, A contrast-enhanced computed tomography image of a different patient shows wall thickening and enhancement (arrow) of the abdominal aorta.
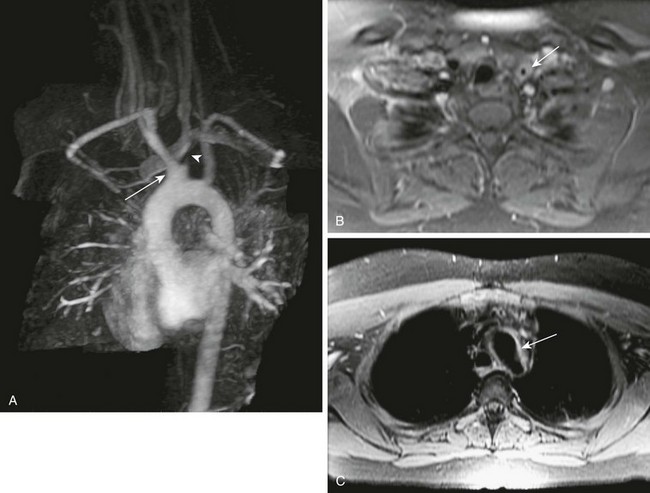
e-Figure 82-4 A 14-year-old girl with active type IIa Takayasu arteritis.
A, An oblique sagittal, thin, maximal intensity projection from a magnetic resonance (MR) angiogram shows a common origin of the left common carotid artery and the brachiocephalic artery (arrow). Severe stenosis of the left common carotid artery is present at its origin (arrowhead), with only a small amount of flow extending superiorly. B, A T1-weighted axial MR image with contrast shows thickening and enhancement of the wall of the left common carotid artery (arrow). C, A T1-weighted axial MR image with contrast shows thickening and enhancement of the wall of the aortic arch (arrow), suggestive of active inflammation.
Other imaging studies can be used to show the abnormalities of Takayasu arteritis. Ultrasound can show vessel wall thickening and CTA may demonstrate mural thickening and wall enhancement, as well as luminal dilation, narrowing, or occlusion (see Fig. 82-5).9 Fluorodeoxyglucose positron emission tomography uptake also may be useful in evaluating active inflammation.9,11
Treatment and Follow-up: Medical therapy of Takayasu arteritis initially focuses on the suppression of the immunologic responses with corticosteroids, methotrexate, azathioprine, and cyclophosphamide. In refractory Takayasu arteritis, the anti–tumor necrosis factor-α agents Etanercept and Infliximab have been used to achieve sustained remission.17 Follow-up with MRA or CTA helps document stability or progression of the vascular abnormalities and their complications.9,14
Patients with renovascular hypertension, severe coarctation of the aorta, claudication, progressive aneurysm enlargement, coronary artery disease, or cervicocranial vessel stenosis may benefit from surgical revascularization.13 Bypass grafts, either synthetic or autologous, can be used, but restenosis has occurred in up to one third of cases in which synthetic grafts were used and in approximately 10% of procedures in which an autologous vessel was used. Other postoperative complications include heart failure, intractable hypertension, and graft deterioration with pseudoaneurysm formation. Postsurgical aneurysm formation at the anastomosis is a complication seen in up to 34% of patients. Angioplasty has been used with success in patients with Takayasu arteritis who have vascular stenosis, with an initial success rate of 92% and a restenosis rate of 22%. Surgical options preferably are performed during disease remission.13
Other Vasculitides
Aneurysmal disease of the aorta, which generally afflicts older patients, is a rare complication of systemic lupus erythematosus. The exact pathophysiology of the aortic aneurysm is unknown. It may be caused directly by the systemic lupus erythematosus–related inflammation of the vessel wall or as a result of chronic steroid treatment and the resultant accelerated aortic atherosclerosis.18 Sarcoidosis has been reported as a rare cause of large-vessel vascular stenosis, and it has been recommended that children with early-onset sarcoidosis be evaluated for occlusive arterial disease.19
Other rare causes of vasculitis, especially in the pulmonary artery, include Behçet syndrome and Wegener granulomatosis.12,20 Behçet syndrome is a multisystem inflammatory disease of unknown cause. Classically, Behçet syndrome describes a combination of recurrent aphthous ulcers, genital ulceration, and uveitis. It also involves the central nervous system in 10% to 30% of cases and the vascular system, including systemic, pulmonary, and coronary vessels, in 10% to 40% of cases. Its prevalence in the United States is probably less than 5 cases per 100,000 people, but it is much more common in Turkey, with an estimated prevalence of 100 cases per 100,000 people. Although Behçet syndrome typically is an adult disease, onset in childhood is well recognized. When the pulmonary artery is affected by Behçet syndrome, the most common lesion is pulmonary artery aneurysm. Other findings include pulmonary artery stenosis and thrombosis, pulmonary infarct, and hemorrhage.21 These lesions are readily evaluated with computed tomography (CT) pulmonary angiography.20 Treatment, as for Takayasu arteritis, is focused on immunosuppression.21 Resolution of aneurysms has been observed after remission of Behçet syndrome.
Malnutrition
Cardiovascular complications occur in up to 80% of patients and are the cause of approximately one third of deaths in this disorder. The most common cardiac abnormality is decreased ventricular wall thickness caused by a loss of cardiac muscle. Other cardiac manifestations include sinus bradycardia, hypotension, arrhythmias, QT interval prolongation, and even sudden death.22 These abnormalities are reversible in the early stages of disease.22,23
Obesity
A worldwide epidemic of obesity is occurring among people of all ages.24 The Obesity Consensus Working Group reported that the prevalence of overweight status has doubled among children 6 to 11 years of age and tripled in those 12 to 17 years of age from 1980 to 2000. Approximately 15% of all 15-year-olds can be classified as obese (defined as a body mass index greater than the 95th percentile) in the United States.
Obesity in children has led to an increase in type 2 diabetes mellitus, hypertension, fatty-related liver disease, and possibly asthma. Heart disease is a significant cause of morbidity in obese persons.24 Excessive adipose accumulation induces increased blood volume and cardiac output, leading to cardiomegaly. Decreased alveolar ventilation and sleep apnea may contribute to both cardiomegaly and pulmonary hypertension (Pickwickian syndrome). Obesity and type 2 diabetes lead to the development of early atherosclerotic disease. It is uncertain whether childhood obesity increases the risk of myocardial infarction or stroke in adulthood.
The high incidence of obesity has led to a corresponding increase in the number of bariatric procedures used to treat patients, including children. Rapid loss of a large amount of weight and decreased absorption of nutrients postoperatively puts individuals at risk for nutritional deficiencies. Beriberi, a disorder of thiamine deficiency, has been reported after gastric bypass surgery.25 The wet form of beriberi is associated with cardiac failure and edema.
Endocrine Disorders
Overview: Type 1 insulin-dependent diabetes mellitus occurs relatively frequently in the United States; it develops in an estimated 3 of 1000 children by age 20 years. Cardiovascular complications are the most common causes of morbidity and mortality in persons with diabetes in childhood and are mainly the result of atherosclerotic disease.26 Persons with diabetes are more likely than nondiabetic individuals to have severe narrowing of the coronary arteries, stenosis in all three major coronary vessels, and disease in more distal segments.
Gestational Diabetes
Gestational diabetes is associated with congenital abnormalities in infants born to affected mothers. The Baltimore-Washington Infant Study found that maternal diabetes was strongly associated with early cardiovascular malformations and with cardiomyopathy.27 Early cardiovascular malformations refer to defects in early cardiac development such as laterality and cardiac looping defects, outflow tract anomalies with or without transposed great vessels, and atrioventricular septal defects. The data from this study also showed that maternal diabetes was not associated with obstructive and simple shunt defects.
Visceromegaly, hypoglycemia, and cardiomegaly occur commonly in neonates born to diabetic mothers (Fig. 82-6). The severity of these findings and of congenital heart defects may be related to how well the maternal blood sugar was controlled during pregnancy.28 Left ventricular septal wall thickening and hypertrophic subaortic stenosis are characteristic and often transient features in affected neonates (see Fig. 82-6).
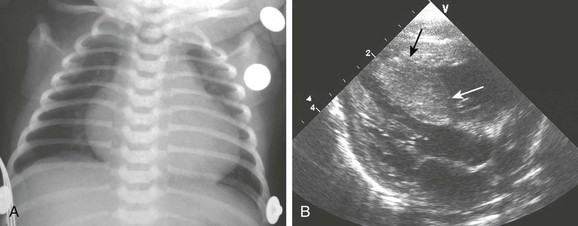
Figure 82-6 A newborn boy with a diabetic mother.
A, A chest radiograph shows an enlarged heart and mild congestive heart failure. A hypoplastic left heart malformation was diagnosed on echocardiography. B, A Long-axis echocardiogram from another patient with neonatal diabetic cardiomyopathy shows septal hypertrophy (arrows), which partially impinges on the left ventricular outflow tract. (B, Courtesy Fred Sherman, MD, Children’s Hospital of Pittsburgh.)
Blood Disorders
Overview: Sickle cell anemia, which is the most common single gene disorder of African Americans, affects 1 in 375 African Americans. Almost 1 in 12 African Americans are heterozygous for the trait. Children with sickle cell anemia constitute the largest subgroup of patients with chronic anemia in the United States. Cardiac enlargement is the most common cardiac feature related to the chronic anemia.29 Cardiomyopathy also may develop from coronary sickling and ischemia, along with cardiac dysfunction resulting from chronic transfusion and myocardial iron deposition.29,30
Imaging: Acute chest syndrome is a common cause of hospitalization, morbidity, and mortality in children with sickle cell disease (Fig. 82-7). Clinical presentation includes chest pain, leukocytosis, and fever. Typical chest radiographic manifestations include cardiomegaly, venous congestion, new radiographic opacity (atelectasis or consolidation), and pleural effusion. The inciting etiology is not always apparent, but possibilities include infection, pulmonary sickling and infarction, and fat emboli from marrow infarction. Infection appears to be a more common underlying factor in younger children, whereas infarction is more common in older persons.
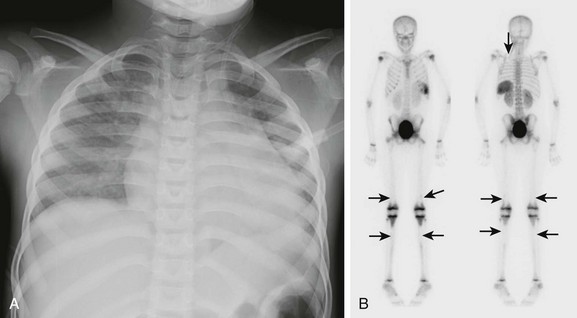
Figure 82-7 Acute chest syndrome in a patient with sickle cell disease.
A, The frontal view of the chest in a patient with sickle cell disease shows a new opacity in the left lower lobe and a mildly enlarged heart. B, Anterior and posterior projection from a technetium-99m methylene-diphosponate bone scan in a different patient shows multiple areas of decreased uptake due to infarcts (arrows) in the distal femurs, proximal tibias, and left posterior third rib.
Imaging of the chest in children with sickle cell anemia depends on the clinical scenario. Plain chest radiographs are the mainstay but can be supplemented with echocardiography, CT, MRI, or a nuclear scan for such concerns as bone infarcts or osteomyelitis, pulmonary emboli, complicated pulmonary infection, myocardial ischemia or dysfunction, and liver/myocardial iron overload related to repeated transfusion.30
Children with sickle cell disease and specific risk factors have been shown by de Montalembert and coworkers31 to be at risk for myocardial ischemia. Consequences of ischemia include include chest pain, heart failure, or a ventricular arrhythmia. Thallium-201 single-photon emission CT scans show that almost one third of symptomatic children have a fixed perfusion defect. These perfusion defects may not have a specific vascular pattern, suggesting involvement of the cardiac microcirculation.31
Thalassemia
Cardiac manifestations of thalassemia are primarily due to chronic anemia and vary with the severity of the disease.32 Thalassemia major (homozygous) has more marked hematologic, cardiac, and bone changes than thalassemia minor (heterozygous) and intermedia (the homozygous, milder form). A hyperdynamic circulation and congestive heart failure are the most common clinical cardiac findings.
Cardiomyopathy may develop as a result of ischemia or myocardial iron overload related to chronic transfusion. Widened and coarsely trabeculated rib changes are common in association with red marrow conversion. Extramedullary hematopoiesis occurs commonly in the liver and spleen as well as paraspinal soft tissues, most often in the lower thoracic region, with resultant visceromegaly and paraspinal soft tissue masses. MR imaging is useful for serial follow-up of children who undergo chronic transfusion to assess their visceral and myocardial iron burden and cardiac function (Fig. 82-8).
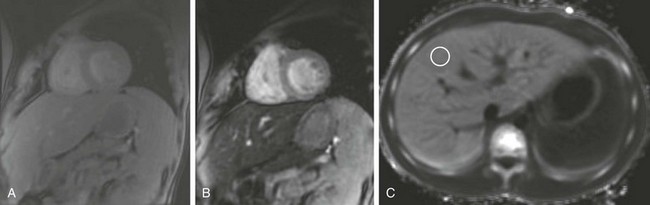
Figure 82-8 A 14-year-old boy with β-thalassemia who has received multiple transfusions.
Magnetic resonance images were obtained of the liver and heart to evaluate iron deposition. A and B, Short axis gated, multiecho gradient echo sequence with increasing echo time shows progressive darkening of the liver but not the myocardium. A, Echo time (TE) 2.2. B, TE 13.4. C, An axial R2* map of the liver that was reconstructed from multiple gradient echo images. A region of interest in the liver as shown gave an R2* reading in the liver parenchyma of 124 Hertz. T2* can be calculated as the inverse of R2*, 8.1 msec. This T2* number reflects moderate iron deposition within the liver. Note the absence of iron in the spleen (dark on this image). T2* in the myocardium was similarly calculated (not shown) to be 30 msec (within normal limits).
Other Systemic Disorders
Many other systemic disorders affect the cardiovascular system. These disorders are listed in Table 82-1 (also see e-Figs. 82-9 to 82-11).
References
Full references for this chapter can be found on www.expertconsult.com.
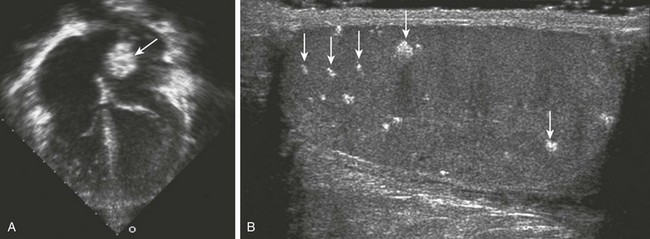
e-Figure 82-9 A 12-year-old boy with a family history of cardiac myxomas and a diagnosis of Carney complex.
A, A four-chamber view from an echocardiogram shows an echogenic mass in the left atrium (arrow) that on resection was a cardiac myxoma. B, An ultrasound of the right testis shows multiple echogenic foci with posterior acoustic shadowing (arrows). Similar findings were present on the left (not shown). Carney complex is a rare autosomal dominant disorder that has a constellation of findings, including spotty skin pigmentation, endocrine hyperactivity, and cardiac myxomas. Common endocrine gland manifestations are acromegaly, thyroid and testicular tumors, and adrenocorticotropic hormone–independent Cushing syndrome. The large-cell calcifying Sertoli cell tumor is the most common testicular tumor and can have macrocalcifications. (A, Courtesy Lizabeth Lanford, MD, Children’s Hospital of Pittsburgh.)
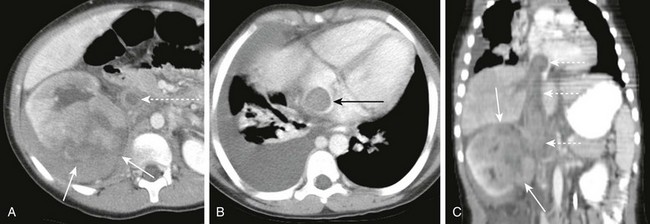
e-Figure 82-10 Wilms tumor with inferior vena cava thrombus.
A, Axial contrast-enhanced computed tomography (CECT) image of the abdomen shows a large infiltrative mass (arrows) of the right kidney. The inferior vena cava is enlarged and thrombosed (dashed arrow). B, Axial CECT of the chest at the level of the heart shows extension of the tumor thrombus (arrow) into the right atrium. C, Coronal CECT shows the infiltrative Wilms tumor (arrows) of the right kidney. The tumor thrombus (dashed arrows) extends from the insertion of the renal vein to the right atrium.
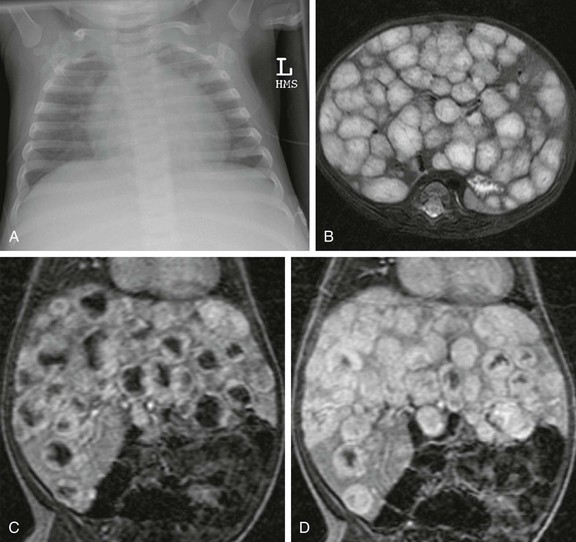
e-Figure 82-11 Infantile hepatic hemangioma.
A, A frontal radiograph of the chest shows an enlarged heart and increased pulmonary vascularity. The abdomen is not completely imaged but hepatomegaly appears to be present. B, An axial T2-weighted image of the liver shows multiple hyperintense lesions replacing the enlarged liver. C, Coronal liver acquisition with volume acquisition (LAVA) in the arterial phase of imaging shows multiple lesions with peripheral nodular enhancement. D, A coronal LAVA image in the portal venous phase of imaging shows that the multiple hepatic lesions have filled in with time, consistent with infantile hepatic hemangioma.
References
1. Bertrand, J, Floyd, LL, Weber, MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep. 2005;54(RR-11):1–14.
2. Chaudhuri, JD. Alcohol and the developing fetus—a review. Med Sci Monit. 2000;6(5):1031–1041.
3. Taybi, H, Lachman, RS. Taybi and Lachman’s radiology of syndromes, metabolic disorders, and skeletal dysplasias. Philadelphia: Mosby; 2007.
4. Barth, H, Moosdorf, R, Bauer, J, et al. Mycotic pseudoaneurysm of the aorta in children. Pediatr Cardiol. 2000;21(3):263–266.
5. Lopes, RJ, Almeida, J, Dias, PJ, et al. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488–490.
6. Knirsch, W, Cavigelli-Brunner, A, Dodge-Khatami, A, et al. Candida mediastinitis with aortic rupture after congenital heart surgery. Int J Cardiol. 2009;134(2):e76–e78.
7. Macedo, TA, Stanson, AW, Oderich, GS, et al. Infected aortic aneurysms: imaging findings. Radiology. 2004;231(1):250–257.
8. Gonzalez-Fajardo, JA, Gutierrez, V, Martin-Pedrosa, M, et al. Endovascular repair in the presence of aortic infection. Ann Vasc Surg. 2005;19(1):94–98.
9. Mason, JC. Takayasu arteritis—advances in diagnosis and management. Nat Rev Rheumatol. 2010;6(7):406–415.
10. Nastri, MV, Baptista, LP, Baroni, RH, et al. Gadolinium-enhanced three-dimensional MR angiography of Takayasu arteritis. Radiographics. 2004;24(3):773–786.
11. Tann, OR, Tulloh, RM, Hamilton, MC. Takayasu’s disease: a review. Cardiol Young. 2008;18(3):250–259.
12. Ozen, S, Ruperto, N, Dillon, MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65(7):936–941.
13. Jain, S, Sharma, N, Singh, S, et al. Takayasu arteritis in children and young Indians. Int J Cardiol. 2000;75(suppl 1):S153–S157.
14. Aluquin, VP, Albano, SA, Chan, F, et al. Magnetic resonance imaging in the diagnosis and follow up of Takayasu’s arteritis in children. Ann Rheum Dis. 2002;61(6):526–529.
15. Nakamura, T, Hayashi, S, Fukuoka, M, et al. Pulmonary infarction as the initial manifestation of Takayasu’s arteritis. Intern Med. 2006;45(11):725–728.
16. Weyand, CM, Goronzy, JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349(2):160–169.
17. Liang, P, Hoffman, GS. Advances in the medical and surgical treatment of Takayasu arteritis. Curr Opin Rheumatol. 2005;17(1):16–24.
18. Ohara, N, Miyata, T, Kurata, A, et al. Ten years’ experience of aortic aneurysm associated with systemic lupus erythematosus. Eur J Vasc Endovasc Surg. 2000;19(3):288–293.
19. Fauroux, B, Clement, A. Paediatric sarcoidosis. Paediatr Respir Rev. 2005;6(2):128–133.
20. Hiller, N, Lieberman, S, Chajek-Shaul, T, et al. Thoracic manifestations of Behçet disease at CT. Radiographics. 2004;24(3):801–808.
21. Uzun, O, Akpolat, T, Erkan, L. Pulmonary vasculitis in Behcet disease: a cumulative analysis. Chest. 2005;127(6):2243–2253.
22. Mont, L, Castro, J, Herreros, B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry. 2003;42(7):808–813.
23. Olivares, JL, Vazquez, M, Fleta, J, et al. Cardiac findings in adolescents with anorexia nervosa at diagnosis and after weight restoration. Eur J Pediatr. 2005;164(6):383–386.
24. Speiser, PW, Rudolf, MC, Anhalt, H, et al. Childhood obesity. J Clin Endocrinol Metab. 2005;90(3):1871–1887.
25. Towbin, A, Inge, TH, Garcia, VF, et al. Beriberi after gastric bypass surgery in adolescence. J Pediatr. 2004;145(2):263–267.
26. Dahl-Jorgensen, K, Larsen, JR, Hanssen, KF. Atherosclerosis in childhood and adolescent type 1 diabetes: early disease, early treatment? Diabetologia. 2005;48(8):1445–1453.
27. Loffredo, CA, Wilson, PD, Ferencz, C. Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology. 2001;64(2):98–106.
28. Lisowski, LA, Verheijen, PM, Copel, JA, et al. Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis. Herz. 2010;35(1):19–26.
29. Batra, AS, Acherman, RJ, Wong, WY, et al. Cardiac abnormalities in children with sickle cell anemia. Am J Hematol. 2002;70(4):306–312.
30. Lonergan, GJ, Cline, DB, Abbondanzo, SL. Sickle cell anemia. Radiographics. 2001;21(4):971–994.
31. de Montalembert, M, Maunoury, C, Acar, P, et al. Myocardial ischaemia in children with sickle cell disease. Arch Dis Child. 2004;89(4):359–362.
32. Ferrara, M, Matarese, SM, Borrelli, B, et al. Cardiac involvement in beta-thalassemia major and beta-thalassemia intermedia. Hemoglobin. 2004;28(2):123–129.