158 Cardiovascular and Endocrinologic Changes Associated with Pregnancy
 Cardiovascular Changes in Pregnancy
Cardiovascular Changes in Pregnancy
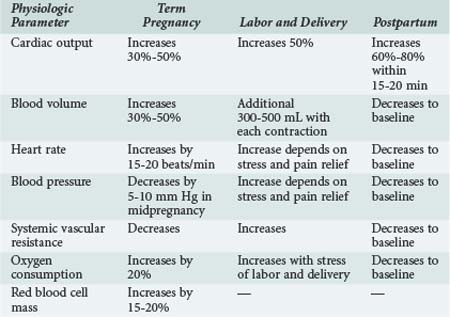
Cardiovascular and blood volume changes are among some of the more dramatic changes that occur in pregnancy (Table 158-1). These changes are primarily adaptive mechanisms, allowing the pregnant woman to accommodate her additional metabolic needs as well as those of the fetus during gestation and immediately after delivery. Cardiac output is significantly increased during pregnancy by as much as 50% compared with nonpregnant values. Cardiac output is further increased in twin pregnancies and multiple gestations.1,2 The dramatic rise in cardiac output is seen as early as the first 6 to 8 weeks of pregnancy. After the 10th week, cardiac output is increased by 1 to 1.5 L/min and reaches a maximum value by approximately the 20th to 24th week of gestation. The early increase in cardiac output is primarily due to a significant increase in stroke volume. However, stroke volume decreases as the pregnancy advances because of aortocaval compression by the uterus and the pressure of the fetal presenting part on the common iliac vein. Caval compression occurs because the large, gravid uterus rests on the vena cava, effectively decreasing venous return to the heart and therefore decreasing ventricular preload. In the latter half of pregnancy, a progressive increase in the maternal heart rate by 15 to 20 beats/min is primarily responsible for maintaining the elevated cardiac output. The additional increase in cardiac output before labor and delivery is caused by a further increase in heart rate. Resting cardiac output either is maintained or decreases slightly as term approaches.3
Influence of Body Position
Venous return is further compromised with changes in body position, particularly if the pregnant patient is supine. As a result, cardiac output can be diminished by as much as 25% to 30%. The effects of changes in body position are most obvious in the latter half of pregnancy when the fetal size and gravid uterus can effectively tamponade the vena cava. This phenomenon is exaggerated in women with poorly developed venous collaterals. With compression of the vena cava in the supine position, these women exhibit signs of severe hypoperfusion (hypotension and bradycardia), a phenomenon described as the supine hypotensive syndrome of pregnancy. Symptoms quickly resolve after the patient is repositioned to the left lateral recumbent position.4 Cardiac output can decrease by 30% to 40% in patients with this syndrome. This vasovagal phenomenon underscores the influence of maternal body position on the hemodynamic alterations occurring in pregnancy.
Hemodynamic changes associated with a decrease in preload and, subsequently, a reduced cardiac output are less pronounced when the gravid uterus is minimally compressing the vena cava. This is optimally achieved by maintaining the pregnant woman with more than 20 weeks gestation in the full left lateral position whenever she is recumbent. Alternatives to this position, less optimal than the left lateral position but preferable to the supine position, are a left lateral tilt to 15 degrees or manual displacement of the gravid uterus. The latter maneuver of left uterine displacement can be performed by manually moving the uterus away from the midline to the left side when the patient is supine. This maneuver is particularly useful when performing cardiac compressions in a pregnant patient. In the supine position, the gravid uterus, which accounts for as much as 10% of the cardiac output, hinders successful resuscitation because of its adverse effects on intrathoracic pressure and venous return. Although hemodynamics are optimized in the left lateral position, it is difficult to achieve optimal chest compressions with the patient tilted all the way into the left lateral decubitus position. Acceptable alternatives are to perform cardiac compressions with the patient supine but with concurrent manual displacement of the uterus to the other side; it is also satisfactory to place a wedge under the right hip of the patient.5,6
Oxygen Consumption and Ventricular Performance
As cardiac output progressively increases, maternal oxygen consumption also increases. However, the increase in cardiac output is seen earlier than the rise in maternal oxygen consumption. Accordingly, the arteriovenous oxygen difference actually narrows early in pregnancy. The arteriovenous oxygen difference widens at the end of gestation. By term, there is a 20% increase in maternal oxygen consumption, mostly as a result of the increase in metabolic needs of the fetus. The increase in oxygen consumption is also a result of the increased work of ventilation during pregnancy, the increase in myocardial oxygen demand, and the increase in renal oxygen consumption. Oxygen extraction also gradually increases throughout gestation. The increase in cardiac output is probably the result of a combination of factors including increased uterine blood flow, increased maternal circulating blood volume (and hence ventricular preload), and possibly estrogen- and prolactin-induced augmentation of myocardial contractility. Ventricular dynamics are improved during pregnancy as a direct result of the action of steroid hormones on the pregnant myocardium. In animal models, estrogens have been shown to increase cardiac output and decrease peripheral vascular resistance.7 Echocardiographic studies performed in healthy pregnant women have demonstrated a decrease in the pre-ejection period of left ventricular systole but an increase in the left ventricular end-diastolic dimension.8–10 It may be that a combination of improved myocardial contractility and increased ventricular diastolic area may be responsible for increases in cardiac output during normal pregnancy.11
Hemodynamic Changes during Labor and Delivery
The hemodynamic changes seen during labor and delivery are influenced by anesthetic and analgesic techniques. The increase in cardiac output is less if caudal anesthesia is used.12,13 Within the first 20 to 30 minutes after delivery of the fetus and placenta, there is an even greater increase in cardiac output, because blood is no longer diverted to the uteroplacental vascular bed. Approximately 500 mL is redirected to the maternal circulation in the so-called autotransfusion effect of pregnancy. This effect can cause cardiac output to increase by 60% to 80% after aortocaval compression is removed and blood volume is increased. Most of the physiologic changes of pregnancy resolve and revert to normal within several days after delivery. Cardiac output returns to normal within 2 weeks to 3 months after delivery as sodium and water balances normalize.
Blood Volume Changes
The changes in maternal blood volume during pregnancy are dramatic. Plasma volume increases by 30% to 50% by the end of gestation. This value is increased in the multigravida patient compared with primigravidas, but the exact mechanism responsible for this effect is unclear. The increase in blood volume can be as high as 70% with twin pregnancies. An increase of 10% to 15% in blood volume is seen as early as the seventh week of gestation. Blood volume is maximal at 30 to 34 weeks, after which the value plateaus until term.14 Ventricular filling pressures do not increase despite the large increases in plasma volume.15 This is most likely the result of concurrent decreases in systemic and pulmonary vascular resistance.
Plasma renin and aldosterone levels are elevated during pregnancy despite expansion of the maternal blood volume. Activation of the renin-angiotensin-aldosterone system may result from the concomitant decrease in peripheral vascular resistance and the increase in vascular capacitance seen as early as the first 6 weeks of pregnancy.2 Both estrogens and progesterone increase aldosterone levels, increasing sodium and water retention.16 At 12 weeks of gestation, atrial natriuretic peptide levels also increase, most likely in response to the increase in plasma volume.
Physiologic Anemia of Pregnancy
The degree of change in RBC mass during pregnancy depends in part on whether iron is supplemented. With the increase in RBC mass, there is a need for additional iron to prevent the development of iron-deficiency anemia. Maternal requirements for iron can increase to 5 to 6 mg/d. The fetus uses iron from maternal stores to prevent fetal anemia, but the presence of significant maternal iron-deficiency anemia has been shown to result in a higher incidence of fetal complications, including preterm labor and late spontaneous abortions.17
Changes in Blood Pressure and Vascular System
Arterial blood pressure decreases as early as the sixth week of pregnancy; the lowest diastolic pressures are recorded during the second trimester. By the eighth week of gestation, diastolic blood pressure decreases by approximately by 10%. Diastolic pressure reaches a nadir at 16 to 24 weeks and is typically 5 to 10 mm Hg less than normal. After the 16th gestational week, blood pressure progressively increases and is back to baseline by term. With the increase in venous return associated with uterine contractions and the additional factors of pain, anxiety, and stress during labor and delivery, an increase in blood pressure usually occurs during this time. The decrease in blood pressure during pregnancy is associated with a significant decrease in peripheral vascular resistance. The decrease in arteriolar tone is influenced by several factors, including hormonal changes that induce vasodilatation and lack of responsiveness to the pressor effect of angiotensin II.18 There is evidence for blood vessel remodeling in pregnancy, leading to increased venous compliance.19,20 During pregnancy, circulating levels of numerous endogenous procoagulant and anticoagulant proteins change, leading to a hypercoagulable state. As a consequence, the risk of venous thrombosis increases during pregnancy. The reported incidence is 0.7 cases per 1000 women, and this rate increases threefold to fourfold in the postpartum period.21
The treatment of choice for severe hypotension resulting from acute hemorrhage, sepsis, or other critical illness during pregnancy is (ideally) aggressive fluid resuscitation. However, in cases of fluid-unresponsive hypotension, vasopressors must be used to prevent detrimental consequences to both the mother and fetus as a result of inadequate uterine blood flow secondary to hypotension. Most vasopressors increase maternal blood pressure at the expense of fetal blood flow, inducing vasoconstriction of the uterine vessels. There are few human studies of these agents in pregnant women. However, animal studies animals indicate that ephedrine and dopamine increase uterine blood flow to the uteroplacental circulation while at the same time increasing maternal blood pressure.22
Structural Remodeling of the Heart
The heart is dramatically remodeled during the first few weeks of pregnancy. There is enlargement of all four chambers. The valvular annular diameters increase, as does the thickness of the left ventricular wall. End-diastolic volume increases, although end-diastolic pressure remains unchanged.10,20 Chamber enlargement, particularly of the left atrium, may be a predisposing factor for supraventricular and atrial arrhythmias. Nonspecific ST-T wave changes may also be found in asymptomatic pregnant woman.
New murmurs often appear during pregnancy. Systolic flow murmurs and a third heart sound are common but are soft. Mild pulmonic and tricuspid regurgitation occurs in more than 90% of healthy pregnant woman.23,24 One-third of pregnant women have evidence of clinically insignificant mitral regurgitation. Diastolic, pansystolic, and late systolic murmurs are rare in normal pregnancy and may indicate underlying heart disease. Bruits originating from the internal mammary artery and venous hums with diastolic components are common during pregnancy. These findings can initially confuse the diagnosis of a more serious underlying cardiac illness.
Cardiac Disease and Pregnancy
In women with significant cardiac pathology, the hemodynamic aberrations associated with pregnancy can be life threatening. The incidence of significant cardiac disease in pregnancy is less than 2% but is increasing.25,26 Advances in medical therapy and in cardiac surgery have allowed female cardiac patients to survive to childbearing age and to have successful term pregnancies.27 For women with severe cardiac problems such as pulmonary hypertension, Eisenmenger’s syndrome, severe mitral stenosis, or Marfan syndrome (in which the risk of aortic dissection is high during pregnancy), the physiologic changes of pregnancy can increase both maternal and fetal morbidity and mortality by transiently or permanently worsening the underlying heart disease.28 Increases in blood volume, stroke volume, cardiac output, and heart rate and the decrease in systemic vascular resistance are poorly tolerated by pregnant women with severe underlying cardiac disease. Maternal mortality is less than 1% for patients with less severe cardiac problems, but it increases to 50% if pregnancy is associated with the presence of underlying primary pulmonary hypertension or cyanotic disorders such as Eisenmenger’s syndrome.29,30
Approximately 90% of pregnant women with cardiac disease are rated as New York Heart Association (NYHA) functional class I or class II. These patients tolerate the hemodynamic changes of pregnancy and can be managed well with medical therapy, although the incidence of heart failure and arrhythmias tends to be higher in this group of patients.31 The 10% of pregnant patients with NYHA functional class III or IV heart disease account for 85% of cardiac deaths.32 Fetal morbidity and mortality are increased in these patients, and there is a higher incidence of prematurity, miscarriage, and intrauterine growth retardation.33 Cardiac telemetry, fetal monitoring, and hemodynamic monitoring are usually necessary for these high-risk patients during labor and delivery and, because of the large changes in intravascular volume after delivery, during the first few postpartum days.
 Endocrine and Metabolic Changes in Pregnancy
Endocrine and Metabolic Changes in Pregnancy
Hypothalamic and Pituitary Alterations
Free and bound cortisol levels are increased in pregnancy, even though circulating ACTH concentrations are elevated. These changes suggest that the normal negative feedback loop between ACTH and cortisol concentrations is altered in the pregnant state.34 Free plasma cortisol concentrations may be two to three times higher than normal at term. Diurnal variation of cortisol is blunted but maintained throughout pregnancy. The clinical signs of weakness, peripheral edema, glucose intolerance, and weight gain associated with Cushing’s disease are sometimes difficult to differentiate from the clinical features of normal gestation. The symptoms of Cushing’s disease are exacerbated by pregnancy but often resolve after delivery. Improved outcomes are seen with surgical therapy intrapartum, if pituitary or adrenal tumors are discovered during the course of the pregnancy.35,28 In normal pregnancy, cortisol release may not be suppressed with a low intravenous dose (1 mg) of dexamethasone. An 8-mg dose of dexamethasone is usually needed to suppress cortisol secretion if a tumor is present. In patients with occult adrenal insufficiency, a life-threatening adrenal crisis may be precipitated by the stress of labor and delivery. During pregnancy, the signs and symptoms may be vague and nonspecific, but with the stress of labor, these symptoms are exaggerated. The clinical diagnosis is made in conjunction with laboratory evidence of a low cortisol level or even a low-normal level and no increase in the plasma cortisol concentration with an ACTH stimulation test. Immediate treatment with stress doses of hydrocortisone is indicated in these patients.
In preparation for lactation, circulating prolactin levels progressively increase to about 10 times normal during the course of pregnancy, secondary to stimulation of the anterior pituitary by placental estrogens and progesterone. The dramatic increase in plasma prolactin concentration may lead to an increase in size of preexisting pituitary adenomas larger than 1 cm.36 Symptoms resulting from an increase in prolactin secretion usually subside within 6 weeks after delivery if the patient is not breastfeeding.
TSH secretion is transiently decreased in the first trimester, but circulating TSH concentrations are usually increased by term. Circulating levels of thyroxine (T4) and triiodothyronine (T3) increase as a result of a twofold estrogen-stimulated increase in the synthesis of thyroxine-binding globulin. Levels of free (dialyzable) T4 and free T3 are unchanged. The thyroid gland does not increase in size, despite the increase in production of thyroid hormones. Pregnant women who obtain sufficient dietary iodine (more than 200 µg daily) have no untoward complications from the changes in thyroid function.37,38
Posterior pituitary hormones are altered in pregnancy. Circulating oxytocin levels increase, but the vasopressin concentration remains essentially unchanged. Plasma osmolality decreases by 5 to 10 mOsm/kg, suggesting that the threshold for secretion of vasopressin decreases during gestation. Although vasopressin levels remain unchanged, some women develop transient diabetes insipidus during pregnancy.39
Changes in Glucose Metabolism
Pregnant women with diabetes mellitus experience more hypoglycemic episodes in the first trimester, because hepatic gluconeogenesis is decreased during this period. Insulin secretion increases during pregnancy. There is a relative state of insulin resistance, as evidenced by postprandial maternal hyperglycemia.40 Normally, women adapt to the state of relative insulin resistance during pregnancy. However, those women with marginal pancreatic reserve or preexisting insulin resistance due to obesity may not produce sufficient insulin, leading to the development of gestational diabetes mellitus. Pregnant women with preexisting diabetes mellitus require as much as 30% more insulin than before pregnancy. There is a close correlation between maternal blood glucose levels and glucose uptake and utilization by the fetus, because glucose crosses the placental barrier. Poor maternal glucose control worsens fetal morbidity. For patients with preexisting insulin-dependent diabetes mellitus, fetal and neonatal mortality rates have decreased significantly, from 65% to between 2% and 5%, as a result of implementing strict metabolic glucose control with insulin.41
Lipid metabolism is accelerated in pregnancy, and the circulating concentrations of triglycerides and cholesterol increase. Increased production of triglycerides allows for maternal consumption while sparing glucose for use by the fetus.42 Lipolysis is stimulated in adipose tissue, and there is a release of glycerol and fatty acids that decreases maternal glucose utilization, additionally sparing glucose for the fetus.
Key Points
2005 2005 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10:8: cardiac arrest associated with pregnancy. Circulation. 2005;112:IV-150.
Clark SL, Cotton DB, Lee W, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439.
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007;146:204.
Burt CC, Durbridge J. Management of cardiac disease in pregnancy. Contin Educ Anaesth Crit Care Pain. 2009;9:44.
Van De Velde M, De Buck F. Anesthesia for non-obstetric surgery in the pregnant patient. Minerva Anestesiol. 2007;73:235-240.
1 Kametas NA, McAuliffe F, Krampl E, et al. Maternal cardiac function in twin pregnancy. Obstet Gynecol. 2003;102:806.
2 Suszuki S, Shinagawa S, Chihara H, et al. Resting oxygen consumption with twin pregnancies. Arch Gynecol Obstet. 2005;271:152-153.
3 Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060.
4 McMahon MA, Fenwick A, Banks A, et al. Prevention of supine hypotensive syndrome in pregnant women undergoing computed tomography—A national survey of current practice. Radiography. 2009;15:97-100.
5 2005 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10:8: Cardiac arrest associated with pregnancy. Circulation. 2005;112:IV-150-IV-153.
6 Campbell TA, Sanson TG. Cardiac arrest and pregnancy. J Emerg Trauma Shock. 2009;2:34-42.
7 Zhang Y, Stewart KG, Davidge ST. Endogenous estrogen mediates vascular reactivity and distensibility in pregnant rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2001;280:H956-H961.
8 Campos O. Doppler echocardiography during pregnancy. Echocardiography. 1996;13:135-146.
9 Dharmarajan L, Hale TM, Velastegui Z, et al. Utility of two-dimensional echocardiography in pregnancy and post-partum period and impact on management in an inner city hospital. J Perinatal Med. 2009;37:663-668.
10 Desai DK, Moodley J, Naidoo DP. Echocardiographic assessment of cardiovascular hemodynamics in normal pregnancy. Obstet Gynecol. 2004;104:20-29.
11 Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370-1380.
12 Kuczkowski KM. The safety of anaesthetics in pregnant women. Expert Opin Drug Saf. 2006;5:251-264.
13 Van De Velde M, De Buck F. Anesthesia for non-obstetric surgery in the pregnant patient. Minerva Anestesiol. 2007;73:235-240.
14 Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393.
15 Clark SL, Cotton DB, Lee W, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439.
16 Anton L, Merrill DC, Neves LA, et al. The uterine placental bed renin-angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009;150:4316-4325.
17 Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71:1280S-1284s.
18 Pipkin FB, Baker PN. Angiotensin II has depressor effects in pregnant and nonpregnant women. Hypertension. 1997;30:1247-1252.
19 Thornburg KL, Jacobson SL, Giraud GD, et al. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24:11.
20 Poppas A, Shroff SG, Korcarz CE, et al. Serial assessment of the cardiovascular system in normal pregnancy: Role of arterial compliance and pulsatile arterial load. Circulation. 1997;95:2407.
21 Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007;146:204-210.
22 Erkinaro T, Mäkikallio K, Kavasmaa T, et al. Effects of ephedrine and phenylephrine on uterine and placental circulations and fetal outcome following fetal hypoxaemia and epidural-induced hypotension in a sheep model. Br J Anaesth. 2004;93:825-832.
23 Scirica BM, O’Gara PT. Valvular heart disease in pregnancy. Curr Cardiol Rep. 2006;8:83-89.
24 Campos O, Andrade JL, Bocanegra J, et al. Physiologic multivalvular regurgitation during pregnancy: A longitudinal Doppler echocardiographic study. Int J Cardiol. 1993;40:265.
25 Steer PJ, Gatzoulis MA, Baker P. Heart Disease and Pregnancy. London: RCOG Press; 2006.
26 Reimold SC, Rutherford JD. Valvular Heart Disease in Pregnancy. New Engl J Med. 2003;349:52-59.
27 Burt CC, Durbridge J. Management of cardiac disease in pregnancy. Contin Educ Anaesth Crit Care Pain. 2009;9:44-47.
28 Nicoletta Polli N, Giraldi FP, Cavagnini F. Cushing’s disease and pregnancy. Pituitary. 2004;7:237-241.
29 Carro-Jiménez EJ, López JE. Primary pulmonary hypertension and pregnancy. Bol Assco Med P R. 2005;97:328-333.
30 Head CEG, Thorne SA. Congenital heart disease in pregnancy. Postgrad Med J. 2005;81:292-298.
31 Gowda RM, Khan IA, Mehta NJ, et al. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol. 2003;88:129-133.
32 Ray P, Murphy GJ, Shutt LE. Recognition and management of maternal cardiac disease in pregnancy. Brit J Anaesth. 2004;93:428-439.
33 Abdel-Hady E-S, El-Shamy M, El-Rifai A-A, et al. Maternal and perinatal outcome of pregnancies complicated by cardiac disease. Int J Gynaecol Obstet. 2005;90:21-25.
34 Mastorakos G, Ilias I. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartum period: Postpartum-related disorders. Ann N Y Acad Sci. 2000;900:95.
35 Tejura H, Weinerr J, Gibby O, et al. Cushing’s syndrome in pregnancy. J Obstet Gynecol. 2005;25:713-714.
36 Bronstein MD. Prolactinomas and pregnancy. Pituitary. 2005;8:31-38.
37 Casey BM, Levenek K. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283-1292.
38 Neale D, Cootauco A, Burrow G. Thyroid disease in pregnancy. Clin Perinatol. 2007;34:543-557.
39 Hague WM. Diabetes insipidus in pregnancy. Obstet Med. 2009;2:138-141.
40 Ahn KJ. Insulin resistance in pregnancy. Korean Diabetes J. 2009;33:77-82.
41 Hod M, Damm P, Kaaja R, et al. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198:186.e1-186.e7.
42 Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynecol. 2005;89:211-215.