17 Cardiopulmonary Bypass and Management
Basic Aspects of Cardiopulmonary Bypass
Special Coagulation and Hematologic Problems
A Perspective on Blood Preservation: Cardiopulmonary Bypass in Jehovah’s Witness Patients
Phases of Cardiopulmonary Bypass
Particular Aspects of Management on Cardiopulmonary Bypass
Pre-Bypass Anesthetic Management
Anesthesia on Cardiopulmonary Bypass
Effects of Cardiopulmonary Bypass
Basic Aspects of Cardiopulmonary Bypass
The basic principles of CPB remain unchanged from when they were first introduced in the 1950s: the CPB machine assumes the functions of the heart and lungs during the time necessary to complete either an intracardiac or an extracardiac repair. A basic bypass circuit (Fig. 17-1) consists of an oxygenator, heat exchanger, and venous reservoir; pump heads for perfusion, cardiotomy suction, and cardioplegia; and appropriate tubing, cannulas, and monitoring and alarm devices.1 Major differences exist between pediatric and adult CPB, stemming from anatomic, metabolic, and physiologic differences in these age groups (Table 17-1).
TABLE 17-1 Comparison of Pediatric Versus Adult Cardiopulmonary Bypass
| Child | Adult | |
|---|---|---|
| Hemodilution | 3-15× adult | Moderate |
| Perfusion pressure | 30-40 mm Hg | Moderate (>50-80 mm Hg) |
| Wide flow rates (0-200 mL/kg/min) | Narrow range (CI 2.0-2.4 L/m2/min) | |
| Blood gas management | pH-stat (Pco2 20-80 mm Hg or greater) | α-stat (Pco2 30-45 mm Hg) |
| Cannulation techniques | Variable | Predictable |
| Aortopulmonary collaterals | Uncommon | |
| Temperature ranges | Variable | DHCA occasionally |
| Glucose management | Predictable | |
| Inotropic response | Negative | Positive |
| Perfusion circuit | Per kilogram weight | Standard |
| Parameters | Hematocrit often >55%-60% | |
| Po2 40-80 mm Hg | ± | |
| Sao2 75%-85% | ||
| Ultrafiltration (MUF/CUF) | ±Ultrafiltration |
CI, Cardiac index; CUF, conventional ultrafiltration; DHCA, deep hypothermic circulatory arrest; MUF, modified ultrafiltration.
The Circuit and Cannulas
Bypass Circuit
Technical advances in the field of oxygenator construction and size, and reduction of priming volumes to as low as 45 mL for neonatal oxygenators, have allowed marked reductions of circuit volumes over the past decade. Also, tubing sizes can be reduced to  -inch diameters, which, in combination with shorter length tubing, allows reduction of priming volumes to the range of 100 to 150 mL for neonates. A summary of the Texas Children’s Hospital sizing chart is shown in Table 17-2.
-inch diameters, which, in combination with shorter length tubing, allows reduction of priming volumes to the range of 100 to 150 mL for neonates. A summary of the Texas Children’s Hospital sizing chart is shown in Table 17-2.
TABLE 17-2 Cardiopulmonary Bypass Circuit Prime Volume and Constituents at Texas Children’s Hospital
| Patient Weight | Prime Volume | Prime Constituents |
|---|---|---|
| <8 kg | 350 mL | Whole blood or PRBC + FFP + crystalloid prime* |
| 8-15 kg | 650 mL | 100 mL albumin 25% ± PRBC + crystalloid prime* |
| 15-25 kg | 900 mL | 100 mL albumin 25% + crystalloid prime* |
| 15-25 kg | 1200 mL | Crystalloid prime* |
FFP, Fresh frozen plasma; PRBC, packaged red blood cells.
*Crystalloid prime: 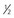 normal serum + Plasmalyte + CaCl2 + KCl.
normal serum + Plasmalyte + CaCl2 + KCl.
Heparin Coated Versus Noncoated Circuits
Young children are more susceptible to the adverse effects of CPB than adults, and the inflammatory response to CPB may have serious consequences for neonatal and pediatric patients.2,3 This is in part related to the surface area of the CPB circuit, which is large relative to the child’s blood volume when compared with an adult’s blood volume. For example, a 3-kg neonate with a blood volume of 90 mL/kg has a total blood volume of approximately 270 mL, and with an average priming volume in many centers of 350 mL, the CPB circuit volume thus causes greater than 100% dilution. A 70-kg adult with 70 mL/kg blood volume has an approximately 5000-mL blood volume, and with a CPB circuit prime of 1500 mL, this results in less than 33% dilution. Contact of blood with the surface of the circuit also plays an important role for activation of coagulation and fibrinolysis. Heparin-coated biocompatible bypass systems reduce this activation in children weighing less than 10 kg undergoing CPB.4 They also have been shown to reduce the activation of factor XII and the complement system.5,6 This results in less production of kallikrein and bradykinin, which in turn reduces the secretion of tissue plasminogen activator from endothelial cells. One study has documented more bleeding with the use of a conventional, non–heparin-coated circuit compared with a heparin-coated circuit.6 Overall, children operated on while supported with heparin-coated circuits have a significant reduction in inflammatory mediator release and fewer consequences thereof, such as prolonged postoperative ventilation and stay in the intensive care unit (ICU).7
Cardiopulmonary Bypass Pumps
The two pumps used most commonly for CPB are roller pumps and centrifugal pumps. Roller pumps have the advantages of simplicity, low cost, ease and reliability of flow calculation, and the ability to pump against high resistance without reducing flow.8 Disadvantages include the need to assess occlusiveness, spallation of the inner tubing surface (potentially producing particulate arterial emboli), capability for pumping large volumes of air, and ability to create large positive and negative pressures. Compared with roller pumps, centrifugal pumps offer the advantages of less air pumping capabilities, less ability to create large positive and negative pressures, less blood trauma, and virtually no spallation. Disadvantages of centrifugal pumps include higher cost, the lack of occlusiveness (creating the possibility of accidental patient exsanguination), and afterload-dependent flow that requires constant flow measurement. In the setting of short-term CPB for cardiac surgery, it remains uncertain whether the selection of a roller pump over a centrifugal pump, or of any specific centrifugal pump over another, has clinical importance. Pulsatile perfusion may prove to be beneficial in the future, but further outcome data and technical improvements are needed.9
Cardiopulmonary Bypass Prime
The optimal priming fluid in cardiac surgery is a topic of enduring debate. Crystalloid solutions, colloids, and mixtures of both are used. Children appear to benefit from a colloid prime. If crystalloid is used for priming, it should not contain lactate or dextrose because CPB induces a metabolic acidosis10 that has been shown to be iatrogenic and not splanchnic in origin.11 The addition of lactate to the prime increases postoperative serum lactate concentrations and should be avoided.12 Hyperchloremic metabolic acidosis is the second contributing component of a metabolic acidosis on CPB. This is often only detected by measuring the strong ion difference via the Stewart approach to the acid-base homeostasis.13 Both acidifying events are attenuated by the dilutional hypoalbuminemia induced by the administration of the pump prime. Because a hyperchloremic acidosis of a mild degree seems to be well tolerated and not associated with a poor outcome, no intervention seems necessary. Understanding the nature of CPB-associated acidosis, however, is likely to prevent unnecessary investigations or interventions.
The proportionally large volume of the bypass circuit compared with the child’s blood volume has a significant impact on the coagulation factors and cellular components. Platelet count decreases and coagulation factors, including fibrinogen, are diluted after bypass; these factors may contribute to a coagulopathy. The fibrinogen concentration at the end of bypass has been shown to correlate with the 24-hour chest drainage in children weighing less than 8 kg.14 This is seen more frequently in infants and neonates in whom an average decrease in plasma concentrations of hemostatic proteins by 56% immediately on initiation of bypass can be observed.15 Overall, younger age represents the single most important risk factor for coagulopathy and bleeding complications.16
One approach to the just-mentioned problems is the addition of whole blood to the circuit prime. Proponents cite two advantages: (1) improved hemostasis and (2) a decreased SIRS with less edema formation and less organ dysfunction. One study has disproved these perceived advantages; the investigators found that the use of fresh whole blood increased perioperative fluid requirements, leading to a longer duration of mechanical ventilation and ICU stay than in the single component group.17 The only advantage found by their study was the lesser number of donor exposures, a problem we try to overcome by matching packed red blood cells (PRBCs) and fresh frozen plasma (FFP) from the same donor.18 In addition, whole blood is frequently not available. An alternative approach is the use of FFP in the prime.19 Other investigators found that the use of FFP led to greater fibrinogen concentrations at the end of surgery. On average, children in the FFP group needed 1.3 fewer donor exposures and tended to need fewer PRBCs. The lower donor exposure was primarily because of fewer transfusions of cryoprecipitate.19 FFP may be safely substituted by 5% albumin in the prime in children with less complex repairs and acyanotic lesions.20 Whenever possible, we prefer fresh blood less than 5 days old. Fresh PRBCs are presumably more balanced metabolically than stored PRBCs; the former contain less potassium, a greater concentrations of glucose, reduced concentrations of lactate, and a greater pH.21 Also postoperative morbidity increases with increasing age of red blood cells.22 Pulmonary complications, acute renal failure, and increased infection rates were among the main complications associated with increased red blood cell storage time. As far as potassium levels and acid-base balance are concerned, PRBC priming can be safely performed with stored PRBCs if the priming solution is circulated for 20 minutes before the initiation of CPB.23
The average prime volume of the circuits we use is shown in Table 17-2. Other prime additives are heparin, antifibrinolytics, antiinflammatory agents (corticosteroids), antibiotics, vasodilators, and, sometimes, diuretics (mannitol, furosemide). At the end of the case and before separation from bypass, blood gas analysis is done to ensure that the electrolytes (including calcium and magnesium ions), glucose, and hematocrit are within a desired range. Acid-base changes and sodium concentration are corrected with sodium bicarbonate, and residual lactate is washed out with the help of the hemofiltration.
Antifibrinolytic Agents (See Chapter 18)
Aprotinin
The first use of aprotinin in pediatric cardiac surgery was reported in 199024; a high-dose regimen was administered to 28 children at increased risk of bleeding. This population included those undergoing transposition of the great arteries or reoperations, and children with endocarditis. No reduction in blood loss or drainage was observed; there were no adverse effects, and chest closure time was reduced.
Despite the high cost of aprotinin, follow-up studies have had more favorable results and its use has been shown to reduce overall costs, with decreased patient charges as a result of reduction in the number of blood products used, operative time, duration of postoperative ventilation, and hospitalization.25,26 This was confirmed in a recent comparative analysis amongst antifibrinolytic medications.27 However, this benefit was observed only in complex repairs and the use of a high-dose regimen.28 The lesser effect of a low-dose regimen may be attributable to the dilutional effects in pediatric surgery compared with the adult population.29 Pediatric lung transplantation has been studied as a potential target group for aprotinin use.30 As in most high-risk groups, a significant benefit was found for children with repeat operations (defined as repeat sternotomies or repeat transplantations), either with a high- or a low-dose regimen. This is consistent with our experience. Also, in general, infants younger than 6 months of age and those with repeat sternotomies seem to get a particular benefit from a high-dose regimen of aprotinin31 compared with reduced doses, despite greater drug costs. Economic studies have shown a cost-effective benefit of aprotinin in repeat cardiac procedures.25,26
Aprotinin seems to have an influence on the inflammatory response to CPB in children.32 Less time mechanically ventilated postoperatively33 and an improved Pao2/Fio2 (ratio of arterial oxygen concentration to the fraction of inspired oxygen, or P/F ratio) as an indicator of an attenuated reperfusion injury of the lung with the use of aprotinin have been reported.34 The clinical relevance of its antiinflammatory action remains unclear but points toward significant antiinflammatory properties.
Although a standard dosing regimen has yet to be defined in children, pediatric studies have demonstrated decreases in operative time post CPB, exposure to donor blood, and postoperative chest tube drainage with the use of aprotinin.33 In vitro plasma concentrations of aprotinin have been related to antifibrinolytic and antiinflammatory activity at concentrations of 50 to 125 kallikrein inhibitor units (KIU)/mL and 200 KIU/mL, respectively.25,35,36 Anaphylactic and anaphylactoid reactions may occur with aprotinin, and a test dose should be given before administration of the loading dose or addition of aprotinin to the CPB circuit. In a retrospective review of 681 children, reactions occurred in 1% of first exposures, 1.3% of second exposures, and 2.9% of more frequent exposures.37
We used aprotinin for complex neonatal repairs, such as arterial switch operations or Norwood procedures, as well as for most reoperative procedures and organ transplantations.38,39 Because of safety concerns in adults, the drug is currently unavailable in the USA and Europe. The decision to withdraw the drug was based on data that were obtained entirely from adults, who present with a different profile of complications following cardiac surgery than do children. Aprotinin has been shown to be safe and efficient in the neonate.40 Furthermore, serious questions have been raised regarding the statistical method used in the sentinel study that questioned the safety of aprotinin. Aprotinin continues to be used in Australia and New Zealand and has been reintroduced for adult coronary artery bypass graft surgery in Canada. Currently, the FDA in the United States is reevaluating the role of aprotinin in anesthesia. Our dosing regimen is weight based, with 60,000 KIU/kg as a loading dose and in the pump prime. Aprotinin is started before the incision and blood levels are maintained with a continuous infusion of 7000 KIU/kg/hour; this is discontinued just before leaving the operating room. Regimens based on body surface area are also used, along with a CPB prime dose based on priming volume designed to achieve a plasma level above 200 KIU/mL. An example is 0.85 to 1.7 × 106 KIU/m2 loading dose, and into the CPB prime, and 2.0 to 4.0 × 105 KIU/m2/hour infusion.25
The Lysine Analogues: Aminocaproic Acid and Tranexamic Acid
Despite meticulous surgical technique, it is still frequently difficult to achieve adequate hemostasis after CPB, particularly in neonates. ε-Aminocaproic acid (EACA) and tranexamic acid (TXA) are analogs of the amino acid lysine. They exert their antifibrinolytic effect by interfering with the binding of plasminogen to fibrin, thereby preventing the activation of the active plasmin. TXA may also improve hemostasis by preventing plasmin-induced platelet activation. Both TXA and EACA exercise some antiinflammatory properties but not to the same extent as aprotinin. In one study, the use of EACA reduced bleeding postoperatively in 25 of 71 children undergoing cardiac surgery on CPB and was found to benefit only the children with cyanotic heart disease.41 The EACA loading dose was 75 mg/kg followed by an infusion of 15 mg/kg/hour; an additional 75 mg/kg was added to the CPB prime. Because the effective dose of EACA is unknown, another study used a regimen of 150 mg/kg bolus followed by an infusion of 30 mg/kg/hour of EACA. Intraoperative blood loss was reduced, but postoperative blood loss was not different between treatment arm and placebo.42 Blood coagulation measured with a thromboelastograph showed less fibrinolysis with EACA.
EACA compares favorably with TXA; a beneficial effect has been reported only in children with cyanotic heart disease.43 Those with acyanotic defects or undergoing repeat sternotomies had no benefit from TXA. Their dosing regimen, however, was only 50 mg/kg as a single bolus before incision. In children, the TXA plasma concentration between the post bolus peak and the end of CPB has an 80% decline when a continuous infusion is not used.44
In conclusion, though less efficient than aprotinin, TXA and EACA are equally effective in reducing perioperative blood loss in pediatric cardiac surgery.45 Given their safety profile, they may be even more appealing in the future. Further studies are needed to delineate their pharmacokinetic profiles and their efficacy. We use EACA based on simulation results from a study by Ririe and coworkers.46 An initial loading dose of 75 mg/kg over 10 minutes and a maintenance infusion rate of 75 mg/kg/hour is used with 75 mg/kg placed in the pump to maintain serum concentrations above the therapeutic concentration (assumed to be 130 µg/mL) in more than 95% of children.
Special Coagulation and Hematologic Problems
Heparin-Induced Thrombocytopenia
The use of unfractionated heparin for anticoagulation for CPB in adults produces antiheparin antibodies in 25% to 50% of patients within 10 days postoperatively. In a small minority of these patients, high-titer IgG platelet-activating antibodies form and make immune complexes with heparin and platelet factor 4 (PF4).47 This results in activation of platelets (via their Fc receptors) and formation of procoagulant platelet microparticles, leading to thrombin generation and thrombosis. Thus, the major problem in heparin-induced thrombocytopenia (HIT) is thrombocytopenia several days after heparin exposure accompanied by thrombosis, often in major vessels or structures. HIT appears to be less common, of milder course, and probably underrecognized in neonates and children. About 1% of children exposed to CPB have PF4 antibodies when tested before their second CPB, and actual HIT is much less common.48 When HIT is suspected, either PF4 enzyme-linked immunosorbent assay or a functional assay for HIT can be used to make the diagnosis; if positive, no further heparin should be given. If CPB is necessary, alternatives, such as the direct thrombin inhibitors argatroban, lepirudin, and bivalirudin, may be used. None of these agents is approved for use in children for anticoagulation for CPB, but case reports and small series have documented their successful use when HIT is diagnosed.49–51 The partial thromboplastin time (PTT), activated clotting time (ACT), and a specialized clotting time called the ecarin clotting time can be used to follow anticoagulation with these agents, but there is no reversal agent for them. Thus, treatment of post-CPB bleeding involves administration of blood products and coagulation factors as well as recombinant factor VIIa (see later discussion).
Antithrombin III Deficiency
Heparin produces anticoagulation by combining in a 1 : 1 ratio with antithrombin III (ATIII), which then binds to and inhibits thrombin, leading to anticoagulation. Of adult patients, 4% to 13% have a resistance to normal doses of heparin for CPB; most instances occur because of a partial deficiency of ATIII, rendering heparin less effective at producing anticoagulation.52 In children this is often unknown, and the first suspicion of ATIII deficiency may occur when the standard heparin dose of 300 to 400 units/kg fails to adequately anticoagulate before CPB, that is, the ACT remains less than 300 seconds. The usual response is to apply another dose of heparin from a different vial and remeasure the ACT, but if the ACT is still not adequately prolonged, a diagnosis of ATIII deficiency may be suspected. Infants less than 6 months of age and children with congenital heart disease have decreased ATIII concentrations.53 Therefore, heparin may not achieve adequate anticoagulation, and disorders in hemostasis and thrombosis and an exaggerated inflammatory response may occur. In this case, blood can be sent for ATIII levels, but to proceed with CPB, the ATIII must be increased. This can be accomplished in two ways: (1) by supplementing ATIII with recombinant ATIII, 75 units/kg, and ensuring that the ACT is adequately prolonged before proceeding with CPB, or (2) by adding FFP (which has ample levels of ATIII) to the CPB prime or administering it to the child.52,54
Recombinant Factor VIIa for Massive Hemorrhage
Recombinant factor VIIa (rFVIIa) was originally approved for use in patients with hemophilia who possess inhibitors to factors VIII or IX, and was shown to be effective at treating bleeding in these patients at doses of 90 µg/kg (see Chapter 10).55 Endogenous factor VII circulates at low concentrations in the plasma. At a site of tissue or blood vessel injury, tissue factor (TF) is exposed, and the extrinsic coagulation pathway is activated by the binding of factor VII to TF, resulting in the activation of factor X to factor Xa, leading to the generation of thrombin from prothrombin, with further activation of platelets and the coagulation cascade.56 High concentrations of rFVIIa result in major activation of the extrinsic pathway at the site of injury, theoretically without resulting in systemic hypercoagulability. However, thrombotic complications are increased after its use. rFVIIa also activates platelets, adding to the potential benefit of this agent in significant hemorrhage. Thus, this therapy seems attractive for the treatment of surgical bleeding. A recent analysis of factor VII concentrations during pediatric cardiac surgery recommended that it be used to treat postoperative coagulopathies that were resistant to conventional therapy, when no identifiable surgical cause of bleeding could be determined.57
Sickle Cell Disease
Sickle cell disease (SCD), one of the most common hemoglobinopathies among patients of African-American or West Indian origin (with a prevalence of 0.2% to 0.3% in that population), results from the substitution of valine for glutamic acid in position 6 of the β-hemoglobin chain. Normal adult hemoglobin is referred to as HbA, whereas hemoglobin containing the mutant β-hemoglobin chains is referred to as HbS. SCD is represented by a homozygous genotype (HbSS) with fractional concentrations of HbS in the range from 70% to 90%. Sickle cell trait, on the other hand, is a heterozygous manifestation (HbAS) with a prevalence of 8% to 10% in the same population. The definitive diagnosis of any sickle cell hemoglobinopathy is confirmed by hemoglobin electrophoresis (see Chapter 9).
Children with SCD are at a particular risk for perioperative complications.58,59 Sickling can be triggered by hypoxia, dehydration, acidosis,60 hypothermia, stress, and infections. Hypoxia induces opening of a Ca2+-activated K+ channel (Gardos channel) that causes intracellular dehydration.61 Chain formation occurs and leads to increased blood viscosity with vaso-occlusion. Opening of the Gardos channel is an important mechanism of sickle cell dehydration, which is temperature dependent, with greater potassium efflux at lower temperatures.62 Shrinkage of sickle erythrocytes may also result from activation of a K+/Cl− cotransport pathway under acidotic conditions.63 Activation of this pathway can be blocked by increasing the abnormally low level of intracellular magnesium in sickle erythrocytes. The use of magnesium and hydroxyurea in the perioperative period therefore seems to be beneficial.64
CPB, particularly for more complex surgical procedures, may involve periods of low flow or even circulatory arrest, as well as hypothermia with consequent local vasoconstriction, hypoxemia, and acidosis. There is some evidence that CPB can be safely undertaken in SCD.65 Flow conditions are an important determinant of sickle erythrocyte adherence to endothelium. Under low-flow conditions sickle cell adhesion to endothelium increases with contact time in the absence of endothelium activation or adhesive proteins, whereas under venular flow conditions sickle cell adhesion occurs only after endothelial activation. During CPB, both low-flow conditions and endothelial activation may occur. Multiple triggers of sickling are likely to occur during CPB, and close attention should be paid to the conduct of all aspects of bypass.
In the past, routine exchange transfusion has been recommended to prevent these complications.66 More recent experience provides evidence that not all children require an exchange transfusion.67 The growing evidence of the harmful effects of blood transfusion adds to the need to carefully reconsider routine exchange transfusion.68 For uncomplicated bypass surgery without periods of cardiac arrest, the omission of exchange transfusion has led to good outcomes.
Guidelines have been proposed for the perioperative management of children with sickle cell disorders.67 It is essential to avoid hypothermia using tepid or warm CPB in its stead; blood transfusion only for a decrease in hematocrit to less than 20%; maintenance of intravascular volume and body temperature while on CPB; the avoidance of vasopressors; the use of postoperative multimodal pain therapy; and early incentive spirometry to prevent pulmonary complications.69 In our practice, we utilize cerebral near-infrared spectroscopy (NIRS) to help determine an acceptable hematocrit for the individual child.
For children undergoing hypothermia, successful management with70 and without71 partial or complete exchange transfusion on bypass has been reported. Exchange transfusion can be performed preoperatively or on initiation of CPB.72 For exchange transfusion during CPB, the extracorporeal circuit is primed with blood and the usual components. When CPB is commenced, the child’s blood volume is drained into storage bags and separated. The platelet-rich plasma is reinfused at the end of CPB, and the concentrated sickle cells are discarded. Platelet and plasma sequestration in conjunction with exchange transfusion reduces the need for postoperative transfusion and protects the platelets from the negative effects of CPB.73
There seems to be no consensus as to a suitable target HbS level. Reducing the absolute level of HbS may be of greater benefit than achieving a particular ratio of HbA to HbS because the remaining sickle-prone cells are still at risk for sickling.74 In SCD, exchange transfusion has been shown to favorably affect cerebral tissue oxygenation.75 Exchange transfusion will decrease both the proportion and absolute amount of HbS, but it does not remove every cell that may sickle; it may also have favorable effects on hypoxic pulmonary vasoconstriction.75 In this context, these children may benefit from continuous hemofiltration to reduce inflammatory mediators and improve pulmonary recovery.76 Inhaled nitric oxide also has been suggested as an adjunct for the prevention of sickle cell crisis. It may improve the binding of oxygen, thereby reducing the formation of sickle cells; reduce pulmonary hypertension; and improve pulmonary function without adverse effects on normal hemoglobin.77
A Perspective on Blood Preservation: Cardiopulmonary Bypass in Jehovah’s Witness Patients
Acute isovolumic reduction of hemoglobin down to 5 g/dL is tolerated in healthy individuals under anesthesia and does not appear to reduce tissue oxygenation significantly.78 Reduction of oxygen delivery to 7 to 8 mL/kg/min under resting conditions does not lead to an oxygen debt and is compensated by increased extraction, an increase in cardiac index, and a subsequent decrease in systemic vascular resistance.79,80 In a retrospective study of the tolerance of reduced hemoglobin concentration in Jehovah’s Witness patients, the hemoglobin concentration of those who died was less than 5 g/dL.81 A safe limit of hemodilution in children has not been established. One report showed that hemodilution up to 50% in acyanotic children appears to be safe.82 In cyanotic children, however, the limit was estimated to be around 40%. If this level of hemodilution is exceeded, hemodynamic instability and inadequate oxygen transfer can occur. Evidence suggests that hematocrit levels of 21.5% in infants on CPB leads to significantly worse psychomotor developmental outcome, compared with 27.8%.83
The most important and simplest way to avoid transfusion in the setting of cardiac surgery is to limit blood loss. Unnecessary and reduced amounts of blood sampling help to preserve blood.84 Pharmacologic agents, such as aprotinin and tranexamic acid, reduce the risk of perioperative blood loss.85 Hormonal stimulation of erythropoiesis with preoperative recombinant erythropoietin is another strategy acceptable to Jehovah’s Witnesses. The administration of erythropoietin in the cardiac surgery setting has been shown to reduce the risk of exposure to allergenic blood.86 However, the associated increase in hematocrit with erythropoietin use may be potentially thrombogenic and could lead to an increase in the incidence of perioperative venous thromboembolism. The cost of using erythropoietin can be large, and cost analysis suggested that its use in cardiac surgery was not cost effective.87
Intraoperative recovery of blood with a cell salvage device is also acceptable to many Jehovah’s Witnesses. This involves the removal by suction of blood from the operative field followed by washing, filtering, and return of red blood cells to the patient. A randomized controlled trial of intraoperative cell salvage in cardiothoracic surgery has demonstrated a reduction in red blood cell transfusion and an increase in postoperative hemoglobin.88
Acute normovolemic hemodilution involves the preoperative removal of a volume of blood from the patient with the simultaneous administration of crystalloid or colloid to maintain circulating volume.89 The collected blood is then reinfused during the operation. Some Jehovah’s Witnesses find this process acceptable, especially if the access line is maintained in continuity with the patient. Acute normovolemic hemodilution has other advantages, including lower costs, because the blood does not need compatibility testing; reduced possibility of administrative error; and a saving in patient time (see Chapter 10). The development of artificial red cell substitutes could potentially abrogate the need for compatibility testing, as well as vastly reduce infection risks, with none of the immunomodulatory side effects of allogeneic blood.90 Some of these products would also be acceptable to Jehovah’s Witness families. Substitutes include perfluorocarbons, hemoglobin solutions, intramolecular cross-linked hemoglobin, and liposome encapsulated hemoglobin. None of these has reached clinical practice. Lastly, autologous retrograde priming has been used in Jehovah’s Witness patients and can further reduce the hemodilutional effects of the prime.89,91
In the great majority of adult patients, open-heart surgery can be performed without administration of blood or blood components. In children and small infants weighing less than 5 kg, bloodless open-heart surgery is more complicated. Preoperative iron supplementation (6 mg/kg/day) and erythropoietin (200 to 400 U/kg/wk) have been used successfully to augment preoperative hemoglobin levels.92
Postoperative care involves minimal blood sampling, and only on special indications. Noninvasive monitoring allows uncomplicated weaning from the ventilator.93 The first report of successful outcomes in Jehovah’s Witness children with congenital cardiac defects was in 198594; 110 children older than 6 months of age successfully underwent operation, with a perioperative mortality rate of 5.3%. Only one death was attributed to blood loss. A weight less than 5 kg is considered by some as a contraindication for open-heart surgery and palliative procedures were advocated in the past.95 For some lesions, however, no palliation is possible. The development of miniaturized circuits, preoperative optimization, use of high-dose aprotinin, vacuum-assisted drainage to allow smaller tubing and cannula sizes, as well as the use of modified ultrafiltration, enabled the safe expansion of surgery into the neonatal population. Individualized heparin level–based anticoagulation management further results in a reduction of coagulation problems, blood loss, and transfusion requirements.96 The addition of desmopressin, 0.3 µg/kg, also not proven, is thought by some to improve platelet activity and stimulate the release of von Willebrand factor after protamine infusion.
Myocardial Protection
Myocardial protection during cardiac surgery has evolved over the years. Melrose and colleagues introduced the concept of chemical cardioplegia in 1955.97 Before the popular use of chemical cardioplegia, topical cardiac hypothermia was used. In the late 1970s and early 1980s, the concept of cold hyperkalemic blood cardioplegia was introduced.98 Potassium concentrations in cardioplegic solutions ranging from 12 to 30 mEq/L are typically used to achieve cardiac standstill within 1 to 2 minutes under hypothermic conditions, with higher concentrations (or longer induction times) required for normothermic conditions. Myocardial edema after bypass and global ischemia can be reduced by a number of strategies that involve modifying the conditions of delivery and composition of cardioplegia solutions as they affect the movement of intracellular and interstitial fluid. In contrast to studies in adults, most studies conducted in neonates have shown little difference between blood and crystalloid cardioplegia.99,100 Hypothermia also decreases myocardial oxygen consumption. The benefits of this approach appear to be optimal at myocardial temperatures between 24° C and 28° C. However, there is growing evidence that warm, intermittent blood cardioplegia may be advantageous to either cold crystalloid or cold blood cardioplegia.101 The benefits of blood cardioplegia are more pronounced in younger, cyanotic children who require longer aortic cross-clamping. For acyanotic children, the cardioplegic technique is probably not as critical.102 Avoidance or reduction of myocardial edema occurs by limiting the pressure of cardioplegia infusions and by providing moderately hyperosmolar cardioplegia solutions that contain blood. Buffering the acidosis that results from ischemia is achieved by including tromethamine, histidine-imidazole, or both in the cardioplegia solution. Close management of myocardial calcium balance to avoid extremes of intracellular hypercalcemia or hypocalcemia, especially during reperfusion, is very important.103,104 The addition of magnesium may solve this dilemma by preventing damage from higher cardioplegic calcium concentrations by its action as a calcium antagonist.104,105 This prevents mitochondrial calcium overload as a consequence of reperfusion injury. Magnesium also prevents the influx of sodium into the postischemic myocardium, which is exchanged for calcium during reperfusion.
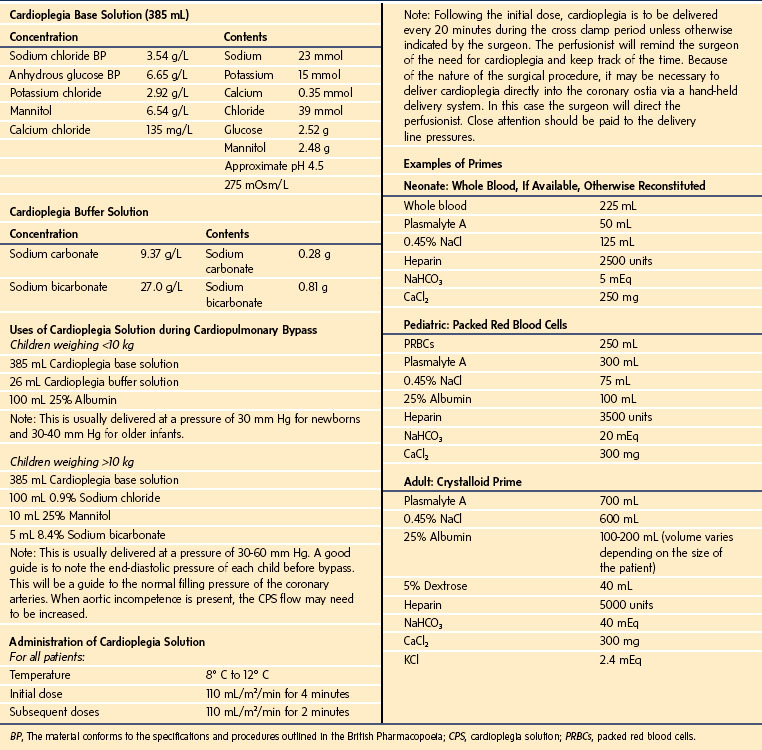
Every cardiac program has its own philosophy regarding cardioplegia and myocardial protection. At Texas Children’s Hospital, plain crystalloid cardioplegia is used. The prime blood gas and electrolytes should mimic physiologically the child’s arterial blood gas as closely as possible. If whole blood or packed cells are added to the prime, the target hemodilution range should be 28% to 30%; the prime should be recirculated continuously and warmed between 35.0° C and 36.5° C before initiation of bypass. In neonates and infants, albumin is added to the cardioplegic solution to maintain an appropriate colloid osmotic pressure. This may decrease edema formation of the arrested heart. In children undergoing circulatory arrest, long cross clamp times, and large pump suction return cases, 20 mg/kg methylprednisolone is used, up to a maximum of 500 milligrams, to reduce the production of inflammatory mediators that result in myocardial dysfunction. Table 17-3 summarizes the Texas Children’s Hospital protocols for cardioplegia and myocardial protection.
Phases of Cardiopulmonary Bypass
Surgical cases requiring CPB are divided into several basic phases.
Cannulation and Initiation of Bypass
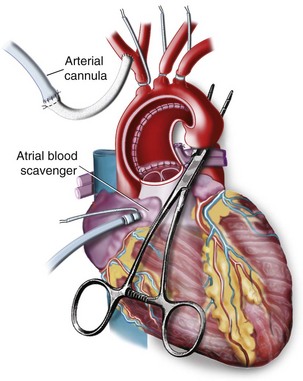
After sternotomy and mediastinal dissection, the aorta is cannulated, along with either the right atrium, if single venous drainage is planned, or the superior and inferior venae cavae for bicaval venous drainage. A large dose of heparin (300 to 400 units/kg) is administered intravenously, and the adequacy of anticoagulation is measured using the ACT before initiating CPB. The target ACT is usually 480 seconds. High ACTs are maintained during CPB with the addition of heparin to the prime as needed, because larger doses of heparin lead to a reduced degree of consumptive coagulopathy, which translates into reduced blood product therapy requirements.96 Other methods of measuring anticoagulation include the Hepcon system (a plasma heparin concentration assay), which may allow for more accurate titration of heparin and protamine dosages.106 The thromboelastogram may also be used as a baseline measure of the coagulation system and then may be repeated during bypass, with heparinase added to more objectively assess each child’s anticipated need for coagulation products.107 An improved preservation of the hemostatic system with subsequent reduction of blood loss and a reduction in transfusion requirements has been demonstrated after maintenance of high heparin levels during CPB.108 The additional maintenance of high ATIII concentrations may further contribute to a reduction of hemostatic activation.109
In most centers, bicaval cannulation is used for all but the smallest children (less than 2 kg) to prevent venous return from interfering with the surgical field. A gradual transition to full CPB is then performed to minimize myocardial stress, using a prime that has essentially the same composition as the child’s blood with regard to temperature, pH, calcium, potassium, and hematocrit. CPB flows of 150 mL/kg/min are used for infants weighing less than 10 kg, and 2.4 L/min/m2 is used for children weighing more than 10 kg. Flow rates may be reduced during periods of hypothermia (see later), although many centers now prefer to maintain greater flows throughout the bypass period. Misplaced cannulas can lead to significant morbidity. Obstruction of the inferior vena cava (IVC) by a misplaced IVC cannula can lead to increased venous pressure, which causes ascites and decreased perfusion pressure in mesenteric, hepatic, and renal vascular beds. Misplacement of the cannula in the superior vena cava can result in cerebral edema from inadequate venous drainage and a subsequent reduction in cerebral blood flow, potentially resulting in ischemia. Arterial cannula misplacement can also occur. If the cannula inadvertently slips beyond the takeoff of the right innominate artery, preferential perfusion to the left side of the brain can be observed. This can be detected on the NIRS monitor, which may be an important monitor, particularly in pediatric cardiac surgery.110
Removal of Aortic Cross Clamp and Rewarming Phase
After completion of the intracardiac repair and de-airing of the heart, the aortic cross clamp is removed, allowing reperfusion of the myocardium. Optimally, normal sinus rhythm and myocardial contractility are restored during this time, while the child is slowly rewarmed. During rewarming, surgery is completed, inotropic and vasoactive agents are started, and ventilation begins. Hemofiltration and blood transfusion are used to achieve the desired hematocrit. Left atrial and/or pulmonary artery monitoring lines, if indicated, are placed at this time, as are temporary atrial and ventricular pacing wires. If the child is incompletely rewarmed before separation from CPB, a significant afterdrop with precipitous post-bypass reduction in core body temperature can occur. This can lead to vasoconstriction, shivering, increased oxygen consumption, and acidosis. However, postischemic hyperthermia can lead to delayed neuronal cell death.111 Mild degrees of hypothermia and certainly the avoidance of hyperthermia are essential in the perioperative period.112 In children, rectal temperature mostly reflects peripheral temperature. One study showed that the temperature of the foot was more sensitive than the temperature of the hand.113 Another study revealed that for anatomic or physiologic reasons, temperature gradients in the toes develop more readily than those in the fingers.114 Several endpoints have been proposed, such as nasopharyngeal temperatures greater than 35.0° C, bladder temperature greater than 36.2° C, or skin temperatures greater than 30° C115,116; we use an endpoint of 35.5° C rectal temperature.117
Post-Bypass Period
This phase lasts until chest closure and transfer to the ICU have been accomplished. During this time, modified ultrafiltration (MUF) may be performed for 10 to 15 minutes after cessation of CPB. Cardiac function and the quality of the surgical repair are assessed via TEE, and, if found to be satisfactory, protamine is administered to neutralize residual heparin. The usual dose of protamine is 1.0 to 1.3 mg/100 units of heparin given at the onset of bypass. Limiting protamine to this dose prevents an overdose with its associated effects on platelet function (reduction of the interaction of glycoprotein Ib receptor interaction with von Willebrand factor).118 If the ACT remains increased or prime blood is given back to the child, an additional 25% of the initial dose of protamine is added and the ACT is rechecked. However, particularly in infants, the administration of protamine and the persistent treatment of a suspected incomplete heparin reversal should not distract and delay the treatment of other commonly associated post-bypass coagulopathies, such as thrombocytopenia, platelet dysfunction, and other coagulation factor deficiencies.
Protamine reactions occur much less frequently in children younger than 16 years of age, approximately 1.76% to 2.88%.119 Independent risk factors are a female gender, a larger protamine dose, and smaller heparin doses. Type I reactions or effects during administration are rare and adding calcium does not change the hemodynamic consequences of injection.120 Fortunately, severe anaphylactic reactions (type II) or catastrophic pulmonary vasoconstriction (type III) are rare but have been observed by us and others.121 Administering the protamine over no less than 5 minutes reduces the severity and precipitous nature of any protamine reaction.
Because CPB can have a multitude of adverse physiologic effects, attempts are made to minimize both the duration of CPB and ischemic (aortic cross clamp) time; thus, as much of the surgery as possible is performed outside of these phases. In general, physiologic responses to bypass are more extreme with decreasing age and size of the child. The neonate experiences a greater degree of hemodilution on bypass and colder temperatures on bypass and frequently requires longer aortic cross clamp times, all of which can result in a greater inflammatory response. Table 17-4 summarizes clinical management issues during the major phases of CPB.
TABLE 17-4 Checklist for Bypass Management
1. Check temperature; maintain normothermia during induction and preparation.
3. Ensure noninvasive monitoring: blood pressure, ECG, pulse oximetry, stethoscope.
4. Perform inhalational induction after preoxygenation; intravenous induction, if cannula is in place.
5. Peripheral intravenous placement(s)
6. Neuromuscular blockade and ventilation
7. Intubation and mechanical ventilation according to shunt lesion (CO2, O2 control)
10. Deepening of anesthetic level
11. Antifibrinolytics and corticosteroids, as indicated
12. Heparin, 300-400 U/kg, before arterial cannulation
1. Stop ventilation and drips when full flow is reached.
3. Evaluate quality of perfusion (perfusion pressure, central venous pressure, diuresis, arterial blood gases, temperature gradient).
5. Set and control temperature and rewarming (heating blanket, room temperature).
7. Check arterial blood gases in preparation for discontinuation of CPB; correct abnormalities.
2. Fine-tune blood pressure; consider direct blood pressure measurement for hypotension at the aortic cannula; volume ± drips.
3. Consider modified ultrafiltration.
4. Check arterial blood gases.
5. Evaluate with transesophageal echocardiography for residual defects.
6. Give protamine, 1-1.3 mg/100 IU of initial heparin.
7. Check activated clotting time and arterial blood gases.
CPB, Cardiopulmonary bypass; ECG, electrocardiogram; TEE, transesophageal echocardiography.
Particular Aspects of Management on Cardiopulmonary Bypass
pH-Stat Versus α-Stat Management
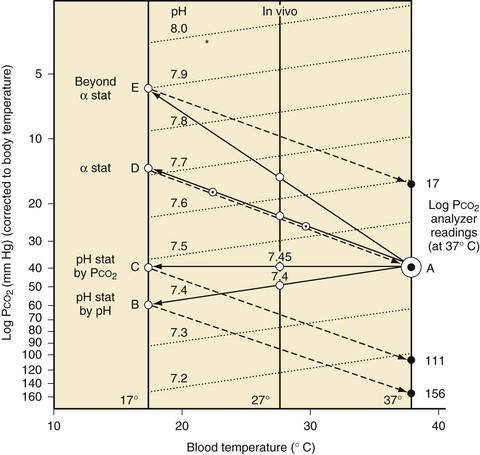
Some degree of hypothermia is used for nearly every cardiac operation to slow the metabolism and oxygen consumption of all organs, particularly the brain and heart.122 During cooling, the carbon dioxide contained in blood becomes more soluble and its partial pressure decreases. The Paco2 sensed by the body decreases as body temperature decreases, with the result that at a core temperature of 17° C to 18° C, if pH and Paco2 have not been corrected for temperature, the body experiences a pH of about 7.6 and Paco2 of 15 to 18 mm Hg (Fig. 17-2).123 This very low Paco2 causes cerebral vasoconstriction, particularly during the cooling phase of bypass, which in turn leads to less cerebral blood flow, less efficient brain cooling, and less cerebral protection at a given temperature.124 Because blood samples are normally heated to 37° C before measurement of pH, Paco2, and Pao2, the use of pH-stat management indicates that blood gases are being corrected for the child’s actual body temperature by increasing the Paco2 during bypass, as it is measured at 37° C, so that the body experiences a Paco2 of approximately 40 mm Hg and a pH of 7.4 at all temperatures. Conversely, α-stat management means not correcting the blood gases for temperature, as if the child’s blood was always at 37° C, with the goal of pH 7.4 and Paco2 40 mm Hg. In the early days of CPB, pH-stat was used to preserve cerebral blood flow at all ages.123 Subsequently, in the 1970s and 1980s, randomized controlled studies in adults undergoing CPB confirmed that acute, post-CPB neurologic problems were worse with the use of pH-stat management.125 α-Stat management was, therefore, adopted for both adult and pediatric CPB. However, studies in a neonatal pig model have challenged this conclusion, proving that neurologic outcomes, both behavioral and neuropathologic, are significantly worse when α-stat management is used in infants.124,126
Advantages of pH-stat CPB have been shown to include:
 A decreased brain metabolic rate127
A decreased brain metabolic rate127
 An increased rate of brain cooling and reperfusion,128 thereby providing better protection through more even and faster cooling and rewarming secondary to increased CBF128,129
An increased rate of brain cooling and reperfusion,128 thereby providing better protection through more even and faster cooling and rewarming secondary to increased CBF128,129
 Molecular effects of altered Pao2 and pH, including changes in cerebral oxygenation and brain enzyme activity, as well as decreased brain electrical activity129–131
Molecular effects of altered Pao2 and pH, including changes in cerebral oxygenation and brain enzyme activity, as well as decreased brain electrical activity129–131
 Decreased oxyhemoglobin affinity132
Decreased oxyhemoglobin affinity132
 Increased cortical oxygenation before arrest (through hypercapnic capillary vasodilation) and decreased oxygen metabolic rate, providing slower deoxygenation compared with α-stat management (approximately 10 vs. approximately 7 minutes).124,133 Cortical anoxia occurs at 36 minutes versus 24 minutes for α-stat management.
Increased cortical oxygenation before arrest (through hypercapnic capillary vasodilation) and decreased oxygen metabolic rate, providing slower deoxygenation compared with α-stat management (approximately 10 vs. approximately 7 minutes).124,133 Cortical anoxia occurs at 36 minutes versus 24 minutes for α-stat management.
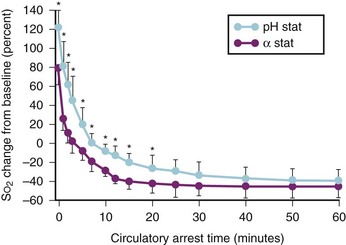
In cyanotic infants with aortopulmonary collaterals, pH-stat management results in significantly improved brain oxygenation as measured by near-infrared cerebral oximetry.134 A retrospective study of 16 infants revealed worse neurodevelopmental outcomes with α-stat management.135 In a randomized prospective trial of pH- versus α-stat management in 182 infants younger than 9 months of age, there was a strong trend toward improved outcomes with pH-stat management, including earlier return of electroencephalographic activity, fewer seizures, and improved psychomotor development index.136 One study examined the effects of α-stat and pH-stat on developmental and neurologic outcomes after deep hypothermic CPB in infants.137 Psychomotor Development Index scores of 110 patients did not differ significantly between the groups (P = .97). The results of the Mental Development Index scores were dependent on diagnosis. In all but the ventricular septal defect subgroup, the pH-stat group did not have statistically greater Mental Development Index scores. Abnormalities on the electroencephalogram (P = .77) and neurologic examination (P = .70) were similar with the two methods of blood gas management. The authors concluded that the use of α-stat or pH-stat strategy is not consistently associated with improved or impaired early neurodevelopmental outcomes in infants undergoing deep hypothermic CPB.137 One reason for the differing results between pediatric and adult studies is that cerebral emboli are more common in the adult population because of the presence of atherosclerotic plaques, which cause microembolic infarcts, and the increased cerebral blood flow produced by pH-stat management, which leads to a greater number of cerebral emboli in adults. Emboli occur much less frequently in children, and the primary cause of neurologic injury from CPB in children is hypoxic-ischemic.138 Thus, the increased cerebral blood flow observed on CPB with pH-stat management lessens this risk in children. Interestingly, this putative mechanism has been recently challenged by a study involving a controlled microembolic load and DHCA in pigs that revealed that pH-stat was still associated with improved outcomes when compared with α-stat.139 pH-stat also improves oxygen delivery by counteracting the pH- and hypothermia-associated leftward shift in the oxyhemoglobin dissociation curve. Studies have also revealed a decrease in peak postoperative troponin levels, reduced ventilator dependence, and reduced ICU stays with pH-stat versus α-stat.140 Most programs specializing in surgery for congenital heart defects currently use pH-stat management. This necessitates careful attention to Paco2 during all phases of bypass, and possibly reducing the sweep gas flow into the CPB oxygenator (to decrease the efficiency of CO2 removal), and often adding inspired CO2 to the sweep gas of the bypass circuit, particularly in small infants.
Hematocrit on Bypass
The relatively small total blood volume in children, along with the volume required to prime the CPB circuit, means that adding blood to the CPB prime is mandatory for small infants. Practice is institution specific; but in many centers adding either whole blood, PRBCs with FFP (for children less than 8 kg), or PRBCs alone (for children less than 12 to 15 kg) is necessary to ensure that the hematocrit on bypass is not less than 20%. As a result of increased transfusion-related concerns from bloodborne viral disease transmission during the 1980s and 1990s, and given that a low hematocrit is thought to be necessary to ensure adequate blood flow through capillary beds (because the blood viscosity increases at low temperatures), hematocrits of 20% or less on CPB with deep hypothermia were frequently tolerated.141 There is increasing evidence that the practice of extreme hemodilution is detrimental to neurologic outcome in children. In a piglet model, one group of investigators determined that the incidence and degree of hypoxic-ischemic brain injury after a period of DHCA was significantly greater with a hematocrit of 20% versus one of 30%, regardless of whether pH- or α-stat strategy was used.142 In another piglet model, using intravital microscopy of pial capillaries during deep hypothermic CPB, a hematocrit of 30% did not impair cerebral microcirculation when compared with a hematocrit of 20%.143 Finally, in a prospective randomized trial of CPB hematocrit of 20% versus 30% at the Children’s Hospital Boston, children in the lower hematocrit group demonstrated significantly reduced psychomotor development index scores 1 year after surgery.83 In a follow-up study of hematocrit 25% vs. 35%, the same group did not observe a difference in neurodevelopmental outcomes.144 However, when they combined all children from both hematocrit trials, they found that a hematocrit less than 24% was associated with lower psychomotor index scores 1 year after surgery.145 The hypoxic-ischemic damage most likely occurs during the cooling and rewarming phases of bypass, when cerebral oxygen metabolism is not suppressed, yet hematocrit and, thus, oxygen delivery, is low. Therefore, many centers are now maintaining higher hematocrits on CPB (at least 25%), which either means using more donor blood products or using hemofiltration to raise the hematocrit during bypass. The current estimate by the American Red Cross of the risk of viral transmission from a single unit of blood is 1 in 205,000 for hepatitis B, 1 in 1.9 million for hepatitis C, and 1 in 2.1 million for human immunodeficiency virus.146 The risk–benefit ratios therefore favor the greater hematocrit approach, a definitive change from previous practice patterns. Balanced against this practice of a greater hematocrit on CPB is the finding that greater transfusion of blood products in the intraoperative, and early postoperative periods, is associated with longer duration of mechanical ventilation in infants undergoing two-ventricle repairs.147 Additional studies are required to optimize strategies for the use of blood products in infants and children undergoing CPB.
Flow Rates on Bypass
The traditional practice in many institutions has been to decrease CPB flows, particularly during hypothermia, to reduce the volume of blood returning to the surgical field and allow more efficient completion of the surgery, particularly in small infants. This concept has been questioned in recent years owing to the inability to determine the safe low-flow bypass rate in the individual child. One report studied 28 neonates who underwent arterial switch operation with α-stat blood gas management during CPB.148 At 14° C to 15° C, bypass flow was sequentially reduced from 150 mL/kg/min to 50 mL/kg/min, and then further decreased in increments of 10 mL/kg/min until circulatory arrest was begun (to 0 mL/kg/min). All neonates had detectable cerebral blood flow by transcranial Doppler at CPB flows above 20 mL/kg/min, but one had no detectable perfusion at 20 mL/kg/min, and eight had none at 10 mL/kg/min, leading the authors to conclude that 30 mL/kg/min was the minimum acceptable flow in this population. A neonatal pig model determined that, at normothermia, bypass flows of at least 150 to 175 mL/kg/min were necessary to ensure full oxygenation of all end organs and tissues.149 Clinical studies of a high-flow bypass strategy, which included flows of 150 mL/kg/min at all phases of bypass except during DHCA, minimal use of DHCA, and α-adrenergic receptor blockade with phenoxybenzamine to produce long-duration systemic vasodilation, demonstrated excellent short- and long-term clinical and neurodevelopmental outcomes, with no child scoring outside normal ranges for testing performed at a mean age of 9 years.150 This strategy also has led to excellent early results for the Norwood operation, with an early perioperative survival of 83% for cases carried out from 1993 to 1999.151 During the same era, one report documented that 26.7% of arterial switch children had neurologic abnormalities and 55% had at least one abnormal area on neurodevelopmental testing (performed at a mean of 10 years) when DHCA and low-flow bypass had been used.151
Vasoconstriction and increased vascular resistance, resulting in uneven regional organ perfusion, are among the undesired side effects of CPB. Endogenous catecholamine production and the alkaline α-stat CPB technique, if used, are responsible for these effects. To be able to run full flow during hypothermic CPB without significant hypertension, vasodilators are often used. Agents currently used to provide systemic vasodilation and more even cooling and rewarming include phentolamine, nitroprusside, or nitroglycerin. Phenoxybenzamine, which is no longer available, was used as part of a treatment strategy after stage 1 palliation for hypoplastic left heart syndrome, and has been associated with improved outcome.152,153 Phenoxybenzamine was more effective than sodium nitroprusside in improving peripheral circulation, as shown by temperature gradients intraoperatively.154 Greater CPB flows are associated with an improved oxygen delivery, which can improve patient outcome.155
Phentolamine is a nonselective competitive α1 and α2 catecholamine receptor blocker. It has a half-life of 19 minutes and is eliminated mainly by the kidneys. Through postsynaptic α1 and α2 receptor inhibition it has a vasodilating and hypotensive effect that can improve cardiovascular parameters and metabolic acidosis during CPB management.156 In children receiving phentolamine, increasing lactate concentrations at the end of the CPB period show a steady state toward the end of the surgery, whereas lactate continues to rise in patients who did not receive phentolamine.156 These findings suggest that the use of phentolamine limits lactic acid production during the hypothermic period and aids the disposal of lactic acid from tissues. Seelye and associates called the physiologic state after hypothermia the “oxygen debt repayment” period in infants.157 Although it has a beneficial effect on CPB management, the potential harmful effects of phentolamine, especially on the brain, have still not been fully elucidated. One study provided evidence that phentolamine increases S100B protein and a parameter indicative of altered cerebrovascular resistance, the pulsatility index in the middle cerebral artery, in infants given phentolamine during open-heart surgery.158
Nitroprusside has been used as an easily titratable agent with α-adrenergic receptor–blocking capacity. One study examined the effect of perioperative sodium nitroprusside application in 25 neonates undergoing an arterial switch operation for transposition of the great arteries.159 In comparison to the pre-bypass values, a similar increase in the concentration of S100B protein was found 2 hours after the termination of CPB in the sodium nitroprusside-treated and nontreated neonates, which decreased over the subsequent 48 postoperative hours. However, significantly reduced post-bypass serum levels of S100B protein were found in the sodium nitroprusside-treated group after 24 and 48 hours of treatment.
Nitroglycerin has been used with the same success. The only proven benefit over other agents is its nitric oxide donation capacity.160 In Japan, high-dose chlorpromazine has been used as part of a low-resistance strategy during CPB for the Norwood procedure.161
We routinely use phentolamine, 0.1 to 0.2 mg/kg, to provide normal CPB flow and mean arterial pressure in the range of the diastolic pressure. If hypotension develops during bypass, the flow should be increased up to 150% of predicted; also one should examine the acid-base status in conjunction with cerebral oxygenation and mixed venous saturations. Often, severe hemodilution with oxygen debt is the cause and should be treated as such. After exclusion, we treat the hypotension carefully with vasoconstrictors, knowing that normal systemic pressures will not restore splanchnic hypoperfusion162 and that vasoconstrictors will often lead to a greater base excess. Excessive α-adrenergic receptor blockade can be antagonized by vasopressin.163 One study demonstrated that vasoconstrictor treatment results in more sodium bicarbonate to treat the acidosis and is associated with a later time to extubation and return of bowel function.164 In conclusion, α-adrenergic receptor blockade during bypass should be considered because of its benefits for tissue perfusion, but carefully executed and balanced against potential drawbacks afterwards.
Conventional Ultrafiltration and Modified Ultrafiltration
Ultrafiltration involves placing a hemofilter (similar to those used for continuous arteriovenous or venovenous hemofiltration in the ICU) in the CPB circuit and has become the standard of care for nearly all programs that specialize in surgery for congenital heart defects.165 Conventional ultrafiltration (CUF) is performed during CPB, with the filter placed between the arterial and venous sides of the CPB circuit. The hemofilter has thousands of fibers with pores, which allow water, electrolytes, and small molecules to be filtered out of the blood. Suction is applied to the hemofilter on CPB, and an ultrafiltrate of plasma is produced. Advantages of ultrafiltration include the ability to increase the hematocrit, fibrinogen, plasma proteins, and platelet count,166,167 without necessitating further blood transfusion, the ability to remove excess free water and sodium (which contribute to excess intravascular volume, tissue edema, pulmonary and myocardial edema), as well as the ability to correct acid-base and electrolyte imbalances, and to remove small molecules, such as interleukins and tumor necrosis factor-α (TNF-α) in particular,168 which are involved in the post-bypass inflammatory process.169,170 This improves systolic and diastolic function of the myocardium and reduces endothelial dysfunction in the systemic and pulmonary vasculature.170,171 Pulmonary function is better preserved, probably owing to a slight reduction in interleukin 6 (IL-6) and thromboxane B2,172 even though this is not a consistent finding in the literature.173,174 Endothelin-1, another mediator of pulmonary damage and hypertension, was not reduced by any filtration method.174 Clinically, however, any ultrafiltration method seems to benefit children, especially those undergoing complex repairs, neonates, and children with preexisting pulmonary hypertension.173
Modified ultrafiltration (MUF) is performed for 10 to 15 minutes immediately after the conclusion of CPB. It can be performed in an arteriovenous manner with a hemofilter placed between the aortic cannula and the IVC cannula, or in a venovenous fashion using bicaval cannulation or an internal jugular venous catheter.175 It was developed in 1991176 as an alternative method to reduce the side effects of CPB. CUF during bypass is often limited by the minimal venous reservoir levels and requires the addition of crystalloid or colloid to be able to continuously remove cytokines during ultrafiltration. During MUF, blood passes out of the aorta, through the hemofilter, and is returned through the IVC cannula. The theoretical advantage of MUF over CUF is that only the child’s blood volume is filtered, yielding a more efficient system for achieving the goals just outlined. The disadvantages are that the child remains heparinized, and body temperature may decrease during the process (unless the circuit is modified to include the heat exchanger).177 It also requires extra time, an aortic cannula is needed that can obstruct the aorta in small infants, and acute intravascular volume shifts may occur at a time when the child is prone to hemodynamic instability. Opposite to the expected effects of fluid removal, MUF actually increases arterial pressures despite decreasing filling pressures and improving myocardial performance.178
There is increasing evidence that the use of ultrafiltration reduces bypass-related postoperative morbidity. Outcome studies have demonstrated that ultrafiltration improves myocardial and pulmonary function, lessens tissue edema, allows faster weaning from mechanical ventilation, and decreases the need for inotropic support.179 In that aspect it may be as efficient as the perioperative application of steroids.180 The reduction of inflammatory transmitters is only temporary, because the levels of cytokines are similar after 24 hours.181
Although each method has its proponents, and some centers perform both techniques in the same children, controlled comparative studies revealed no difference in outcome between MUF and CUF.179,182 We routinely use a balanced ultrafiltration technique for all cases on CPB because it removes fluids and cytokines, as well as reduces lactate, which can aggravate reperfusion injury.183
Anesthesia on Cardiopulmonary Bypass
Changes in Pharmacokinetics
The initiation of CPB introduces additional volume to the intravascular space (hemodilution). This greatly affects drug distribution, plasma concentrations, and elimination. The major factors responsible for this are hemodilution and altered plasma protein binding,184 hypotension, hypothermia,185 pulsatility,186 isolation of the lungs from the circulation, and uptake of anesthetic drugs by the bypass circuit.187,188 Drugs in the blood exist in the free (unbound and therefore the active form) or plasma bound (inactive form bound to protein, e.g., albumin) forms and therefore are subject to marked changes with alterations in plasma protein levels. CPB alters all these factors, which makes description of pharmacokinetic parameters during CPB problematic. The greatest changes occur within 5 minutes of initiation of CPB. The addition of the prime volume immediately reduces the protein concentration, and the ratio of bound-to-free drug in the circulation changes. A reduction in red blood cell concentration occurs, and this reduces the free drug concentrations. This will reduce the amount of drug available for interaction with the receptors. Most studies show a reduction in total drug concentration in plasma with little change in unbound drug concentration over time, whereas on CPB other than the transient (less than 5 minutes) reduction at initiation of CPB189 it would appear that the greatest risk for unwanted “lightening of anesthesia” is within this time frame, and additional doses of fentanyl, muscle relaxant, and midazolam are generally administered just before or with the onset of CPB. The explanation for why unbound drug concentrations are sustained during CPB is that the volume of distribution for most anesthetic agents is large relative to the volume of the CPB prime and serves as a huge reservoir for drug after intravenous administration. A decrease in the plasma concentrations of medications as a result of hemodilution shifts drugs down their concentration gradient from tissue to plasma. Hypothermia contributes to the changes in plasma concentrations primarily by depressing enzyme function and slowing the metabolism of medications. Drug metabolism is diminished during hypothermia; enzyme activity is approximately halved for every 10° C reduction in temperature. This may increase the free drug available for binding. When normothermia is reestablished, reperfusion of tissues might lead to washout of drug sequestered during the hypothermic CPB period. This may explain the secondary increases in plasma concentrations of opioids reported during the rewarming phase. pH-stat management also affects the degree of ionization and protein binding of certain medications, leading to increased unbound drug. During CPB, the lungs are out of circuit and medications that are taken up by the lungs (e.g., opioids) are sequestered during CPB. These medications are released when systemic reperfusion is established and concentrations are transiently increased. The volume of distribution of many drugs is expanded because of the priming volume of the bypass circuit, especially with neonates and small infants, where the priming volume is often greater than the child’s blood volume. Finally, medications may be taken up by various components of the CPB circuit itself.
Changes in Pharmacodynamics
The pharmacodynamic effects of anesthetic agents are affected primarily via the central nervous system, which undergoes major changes during CPB. For example, hypothermia during CPB reduces anesthetic requirements. Hypothermia causes a host of other effects, including decreases in receptor affinity (e.g., decreased opioid receptor affinity190 and nicotinic acetylcholine receptor sensitivity191), enhanced effects of neuromuscular receptor blocking drugs at the neuromuscular junction,192,193 and alterations in tissue blood flow that may affect the response to catecholamines.194
CPB also affects the degree of ionization and protein binding (hence free or unbound drug concentrations) of weak acids and bases, as well as the electrolyte balance achieved by the blood gas management strategy used during CPB. Plasma concentrations of calcium, magnesium, and potassium decrease during CPB,195,196 and these changes may lead to muscle weakness, dysrhythmias, and digitalis toxicity. The number of receptors available for interaction with a ligand will determine the subsequent magnitude of a drug effect. A reduction in the number of cardiac receptors has been observed in congestive heart failure, and defects in receptor transduction, as well as impairment of synthesis and reuptake of norepinephrine occur.
Administration of β-adrenergic agonists in this condition has been associated with further reductions in β-receptor numbers, with diminished pharmacologic effect. Removal of β-adrenergic blockade may lead to β-adrenergic receptor upregulation and increased adrenergic responsiveness.197 Changes in receptor density and function may occur very quickly and have been observed to occur during cardiac surgery. Many perfusionists, under the direction of the anesthesiologist, can also administer inhalation agents via a separate vaporizer mounted on the bypass machine. Anesthetic requirements decrease with systemic hypothermia,198 but as rewarming is initiated, additional anesthetic drugs, including a benzodiazepine, are added to the pump to ensure that amnesia is maintained. Further work is required to elucidate the mechanisms and clinical implications of these acute changes in receptor density and function.
Special Techniques
Management of Deep Hypothermic Circulatory Arrest
In the early days of cardiac surgery and CPB, hypothermia was used to improve intracardiac surgical exposure. In 1950, Bigelow and colleagues were the first to show that hypothermia decreases the metabolic rate.199 Since then, we have discovered other advantages of hypothermia, including decreases in the inflammatory response of CPB,200 decreases in blood loss,201 myocardial protection,202 and neuroprotection.203 The last effect relates primarily to the decrease in the metabolic rate (by approximately 64%) that is achieved by cooling from 37° C to 27° C. The disadvantages of DHCA include a prolongation of CPB and a greater tendency toward postoperative bleeding.204 Postoperative recovery, however, is not prolonged by hypothermia.205 The rate of wound infection is uninfluenced by hypothermic bypass.206
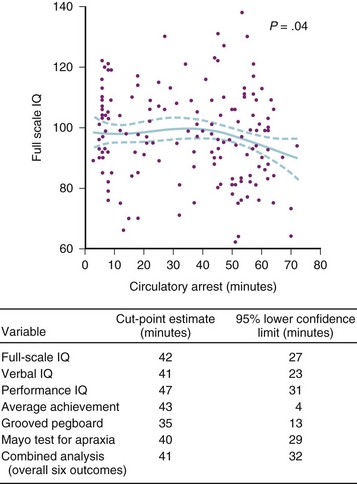
Hypothermia during cardiac surgery gained widespread acceptance only after the development of a heat exchanger that could be integrated into the CPB machine in 1959.207 Deep hypothermic circulatory arrest involves cooling the child’s body temperature during CPB to 17° C to 18° C, stopping the bypass machine, draining the blood from the child into a venous reservoir, and removing the cannulas from the heart. After the first reports of DHCA in the 1960s, this technique gained popularity in the 1970s and 1980s because the bloodless field it provided, thus facilitating complex intracardiac and aortic repairs in neonates and small infants,208 as well as reducing myocardial edema. However, it soon became evident that DHCA was associated with neurologic morbidity. Choreoathetosis, seizures, coma, and hemiparesis were all noted, especially with prolonged (>60 min) DHCA. The incidence of these acute morbidities seemed to increase when α-stat management became the accepted standard in many centers. Long-term adverse neurodevelopmental outcomes have also been associated with long periods of DHCA, including abnormalities in mental development and in fine and gross motor skills.209 The Boston Circulatory Arrest Study is a remarkable achievement in which 155 neonates undergoing the arterial switch operation from 1988 to 1992 were studied, with follow-up complete to 8 years of age.210 The CPB protocol in those years included α-stat management, routine hemodilution to a hematocrit of 20%, and the absence of an arterial filter on the CPB circuit. A DHCA time of greater than 40 minutes was associated with a significant increase in adverse long-term neurologic outcomes (Fig. 17-3). Although the 40-minute cutoff is now well-accepted in surgery for congenital heart defects, a number of changes have subsequently been made to bypass protocols. Results from animal experiments using a neonatal pig model of DHCA, as well as data from the Boston Circulatory Arrest Study, led to the following recommendations for increasing the child’s safety margin when using DHCA:
 Hematocrit of 30% should be the target.83
Hematocrit of 30% should be the target.83
 Systemic hypothermia should be achieved slowly, over no less than 20 minutes.211
Systemic hypothermia should be achieved slowly, over no less than 20 minutes.211
 pH-stat blood gas management should be used, at least for cooling (Fig. 17-4).124,126
pH-stat blood gas management should be used, at least for cooling (Fig. 17-4).124,126
 Core body temperatures of 17° C to 18° C should be used, and ice bags should be applied to the head.212
Core body temperatures of 17° C to 18° C should be used, and ice bags should be applied to the head.212
 DHCA should be divided into periods of less than 20 minutes, allowing a reperfusion period of at least 2 minutes between each segment of DHCA, to improve neurologic outcome.213
DHCA should be divided into periods of less than 20 minutes, allowing a reperfusion period of at least 2 minutes between each segment of DHCA, to improve neurologic outcome.213
 Low-flow CPB is better than DHCA. Selective regional cerebral perfusion may be better than full body low-flow CPB.214
Low-flow CPB is better than DHCA. Selective regional cerebral perfusion may be better than full body low-flow CPB.214
 Normoxemia should be maintained to decrease exacerbation of brain injury after DHCA.215
Normoxemia should be maintained to decrease exacerbation of brain injury after DHCA.215
Neurologic monitoring (see later discussion) may be useful in the individual child to aid in determining the safe duration of DHCA.124,216
Regional Cerebral Perfusion
To avoid the use of DHCA, several novel CPB techniques have been developed. The purpose of these techniques is to allow perfusion of the brain during critical periods of surgery, such as aortic reconstruction during the Norwood operation.217,218 These techniques are collectively referred to as selective cerebral perfusion. Regional cerebral perfusion (RCP) is one variation in which a small Gore-Tex graft of 3 to 4 mm is sewn onto the innominate artery before initiation of CPB and is then used as the aortic cannula during CPB (Fig. 17-5). During aortic reconstruction, snares are placed around the brachiocephalic vessels and CPB flow is decreased, with only the brain receiving perfusion via the right carotid artery during this period. In this way, a bloodless operative field is achieved, just as if DHCA was being performed, yet the brain is still receiving blood flow and oxygen, theoretically increasing protection from hypoxic ischemic brain injury. Another potential advantage of this technique occurs in neonates, who frequently have extensive arterial collaterals between the proximal branches of the aorta and the lower body via the internal mammary and long thoracic arteries. In this instance, the use of selective cerebral perfusion also provides some blood flow to the lower body, protecting renal, hepatic, and gastrointestinal systems from hypoxic damage as well.219 This protection is, however, incomplete and RCP at 25° C is no more protective than DHCA.220 Also, the ongoing perfusion prolongs the effective bypass time, leading to more cytokine release and capillary leakage, with worse pulmonary function, more weight gain, and decreased right ventricular function.221
Despite the theoretical advantages of selective cerebral perfusion and a study demonstrating that selective cerebral perfusion does provide oxygenated blood flow to both cerebral hemispheres,222 no long-term outcome studies have been performed that prove it is superior to standard techniques. This may in part be related to the novelty of this procedure. Neurologic monitoring has been used to determine the flow rate that is necessary during RCP.222,223 We use 40% to 50% of full flow and adjust it according to brain saturation or Doppler measurements, maintaining baseline saturation before the onset of RCP. If a left radial arterial line or a femoral arterial line (or umbilical arterial line) is in place, an abdominal perfusion pressure of about 12 mm Hg, which correlates with radial artery pressures of 25 to 30 mm Hg, is the goal.222
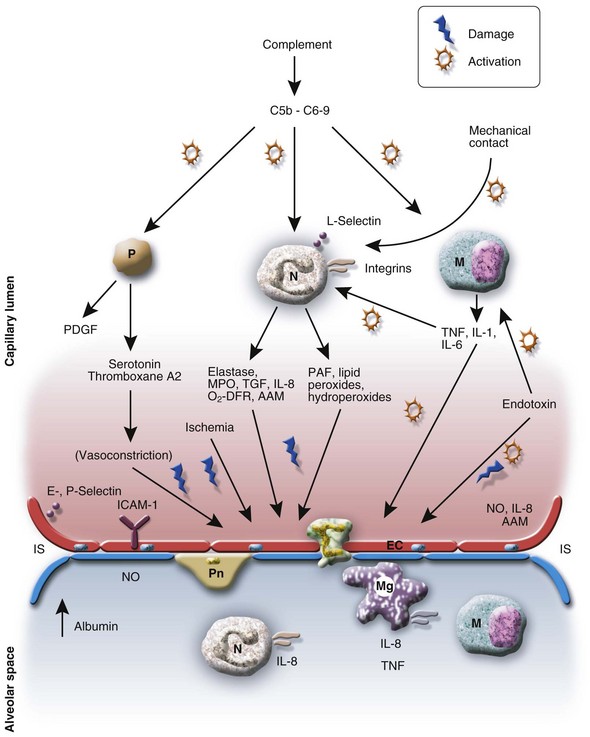
Effects of Cardiopulmonary Bypass
Cardiac Effects
In addition to myocardial ischemic injury secondary to aortic cross clamping, several other factors can contribute to perioperative myocardial dysfunction. The first is entrainment of air into the coronary arteries, which frequently occurs during weaning from bypass.224 Despite meticulous de-airing of the heart, air may enter the right coronary artery, producing ischemia that is heralded by a pale myocardium, poor contractility, and ST-segment elevation of the ECG. Should this occur, appropriate management involves remaining on CPB, increasing perfusion pressure, and “milking” the air through the coronary arteries, allowing time for recovery of the ECG and ventricular function before attempting to wean from bypass. Surgical factors, such as reimplantation of coronary arteries with possible resultant ischemia or residual surgical defects, can also occasionally contribute to myocardial dysfunction.
The inflammatory response to CPB (see later discussion) has important implications for cardiac function.225 This systemic response results in a capillary leak syndrome, which in turn leads to accumulation of edema fluid in interstitial and extravascular spaces, including the myocardium.226 Myocardial edema can contribute to post-CPB myocardial dysfunction by impairing diastolic function and causing mechanical limitation of cardiac filling and outflow in small infants whose sternums have been closed. Additionally, myocardial edema has been implicated as a causative factor in the frequent decline in myocardial function that occurs 6 to 12 hours after conclusion of CPB. Inflammatory mediators also affect the responsiveness of the myocardium to catecholamines by interfering with their binding to the cell surface receptors,227 rendering exogenously administered drugs, such as dopamine and epinephrine, as well as the child’s endogenous catecholamines, less effective at increasing cardiac output in the perioperative period.
Mechanisms for prevention and treatment of myocardial dysfunction include the use of ultrafiltration and antiinflammatory drugs, such as corticosteroids and aprotinin.228,229 The prophylactic use of noncatecholamine inotropic agents, such as milrinone, has also been shown to prevent low cardiac output syndrome in infants, even if cardiac function is adequate in the immediate postoperative period.230
Systemic and Pulmonary Vasculature Effects
The inflammatory response to CPB often produces mediators that directly increase pulmonary and systemic vascular resistance. These include interleukins, leukotrienes, and endothelin.231 Indeed, when pulmonary artery pressure is measured directly, it is often significantly increased immediately after bypass, even if surgical results are optimal. This increase can be extremely detrimental in children with large left-to-right shunts, those undergoing cardiac transplantation secondary to dilated cardiomyopathy, and those undergoing bidirectional cavopulmonary anastomosis, where right ventricular output depends on maintaining low PVR. Prevention and treatment of increases in PVR include maintaining an adequate depth of anesthesia, ventilating with 100% oxygen, and judicious use of hyperventilation. Milrinone will increase right-sided heart output via its actions as both an inotropic agent and a pulmonary vasodilator. When PVR is significantly increased, inhaled nitric oxide is often used to assist in the early postoperative period.232 Although effective, its cost is not inconsequential, and because PVR almost always decreases with time, nitric oxide is generally reserved for selected cases of pulmonary hypertension. Other simpler, less expensive treatments include oral or intravenous sildenafil233,234 and inhaled nebulized prostacyclin.235
Pulmonary Effects
The lungs are not ventilated during CPB and are usually totally collapsed by intention, with the ventilator circuit disconnected, especially in small infants. This leads to significant atelectasis. The lungs are also at least partially ischemic during the bypass period, resulting in decreased production and alveolar levels of surfactant after CPB.236 In addition, reperfusion injury (pulmonary edema or hemorrhage after a sudden increase in pulmonary flow) can also occur after creation of a systemic-to-pulmonary artery shunt or pulmonary artery unifocalization. Inflammatory mediators liberated by the bypass run, also predispose to increases in smooth muscle tone and resistance, and can result in bronchospasm.237
In addition to complement, endotoxins and certain cytokines can also activate neutrophils and attract them toward sites of inflammation.238 In animal studies, endotoxin-induced lung injury can lead to rapid (within 45 minutes) accumulation of neutrophils within lung capillaries. Activation of neutrophils, with upregulation of adhesion molecules, neutrophil adhesion to the endothelium of lung vessels, and endothelial damage through proteases, appears to be the main step of the underlying pathophysiologic mechanism (Fig. 17-6). Macrophages play an important role in the evolution of the inflammatory acute lung injury through the secretion of cytokines, cytotoxic metabolites, and chemoattractants for leukocytes. At the clinical level, acute respiratory distress syndrome (ARDS) is often only one part of multiorgan failure, and lung injury should be seen as part of a more general state of systemic inflammation. The reported prevalence of ARDS after CPB in adults is 0.5% to 1.7%; the incidence in children is unknown. Interestingly, general hypothermia at 28° C failed to prevent the loss of ATP and the accumulation of lactate in lungs.239 Other methods that aim to protect the lungs during CPB, such as continuous lung perfusion, pneumoplegia, and nitric oxide ventilation at lung reperfusion, prevent more severe hemodynamic deterioration and preserve reactivity of the pulmonary vasculature, but fail to prevent pulmonary dysfunction.
The severity of pulmonary dysfunction after CPB can be measured via changes in the alveolar-arterial oxygenation gradient, intrapulmonary shunt, degree of pulmonary edema, pulmonary compliance, and PVR. Treatment of pulmonary atelectasis includes careful reinflation of the lungs when weaning from bypass (by administering several vital capacity breaths), gentle but thorough suctioning of the tracheal tube, and prophylactic use of inhaled bronchodilators before separation from CPB. Using these measures, pulmonary function has been shown to improve immediately in most children with large left-to-right shunts, with the duration of CPB seemingly having little effect on pulmonary outcomes.240 Thus, CPB itself has little effect on pulmonary function in most children. There is still an occasional child, however, who experiences classic “pump lung” ARDS, caused by the factors noted earlier. Treatment is supportive as for anyone with ARDS.
Neurologic Monitoring and Effects of Cardiopulmonary Bypass on the Brain
Cerebral monitoring can help to detect those children who are at risk for neurologic sequelae after bypass, promptly recognize and treat changes in cerebral blood flow/oxygenation, evaluate the effect of therapeutic interventions on cerebral physiology, optimize brain protection during the vulnerable periods of CPB, and potentially improve short- and long-term neurologic outcomes.241
The cerebral NIRS monitor measures brain tissue oxygenation. This device noninvasively measures the cerebral tissue oxygen saturation and displays a numerical value for the cerebral regional oxygen saturation (rSo2), the ratio of oxyhemoglobin to total hemoglobin in the light path. rSo2 is a measure of local microcirculatory oxygen supply-and-demand balance and is reported on a scale from 15% to 95%. It has been assumed from anatomic models that 75% of the cerebral blood volume in the light path is venous and 25% is arterial. One study verified this in children with congenital heart disease by directly measuring the jugular venous bulb and arterial oxygen saturations and comparing these with the cerebral oxygen saturation measured with NIRS.242 The actual ratio in children varied widely, but on average the venous to arterial ratio was 85 : 15. All devices measure both the arterial and venous blood oxygen saturations. Accordingly, this device does not provide a measure of the jugular venous bulb oxygen saturation (Sjvo2). A corollary of this is that maneuvers that increase arterial oxygen saturation (e.g., increasing Fio2) increase cerebral oxygenation as measured by these devices, although the Sjvo2 may remain unchanged. In a study of 40 infants and children with congenital heart disease who were undergoing cardiac surgery or catheterization, NIRS correlated poorly with Sjvo2 measurements, except in infants younger than 1 year of age.243 In contrast, in a study of 30 children undergoing cardiac catheterization, NIRS correlated very well with Sjvo2 (r = .93).244 These data suggest that NIRS is a useful indicator of trends in cerebral oxygenation in individual infants and children. NIRS values also correlate with long-term neurodevelopmental outcomes in infant heart surgery. In a prospective study of 104 two-ventricle repairs, low rSo2 in the intraoperative period did not correlate with death or major morbidity.245 However, when these children underwent neurodevelopmental testing at 1 year of age, lower average and minimum rSo2 in the 60 minute period immediately after CPB correlated with worse psychomotor development index scores. Low rSo2 also correlated with remote ischemic changes on brain MRI at 1 year of age.246
Neurologic Monitoring for Low-Flow Hypothermic Bypass
Transcranial Doppler (TCD) ultrasonography has been used to determine the threshold of detectable cerebral perfusion during low-flow CPB. TCD velocities reveal trends or changes in cerebral blood flow and not absolute values. One report studied 28 neonates undergoing the arterial switch operation using α-stat blood gas management.148 Their study suggested that NIRS and TCD may be useful to determine the minimum acceptable bypass flow rate for an individual neonate during low-flow hypothermic bypass. Blood flow becomes insufficient at bypass flow rates less than 30 mL/kg/min.148,247 Inadequate blood flow to the brain during this technique could be undetected without such monitoring, and low-flow bypass may confer no advantage to the brain over DHCA in some children. Long-term outcome studies of this monitoring strategy are not available.
Neurologic Monitoring for Deep Hypothermic Circulatory Arrest
Despite clinical and experimental evidence that periods of DHCA that exceed approximately 40 minutes are associated with an increased risk of adverse long-term neurologic and developmental outcomes, this technique is still widely used in surgery to correct congenital heart defects. Recent recommendations for improving outcome after DHCA, based on both animal and clinical studies, were described previously. During DHCA, rSo2 predictably decreases to a nadir 60% to 70% (relative change) below baseline values obtained before bypass. The nadir is reached at 10 to 20 minutes, after which there is no further decrease.248 At this point, it appears that there is no additional oxygen uptake by the brain. Several studies suggest the potential for near-infrared cerebral oximetry to determine the safe conduct and duration of DHCA in the individual child. In a study of infants and children undergoing surgery with bypass and DHCA, three children with low rSo2 developed acute postoperative neurologic changes—seizures in one, and prolonged coma in two.248 In these three children, the increase in rSo2 after the onset of CPB was much less (average 3% relative increase vs. 33% increase in children without neurologic deficit) and the duration of cooling before DHCA shorter than in the remaining 23 children who did not develop neurologic changes. In a neonatal pig model, the timing of the nadir of rSo2 values during DHCA correlated with neurologic outcome: a more prolonged period without oxygen uptake by the brain correlated with a greater incidence of adverse neurologic outcome. The maximum safe duration at 17° C without additional brain oxygen uptake was 30 minutes.142 Interestingly, this time period correlates with clinical and experimental studies, suggesting that 40 minutes is the safe duration for circulatory arrest (see Fig. 17-3). When circulatory arrest is initiated at greater temperatures (e.g., 25° C), the rSo2 decreases more rapidly, and the nadir is achieved sooner, than at lower temperatures.249 Reperfusion results in an increase in rSo2 to levels observed at full bypass flow before DHCA, with a subsequent decrease during rewarming. Based on these data, our current practice is to reperfuse after the NIRS nadir has been reached for a period of 20 to 25 minutes.
Neurologic Monitoring for Regional Cerebral Perfusion
RCP (also known as selective cerebral perfusion or antegrade cerebral perfusion) uses a polytetrafluoroethylene (PTFE) graft or a small aortic cannula as arterial inflow to the right innominate artery for neonatal aortic surgery, such as the Norwood stage operation or aortic arch advancement. The other brachiocephalic vessels and descending thoracic aorta are snared, resulting in a bloodless operating field. The brain is perfused through the right innominate and right vertebral arteries only. This approach significantly reduces or eliminates the use of DHCA for these operations and preserves brain perfusion, potentially improving neurologic outcome. Initial descriptions of this technique used the pressure in the radial artery or a predetermined bypass rate of 25 to 30 mL/kg/min as an estimate for the bypass flow during RCP without neurologic monitoring. When flow rate was estimated on the basis of NIRS monitoring in individual children, it was determined that 20 to 25 mL/kg/min was required.217 However, NIRS was applied only to the right side of the skull (i.e., brain), the same side as the sole arterial inflow. Using a pH-stat blood gas strategy for RCP, we noted that the majority of our children had an rSo2 of 95% (the maximum reading on the rSo2 scale) when we used the left radial artery pressure of 20 to 25 mm Hg as the target for bypass flow. These children were theoretically at risk for excessive cerebral perfusion. Therefore, we performed a study using both NIRS and TCD of the right cerebral hemisphere, to determine if TCD could be used as a guide to RCP flow rate.222 Bypass flow rate was adjusted to achieve a cerebral blood flow volume within 10% of baseline (e.g., TCD was used to determine necessary flow). The estimated flow rate, 63 mL/kg/min (range, 24 to 94 mL/kg/min), proved to be significantly greater than that estimated in the earlier studies. This flow rate did not correlate with the pressure in the right or left radial artery. The rSo2 was well maintained in all children, leading us to conclude that TCD was a useful monitor to ensure adequate but not excessive cerebral blood flow during RCP. Because RCP perfuses the brain through a single arterial inflow vessel, questions have arisen about the adequacy of cerebral blood flow and oxygenation to the left cerebral hemisphere. Although the circle of Willis is expected to be intact without stenoses in neonates, 10% of healthy full-term neonates exhibit deviations from normal flow patterns. Two studies concluded that although cerebral blood flow and oxygenation were adequate to both cerebral hemispheres in neonates during RCP, bilateral monitoring, at least of NIRS, may be warranted.222,250
Systemic Inflammatory Response Syndrome
Exposure of blood to the artificial materials in the bypass circuit—plastics, polypropylene oxygenator fibers, and metal suction devices—initiates a cascade of inflammatory responses, including activation of the complement system, the kallikrein system, and the coagulation system.226 As a result, interleukins, tumor necrosis factor, endotoxin, heat shock protein, and many other inflammatory mediators are released into the circulation. Leukocyte activation also results in secretion of inflammatory mediators, such as proteases and cytokines (e.g., TNF-α and IL-1), which are secreted early in the evolution of the inflammatory process. This chemokine-mediated increased leukocyte activation constitutes an important link in the chain of the propagation of the inflammatory response (see Fig. 17-6).
This inflammatory response is counterbalanced by a complex system of inhibitors, such as IL-10 and soluble cytokine receptors.251 Also, the inflammatory response of the neonate may be more exaggerated than that of the infant or older child,252 justifying a more aggressive approach to its modulation in the neonate (see later discussion).
Effective treatments used every day in the operating room and ICU include:
 Ultrafiltration228 (see earlier discussion)
Ultrafiltration228 (see earlier discussion)
 Aprotinin229 (see earlier discussion)
Aprotinin229 (see earlier discussion)
 Leukocyte depletion254: leukocyte-depleted blood in prime and in-line arterial filter; initiate bypass using normoxic management (Fio2 of 21%) in severely cyanotic infants.
Leukocyte depletion254: leukocyte-depleted blood in prime and in-line arterial filter; initiate bypass using normoxic management (Fio2 of 21%) in severely cyanotic infants.
Corticosteroids interrupt the inflammatory response at several levels by entering cell nuclei and changing the rate of transcription of inflammatory molecules. Increasing evidence suggests that glucocorticoids act by regulating transcription or translation of antiinflammatory cytokines, such as IL-10, and altering expression of other proteins, such as endothelin-1 and inhibitor NF-κβ.255,256 Because these processes take time to develop, the effects of corticosteroids are not immediate, taking up to several hours.257 Thus, the common practice of adding corticosteroids to the CPB prime will not fully prevent the inflammatory response258; to be effective, corticosteroids may need to be administered 4 or more hours before the onset of CPB.259
Despite these theoretical advantages of using corticosteroids to modulate the inflammatory response, a recent large discharge database review of over 46,000 infants and children, in which 54% did receive corticosteroids, demonstrated no difference in mortality. Using propensity score matching the authors concluded that corticosteroids were associated with greater length of hospital stay, greater rate of infection, and greater use of insulin. There was no difference in duration of ventilation. Steroids conferred no significant benefit; conversely in the simpler surgery categories, there was increased morbidity with these drugs.260 Although inflammatory activation from CPB definitely occurs, and it would seem intuitive that this would lead to worse outcomes in those patients with excessive inflammation, in contemporary practice, the correlation of the magnitude of this response and length of ICU stay and blood product administration, is statistically significant, but clinically modest, accounting for only 4% to 9% of the difference in these variables, in a large study of infants undergoing two-ventricle repairs.261
Coagulation Effects
Blood coagulation is frequently abnormal after CPB for several reasons. The inflammatory cascade activates the coagulation system, resulting in factor consumption and fibrinolysis, which, in turn, breaks down existing blood clots, leading to increased bleeding.16 Treatment is adequate heparinization, reversal with protamine, and the use of an antifibrinolytic to inhibit fibrinolysis and improve platelet function.33 In addition, the smaller the child, the greater the dilution of clotting factors by the bypass prime, and the greater the risk for low concentrations of clotting proteins and fibrinogen postoperatively. Platelets are also degranulated and consumed by the CPB circuit, leading both to low platelet counts and nonfunctioning platelets.16 The smaller the infant, the greater the duration of bypass, and the more complicated the surgery, the greater the incidence of coagulopathy after bypass. Efforts to minimize the post-bypass coagulopathy in infants includes priming the CPB circuit with fresh whole blood for small infants, if available, or packed cells plus fresh frozen plasma (FFP) if fresh whole blood cannot be obtained.19,262 Treatment involves administration of platelets to small infants as the first line of therapy, followed by the replacement of fibrinogen and FFP to replace clotting factors. If these factors are not effective after correcting coagulation parameters, such as platelet count, prothrombin/PTT, fibrinogen, and thromboelastogram, then surgical bleeding may be the cause and surgical reexploration may be warranted.263 Factor VIIa has also been used as a last resort in children who have significant post-bypass bleeding unresponsive to standard measures.56
Hepatic, Renal, and Gastrointestinal Effects
The liver, kidneys, and gastrointestinal tract, like the brain and heart, may be rendered ischemic by prolonged CPB, DHCA, or low cardiac output syndrome. Renal function is compromised on CPB. This is manifested by the appearance of proteinuria and impaired tubular cellular function immediately after CPB. Renal dysfunction from ischemia is also common. Low urine output may occur secondary to secretion of antidiuretic hormone, a response to surgical stress. However, the latter appears to be transitory and usually resolves spontaneously.264 The incidence of acute renal dysfunction after surgery with bypass to correct congenital heart defects is 17%, ranging from 0.7% for arterial septal defect closure to 59% for arterial switch operations.265 Deep hypothermic cardiac arrest subjects the kidney to additional ischemia reperfusion injury.266Acute renal failure after CPB is uncommon in children, with fewer than 3% requiring dialysis perioperatively.265,267 Infants who undergo cardiac surgery routinely receive diuretics or a peritoneal dialysis catheter, the latter prophylactically in some instances.268,269 Although some have attributed the improved survival with early peritoneal dialysis to the prevention of fluid overload, others have attributed it to a more rapid clearance of CPB-induced proinflammatory cytokines.270 Further study is required to clarify the mechanism of action of early peritoneal dialysis. In our center, neonates and children with a complex heart defect usually receive peritoneal dialysis immediately postoperatively to prevent fluid overload.
Recovery of hepatic and gastrointestinal function follows hemodynamic recovery, but may require several days. Therapy is mainly supportive. Splanchnic and renal perfusion can be monitored noninvasively using somatic oximetry. Somatic oxygenation may predict renal dysfunction and predict organ failure. Interventions based on the somatic NIRS may improve outcome.271
Immune System Effects
Leukocytes are activated by the CPB circuit, although their numbers may be depleted by leukocyte filters, which are sometimes used to attenuate the inflammatory response. Despite the theoretical possibility that this may increase the risk of infection or neutrophil dysfunction, this has not been observed in published studies or clinical practice.272
Endocrine System Effects
The magnitude of the inflammatory and endocrine responses after cardiac surgery depends in part on the duration of the surgical procedure and CPB.273 In children undergoing brief operating times, postoperative blood concentrations of cortisol, adrenocorticotropic hormone, and β-endorphins are significantly greater than those in children undergoing prolonged operation times. In contrast, the serum concentrations of the proinflammatory cytokines IL-6, IL-1β, and TNF-α are similar in the two groups. Adrenocorticotropic hormone and cortisol concentrations correlated positively with the blood concentrations of IL-1β, IL-6, and TNF-α in the group of children with prolonged operation times.
The plasma concentrations of both epinephrine and cortisol increase after cardiac surgery.274 In children, pre- and post-bypass cortisol and norepinephrine increase significantly during isoflurane anesthesia when 2 µg/kg of fentanyl is used rather than 25, 50, 100, or 150 µg/kg.275 No significant increase in the blood concentrations of these hormones occurred with any of the fentanyl doses of 25 µg/kg or greater. In addition to cardiovascular stability, continued use of larger doses of opioids during bypass minimizes the stress responses and stabilizes hemodynamics during and after bypass, but may delay recovery.276 Also, growth hormone, glucose and insulin, lactate, glutamate, aspartate, and free fatty acid concentrations increase after cardiac surgery, whereas total triiodothyronine concentrations decrease.277 Limiting the amount of opioids balances the negative effects of inflammation and stress with the opportunity to fast-track children’s recovery after surgery for congenital heart disease.278,279
Andropoulos DB, Stayer SA, McKenzie ED, Fraser CD, Jr. Regional low-flow perfusion provides comparable blood flow and oxygenation to both cerebral hemispheres during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2003;126:1712–1717.
du Plessis AJ, Jonas RA, Wypij D, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1000.
Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765–1774.
Miller BE, Mochizuki T, Levy JH, et al. Predicting and treating coagulopathies after cardiopulmonary bypass in children. Anesth Analg. 1997;85:1196–1202.
Newburger JW, Jonas RA, Soul J, et al. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135:347–354.
Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1403.
Wypij D, Jonas RA, Bellinger DC, et al. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg. 2008;135:355–360.
1 Hessel EA, Hill AG. Circuitry and cannulation techniques. In: Gravlee GP, Davis RF, Kurusz M, Utley JR, eds. Cardiopulmonary bypass: principles and practice. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:69–97.
2 Larson DF, Bowers M, Schechner HW. Neutrophil activation during cardiopulmonary bypass in paediatric and adult patients. Perfusion. 1996;11:21–27.
3 Butler J, Pathi VL, Paton RD, et al. Acute-phase responses to cardiopulmonary bypass in children weighing less than 10 kilograms. Ann Thorac Surg. 1996;62:538–542.
4 Jensen E, Andreasson S, Bengtsson A, et al. Changes in hemostasis during pediatric heart surgery: impact of a biocompatible heparin-coated perfusion system. Ann Thorac Surg. 2004;77:962–967.
5 te Velthuis H, Baufreton C, Jansen PG, et al. Heparin coating of extracorporeal circuits inhibits contact activation during cardiac operations. J Thorac Cardiovasc Surg. 1997;114:117–122.
6 Sanchez J, Elgue G, Riesenfeld J, Olsson P. Studies of adsorption, activation, and inhibition of factor XII on immobilized heparin. Thromb Res. 1998;89:41–50.
7 Miyaji K, Hannan RL, Ojito J, et al. Heparin-coated cardiopulmonary bypass circuit: clinical effects in pediatric cardiac surgery. J Card Surg. 2000;15:194–198.
8 Cooley DA. Development of the roller pump for use in the cardiopulmonary bypass circuit. Tex Heart Inst J. 1987;14:112–118.
9 Haines N, Wang S, Kunselman A, Myers J, Undar A. Comparison of pumps and oxygenators with pulsatile and nonpulsatile modes in an infant cardiopulmonary bypass model. Artif Organs. 2009;33:993–1001.
10 Harris EA, Seelye ER, Barratt-Boyes BG. Respiratory and metabolic acid-base changes during cardiopulmonary bypass in man. Br J Anaesth. 1970;42:912–922.
11 Hayhoe M, Bellomo R, Liu G, et al. Role of the splanchnic circulation in acid-base balance during cardiopulmonary bypass. Crit Care Med. 1999;27:2671–2677.
12 McKnight CK, Elliott MJ, Pearson DT, et al. The effects of four different crystalloid bypass pump-priming fluids upon the metabolic response to cardiac operation. J Thorac Cardiovasc Surg. 1985;90:97–111.
13 Liskaser FJ, Bellomo R, Hayhoe M, et al. Role of pump prime in the etiology and pathogenesis of cardiopulmonary bypass-associated acidosis. Anesthesiology. 2000;93:1170–1173.
14 Miller BE, Mochizuki T, Levy JH, et al. Predicting and treating coagulopathies after cardiopulmonary bypass in children. Anesth Analg. 1997;85:1196–1202.
15 Chan AK, Leaker M, Burrows FA, et al. Coagulation and fibrinolytic profile of paediatric patients undergoing cardiopulmonary bypass. Thromb Haemost. 1997;77:270–277.
16 Williams GD, Bratton SL, Ramamoorthy C. Factors associated with blood loss and blood product transfusions: a multivariate analysis in children after open-heart surgery. Anesth Analg. 1999;89:57–64.
17 Mou SS, Giroir BP, Molitor-Kirsch EA, et al. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351:1635–1644.
18 Hamilton MS, Menitove JE. Pump priming in heart surgery in infants. N Engl J Med. 2005;352:731.
19 McCall MM, Blackwell MM, Smyre JT, et al. Fresh frozen plasma in the pediatric pump prime: a prospective, randomized trial. Ann Thorac Surg. 2004;77:983–987.
20 Oliver WC, Jr., Beynen FM, Nuttall GA, et al. Blood loss in infants and children for open heart operations: albumin 5% versus fresh-frozen plasma in the prime. Ann Thorac Surg. 2003;75:1506–1512.
21 Sumpelmann R, Schurholz T, Thorns E, Hausdorfer J. Acid-base, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11:169–173.
22 Ranucci M, Carlucci C, Isgró G, et al. Duration of red blood cell storage and outcomes in pediatric cardiac surgery: an association found for pump prime blood. Crit Care. 2009;13:R207.
23 Keidan I, Amir G, Mandel M, Mishali D. The metabolic effects of fresh versus old stored blood in the priming of cardiopulmonary bypass solution for pediatric patients. J Thorac Cardiovasc Surg. 2004;127:949–952.
24 Elliott MJ, Allen A. Aprotinin in pediatric cardiac surgery. Perfusion. 1990;5:73–76.
25 D’Errico CC, Shayevitz JR, Martindale SJ, et al. The efficacy and cost of aprotinin in children undergoing reoperative open heart surgery. Anesth Analg. 1996;83:1193–1199.
26 Miller BE, Tosone SR, Tam VK, et al. Hematologic and economic impact of aprotinin in reoperative pediatric cardiac operations. Ann Thorac Surg. 1998;66:535–540.
27 Pasquali SK, Li JS, He X, et al. Comparative analysis of antifibrinolytic medications in pediatric heart surgery. J Thorac Cardiovasc Surg. 2012;143:550–557.
28 Carrel TP, Schwanda M, Vogt PR, Turina MI. Aprotinin in pediatric cardiac operations: a benefit in complex malformations and with high-dose regimen only. Ann Thorac Surg. 1998;66:153–158.
29 Speekenbrink RG, Wildevuur CR, Sturk A, Eijsman L. Low-dose and high-dose aprotinin improve hemostasis in coronary operations. J Thorac Cardiovasc Surg. 1996;112:523–530.
30 Spray TL. Use of aprotinin in pediatric organ transplantation. Ann Thorac Surg. 1998;65:S71–S73.
31 Costello JM, Backer CL, de Hoyos A, et al. Aprotinin reduces operative closure time and blood product use after pediatric bypass. Ann Thorac Surg. 2003;75:1261–1266.
32 Graham EM, Atz AM, Gillis J, et al. Differential effects of aprotinin and tranexamic acid on outcomes and cytokine profiles in neonates undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2012;143:1069–1076.
33 Mossinger H, Dietrich W, Braun SL, et al. High-dose aprotinin reduces activation of hemostasis, allogeneic blood requirement, and duration of postoperative ventilation in pediatric cardiac surgery. Ann Thorac Surg. 2003;75:430–437.
34 Rahman A, Ustunda B, Burma O, et al. Does aprotinin reduce lung reperfusion damage after cardiopulmonary bypass? Eur J Cardiothorac Surg. 2000;18:583–588.
35 Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs. 1985;29:236–261.
36 Royston D. High-dose aprotinin therapy: a review of the first five years’ experience. J Cardiothorac Vasc Anesth. 1992;6:76–100.
37 Jaquiss RD, Ghanayem NS, Zacharisen MC, et al. Safety of aprotinin use and reuse in pediatric cardiothoracic surgery. Circulation. 2002;106:I90–I94.
38 D’Errico CC, Munro HM, Bove EL. Pro: the routine use of aprotinin during pediatric cardiac surgery is a benefit. J Cardiothorac Vasc Anesth. 1999;13:782–784.
39 Williams GD, Ramamoorthy C. Con: the routine use of aprotinin during pediatric cardiac surgery is not a benefit. J Cardiothorac Vasc Anesth. 1999;13:785–788.
40 Wilder NS, Kavarana MN, Voelpel Lewis T, et al. Efficacy and safety of Aprotinin in neonatal congenital heart operations. Ann Thorac Surg. 2011;92:958–963.
41 McClure PD, Izsak J. The use of epsilon-aminocaproic acid to reduce bleeding during cardiac bypass in children with congenital heart disease. Anesthesiology. 1974;40:604–608.
42 Williams GD, Bratton SL, Riley EC, Ramamoorthy C. Efficacy of epsilon-aminocaproic acid in children undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1999;13:304–308.
43 Zonis Z, Seear M, Reichert C, et al. The effect of preoperative tranexamic acid on blood loss after cardiac operations in children. J Thorac Cardiovasc Surg. 1996;111:982–987.
44 Isetta C, Garraffo R, Merville C, et al. Pharmacokinetic study of tranexamic acid during cardiac surgery with cardiopulmonary bypass. Anesth Analg. 1996;82:S199.
45 Martin K, Breuer T, Gertler R, et al. Tranexamic acid versus ε-aminocaproic acid: efficacy and safety in paediatric cardiac surgery. Eur J Cardiothorac Surg. 2011;39:892–897.
46 Ririe DG, James RL, O’Brien JJ, et al. The pharmacokinetics of epsilon-aminocaproic acid in children undergoing surgical repair of congenital heart defects. Anesth Analg. 2002;94:44–49.
47 Alsoufi B, Boshkov LK, Kirby A, et al. Heparin-induced thrombocytopenia (HIT) in pediatric cardiac surgery: an emerging cause of morbidity and mortality. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:155–171.
48 Boning A, Morschheuser T, Blase U, et al. Incidence of heparin-induced thrombocytopenia and therapeutic strategies in pediatric cardiac surgery. Ann Thorac Surg. 2005;79:62–65.
49 Mejak B, Giacomuzzi C, Shen I, et al. Cardiopulmonary bypass using argatroban as an anticoagulant for a 6.0-kg pediatric patient. J Extra Corpor Technol. 2005;37:303–305.
50 Dyke PC, Russo P, Mureebe L, et al. Argatroban for anticoagulation during cardiopulmonary bypass in an infant. Paediatr Anaesth. 2005;15:328–333.
51 Iannoli ED, Eaton MP, Shapiro JR. Bidirectional Glenn shunt surgery using lepirudin anticoagulation in an infant with heparin-induced thrombocytopenia with thrombosis. Anesth Analg. 2005;101:74–76.
52 Avidan MS, Levy JH, van Aken H, et al. Recombinant human antithrombin III restores heparin responsiveness and decreases activation of coagulation in heparin-resistant patients during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;130:107–113.
53 Odegard KC, Zurakowski D, DiNardo JA, et al. Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage I through Fontan completion. J Thorac Cardiovasc Surg. 2009;137:934–941.
54 Despotis GJ, Levine V, Joist JH, et al. Antithrombin III during cardiac surgery: effect on response of activated clotting time to heparin and relationship to markers of hemostatic activation. Anesth Analg. 1997;85:498–506.
55 Kovesi T, Royston D. Pharmacological approaches to reducing allogeneic blood exposure. Vox Sang. 2003;84:2–10.
56 Leibovitch L, Kenet G, Mazor K, et al. Recombinant activated factor VII for life-threatening pulmonary hemorrhage after pediatric cardiac surgery. Pediatr Crit Care Med. 2003;4:444–446.
57 Warren OJ, Rogers PL, Watret AL, et al. Defining the role of recombinant activated factor VII in pediatric cardiac surgery: where should we go from here? Pediatric Critical Care Medicine. 2009;10:572–582.
58 Holzmann L, Finn H, Lichtman HC, Harmel MH. Anesthesia in patients with sickle cell disease: a review of 112 cases. Anesth Analg. 1969;48:566–572.
59 Koshy M, Weiner SJ, Miller ST, et al. Surgery and anesthesia in sickle cell disease. Cooperative Study of Sickle Cell Diseases. Blood. 1995;86:3676–3684.
60 Manno M, San Biagio PL, Palma MU. The role of pH on instability and aggregation of sickle hemoglobin solutions. Proteins. 2004;55:169–176.
61 McGoron AJ, Joiner CH, Palascak MB, et al. Dehydration of mature and immature sickle red blood cells during fast oxygenation/deoxygenation cycles: role of KCl cotransport and extracellular calcium. Blood. 2000;95:2164–2168.
62 Hoffman JF, Joiner W, Nehrke K, et al. The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells. Proc Natl Acad Sci USA. 2003;100:7366–7371.
63 Brugnara C, Van HT, Tosteson DC. Acid pH induces formation of dense cells in sickle erythrocytes. Blood. 1989;74:487–495.
64 Brugnara C. Sickle cell disease: from membrane pathophysiology to novel therapies for prevention of erythrocyte dehydration. J Pediatr Hematol Oncol. 2003;25:927–933.
65 Leachman RD, Miller WT, Atias IM. Sickle cell trait complicated by sickle cell thrombi after open-heart surgery. Am Heart J. 1967;74:268–270.
66 Heiner M, Teasdale SJ, David T, et al. Aorta-coronary bypass in a patient with sickle cell trait. Can Anaesth Soc J. 1979;26:428–434.
67 Djaiani GN, Cheng DC, Carroll JA, et al. Fast-track cardiac anesthesia in patients with sickle cell abnormalities. Anesth Analg. 1999;89:598–603.
68 Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.
69 Bellet PS, Kalinyak KA, Shukla R, et al. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. N Engl J Med. 1995;333:699–703.
70 Balasundaram S, Duran CG, al-Halees Z, Kassay M. Cardiopulmonary bypass in sickle cell anaemia: report of five cases. J Cardiovasc Surg (Torino). 1991;32:271–274.
71 Frimpong-Boateng K, Amoah AG, Barwasser HM, Kallen C. Cardiopulmonary bypass in sickle cell anaemia without exchange transfusion. Eur J Cardiothorac Surg. 1998;14:527–529.
72 Madan AK, Hartz RS, Major C, et al. Mitral valve replacement in Sickle C disease using intraoperative exchange transfusion. J Card Surg. 1998;13:48–50.
73 Kingsley CP, Chronister T, Cohen DJ, et al. Case 2-1996: anesthetic management of a patient with hemoglobin SS disease and mitral insufficiency for mitral valve repair. J Cardiothorac Vasc Anesth. 1996;10:419–424.
74 Schmalzer EA, Lee JO, Brown AK, et al. Viscosity of mixtures of sickle and normal red cells at varying hematocrit levels: implications for transfusion. Transfusion. 1987;27:228–233.
75 Aldrich TK, Dhuper SK, Patwa NS, et al. Pulmonary entrapment of sickle cells: the role of regional alveolar hypoxia. J Appl Physiol. 1996;80:531–539.
76 Sutton SW, Hunley EK, Duncan MA, et al. Sickle cell disease and aortic valve replacement: use of cardiopulmonary bypass, partial exchange transfusion, platelet sequestration, and continuous hemofiltration. Tex Heart Inst J. 1999;26:283–288.
77 Head CA, Brugnara C, Martinez-Ruiz R, et al. Low concentrations of nitric oxide increase oxygen affinity of sickle erythrocytes in vitro and in vivo. J Clin Invest. 1997;100:1193–1198.
78 Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221.
79 Shibutani K, Komatsu T, Kubal K, et al. Critical level of oxygen delivery in anesthetized man. Crit Care Med. 1983;11:640–643.
80 Lieberman JA, Weiskopf RB, Kelley SD, et al. Critical oxygen delivery in conscious humans is less than 7.3 mL O2 × kg(−1) × min(−1). Anesthesiology. 2000;92:407–413.
81 Viele MK, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah’s Witnesses. Transfusion. 1994;34:396–401.
82 Kawamura M, Minamikawa O, Yokochi H, et al. Safe limit of hemodilution in cardiopulmonary bypass—comparative analysis between cyanotic and acyanotic congenital heart disease. Jpn J Surg. 1980;10:206–211.
83 Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765–1774.
84 Eyster E, Bernene J. Nosocomial anemia. JAMA. 1973;223:73–74.
85 Laupacis A, Fergusson D. Drugs to minimize perioperative blood loss in cardiac surgery: meta-analyses using perioperative blood transfusion as the outcome. The International Study of Perioperative Transfusion (ISPOT) Investigators. Anesth Analg. 1997;85:1258–1267.
86 Laupacis A, Fergusson D. Erythropoietin to minimize perioperative blood transfusion: a systematic review of randomized trials. The International Study of Perioperative Transfusion (ISPOT) Investigators. Transfus Med. 1998;8:309–317.
87 Coyle D, Lee KM, Fergusson DA, Laupacis A. Cost effectiveness of epoetin-alpha to augment preoperative autologous blood donation in elective cardiac surgery. Pharmacoeconomics. 2000;18:161–171.
88 Bell K, Stott K, Sinclair CJ, et al. A controlled trial of intraoperative autologous transfusion in cardiothoracic surgery measuring effect on transfusion requirements and clinical outcome. Transfus Med. 1992;2:295–300.
89 Napier JA, Bruce M, Chapman J, et al. Guidelines for autologous transfusion. II. Perioperative haemodilution and cell salvage. British Committee for Standards in Haematology Blood Transfusion Task Force. Autologous Transfusion Working Party. Br J Anaesth. 1997;78:768–771.
90 Klein HG. The prospects for red-cell substitutes. N Engl J Med. 2000;342:1666–1668.
91 Cormack JE, Forest RJ, Groom RC, Morton J. Size makes a difference: use of a low-prime cardiopulmonary bypass circuit and autologous priming in small adults. Perfusion. 2000;15:129–135.
92 Alexi-Meskishvili V, Stiller B, Koster A, et al. Correction of congenital heart defects in Jehovah’s Witness children. Thorac Cardiovasc Surg. 2004;52:141–146.
93 Nadkarni UB, Shah AM, Deshmukh CT. Noninvasive respiratory monitoring in paediatric intensive care unit. J Postgrad Med. 2000;46:149–152.
94 Henling CE, Carmichael MJ, Keats AS, Cooley DA. Cardiac operation for congenital heart disease in children of Jehovah’s Witnesses. J Thorac Cardiovasc Surg. 1985;89:914–920.
95 van Son JA, Hovaguimian H, Rao IM, et al. Strategies for repair of congenital heart defects in infants without the use of blood. Ann Thorac Surg. 1995;59:384–388.
96 Codispoti M, Ludlam CA, Simpson D, Mankad PS. Individualized heparin and protamine management in infants and children undergoing cardiac operations. Ann Thorac Surg. 2001;71:922–927.
97 Melrose DG, Dreyer B, Bentall HH, Baker JB. Elective cardiac arrest. Lancet. 1955;269:21–22.
98 Follette D, Fey K, Mulder D, et al. Prolonged safe aortic clamping by combining membrane stabilization, multidose cardioplegia, and appropriate pH reperfusion. J Thorac Cardiovasc Surg. 1977;74:682–694.
99 Corno AF, Bethencourt DM, Laks H, et al. Myocardial protection in the neonatal heart: a comparison of topical hypothermia and crystalloid and blood cardioplegic solutions. J Thorac Cardiovasc Surg. 1987;93:163–172.
100 Pearl JM, Laks H, Drinkwater DC, et al. Normocalcemic blood or crystalloid cardioplegia provides better neonatal myocardial protection than does low-calcium cardioplegia. J Thorac Cardiovasc Surg. 1993;105:201–206.
101 Durandy Y. Pediatric myocardial protection. Curr Opin Cardiol. 2008;23:85–90.
102 Raja S. Is blood cardioplegia superior to crystalloid cardioplegia in pediatric cardiac surgery? Interact Cardiovasc Thorac Surg. 2008;7:498–499.
103 Aoki M, Nomura F, Kawata H, Mayer JE, Jr. Effect of calcium and preischemic hypothermia on recovery of myocardial function after cardioplegic ischemia in neonatal lambs. J Thorac Cardiovasc Surg. 1993;105:207–212.
104 Kronon M, Bolling KS, Allen BS, et al. The relationship between calcium and magnesium in pediatric myocardial protection. J Thorac Cardiovasc Surg. 1997;114:1010–1019.
105 Lareau S, Boyle AJ, Stewart LC, et al. The role of magnesium in myocardial preservation. Magnes Res. 1995;8:85–97.
106 Martindale SJ, Shayevitz JR, D’Errico C. The activated coagulation time: suitability for monitoring heparin effect and neutralization during pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:458–463.
107 Miller BE, Guzzetta NA, Tosone SR, et al. Tissue factor-activated thromboelastograms in children undergoing cardiac surgery: baseline values and comparisons. Anesth Analg. 2003;97:1289–1293.
108 Despotis GJ, Joist JH, Hogue CW, Jr., et al. More effective suppression of hemostatic system activation in patients undergoing cardiac surgery by heparin dosing based on heparin blood concentrations rather than ACT. Thromb Haemost. 1996;76:902–908.
109 Koster A, Chew D, Kuebler W, et al. High antithrombin III levels attenuate hemostatic activation and leukocyte activation during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;126:906–907.
110 Gottlieb EA, Fraser CD, Jr., Andropoulos DB, Diaz LK. Bilateral monitoring of cerebral oxygen saturation results in recognition of aortic cannula malposition during pediatric congenital heart surgery. Paediatr Anaesth. 2006;16:787–789.
111 Chopp M, Chen H, Dereski MO, Garcia JH. Mild hypothermic intervention after graded ischemic stress in rats. Stroke. 1991;22:37–43.
112 Shum-Tim D, Nagashima M, Shinoka T, et al. Postischemic hyperthermia exacerbates neurologic injury after deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1998;116:780–792.
113 Bridges KG, Reichard GA, Jr., MacVaugh H, III., et al. Effect of phentolamine in controlling temperature and acidosis associated with cardiopulmonary bypass. Crit Care Med. 1985;13:72–76.
114 Stoen R, Sessler DI. The thermoregulatory threshold is inversely proportional to isoflurane concentration. Anesthesiology. 1990;72:822–827.
115 Muravchick S, Conrad DP, Vargas A. Peripheral temperature monitoring during cardiopulmonary bypass operation. Ann Thorac Surg. 1980;29:36–41.
116 Ramsay JG, Ralley FE, Whalley DG, et al. Site of temperature monitoring and prediction of afterdrop after open heart surgery. Can Anaesth Soc J. 1985;32:607–612.
117 Kim WG, Yang JH. End-point temperature of rewarming after hypothermic cardiopulmonary bypass in pediatric patients. Artif Organs. 2005;29:876–879.
118 Barstad RM, Stephens RW, Hamers MJ, Sakariassen KS. Protamine sulphate inhibits platelet membrane glycoprotein Ib-von Willebrand factor activity. Thromb Haemost. 2000;83:334–337.
119 Seifert HA, Jobes DR, Ten HT, et al. Adverse events after protamine administration following cardiopulmonary bypass in infants and children. Anesth Analg. 2003;97:383–389.
120 Muangmingsuk V, Tremback TF, Muangmingsuk S, et al. The effect on the hemodynamic stability of varying calcium chloride administration during protamine infusion in pediatric open-heart patients. Anesth Analg. 2001;93:92–95.
121 Boigner H, Lechner E, Brock H, et al. Life threatening cardiopulmonary failure in an infant following protamine reversal of heparin after cardiopulmonary bypass. Paediatr Anaesth. 2001;11:729–732.
122 Greeley WJ, Kern FH, Ungerleider RM, et al. The effect of hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral metabolism in neonates, infants, and children. J Thorac Cardiovasc Surg. 1991;101:783–794.
123 Swan H. The importance of acid-base management for cardiac and cerebral preservation during open heart operations. Surg Gynecol Obstet. 1984;158:391–414.
124 Kurth CD, O’Rourke MM, O’Hara IB. Comparison of pH-stat and alpha-stat cardiopulmonary bypass on cerebral oxygenation and blood flow in relation to hypothermic circulatory arrest in piglets. Anesthesiology. 1998;89:110–118.
125 Patel RL, Turtle MR, Chambers DJ, et al. Alpha-stat acid-base regulation during cardiopulmonary bypass improves neuropsychologic outcome in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1996;111:1267–1279.
126 Priestley MA, Golden JA, O’Hara IB, et al. Comparison of neurologic outcome after deep hypothermic circulatory arrest with alpha-stat and pH-stat cardiopulmonary bypass in newborn pigs. J Thorac Cardiovasc Surg. 2001;121:336–343.
127 Hindman BJ, Dexter F, Cutkomp J, Smith T. pH-stat management reduces the cerebral metabolic rate for oxygen during profound hypothermia (17 degrees C): a study during cardiopulmonary bypass in rabbits. Anesthesiology. 1995;82:983–995.
128 Aoki M, Nomura F, Stromski ME, et al. Effects of pH on brain energetics after hypothermic circulatory arrest. Ann Thorac Surg. 1993;55:1093–1103.
129 Hiramatsu T, Miura T, Forbess JM, et al. pH strategies and cerebral energetics before and after circulatory arrest. J Thorac Cardiovasc Surg. 1995;109:948–957.
130 Miller AL, Corddry DH. Brain carbohydrate metabolism in developing rats during hypercapnia. J Neurochem. 1981;36:1202–1210.
131 Yoshioka H, Miyake H, Smith DS, et al. Effects of hypercapnia on ECoG and oxidative metabolism in neonatal dog brain. J Appl Physiol. 1995;78:2272–2278.
132 Callaghan PB, Lister J, Paton BC, Swan H. Effect of varying carbon dioxide tensions on the oxyhemoglobin dissociation curves under hypothermic conditions. Ann Surg. 1961;154:903–910.
133 Duelli R, Kuschinsky W. Changes in brain capillary diameter during hypocapnia and hypercapnia. J Cereb Blood Flow Metab. 1993;13:1025–1028.
134 Sakamoto T, Kurosawa H, Shin’oka T, et al. The influence of pH strategy on cerebral and collateral circulation during hypothermic cardiopulmonary bypass in cyanotic patients with heart disease: results of a randomized trial and real-time monitoring. J Thorac Cardiovasc Surg. 2004;127:12–19.
135 Jonas RA, Bellinger DC, Rappaport LA, et al. Relation of pH strategy and developmental outcome after hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1993;106:362–368.
136 du Plessis AJ, Jonas RA, Wypij D, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1000.
137 Bellinger DC, Wypij D, du Plessis AJ, et al. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2001;121:374–383.
138 Scallan MJ. Brain injury in children with congenital heart disease. Paediatr Anaesth. 2003;13:284–293.
139 Dahlbacka S, Heikkinen J, Kaakinen T, et al. pH-stat versus alphastat acid-base management strategy during hypothermic circulatory arrest combined with embolic brain injury. Ann Thorac Surg. 2005;79:1316–1325.
140 Nagy ZL, Collins M, Sharpe T, et al. Effect of two different bypass techniques on the serum troponin-T levels in newborns and children: does pH-stat provide better protection? Circulation. 2003;108:577–582.
141 Cook DJ, Orszulak TA, Daly RC. Minimum hematocrit at differing cardiopulmonary bypass temperatures in dogs. Circulation. 1998;98:II170–II174.
142 Sakamoto T, Zurakowski D, Duebener LF, et al. Combination of alpha-stat strategy and hemodilution exacerbates neurologic injury in a survival piglet model with deep hypothermic circulatory arrest. Ann Thorac Surg. 2002;73:180–189.
143 Duebener LF, Sakamoto T, Hatsuoka S, et al. Effects of hematocrit on cerebral microcirculation and tissue oxygenation during deep hypothermic bypass. Circulation. 2001;104:1260–1264.
144 Newburger JW, Jonas RA, Soul J, et al. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135:347–354.
145 Wypij D, Jonas RA, Bellinger DC, et al. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: Results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg. 2008;135:355–360.
146 Dodd RY, Notari EP, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975–979.
147 Kipps AK, Wypij D, Thiagarajan RR, Bacha EA, Newburger JW. Blood transfusion is associated with prolonged duration of mechanical ventilation in infants undergoing reparative cardiac surgery. Pediatr Crit Care Med. 2011;12:52–56.
148 Zimmerman AA, Burrows FA, Jonas RA, Hickey PR. The limits of detectable cerebral perfusion by transcranial Doppler sonography in neonates undergoing deep hypothermic low-flow cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1997;114:594–600.
149 Schears G, Schultz SE, Creed J, et al. Effect of perfusion flow rate on tissue oxygenation in newborn piglets during cardiopulmonary bypass. Ann Thorac Surg. 2003;75:560–565.
150 Karl TR, Hall S, Ford G, et al. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2004;127:213–222.
151 Hovels-Gurich HH, Seghaye MC, Schnitker R, et al. Long-term neurodevelopmental outcomes in school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2002;124:448–458.
152 Tweddell JS, Hoffman GM, Fedderly RT, et al. Phenoxybenzamine improves systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg. 1999;67:161–167.
153 Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106:I82–I89.
154 Motta P, Mossad E, Toscana D, et al. Comparison of phenoxybenzamine to sodium nitroprusside in infants undergoing surgery. J Cardiothorac Vasc Anesth. 2005;19:54–59.
155 Hoffman GM, Mussatto KA, Brosig CL, et al. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2005;130:1094–1100.
156 Koner O, Tekin S, Koner A, et al. Effects of phentolamine on tissue perfusion in pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 1999;13:191–197.
157 Seelye ER, Harris EA, Squire AW, Barratt-Boyes BG. Metabolic effects of deep hypothermia and circulatory arrest in infants during cardiac surgery. Br J Anaesth. 1971;43:449–459.
158 Gazzolo D, Masetti P, Kornacka M, et al. Phentolamine administration increases blood S100B protein levels in pediatric open-heart surgery patients. Acta Paediatr. 2003;92:1427–1432.
159 Abdul-Khaliq H, Schubert S, Fischer T, et al. The effect of continuous treatment with sodium nitroprusside on the serum kinetics of the brain marker protein S-100beta in neonates undergoing corrective cardiac surgery by means of hypothermic cardiopulmonary bypass. Clin Chem Lab Med. 2000;38:1173–1175.
160 Hatsuoka S, Sakamoto T, Stock UA, et al. Effect of L-arginine or nitroglycerine during deep hypothermic circulatory arrest in neonatal lambs. Ann Thorac Surg. 2003;75:197–203.
161 Nakano T, Kado H, Shiokawa Y, et al. The low resistance strategy for the perioperative management of the Norwood procedure. Ann Thorac Surg. 2004;77:908–912.
162 O’Dwyer C, Woodson LC, Conroy BP, et al. Regional perfusion abnormalities with phenylephrine during normothermic bypass. Ann Thorac Surg. 1997;63:728–735.
163 O’Blenes SB, Roy N, Konstantinov I, et al. Vasopressin reversal of phenoxybenzamine-induced hypotension after the Norwood procedure. J Thorac Cardiovasc Surg. 2002;123:1012–1013.
164 Sato K, Watanabe H, Sogawa M, et al. Vasoconstrictor administration during cardiopulmonary bypass affects acid-base balance in infants and children. Artif Organs. 2006;30:101–105.
165 Journois D, Israel-Biet D, Pouard P, et al. High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology. 1996;85:965–976.
166 Friesen RH, Campbell DN, Clarke DR, Tornabene MA. Modified ultrafiltration attenuates dilutional coagulopathy in pediatric open heart operations. Ann Thorac Surg. 1997;64:1787–1789.
167 Ootaki Y, Yamaguchi M, Oshima Y, et al. Effects of modified ultrafiltration on coagulation factors in pediatric cardiac surgery. Surg Today. 2002;32:203–206.
168 Wang MJ, Chiu IS, Hsu CM, et al. Efficacy of ultrafiltration in removing inflammatory mediators during pediatric cardiac operations. Ann Thorac Surg. 1996;61:651–656.
169 Bando K, Turrentine MW, Vijay P, et al. Effect of modified ultrafiltration in high-risk patients undergoing operations for congenital heart disease. Ann Thorac Surg. 1998;66:821–827.
170 Bando K, Vijay P, Turrentine MW, et al. Dilutional and modified ultrafiltration reduces pulmonary hypertension after operations for congenital heart disease: a prospective randomized study. J Thorac Cardiovasc Surg. 1998;115:517–525.
171 Davies MJ, Nguyen K, Gaynor JW, Elliott MJ. Modified ultrafiltration improves left ventricular systolic function in infants after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1998;115:361–369.
172 Huang H, Yao T, Wang W, et al. Continuous ultrafiltration attenuates the pulmonary injury that follows open heart surgery with cardiopulmonary bypass. Ann Thorac Surg. 2003;76:136–140.
173 Chew MS, Brix-Christensen V, Ravn HB, et al. Effect of modified ultrafiltration on the inflammatory response in paediatric open-heart surgery: a prospective, randomized study. Perfusion. 2002;17:327–333.
174 Pearl JM, Manning PB, McNamara JL, et al. Effect of modified ultrafiltration on plasma thromboxane B2, leukotriene B4, and endothelin-1 in infants undergoing cardiopulmonary bypass. Ann Thorac Surg. 1999;68:1369–1375.
175 Kiziltepe U, Uysalel A, Corapcioglu T, et al. Effects of combined conventional and modified ultrafiltration in adult patients. Ann Thorac Surg. 2001;71:684–693.
176 Naik SK, Knight A, Elliott M. A prospective randomized study of a modified technique of ultrafiltration during pediatric open-heart surgery. Circulation. 1991;84:III422–III431.
177 Groom RC, Akl BF, Albus RA, et al. Alternative method of ultrafiltration after cardiopulmonary bypass. Ann Thorac Surg. 1994;58:573–574.
178 Elliott MJ. Ultrafiltration and modified ultrafiltration in pediatric open heart operations. Ann Thorac Surg. 1993;56:1518–1522.
179 Thompson LD, McElhinney DB, Findlay P, et al. A prospective randomized study comparing volume-standardized modified and conventional ultrafiltration in pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2001;122:220–228.
180 Liu J, Ji B, Long C, Li C, Feng Z. Comparative effectiveness of methylprednisolone and zero-balance ultrafiltration on inflammatory response after pediatric cardiopulmonary bypass. Artif Organs. 2007;31:571–575.
181 Sever K, Tansel T, Basaran M, et al. The benefits of continuous ultrafiltration in pediatric cardiac surgery. Scand Cardiovasc J. 2004;38:307–311.
182 Maluf MA, Mangia C, Silva C, et al. Conventional and conventional plus modified ultrafiltration during cardiac surgery in high-risk congenital heart disease. J Cardiovasc Surg (Torino). 2001;42:465–473.
183 Samaja M, Allibardi S, Milano G, et al. Differential depression of myocardial function and metabolism by lactate and H+. Am J Physiol. 1999;276:H3–H8.
184 Dawson PJ, Bjorksten AR, Blake DW, Goldblatt JC. The effects of cardiopulmonary bypass on total and unbound plasma concentrations of propofol and midazolam. J Cardiothorac Vasc Anesth. 1997;11:556–561.
185 d’Hollander AA, Duvaldestin P, Henzel D, et al. Variations in pancuronium requirement, plasma concentration, and urinary excretion induced by cardiopulmonary bypass with hypothermia. Anesthesiology. 1983;58:505–509.
186 Weiner B, Melby MJ, Faraci PA, et al. Cefamandole pharmacokinetics during standard and pulsatile cardiopulmonary bypass. J Clin Pharmacol. 1988;28:655–659.
187 Hynynen M. Binding of fentanyl and alfentanil to the extracorporeal circuit. Acta Anaesthesiol Scand. 1987;31:706–710.
188 Hynynen M, Hammaren E, Rosenberg PH. Propofol sequestration within the extracorporeal circuit. Can J Anaesth. 1994;41:583–588.
189 Kussman BD, Zurakowski D, Sullivan L, et al. Evaluation of plasma fentanyl concentrations in infants during cardiopulmonary bypass with low-volume circuits. J Cardiothorac Vasc Anesth. 2005;19:316–321.
190 Puig MM, Warner W, Tang CK, et al. Effects of temperature on the interaction of morphine with opioid receptors. Br J Anaesth. 1987;59:1459–1464.
191 Holmes PE, Jenden DJ, Taylor DB. The analysis of the mode of action of curare on neuromuscular transmission; the effect of temperature changes. J Pharmacol Exp Ther. 1951;103:382–402.
192 Buzello W, Schluermann D, Schindler M, Spillner G. Hypothermic cardiopulmonary bypass and neuromuscular blockade by pancuronium and vecuronium. Anesthesiology. 1985;62:201–204.
193 Smeulers NJ, Wierda JM, van den Broek L, et al. Effects of hypothermic cardiopulmonary bypass on the pharmacodynamics and pharmacokinetics of rocuronium. J Cardiothorac Vasc Anesth. 1995;9:700–705.
194 Hindman BJ. Sodium bicarbonate in the treatment of subtypes of acute lactic acidosis: physiologic considerations. Anesthesiology. 1990;72:1064–1076.
195 Scheinman MM, Sullivan RW, Hyatt KH. Magnesium metabolism in patients undergoing cardiopulmonary bypass. Circulation. 1969;39:I235–I241.
196 Romero EG, Castillo-Olivares JL, O’Connor F, et al. The importance of calcium and magnesium ions in serum and cerebrospinal fluid during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1973;66:668–672.
197 Alderman EL, Coltart DJ, Wettach GE, Harrison DC. Coronary artery syndromes after sudden propranolol withdrawal. Ann Intern Med. 1974;81:625–627.
198 Neumeister MW, Li G, Williams G, et al. Factors influencing MAC reduction after cardiopulmonary bypass in dogs. Can J Anaesth. 1997;44:1120–1126.
199 Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia: its possible role in cardiac surgery. An investigation of factors governing survival in dogs at low body temperatures. Ann Surg. 1950;132:849–866.
200 Tassani P, Barankay A, Haas F, et al. Cardiac surgery with deep hypothermic circulatory arrest produces less systemic inflammatory response than low-flow cardiopulmonary bypass in newborns. J Thorac Cardiovasc Surg. 2002;123:648–654.
201 Rasmussen LS, Sztuk F, Christiansen M, Elliott MJ. Normothermic versus hypothermic cardiopulmonary bypass during repair of congenital heart disease. J Cardiothorac Vasc Anesth. 2001;15:563–566.
202 Kuniyoshi Y, Koja K, Miyagi K, et al. Myocardial protective effect of hypothermia during extracorporeal circulation—by quantitative measurement of myocardial oxygen consumption. Ann Thorac Cardiovasc Surg. 2003;9:155–162.
203 Croughwell N, Smith LR, Quill T, et al. The effect of temperature on cerebral metabolism and blood flow in adults during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1992;103:549–554.
204 Felfernig M, Blaicher A, Kettner SC, et al. Effects of temperature on partial thromboplastin time in heparinized plasma in vitro. Eur J Anaesthesiol. 2001;18:467–470.
205 Birdi I, Regragui I, Izzat MB, et al. Influence of normothermic systemic perfusion during coronary artery bypass operations: a randomized prospective study. J Thorac Cardiovasc Surg. 1997;114:475–481.
206 Randomised trial of normothermic versus hypothermic coronary bypass surgery. The Warm Heart Investigators. [No authors listed]. Lancet. 1994;343:559–563.
207 Sealy WC, Brown IW, Jr., Young WG, et al. Hypothermia and extracorporeal circulation for open heart surgery: its simplification with a heat exchanger for rapid cooling and rewarming. Ann Surg. 1959;150:627–639.
208 Barratt-Boyes BG, Simpson M, Neutze JM. Intracardiac surgery in neonates and infants using deep hypothermia with surface cooling and limited cardiopulmonary bypass. Circulation. 1971;43:I25–I30.
209 Wong PC, Barlow CF, Hickey PR, et al. Factors associated with choreoathetosis after cardiopulmonary bypass in children with congenital heart disease. Circulation. 1992;86:II118–II126.
210 Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1403.
211 Bellinger DC, Wernovsky G, Rappaport LA, et al. Rapid cooling of infants on cardiopulmonary bypass adversely affects later cognitive function. Circulation. 1988;78:358–363.
212 Brooker RF, Zvara DA, Velvis H, Prielipp RC. Topical ice slurry prevents brain rewarming during deep hypothermic circulatory arrest in newborn sheep. J Cardiothorac Vasc Anesth. 1997;11:591–594.
213 Langley SM, Chai PJ, Miller SE, et al. Intermittent perfusion protects the brain during deep hypothermic circulatory arrest. Ann Thorac Surg. 1999;68:4–12.
214 Hickey PR. Neurologic sequelae associated with deep hypothermic circulatory arrest. Ann Thorac Surg. 1998;65:S65–S69.
215 Tsui SS, Schultz JM, Shen I, Ungerleider RM. Postoperative hypoxemia exacerbates potential brain injury after deep hypothermic circulatory arrest. Ann Thorac Surg. 2004;78:188–196.
216 Sakamoto T, Hatsuoka S, Stock UA, et al. Prediction of safe duration of hypothermic circulatory arrest by near-infrared spectroscopy. J Thorac Cardiovasc Surg. 2001;122:339–350.
217 Pigula FA, Nemoto EM, Griffith BP, Siewers RD. Regional low-flow perfusion provides cerebral circulatory support during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2000;119:331–339.
218 Tchervenkov CI, Korkola SJ, Shum-Tim D, et al. Neonatal aortic arch reconstruction avoiding circulatory arrest and direct arch vessel cannulation. Ann Thorac Surg. 2001;72:1615–1620.
219 Pigula FA, Gandhi SK, Siewers RD, et al. Regional low-flow perfusion provides somatic circulatory support during neonatal aortic arch surgery. Ann Thorac Surg. 2001;72:401–406.
220 Roerick O, Seitz T, Mauser-Weber P, et al. Low-flow perfusion via the innominate artery during aortic arch operations provides only limited somatic circulatory support. Eur J Cardiothorac Surg. 2006;29:517–524.
221 Schultz JM, Karamlou T, Swanson J, et al. Hypothermic low-flow cardiopulmonary bypass impairs pulmonary and right ventricular function more than circulatory arrest. Ann Thorac Surg. 2006;81:474–480.
222 Andropoulos DB, Stayer SA, McKenzie ED, Fraser CD, Jr. Regional low-flow perfusion provides comparable blood flow and oxygenation to both cerebral hemispheres during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2003;126:1712–1717.
223 Andropoulos DB, Stayer SA, McKenzie ED, Fraser CD, Jr. Novel cerebral physiologic monitoring to guide low-flow cerebral perfusion during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2003;125:491–499.
224 Greeley WJ, Kern FH, Ungerleider RM, Kisslo JA. Intramyocardial air causes right ventricular dysfunction after repair of a congenital heart defect. Anesthesiology. 1990;73:1042–1046.
225 Hammer S, Loeff M, Reichenspurner H, et al. Effect of cardiopulmonary bypass on myocardial function, damage and inflammation after cardiac surgery in newborns and children. Thorac Cardiovasc Surg. 2001;49:349–354.
226 Seghaye MC, Grabitz RG, Duchateau J, et al. Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg. 1996;112:687–697.
227 Paulus WJ. Cytokines and heart failure. Heart Fail Monit. 2000;1:50–56.
228 Journois D, Pouard P, Greeley WJ, et al. Hemofiltration during cardiopulmonary bypass in pediatric cardiac surgery: effects on hemostasis, cytokines, and complement components. Anesthesiology. 1994;81:1181–1189.
229 Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:745–754.
230 Hoffman TM, Wernovsky G, Atz AM, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRI-MACORP) study. Prophylactic Intravenous Use of Milrinone After Cardiac Operation in Pediatrics. Am Heart J. 2002;143:15–21.
231 Schulze-Neick I, Li J, Reader JA, et al. The endothelin antagonist BQ123 reduces pulmonary vascular resistance after surgical intervention for congenital heart disease. J Thorac Cardiovasc Surg. 2002;124:435–441.
232 Kadosaki M, Kawamura T, Oyama K, et al. Usefulness of nitric oxide treatment for pulmonary hypertensive infants during cardiac anesthesia. Anesthesiology. 2002;96:835–840.
233 Shekerdemian LS, Ravn HB, Penny DJ. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2002;165:1098–1102.
234 Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91:307–310.
235 Haraldsson A, Kieler-Jensen N, Ricksten SE. Inhaled prostacyclin for treatment of pulmonary hypertension after cardiac surgery or heart transplantation: a pharmacodynamic study. J Cardiothorac Vasc Anesth. 1996;10:864–868.
236 Griese M, Wilnhammer C, Jansen S, Rinker C. Cardiopulmonary bypass reduces pulmonary surfactant activity in infants. J Thorac Cardiovasc Surg. 1999;118:237–244.
237 Gilliland HE, Armstrong MA, McMurray TJ. The inflammatory response to pediatric cardiac surgery: correlation of granulocyte adhesion molecule expression with postoperative oxygenation. Anesth Analg. 1999;89:1188–1191.
238 Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999;68:1107–1115.
239 Serraf A, Robotin M, Bonnet N, et al. Alteration of the neonatal pulmonary physiology after total cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1997;114:1061–1069.
240 Stayer SA, Diaz LK, East DL, et al. Changes in respiratory mechanics among infants undergoing heart surgery. Anesth Analg. 2004;98:49–55.
241 Andropoulos DB, Stayer SA, Diaz LK, Ramamoorthy C. Neurological monitoring for congenital heart surgery. Anesth Analg. 2004;99:1365–1375.
242 Watzman HM, Kurth CD, Montenegro LM, et al. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93:947–953.
243 Daubeney PE, Pilkington SN, Janke E, et al. Cerebral oxygenation measured by near-infrared spectroscopy: comparison with jugular bulb oximetry. Ann Thorac Surg. 1996;61:930–934.
244 Abdul-Khaliq H, Troitzsch D, Berger F, Lange PE. [Regional transcranial oximetry with near infrared spectroscopy (NIRS) in comparison with measuring oxygen saturation in the jugular bulb in infants and children for monitoring cerebral oxygenation]. Biomed Tech (Berl). 2000;45:328–332.
245 Kussman BD, Wypij D, DiNardo JA, et al. Cerebral oximetry during infant cardiac surgery: evaluation and relationship to early postoperative outcome. Anesth Analg. 2009;108:1122–1131.
246 Kussman BD, Wypij D, Laussen PC, et al. Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation. 2010;122:245–254.
247 Nara M, Chiba Y, Niwa H, et al. Experimental determination of the safe minimum perfusion flow rate for low-flow hypothermic cardiopulmonary bypass. Cardiovasc Surg. 1999;7:715–722.
248 Kurth CD, Steven JM, Nicolson SC. Cerebral oxygenation during pediatric cardiac surgery using deep hypothermic circulatory arrest. Anesthesiology. 1995;82:74–82.
249 Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–I114.
250 Hofer A, Haizinger B, Geiselseder G, et al. Monitoring of selective antegrade cerebral perfusion using near infrared spectroscopy in neonatal aortic arch surgery. Eur J Anaesthesiol. 2005;22:293–298.
251 Dehoux MS, Hernot S, Asehnoune K, et al. Cardiopulmonary bypass decreases cytokine production in lipopolysaccharide-stimulated whole blood cells: roles of interleukin-10 and the extracorporeal circuit. Crit Care Med. 2000;28:1721–1727.
252 Ashraf SS, Tian Y, Zacharrias S, et al. Effects of cardiopulmonary bypass on neonatal and paediatric inflammatory profiles. Eur J Cardiothorac Surg. 1997;12:862–868.
253 Langley SM, Chai PJ, Jaggers JJ, Ungerleider RM. Preoperative high dose methylprednisolone attenuates the cerebral response to deep hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2000;17:279–286.
254 Allen BS, Ilbawi MN. Hypoxia, reoxygenation and the role of systemic leukodepletion in pediatric heart surgery. Perfusion. 2001;16(Suppl):19–29.
255 Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–692.
256 Frangogiannis NG, Mendoza LH, Lindsey ML, et al. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–2808.
257 Shum-Tim D, Tchervenkov CI, Laliberte E, et al. Timing of steroid treatment is important for cerebral protection during cardiopulmonary bypass and circulatory arrest: minimal protection of pump prime methylprednisolone. Eur J Cardiothorac Surg. 2003;24:125–132.
258 Gessler P, Hohl V, Carrel T, et al. Administration of steroids in pediatric cardiac surgery: impact on clinical outcome and systemic inflammatory response. Pediatr Cardiol. 2005;26:595–600.
259 Clarizia NA, Manlhiot C, Schwartz SM, et al. Improved outcomes associated with intraoperative steroid use in high-risk pediatric cardiac surgery. Ann Thorac Surg. 2011;91:1222–1227.
260 Pasquali SK, Hall M, Li JS, et al. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation. 2010;122:2123–2130.
261 Allan CK, Newburger JW, McGrath E, et al. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. 2010;111:1244–1251.
262 Manno CS, Hedberg KW, Kim HC, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991;77:930–936.
263 Miller BE, Guzzetta NA, Tosone SR, Levy JH. Rapid evaluation of coagulopathies after cardiopulmonary bypass in children using modified thromboelastography. Anesth Analg. 2000;90:1324–1330.
264 Dittrich S, Priesemann M, Fischer T, et al. Hemorheology and renal function during cardiopulmonary bypass in infants. Cardiol Young. 2001;11:491–497.
265 Kist-van Holthe tot Echten JE, Goedvolk CA, Doornaar MB, et al. Acute renal insufficiency and renal replacement therapy after pediatric cardiopulmonary bypass surgery. Pediatr Cardiol. 2001;22:321–326.
266 Dittrich S, Priesemann M, Fischer T, et al. Circulatory arrest and renal function in open-heart surgery on infants. Pediatr Cardiol. 2002;23:15–19.
267 Picca S, Principato F, Mazzera E, et al. Risks of acute renal failure after cardiopulmonary bypass surgery in children: a retrospective 10-year case-control study. Nephrol Dial Transplant. 1995;10:630–636.
268 Sorof JM, Stromberg D, Brewer ED, et al. Early initiation of peritoneal dialysis after surgical repair of congenital heart disease. Pediatr Nephrol. 1999;13:641–645.
269 Stromberg D, Fraser CD, Jr., Sorof JM, et al. Peritoneal dialysis: an adjunct to pediatric postcardiotomy fluid management. Tex Heart Inst J. 1997;24:269–277.
270 Bokesch PM, Kapural MB, Mossad EB, et al. Do peritoneal catheters remove pro-inflammatory cytokines after cardiopulmonary bypass in neonates? Ann Thorac Surg. 2000;70:639–643.
271 Hoffman GM, Ghanayem NS, Mussatto KA, Musa N. Perioperative perfusion assessed by somatic NIRS predicts postoperative renal dysfunction. Anesthesiology. 2005;103:A1327.
272 Tarnok A, Schneider P. Pediatric cardiac surgery with cardiopulmonary bypass: pathways contributing to transient systemic immune suppression. Shock. 2001;16(Suppl 1):24–32.
273 Roth-Isigkeit A, Dibbelt L, Schmucker P, Seyfarth M. The immune-endocrine interaction varies with the duration of the inflammatory process in cardiac surgery patients. J Neuroendocrinol. 2000;12:546–552.
274 Vogeser M, Felbinger TW, Kilger E, et al. Corticosteroid-binding globulin and free cortisol in the early postoperative period after cardiac surgery. Clin Biochem. 1999;32:213–216.
275 Duncan HP, Cloote A, Weir PM, et al. Reducing stress responses in the pre-bypass phase of open heart surgery in infants and young children: a comparison of different fentanyl doses. Br J Anaesth. 2000;84:556–564.
276 Anand KJ, Hansen DD, Hickey PR. Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology. 1990;73:661–670.
277 Rumelin A, Nietgen G, Pirlich M, et al. Postoperative pattern of various hormonal and metabolic variables: a pilot study in patients without complications following cardiac surgery. Curr Med Res Opin. 1999;15:339–348.
278 Yamasaki Y, Shime N, Miyazaki T, et al. Fast-track postoperative care for neonatal cardiac surgery: a single-institute experience. J Anesth. 2011;25:321–329.
279 Alghamdi AA, Singh SK, Hamilton BC, et al. Early extubation after pediatric cardiac surgery: systematic review, meta-analysis, and evidence-based recommendations. J Card Surg. 2010;25:586–595.

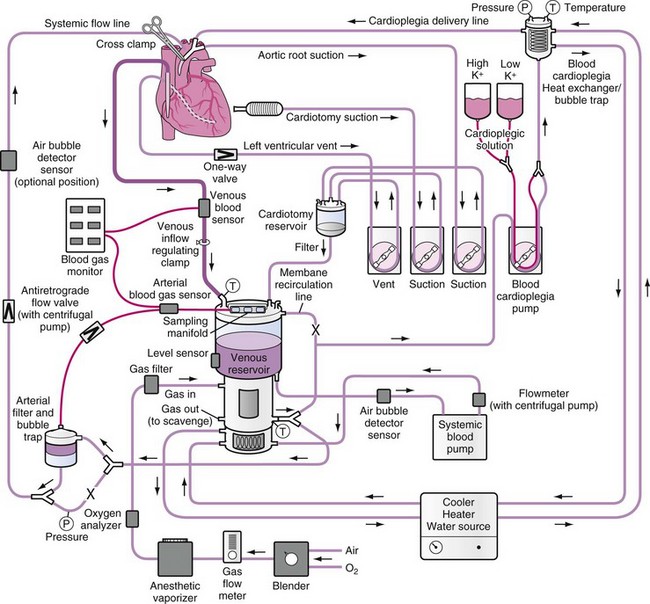

 -inch tubing to 0.75 mL per 10 cm of a
-inch tubing to 0.75 mL per 10 cm of a  -inch tubing. The limiting factor, however, is the necessary flow. For a
-inch tubing. The limiting factor, however, is the necessary flow. For a  -inch arterial line, a maximum flow of 1.8 L/min was established as the point at which the Reynolds number reaches a value of 1000, indicating a change to turbulent flow. Modified ultrafiltration at the end of CPB through a fluid warmer line to prevent heat loss or continuous ultrafiltration has been used. The venous line and the reservoir are emptied before discontinuation of bypass, the field is suctioned, and all blood is retransfused through the arterial line. Decannulation is achieved and protamine is given as usual. Crystalloid cardioplegia solution should be evacuated from the field by an external sucker to prevent dilution of the pump volume.
-inch arterial line, a maximum flow of 1.8 L/min was established as the point at which the Reynolds number reaches a value of 1000, indicating a change to turbulent flow. Modified ultrafiltration at the end of CPB through a fluid warmer line to prevent heat loss or continuous ultrafiltration has been used. The venous line and the reservoir are emptied before discontinuation of bypass, the field is suctioned, and all blood is retransfused through the arterial line. Decannulation is achieved and protamine is given as usual. Crystalloid cardioplegia solution should be evacuated from the field by an external sucker to prevent dilution of the pump volume.