Chapter 2. Cardiology
Introduction
In many cases, however, the diagnosis will not be clear-cut. This is particularly true in the elderly, in whom the presentation of acute cardiac problems ranges from sudden confusion to abdominal pain and vomiting. Often, the clinical picture may overlap with non-cardiac conditions. The classic situation is difficulty in differentiating cardiac and respiratory breathlessness in patients with the common combination of COPD and ischaemic heart disease. Other examples include abdominal symptoms in congestive cardiac failure, and cachexia with weight loss in advanced decompensated valvular heart disease.
All these patients will be admitted to the Acute Medical Unit, where the expectation is that they will be assessed, diagnosed and appropriately treated with the minimum of delay. The patients, particularly those who are older than 75 years, frequently have several medical conditions and when admitted are receiving complex treatment regimens. It is often the case that cardiac problems complicate other diseases that themselves were the reason for admission: the patient with anaemia from GI blood loss who develops angina after admission is a typical example.
The task for the nurse is to perform an accurate assessment that takes into account both cardiac and non-cardiac aspects of the patient’s medical history. The patient’s problems must be prioritised: for example, increasing angina will take priority over a recent change in bowel habit, and attacks of nocturnal breathlessness will be more urgent to address than newly diagnosed but stable Type II (non-insulin-dependent) diabetes.
The management of heart disease must be based on an understanding of the underlying conditions, particularly ischaemic heart disease (coronary artery disease), cardiac failure and atrial fibrillation. There have been important advances in the diagnosis and management of these conditions that have altered our approach to patients:
• Active management of myocardial infarction
— the use of troponin-T and other markers for risk stratification
— urgent re-perfusion by medical intervention and/or balloon angioplasty in acute myocardial infarction
— aspirin prophylaxis
— referral of unstable patients for CABG or angioplasty
• New approaches to cardiac failure
— the use of ACE inhibitors (e.g. enalapril)
— the use of low-dose beta-blockers
— new pacing and electrophysiological techniques
— effective antiarrhythmic drugs: amiodarone and flecainide
• Recognition of the frequency and significance of atrial fibrillation
— increased use of anticoagulation in atrial fibrillation
This chapter aims to provide the basis for this understanding by looking at disease mechanisms and by providing a logical approach to the patient with possible heart disease.
Common Clinical Problems
Acute severe breathlessness
Acute severe breathlessness is a common problem on the Acute Medical Unit, but there is often a diagnostic dilemma in deciding between pulmonary oedema and asthma or COPD (→Chapter 3; The breathless patient: the general approach). This chapter looks at the mechanisms and causes of cardiac failure, which will put into context both the clinical features and the management strategy of the patient with pulmonary oedema or congestive failure.
Chest pain and atypical myocardial infarction
The various clinical problems associated with ischaemic heart disease – infarction, angina and silent myocardial ischaemia – will be described. The chapter aims to clarify the management of chest pain, in particular how to assess the urgency of the situation in a new patient.
Atrial fibrillation
Atrial fibrillation is the most common arrhythmia encountered on the emergency ward. It causes cardiac failure, syncope and unexplained dizziness, and can complicate any severe febrile illness such as pneumonia or exacerbations of COPD. Atrial fibrillation is a common cause of stroke and is one of the major indications for anticoagulant therapy. The management of atrial fibrillation encompasses the use of a good assessment technique, a working knowledge of modern cardiac drugs, and the ability to recognise and deal with complications such as cardiac failure and angina in an acutely distressed and anxious patient.
Immediate relevance of heart disease to other acute medical conditions
When assessing an unstable patient with any acute medical condition, it is important to consider the risk posed by any coexisting cardiac problem to the safety of the patient:
• Medium risk
— stable angina
— previous myocardial infarction
— previous cardiac failure
• Low risk
— poorly controlled hypertension
— previous CABG, but now asymptomatic
— angina, but comfortable at a normal walking speed
Any patient within the first category needs to be assessed immediately and with particular care, and there should be no hesitation in involving medical staff if problems arise. In contrast, the cardiac conditions in patients in the medium- and low-risk categories are less likely to be immediately relevant to an acute admission, particularly during the first 24h of their admission. Box 2.1 lists some key questions that arise in the treatment of cardiac patients.
Box 2.1
• Is this chest pain cardiac in origin?
• What is the difference between cor pulmonale and congestive cardiac failure?
• How do I approach acute dyspnoea in an asthmatic with known heart disease?
• The pulse is 135 beats/min: what is the next step?
• Why is this patient cold, clammy and shut down?
• Why are nitrates used to treat angina as well as pulmonary oedema?
• Why can morphine be used for acute LVF but not for treating asthma?
• Does this patient need urgent medical attention?
• How can I make sense of this patient’s drug regimen?
• What can I say to the relatives of this patient with acute pulmonary oedema?
Core Nursing Skills
Application of triage using ABCDE (→p. 2; primary assessment)
The essential skill of cardiac nursing is to establish the safety of the patient. As with any acute illness, this is based on basic observations of the vital signs. The immediate priority is to establish adequate oxygenation and an effective circulation. In pulmonary oedema, for example, the patient can be so ill that other nursing considerations are overshadowed by the need to administer opiates, oxygen and intravenous (i.v.) frusemide to save the patient’s life.
Reassurance of acutely distressed and anxious patients
The patient will be acutely unwell, with a combination of unpleasant and frightening symptoms: severe breathlessness, chest pain, rapid irregular palpitations and overwhelming fatigue. Not surprisingly, cardiac patients are among the most frightened and anxious, and this will not be helped by hearing staff talk of heart ‘failure’ and ‘unstable’ angina. Anxiety worsens breathlessness, can exacerbate angina and will put an extra load on the heart. Reassurance is vital and this is best provided by nurses who understand exactly what is wrong and why and who can explain the details of their management. It is easy to forget, for example, that patients who are having intensive nursing observations and complex tests carried out may feel very anxious that they are not being told what all this information means and whether they are in fact going to recover.
Resuscitation skills
Acute cardiac illness is characterised by rapid changes in the patient’s condition. The most difficult problems encountered on the Acute Medical Unit are fulminating pulmonary oedema with hypoxia and hypotension and the unexpected cardiac arrest. It is therefore important that all the staff are fully trained in CPR and are familiar with the resuscitation equipment.
Coordinated care with the medical staff
There is considerable overlap between nursing and medical responsibilities in the care of patients with acute heart disease. The initial nursing assessment will include important diagnostic information: the nature of the predominant symptoms and the identification of ischaemic chest pain are two obvious examples.
Subsequently, documentation of the trends in the vital signs, urine output and symptomatic improvement is the main way that the patient’s response to treatment is evaluated. It is important that all this information is shared constructively with the medical staff and that the duplication of tasks such as history taking and observations is kept to the absolute minimum, both to save time and to reduce the burden to the patient.
Anticipating problems
With experience backed by a good understanding of the underlying disease mechanisms, some problems can be anticipated and often forestalled.
Obvious examples include the hypotension that can complicate the use of several cardiac drugs and the development of ischaemic chest pain in a patient who develops rapid atrial fibrillation. It can be extremely helpful to assess the way in which the patient has responded to similar situations in the past by examining the hospital records and noting what the patient and their relatives have to say about it.
Cardiac Failure
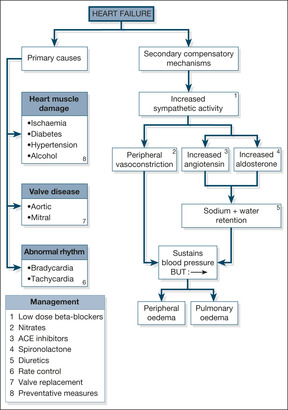
There are two components to heart failure: the primary problem, which damages the function of the heart, and the secondary mechanisms, which are activated to try and compensate for the impaired cardiac function. These are summarised in Fig. 2.1.
Primary Problems in the Heart
Heart muscle damage
The most common cause of chronic heart failure is heart muscle damage from myocardial ischaemia due to coronary artery disease. Most patients give a history of long-standing angina and recurrent myocardial infarctions, but a significant proportion of cases, particularly patients with diabetes, have unrecognised silent ischaemia that presents for the first time as cardiac failure. Hypertension is also an important cause of cardiac failure. The response to long-standing, uncontrolled hypertension is an increase in the bulk and strength of the heart muscle in order to drive blood out of the heart against the increased pressure. In time, however, the muscle fatigues and weakens, leading to reduced cardiac reserve and eventual failure.
Other causes of heart muscle disease are listed in Fig. 2.1. Of these, alcoholic cardiomyopathy is probably the most important, as it is common and will respond to the combination of alcohol withdrawal and thiamine (vitamin B) replacement.
Valvular disease
Although not as common as heart muscle damage, valvular disease can lead to chronic cardiac failure that, with correct management, can be alleviated or controlled. Heart valves normally act as one-way doors which ensure that, when the heart contracts, blood can pass in only one direction. To continue the analogy with a door – as a result of damage (e.g. past rheumatic fever or a congenital fault in the valve’s structure) the valve can become permanently stuck half-open (stenosis), in which case the heart has difficulty expelling blood through it. This has two important consequences: the heart may become overworked, and blood may dam up behind the stenosed valve.
Alternatively, a valve can lose its one-way facility so that, instead of its being normally closed in either systole or diastole (depending on which valve is being considered), blood can pass through it throughout the cardiac cycle: this is known as valve incompetence or valve regurgitation. In valve incompetence/regurgitation, the affected chamber not only has its normal quota of blood to expel each beat, but it also has the added amount that comes back to it as a result of the leaking valve. Thus, in mitral incompetence, during left ventricular contraction blood leaves by the usual route into the aorta, but some blood is also forced back into the left atrium through the incompetent mitral valve. This blood then returns, along with the normal quota of blood, during the subsequent diastole. In aortic incompetence, blood leaks back into the left ventricle during diastole, when the aortic valve should normally be closed. The left ventricle has to deal with this back-flow in addition to the normal amount coming to it from the left atrium. In time the heart muscle fatigues, either because of the extra pressure it has to generate (in, for example, aortic valve stenosis) or because of the extra volume of blood it has to cope with (in, for example, mitral valve incompetence). This fatigue, in turn, in time leads to clinical cardiac failure.
Rhythm disturbance
Abnormally slow (bradyarrhythmias) or, more commonly, abnormally fast (tachyarrhythmias) cardiac rhythms can result in heart failure. A slow pulse leads to a fall-off in the cardiac output, simply because the heart beats less often per minute. A healthy heart compensates for a slow pulse by having more time to fill before each contraction; the heart chamber responds to such stretching by producing a more forceful contraction, expelling an increased amount of blood. A diseased heart is unable to respond in this way, so that a reduced heart rate causes a reduced cardiac output. Fast rhythms disrupt the events that occur during diastole – filling of the heart chambers and blood flow down the coronary arteries. Once again, a healthy heart can cope with a rapid rate, but a diseased heart can soon be compromised, particularly if the coronary arteries are narrowed. The most common rhythm disturbance to cause or contribute to cardiac failure is uncontrolled atrial fibrillation, in which the rate is both rapid and irregular. The onset of rapid atrial fibrillation can often trigger acute heart failure.
Secondary Compensatory Mechanisms in Cardiac Failure
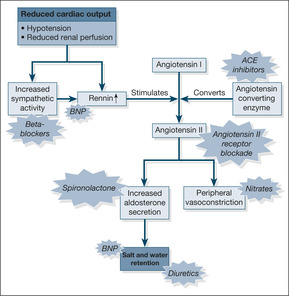
In response to the basic problem of reduced cardiac output, two compensatory mechanisms are activated to try and maintain a normal blood pressure:
1. increased sympathetic nervous system activity (exaggerated stress reaction)
2. activation of the renin-angiotensin-aldosterone hormone system (salt and water retention)
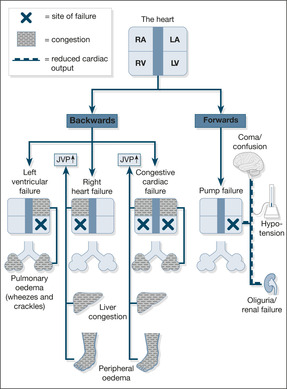
These two mechanisms respectively increase the narrowing of the peripheral arterioles and increase the circulating blood volume – a strategy designed to keep the blood pressure up and maintain a good blood supply to the kidneys. Although this may work for a while, ultimately it leads to an increased load against which the heart must pump (in the case of the narrowed arterioles) and pulmonary congestion and ankle oedema (in the case of the increased circulating blood volume). Pumping against an abnormally high load eventually leads to heart muscle fatigue, so-called pump failure or ‘forward’ heart failure (low blood pressure and poor organ perfusion). Conversely, the congestive component is known as ‘backward’ failure (pulmonary and peripheral oedema). These two components of cardiac failure are summarised in Fig. 2.2.
Modern management of cardiac failure aims to reverse these mechanisms by decreasing the resistance against which the heart must pump and unloading the congestion behind the failing heart. As shown in Fig. 2.3, this is achieved by:
• dilating the arterioles (e.g. GTN, isosorbide mononitrate, hydralazine)
• reducing the salt and water retention (e.g. frusemide and bumetanide)
• inhibiting the renin-angiotensin-aldosterone system (ACE inhibitors, angiotensin receptor blockers, aldosterone antagonists)
• reducing sympathetic activity – the cautious use of sympathetic blockade (beta-blockers) e.g. carvedilol
An important new biomarker of cardiac failure: B-natriuretic peptide
There are two important clinical implications. First, the blood test can help diagnose cardiac failure and will become particularly helpful in patients in whom the underlying cause of their acute breathlessness is unclear. Normal BNP levels in a patient with acute severe breathlessness rules out cardiac failure as the underlying cause. Secondly, drugs with the same chemical properties as BNP are being developed to exploit its effects on reversing the underlying mechanisms in cardiac failure.
Types of Heart Failure
Left heart failure
In left heart failure, the clinical problem is dominated by the build-up of fluid in the lungs, because the failing left ventricle cannot cope with the increased circulating blood volume – blood dams up behind the left side of the heart, producing pulmonary congestion and pulmonary oedema. The symptoms of left heart failure are:
• breathlessness on lying flat (orthopnoea)
• PND
• coughing frothy pink sputum (alveolar oedema)
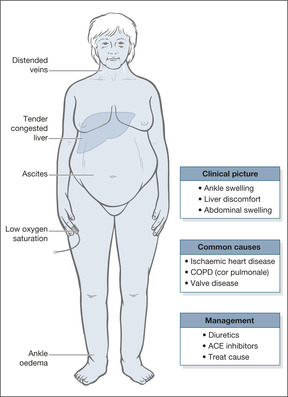
Right heart failure
In right heart failure, the right ventricle cannot cope with the increased circulating blood volume and blood dams up in the veins, leading to congestion of the liver, ankle oedema and oedema over the sacrum. The symptoms of right heart failure are:
• swollen ankles
• liver discomfort
• abdominal swelling (ascites)
Congestive cardiac failure
In congestive cardiac failure, there is a combination of left- and right-sided failure, giving a constellation of symptoms that include:
• breathlessness
• fatigue
• pulmonary and ankle oedema
Diastolic heart failure
Diastolic heart failure is a newly recognised condition which may account for a fifth or more cases of cardiac failure. Typically seen in elderly, obese, hypertensive and diabetic women, diastolic failure results from an abnormally hypertrophied and stiffened ventricle, which contracts well enough but in diastole cannot relax sufficiently to accommodate returning blood. The symptoms – pulmonary and peripheral oedema – are the same as in other forms of failure; the diagnosis relies on the characteristic changes on an echocardiogram.
Clinical Features and Management of Cardiac Failure
Characteristics of patients admitted acutely with cardiac failure
In general, patients with heart failure comprise an elderly group of patients, average age around 75 years, with a strong preponderance of ischaemic heart disease – half will have had a previous infarct. At least half of all these patients will be in atrial fibrillation, and for about a tenth the heart failure will have been triggered by their first attack of atrial fibrillation. One-third will also have COPD and a fifth will be diabetic. In the majority, around 80%, the cause of the failure will be associated with poor heart muscle function; valve problems will only be seen in about 10% of the patients.
It can be helpful to characterise the patient’s pre-existing level of disability. The NYHA classification is the one most commonly used:
• Class I – no limitations (no fatigue, breathlessness or palpitations)
• Class II – slight limitations, fine at rest, but ordinary exertion leads to symptoms
• Class III – marked limitations, fine at rest, but symptoms on less than ordinary exertion
• Class IV – symptoms at rest
Patients in NYHA Classes III and IV have a poor outlook, with 5-year mortality rates approaching 50%.
Principles of managing cardiac failure
2. In addition, or as an alternative, the compensatory mechanisms that are responsible for the clinical picture of chronic cardiac failure are addressed:
— reducing fluid retention (diuretics)
— reducing the load on the heart (vasodilators)
— counteracting excessive sodium retention (ACE inhibitors such as enalapril, angiotensin receptor blockers such as losartan)
— counteracting increased sympathetic activity (low dose beta-blockers).
There is an obvious risk in correcting what are supposed to be compensatory mechanisms – in particular, over-diuresis and excessive use of vasodilators can produce hypotension, with a worsening of tissue perfusion.
3. Cardiac resynchronisation therapy
In some patients with cardiac failure, delayed electrical activation of the left ventricle (recognised on an ECG by the pattern of left bundle branch block) produces a disjointed and inefficient cardiac contraction. If severely impaired, the contractile state can be resynchronised and improved using a novel permanent pacemaker which, unlike conventional pacemakers, paces the left as well as the right ventricle. This technique, termed dual-chamber pacing, will have an increasing role in selected cases of cardiac failure, although the effects on quality of life surpass those on actual survival.
Patients with heart failure are at least six times more likely to die suddenly than the general population. In carefully selected patients, ICDs can be fitted to reduce mortality by treating any lethal ventricular arrhythmia.
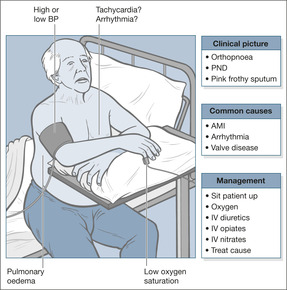
Acute left ventricular failure
Acute LVF (→Fig. 2.4) is a common cause for acute life-threatening breathlessness (the others being acute asthma, tension pneumothorax and pulmonary embolism). Case Study 2.1 illustrates the typical picture in heart failure due to ischaemic heart disease. Interestingly, the patient did not receive an ACE inhibitor after his infarct; these are now given routinely as post-infarct prophylaxis to lessen the risk of developing heart failure.
Case Study 2.1
A 62-year-old man with a previous myocardial infarct was admitted with a 3-week history of increasing breathlessness. He had no other illnesses and was only taking low-dose aspirin. His blood pressure on admission was 180/120 mmHg, the ECG showed heart strain and the chest film showed LVF. Urine testing showed proteinuria. His kidney function was normal.
Initial assessment was of a distressed and overweight man with a resting pulse of 120 beats/min which felt regular, a respiratory rate of 35 breaths/min with an audible wheeze, oxygen saturation on air of 85% and a blood pressure of 180/120 mmHg. His peripheries were cold and clammy and capillary refill time was prolonged. He complained of breathlessness but no chest pain.
The patient was propped upright and given high-flow oxygen; an intravenous cannula was inserted and blood was taken, primarily to check the kidney function, cardiac enzymes, sugar and full blood count.Arterial blood gases were checked.An ECG monitor to identify arrhythmias and an oximeter to confirm adequate oxygenation (saturations more than 94%) were attached to the patient. He was given:
• i.v. frusemide 80mg
• i.v. diamorphine 2.5mg
• cyclizine as an antiemetic
Oxygen saturation increased to 94% and the respiratory rate decreased to 25 breaths/min; his pulse remained high at 120 beats/min.The patient passed 1L of urine in the first 1h of admission, but remained breathless.
An infusion of GTN was started and plans were made for a trial of an ACE inhibitor.
Critical nursing tasks in acute LVF
Ensure the patient is adequately oxygenated
Patients in acute LVF die of hypoxia due to pulmonary oedema. Therefore the safest practice is to start with high-flow oxygen or a non-rebreathing mask, aiming to give the patient at least 60% oxygen. This can be modified in the light of the oxygen saturations or blood gas measurements. The target oxygen saturation is between 94 and 98%. Patients must be propped upright – even if they are hypotensive. Their symptoms will improve because pulmonary oedema lessens in the sitting position.
The Boussignac CPAP mask in acute left ventricular failure
Acute pulmonary oedema with resistant hypoxia which is unresponsive to high-flow oxygen and diuretics may respond to short term (two hours) treatment with CPAP (continuous positive airway pressure). The Boussignac CPAP system uses a small lightweight plastic cylinder that is directly connected to a tight fitting face mask. It requires a standard oxygen supply to administer CPAP pressures up to 10 cm of water and inspired oxygen levels approaching 100%. It is suitable for use on acute medical wards providing there are adequate numbers of appropriately trained staff.
Measure the patient’s blood pressure
Hypertension. Many patients with LVF become acutely hypertensive as a stress response; the blood pressure settles as their condition improves. Less commonly, hypertension is believed to be the cause, rather than the effect, of a patient’s LVF. In such cases it is usually severe (e.g. 210/140 mmHg) and persistent, and has to be lowered as part of the patient’s immediate management. Fortunately some of the drugs used to treat LVF, such as nitrates and diuretics, have a dual effect: they both eliminate pulmonary oedema and reduce the blood pressure.
Hypotension. Hypotension in the setting of LVF is an unfavourable sign, particularly if blood pressure remains low after any abnormal cardiac rhythm has been corrected. It can indicate heart muscle damage, it limits the type and quantity of drugs that can be used – because several cause hypotension themselves – and it can put kidney function at risk. Persistent hypotension is an indication for inotropic support (drugs such as dopamine, which improve cardiac performance), particularly if the urine output is inadequate.
Evaluate the patient’s pulse rate. The analysis of the patient’s pulse rate is not easy from bedside observation, particularly if the patient is acutely ill, and a monitor and 12-lead ECG will be required. Nonetheless, atrial fibrillation (or its close relation, atrial flutter) is likely to be the cause of any rapid pulse of greater than 130 beats/min.
Occasionally, LVF can be accompanied by, and even caused by, an abnormally slow pulse. Drug treatment, in particular with beta-blockers, can be an important cause of this. The bradycardia resolves once the drug is withdrawn.
Important nursing tasks in acute LVF
Does the patient understand and comply with the oxygen therapy?
Ensure that the patient understands the need for continuous (as opposed to symptomatic) administration of oxygen. Is this reflected by appropriate improvements in the oxygen saturation?
Has the patient responded to the diuretic?
An immediate diuresis is a good prognostic sign, whereas failure to establish a diuresis, especially if it is accompanied by hypotension, suggests a problem with maintaining blood flow through the kidneys. It is important to exclude retention as a cause for a low output, particularly in older male patients. It is common practice to obtain an exact assessment of the urine output in critically ill patients by using a urethral catheter.
Is the patient less breathless and in a better clinical condition?
As the treatment takes effect, the patient should become less breathless; he will be less distressed and his respiratory rate will decrease.
As the heart picks up the peripheral circulation will also improve: extremities will be less clammy and the feet and hands will warm up – capillary filling should also improve.
Acute myocardial infarction can present as acute LVF and the chest pain may be ignored in the presence of severe breathlessness. It is therefore important to enquire specifically about pain and ensure that appropriate analgesia – morphine or diamorphine – is prescribed.
Have the blood pressure and pulse returned towards normal?
Acute on chronic congestive cardiac failure
The main clinical features of acute on chronic congestive cardiac failure are bilateral ankle oedema, debilitating tiredness and breathlessness on exertion (→Fig. 2.5). The dominant problem is usually a progressive reduction in exercise tolerance, but in advanced failure there is overwhelming fatigue: patients can be too tired to eat, they cannot cope with personal care and even mild constipation can prove a serious challenge. Patients are often increasingly dependent and depressed, sleep patterns are disturbed (frequent arousals are a consequence of Cheyne-Stokes respiration and paroxysmal nocturnal breathlessness) and the appetite is poor. Cardiac cachexia is a state of malnutrition caused by a combination of anorexia and impaired food absorption in advanced cardiac failure. The weight loss is often accompanied by abdominal pain and swelling due to liver congestion and ascites. Nausea and vomiting can have the same cause, but are also seen in both hypovolaemia and diuretic-induced electrolyte disturbances, particularly hyponatraemia (low sodium).
Important nursing tasks in congestive cardiac failure
Identify possible causes of cardiac failure
Uncontrolled hypertension. Hypertension in the range 160/110 mmHg or higher can lead to cardiac failure, but unfortunately may be ignored or inadequately treated. Contrary to earlier views, systolic and diastolic hypertension are equally damaging – treatment is started at sustained levels of 140-160 mmHg systolic or 90-100 mmHg diastolic. If hypertension is identified, the urine should be checked for protein (hypertensive kidney damage) and a family history should be taken to identify familial kidney diseases (especially polycystic kidneys) and unexplained hypertension. Is the patient’s hypertension compounded by the presence of the other main cardiac risk factors: smoking, high cholesterol or diabetes? Is there glycosuria?
Ischaemic heart disease. A history of a previous infarct, angina or intermittent claudication makes it very likely that the basis for the cardiac failure is ischaemia.
Alcohol-induced cardiac failure. Chronic alcohol abuse often leads to heart muscle damage. The importance in recognising this lies in the good response that is seen with a change in lifestyle and treatment with vitamin replacements. Chronic rheumatic valve disease. Forty years ago, rheumatic fever with cardiac involvement was common in adolescents in the UK. These patients are now in their 60s and 70s, with ageing valve replacements, chronic cardiac failure, complex drug regimens and extensive hospital notes. Hospital records make extremely interesting reading in these patients and are a useful starting point.
Often, the patient’s current problems have been encountered and solved before and will have been documented fully. Examples may include:
• previous episodes of cardiac failure with a documented cause (e.g. attempting to reduce diuretics)
• previous episodes of renal insufficiency triggered by drug interactions (in particular the use of NSAIDs)
• the results of a previous ACE inhibitor trial (in general, the nursing notes give a more detailed account of individual problem episodes during the course of a patient’s stay than do the medical notes)
• previous experience with recurrent arrhythmias and their management
• previous problems with anticoagulant control because of intercurrent illness, antibiotic administration or some other drug interaction
Establish the exact preadmission drug regimen
Patients with chronic heart failure are usually receiving complicated drug regimens that have undergone serial changes as the clinical condition altered. The general practitioner and the hospital clinic may have been adjusting the drugs independently, so that hospital letters and so forth may not reflect the actual situation. Even the instructions on the drug containers may not coincide with what the patient really takes. Important drug toxicity and interactions are common and often unrecognised. Examples include:
• digoxin toxicity, especially in the elderly
• warfarin overactivity due to antibiotic treatment or worsening CCF
• electrolyte imbalance from diuretic therapy
• worsening heart failure due to the use of NSAIDs
• dangerous hyperkalaemia with spironolactone, particularly when combined with ACE inhibitors in diabetes
Recognise significant hypotension
Hypotension is common and important in patients with acute on chronic cardiac failure: a reduced blood flow to the brain and kidneys causes lethargy, near syncope and renal failure. The two most common causes of hypotension are fluid depletion and drug therapy. Fluid depletion may be caused by:
• over-diuresis
• vomiting and poor intake due to congestion of the gastrointestinal tract and liver
• ‘concealed’ gastrointestinal bleeding (patients are usually receiving aspirin/warfarin)
Diuretics, nitrates, ACE inhibitors and virtually all antiarrhythmic drugs can induce postural hypotension, as can other classes of drug that may have been ‘added in’, notably any antidepressant.
In any cardiac patient with a low blood pressure, it is also critical to exclude two other causes: sepsis and acute myocardial ischaemia. The most common sources of infection are Venflon® sites, the respiratory tract (ask the patient to cough and inspect the sputum) and the urine. A rare but serious source of sepsis in these patients is infection of the heart valves (endocarditis). An unrecognised infarct may produce a combination of heart failure and hypotension, which will usually be diagnosed from the ECG and cardiac enzyme results.
Assess the lower limbs and pressure areas
Oedematous legs and a poor cardiac output are potent causes of poor skin nutrition, soft tissue infection and damage to pressure areas, particularly in the immobile elderly patient. In severe congestive failure, the legs may be blistered and weeping fluid, frequently on a background of chronically inflamed and eczematous ankles and calves. The feet should be examined with care, because the poor blood supply and impaired nutrition can lead to fungal infections between the toes (soggy inflamed looking skin) and even areas of local gangrene. Early dermatological advice can be extremely useful in these situations. Patients who are immobile and oedematous develop oedema over the sacrum and are very susceptible to pressure sores. This area must be assessed with care so that appropriate pressure-relieving measures can be introduced.
Assess the fluid balance
This is a problematic area of management in patients with cardiac failure, because there is a difficult balance to be maintained between removing unwanted fluid from congested lungs while retaining enough fluid to support the circulation. The acute nursing assessment will help to differentiate fluid overload from diuretic-induced fluid depletion.
• Symptoms of congestion – breathlessness, PND, orthopnoea, abdominal discomfort, weight gain
• Signs of congestion – increased respiratory rate, ankle oedema, abdominal distension (ascites)
• Symptoms of fluid depletion – thirst, nausea, lethargy, postural dizziness, weight loss
• Signs of fluid depletion – postural hypotension, poor capillary refill, poor urine output
Answering Patients’ and Relatives’ Questions in Cardiac Failure
What does ‘failure’ mean? The heart has been put under temporary strain as a result of infection/angina/blockage of the heart’s circulation. As a result, its efficiency as a pump has tired. We are therefore trying to take the strain off the heart with a variety of drugs, bed rest and oxygen.
What will be the signs of improvement? The observations such as pulse rate and blood pressure will start to return towards normal. The patient will become less breathless. There will be an increase in the urine output as we get rid of some of the excess fluid on the lungs and in the legs.
How can we help? The patient needs complete rest – keep visitors to a minimum to start with. Encourage the patient to keep the oxygen on at all times and to stay propped up in bed. Fluids are likely to be rationed for a time.
What about the diet and fluid intake once he is discharged? Salt-rich foods should be avoided and salt should not be on the table or in the cooking. The main salt-rich foods are cheese, crisps, bacon, sausages and processed meat foods such as pies. Fluid intake is not usually restricted unless persistent fluid retention becomes a particular problem.
What about weight? If there is obesity, aim for a reduction of around 2 lb (1 kg) or so per week towards a realistic target. Weight gain can be the first sign of fluid retention (worsening heart failure) and regular weighing can be very helpful as part of the patient’s follow-up care.
Provide small frequent meals (perhaps with calorie supplements if there is cachexia) and consider water-soluble vitamin supplements (B complex and C). Alcohol may be taken in moderation unless there is an alcoholic myopathy.
What about smoking? Smoking must be stopped immediately.
What about exercise? Regular non-tiring exercise should be encouraged. ‘Reconditioning’ with regular graded exercise will help to improve endurance in some patients with cardiac failure. This probably needs to be supervised in some form of hospital-based programme.
Addressing compliance problems
Lack of compliance is a major factor in triggering readmissions in cardiac disease, especially in the elderly who are often on complex regimens. Side-effects, notably constipation, anorexia and confusion, make the drugs unpopular with the patients and this may be compounded by forgetfulness. It is therefore vital to discuss details of the patient’s regimen with the relatives and carers.
Ischaemic Heart Disease
More patients present to the Acute Medical Unit with the consequences of ischaemic heart disease than with any other single condition. Atheromatous narrowing of the coronary arteries leads to cardiac failure, angina, myocardial infarction and cardiac arrhythmias (→Fig. 2.6). Fortunately, the combination of improved treatment for established disease and the widespread introduction of preventative measures has reduced mortality from coronary artery disease by 10% in the past decade. However, cardiac ischaemia continues to present major issues for the Acute Medical Unit: recognising its various manifestations (heart failure, chest pain, arrhythmias), dealing with atypical clinical features in the elderly, and identifying those patients who need urgent coronary care.
Chest Pain
Approach to the patient who has chest pain on the Acute Medical Unit (→Case Studies 2.2)
Case Studies 2.2
CASE 1
A 75-year-old woman presented with acute sweating, malaise and mild heart burn. She denied chest pain.The ECG was done on admission but not seen for several hours: it showed an acute inferior myocardial infarction. Patients with an atypical history slip through the net. On a CCU the emphasis is on urgent revascularisation for the true infarcts. In the Acute Medical Unit there will be delays because, by their nature, these patients are atypical.A system of triage is needed.
CASE 2
A 90-year-old lady awoke with a left-sided stroke. She was alert and denied any chest pain. Her ECG showed a recent inferior infarction and her cardiac enzymes were elevated.
Context
Chest pain in a patient with a history of angina, previous myocardial infarction or any serious cardiovascular disease should be assumed to come from the heart until proved otherwise. Whereas pain in a young man with a recent chest infection and no risk factors is likely to be pleurisy, in the case of an elderly woman with a recent fall and vertebral collapse, chest pain is as likely to be referred from the back as it is to be angina. The situation in which the pain arises is as important as the nature of the pain in establishing the diagnosis.
Chest pain can be so difficult to describe that it is preferable to be overcautious in your management. After all, if the history was crystal clear, the patient would not be on the Acute Medical Unit in the first place, but in the CCU. This seems to be a particular issue in female patients and in patients from ethnic minorities in whom, for some reason, cardiac symptoms are all too often dismissed as insignificant, leading to diagnostic delays.
Onset
Classically, angina builds up over a few minutes into a dull, heavy, central chest ache and spreads into the jaw, upper arm or wrists. It is not usually localised to a single spot on the chest or associated with local tenderness; however, so-called ‘atypical angina’ can be positional, worsened by breathing, and may even radiate through into the back and to the upper abdomen.
Commonly used descriptions in angina include pressure, weight, chest tightness and constriction. Patients often also describe a feeling of breathlessness. Triggers on the ward include showering, temperature changes and strong emotion.
Common reasons for worsening angina on the Acute Medical Unit are:
• anaemia (e.g. recent upper GI bleed, anaemia due to cancer or chemotherapy)
• sudden onset of atrial fibrillation
• stress of acute illness (especially exacerbations of COPD)
Other important symptoms and signs
Serious anginal episodes are accompanied by pallor, sweating and hypotension. Prolonged pain, lasting for more than 15min, especially if it is accompanied by nausea and vomiting, is more in-keeping with an infarction than with angina, although the distinction can be difficult, particularly in the elderly.
In the elderly, the symptoms of acute ischaemic heart disease are often atypical and include:
• breathlessness without pain
• acute confusion
• dizziness
• abdominal pain
• syncope
It should be remembered that around one in four acute myocardial infarcts are painless or unrecognised by the patient. This is a particularly common situation in patients with diabetes.
Why obtain an ECG while the patient is in pain?
A resting ECG should be recorded during any episode of acute chest pain or discomfort. There will usually be changes during an attack of angina indicating a temporary reduction in the blood supply to the heart muscle. Persistence of ischaemic changes after the pain has resolved suggests either unstable angina or an actual infarction.
Acute Coronary Syndromes, Nstemis and Unstable Angina (→Fig. 2.6)
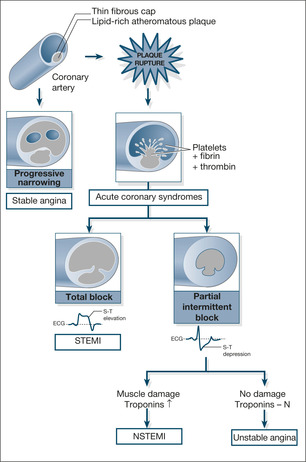
Ischaemic heart disease occurs when the enlargement of cholesterol-rich plaques within the coronary artery walls leads to arterial narrowing, which is sufficient to starve the heart muscle of oxygen. Slow progressive narrowing results in stable angina. However if a plaque becomes unstable and ruptures, there is a sudden combination of platelet clumping and thrombosis which clots off the coronary artery. The resulting clinical conditions are termed Acute Coronary Syndromes (ACS). There are three types of ACS dependant on the extent of the blockage and the degree of resulting heart muscle damage: STEMI, NSTEMI and unstable angina.
A complete block results in severe damage and then, within 45 minutes, irreversible death of the cardiac muscle. Troponin levels are markedly increased and the ECG reflects damage to the full thickness of the heart muscle wall with elevation of the ST-segments (ST-elevation myocardial infarction or STEMI). A partial or intermittent block results in depression of the ST segments accompanied by evidence of heart muscle damage and elevated Troponin levels (non ST-elevation myocardial infarction or NSTEMI) or the same ECG changes with no heart muscle damage and normal Troponin levels (unstable angina).
The clinical picture in a STEMI is most commonly that of the classic ‘heart attack’: severe crushing pain, sweating and nausea; in NSTEMI and Unstable Angina it is usually one of intermittent pain or increasing exertional pain culminating in episodes of prolonged pain at rest.
The sooner the coronary artery can be re-opened (re-perfusion) the less the damage and the lower the mortality risk. STEMIs were previously treated with urgent thrombolytics but should now receive immediate Percutaneous Coronary Intervention (PCI) in the form of a balloon angioplasty and stent to unblock the coronary artery and salvage any damaged heart muscle. NSTEMIs and Unstable Angina need timely diagnosis and urgent risk assessment to identify the level of intervention required to prevent progression from partial to complete coronary artery blockage. This will range from immediate treatment combining anti-platelet drugs (aspirin and clopidogril) with anti-thrombin agents (subcutaneous fondaparinux or enoxaparin) escalating, in the high-risk patient, to intravenous glycoprotein IIb/IIIb inhibitors (tirofiban and eptifabatide) and urgent re-vascularisation with angioplasty or coronary artery bypass.
The current organisation of care for a classic STEMI (severe pain at home – ambulance – diagnostic ECG – PCI unit) means that they are seen with increasing rarity outside the specialist centres. Unstable Angina and NSTEMIs however can have a less straightforward presentation (often initially labelled as ‘atypical chest pain’) and both can present to the AMU, where early recognition and treatment are vital to lessen the risk of further life-threatening cardiac damage.
Troponin T and Troponin I in the assessment of chest pain
The diagnosis of a myocardial infarction is based on three elements: the history, the ECG, and the measurement of bio-markers in the blood, Troponin T and Troponin I, which are released from heart muscle when it is damaged. Troponins are highly specific for cardiac muscle and are sensitive to even minor degrees of damage. Troponin levels rise three to six hours after the onset of damage, peak at 24 hours and remain elevated for up to 10 days. As a general rule, a normal Troponin T level (less than 0.01 mcg/L) six hours after the onset of suspicious chest pain rules out a myocardial infarction (but not unstable angina, see later). Levels in the intermediate range (0.01 to 0.03 mcg/L) need follow-up bloods to identify rising values as even small increases can indicate damage, while cases where the levels are over 0.03 mcg/L have definite damage and, depending on other risk factors, need urgent damage limitation with anti-platelet drugs, anti-thrombins and possible angioplasty.
Risk stratification in the management of chest pain
Chest pain is a very common medical emergency – accounting for around a quarter of all emergency medical admissions. It is vital to recognise those patients, many of whom will not have ‘classic’ cardiac pain, who are at particular risk of progressing to acute myocardial infarction, or who may need emergency re-vascularisation or who may even suffer sudden death. It is now recognised that a reliance on the history, or the ECG changes or even Troponin levels is insufficient to accurately identify those at risk – patients will be missed and either falsely re-assured or managed inappropriately. Reliable scoring techniques are now available which stratify patients with acute chest pain by their risk of a subsequent myocardial infarction or sudden death – and which therefore determine the degree and urgency of preventative action required. Most reliable is the GRACE system of risk stratification which is used to grade patients with acute chest pain according to age, ECG ST segment changes, Troponin levels, admission heart rate, admission systolic blood pressure, degree of heart failure, renal function and whether or not the patient suffered a cardiac arrest on admission.
Cocaine and the heart
Cocaine is a potent cause of cardiac damage – the catecholamine surge, which is seen within an hour of ingestion, can trigger acute hypertension and spasm of the coronary arteries leading to an acute coronary syndrome or myocardial infarction. Cocaine use is important to recognise as a cause of acute heart damage as it requires specific management. Treatment is aimed at sedating the patient with diazepam and reducing the spasm with i.v. nitrates and diltiazem – beta-blockers worsen the situation and drugs conventionally used for ACS, aimed at ‘plaque stabilisation’, such as aspirin and clopidogrel are of no value.
When to call the medical staff
At-risk patients have cardiac-sounding pain, may be in the convalescent period from a recent infarct, or may have a definite past history of cardiovascular disease. If these patients have rest pain, there should be liaison with the medical staff, particularly if the pain is associated with ECG changes or a decrease in blood pressure. Unstable angina needs active and immediate intervention, probably in a CCU. Such patients, if left on a general ward, do worse in terms of time to angioplasty, treatment of serious arrhythmias, management of acute haemodynamic changes and their chances of survival from a cardiac arrest.
When to try GTN
A patient with known angina will be familiar with their pain and can advise you whether any particular episode is unusually severe or prolonged. Their ‘usual’ angina can be treated with simple sublingual GTN, but the patient must first be assessed for the severity and frequency of their attacks.
What to say to the patient
‘We may have to “nip this problem in the bud” using anticoagulants, i.v. nitrates and so forth. You must report any further chest pain to the nursing staff immediately.’
Case Study 2.3 illustrates the management of a patient with breathlessness and chest pain.
Case Study 2.3
An 83-year-old man, an independent retired widower, was admitted at 18.00h with a 4-h history of increasing breathlessness.There had been no response to nebulised salbutamol, which he had tried at home.
He had COPD and, because of an exacerbation, he had been receiving antibiotics and oral steroids for 3 weeks prior to admission.
Initial diagnosis
• Exacerbation of COPD
• Possible left ventricular failure
Correctly, the paramedics had considered a cardiac cause for his symptoms and tried GTN. A painless myocardial infarction would have to be excluded with ECGs and cardiac enzymes.
It can be difficult to decide in the emergency situation what is the relative contribution from COPD and the initial management may have to target both conditions.The priority at this stage is:
• to maintain oxygenation
• to correct any reversible underlying cause (LVF, arrhythmia, bronchoconstriction)
Management. The patient was propped upright and reassured. 28% oxygen was administered and a cardiac monitor was put in place. He was given:
• i.v. frusemide 80mg
• nebulised salbutamol 5mg (caution is needed if acute cardiac ischaemia is a suspected possible diagnosis as salbutamol can overstimulate the heart – this dose may have been too large)
• i.v. hydrocortisone (for presumed bronchoconstriction)
• oral ampicillin (if there had been pneumonia on the chest film this would have been given intravenously to a patient as ill as this)
Theophylline levels were checked urgently:
• to guide the further aminophylline doses if they are needed
• to exclude theophylline toxicity as the cause for his marked tachycardia The patient had an immediate diuresis; this was charted and a strict fluid balance chart was started.
Assessment. The patient was severely distressed. His saturations were 75% on 28% oxygen, respiratory rate 40 breaths/min, pulse 140 beats/min and regular, and blood pressure 170/75 mmHg. His abdomen was soft and non-tender and there was no sign of acute urinary retention. (Intra-abdominal catastrophes such as a ruptured aortic aneurysm can present in the elderly with acute sudden cardiovascular deterioration. Acute retention is common in men of this age after i.v. diuretics.)
The ECG had not changed, neither had the chest film.The possibilities at this stage were:
• worsening respiratory failure
• increasing LVF
• possible acute myocardial infarction
The oxygen delivered was changed to 60% (in the acutely deteriorating patient, correcting hypoxia takes precedence over all other considerations).The patient was given:
• i.v. frusemide 120mg
• i.v. diamorphine 2.5mg for chest pain and LVF (this must be used with extreme caution in patients with a possible respiratory component to their illness as it will depress ventilation)
• an aminophylline infusion was started (the initial theophylline level was in the normal range so a loading dose was not given).Aminophylline has the advantage of working in both pulmonary oedema and bronchoconstriction, although it is not generally used as first-line treatment in either situation.
A decision was made to consider ventilation if the patient did not improve (using information about his previous level of independence and health from the admission assessment). His relatives were contacted and brought in.
The patient’s arterial blood gases at this stage showed an acceptable oxygen level but, possibly as a result of the diamorphine, an increasing pCO2. His oxygen mask was changed again to 28% and he was given two further 120mg doses of i.v. frusemide. By 07.00h after a significant diuresis there was notable and continued improvement. He was eventually well enough to be discharged back to his home.
Subsequent cardiac enzymes (Table 2.1) showed that he had suffered an acute myocardial infarction, which had presumably caused the sudden deterioration in the early hours of the first morning after his admission.
| Enzyme level (IU/L) | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| AST | 30 | 129 | 93 |
| CK | 114 | 559 | 354 |
Critical nursing tasks in the patient with chest pain
ABC triage Chest pain with hypotension and arterial desaturation needs urgent/immediate medical attention. The possible causes in a collapsed patient include a myocardial infarct and a pulmonary embolus; a less common cause is acute aortic dissection.
Monitor oxygen saturation
Maintain saturations above 90% using high-flow oxygen.
Obtain a 12-lead ECG
This is useful to confirm a myocardial infarction or unstable angina. A normal ECG is not particularly helpful in ruling out other conditions. The way to gain the most information from an ECG is to compare it with others from the case record.
Take an overall look at the patient
Severe angina, myocardial infarction or pulmonary embolus produce a pale, sweaty and clammy patient. A patient who cannot take a full breath because of pain probably has pleurisy. Biliary colic makes the patient roll around the bed in pain.
Ask key questions
• Where is the pain and where does it radiate?
• What makes it worse (deep inspiration, minor exertion such as showering)?
• What relieves it (e.g. GTN, breathing shallowly)?
• Has it occurred before? Is it like your usual angina/previous heart attack, etc.?
Sudden Cardiac Death
Sudden cardiac death describes a sudden collapse, usually in ventricular fibrillation, ventricular tachycardia or asystole. There are few warning symptoms, perhaps a few seconds of chest tightness, palpitations or weakness. The outlook is variable, with the best survival rates seen in patients experiencing witnessed in-hospital arrests in which the rhythm is ventricular fibrillation or ventricular tachycardia – of whom 20% will leave hospital alive.
Ischaemic heart disease is responsible for 70% of SCDs, and although it may be the first symptom, more commonly it occurs during the first 12 months after an acute myocardial infarction. The use of preventative measures post-infarct, particularly in patients with poor left ventricular function, has had a significant impact on reducing the incidence of SCD. A typical regime in a high-risk case would consist of:
• an ACE inhibitor or an angiotensin II receptor antagonist
• a beta-blocker
• spironolactone
• intensive statin therapy
• aspirin
There is increasing interest in preventing SCDs with implantable cardioverter defibrillators (ICDs). These would be considered, for example, in high-risk survivors of cardiac arrest such as those with poor left ventricular function and in whom electrophysiological testing demonstrates instability of their cardiac rhythm.
Atrial Fibrillation and Arterial Emboli
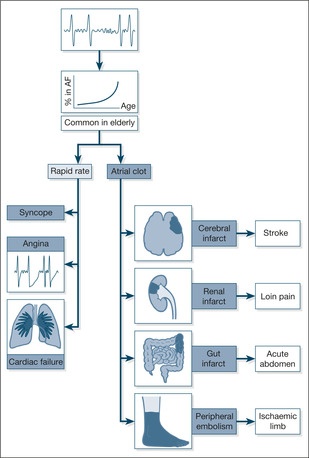
Normally, the heart beats in an orderly fashion and is led in this by the rhythmic activity of the SA node. Each atrial contraction is followed immediately by that of the ventricles. The SA node establishes the heart rate through the rhythmic discharge of a small electrical impulse that spreads, wave-like, through each atrium, producing atrial contraction. The impulse then passes through the AV node and continues onwards to initiate ventricular contraction. Thus, if the SA node is firing regularly 75 times per minute, the AV node will in turn be activated at the same rate, leading to a regular ventricular response (and, of course, pulse rate) of 75 contractions per minute.
In atrial fibrillation (→Fig. 2.7), organised atrial activity is replaced by random electrical impulses which spread chaotically through the atrium. The atrium responds by muscular activity that can be described as a shivering motion (fibrillation), rather than a coordinated contraction. There are two important consequences:
• The atrial ‘squeeze’, which expels the final 10% of the blood into the ventricle at the end of diastole, is lost. This loss can be important, particularly if the ventricle relies on atrial contraction to ensure it is properly filled, for example when the valve between the atrium and ventricle is already narrowed (as in mitral stenosis).
• The AV node is activated at random by the chaotic atrial electrical activity at rates of anything between 60 and 140 times per minute. The ventricles respond to this random activation accordingly, and produce irregular pulse rates within this wide range. Clearly, an irregular heart rate of 140 beats/min will not be tolerated by the heart for long.
 |
| Fig. 2.7 |
Thus atrial fibrillation reduces cardiac output (→Box 2.2).
Box 2.2
• Loss of atrial contraction
• Ventricular response too rapid
• Irregular ventricular rate
Causes of Atrial Fibrillation
Cardiac failure from whatever cause can be associated with atrial fibrillation. The most common situations in which atrial fibrillation is seen on the Acute Medical Unit are:
• the ageing heart (8% of all those older than 80 years old are in atrial fibrillation)
• ischaemic and hypertensive heart disease
• chronic heart-valve disease
• binge alcohol drinking
• atrial fibrillation triggered by hypoxia or infection in COPD
Atrial fibrillation can also be triggered by an acute myocardial infarction and by a pulmonary embolus.
Importance of Atrial Fibrillation
Uncontrolled atrial fibrillation
Cardiac failure
Atrial fibrillation itself can cause right and left heart failure if the ventricular rate is excessive. This is particularly the case in elderly patients. More commonly, the onset of atrial fibrillation in someone with pre-existing valvular or heart muscle disease will tip the patient into cardiac failure (→Case Study 2.4).
Case Study 2.4
A 68-year-old woman was admitted severely breathless, coughing up copious frothy pink sputum characteristic of pulmonary oedema. Her pulse rate was 145 beats/min and irregular and her blood pressure was 70/40 mmHg. Her oxygen saturation was 70%.
The patient was treated with high-flow oxygen and i.v. diuretics. Her atrial fibrillation was controlled with i.v. digoxin (electrical cardioversion was considered but she improved rapidly with initial treatment and it was unclear from the history how long the atrial fibrillation had been present). Subsequent examination and investigation demonstrated tight mitral stenosis.There was no history of cardiac disease but she had had rheumatic fever as a teenager.
Angina
Uncontrolled atrial fibrillation can reduce the blood supply to the heart muscle and trigger angina simply because there is not enough time for adequate coronary artery blood flow to occur. The reverse may also occur – an attack of ischaemia can trigger atrial fibrillation – so it is important to take a good history to see which came first, the pain or the palpitations.
Syncope and near syncope
The cardiac output may decrease suddenly in acute atrial fibrillation, sufficiently to reduce cerebral blood flow, leading to syncope or presyncope. This is a particular risk if there are existing problems with blood flow because of either aortic valve stenosis or disease in the carotid arteries (→Case Study 2.5).
Case Study 2.5
A 68-year-old man was admitted as an emergency with a 4-month history of feeling unwell and collapsing to the floor. Some of the episodes were accompanied by fast palpitations. On the day of admission he had a rushing feeling in his head and collapsed to the floor. His wife said that he was grey and clammy and stayed unconscious for around 10min. There were no convulsive movements.When he came round he was slightly confused and had been incontinent of urine.
The only finding on admission was a murmur coming from what was thought to be a stenosed aortic valve.The patient’s pulse was normal at 86 beats/min. Three hours after admission he suddenly went into rapid atrial fibrillation and felt very faint. His atrial fibrillation stopped without treatment after 2min but it was followed by a long pause on the ECG during which time the patient felt worse.
He was referred to the cardiologists and urgently transferred to the CCU. A cardiac echo showed severe aortic stenosis.
There are several important issues in Case Study 2.5. The patient’s old hospital records showed he had previously been investigated for unexplained blackouts. Some had been associated with transient atrial fibrillation, but his heavy alcohol intake had been blamed for this. The murmur had been heard, but not investigated. Aortic stenosis is a common valve problem and usually results from degenerative calcification of a congenitally abnormal valve. Rheumatic valve disease is a much less common cause of aortic stenosis. The stenosis leads to three symptoms: increasing angina, recurrent syncope and sudden death. All are a manifestation of obstruction to the flow of blood out of the left side of the heart. If undiagnosed, it can eventually lead to LVF. Critical stenosis is potentially lethal, yet easily managed with valve replacement – a diagnosis not to be missed. In this patient, the atrial fibrillation combined with the stenosis led to syncope and the excess alcohol intake may well not have been of immediate relevance, except for obscuring the true nature of the problem.
Sudden atrial fibrillation causing hypotension during severe illness (→Case Study 2.6)
Acute atrial fibrillation can cause a sudden decrease in the blood pressure and, if this decrease occurs in the setting of a severe illness, it can be misinterpreted as hypovolaemia or sepsis.
Case Study 2.6
A patient was admitted with severe diabetic ketoacidosis: blood sugar 55 mmol/L, pH 6.85 and an unrecordable blood pressure. His pulse rate was 140 beats/min and was initially misinterpreted as a normal rhythm. He was given aggressive fluid replacement with no improvement in his blood pressure. After 4h, his heart rhythm suddenly reverted from what was now clearly rapid atrial fibrillation to sinus rhythm. His blood pressure immediately increased to 110/70 mmHg.
In a sick patient, it can be difficult to tell whether a severe tachycardia is the primary cause of a low blood pressure or whether, along with the hypotension, it is one of the effects of the acute illness.
Complications of atrial fibrillation
Atrial fibrillation and the formation of left atrial clots
Unfortunately, blood stagnates in a fibrillating atrium and is liable to clot. A clot that breaks loose (embolus) can, in the case of those originating in the left atrium, have disastrous consequences: stroke as a result of cerebral embolus, kidney damage as a result of renal emboli, and acutely ischaemic limbs as a result of peripheral vessel emboli. Transthoracic or transoesophageal echocardiography can help to assess the risk of embolisation by examining overall cardiac function, looking for enlargement of the left atrium and identifying left atrial clot.
Of these complications, the risk of embolic stroke is much the most important and is quite common – approaching 5% of patients in atrial fibrillation per year. The risk of stroke is particularly high in those older than 65 years, especially if there are other risk factors present such as diabetes, cardiac failure, hypertension and previous cerebrovascular disease. This group of patients will be considered for anticoagulation as soon as atrial fibrillation has been diagnosed.
Diagnosis of Atrial Fibrillation
Atrial fibrillation causes a chaotically irregular pulse, which can be fast or slow. If the ventricle is contracting quickly and irregularly, the amount of blood expelled with each beat will vary and the very weak beats will not be palpable at the wrist. This produces a deficit between the apical rate (measured with a stethoscope at the apex) and the rate measured by palpation at the wrist.
This is a common pitfall in uncontrolled atrial fibrillation – the pulse can be 80 beats/min at the wrist and 130 beats/min at the apex. If the pulse feels irregular, measure both the apical and the radial rates.
Management of Atrial Fibrillation
Unless there is a contraindication, most patients in atrial fibrillation will be started immediately on anticoagulation using low-molecular weight heparin. The choice then lies between:
• converting the atrial fibrillation to a normal rhythm (cardioversion)
• controlling the atrial fibrillation by slowing the ventricular rate
Management options are summarised in Box 2.3.
Box 2.3
• Immediate anticoagulation and observation
• Immediate anticoagulation and rate control with digoxin/beta-blocker/diltiazem
• Immediate anticoagulation and cardioversion
• Immediate anticoagulation and planned cardioversion in 3 weeks
Observation
This would be appropriate for the first 24h in a patient with slow atrial fibrillation in whom the rhythm was not compromising cardiac function. If the patient is acutely unwell due to atrial fibrillation urgent action is required to restore a normal heart rhythm or to control the heart rate.
Cardioversion
It is possible to convert atrial fibrillation to a normal rhythm by using drugs (chemical cardioversion) or by a synchronised shock (electrical cardioversion). In general, in rapid atrial fibrillation of recent onset (less than 48h), cardioversion is the treatment of choice, particularly if the abnormal rhythm is having an adverse effect on the patient. The sooner that cardioversion is tried, the better the chances of success. Unless the situation is critical, drugs are tried before a synchronised shock is attempted – the two drugs that are most commonly used are i.v. flecainide and i.v. amiodarone.
If atrial fibrillation has been present for longer than 48h, it is essential to anticoagulate the patient for at least 3 weeks before attempting cardioversion, because of the risk of dislodging a clot from the left atrium. Rather surprisingly, the evidence suggests that patients with well established atrial fibrillation fare just as well from control of the ventricular rate as they do from re-establishing normal rhythm with cardioversion.
Controlling atrial fibrillation
In many acute situations, the duration of the atrial fibrillation will be unclear, and urgent cardioversion could put the patient at risk from an embolus. As untreated, rapid atrial fibrillation compromises the heart, in an unstable patient it is important to use a drug which acts quickly. Where there is severe heart failure (hypotension, pulmonary/ peripheral oedema), intravenous digoxin or amiodarone is used, otherwise the best option is intravenous diltiazem.
To prevent emboli, once the atrial fibrillation is controlled the choice is between aspirin and warfarin as long-term therapy. Warfarin is twice as effective as aspirin in lowering the risk of stroke (70% versus 35% risk reduction) but it is twice as likely to cause bleeding as a complication. Low-risk patients are given aspirin, as their chances of emboli are very small.
High-risk patients are treated with warfarin long-term anticoagulation, as there is a high incidence of emboli.
• High-risk patients (annual risk of stroke 10%)
— valvular heart disease
— previous emboli, cerebral infarction, TIA
— hypertension
— diabetic
— those with heart failure
— age over 75 years
— cardio-vascular arterial disease
Patients in atrial fibrillation over the age of 65 with no risk factors and those between 65 and 75 who have hypertension, diabetes or vascular disease are classed as intermediate risk – in these patients the risks and benefits of warfarin versus aspirin are less clear.
Atrial fibrillation in the elderly. Atrial fibrillation is present in 8% of the population over 80 years of age and is associated with a high risk of complications – there is a sixfold increased risk of emboli and atrial fibrillation is implicated in one-third of all strokes in this age group. Although long-term warfarin therapy has dangers in the elderly, particularly in those experiencing confusion and recurrent falls, it also has major benefits, with large reductions in the risk of stroke, provided that there is careful selection of the patient and monitoring of the anticoagulant control. The risk of warfarin-induced haemorrhage (in particular, intracerebral haemorrhage) can be reduced by maintaining an INR between 2.0 and 3.0, avoiding the combined use of aspirin, falls-reduction strategies and careful blood pressure control.
Atrial fibrillation induced by alcohol.Case Study 2.7 describes the management of a patient whose atrial fibrillation was caused by excess alcohol intake. The important points from this case study are:
• Alcohol itself can directly trigger atrial fibrillation and is the precipitating factor in up to a quarter of cases of acute atrial fibrillation (it can also eventually damage the heart muscle by causing thiamine deficiency).
• Up to half of all cases of first-time atrial fibrillation will return to a normal rhythm without treatment.
• If the atrial fibrillation is new (less than 48h old), there is little risk of a clot having formed in the left atrium and cardioversion can be tried without first giving prolonged anticoagulation.
• The alternative to cardioversion would have been to control the rate of atrial fibrillation initially with digoxin, if necessary adding a beta-blocker or verapamil.
Case Study 2.7
A 43-year-old man was admitted to hospital as an emergency at 03.00h on a Sunday morning, complaining of palpitations and chest tightness. He had consumed two bottles of red wine and three pints of beer during the course of a dinner party the evening before. He was awoken at 02.00h with rapid palpitations and chest tightness.The past history was negative but he drank on average, 30 units of alcohol per week and smoked 20 cigarettes per day.
On admission the patient was anxious with an irregular pulse of 110 at the wrist and 140 beats/min at the apex. Blood pressure was 160/90 mmHg and full examination was negative.The chest film was clear and the 12-lead ECG confirmed atrial fibrillation with no evidence of a myocardial infarction.
He and his wife were reassured that this was probably not a heart attack, he was monitored and blood was taken for cardiac enzymes and biochemistry. He was started on subcutaneous low-molecular weight heparin and a decision was made that, as this was a short-lived arrhythmia (and hence there had not been time for a clot to form in the left atrium), it would be safe to try cardioversion, initially with an i.v. infusion of the drug, flecainide. Fortunately, while waiting for the results of his blood tests his monitor showed a sudden return to sinus rhythm with immediate relief of his symptoms. Cardioversion was not needed.
A cardiac echogram was arranged, to look at the heart valves and heart muscle function, and he was discharged home with an appointment for the cardiology clinic. He was strongly advised to moderate his alcohol consumption to prevent any recurrence.
Critical nursing tasks in atrial fibrillation
The aim of the nursing assessment is to document the effect that the atrial fibrillation is having on the patient, and hence to determine the urgency of the situation. If atrial fibrillation is causing angina, hypotension or heart failure, then corrective action is necessary as soon as possible. Slow atrial fibrillation with no acute complication may simply require a period of observation.
Assess the rate of the atrial fibrillation
Atrial fibrillation at a pulse rate of 80-100 beats/min is unlikely to cause acute cardiac decompensation, whereas at 130-140 beats/min problems will arise. It is important to document both the apical and the radial rate. In uncontrolled atrial fibrillation, not all the apical impulses are conducted to the radial pulse, which leads to a difference between the rates (pulse deficit); this gap narrows as the heart rate comes under control with treatment.
Identify chest pain
A history of chest pain may mean that the atrial fibrillation has been triggered by an ischaemic episode or even by a myocardial infarction. More commonly, however, rapid atrial fibrillation can cause rate-related anginal pain.
Look for heart failure
Symptoms of breathlessness, particularly on lying flat, and associated with signs of an increase in respiratory rate and bilateral ankle oedema, suggest that the atrial fibrillation is causing, or contributing to, cardiac failure.
Document hypotension
Sudden rapid atrial fibrillation can decrease the blood pressure acutely. This can have a critical impact, especially if the atrial fibrillation is complicating further major acute illness such as pneumonia or diabetic ketoacidosis.
Important nursing tasks in atrial fibrillation
Watch for emboli caused by the atrial fibrillation
The three areas particularly prone to emboli are the brain, limbs and intestine – presenting as stroke-like illness or sudden confusion, acute painful, pale and pulseless limbs and, particularly in the elderly, an acute abdomen.
Identify factors that could have triggered the atrial fibrillation
Common non-cardiac causes of acute atrial fibrillation are pneumonia, exacerbations of COPD and any source of sepsis, particularly if there is pyrexia.
Obtain an accurate drug history
The name and doses of all cardiovascular drugs need to be recorded. This is especially important for digoxin (under- or over-treatment can cause problems with uncontrolled atrial fibrillation), oral theophyllines (over-treatment is a potent cause of any arrhythmia) and anticoagulants. Regimens will often need to be cross-referenced with information from relatives and from the GP’s surgery.
Answering Relatives’ Questions in Atrial Fibrillation
Atrial fibrillation is a prolonged irregular heart rhythm. It makes the heart pump less efficiently and can cause the blood to stagnate and form potentially dangerous clots. It is often temporary, especially if it has been triggered by some other acute illness.
How will it be treated? If it is troublesome, we can slow the pulse down to normal with drugs or try and jolt the heart back into a normal rhythm using either an injectable drug (flecainide/amiodarone) or a small electrical impulse delivered under an anaesthetic (‘synchronised DC shock’). While the pulse is irregular, we will keep the blood watered down with heparin/warfarin to prevent it clotting.
Infective Endocarditis
Infective endocarditis is a serious condition in which there is infection of the heart valves, usually the mitral or aortic valve. In the past, only damaged or mechanical valves have been involved, but the increasing incidence of i.v. drug abuse has put even normal heart valves at risk. Other major risk factors are advanced age, diabetes and recent dental, operative and endoscopic procedures, which can result in bacteria entering the bloodstream.
Infective endocarditis is a serious acute medical problem. The affected valve may fail suddenly as it is destroyed by infection. Pieces of infected material can break off and spread via the blood to vital organs, resulting in:
• foci of sepsis, especially in the kidneys
• septic cerebral emboli (stroke-like illness)
How Infective Endocarditis Presents
Pyrexia of unknown origin
The picture is of severe malaise associated with night sweats and generalised aches and pains. Usually there will be a known valve lesion, valve replacement or previously diagnosed congenital heart defect.
Catastrophic valve failure
Infection can destroy a cardiac valve and even form an abscess within the heart muscle or in the conducting system. This can lead to devastating cardiac failure, particularly in the case of the aortic valve, and the patient can deteriorate acutely, with sudden pulmonary oedema and hypotensive shock.
Infected emboli
The history here is of a recent feverish illness complicated by the acute onset of a stroke (cerebral embolus), ischaemic limb (large vessel embolus) or, in the case of right-sided endocarditis, infected pulmonary emboli as described in Case Study 2.8.
Case Study 2.8
The patient’s condition deteriorated suddenly and, on the basis that this could be pneumonia, i.v. ampicillin and erythromycin were started. He remained severely hypoxic, deteriorated further and died.
The autopsy finding was a staphylococcal endocarditis involving the tricuspid valve – a typical finding in bacterial endocarditis caused by the use of infected intravenous needles (the infection passes through the veins to the right side of the heart, and when infected particles break off the tricuspid valve, they embolise to the lungs).
Role of the Acute Medical Unit in Suspected Endocarditis
Prompt investigation of any illness accompanied by fever
Infected heart valves shed bacteria into the bloodstream, resulting in positive blood cultures. If endocarditis is suspected, then three sets of blood cultures taken at 5-min intervals on two consecutive days should be taken. The urine should also be cultured.
Recognition of high-risk patients
These include patients with valve disease or congenital heart disease admitted to hospital with fever or unexplained deterioration, and illicit drug users with breathlessness and fever.
Appropriate action on finding proteinuria and haematuria
In endocarditis, microemboli of infected material lodge in the kidneys and set up an inflammatory response. As a result, blood and protein appear in the urine. Clearly, in patients with fever, haematuria and proteinuria are more likely to indicate a simple urinary infection; nonetheless, if other risk factors are present this finding can be critical in guiding the medical staff towards the correct diagnosis.
Principles of management in the first 24h
Ensure the safety of the patient
Secure the diagnosis
• identify at-risk patients
• take several blood cultures (avoid the casual use of antibiotics on the ward)
• conduct urine testing and culture
• examine the valves (cardiac echo)
Initiate antibiotic therapy
• insert i.v. lines
• monitor antibiotic levels
Prepare the patient for a ‘long haul’ of antibiotic therapy (several weeks)
• take particular care with Venflon® sites
Observe for complications
• look for antibiotic allergy
• look for left heart failure (breathlessness, respiratory rate, oxygen saturations, pulse, blood pressure)
• monitor temperature
• measure urine output
• look for septic emboli (any sudden event, e.g. confusion, stroke, cold limb)
Drugs in the Management of Acute Heart Disease
Intravenous frusemide
Lasix®
Indications
Acute pulmonary oedema
Severe congestive cardiac failure
• Frusemide is a well-tolerated and powerful diuretic with a very wide dose range – from 20mg to 250mg or more. Low doses are used in patients not previously exposed to the drug; higher doses are needed with patients taking oral frusemide, patients with renal failure and patients with severe heart failure.
• As well as advantages, there are also dangers of a sudden diuresis: this may precipitate acute retention, causing hypovolaemic hypotension and sudden electrolyte changes (predominantly urinary potassium loss).
• For doses greater than 50mg, the rate of i.v. administration should not exceed 4mg/min, to avoid the risk of acute hearing disturbance.
Intravenous nitrates
Isosorbide dinitrate (Isoket®), glyceryl trinitrate (Nitrocine®)
Indications
Unstable angina
Acute left ventricular failure
• Nitrates have three important actions: they dilate the coronary arteries, they unload the heart by reducing the load against which it pumps (peripheral arterial dilatation), and they increase pooling of blood in the veins (which unloads the right side of the heart). They are therefore useful in both angina and cardiac failure.
• Patients rapidly develop tolerance to nitrates, so the initial dose may have to be increased repeatedly to overcome this (→Table 2.2).
| *Data sheets must be used with care when preparing the admixture of the drugs and the saline or dextrose vehicle. For example, Isoket® is provided in 0.05% and 0.1% strength ampoules and the examples used in the preparation instructions express the doses in mg per hour (2-20mg per hour). In contrast, the doses for Nitrocine® are expressed in mcg per minute. Local protocols are invaluable in these situations. |
||
| Drug parameters | Nitrocine® | Isoket® |
|---|---|---|
| Infusion strength* | 100 μg /ml | 100 μg /ml |
| Starting rate angina | 10 μg /min | 15 μg /min |
| Starting rate LVF | 20 μg /min | 30 μg /min |
| Step up rate every 30min | 10-20 μg /min | 15-30 μg /min |
| Maximum dose | 100-200 μg /min | 300 μg /min |
• There is a risk of hypotension with i.v. nitrates, particularly when the heart is already in a parlous state. However, this property can be useful in acute LVF associated with hypertension.
• The advantage of the i.v. infusion of nitrates is that the onset of action is immediate and the dose can be titrated continuously to balance haemodynamics (the blood pressure and pulse) with the beneficial clinical response.
• As i.v. nitrates are incompatible with PVC infusion sets (Viaflex®, Steriflex®) they must be administered through polyethylene or glass containers.
Digoxin
Indications
Atrial fibrillation
Cardiac failure
• Digoxin increases, albeit to a modest degree, the strength of the heartbeat.
• Rapid control of atrial fibrillation requires i.v. loading:
0.75mg to 1mg of digoxin in 50ml of fluid over 2h
• Lower doses are needed in the elderly, in renal failure and in those who have received digoxin within the past 2 weeks.
• Oral digitalisation can be achieved over 24h using divided doses of the total loading dose of 1.0-1.25mg.
• The symptoms of digoxin toxicity (drug levels more than 3mg/L) are anorexia, nausea, vomiting and an abnormally slow pulse (less than 50 beats/min). Toxicity is more likely if there is potassium deficiency from diuretic therapy.
Intravenous diltiazem
Indications
Rapid control of atrial fibrillation
• Intravenous 0.25mg/kg up to three doses then 120 to 300mg orally daily in three doses.
• Diltiazem can depress cardiac function and worsen heart failure.
ACE inhibitors
Captopril, enalapril, lisinopril
Indications
Cardiac failure
Hypertension
Post myocardial infarct
Diabetic renal disease
• ACE inhibitors have proved an important advance in the treatment of heart failure and hypertension. They improve survival and symptoms in heart failure and after myocardial infarction, and they have a beneficial effect on diabetic renal disease.
• Their use requires close supervision – particularly in the elderly, those with intercurrent illness and those in cardiac failure – because they can adversely affect kidney function through their mode of action, which is to inhibit mechanisms that are normally in place to protect renal blood flow (the renin-angiotensin system).
• First-dose hypotension is a recognised problem, but is usually relieved by lying the patient down. The hypotensive response can be exaggerated in the patient who has severe cardiac failure and is already receiving high-dose diuretics. A small test dose should be given and the blood pressure monitored for 2h.
Intravenous amiodarone
Indications
Rapid termination of serious arrhythmias (rapid atrial fibrillation/flutter, ventricular tachycardia)
• The drug causes local phlebitis. Although the loading dose can be given through a peripheral vein, the follow-up 24h continuous infusion needs a central line.
• The loading dose is 5mg/kg (usually 300mg) in 250ml 5% dextrose over 20min.
• The infusion dose is 15mg/kg (usually 1.2 g) in 500ml 5% dextrose over 24h.
• Once there is a response, the infusion is replaced, if indicated, by oral amiodarone.
• There are several important drug interactions, notably with warfarin and digoxin, and some potentially serious side-effects on the thyroid gland, lungs and liver. Most of these are associated with long-term rather than acute therapy.
Further Reading
Heart Failure
Lonn, E.; McKelvie, R., Drug treatment in heart failure, British Medical Journal 320 (2000) 1188–1192.
Millane, T.; Jackson, G.; Gibbs, C.R.; et al., ABC of heart failure: acute and chronic management strategies, British Medical Journal 320 (2000) 559–562.
Stewart, S.; Marley, J.E.; Horowitz, J.D., Effects of a multidisciplinary home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study, Lancet 354 (1999) 1077–1083.
Atrial Fibrillation
Lafuente-Lafuente, C.; Mahe, I.; Extramiana, F., Management of atrial fibrillation, British Medical Journal 340 (2010) 40–45.
Ischaemic Heart Disease
Edmondstone, W.M., Cardiac chest pain: does body language help the diagnosis? British Medical Journal 311 (1995) 1660–1661.