Chapter 19
Cardiogenic Shock
2. What are the various types of shock?
3. Describe the clinical signs observed in cardiogenic shock and other types of shock?
The medical history and clinical examination help in making the diagnosis of cardiogenic shock. Feeling the extremities and examining the jugular veins provide vital clues: warm skin is suggestive of a vasogenic cause; cool, clammy skin reflects enhanced reflex sympathoadrenal discharge leading to cutaneous vasoconstriction, suggesting hypovolemia or cardiogenic shock. Distended jugular veins, rales, and an S3 gallop suggest a cardiogenic cause rather than hypovolemia. Figure 19-1 presents an algorithm for the evaluation and treatment of cardiogenic shock.
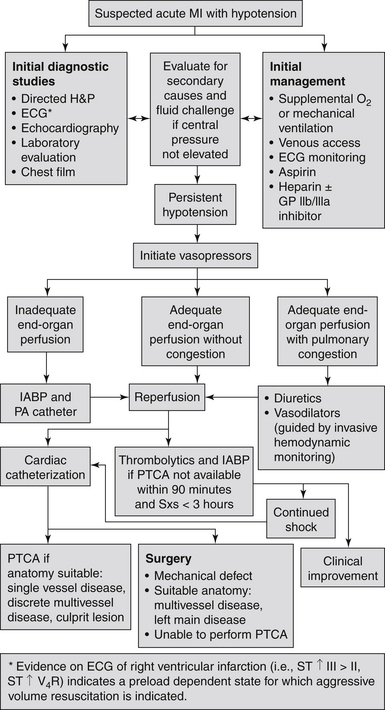
Figure 19-1 Evaluation and treatment algorithm for cardiogenic shock. (Modified from Hollenberg SM, Kavinsky CJ, Parillo JE: Cardiogenic shock. Ann Intern Med 131:47-59, 1999.) ECG, Electrocardiogram; GP, glycoprotein; H&P, history and physical examination; IABP, intraaortic balloon pump; MI, myocardial infarction; PA, pulmonary artery; PTCA, percutaneous transluminal coronary angioplasty; Sxs, symptoms.
4. Do all patients with cardiogenic shock have an increased heart rate?
5. What are the determinants of central venous pressure (CVP)?
The normal CVP is 5 to 12 cm H2O. Intravascular volume, intrathoracic pressure, right ventricular function, and venous tone all affect the CVP. To reduce variability caused by intrathoracic pressure, CVP should be measured at the end of expiration.
6. What is the significance of a loud holosystolic murmur in a patient with shock after acute myocardial infarction?
7. How can one differentiate cardiogenic from septic shock?
8. What is the most common cause of cardiogenic shock?
9. Describe the pathophysiology of cardiogenic shock among patients with acute myocardial infarction?
10. Describe other mechanisms that cause or contribute to cardiogenic shock after myocardial infarction.
It is critical to exclude mechanical complications after MI, which may cause or exacerbate cardiogenic shock in some patients. These mechanical complications include ventricular septal rupture, ventricular free wall rupture, and papillary muscle rupture. Two-dimensional (2-D) echocardiography is the preferred diagnostic modality and should be promptly performed when mechanical complications are suspected. Among STEMI patients with suspected mechanical complications in whom a 2-D echocardiogram is not available, diagnostic pulmonary artery catheterization should be performed (class I recommendation by the 2004 American College of Cardiology/American Heart Association [ACC/AHA] guidelines). The detection of mechanical complications before coronary angiography may help in dictating the revascularization strategy (surgical revascularization with mechanical repair of the complication rather than primary PCI of the infarct vessel without mechanical repair of the complication).
11. Can right ventricular (RV) dysfunction result in cardiogenic shock?
12. List the other major causes of cardiogenic shock.
Cardiovascular causes of cardiogenic shock are given in Table 19-1.
TABLE 19-1
CARDIOVASCULAR CAUSES OF CARDIOGENIC SHOCK
 Acute myocardial infarction with severe left ventricular dysfunction
Acute myocardial infarction with severe left ventricular dysfunction
 Acute myocardial infarction with mechanical complication (ruptured papillary muscle, ventricular septal defect, free wall rupture)
Acute myocardial infarction with mechanical complication (ruptured papillary muscle, ventricular septal defect, free wall rupture)
 Aortic dissection (+/− acute aortic regurgitation)
Aortic dissection (+/− acute aortic regurgitation)
 Endocarditis leading to mitral and/or aortic regurgitation
Endocarditis leading to mitral and/or aortic regurgitation
 Chronic congestive cardiomyopathy
Chronic congestive cardiomyopathy
 Critical mitral or aortic stenosis
Critical mitral or aortic stenosis
 Toxins or drugs (negative inotropes, negative chronotropes, vasodilators)
Toxins or drugs (negative inotropes, negative chronotropes, vasodilators)
 Traumatic cardiogenic shock (cardiac penetration with subsequent tamponade, myocardial contusion, or tension pneumothorax)
Traumatic cardiogenic shock (cardiac penetration with subsequent tamponade, myocardial contusion, or tension pneumothorax)
13. What is the mainstay therapy for patients with cardiogenic shock complicating myocardial infarction?
Acute reperfusion and prompt revascularization for cardiogenic shock improves survival substantially and is considered the mainstay therapy for cardiogenic shock after acute MI. The Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) study was a landmark trial conducted in the 1990s enrolling patients with acute MI complicated by cardiogenic shock and randomizing patients to emergency revascularization or initial medical stabilization. Six-month mortality was lower in the early revascularization group than in the medical stabilization group (50% versus 63%, P = 0.03). At 1 year, survival was 47% for patients in the early revascularization group compared with 34% in the initial medical stabilization group (P < 0.03). The benefits of early revascularization persisted at long-term follow-up, and the strategy of early revascularization was associated with 67% relative improvement in 6-year survival compared with initial medical stabilization. Currently, the ACC/AHA guidelines recommend early revascularization in cardiogenic shock for those aged 75 years or younger.
14. Which is the best revascularization strategy in patients with cardiogenic shock complicating myocardial infarction?
15. Does the timing of revascularization matter in the treatment of cardiogenic shock?
16. Describe the common medical therapies for cardiogenic shock.
17. What is the mainstay mechanical therapy for cardiogenic shock?
Intraaortic balloon pump counterpulsation (IABP) has long been the mainstay of mechanical therapy for cardiogenic shock. IABP support should be instituted promptly, even before transfer for revascularization. IABP improves coronary and peripheral perfusion via diastolic balloon inflation (perfusion augmentation) and augments LV performance via systolic balloon deflation (afterload reduction). Thus, accurate timing of IABP inflation and deflation is important for the optimal hemodynamic support of the failing heart. IABP was performed in 86% of patients in the SHOCK trial. The overall and major complication rates were 7% and 3%, respectively, and were more evident among women and in patients with small body size and peripheral arterial disease.
18. What is the role of total circulatory support in cardiogenic shock?
Percutaneous LVADs are currently available and can be placed in the cardiac catheterization laboratory. The TandemHeart (CardiacAssist, Inc., Pittsburgh) removes blood from the left atrium using a cannula placed through the femoral vein and into the left atrium via transseptal puncture. Blood is then returned to a systemic artery, usually the femoral, with retrograde perfusion of the abdominal and thoracic aorta. Another percutaneous device, the Impella (Abiomed, Danvers, Mass.), is a novel intravascular microaxial blood pump, which can be introduced via the femoral artery and placed across the aortic valve into the left ventricle to unload the left ventricle and provide a short-term mechanical support for the failing heart (Fig. 19-2). Extracorporeal life support (ECLS) involves extracorporeal circulation of blood through a membrane oxygenator, which relieves both the right side and left side of the heart and the lungs of part of their workload.
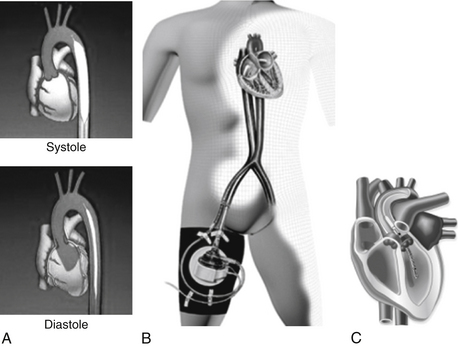
Figure 19-2 Percutaneous left ventricular support devices. A, Intraaortic balloon counterpulsation device inflating in systole and deflating during diastole. B, The TandemHeart inflow cannula enters the left atrium transseptally; its outflow cannula is inserted into the arterial circulation. C, The Impella 2.5 L/min device enters the ventricle in a retroaortic manner.
19. What are the mortality and morbidity rates of cardiogenic shock?
Bibliography, Suggested Readings, and Websites
1. Anderson, J.L., Adams, C.D., Antman, E.M., et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction. J Am Coll Cardiol. 2007;50:e1–e157.
2. Hochman, J.S., Sleeper, L.A., Webb, J.G., et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634.
3. Hochman, J.S., Sleeper, L.A., Webb, J.G., et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295:2511–2515.
4. Jneid, H., Anderson, J.L., Wright, R.S., et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60(7):648–681.
5. Levine, G.N., Bates, E.R., Blankenship, J.C., et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651.
6. O’Gara, P.T., Kushner, F.G., Ascheim, D.D., et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425.