Chapter 29
Cardiac Transplantation
1. How many heart transplantations are performed in the U.S. each year? What are the most frequent causes of heart disease requiring cardiac transplantation?
2. List common indications for heart transplantation.
 Severe heart failure (New York Heart Association [NYHA] class III or IV) with poor short-term prognosis despite maximal medical therapy, requiring continuous inotropic therapy, requiring mechanical support (e.g., balloon pump, left ventricular assist device [LVAD], extracorporeal membrane oxygenation [ECMO])
Severe heart failure (New York Heart Association [NYHA] class III or IV) with poor short-term prognosis despite maximal medical therapy, requiring continuous inotropic therapy, requiring mechanical support (e.g., balloon pump, left ventricular assist device [LVAD], extracorporeal membrane oxygenation [ECMO])
 Restrictive or hypertrophic cardiomyopathy with NYHA class III or IV symptoms
Restrictive or hypertrophic cardiomyopathy with NYHA class III or IV symptoms
 Refractory angina despite medical therapy, not amenable to revascularization, with poor short-term prognosis
Refractory angina despite medical therapy, not amenable to revascularization, with poor short-term prognosis
 Recurrent or refractory ventricular arrhythmias, despite medical and/or device therapy
Recurrent or refractory ventricular arrhythmias, despite medical and/or device therapy
 Complex congenital heart disease with progressive ventricular failure not amenable to surgical or percutaneous repair
Complex congenital heart disease with progressive ventricular failure not amenable to surgical or percutaneous repair
 Unresectable low-grade tumors confined to the myocardium, without evidence of metastasis
Unresectable low-grade tumors confined to the myocardium, without evidence of metastasis
3. What baseline evaluations are obtained in the pretransplantation workup?
Pretransplantation evaluation serves the purpose of assessing a patient’s severity of heart failure, mortality benefit from surgery, comorbidities, and potential contraindications to surgery. Factors important in transplantation evaluation are given in Box 29-1.
4. What are contraindications to heart transplantation?
Contraindications include any noncardiac conditions that may decrease a patient’s survival, and increase risk of rejection or infection, and are listed in Box 29-2.
5. Define allotransplantation versus xenotransplantation, and orthotopic versus heterotopic transplantation.
Allotransplantation involves transplantation of cells, tissue, or organs between same species Xenotransplantation involves transplantation of cells, tissue, or organs between different species. During orthotopic heart transplantation, the donor heart is transplanted in place of the recipient’s heart. There are two anastomotic approaches used (Fig. 29-1).
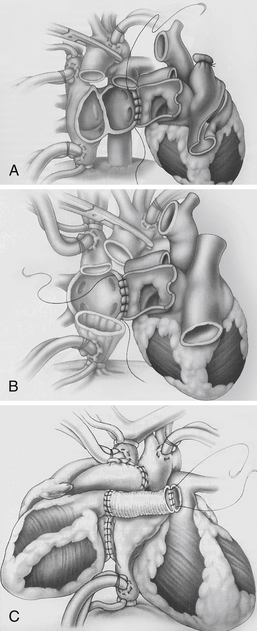
Figure 29-1 Anastomotic approaches used in cardiac transplantation. A, Biatrial approach. The donor atrial cuff is anastomosed to the recipient left atrium, right atrium, followed by aortic and pulmonary artery anastomosis. B, Bicaval approach. The donor left atrial cuff is anastomosed to the recipient left atrium, followed by inferior vena cava, superior vena cava, aortic, and pulmonary artery anastomosis. This approach may be associated with improved atrial function, lower incidence of atrial arrhythmias, sinus node dysfunction, and tricuspid insufficiency. C, Heterotopic transplantation. The donor-recipient left atrial cuff is anastomosed, followed by superior vena cava, aortic, and pulmonary artery anastomosis via Dacron graft. This procedure is considered in donor-recipient body-size mismatch, when the recipient pulmonary artery systolic pressure is greater than 60 mm Hg, or when there is suboptimal donor heart systolic function. (From Kirklin JK, Young JB, McGiffin DC: Heart transplantation, Philadelphia, 2002, Churchill Livingstone.)
 Biatrial approach: The donor atrial cuff is anastomosed to the recipient left atrium, right atrium, followed by aortic and pulmonary artery anastomosis.
Biatrial approach: The donor atrial cuff is anastomosed to the recipient left atrium, right atrium, followed by aortic and pulmonary artery anastomosis.
 Bicaval approach: The donor left atrial cuff is anastomosed to the recipient left atrium, followed by inferior vena cava, superior vena cava, aortic, and pulmonary artery anastomosis. This approach may be associated with improved atrial function, lower incidence of atrial arrhythmias, sinus node dysfunction, and tricuspid insufficiency.
Bicaval approach: The donor left atrial cuff is anastomosed to the recipient left atrium, followed by inferior vena cava, superior vena cava, aortic, and pulmonary artery anastomosis. This approach may be associated with improved atrial function, lower incidence of atrial arrhythmias, sinus node dysfunction, and tricuspid insufficiency.
During heterotopic heart transplantation, the recipient’s heart is left in the mediastinum, and the donor heart is attached “parallel” to the recipient heart (see Fig. 29-1).
6. Define ischemic time of the donor heart. Why is it important?
The cold ischemic time is the time interval between removal of the donor heart and the implantation in the recipient. During this interval, ischemic injury can occur to the heart due to lack of perfusion. Myocardial preservation is achieved with hypothermia and placement of the heart in a solution mimicking intracellular milieu to prevent cellular edema and/or acidosis, and maintain ATP supply for membrane function. A prolonged ischemic time can lead to irreversible damage to the harvested organ. A cold ischemic time of more than 5 hours is associated with a higher incidence of cardiac allograft dysfunction and decreased transplant recipient survival.
7. What is the estimated graft survival at 1 year, 3 years, 5 years, and 10 years posttransplantation? What are the common causes of death?
The major causes of death posttransplantation are as follows:
 Less than 30 days: graft failure, multiorgan failure, infection
Less than 30 days: graft failure, multiorgan failure, infection
 Less than 1 year: infection, graft failure, acute allograft rejection
Less than 1 year: infection, graft failure, acute allograft rejection
 More than 5 years: allograft vasculopathy, late graft failure, malignancies, infection
More than 5 years: allograft vasculopathy, late graft failure, malignancies, infection
8. What is cardiac allograft vasculopathy (CAV)? Describe its pathophysiology, incidence, risk factors, and outcome.
Also known as transplant vasculopathy or transplant coronary artery disease (CAD), CAV is the progressive narrowing of the coronary arteries of the transplanted heart. Angiographic incidence of CAV is approximately 30% at 5 years and 50% at 10 years. CAV is associated with a significantly increased risk of death. After the first year posttransplantation, CAV is the second most common cause of death (after malignancy). In CAV, there is diffuse, concentric proliferation of the intimal smooth muscle cells and it typically involves the entire length of the coronary artery. In contrast, conventional atherosclerosis results from fibrofatty plaque resulting in concentric or eccentric focal lesions. The etiology of CAV remains unclear, but both immunologic (cellular and/or humoral rejection, human leukocyte antigen [HLA] mismatch) and nonimmunologic (cytomegalovirus [CMV] infection, hypercholesterolemia, older age and/or male donors, younger recipients, history of CAD, diabetes mellitus [DM], and insulin resistance) factors have been implicated
 Progression of preexisting donor CAD, and
Progression of preexisting donor CAD, and
 De novo development as a result of transplantation-related hypertension, DM, or dyslipidemia.
De novo development as a result of transplantation-related hypertension, DM, or dyslipidemia.
9. List infections that are encountered early and late after cardiac transplantation.
 Nosocomial infections related to surgery or invasive procedures (mediastinitis, wound/line/urinary tract infections, ventilator-associated pneumonia)
Nosocomial infections related to surgery or invasive procedures (mediastinitis, wound/line/urinary tract infections, ventilator-associated pneumonia)
 Early virus reactivation (typically herpes simplex virus, human herpesvirus 6)
Early virus reactivation (typically herpes simplex virus, human herpesvirus 6)
 Most patients have stable graft function by this time, therefore the immunosuppressive regimen is reduced, and the risk for opportunistic infection decreases. Typically encountered infections include common viral and bacterial respiratory pathogens, however, some patients may develop chronic or recurrent opportunistic infections (i.e., CMV-related superinfection, EBV-associated lymphoproliferative disease).
Most patients have stable graft function by this time, therefore the immunosuppressive regimen is reduced, and the risk for opportunistic infection decreases. Typically encountered infections include common viral and bacterial respiratory pathogens, however, some patients may develop chronic or recurrent opportunistic infections (i.e., CMV-related superinfection, EBV-associated lymphoproliferative disease).
10. What type of malignancies are encountered posttransplantation? List incidence, time course, and prognosis.
11. Describe potential arrhythmias encountered posttransplantation.
 Sinus node dysfunction occurs in up to 50% of cardiac transplant recipients. Sinus node dysfunction early after transplantation does not appear to affect mortality, but has been associated with increased morbidity. Treatment of sinus bradycardia includes temporary pacing, intravenous (isoproterenol or dobutamine) or oral (theophylline or terbutaline) therapy in the immediate postoperative period. However, severe persistent bradycardia may require permanent pacemaker placement (up to 15% of patients).
Sinus node dysfunction occurs in up to 50% of cardiac transplant recipients. Sinus node dysfunction early after transplantation does not appear to affect mortality, but has been associated with increased morbidity. Treatment of sinus bradycardia includes temporary pacing, intravenous (isoproterenol or dobutamine) or oral (theophylline or terbutaline) therapy in the immediate postoperative period. However, severe persistent bradycardia may require permanent pacemaker placement (up to 15% of patients).
 AV nodal block is rarely encountered; its occurrence may indicate the presence of transplant vasculopathy and has been associated with increased mortality.
AV nodal block is rarely encountered; its occurrence may indicate the presence of transplant vasculopathy and has been associated with increased mortality.
 Atrial arrhythmias: Transient atrial arrhythmias, especially premature atrial contractions (PACs) are common in the early postoperative period; their clinical significance remains unclear, but frequent occurrences should prompt evaluation for rejection. Atrial fibrillation or flutter can occur in up to 25% of cardiac transplant recipients; late occurrence warrants evaluation for rejection. Treatment includes rate control with beta-adrenergic blocking agents (β-blockers), calcium channel blockers, cardioversion, overdrive pacing, and treatment for rejection. Atrial flutter can be treated with radiofrequency ablation.
Atrial arrhythmias: Transient atrial arrhythmias, especially premature atrial contractions (PACs) are common in the early postoperative period; their clinical significance remains unclear, but frequent occurrences should prompt evaluation for rejection. Atrial fibrillation or flutter can occur in up to 25% of cardiac transplant recipients; late occurrence warrants evaluation for rejection. Treatment includes rate control with beta-adrenergic blocking agents (β-blockers), calcium channel blockers, cardioversion, overdrive pacing, and treatment for rejection. Atrial flutter can be treated with radiofrequency ablation.
 Ventricular arrhythmias: Premature ventricular contractions (PVCs) are not uncommon early after cardiac transplantation, and their clinical significance is unknown. However, nonsustained ventricular tachycardia (>3 consecutive PVCs) has been associated with rejection and transplant vasculopathy. Sustained ventricular tachycardia or ventricular fibrillation are associated with poor prognosis and indicate severe transplant vasculopathy or high-grade rejection. Treatment includes correcting electrolyte abnormalities, intravenous amiodarone or lidocaine, defibrillation, and prompt evaluation for rejection and transplant vasculopathy.
Ventricular arrhythmias: Premature ventricular contractions (PVCs) are not uncommon early after cardiac transplantation, and their clinical significance is unknown. However, nonsustained ventricular tachycardia (>3 consecutive PVCs) has been associated with rejection and transplant vasculopathy. Sustained ventricular tachycardia or ventricular fibrillation are associated with poor prognosis and indicate severe transplant vasculopathy or high-grade rejection. Treatment includes correcting electrolyte abnormalities, intravenous amiodarone or lidocaine, defibrillation, and prompt evaluation for rejection and transplant vasculopathy.
12. What are the clinical signs and symptoms associated with acute cardiac transplant rejection (allograft rejection)?
13. List the different types of acute allograft rejection.
Allograft rejection occurs as a result of recipient immune response to donor heart antigens.
 Hyperacute rejection occurs within minutes to hours of transplantation due to preformed recipient antibodies against donor ABO, HLA, and endothelial cell antigens. Hyperacute rejection often results in loss of the graft.
Hyperacute rejection occurs within minutes to hours of transplantation due to preformed recipient antibodies against donor ABO, HLA, and endothelial cell antigens. Hyperacute rejection often results in loss of the graft.
 Cellular rejection is a T lymphocyte predominant, mononuclear inflammatory response directed against the allograft.
Cellular rejection is a T lymphocyte predominant, mononuclear inflammatory response directed against the allograft.
 Noncellular or humoral/antibody-mediated rejection is the result of de novo antibodies formed by the donor against recipient HLA antigens expressed on the vascular endothelium of the graft. Complement-mediated cytokine release leads to microvascular damage to the donor heart.
Noncellular or humoral/antibody-mediated rejection is the result of de novo antibodies formed by the donor against recipient HLA antigens expressed on the vascular endothelium of the graft. Complement-mediated cytokine release leads to microvascular damage to the donor heart.
14. Describe the grading and immunohistologic findings of acute cellular rejection (ACR) and acute antibody-mediated rejection (AMR).
 The rejection grades and corresponding histologic findings for ACR are presented in Table 29-1.
The rejection grades and corresponding histologic findings for ACR are presented in Table 29-1.
TABLE 29-1
ACUTE CELLULAR REJECTION (ACR)
| Rejection Grade | Histologic Findings |
| Grade 0R | No rejection |
| Grade 1R, mild | Interstitial and/or perivascular infiltrate with ≤1 focus of myocyte damage |
| Grade 2R, moderate | ≥2 Foci of infiltrate with associated myocyte damage |
| Grade 3R, severe | Diffuse infiltrate with multifocal myocyte damage, ± edema, hemorrhage, or vasculitis |
 The rejection grades and corresponding histologic findings for AMR are presented in Table 29-2.
The rejection grades and corresponding histologic findings for AMR are presented in Table 29-2.
TABLE 29-2
ACUTE ANTIBODY-MEDIATED REJECTION (AMR)
| Rejection Grade | Histologic Findings |
| AMR 0 | Negative for acute AMR No histologic or immunopathologic features of AMR |
| AMR 1 | Positive for AMR Histologic∗ features of AMR Positive immunofluorescence/immunoperoxidase† staining (+CD68, C4d) |
∗Histologic features of AMR: myocardial capillary injury with endothelial swelling and accumulation of perivascular macrophage.
†Immunohistochemistry shows deposition of immunoglobulin (IgG, M, A), complement (C3d, C4d, C1q), and CD68 staining for macrophage in capillaries (using CD31 or CD34 vascular markers).
15. How is allograft rejection diagnosed?
 Echocardiogram may reveal increased ventricular wall thickness due to edema, alterations in diastolic function (decreased early diastolic filling velocity [Ea], pseudonormal and/or restrictive filling pattern, shortened isovolumic relaxation time), decreased systolic function, and pericardial effusion.
Echocardiogram may reveal increased ventricular wall thickness due to edema, alterations in diastolic function (decreased early diastolic filling velocity [Ea], pseudonormal and/or restrictive filling pattern, shortened isovolumic relaxation time), decreased systolic function, and pericardial effusion.
 Endomyocardial biopsy (EMB) remains the gold standard for the diagnosis of allograft rejection. ACR is characterized by lymphocytic infiltration and myocyte damage. AMR is supported by findings of myocardial capillary injury with intravascular macrophages; immunologic staining will identify antibody and complement deposits within capillaries.
Endomyocardial biopsy (EMB) remains the gold standard for the diagnosis of allograft rejection. ACR is characterized by lymphocytic infiltration and myocyte damage. AMR is supported by findings of myocardial capillary injury with intravascular macrophages; immunologic staining will identify antibody and complement deposits within capillaries.
 Rejection is a clinical event. Even if biopsies do not support rejection, treatment can still be initiated on the basis of symptoms.
Rejection is a clinical event. Even if biopsies do not support rejection, treatment can still be initiated on the basis of symptoms.
16. How is an EMB performed and what are potential complications?
Potential complications of biopsies include:
 Tricuspid valve or subvalvular damage, and chordal rupture can occur, resulting in flail leaflets and severe regurgitation
Tricuspid valve or subvalvular damage, and chordal rupture can occur, resulting in flail leaflets and severe regurgitation
 Right ventricular wall perforation resulting in cardiac tamponade
Right ventricular wall perforation resulting in cardiac tamponade
 Transient heart block and ventricular arrhythmia
Transient heart block and ventricular arrhythmia
 Complications associated with venous access that include hematomas, nerve paresis, pneumothorax, thrombosis, and thromboembolism
Complications associated with venous access that include hematomas, nerve paresis, pneumothorax, thrombosis, and thromboembolism
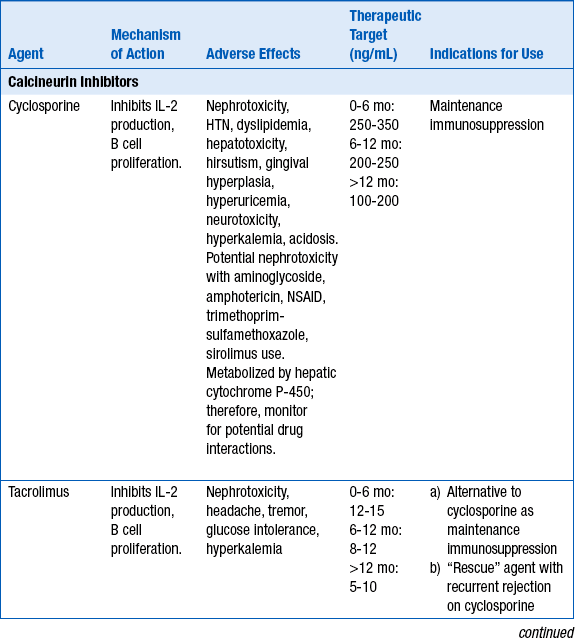
17. What is induction therapy? Describe its role in cardiac transplantation.
 Steroid-refractory, recurrent rejection
Steroid-refractory, recurrent rejection
 Inability to use calcineurin inhibitors in setting of profound renal insufficiency in the immediate postoperative period. Antilymphocyte antibodies can provide immunosuppression for at least 10 to 14 days, until recovery of renal function (Table 29-3).
Inability to use calcineurin inhibitors in setting of profound renal insufficiency in the immediate postoperative period. Antilymphocyte antibodies can provide immunosuppression for at least 10 to 14 days, until recovery of renal function (Table 29-3).
18. What is the incidence of ACR? Describe predisposing factors and treatment.
Approximately 40% of cardiac transplant recipients have ACR (grade >1R) in the first year after transplantation. Rejection frequency declines after the first year posttransplantation. Risk factors for ACR include early posttransplantation period; female donor; young, African American, or female recipients; and HLA mismatches. Treatment generally consists of high-dose corticosteroids, antithymocyte globulin (ATG), or muromonab-CD3 (OKT3) (Table 29-4).
TABLE 29-4
ACUTE CELLULAR REJECTION THERAPIES
| Rejection Grade | Therapy |
| 1R | No treatment |
| 1R + sx/hemodynamic compromise | IV steroids followed by oral taper, repeat biopsy in 1 week |
| 2R | High dose oral or IV steroids |
| 2R + sx/hemodynamic compromise | IV steroids followed by taper, repeat biopsy in 1 week ATG or muromonab-CD3 if persistent rejection despite 2 courses of steroid therapy |
| 3R | ATG or muromonab-CD3, repeat biopsy after course and again in 1 week |
| Recalcitrant rejection | Photopheresis, total lymphoid radiation |
19. Describe predisposing risk factors and treatment for AMR?
20. Describe typical maintenance immunosuppression therapy.
 Calcineurin inhibitors: cyclosporine or tacrolimus
Calcineurin inhibitors: cyclosporine or tacrolimus
 Antimetabolites or cell cycle modulators: mycophenolate mofetil (MMF) or azathioprine
Antimetabolites or cell cycle modulators: mycophenolate mofetil (MMF) or azathioprine
Approximately 60% of cardiac transplant recipients will not require long-term steroid use. Steroid withdrawal may be attempted after 1 year posttransplantation in patients without episodes of rejection. Newer agents include the TOR inhibitors (sirolimus, everolimus). Currently, the TOR inhibitors are not used as part of the standard maintenance therapy but are added in patients with accelerated transplant vasculopathy, worsening renal function on calcineurin inhibitors, and frequent rejections on standard triple maintenance therapy. Note that mycophenolate mofetil (MMF) or azathioprine are stopped when sirolimus or everolimus are added, to avoid excessive immunosuppression (Table 29-5).
TABLE 29-5
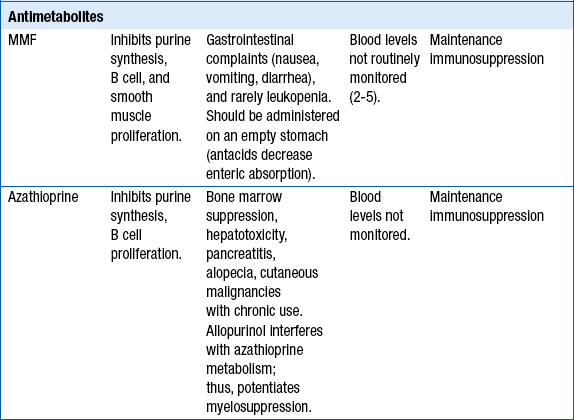
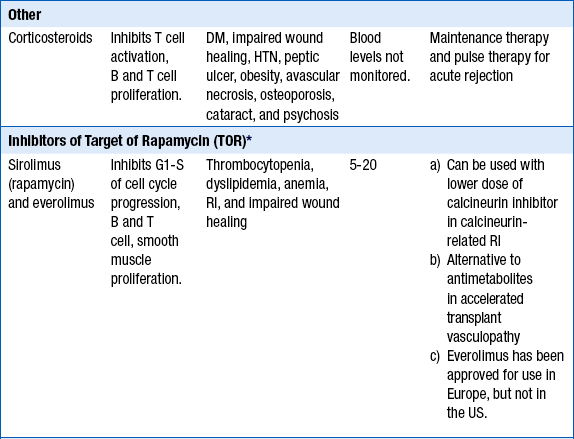
∗Not part of routine maintenance therapy. TOR inhibitors are added in certain situations (see text).
21. What are common medical conditions encountered in posttransplantation patients?
 Hypertension: attributed to sympathetic stimulation, neurohormonal activation, renal vasoconstriction by calcineurin inhibitors and mineralocorticoid effect of steroids)
Hypertension: attributed to sympathetic stimulation, neurohormonal activation, renal vasoconstriction by calcineurin inhibitors and mineralocorticoid effect of steroids)
 Renal impairment: as a result of low cardiac output pretransplantation, ischemic injury during transplantation, and calcineurin-related renal arteriolar vasoconstriction and tubulointerstitial fibrosis)
Renal impairment: as a result of low cardiac output pretransplantation, ischemic injury during transplantation, and calcineurin-related renal arteriolar vasoconstriction and tubulointerstitial fibrosis)
 Dyslipidemia: as a result of weight gain, corticosteroid, and cyclosporine use
Dyslipidemia: as a result of weight gain, corticosteroid, and cyclosporine use
 Diabetes: as a result of corticosteroid use, weight gain
Diabetes: as a result of corticosteroid use, weight gain
 Osteoporosis: due to corticosteroid use
Osteoporosis: due to corticosteroid use
 Gout: hyperuricemia from decreased uric acid clearance with cyclosporine use
Gout: hyperuricemia from decreased uric acid clearance with cyclosporine use
22. Describe adverse effects encountered with calcineurin inhibitor use and potential drug interactions that may lead to calcineurin toxicity.
 Hypertension: greater than 70% by 1 year and 95% by 5 years after transplantation
Hypertension: greater than 70% by 1 year and 95% by 5 years after transplantation
 Renal dysfunction: greater than 25% by 1 year and 5% progress to end-stage renal disease within 7 years after transplantation
Renal dysfunction: greater than 25% by 1 year and 5% progress to end-stage renal disease within 7 years after transplantation
 Rhabdomyolysis when used concurrently with HMG-CoA reductase inhibitors (statins), because calcineurin inhibitors inhibit metabolism of certain statins (lovastatin, simvastatin, cerivastatin, and atorvastatin). Fluvastatin, pravastatin, and rosuvastatin are less likely to be involved in this type of interaction.
Rhabdomyolysis when used concurrently with HMG-CoA reductase inhibitors (statins), because calcineurin inhibitors inhibit metabolism of certain statins (lovastatin, simvastatin, cerivastatin, and atorvastatin). Fluvastatin, pravastatin, and rosuvastatin are less likely to be involved in this type of interaction.
 Calcineurin toxicity is characterized by neurologic symptoms (headaches, tremor, confusion, agitation, delirium, expressive aphasia, and seizures), nephrotoxicity, and hypertension. The enzyme CYP3A4 metabolizes calcineurin inhibitors, and inhibitors of CYP3A4 can lead to increased drug levels and adverse effects. CYP3A4 inhibitors include:
Calcineurin toxicity is characterized by neurologic symptoms (headaches, tremor, confusion, agitation, delirium, expressive aphasia, and seizures), nephrotoxicity, and hypertension. The enzyme CYP3A4 metabolizes calcineurin inhibitors, and inhibitors of CYP3A4 can lead to increased drug levels and adverse effects. CYP3A4 inhibitors include:
23. How do patients with transplant vasculopathy clinically present? What invasive and noninvasive tests are used to assist in the diagnosis of transplant vasculopathy?
 Coronary angiography: Most centers have adopted surveillance angiographies for early diagnosis of CAV. However, CAV is often diffuse and concentric in its distribution and may be underestimated by angiography. To improve detection and sensitivity, intravascular ultrasound, and/or quantitative coronary angiography are used as adjunctive modalities.
Coronary angiography: Most centers have adopted surveillance angiographies for early diagnosis of CAV. However, CAV is often diffuse and concentric in its distribution and may be underestimated by angiography. To improve detection and sensitivity, intravascular ultrasound, and/or quantitative coronary angiography are used as adjunctive modalities.
 Noninvasive stress testing: Dobutamine stress echo or myocardial perfusion imaging can be used to diagnose CAV, but have a lower sensitivity when compared to angiography. To avoid contrast-induced nephropathy, noninvasive stress testing is used in patients with renal insufficiency.
Noninvasive stress testing: Dobutamine stress echo or myocardial perfusion imaging can be used to diagnose CAV, but have a lower sensitivity when compared to angiography. To avoid contrast-induced nephropathy, noninvasive stress testing is used in patients with renal insufficiency.
24. Describe strategies to prevent and treat cardiac allograft vasculopathy.
 Lipid-lowering therapy (pravastatin has fewer drug interactions and is better tolerated than other statins)
Lipid-lowering therapy (pravastatin has fewer drug interactions and is better tolerated than other statins)
 TOR inhibitors (may decrease CAV incidence)
TOR inhibitors (may decrease CAV incidence)
 Blood pressure control (diltiazem may also limit intimal thickening)
Blood pressure control (diltiazem may also limit intimal thickening)
Treatment of cardiac allograft vasculopathy may include the following:
 Percutaneous and surgical revascularization have limited role in the setting of diffuse vasculopathy.
Percutaneous and surgical revascularization have limited role in the setting of diffuse vasculopathy.
 Retransplantation is the only definitive therapy.
Retransplantation is the only definitive therapy.
 Immunosuppression regimen adjustment and trial of TOR inhibitor may be attempted.
Immunosuppression regimen adjustment and trial of TOR inhibitor may be attempted.
25. When should mechanical circulatory support device (MCSD) implantation be considered?
Indications for LVAD implantation include:
 An attempt to extend life in a deteriorating transplantation candidate who is listed for a donor heart (bridge-to-transplant)
An attempt to extend life in a deteriorating transplantation candidate who is listed for a donor heart (bridge-to-transplant)
 In patients with multiorgan failure, an LVAD may help determine transplantation eligibility. If pulmonary hypertension or renal insufficiency improves after LVAD, a heart transplantation would be more likely of benefit (bridge-to-decision).
In patients with multiorgan failure, an LVAD may help determine transplantation eligibility. If pulmonary hypertension or renal insufficiency improves after LVAD, a heart transplantation would be more likely of benefit (bridge-to-decision).
 Support for patients whose surgery is complicated by cardiogenic shock
Support for patients whose surgery is complicated by cardiogenic shock
 Permanent support for patients who are not transplantation candidates (destination therapy)
Permanent support for patients who are not transplantation candidates (destination therapy)
26. What is gene expression profiling (GEP) and how is it used in the diagnosis of rejection?
Bibliography, Suggested Readings, and Websites
1. Department of Transplantation Immunology, University of Heidelberg: Collaborative Transplant Study website. Available at: www.ctstransplant.org. Accessed May 1, 2012.
2. Eisen, H.J. Immunosuppression on the horizon. Heart Fail Clin. 2007;3:43–49.
3. The International Society for Heart and Lung Transplantation: ISHLT website. Available at: www.ishlt.org. Accessed May 1, 2012.
4. Jessup, M., Banner, N., Brozena, S., et al. Optimal pharmacologic and non-pharmacologic management of cardiac transplant candidates: approaches to be considered prior to transplant evaluation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant. 2006;25:1003–1023.
5. The Journal of Heat and Lung Transplantation: JHLT website. Available at: www.jhltonline.org. Accessed May 1, 2012.
6. Kirklin, J.K., Young, J.B., McGiffin, D.C. Heart transplantation. Philadelphia: Churchill Livingstone; 2002.
7. Kobashigawa, J.A. Contemporary concepts in noncellular rejection. Heart Fail Clin. 2007;3:11–15.
8. OPTN/SRTR: 2009 U.S. Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) Annual Report: Transplant Data 1999-2008. Available at: http://optn.transplant.hrsa.gov/ar2009/default.htm. Accessed May 1, 2012.
9. Sipahi, I., Starling, R.C. Cardiac allograft vasculopathy: an update. Heart Fail Clin. 2007;3:87–95.
10. Steinman, T.I., Becker, B.N., Frost, A.E., et al. Guidelines for the referral and management of patients eligible for solid organ transplantation. Transplantation. 2001;71:1189–1204.
11. Stewart, S., Winters, G.L., Fishbein, M.C., et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720.
12. Stehlik, J., Edwards, L.B., Kucheryavaya, A.Y., et al. The Registry of the International Society of Heart and Lung Transplantation: Twenty-eighth official adult heart transplant report—2011. J Heart Lung Transplant. 2011;30:1078–1094.
13. United Network for Organ Sharing: UNOS website. Available at: www.unos.org. Accessed May 1, 2012.