12 Cardiac Surgery and Transplantation
After reading this chapter, you should be able to:
• outline cardiac surgical procedures including coronary artery bypass graft surgery and valve repair and replacement
• describe the indications, advantages and disadvantages of using cardiopulmonary bypass
• outline methods of myocardial preservation during cardiac surgery
• outline immediate postoperative management of cardiac surgical patients including haemodynamic, rhythm monitoring, ventilatory support, postoperative bleeding including pericardial tamponade, postoperative pain, fluid and electrolyte and emotional and family support
• outline the principles of counterpulsation in intra-aortic balloon pumping
• outline the benefits and timing of balloon inflation and deflation, conventional and real timing, management and assessment of timing and timing errors
• describe the nursing management of IABP complications, including limb perfusion, bleeding and immobility-related complications
• discuss methods of weaning IABP and management of IABP removal
• discuss the immediate postoperative care of heart transplant recipients
• describe the clinical manifestations of postoperative complications in heart transplant recipients
• identify signs and symptoms of rejection in heart transplant recipients
• evaluate the effectiveness of nursing interventions in the postoperative management of heart transplant recipients.
Introduction
Many critically ill patients experience compromised cardiac function, as either a primary or secondary condition. This chapter follows on from those situations examined in Chapter 10, and reviews patients with conditions that tend to be cared for in specialised critical care units. In this chapter, the management of a patient who requires cardiac surgery for coronary artery disease or valvular disease will be discussed, including the use of cardiopulmonary bypass. In addition, the use and management of intra-aortic balloon pumping in cardiac surgical and medical patients will be outlined. The management of patients following heart transplantation will be identified including the immediate postoperative complications and their prevention and management.
Cardiac Surgery
Structural Abnormalities
Some structural abnormalities result from myocardial infarction, and have been described in Chapter 10. Other structural abnormalities result in valvular disease (mitral, aortic, tricuspid, pulmonic) and ventricular defects.
Valvular Disease
The incidence and types of valvular disease have changed over the past 50 years.1 Valvular disorders, such as mitral stenosis and aortic regurgitation, often arise from infectious diseases like rheumatic fever and syphilis, which are much less common today. Conversely, there has been a rise in the rate of aortic stenosis, which is due to degenerative disease common in ageing. In contrast to these trends, the prevalence of rheumatic fever and rheumatic heart disease among Indigenous Australians is one of the highest in the world.2 Also, Pacific Islanders living in New Zealand have much higher rates of rheumatic fever than the general population.3 As a result, valvular disorders are much more common in these groups. Rheumatic fever is discussed under infective endocarditis in Chapter 10.
Stenotic valves have a tightened, restricted orifice, so that blood must be forced through at higher pressure (Figure 12.1). In regurgitation, also called valvular incompetence or insufficiency, incomplete closure of the valve leaflets results in backflow of blood. Valvular conditions can result from congenital deformities, but also from the degenerative changes associated with ageing, from infection and rheumatic diseases. When a valve is stenosed, higher pressure is required to push blood through the narrow opening and the heart compensates by hypertrophy and dilation. When a valve is incompetent the heart does not empty sufficiently, so again the heart compensates by hypertrophy and dilation. In both these conditions heart failure may result; however, in regurgitation, pressure in the ventricles and atrium grows and this pressure is reflected back into the pulmonary or venous system. Although the heart contains four valves, the majority of disorders affect the mitral and aortic valves in the left side of the heart.
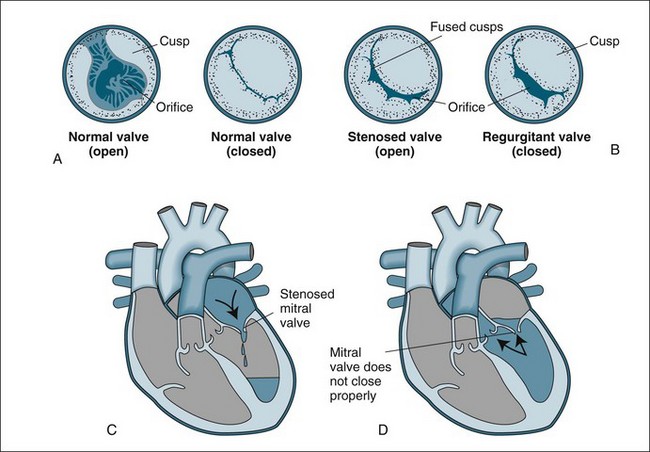
FIGURE 12.1 Valvular stenosis and regurgitation: (A) normal position of valve leaflets (cusps) when the valve is open and closed; (B) open position of a stenosed valve (left) and closed position of a regurgitant valve (right); (C) haemodynamic effect of mitral stenosis shows the mitral valve is unable to open completely during left atrial systole, limiting left ventricular filling; (D) haemodynamic effect of mitral regurgitation shows the mitral valve does not close completely during left ventricular systole, allowing blood to re-enter the left atrium.43
Aortic valve disease
Aortic stenosis is a narrowing of the opening of the valve between the left ventricle and aorta (Figure 12.1). This stenosis often results from degenerative changes that occur with age or as a result of congenital abnormalities such as a bicuspid aortic valve (prevalence of bicuspid aortic valve in the general population is 0.5% and may cause aortic stenosis or regurgitation). Aortic stenosis is usually associated with left ventricular hypertrophy in response to the high pressure needed to push blood into the aorta. Increased myocardial oxygen demands from the hypertrophied muscle also mean that angina is common. Often the first sign of aortic stenosis is left heart failure, which is a culmination of these two effects and adaptive dilation.4 On auscultation, additional heart sounds are heard as a systolic murmur and a loud S4.
Aortic regurgitation may occur acutely when the aortic valve is damaged by endocarditis, trauma or aortic dissection, and presents as a life-threatening emergency. Chronic aortic regurgitation usually results from rheumatic heart disease, syphilis, chronic rheumatic conditions or congenital conditions. Again the left ventricle compensates by hypertrophy and dilation, which ultimately can result in left heart failure. When left heart failure occurs, left atrial pressure rises and may cause pulmonary hypertension. In the acute situation, the patient presents with collapse, severe hypotension and dyspnoea.4 Patients with chronic regurgitation may remain asymptomatic for years, finally presenting with signs of left heart failure. On auscultation, a diastolic murmur can be heard.
Mitral valve disease
Mitral valve stenosis often occurs as a result of rheumatic heart disease and less often from systemic lupus erythromatosus. These diseases cause damage to the leaflets and chordae tendineae, so that during healing the scars contract and seal, restricting the aperture. Left atrial pressure rises with resultant pulmonary hypertension. In chronic conditions, this pressure may also affect the right ventricle.5 Lung compliance is also reduced, causing dyspnoea. On auscultation a low-pitched diastolic murmur and an opening snap can be heard.
Mitral valve regurgitation results when the mitral valve and chordae tendineae are damaged, often due to myocardial infarction, rheumatic disease and infectious endocarditis. Backflow into the left atrium during systole creates elevated atrial and pulmonary pressures, and pulmonary oedema can result.5 On auscultation, a third heart sound and a pansystolic murmur can be heard.
Surgical Procedures
Coronary Artery Bypass Graft Surgery
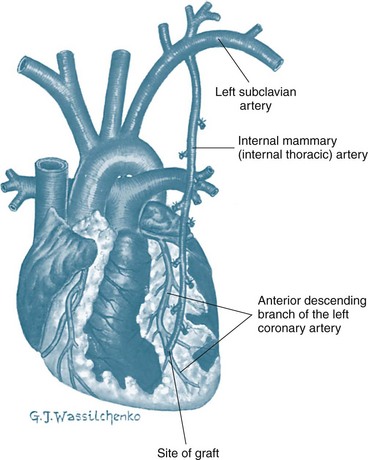
CABG uses a section of vein or artery to bypass a blockage in the patient’s coronary artery. The vessels used for grafting arise from the internal mammary artery, or are taken from the saphenous vein or radial artery. Saphenous veins are removed from the legs, and the radial artery from the forearm and used as a free graft with anastomoses at the ascending aorta and distally to one or more coronary arteries. When saphenous veins are used as grafts (SVG), they often develop diffuse intimal hyperplasia, which ultimately contributes to restenosis. Patency rates are lowest in saphenous vein grafts attached to small coronary arteries or coronary arteries supplying myocardial scars. Consequently, arterial grafts are used more often, as they are more resistant to intimal hyperplasia. Internal mammary arteries (IMAs) and radial artery grafts may be used.6 The IMA remains attached to the subclavian artery and is mobilised from the chest wall and anastomosed to the coronary artery distal to the occlusion (Figure 12.2). If the radial artery is being harvested for grafting, the collateral circulation in the forearm is assessed. Echo colour Doppler provides best accuracy of forearm circulation, although the clinical Allen test is quite commonly used. The disadvantage of the Allen test is that it has around 5% false patency result.7 A selection of IMA, SVG and radial artery grafts may be necessary over time as repeat procedures are needed or in patients with extensive disease requiring multiple grafts.
Over recent years a new approach to CABG – minimally invasive direct coronary artery bypass grafting (MIDCABG) – has been used. This procedure uses intercostal incisions and a thorascope instead of a sternotomy to access the heart and coronary arteries. MIDCABG is also often performed without cardiopulmonary bypass (off pump coronary artery bypass, OPCAB); instead, the heart is slowed with beta-blockers to allow the surgery to be performed on a beating heart.8 OPCAB procedures may also be performed using full or partial sternotomy to provide access for multiple vessels grafting. Both procedures have been successful responses to the drive to reduce recovery times, patient stays in hospital and costs.8 MIDCABG is currently only used in single-vessel disease, particularly the left anterior descending (LAD) artery. More recently, robotically-assisted cardiac surgery has been performed in America and Europe and has been introduced at a small number of Australian hospitals for CABG and mitral valve surgery. This technique has further reduced the invasiveness of cardiac surgery, as little more than stab wounds are required in the right chest for thoroscopy and the robotic instruments. Avoiding true thoracotomy or sternotomy improves postoperative pain experiences and shortens length of stay.9
Although CABG is the most common cardiac surgical procedure undertaken in Australia, the incidence has declined since 2005/06 to be 61 procedures/100,000 population in 2007–08.10 The decline in surgery rates is due to changes in the treatment of CHD, including the advent of percutaneous coronary intervention (PCI). More procedures are now being performed in older patients, with 73% of current patients aged over 60 years.1 CABG is used to relieve the symptoms of angina by increasing coronary blood flow distal to occlusive coronary lesions. It is a palliative, not curative, treatment as the underlying disease process continues.11 CABG is more effective than PTCA in patients with extensive, multi-vessel disease.9,11 CABG is also used in left main vessel lesions due to the high risk of extensive infarction associated with PTCA in this area. Women do not appear to have the same access to CABG surgery, as men are three times more likely to have surgery, although only twice as likely as women to have CHD.12 CABG surgery is commonplace, and many cardiothoracic centres have highly efficient, effective systems in place with mortality rates as low as 2%.
Valve Repair and Replacement
Valve surgery is usually undertaken to repair the patient’s valve or, more often, to replace the valve with either a mechanical or tissue prosthesis. The clinical decision for valve surgery is primarily based on the clinical state of the patient using the New York Heart Association (NYHA) classification system and echocardiographic findings.5 The type of surgery used will depend on the valves involved, the valvular pathology, the severity of the condition and the patient’s clinical condition. Often valve surgery is not a single procedure, and it may involve multiple valves, CABG and implantable cardioverter defribillator (ICD). Valve surgery is palliative, not curative, and patients will require lifelong health care.
Valve replacement may be necessary, but could be associated with higher risks due to long-term disease process and poor underlying left ventricular function. The most common indication for valve replacement is aortic stenosis, and accounts for 60–70% of valve surgery.13 Prosthetic valves may be mechanical or biological. Mechanical valves are made of metal alloys, pyrolite carbon and Dacron (Figure 12.3). Biological valves are constructed from porcine, bovine or human cardiac tissue. Mechanical valves are more durable but have an increased risk of thromboembolism, so lifelong anticoagulation is required. Biological valves suffer from the same problems as the patient’s valve (i.e. calcification and degeneration). The choice of valve depends on the age of the patient and potential difficulties with taking anticoagulants.
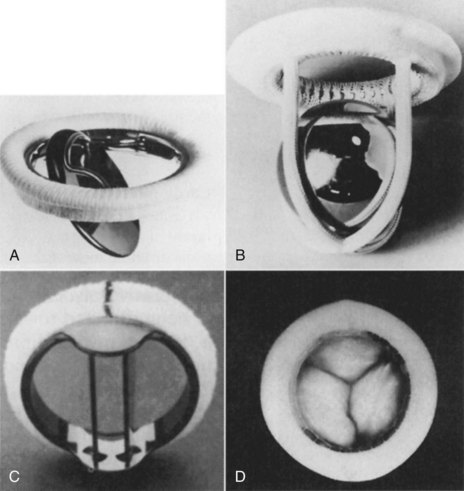
FIGURE 12.3 Prosthetic valves: (A) the Bjork-Shiley valve, with a pyrolyte-carbon disc that opens to 60 degrees; (B) the Starr-Edwards caged-ball valve model 6320, with satellite ball; (C) the St Jude Medical mechanical heart valve, with a mechano-central flow disc; (D) the Hancock II porcine aortic valve, with stent and sewing ring covered in Dacron cloth.5
Mortality for valvular surgery is higher than for CABG, reflecting the underlying loss of ventricular function and additional procedures that are common. Risk stratification models have been developed to help determine the patients that are most likely to have poor recovery and outcomes.14 The major factors that contribute to poor outcomes are worse left ventricular function and age over 70 years old.
Cardiopulmonary Bypass
Adverse effects of CPB are diverse, and include the following:15
• Haematological effects due to exposure of the blood to tubing and gas exchange surfaces, which initiates surface activation of the clotting cycle. Also blood component damage due to shear stress from the roller action of the pump, which reduces haematocrit, leucocyte and platelet counts.
• Pulmonary effects due to activation of systemic inflammatory response syndrome (SIRS) that increases capillary leakage, and lung deflation during surgery leading to post-operative atelectasis.
• Cardiovascular effects due to volume changes, fluid shifts and decreased myocardial contractility, which decreases cardiac output. This is most severe during the first 6 hours, but usually resolves within 48–72 hours.
• Neurological effects due to poor cerebral perfusion and generation of thromboemboli from aortic cannulation, which can lead to cerebrovascular accidents.
• Renal effects due to decreases in cardiac output during initiation of CPB, which decreases renal perfusion.
• Post-pump delirium or psychosis, which occurs in 32% of CPB patients although the cause has not been identified. Symptoms include short-term memory deficit, decreased attention, and inability to respond to and integrate sensory information.
• Activation of a systemic inflammatory response, which may cause vasodilation and increased cardiac output.
Nursing Management
The Immediate Postoperative Period
Patients should be transported to intensive care accompanied by at least an anaesthetist, an appropriately qualified nurse and transport personnel under continuous cardiac monitoring and assisted ventilation. It is prudent to include capnography during patient transport to detect ventilator disconnection, dysfunction, or endotracheal tube migration. Intensive care or theatre nursing staff may be a component of the transport team. The admission to intensive care requires a team approach, with the participation of intensive care nursing and medical staff and/or technician input. The immediate postoperative decision making on patient management is influenced by handover from anaesthetists, settling in procedures and collegial assistance.16 Admission activities are commonly divided between nurses, with one nurse taking responsibility for establishing monitoring and haemodynamic assessment and management, and a second nurse managing ventilation and endotracheal tube security, as well as managing chest drains, gastric tube and urinary catheter. If staffing permits, additional nurses may take responsibility for documentation, performing arterial blood gases, 12-lead ECG and providing assistance as required.
The objectives of immediate post operative management of cardiac surgical patients may include:
Haemodynamic Monitoring and Support
Typical haemodynamic monitoring includes an intra-arterial catheter for continuous blood pressure monitoring and arterial blood sampling. Cardiac output and preload measurement are achieved most commonly with either a pulmonary artery or central venous catheter configured for pulse contour cardiac output (PiCCO) monitoring (see Chapter 9).
Preload measures provided by the pulmonary artery catheter include right atrial pressure (RAP) to approximate right ventricular filling, and pulmonary artery pressure (PAP) to approximate right ventricular systole and provide insight into pulmonary vascular resistance and left heart function. The pulmonary capillary wedge pressure (PCWP) is available to approximate left ventricular filling and left heart function. Alternatively, the PiCCO monitoring system represents preload by intrathoracic blood volume index (ITBVI) and global end-diastolic volume index (GEDVI). In addition, the extravascular lung water index (EVLWI) can demonstrate the accumulation of interstitial lung water.17
Certain common haemodynamic patterns are seen in the early postoperative phase. These must be detected through thorough monitoring and interpretation of variables, and managed according to specific needs. During the initial two hours of recovery period, 95% of patients will experience haemodynamic instability.18
Hypertension
Hypertension is present in up to 30% of patients initially,19 as hypothermia, stress responses, pain and hypovolaemia contribute to vasoconstriction.19–21 When the systemic vascular resistance is excessive, the high afterload may contribute to low cardiac output.19 Rewarming to normothermia with space blankets or heated air blankets, fluid administration, administration of sedation or analgesics, and infusion of IV vasodilators (glyceryl trinitrate or sodium nitroprusside) are all commonly used to overcome vasoconstriction when contribut-ing to hypertension.19–21 Occasionally beta-blockers are used. Hypertension increases myocardial workload and contributes to bleeding.
Hypotension
Transient hypotension requiring treatment is common at some stage during the postoperative period. Contributing factors to hypotension include hypovolaemia and decreased venous return (from polyuria, bleeding, ventilation and positive end-expiratory pressure, and excess vasodilation), contractile impairment (from ischaemia or infarction, hypothermia, and negative inotropic influences), pericardial tamponade, and vasodilation (from excess vasodilator therapy, or as part of an inflammatory response to cardiopulmonary bypass).22
Hypotension may present with reduced or elevated preload, reduced or elevated cardiac output, and reduced or elevated systemic vascular resistance (SVR). When hypovolaemia is present, cardiac output will be low and SVR usually high. Hypovolaemia is diagnosed by measuring preload indicators, as pressure (RAP, PAP, PCWP) or volume (ITBVI, GEDVI).17,19 Colloids (e.g. normal serum albumin) are generally preferred for volume restoration in the postoperative period.18 Blood returned from the cardiopulmonary bypass circuit (‘pump blood’) usually accompanies the patient to ICU, and this should be readministered at a rate suitable to filling indices and blood pressure.
Hypotension accompanied by elevated preload and low cardiac output usually represents cardiac dysfunction or pericardial tamponade, and the distinction should be quickly sought.20,23 When such left ventricular dysfunction is present, there is usually compensatory vasoconstriction and tachycardia, although heart rate responses may be unreliable due to cardioplegia, cold, conduction disease21 and preoperative beta-blocking agents. Inotropic agents, including milrinone hydrochloride, adrenaline, dopamine or dobutamine, may become necessary (these are covered more completely in Table 20.7 and its accompanying text). When the profile of severe left ventricular dysfunction is persistent (either at the time of coming off bypass or later in intensive care), intra-aortic balloon pumping may be instituted. ECG assessment for new ischaemia or infarction should be made, which if of significant size, may warrant surgical re-exploration or angiographic investigation. Pericardial tamponade is also a cause of hypotension (covered later in this chapter).
A fourth common postoperative profile is hypotension with normal or elevated cardiac output in the presence of low SVR. This may occur with excess vasodilator administration, the use of postoperative epidural infusions, and vasodilation from a systemic inflammatory response to cardiopulmonary bypass and other factors such as reinfusion of collected operative site blood.24 The inotrope milrinone hydrochloride is popular in the postoperative phase because of its dilating effect on radial artery grafts,25 but often contributes to hypotension through its systemic vasodilator properties. When hypotension is attributable to vasodilation, metaraminol or noradrenaline may be used.19 Arginine vasopressin, by infusion, has more recently emerged as an effective alternative vasoconstrictor for cardiac surgical patients.26
A mean arterial pressure of 70–80 mmHg is generally targeted in the postoperative period.21 This can sometimes be reduced if there has been ventriculotomy or if there is concern about the status of the aorta.20 The cardiac index should be maintained above 2.2 L/min/m2, as hypoperfusion develops below these values. When at these levels, additional assessments are often undertaken, such as mixed venous oxygen saturation measurement (to assess oxygen delivery deficits) and arterial pH and lactate measurements (to detect metabolic acidosis from anaerobic metabolism).
In addition to assessment of preload, contractility and afterload, heart rate and rhythm should be assessed for their input into cardiac output and blood pressure. Extremes of rate and arrhythmias alter ventricular filling and may need correction. If temporary pacing wires are present, pacing strategies for haemodynamic improvement include rate rises (even if already in the normal range)21 and the provision of dual-chamber or atrial pacing as alternatives to just ventricular pacing. Alternatively, if ventricular pacing is present, reducing the rate to permit expression of a slower sinus rhythm may, with the provision of atrial kick, improve cardiac output and blood pressure (refer to Chapter 11 for more information on pacing).
Rhythm monitoring and postoperative arrhythmias
Continuous rhythm monitoring is necessary while in intensive care, and telemetry monitoring is usually continued until discharge from hospital. Lead selection is often haphazard, but a chest lead in the V1 position (or lead MCL1) generally provides best information on atrial and ventricular activity.27 Unlike many leads, these two leads reliably demonstrate normal rhythms, bundle branch block and ventricular rhythms,27 and may be useful in confirming pulmonary artery catheter irritation as the cause of ventricular arrhythmias.28
A 12-lead ECG is performed on admission to the ICU and should be compared with the preoperative ECG. It should be assessed for signs of new ischaemia or infarction, new bundle branch block and arrhythmias or conduction disturbances. Pericarditis, a frequent complication of surgery, appears as ST segment elevation (often, but not always, in many leads), and may mask or mimic myocardial infarction. The nurse should look for the classic concave upward, or ‘saddle-shaped’ ST segment, to distinguish pericardial changes from the more convex upward ST segment of infarction. Worsening of pain on inspiration and a pericardial rub help to confirm pericarditis.27
Atrial fibrillation is the most common postoperative arrhythmia and contributes significantly to postoperative morbidity and hospital length of stay.29 It occurs in up to 30–50% of patients, most often on days 2 to 3 postoperatively.15,29 Many patients revert without treatment,19 but when treatment becomes necessary beta-blockers and amiodarone appear the most successful agents for correction.29 Digitalis is effective for rate control and IV magnesium is often used, although further evidence for its use is needed. Atrial pacing to prevent atrial fibrillation is being increasingly explored but a clear recommendation on pacing sites and protocols has yet to emerge. By contrast, atrial overdrive pacing can be an effective means to immediately and safely interrupt atrial flutter.29
Ventricular ectopic beats are common and by themselves do not require treatment unless they accompany ischaemia or biochemical disturbance,19 in which case they may progress to more complex arrhythmias. Consideration should always be given to the pulmonary artery catheter as the cause (including both correctly and malpositioned catheters),28 as this is an easily corrected influence. Ventricular tachycardia and fibrillation are uncommon and usually denote myocardial disturbance such as ischaemia or infarction, shock, electrolyte disturbance, hypoxia, or increased excitation by high circulating catecholamine levels.17 Standard approaches to resuscitation according to protocols in Chapter 24 apply, including standard CPR over the recent sternotomy. When ventricular fibrillation cannot be corrected, consideration is often given to re-exploration of the chest to examine graft patency and/or provide internal cardiac massage. The cardiac surgical intensive care unit should be equipped to enable emergency re-exploration for such purposes.
Ventilatory Support
Ventilation should be approached according to the general principles described in Chapter 15. As anaesthesia is not typically reversed at the end of the operation, patients are generally admitted apnoeic, and within 1–3 hours return to wakefulness and spontaneous breathing.
Ensuring a secure airway is an initial priority; the following should be undertaken:
• Confirmation of endotracheal tube position and its security immediately on admission:
There has been a general trend to more rapid ventilatory weaning in recent years, and in some centres ‘fast-track’ cardiac surgical recovery includes extubation at the end of the operation before transfer to a recovery unit for suitable patients. Indices of respiration show no improvement when intubation is maintained for longer compared with early extubation,30 and pooled results from randomised early extubation trials show earlier ICU discharge and shorter lengths of stay (by 1 day) when early extubation is undertaken.31
• intraoperative neurological event
• gas exchange deficit with unresolved hypoxaemia
• significant haemodynamic insufficiency
• patients returning from theatre late in the evening may sometimes continue ventilation overnight to optimise postextubation breathing ability.
For many patients, ventilation is provided purely for initial airway and apnoea protection rather than for treatment of pulmonary deficits. In the absence of pulmonary disease, many centres provide fairly uniform approaches to parameter settings that aim at sustaining ventilation and oxygenation, while limiting traumatic risk to the lungs (see Table 12.1). However, approaches to ventilation will need to be tailored in the presence of operative complications or coexisting lung disease.
| Nominal or generally acceptable settings | Alternatives to nominal settings and reasons for variation |
|---|---|
| SIMV with volume control ventilation | Pressure control suitable. Generally used only if there is significant hypoxaemia or the need to exert greater control on pulmonary pressure. Hybrid modes such as Autoflow, pressure-regulated volume control or volume control plus (VC+) are also suitable, generally for same indications as pressure control. |
| Tidal volume 8–10 mL/kg | Lower tidal volumes (6–8 mL/kg) when there is known compliance disorder (atelectasis, pulmonary oedema, fibrosis) or unexplained high plateau pressures. |
| Mandatory rate 10 L/min | Faster rates may be necessary if low tidal volume strategies become necessary. Lower rates if gas trapping risk due to airways disease. |
| Inspiratory flow 30–50 L/min to provide I : E ratio of 1 : 2 to 1 : 4 acceptable | Slower flows to prolong the inspiratory time may be necessary if there is atelectasis and hypoxaemia, or if there is a desire to lessen inspiratory pressures. Faster flows to enhance expiratory time necessary only if gas-trapping risk. |
| PEEP minimum levels of 5 cmH2O | Higher levels of PEEP according to severity of hypoxaemia. |
| Pressure support 5–10 cmH2O | Automated pressure support modes such as automatic tube compensation (autoadjusted pressure support according to overcome flow resistance of tracheal tubes) or volume support (autoadjusted pressure support to achieve target tidal volume on spontaneous breaths) exist. There is no pressing indication for their use in uncomplicated cardiac surgical patients. |
| Permissive hypercapnoea rarely necessary | Particularly important to avoid if existing pulmonary hypertension, as may worsen acutely with respiratory acidosis. |
| FiO2 initially 1.0 then wean down according to PaO2/SaO2 | According to PaO2/SaO2. |
Ventilation challenges specific to the postcardiac surgical setting include:
• atelectasis due to operative access
• pneumothorax (pleural opening for grafts, or ventilation-induced trauma)
• pulmonary hypertension from cardiac failure or valve disease
• cardiogenic shock/post-pump failure
• systemic inflammatory response syndrome due to cardiopulmonary bypass
• early or rapid weaning that is undertaken before complete readiness, leading to failure at weaning attempt
• surgical pain limiting spontaneous effort and potentially leading to atelectasis or sputum retention.
Approaches to weaning
As patients often have no underlying pulmonary pathology, and have been ventilated for brief periods only, rapid weaning phases have become the norm in most centres. In many instances, as soon as the patient wakes and begins spontaneous breathing activity he/she may be suitable for at least a trial of spontaneous breathing in CPAP mode, usually with some modest level of pressure support (e.g. 5–10 cm H2O). If tolerated and the patient maintains an adequate minute volume, SpO2 and PaCO2, then extubation may be considered within as little as another 30 minutes. Normal demonstrations of airway protection capability (e.g. neuromuscular control, gag, swallow, cough and patient strength) should be sought before extubation (see Chapter 15 for details).
Where ventilation has been more prolonged due to postoperative pulmonary problems, weaning may be approached more cautiously, as might be applied to the general longer-term ventilated patient. Gradual mandatory rate reduction or increasing periods of spontaneous ventilation interspersed with periods of greater assistance have been used.31
Assessment and Management of Postoperative Bleeding
Blood transfusions are not aimed at restoring haemoglobin to normal levels, and, despite variations in acceptable levels, relative anaemia is almost universally tolerated. Haemoglobin levels are thus not routinely treated unless below 80 g/L, except in the elderly or when there are significant comorbidities.19,32 From these levels patients return to normal haemoglobin status within 1 month postoperatively.32
Contributors to impaired haemostatic capability
• cardiopulmonary bypass influences:
• preoperative anticoagulant/antiplatelet medications commonly encountered
• increased fibrinolytic activity
• surgical defects such as failure of access site closure, or vascular anastomosis defects.
Bedside assessment of bleeding
The activated clotting time (ACT) is the most commonly used assessment of coagulation and heparin activity during cardiac surgery and subsequently in intensive care. It measures the time to onset of fibrin formation (initial clot development). The ACT has been valuable because it can be inexpensively and efficiently performed at the bedside, providing prompt results and requiring only modest personnel training. Bleeding patients with a prolonged ACT come under consideration for administration of protamine or other agents.32 Treatable levels vary from greater than 120 sec to greater than 150 sec among different centres.
A limitation of ACT measurements is that they provide no information about clotting processes beyond initial fibrin formation, so clotting deficits such as impaired clot strength or the presence of significant fibrinolysis as contributors to bleeding are not revealed by this test.33 By contrast, the thromboelastograph (TEG) measures the clotting process as it proceeds over time.33 TEG monitoring not only reveals abnormalities early in the clot process (time to fibrin formation, as would be demonstrated by the ACT) but also the subsequent development of clot strength, clot retraction, and finally fibrinolytic activity for each of their contributions in the bleeding patient.33 TEG monitoring, although considerably more expensive than the ACT, is now available as a bedside or operating room technology and offers better insight into bleeding causes. In addition, because TEG monitoring identifies deficiencies at the various stages of clot formation, development of clot strength and the presence of undue fibrinolytic activity, it may permit better matching of procoagulant, blood product or antifibrinolytic therapy to needs.33
Heparin reversal
Cardiopulmonary bypass requires full heparinisation (initially 300 IU/kg), which is reversed at end-operation.32 The specific antidote, protamine sulphate, is administered as bypass is ceased, at a dose of 1 mg per 100 units heparin used (i.e. 3 mg/kg).32 If reversal is less than complete, as evidenced by a prolonged ACT, further protamine sulphate (at doses of 25–50 mg over 5–10 minutes) may be necessary.
Management of bleeding
Treatment approaches to bleeding once the patient is in intensive care include further protamine administration if the ACT remains prolonged, blood and blood product administration (platelets, clotting factors, fresh frozen plasma), procoagulants (desmopressin acetate) and antifibrinolytic agents (see Table 12.2 for more details). Other general measures such as rewarming the patient and preventing or treating hypertension should be undertaken.
TABLE 12.2 Management of the bleeding patient post-cardiac surgery21,22,25,33,34,44
| Therapy | Dose | Comments/issues |
|---|---|---|
| Protamine sulphate | 25–50 mg slow IV (<10 mg/min); may be repeated if ACT prolonged | Specific antidote to heparin. May cause hypotension. Contraindicated in patient with seafood allergy. |
| Aprotinin (Trasylol) | continuous infusion of 2 million units over 30 min, then 500,000 units per hour | Antifibrinolytic. Proteinaceous. Anaphylaxis risk on re-exposure. Alert should be posted on history. |
| Desmopressin acetate (DDAVP) | 0.4 mcg/kg IV | Promotes factor VIII release; limited evidence for use. |
| ‘Pump blood’ (blood retrieved from bypass circuit at end-operation) | often 400–800 mL | This is the remaining blood in bypass circuit; usually centrifuged before returning to patient; note: this blood contains heparin from CPB. |
| Whole blood/packed cells | as necessary to achieve Hb >80 g/L or more according to needs | Autologous blood sometimes available when patients have donated blood preoperatively. |
| Fresh frozen plasma | as necessary | ‘Broad-spectrum’ factor replacement; contains most factors. Useful adjunct to massive blood transfusion. |
| Platelet concentrates | as necessary | Generally ABO and Rh compatible preferred. |
| Epsilon-aminocaproic acid (Amicar) | 100 mg/kg IV followed by 1–2 g/h | Antifibrinolytic. Inhibits plasminogen activation. |
| Cryoprecipitate | 10 units IV | Contains factor VIII and fibrinogen (factor I). |
| Calcium chloride or gluconate | 10 mL 10% solution | Used to offset citrate binding of calcium in stored blood. |
| Prothrombinex | 20–50 IU/kg IV | Contains factors II, IX and X. |
Assessment and Management of Pericardial Tamponade
Described as one of the extra-cardiac obstructive shocks, pericardial tamponade often resembles cardiogenic shock. The low cardiac output and hypotension result in oliguria, altered mentation, peripheral hypoperfusion and development of lactic acidosis. Compensation includes tachycardia and marked vasoconstriction, elevating the systemic vascular resistance. As in cardiogenic shock, there is usually elevation of the filling pressures (right atrial, pulmonary artery and pulmonary capillary wedge pressures), sometimes with a particularly suggestive merging of the pulmonary artery diastolic, right atrial and pulmonary artery wedge pressures.23 Additional features that may be present include muffled heart sounds, decreased QRS voltage, electrical alternans, narrowing pulse pressure and pulsus paradoxus, along with features of increasing anxiety and/or dyspnoea in the awake patient.
Importantly, the ‘classic’ or typical haemodynamic profile described above is not uniformly seen in tamponade, and tamponade should never be excluded because the haemodynamic status does not match this profile. This may be because classic tamponade implies uniform compression of the entire heart, which may not be the case with haemorrhagic tamponade. A clot may develop over just one chamber rather than occupying the entire pericardium, and so there may be compromise to only a single chamber rather than the whole heart.21,23
Management of pericardial tamponade
Steps to control bleeding and blood pressure as described above may limit further losses into the pericardium. All steps should be taken to maintain or re-establish chest tube patency (crushing clots within tubing, ‘milking’ when it is truly necessary) and to ensure free flow of blood from the chest by avoiding dependent tubing loops, or side-to-side rolling of the patient to possibly bring collections into proximity of drain tubes. When tube patency is in doubt, the surgeon may even pass a suction catheter through the chest drain under aseptic conditions in an attempt to remove clots at the drain tip.23 If the above measures do not relieve tamponade, consideration is given to re-exploring the pericardium, either by returning to the operating theatre or, in an emergency, to the intensive care unit, although this is less preferable.
Assessment and Management of Postoperative Pain
As much an art as a science, pain control in the cardiac surgical patient remains a major challenge and continues to provide uncertainty and opportunities for nursing clinicians and researchers. Principles are similar to those outlined in Chapter 7. Surgical pain, often complicated by pericardial inflammation, results in differing pain types, requiring different approaches.34 These different approaches to pain management must be balanced against the promotion of spontaneous breathing, chest physiotherapy, mobilisation, and participation in education and lifestyle modification programs.
Analgesic options include intravenous, oral or rectal analgesics, antiinflammatories and, less commonly, epidural therapies and nerve blocks. Intravenous opiates and codeine/paracetamol preparations provide the mainstay of postoperative analgesia. When insufficient, or when clinical and electrocardiographic features suggest pericarditis, antiinflammatory agents such as rectal indomethacin are appropriate. The place of IV COX-2 inhibitors such as parecoxib appear uncertain, as analgesic efficacy now must be weighed against emerging data suggesting increased thrombotic complications.35
Fluid and Electrolyte Management
Fluid therapy in the postoperative period is aimed at maintaining blood volume, replacing recorded and insensible losses, and providing adequate preload to sustain haemodynamic status. Isotonic dextrose solutions (5%) or dextrose 4% + saline 0.18% are commonly used at approximately 1.5 L/day as maintenance fluids.14
Potassium replenishment is generally necessary according to measured serum potassium. Polyuria is usually evident in the early postoperative period due to deliberate haemodilution while on cardiopulmonary bypass. With polyuria comes potassium losses, which must be treated to avert atrial or ventricular ectopy and tachyarrhythmias. Because of these predictable potassium losses, protocols for potassium replacement may be instituted, with standing orders for potassium replacement (e.g. 10 mmol over 1 hour if the serum potassium is <4.5 mmol/L, or 20 mmol over 2 hours if <4.0 mmol/L). Main line hydration infusions may also have added potassium to avoid hypokalaemia. Hypomagnesaemia may also develop due to polyuria, and is likewise proarrhythmic. Supplementation (magnesium chloride) is often used for arrhythmia management postoperatively, but its effectiveness has been questioned in many trials.36
Emotional Responses and Family Support
The experience of being diagnosed with a cardiac disorder, waiting for surgery, the surgical experience and recovery is an emotional journey for patients and their families. Regardless of low mortality rates, the possibility of death and painful wounds can concern patients. Consequently, patients undergoing cardiac surgery often experience anxiety and depression, which can be distressing for patient and family.37–39 Women appear to be more vulnerable to these emotions in relation to cardiac surgery than men.40 Although it is normal and potentially protective to experience anxiety, higher levels of these emotions can be destructive. Anxiety and depression are predictive of worse postoperative outcomes, including poorer psychosocial adjustment and quality of life, more cardiac symptoms and readmissions.41 Therefore, it is essential to consider and address anxiety and depression when providing care for cardiac surgical patients.
Preoperative preparation provided by nurses usually incorporates information and support, so that patients and their families are familiar with procedures and can cooperate during recovery.42 However, seeing a patient who is successfully recovering from surgery may instil more confidence. Patients who have had their surgery postponed or who have been operated on in an emergency setting may need additional support. For many patients, fast-track procedures, including admission on day of surgery, early extubation and early discharge processes, decrease the discomfort associated with being away from home and surgical costs. For other patients there is too little time to be informed and understand postoperative and post-discharge care. Also, critical pathways for cardiac surgery do not include assessing the patient’s psychological state, so nurses must take care to consider this aspect. Consequently, family members assume an important role in supporting patients and helping them understand recovery requirements. It is vital that family members understand and anticipate a certain amount of anxiety and depression, particularly in the first week post-discharge. Family members may also be distressed by seeing their loved one ill and the unfamiliar ICU environment and equipment, so the additional requirement for them to assess and support the patient may be onerous. Printed information regarding the surgery, recovery and emotions will be useful for the patient and family.
Intra-Aortic Balloon Pumping
Intra-aortic balloon pumping (IABP) is a widely-used circulatory assist therapy that has become straightforward in application and relatively free of complications.43,44 The primary aim of IABP is to assist restoring an existing imbalance between myocardial oxygen supply and demand. The main indications are for cardiogenic shock, myocardial infarction or ischaemia and weaning from cardiopulmonary bypass. The combined effects of increasing cardiac output and mean arterial pressure (increasing oxygen supply) and decreasing myocardial workload (reducing oxygen demand) make IABP therapy ideal for the management of infarct-related cardiogenic shock,45 for which IABP should be regarded as a standard management.
IABP therapy involves placement of a balloon catheter in the descending thoracic aorta. This catheter is most commonly advanced from a percutaneous femoral artery access until the tip of the catheter is situated just below the left subclavian artery (Figure 12.4). A chest X-ray or fluoroscopy should reveal the catheter tip just below the aortic arch, or at the level of the second anterior intercostal space or fifth posterior intercostal space (Figure 12.5). The catheter has two lumens – a monitoring lumen, which opens at the catheter tip from which the aortic pressure waveform is monitored; and a helium drive lumen, through which the helium is shuttled from the pumping console to the catheter balloon. Balloon volumes range from 25 mL (paediatric use) and 34–50 mL in adults (most commonly used is 40 mL balloon) and selected according to patient height (40 mL balloon is used for a patient height of 162–183 cm).
Principles of Counterpulsation
Balloon Inflation
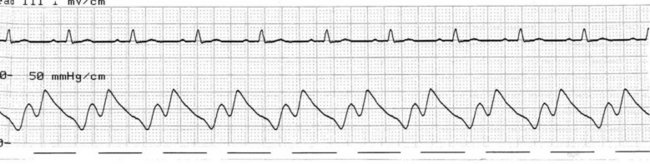
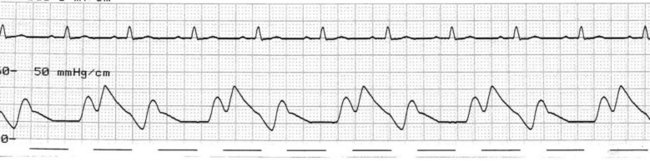
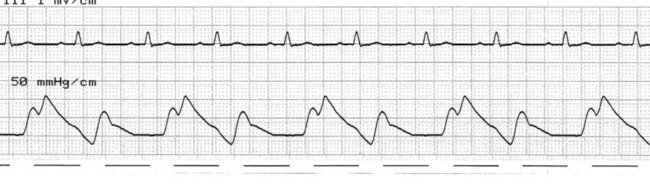
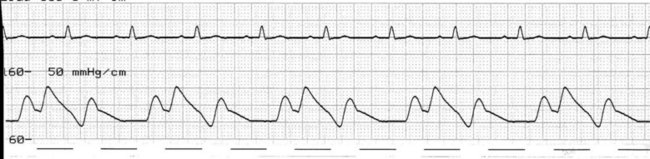
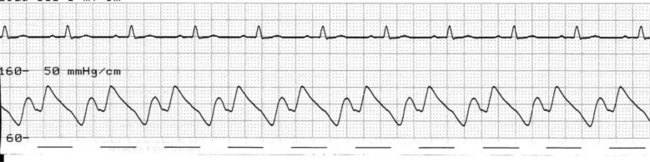
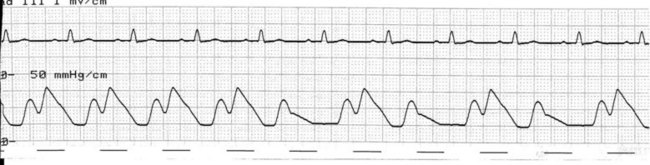
At the onset of diastole, the balloon is rapidly inflated with (most commonly) 40 mL helium. This inflation causes a sudden rise in pressure in the aortic root during diastole, raising mean arterial pressure and, importantly, coronary perfusion pressure. The blood displaced by the balloon expansion improves blood flow into the coronary circulation (which fills largely during diastole), as well as to the brain and systemic circulation. Thus there is improved myocardial oxygen supply, increased mean arterial pressure, as well as improved systemic perfusion.46 The balloon remains inflated for the duration of diastole. The arterial pressure wave should reveal a sharp rise in pressure at the dicrotic notch, with a second pressure peak now appearing on the waveform, described as the ‘augmented diastolic’ or ‘balloon-assisted peak diastolic’ pressure. This peak is usually at least 10 mmHg higher than the systolic pressure (Figure 12.6).
Balloon Deflation
Conventional timing
In conventional timing, the balloon is deflated immediately prior to systole. Rapid deflation induces a precipitous drop in aortic pressure at the end of diastole (a reduced aortic end-diastolic pressure). This reduces the duration of the left ventricle isovolumetric contraction phase of cardiac cycle (most oxygen consuming phase of cardiac cycle), left ventricular afterload and improves left ventricular emptying, improving stroke volume and cardiac output.46,47 In addition, less pressure is required for left ventricular emptying, so systolic work and oxygen demands on the myocardium are reduced.47 Thus deflation during conventional timing should see the aortic pressure drop to below normal at end-diastole, just in advance of the subsequent systole. Systolic pressure should be lower than during non-assisted beat.
Real timing
In contrast to conventional timing, during real timing (also referred to as R wave deflate) the balloon remains inflated for slightly longer, and is deflated not before but at the same time as systole. The reduction in aortic end-diastolic pressure is therefore not seen, but deflating simultaneously with left ventricular contraction still favourably effects left ventricular emptying.48 Thus there is improved stroke volume, systolic pressure reduction, and decreased ventricular work and oxygen demands as seen during conventional timing.47,49 Box 12.1 summarises the impact of balloon inflation and deflation on haemodynamic status and the oxygen supply:demand balance.
The arterial pressure wave reveals the impact of IABP therapy on haemodynamic status. Placing the pump into 1 : 2 assist (balloon pumping on only every second beat) is useful to highlight balloon pump impact and how assisted beats vary from the normal pressure cycle during systole and diastole. Figure 12.6 depicts the impact of IABP on haemodynamic status and the arterial pressure waveform.
Complications of Intra-Aortic Balloon Pumping
Serious complications are uncommon during IABP treatment and continue to decrease in frequency in the last decade with advances in pump technology and smaller catheter size.50 Limb ischaemia remains the commonest serious complication, especially in patients with existing vasculopathy,51 providing impetus to the development of smaller catheters, which have now reached 7.5 French gauge. Additional complications, such as bleeding, catheter migration, thromboembolism, insertion-site vascular damage, thrombocytopenia and device-related problems such as timing inaccuracy, device failure and gas leaks, also occur but are less common. These are described below.
Nursing Management
Prevention and Treatment of Bleeding
Significant bleeding is uncommon,51 but blood loss may occur from the femoral arterial access site. In addition to physical factors at the insertion site, contributors to bleeding include heparinisation, thrombocytopenia from the physical effect of the pump on platelets, and/or other anticoagulants or antiplatelet agents used for the primary disease. Regular observation should be made of the insertion site for bruising or external bleeding, as well as other possible sites of bleeding due to heparinisation. Treatment includes pressure at the insertion site (including the use of sandbags), reinforcement of dressings, and/or topical procoagulant agents. Monitoring of coagulation status and haemoglobin should be undertaken and blood or blood products may (uncommonly) be required.
Weaning of IABP
Weaning of intra-aortic balloon pumping therapy is generally undertaken once the patient has stabilised, is free of ischaemic signs and symptoms and is on minimum or no inotropic support. Algorithms have been offered for approaches to weaning therapy,52 but their impact on weaning duration or success has not been studied. Weaning is carried out by either gradual reductions in balloon inflation volume (volume weaning) or gradual reductions in assist frequency from 1 : 1 through 1 : 2 and 1 : 4 (ratio weaning). Hybrids of the two approaches are sometimes used. Support is reduced at intervals while the patient is observed for haemodynamic deterioration, pulmonary congestion, or the return of ischaemic signs and symptoms.
Assessment of Timing and Timing Errors
Early inflation
Early inflation will at times be difficult to differentiate from correct inflation timing but is recognised by the onset of inflation soon after the peak systolic pressure, before the pressure has declined to the level of the dicrotic notch (Figure 12.7). Early inflation may limit the stroke volume and cardiac output, as terminal systole is impeded and may result in increased myocardial oxygen demands. The inflation point should be adjusted (to later) until the inflation upstroke emerges smoothly out of the dicrotic notch.
Late inflation
The arterial pressure waveform reveals the onset of diastole (the dicrotic notch) before balloon inflation commences (Figure 12.8). This generally results in a lower augmented diastolic pressure than could otherwise be achieved. As the duration of balloon inflation is lessened, the desired rise in mean arterial pressure and coronary perfusion will not be achieved. The inflation marker should be set to ‘earlier’ until the inflation upstroke emerges smoothly out of the dicrotic notch.
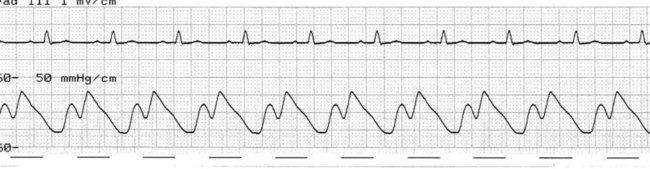
Early deflation
Deflating the balloon earlier than necessary shortens the duration for which the balloon remains inflated and therefore limits the benefit of IABP. When deflation is very early, it may cause harm. Deflation sees the aortic pressure drop markedly but there is now time for blood to fill the space left by the balloon before systole commences. Aortic end-diastolic pressure increases and may even exceed the normal end-diastolic pressure, increasing the duration of isovolumetric phase, worsening left ventricular afterload and increasing myocardial oxygen demand (Figure 12.9). Correction is achieved by setting deflation to later until the pressure drop of deflation occurs just in advance of the succeeding systole.
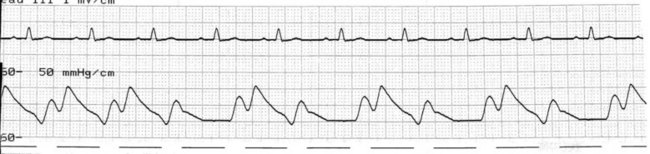
Late deflation
When deflation begins too late, systole commences before complete emptying of the intra-aortic balloon. The typical reduction of aortic end-diastolic pressure is not seen. When significantly late, the end-diastolic pressure may even be increased prolonging the duration of the isovolumetric contraction phase, and worsening afterload. As systole occurs against an incompletely deflated balloon, the stroke volume and cardiac output suffer and ventricular work and oxygen demand increases (Figure 12.10). Deflation should be set to earlier until the systolic upstroke emerges out of the reduced end-diastolic pressure dip.
Alarm States
Alarm functions vary according to manufacturer and model. The main alarm states common to most devices, and their causes and significance, are shown in Table 12.3. Importantly, in most alarm states the pump consoles will revert to standby, suspending pumping. The balloon is at risk of developing thrombi within the folds of the balloon while deflated, and these can be liberated as arterial emboli on recommencement of pumping. It is important to treat alarm states promptly, to limit the duration of balloon stasis. If interruption to pumping is prolonged, intermittent manual inflation of the balloon with a syringe is recommended (e.g. once every 5–10 minutes).
| Alarm state | Causes/significance |
|---|---|
| Catheter alarm |
• ECG trigger: signal disrupted or low in amplitude, or asystole
• Pressure trigger: pulse pressure below threshold for detection
• Pacer trigger: pacing spikes not detected or absent (including demand pacing)
• Device reverts to standby until restoration of trigger; alternative trigger selection may be necessary
Gas loss alarms
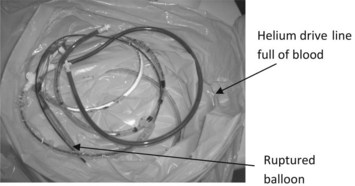
Most devices will determine the severity of gas losses and classify them as slow, rapid or disconnect. In all gas loss states it is imperative that assessments be made to exclude balloon rupture and helium leak into the arterial circulation. Small gas losses of helium may or may not be of clinical significance, but the delivery of sizeable helium volumes may behave as gas emboli, and if delivered into the coronary circulation may result in lethal arrhythmias or result in neurological complications if delivered into the cerebral circulation. In all gas loss alarm states, the helium drive line should be inspected for the presence of blood to indicate loss of integrity of the balloon. If blood is present in the drive line (Figure 12.11), pumping should be suspended and no attempts at recommencing balloon pumping should be made. Prompt removal and/or replacement, along with thorough patient assessment, is essential.
Heart Transplantation
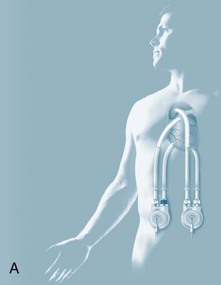
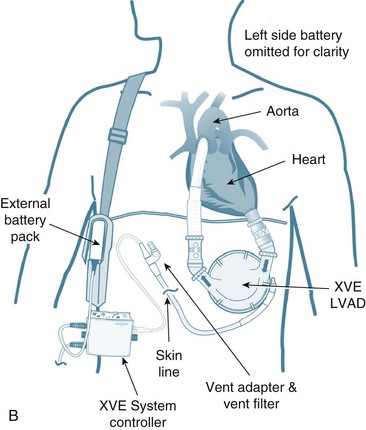
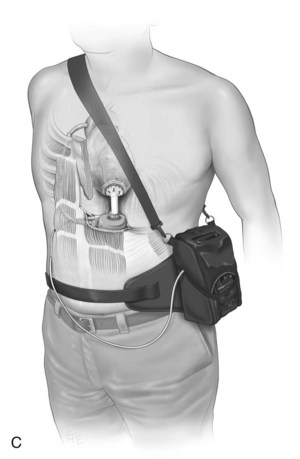
The immediate period following surgery is commonly the first contact that critical care clinicians have with transplant recipients and their families. The exception may be patients awaiting heart transplantation who are supported by an intra-aortic balloon pump or mechanical circulatory support (MCS) also known as a ventricular assist device (VAD) as a ‘bridge to transplantation’ (see Figure 12.12). Ideally, patients with MCS are returned to a sound physical, mental and nutritional state prior to receiving a transplant, and, as part of their recovery, await transplantation in the ward or home setting. For specific management of patients on MCS, readers are referred to specific resources (e.g. websites and operating manuals for individual MCS: HeartMate, Throratec, VentrAssist and DuraHeart).
Heart transplantation is a life-saving and cost-effective form of treatment that enhances the quality of life for many people with chronic heart failure. Legislation that defined brain death and enabled beating-heart retrieval was enacted in Australia from 1982. This legislation heralded the establishment of formal transplant programs. In Australia, the first heart program commenced in 1983.53,54 The success of transplantation in the current era as a viable option for end-stage organ failure is primarily due to the discovery of the immunosuppression agent cyclosporin A.55 In this section, heart transplantation as a component of critical care nursing is discussed, with reference to evidence-based practices.
History
Heart transplant surgery for refractory heart failure was first performed in Australia in 1968, only months after the first heart transplant was performed in South Africa in December 1967.56 However, high mortality rates associated with severe acute rejection and infection within months of surgery led to a reduction in the number of heart transplants performed worldwide, and in effect a moratorium occurred with the procedure. Heart transplantation was finally established in the modern era as a viable treatment option for end-stage heart failure during the early 1980s when cyclosporin A, a then-novel immunosuppressive agent, dramatically improved patients’ survival rates by reducing episodes of acute rejection and lowering attendant infectious complications.57
Incidence
Heart transplants in the modern era have been performed in Australia since 1986 and in New Zealand since 1987. In 2009, 72 heart transplants were performed in Australia and New Zealand.58 As the annual number of transplants globally is likely to remain relatively stable because of limited organ availability, future routine management of end-stage heart failure may involve the insertion of a left ventricular assist device (LVAD) designed for long-term permanent mechanical circulatory support, so-called ‘destination therapy’. Indeed, there have been clinical trials that include destination therapy since the success of LVADs in the REMATCH study.59 In the past decade, LVADs available have been used primarily as ‘bridge to transplantation’ therapy (i.e. support for a failing native heart until a suitable heart becomes available), not ‘destination therapy’. The implementation of destination therapy will require nurses to gain skills in the long-term management of patients and their carers.60 Advances in device design and capability, e.g. fully implantable with internal batteries, are likely to be required for this option to be truly viable.
Outcomes from Heart Transplantation
Currently, the top centres around the world achieve survival rates for heart transplant patients approaching 80–90% at one year, with more than 50% of patients surviving longer than 11 years.61 In Australia and New Zealand, approximately 85% of heart transplant patients survive to 1 year and 75–80% survive more than 5 years.62,63
Indications
The vast majority of patients referred for heart transplantation have NYHA functional class III or IV symptoms (see Chapter 10), secondary to ischaemic heart disease or some form of dilated cardiomyopathy.64,65 Commonly, patients listed for transplantation have a life expectancy of less than 2 years without transplantation. Accepted contraindications for heart transplantation include active malignancy,66 complicated diabetes,67 morbid obesity,68 uncontrolled infection, active substance abuse and an inability to comply with complex medical regimens.69,70 Age has become a relative contraindication, with 16 days old being the youngest and 71 years of age being the oldest.64 However, the presence of multiple comorbidities in patients over 70 years of age would be expected to exclude the majority of such patients from consideration.66,71 Other relative contraindications include renal failure and an irreversible high transpulmonary gradient (mean pulmonary artery pressure minus pulmonary artery wedge pressure) of greater than 15 mmHg72 (see section on Early allograft dysfunction and failure later in this chapter). In the context of a rigorous postoperative regimen of polypharmacy, frequent follow-up medical appointments and routine cardiac biopsies, a strong social support network, absence of psychiatric illnesses and a willingness to participate actively in the recovery process are highly desirable characteristics of prospective recipients.72
Patients referred for heart transplant assessment must have exhausted all other accepted pharmacological and surgical treatment options for end-stage heart failure, such as optimal therapeutic doses of common heart failure medications; revascularisation via coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty; continuous IV infusions of dobutamine in the community/home setting; IV levosimendan (a calcium sensitiser); antiarrhythmic drugs to suppress, or an internal cardiac defibrillator to treat, potentially lethal arrhythmias; and insertion of a biventricular pacemaker (i.e. chronic resynchronisation therapy) to re-establish atrioventricular synchrony (see Chapter 11).
The average costs associated with heart transplantation are high, at approximately $A35,000 for the first year and $A15,000 for each ongoing year.58 However, the high incidence of chronic heart failure and associated hospitalisation costs are also considerable. During 2000, it was estimated that over half a million Australians had chronic heart failure (CHF), with 325,000 patients per annum experiencing symptoms.73 Hospital admissions for heart failure were estimated at 100,000, totalling more than 1.4 million days, figures that represent prevalence rates of 526 hospitalisations and 7400 days per 100,000/annum.73 The cost of a single hospital admission for CHF in Australia is currently approximately $A6000.74 In 2006, approximately 263,000 Australians experienced chronic heart failure, with 2350 dying from end-stage heart disease.75 In New Zealand, hospital admissions for heart failure consume approximately 1% of the healthcare budget.76 In the context of a 50% mortality rate within 4 years of being diagnosed with chronic heart failure, a 50% mortality rate within 1 year for patients with severe heart failure,77 and the burden of care associated with heart failure exceeding that of all types of cancer,78 transplantation for end-stage heart failure is actually a viable and economical treatment option for individuals and society; it is, however, a limited resource, available to only a few recipients.
Forms of Heart Transplant Surgery
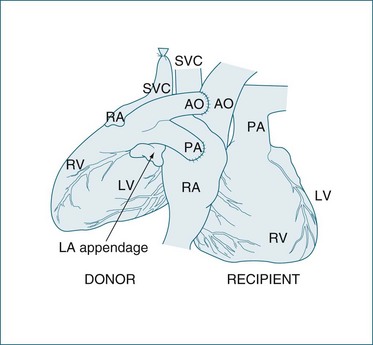
The most common heart transplant surgery is orthotopic transplantation, with two surgical techniques used: the standard or bicaval approaches. The standard technique has been used since the 1960s and involves anastomoses of the donor and native atria.79 Complications associated with the standard technique can include abnormal atrial contribution to ventricular filling, and tricuspid and mitral valve insufficiency.80,81 Since the mid-1990s, the bicaval technique as described by Dreyfus et al.82 has gained favour. The main advantage of the bicaval approach is the maintenance of atrial conducting pathways and the likelihood of promoting sinus rhythm and its associated superior atrial haemodynamics82 (see Figure 12.13). Reported potential disadvantages include stenoses in the inferior and superior vena cava at the anastomosis sites.82
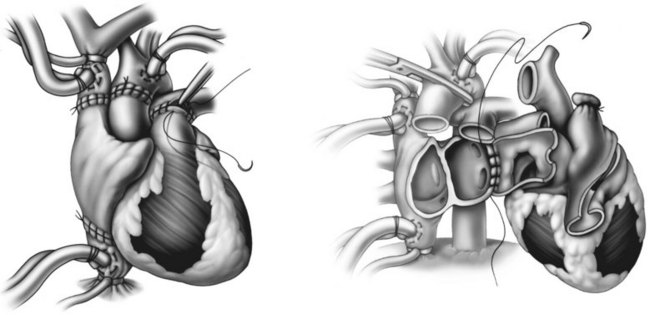
FIGURE 12.13 LEFT Completion of bicaval transplant technique, showing the interior vena caval, superior vena caval, aortic, and pulmonary artery anastomoses RIGHT Commencement of the left artrial anastomosis.79
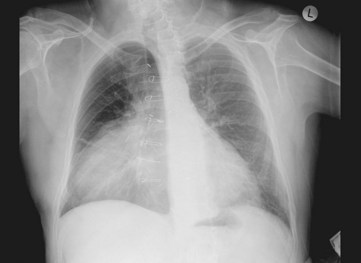
The second form of heart transplant surgery is heterotopic transplantation, although these account for less than 0.5% of heart transplants in Australasia.83 In this procedure, the donor heart is implanted in the right side of the chest next to the native heart84 to augment native systolic function. Figure 12.14 illustrates a chest X-ray of the donor heart next to the native heart.
Heterotopic heart transplantation is primarily indicated in patients with pulmonary hypertension refractory to pulmonary vasodilator therapies. It may also be considered in patients with a large body surface area that are unlikely to receive a suitably large-sized donor heart to enable an orthotopic procedure to take place,79,85 or when the donated organ is unsuitable as an orthotopic graft.85 Heterotopic transplantation is usually performed to support the left ventricle (LVAD configuration), but can be configured to support biventricular function (BiVAD configuration). The LVAD configuration for heterotopic heart transplantation is illustrated in Figure 12.15.
Clinical Practice
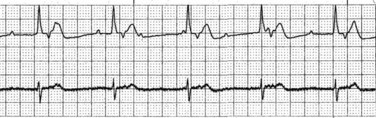
Postoperative nursing and collaborative management of orthotopic heart transplant recipients involves full haemodynamic monitoring with a pulmonary artery catheter (PAC), a triple- or quad-lumen central venous catheter (CVC), arterial line, indwelling urinary catheter, and 5-lead cardiac monitoring to assist in dysrhythmia discrimination. A 12-lead ECG is also recorded. If the orthotopic transplant is performed with the standard technique, a remnant P wave from the native heart may be visible on the ECG or cardiac monitor (see Figure 12.16). As the native sinus node cannot conduct across the right atrial suture line, the recipient’s heart rate is determined by the conduction system of the donor heart, not the native heart. Of interest, it is possible for the native heart to generate a P wave while the donor heart is in atrial fibrillation or other dysrhythmia. (More detailed discussion of cardiac monitoring and haemodynamic management of patients with a heterotopic heart transplant is available.78,86) Monitoring data are combined with physical assessment information from all body systems to determine nursing and collaborative interventions. Intensive continuous monitoring and assessment of haemodynamic parameters according to evidence based practices87–89 and overall clinical status allows nurses to detect and subsequently respond to emergent postoperative complications.
Full ventilatory support is required until the patient’s haemodynamic status is stable. Respiratory status is monitored via clinical, radiological and laboratory-derived data (see Chapter 13). Enteral feeding is usually commenced on the day of admission. Renal and neurological function are closely monitored, as cyclosporin has a deleterious effect on renal function and can lead to failure90 as well as neurotoxicity.91 For the small number of patients who develop allograft dysfunction requiring mechanical circulatory support (i.e. IABP, ECMO or Thoratec LVAD), or acute renal failure requiring haemofiltration, hospitalisation in the critical care unit tends to last weeks rather than days.
The immediate period after transplantation can be a time of great hope and joy for recipients and their family and friends; however, complications and setbacks can make the path to recovery prolonged, unpredictable and difficult. The provision of psychosocial support by all members of the transplant/critical care team to family members and friends is an important part of patients’ recovery from organ transplantation. Meetings with family that convey honest and open information about patient progress need to be conducted regularly. Supporting and managing patient and families following transplant is consistent with support provided to other critically ill patients (see Chapter 8). In addition, there is the issue of dealing with lost hope if the transplant fails; a very distressing time for all involved. In the immediate postoperative period, transplant recipients are at risk of developing complications that include hyperacute rejection, acute rejection, infection, haemorrhage and renal failure. In the immediate postoperative period, heart transplant recipients may experience morbidity specific to the heart transplant procedure, such as early allograft dysfunction (i.e. organ failure due to preservation injury), bleeding, right ventricular failure and acute rejection. Long-term complications include chronic renal failure, hypertension, malignancy and cardiac allograft vasculopathy. The common immediate potential complications and associated clinical management for heart recipients are discussed below.
Hyperacute Rejection
Hyperacute rejection is now a rare form of humoral rejection that occurs minutes to hours after transplantation and results from ABO blood group incompatibility or the recipient having preformed, donor-specific antibodies.92 ABO blood group and panel reactive screening of anti-human lymphocyte antigen (anti-HLA) antibodies preoperatively minimises the possibility of hyperacute rejection, particularly in health care systems where blood that has been prospectively cross-matched is routinely used. If it occurs, hyperacute rejection leads to organ failure and rapid activation of the complement cascade, producing severe damage to endothelial cells, platelet activation, initiation of the clotting cascade, and extensive microvascular thrombosis.78 There is no effective treatment for hyperacute rejection apart from mechanical circulatory support or interim retransplantation.
Acute Rejection
Acute rejection can be classified as either cellular or humoral.93 Cellular rejection involves T-cell infiltration of the allograft. Cellular rejection occurs much more commonly than humoral rejection, but both may occur simultaneously.94 Humoral or microvascular rejection is thought to be primarily mediated by antibodies. Humoral rejection may occur due to the presence of a positive donor-specific cross-match, or in a sensitised recipient with preformed anti-HLA antibodies.95
Percutaneous transvenous endomyocardial biopsy is considered the gold standard for detecting cardiac rejection.96 Grading of cardiac rejection is noted in Table 12.4.97 In humoral rejection, endomyocardial biopsy reveals increased vascular permeability, microvascular thrombosis, interstitial oedema and haemorrhage, and endothelial cell swelling and necrosis.78 An echocardiogram is also performed to evaluate systolic cardiac function.
| Grade | Nomenclature |
|---|---|
| 0 | No rejection |
| 1 | A. Focal (perivascular or interstitial) infiltrate without necrosis B. Diffuse but sparse infiltrate without necrosis |
| 2 | One focus only with aggressive infiltration and/or focal myocyte damage |
| 3 | A. Multifocal aggressive infiltrate and/or myocyte damage B. Diffuse inflammatory process with necrosis |
| 4 | Diffuse, aggressive, polymorphous process with necrosis, with or without any of the following: infiltrate, oedema, haemorrhage, vasculitis |
Therapeutic interventions for rejection vary between centres and are based on the grade of rejection, degree of haemodynamic compromise, clinical findings and time elapsed since transplantation. Asymptomatic mild rejection (grade 1) is rarely treated, and only 20–40% of mild cases progress to moderate rejection (grade 3A), usually requiring treatment.95,98 Grades 3B and 4 rejection are always treated, as they represent myocyte necrosis. Cellular rejection is usually treated with higher doses of corticosteroids, such as ‘pulse’ doses of methylprednisolone (1–3 g IV over 3 days), and antilymphocyte antibody agents (ATG, ATGAM or OKT3). Humoral rejection is treated with plasmapheresis, high-dose corticosteroids, cyclosphosphamide therapy and antilymphocyte antibody therapy.99,100 It may be judicious to review the patient’s medications during periods of rejection to ensure that drugs capable of reducing cyclosporin or tacrolimus serum levels such as certain anticonvulsants and antibiotics have not been taken. In addition to augmentation of immunosuppression therapy, fluid, pharmacological and mechanical therapeutic interventions are instituted to support cardiac function, depending on the degree of ventricular dysfunction.
Immunosuppression Therapy
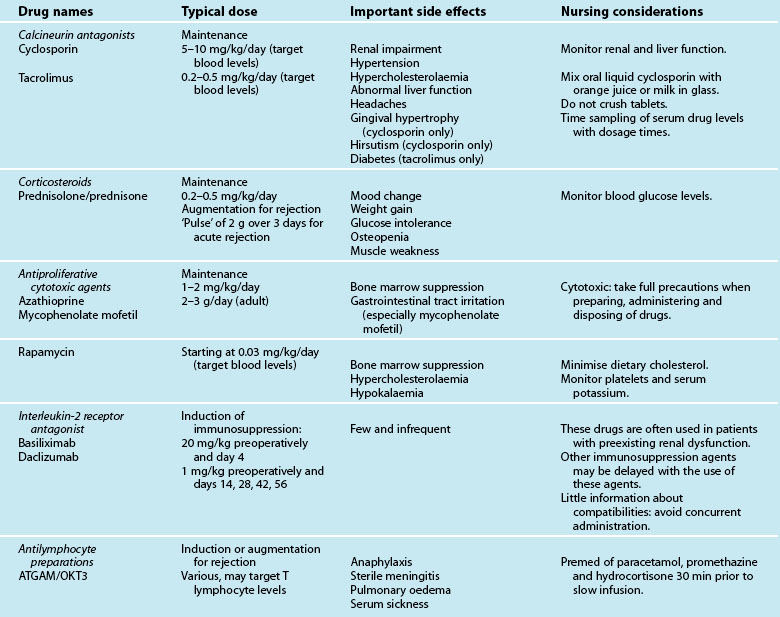
In this section, a brief discussion of immunosuppression therapies and associated nursing implications is provided. To prevent rejection of the transplanted organ, recipients receive a triple-therapy regimen of immunosuppression agents for the remainder of their life. Triple-therapy usually consists of corticosteroids (prednisolone or prednisone), a calcineurin antagonist (cyclosporine or tacrolimus [FK506]) and an antiproliferative cytotoxic agent (mycophenolate mofetil, azathioprine or sirolimus/rapamycin).101,102 For heart patients, sirolimus or rapamycin may become the cytotoxic drug of choice following findings of a recent study that demonstrated a lower incidence of cardiac allograft vasculopathy at 6 and 24 months, and lower rejection rates with sirolimus compared with azathioprine.103
Immunosuppression therapy is commenced preoperatively or in operating theatre. Maintenance immunosuppression regimen is usually instituted within hours of admission to ICU, with each patient’s immunosuppressive needs individually assessed. For instance, the administration time for introduction of the selected immunosuppressive agent(s) may be delayed in patients with preexisting renal dysfunction. When the administration of the usual regimen of immunosuppression is delayed, induction therapy with anti-lymphocyte agents (anti-thymocyte globulin (ATG), ATGAM or OKT3) or interleukin-2 receptor antagonists (basiliximab, daclizumab) may be used in the immediate postoperative period.104,105 Induction therapy may be used in circumstances of primary allograft failure perioperatively, e.g. HLA mismatch (rare), or early humoral rejection, or to allow for a delay in initiating cyclosporine in patients at risk of renal failure.106,107 The common drugs used to suppress the immune system and the nursing implications are illustrated in Table 12.5. As highlighted in the table, some immunosuppressive agents are cytotoxic (e.g. mycophenolate mofetil), requiring safety measures during preparation, delivery and disposal. Likewise, some immunosuppressive agents will be given IV (e.g. azathioprine) until patients can eat and drink as they cannot be crushed for naso-gastric administration. In addition, as blood levels of some immunosuppression agents (e.g. cyclosporine, sirolimus) are taken regularly to assess efficacy, nurses need to be aware of timing blood sampling to dosage times in order to obtain accurate data to inform doses.
Nursing practice
Nurses have an important role in detecting acute rejection, as it is diagnosed by clinical signs and supported by histological findings from an endomyocardial biopsy. Low-grade rejection can be suspected when non-specific signs such as malaise, lethargy, low-grade fever and mood changes are present. Acute rejection causing cardiac irritation is revealed by a sinus tachycardia greater than 120 beats/min; a pericardial friction rub; or new-onset atrial dysrhythmias such as premature atrial contractions, atrial flutter or fibrillation.98,107 More severe forms of acute rejection are suspected when signs and symptoms of varying degrees of heart failure emerge. If patients are awake and alert, they may complain of severe fatigue, sudden onset of dyspnoea during minimal physical effort, syncope or orthopnoea. Physical assessment and haemodynamic monitoring will reveal clinical signs of left and right cardiac failure (see Chapter 9).
Infection
Infection is a major risk factor for transplant recipients due to their immunosuppressed state. The periods of greatest risk for patients are the first 3 months after transplantation, and after episodes of acute rejection when immunosuppression agents are increased.108,109 In addition to the nosocomial bacterial infections that all surgical patients are exposed to in critical care (see Chapter 6), immunosuppressed transplant recipients are at risk of acquiring opportunistic bacterial, viral or fungal infections; latent infections acquired from the donor organ such as cytomegalovirus (CMV); or reactivation of their own latent infections (e.g. CMV or Pneumocystis carinii). To combat Pneumocystis carinii, patients receive trimethoprim with sulfamethoxazole twice weekly.110 Despite preoperative screening for CMV, the shortage of donor organs often necessitates CMV mismatching. Effective prophylaxis for CMV infection is provided by administering CMV hyperimmune globulin to CMV-positive and CMV-negative recipients who receive a heart from a seropositive donor.111 This commences within 24–48 hours of surgery.105 For CMV-negative recipients of organs from seropositive donors, ganciclovir for 1–2 weeks followed by oral therapy for 3 months is required in addition to CMV hyperimmune globulin.111–113
Nursing practice
To prevent infection, standard precautions and meticulous hand-washing (see Chapter 6) are performed, rather than isolation procedures.114 Mandatory measures to prevent overwhelming sepsis are a high level of vigilance by clinicians for signs of infection; obtaining empirical evidence from blood, sputum, urine, wound and catheter-tip cultures; and aggressive and prompt treatment for specific organisms. Although typical signs and symptoms of infection are blunted in transplant recipients, clinicians should suspect infections when patients have a low-grade fever, hypotension, tachycardia, a high cardiac output/index, a decrease in systemic vascular resistance (SVR), changes in mentation, a new cough or dyspnoea.115,116 Elevated white cell count, the presence of dysuria, purulent discharge from wounds, infiltrates on chest X-ray, sputum production or pain also indicate infection.
Prior to administering blood products, nurses must ascertain the CMV status of the patient and donor. Recipients who are seronegative for CMV and who receive a heart from a seronegative donor must receive whole blood, packed/red cells or platelets that are CMV-negative, leuco-depleted or both in order to avoid development of a primary CMV infection.79,112,117
Haemorrhage/Cardiac Tamponade
The risk of haemorrhage or cardiac tamponade is greater for heart transplant recipients than for patients undergoing coronary artery bypass graft or valvular surgery. Preoperative anticoagulation for end-stage heart failure or atrial fibrillation, impairment of hepatic function secondary to right heart failure, redo surgery, surgical suture lines connecting major vessels and atria, and a larger than usual pericardium are all contributing factors. Good surgical technique is mandatory in preventing postoperative bleeding. As the promotion of haemostasis is a major therapeutic goal postoperatively, blood products, procoagulants and antifibrinolytics are commonly administered according to laboratory and clinical data. Postoperative mortality from bleeding has been reported to occur in up to 6.7% of cases.118
Acute Renal Failure
Acute renal failure or varying degrees of renal dysfunction can occur in the initial postoperative period due to preexisting renal dysfunction, cyclosporin, nephrotoxic antibiotics, or sustained periods of hypotension secondary to cardiopulmonary bypass or allograft dysfunction. Diuretic therapy is invariably needed in the initial postoperative period due to these factors, as well as the fluid retention effects of corticosteroids and raised filling pressures secondary to a transient loss of right and/or left ventricular compliance.119 High doses or continuous infusions of diuretics may be required in patients who were on diuretic therapy preoperatively. Close monitoring of serum electrolyte levels will indicate the need for any supplements.
Nursing practice
In addition to all the usual nursing and collaborative measures that are taken to prevent, detect and support renal dysfunction/failure in patients following cardiac surgery on cardiopulmonary bypass (see earlier in this chapter and Chapter 18), the type and dose of immunosuppressive agents in the postoperative period are carefully selected and initiated according to individual risk factors and clinical status. Experience suggests that early intervention with haemofiltration to support renal function is preferable to continued use of high-dose diuretics and deferred haemofiltration. This is because there is little scope to maintain low doses of renal toxic immunosuppressants for weeks given the imminent risk of rejection and resultant allograft failure.
Early Allograft Dysfunction and Failure
Primary allograft failure is the leading cause of death in the first month and year after surgery.120,121 In the immediate postoperative period, myocardial performance is depressed due to the clinical sequelae of cardiopulmonary bypass and ischaemic injury associated with surgical retrieval, hypothermic storage, prolonged ischaemic times, and reperfusion. Despite a preferred time period between organ retrieval and reimplantation of 2–6 hours, the vast distances between capital cities (up to 3000 km) over which donor hearts may be transported, and a decision to accept marginal, suboptimal organs, led Australian researchers and transplant teams to pioneer prolonged ischaemic times of up to 8 hours (New Zealand, 7 hours).122
Heart transplants have been, and are likely to continue to be, performed in Australia and New Zealand and other countries that encompass long distances with ischaemic periods beyond 6 hours, as excellent short-term (30-day mortality) and long-term (ejection fraction at 1 year) outcomes have been reported.122 These outcomes were achieved by using innovative preservation techniques and postoperative mechanical assistance in the form of intra-aortic balloon counterpulsation and/or a right ventricular assist device.122,123 Adrenaline is invariably commenced intraoperatively, irrespective of ischaemic time, to provide inotropic support to the transplanted heart.
To prevent right ventricular dysfunction and failure secondary to raised pulmonary pressures, prospective heart transplant recipients are screened preoperatively for the degree and reversibility of pulmonary hypertension. Reversible pulmonary hypertension is a transpulmonary gradient less than 15 mmHg that responds to pulmonary vasodilator therapies, such as prostaglandin E1, prostacyclin or inhaled nitric oxide (NO).124 Right ventricular dysfunction or failure can also occur in the postoperative context due to ischaemic injury, an undersized heart (greater than 20% difference in body surface area between donor and recipient), or hypoxic pulmonary vasoconstriction.79 Isoprenaline or milronine, dobutamine and adrenaline are administered in this situation.112
Left ventricular dysfunction cannot be anticipated preoperatively, so when signs first emerge peri- or post-operatively, fluid management strategies (filling or diuresis as deemed appropriate) and inotropic agents are commenced immediately.112 In patients with prolonged ischaemic times, mechanical assistance in the form of an IABP is invariably instituted perioperatively.
In the initial postoperative period, cardiac dysfunction can also occur as a result of a low systemic vascular resistance (SVR) syndrome, characterised by a calculated SVR of less than 750 dynes/sec/cm−5 in the presence of an unsustainable high cardiac output.125,126 The cause of low SVR syndrome is not fully understood, although it has been linked with systemic inflammatory response syndrome (SIRS) associated with cardiopulmonary bypass (see Chapter 20), the chronic use of angiotensin-converting enzyme inhibitors for end-stage heart failure (see Chapter 10), and a deficiency of vasopressin.125,127 Noradrenaline is titrated to achieve a calculated SVR within normal parameters and to lower the unsustainably high cardiac index. In severe cases, vasopressin may be infused at doses of 0.04–0.1 units/min concurrently with noradrenaline.128 Experience suggests that the dose of adrenaline should be minimised in the presenceof metabolic acidosis, and the noradrenaline infusion increased to achieve normotension, a calculated SVR higher than 900 dynes/sec/cm-5 and a sustainable cardiac index.
Nursing practice
Depressed left ventricular compliance and contractility due to cardiac dysfunction presents clinically with reduced cardiac index, bradycardia, reduced tissue and end-organ perfusion (decreased mental status, oliguria, poor peripheral perfusion, slow capillary refill and raised serum lactate), low systemic venous oxygenation (SvO2), and dyspnoea. Bradycardia may not be evident due to chronotropic support of the denervated heart with atrial pacing and/or isoprenaline. The following discussion focuses on management of right heart dysfunction/failure and left heart dysfunction/failure (see also Chapter 10).
Right heart dysfunction/failure is suspected in patients with preexisting pulmonary hypertension or a haemodynamic profile in the intra- or postoperative context that includes a rising CVP, low-to-normal PAWP, high calculated pulmonary vascular resistance, raised pulmonary artery pressures, systemic hypotension, and oliguria. The haemodynamic management of patients with right ventricular dysfunction/failure involves optimising right ventricular preload and afterload by titrating fluid and pharmacological therapies to achieve adequate tissue and end-organ perfusion. Fluid resuscitation to a CVP between 14 and 20 mmHg and inotropic therapy is necessary to ensure that the failing right ventricle continues to act as a conduit for the left ventricle. Nitric oxide by inhalation is the therapy of choice, as it provides selective pulmonary vasodilation at doses of 20–40 ppm, thereby reducing right ventricular afterload without producing systemic hypotension.124,129 A secondary benefit of inhaled NO is improved oxygenation due to reduced mismatching of ventilation/perfusion.130 If inhaled NO is not available, IV prostaglandin E1 or prostacyclin may be used to reduce right ventricular afterload when pulmonary pressures exceed 50 mmHg.131
The immediate haemodynamic management of left ventricular dysfunction/failure secondary to acute rejection or ischaemic injury often involves fluid resuscitation to a PAWP of 14–18 mmHg, high-dose inotropes, vasodilator agents and insertion of an IABP to achieve a cardiac index greater than 2.2 L/min/m2 and adequate end-organ perfusion. The insertion of an LVAD (e.g. Biomedicus centrifugal pump) or full mechanical circulatory support with extracorporeal membrane oxygenation (ECMO) is indicated when aggressive therapeutic regimens fail to produce a cardiac output that provides adequate end-organ perfusion.112,132 As noted earlier, augmentation of the immunosuppression regimen may also be necessary to manage the acute rejection.
Denervation
Donor heart implantation severs both afferent and efferent nervous system connections to the heart. Hence, the transplanted heart has no direct autonomic nervous system innervation but is responsive to circulating catecholamines. Denervation impairs circulatory system homeostasis, as evidenced by: a volume-expanded state; a tendency to hypertension; no sensation of angina pectoris; a high resting heart rate; a slow or absent baroreceptor reflex (to increase heart rate/cardiac output in response to hypotension); and no rises in heart rate and contractility due to hypovolaemia or vasodilation.79 As the cardiac allograft is dependent on an adequate preload, the effects of postural changes in recipients are important. (A detailed discussion of physiology of the transplanted heart is provided elsewhere.79)
Nursing practice
There are four important clinical manifestations of denervation in the early postoperative period. First, drugs that act directly on the autonomic nervous system to modify heart rate (e.g. atropine, digoxin) and vagal manoeuvres (carotid sinus massage) are ineffective. Amiodarone and adenosine are effective antiarrhythmic agents. Neither amiodarone nor sotalol interact with immunosuppressive agents.112 However, as the denervated donor sinus node is more sensitive to exogenous adenosine than a sinus node innervated in the normal way,133 it has been suggested that adenosine be avoided.79 That is, a usual adenosine dose may produce toxic-like effects in the context of a denervated heart. Overdrive atrial pacing is a viable alternative to drug therapy to treat a tachyarrhythmia such as atrial flutter.134
Second, although a high resting heart rate is possible from efferent cardiac denervation, sinus or junctional bradycardias may occur in the early postoperative period due to transient sinus node dysfunction or preoperative amiodarone. Studies suggest that sinus node dysfunction occurs in about 20% of cases,135 although anecdotal experience suggests a higher percentage. To prevent low cardiac output secondary to bradycardias, atrial and ventricular epicardial pacing wires are inserted and atrial pacing of >90 beats/min,112 and often at 110 beats/min, is commenced. Atrial pacing at 110 beats/min appears to ‘train’ the sinus node to conduct at rates of 70–100 beats/min in the long term. A resting sinus or junctional heart rate below 70 beats/min prior to hospital discharge is predictive of long-term sinus node dysfunction.79 Insertion of a permanent pacemaker for long-term heart rate control is rarely required. Isoprenaline infusions at doses of 0.5–2 µg/min may be used for chronotropy in combination with atrial pacing. As noted earlier, atrial dysrhythmias such as atrial flutter may be an early indication of acute rejection. Ventricular arrhythmias are rare and often lethal in spite of aggressive resuscitation attempts. Persistent arrhythmias should always prompt investigation of the patient’s rejection level.112
Third, as patients rely on circulating catecholamines, orthostatic hypotension is common. Patients are educated to sit up slowly from a lying position. Fourth, patients rarely feel anginal pain after surgery; however, there are some reports of patients regaining feelings of angina pectoris.136 The inability of patients to feel angina pectoris is important, because all heart transplant recipients are at risk of developing accelerated allograft coronary artery disease.137 As part of discharge education, patients are taught to identify clinical signs of angina other than chest pain, such as shortness of breath and sweating. A summary of the main clinical manifestations and nursing practice issues for patients following heart transplantation is included in Table 12.6.
TABLE 12.6 Summary of nursing practice for patients after heart transplantation
• Standard infection control precautions and meticulous hand-washing is required.
• Observe for signs of infection: low-grade fever, hypotension, tachycardia, a high cardiac output/index, a decrease in systemic vascular resistance, changes in mentation, a new cough, dyspnoea, dysuria, sputum production, or pain.
• Monitor blood, sputum, urine, wound and catheter-tip cultures, infiltrates on chest X-ray, and institute aggressive and prompt treatment for specific infective organisms.
• Observe for depressed left ventricular compliance and contractility: reduced cardiac index, possible bradycardia (may not be evident due to atrial pacing and/or isoprenaline), decreased mental status, oliguria, poor peripheral perfusion, slow capillary refill and raised serum lactate, low systemic venous oxygenation, and dyspnoea.
• Fluid resuscitate to a PAWP of 14–18 mmHg, high-dose inotropes, vasodilator agents, IABP to achieve a cardiac index >2.2 L/min/m2 with adequate end-organ perfusion.
• Insertion of full mechanical circulatory support (ECMO or LVAD) is indicated when other interventions do not provide adequate end-organ perfusion.
• Observe for right heart dysfunction/failure: rising CVP, low to normal PAWP, high calculated pulmonary vascular resistance, raised pulmonary artery pressures, systemic hypotension, and oliguria.
• Optimise right ventricular preload and afterload: titrate fluid and medications to achieve adequate end-organ perfusion; fluid resuscitate to a CVP of 14–20 mmHg; consider inhaled NO (selective pulmonary vasodilation and improved oxygenation from reduced ventilation/perfusion mismatch), prostaglandin E1 or prostacyclin, milrinone, or drug combinations with afterload reduction and inotropic support (e.g. sodium nitroprusside and adrenaline).
• Institute appropriate respiratory management to minimise hypoxaemia and metabolic or respiratory acidosis.
• If no sustained improvement in right ventricular performance, a right VAD is indicated for temporary support.
• Drugs with direct autonomic nervous system actions on heart rate (e.g. atropine, digoxin) and vagal manoeuvres (carotid sinus massage) are ineffective.
• Use overdrive atrial pacing to treat tachyarrhythmias.
• Sinus or junctional bradycardias may occur, and atrial/ventricular epicardial pacing is used to ‘train’ the sinus node.
• Orthostatic hypotension is common: patients should sit up slowly from a lying position.
• Patients rarely feel anginal pain after surgery: they need to identify other clinical signs of angina, such as shortness of breath and sweating.
Medium- to Long-Term Complications
There are four long-term complications associated with heart transplantation: (1) cardiac allograft vasculopathy; (2) malignancy; (3) renal dysfunction; and (4) hypertension.138 Cardiac allograft vasculopathy (CAV) is a diffuse, proliferative form of obliterative coronary arteriosclerosis that affects 30–60% of heart transplant recipients in the first 5 years after surgery.139 Sudden death, ventricular arrhythmias and symptoms of congestive heart failure may be the first signs of significant CAV. The aetiology of CAV is multifactorial, including immunological factors (e.g. episodes of acute rejection and anti-HLA antibodies), non-immunological cardiovascular risk factors (e.g. hypertension, hyperlipidaemia, preexisting diabetes and new-onset diabetes), the surgical procedure (e.g. donor age, ischaemic time and reperfusion injury), and side effects of immunosuppression drugs such as cyclosporin and corticosteroids (e.g. CMV infection and nephrotoxicity).112,139–141 Statins at doses less than that prescribed for hyperlipidaemia are commenced within 2 weeks of surgery irrespective of cholesterol levels to reduce episodes of rejection and CAV.112 Standard use of cyclosporine may be augmented by mycophenolate mofetil, everolimus or sirolimus as they have been shown to reduce the onset and progression of CAV.112 Diagnosis of CAV is difficult, due to allograft denervation, and because coronary angiogram underestimates the extent of the disease and is insensitive to early lesions.142 Currently, intravascular ultrasound (IVUS) provides the most reliable quantitative information about the degree of CAV.112 As the definitive treatment for CAV is retransplantation, ongoing research into the prevention of CAV143 will be the most important factor in reducing the incidence and associated mortality.
All heart transplant recipients are at a greater risk of developing malignancies than the general population, particularly carcinoma of the skin144–146 and lympho-proliferative disorders147,148 as a consequence of long-term immunosuppression therapy.149,150 Nurses play an important role in educating patients about how to avoid and reduce the risks of sun exposure. Treatment options in transplant recipients are the same as for the general population (e.g. chemotherapy, radiation therapy and surgical excision), in addition to a reduction in immunosuppression therapy; however, outcomes remain poor.146
Long-term renal dysfunction occurs primarily post-transplantation due to cyclosporin nephrotoxicity. Careful monitoring of cyclosporin levels, and avoidance of hypovolaemia and other nephrotoxic drugs are important measures in reducing progression to renal failure. Importantly, findings from recent research indicate that chronic cyclosporin nephrotoxicity can be reversed by eliminating cyclosporin from immunosuppression regimens.92 End-stage renal failure requiring dialysis or renal transplantation has been reported in 3–10% of patients.151
Systemic hypertension following transplantation has been linked with cyclosporin-induced tubular nephrotoxicity, peripheral vasoconstriction and fluid retention.152 Lifestyle modifications such as weight loss, low sodium diet and exercise are recommended along with optimal therapeutic doses of cyclosporin, and combinations of calcium channel blockers and angiotensin-converting enzyme inhibitors and blockers.112 Such approaches have been reported to achieve blood pressure control in up to 65% of patients.153
Lifestyle Issues
Following such momentous surgery, patients require sound advice regarding returning to driving, work, exercise and sexual activity. Cardiac rehabilitation with aerobic and resistance exercise is recommended to prevent short-term weight gain and glucose intolerance, as well as adverse effects of immunosuppressive therapy on skeletal muscle.113 Return to work or education is expected and encouraged after surgery. Driving a vehicle can be considered once the patient’s gait, tremor and other neurological issues are normalised, and any bradycardia managed by pacemaker implantation.113 Pregnancy is possible after one year following transplantation; but only under the management of the multidisciplinary team who will explain the considerable risks involved.113
Summary
Cardiac Surgery
• Surgical procedures may be performed as treatment for structural abnormalities, ischaemic lesions within coronary arteries, and repair or replacement of cardiac valves.
• Haemodynamic stability constitutes the most common challenge in the postoperative period and may be managed with fluids, cardiovascular medications, cardiac pacing and intra-aortic balloon pumping.
• Bleeding in the postoperative period may be due to inadequate reversal or heparin, coagulopathy or surgical bleeding; therefore, appropriate diagnosis must occur before relevant treatment is instigated.
Intra-Aortic Balloon Pumping
• Major benefits include increasing cardiac output, increasing myocardial oxygen supply and decreasing myocardial oxygen demand.
• Appropriate timing is essential to obtain maximum benefits, so correction of timing errors forms a central component of care.
• Assessment of limb perfusion, with timely intervention when perfusion is inadequate, is essential to prevent limb ischaemia.
Heart Transplant
• A triple-therapy regimen consisting of corticosteroids, a calcineurin antagonist and an antiproliferative cytotoxic agent is used to suppress the immune system after organ transplantation. All cytotoxic agents necessitate specific administration and disposal procedures.
• Indications for heart transplantation include end-stage heart failure secondary to ischaemic heart disease and cardiomyopathy. Possible complications in the early postoperative period include acute rejection, infection, haemorrhage, renal failure, right ventricular failure and allograft dysfunction (left ventricular dysfunction/failure).
• Although early signs of low-grade rejection can be non-specific, signs of moderate rejection usually present as organ dysfunction/failure.
• The CMV status of the donor and recipient must be known so that blood products with an appropriate CMV status are administered.
• Denervation of the heart renders vagal manoeuvres (e.g. carotid sinus massage), and drugs that act directly on the autonomic system (e.g. atropine, digoxin) to modify heart rate, ineffective.
• Nursing practices for managing patients with heart transplantation focus on prevention and management of complications, maintenance of comfort and promotion of long term recovery.
Case study
The main dimensions of Mr Martin’s progress, care and management follow.
Learning activities
1. Discuss initial assessment of IABP timing and alarms with a senior colleague.
2. Outline nursing assessment and management of patient with IABP in situ, including measures to prevent complications.
3. Identify the model of IABP that your critical care unit uses, and review the alarms that are present, the causes of those alarms and the response of the pump when it senses an alarm situation. With one of the senior staff, discuss the mechanisms that you should undertake to correct each of the alarm situations.
4. Identify any abnormalities on the ABGs in the case study and discuss corrective treatment.
5. Describe the assessment needed throughout a ventilation weaning process, in preparation for extubation and post-extubation. Identify any factors that would identify a patient that is not ready for extubation.
6. Identify the possible causes of hypotension and low cardiac output in the postoperative cardiac surgical patient. Outline the management options for each of these causes.
7. Consider the possible causes of bleeding in the postoperative cardiac surgical patient and outline the appropriate assessment and management for each of these causes.
Australian & New Zealand Intensive Care Society (ANZICS). www.anzics.com.au.
Australian Institute of Health and Welfare. www.aihw.gov.au.
Australian Organ Donor Register. http://www.medicareaustralia.gov.au/public/services/aodr/index.jsp.
Donate Life. www.donatelife.gov.au.
Transplant Nurses’ Association (TNA). www.tna.asn.au.
Transplantation Society of Australia and New Zealand (TSANZ). www.tsanz.com.au.
The International Society for Heart and Lung Transplantation (ISHLT). www.ishlt.org.
National Heart Foundation of Australia. www.heartfoundation.com.au.
National Heart Foundation of New Zealand. www.heartfoundation.org.nz.
Cardiac Society of Australia and New Zealand. www.csanz.edu.au.
National Health Priorities and Quality. www.health.gov.au/internet/wcms/publishing.nsf/content/pq-cardio/.
1 Australian Institute of Health and Welfare. Heart, stroke and vascular diseases: Australian facts [AIHW cat. no. CVD 27]. AIHW and National Heart Foundation of Australia: Canberra, 2004.
2 Groves AM. Rheumatic fever and rheumatic heart disease: an overview. Trop Doct. 1999;29:129–132.
3 Hay DR. Cardiovascular disease in New Zealand, 2004: Technical report. Auckland: National Heart Foundation of New Zealand; 2004.
4 Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91(7):899–906.
5 Urden L, Stacy K, Lough M. Thelan’s critical care nursing: diagnosis and management, 5th edn. St Louis: Mosby; 2006.
6 Modine T, Al-Ruzzeh S, Mazrani W, Azeem F, Bustami M, Ilsley A. Use of radial artery graft reduces the morbidity of coronary artery bypass graft surgery in patients aged 65 years and older. Ann Thorac Surg. 2002;74(4):1144–1147.
7 Agrifoglio M, Dainese L, Pasotti S, Galanti A, Cannata A, et al. Preoperative assessment of the radial artery for coronary artery bypass grafting: is the Clinical Allen Test adequate? Ann Thorac Surg. 2005;79(2):570–572.
8 Kettering K, Dapunt O, Baer FM. Minimally invasive direct coronary artery bypass grafting: a systematic review. J Cardiovasc Surg. 2004;45(3):255–264.
9 Jones BA, Krueger S, Howell D, et al. Robotic mitral valve repair: a community hospital experience. Tex Heart Inst J. 2005;32(2):143–146.
10 Australian Institute of Health and Welfare. Australia’s health 2010 [Australia’s health series no. 12. Cat. no. AUS 122]. AIHW: Canberra, 2010.
11 NHS Centre for Review and Dissemination (NHSCRD). Management of stable angina. Effect Health Care. 1997;3(5):1–8.
12 Petticrew M, McKee M, Jones J. Coronary artery surgery: are women discriminated against? BMJ. 1993;306(6686):1164–1166.
13 Pretre R, Turina M. Valve disease: cardiac surgery in the octogenerarian. Heart. 2000;83(1):116–121.
14 Parsonnet V, Dean D, Bernstein A. A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79(Suppl):3–12.
15 Fisher S, Walsh G, Cross N. Nursing management of the cardiac surgical patient. In: Hatchett R, Thompson D. Cardiac nursing: a comprehensive guide. Edinburgh: Churchill Livingstone Elsevier, 2002.
16 Currey J, Browne J, Botti M. Haemodynamic instability after cardiac surgery: nurses’ perceptions of clinical decision-making. J Clin Nurs. 2006;15(9):1081–1090.
17 Reuter DA, Felbinger TW, Moerstedt K, et al. Intrathoracic blood volume index measured by thermodilution for preload monitoring after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16(2):191–195.
18 Currey J, Botti M. The haemodynamic status of cardiac surgical patients in the initial 2-h recovery period. Eur J Cardiovasc Nurs. 2005;4(3):207–214.
19 Doolan L, Gaer J, Bellomo R, et al. Postoperative care. In: Buxton B, Frazier OH, Westaby S. Ischemic heart disease: surgical management. London: Mosby, 1999.
20 Marolda D, Finkelmeier BA. Postoperative patient management. Finkelmeier BA, ed. Cardiothoracic surgical nursing, 2nd edn, Philadelphia: Lippincott: Williams & Wilkins, 2000.
21 St Andre AC, DelRossi A. Hemodynamic management of patients in the first 24 hours after cardiac surgery. Crit Care Med. 2005;33(9):2082–2093.
22 Larmann J, Theilmeier G. Inflammatory response to cardiac surgery: cardiopulmonary bypass versus non-cardiopulmonary bypass surgery. Best Pract Res Clin Anaesthesiol. 2004;18(3):425–438.
23 Buxton B, Calafiore P, Doolan L. Cardiac tamponade. In: Buxton B, Frazier OH, Westaby S. Ischemic heart disease: surgical management. London: Mosby, 1999.
24 Westerberg M, Bengtsson A, Jeppsson A. Coronary surgery without cardiotomy suction and autotransfusion reduces the postoperative systemic inflammatory response. Ann Thorac Surg. 2004;78(1):54–59.
25 Garcia-Rinaldi R, Soltero ER, Carballido J, et al. Intraluminal milrinone for dilation of the radial artery graft. Tex Heart Inst J. 1999;26(3):189–191.
26 Albright TN, Zimmerman MA, Selzman CH. Vasopressin in the cardiac surgery intensive care unit. Am J Crit Care. 2002;11(4):326–330.
27 Wagner GS, Marriott HJL. Marriott’s practical electrocardiography, 10th edn. Baltimore: Lippincott, Williams & Wilkins; 2000.
28 Dennis MJ. ECG criteria to differentiate pulmonary artery catheter irritation from other proarrhythmic influences as the cause of ventricular arrhythmias. [abstract]. Am Coll Cardiol. 2002;39(9):2B. SupplB
29 Mitchell LB, Crystal E, Heilbron B, Page P. Atrial fibrillation following cardiac surgery. Can J Cardiol. 2005;21(SupplB):45B–50B.
30 Quasha AL, Loeber N, Feely TW, et al. Postoperative respiratory care: a controlled trial of early and late extubation following coronary artery bypass grafting. Anaethesia. 1980;52(2):135–141.
31 Meade MO, Guyatt G, Butler R, et al. Trials comparing early vs late extubation following cardiovascular surgery. Chest. 2001;120(6Suppl):S445–S453.
32 Edmunds LH, Jr. Blood conservation. In: Buxton B, Frazier OH, Westaby S. Ischemic heart disease: surgical management. London: Mosby, 1999.
33 Mallett SV, Cox DJA. Thromboelastography. Br J Anaesth. 1992;69(3):307–313.
34 Botti M, Bucknall T, Manias E. The problem of postoperative pain: issues for future research. Int J Nurs Pract. 2004;10(6):257–263.
35 Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. New Engl J Med. 2005;352(11):1081–1091.
36 Bradley D, Cresswell LL, Hogue CW, et al. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128(2Suppl):S39–S47.
37 Koivula M, Paunonen-Ilmonen M, Tarkka M, Laippala P. Fear and anxiety in patients awaiting coronary artery bypass grafting. Heart Lung. 2001;30(4):302–311.
38 Duits A, Boeke S, Taams M, Passchier J, Erdman R. Prediction of quality of life after coronary artery bypass graft surgery: a review and evaluation of multiple, recent studies. Psychosom Med. 1997;59(3):257–268.
39 Gallagher R, McKinley S, Dracup K. Evaluation of telephone follow-up to promote psychosocial adjustment and risk factor modification in women with coronary heart disease. Heart Lung. 2003;32(2):79–87.
40 King K. Emotional and functional outcomes in women with coronary heart disease. J Cardiovasc Nurs. 2001;15(3):54–70.
41 Jenkins C, Jono R, Stanton B. Predicting completeness of symptom relief after major heart surgery. Behav Med. 1996;22(2):45–57.
42 Dusseldorp E, van Elderen T, Maes S. A meta-analysis of psychoeducational programs for coronary heart disease patients. Health Psychol. 1999;18(5):506–519.
43 Huether S, McCance K. Understanding pathophysiology. St Louis: Mosby; 1996.
44 Bryant B, Knights K, Salerno E. Pharmacology for health professionals. Sydney: Mosby; 2003.
45 Duvernoy CS, Bates ER. Management of cardiogenic shock attributable to acute myocardial infarction in the reperfusion era. J Intens Care Med. 2005;20(4):188–198.
46 Richenbacher WE, Pierce WE. Treatment of heart failure: assisted circulation. Braunwald E, Dipes DP, Libby P. Heart disease: a textbook of cardiovascular medicine, 6th edn, Philadelphia: WB Saunders, 2001.
47 Frazier OH, Westaby S. Mechanical circulatory support. In: Buxton B, Frazier OH, Westaby S. Ischemic heart disease: surgical management. London: Mosby, 1999.
48 Cadwell CA, Hobson KS, Pettis S, Blackburn A. Clinical observations with real timing. Crit Care Nurs Clin North Am. 1996;8(4):357–370.
49 Cadwell CA, Quaal SJ. Intra-aortic balloon counterpulsation timing. Am J Crit Care. 1996;5(4):254–261.
50 Elahi MM, Chetty GK, Kirke R, Azeem T, et al. Complications related to intra-aortic balloon pump in cardiac surgery: a decade later. Eur J Vasc Endovasc Surg. 2005;29(6):591–594.
51 Meco M, Gramegna G, Yassini A, et al. Mortality and morbidity from intra-aortic balloon pumps: risk analysis. J Cardiovasc Surg. 2002;43(1):17–23.
52 Krau SD. Successfully weaning the intra-aortic balloon pump patient: an algorithm. Dimens Crit Care Nurs. 1993;18(3):2–11.
53 Chapman JR. Transplantation in Australia – 50 years in progress. Med J Aust. 1992;157(1):46–50.
54 McBride M, Chapman JR. An overview of transplantation in Australia. Anaesth Intens Care. 1995;23(1):60–64.
55 Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin-A: a new antilymphocytic agent. Agents Actions. 1976;6(4):468–475.
56 Barnard CN. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Capetown. S Afr Med J. 1967;41(48):1271–1274.
57 Oyer PE, Stinson EB, Jamieson SA, et al. Cyclosporin A in cardiac allografting: a preliminary experience. Transplant Proc. 1983;15:1247–1252.
58 Excell L, Hee K, Russ GR. Australia and New Zealand organ donation registry 2010. [Cited Sept 2010]. Available from http://www.anzdata.org.au/anzod/ANZODReport/2010/2010Contents.pdf
59 Rose EA, Moskowitz AJ, Packer M, Sollano JA, Williams DL, et al. The REMATCH trial: rationale, design, and end points. Randomized evaluation of mechanical assistance for the treatment of congestive heart failure. Ann Thorac Surg. 1999;67(3):723–730.
60 Mason VF, Konicki AJ. Left ventricular assist devices as destination therapy. AACN Clin Iss. 2003;14(4):488–497.
61 Hertz MI, Aurora P, Christie JD, Dobbels F, Edwards LB, et al. Scientific Registry of the International Society for Heart and Lung Transplantation: Introduction to the 2009 Annual Reports. J Heart Lung Transplantation. 2009;28(4):989–992.
62 National Heart Foundation. Heart transplants and organ donation [Cited Sept 2010]. Available from http://www.heartfoundation.org.au/SiteCollectionDocuments/A_HeartTransplantsDonations_ISC_FINAL.pdf
63 Organ Donation New Zealand [Cited Sept 2010]. Available from http://www.donor.co.nz/donor/transplants/success_rates.php
64 Keogh A, Pettersson R. Australia and New Zealand Cardiothoracic Organ Transplant Registry: 2008 Report. Darlinghurst: Australia and New Zealand Cardiothoracic Organ Transplant Registry; 2008.
65 Taylor DO, Stehlik J, Edwards LB, et al. Registry of the international society for heart and lung transplantation: twenty-sixth official adult heart transplant report 2009. J Heart Lung Transplant. 2009;28(10):1007–1022.
66 Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates, 2006. J Heart Lung Transplant. 2006;25(9):1024–1042.
67 Russo MJ, Chen JM, Hong KN, et al. Survival after heart transplantation is not diminished among recipients with uncomplicated diabetes mellitus: an analysis of the United Network of Organ Sharing database. Circulation. 2006;114(21):2280–2287.
68 Grady KL, White-Williams C, Naftel D, et al. Are preoperative obesity and cachexia risk factors for post heart transplant morbidity and mortality: a multi-institutional study of preoperative weight-height indices. Cardiac Transplant Research Database (CTRD) Group. J Heart Lung Transplant. 1999;18(8):750–763.
69 Chacko RC, Harper RG, Gotto J, et al. Psychiatric interview and psychometric predictors of cardiac transplant survival. Am J Psychiatry. 1996;153(12):1607–1612.
70 Shapiro PA, Williams DL, Foray AT, et al. Psychosocial evaluation and prediction of compliance problems and morbidity after heart transplantation. Transplantation. 1995;60(12):1462–1466.
71 Macdonald P. Heart transplantation: who should be considered and when? Intern Med J. 2008;38(12):911–917.
72 The Transplantation Society of Australia and New Zealand. Eligibility guidelines and allocation protocols. Draft; 22 March 2010. Available from http://www.tsanz.com.au/organallocationprotocols/downloads/TSANZ%20Elibility%20and%20Allocation%202nd%20Draft.pdf
73 Clark RA, McLennan S, Dawson A, Wilkinson D, Stewart S. Uncovering a hidden epidemic: A study of the current burden of heart failure in Australia. Heart, Lung Circ. 2004;13(2):166–173.
74 Chronic Heart Failure Working Party. Hospital admission risk program (HARP): chronic heart failure working group report. Melbourne: Victorian Government Department of Human Services; 2003.
75 Australian Institute of Health and Welfare. Australia’s Health 2008. Canberra: AIHW; Report No. 11.
76 Doughty R, Yee T, Sharpe N, MacMahon S. Hospital admissions and deaths due to congestive heart failure in New Zealand, 1988–91. NZ Med J. 1995;108(1012):473–475.
77 Garg R, Packer M, Pitt B, Yusuf S. Heart failure in the 1990s: evolution of a major public health problem in cardiovascular medicine. J Am Coll Cardiol. 1993;22(4 Suppl A):3A–5A.
78 Australian Institute of Health and Welfare. Heart, stroke and vascular disease: Australian Facts 2001. Perth: Australian Institute of Health and Welfare, National Heart Foundation and National Stroke Foundation of Australia; 2001.
79 Kirklin JK, Young JB, McGiffin DC. Heart transplantation. Philadelphia: Churchill Livingstone; 2002.
80 Angermann CE, Spes CH, Tammen A, Stempfle HU, Schutz A, et al. Anatomic characteristics and valvular function of the transplanted heart: transthoracic versus transesophageal echocardiographic findings. J Heart Transplant. 1990;9(4):331–338.
81 Kendall SWH, Ciulli F, Mullins PA, Biocina B, Dunning JJ, Large SR. Total orthotopic heart transplantation: An alternative to the standard technique. Ann Thorac Surg. 1992;54(1):187–192.
82 Dreyfus G, Jebara V, Mihaileanu S, Carpentier AF. Total orthotopic heart transplantation: an alternative to the standard technique. Ann Thorac Surg. 1991;52(5):1181–1184.
83 International Society for Heart Lung Transplantation (ISHLT) Transplant Registry Quarterly Reports for Heart in Australasia. 2004 [Cited Dec 2004]. Available from http://www.ishlt.org
84 Nakatani T, Frazier OH, Lammermeier DE, Marcis MP, Radovancevic B. Heterotopic heart transplantation: a reliable option for a select group of high-risk patients. J Heart Lung Transplant. 1989;8(1):40–47.
85 Newcomb AE, Esmore DS, Rosenfeldt FL, Richardson M, Marasco SF. Heterotopic heart transplantation: an expanding role in the twenty-first century? Ann Thorac Surg. 2004;78(4):1345–1350.
86 Neerukonda SK, Schoonmaker FW, Nampalli VK, Narrod JA. Ventricular dysrthythmia and heterotopic heart transplantation. J Heart Lung Transplant. 1992;11(4 Pt 1):793–796.
87 Bridges EJ. Monitoring pulmonary artery pressures: just the facts. Crit Care Nurse. 2000;20(6):59–78.
88 Cathelyn J. Avoiding respiratory excursions: obtaining reliable pulmonary capillary wedge pressures. Dimens Crit Care Nurs. 1997;16(1):2–7.
89 Sommers MS, Woods SL, Courtade MA. Issues in methods and measurement of thermodilution cardiac output. Nurs Res. 1993;42(4):228–233.
90 Busauschina A, Schnuelle P, van der Woude FJ. Cyclosporine nephrotoxicity. Transplant Proc. 2004;36(2 Suppl):S229S–33.
91 Serkova NJ, Christians U, Benet LZ. Biochemical mechanisms of cyclosporine neurotoxicity. Mol Interv. 2004;4(2):97–107.
92 Trento A, Hardesty RL, Griffith BP, Zerbe T, Kormos RL, Bahnson HT. Role of the antibody to vascular endothelial cells in hyperacute rejection in patients undergoing cardiac transplantation. J Thorac Cardiovasc Surg. 1988;95(1):37–41.
93 Stevenson LW, Miller LW. Cardiac transplantation as therapy for heart failure. Curr Probl Cardiol. 1991;16(4):217–305.
94 Hammond EH. Pathology of cardiac allograft rejection. In: Cooper DKC, Miller LW, Patterson GA. The transplantation and replacement of thoracic organs. 2nd edn. Boston: Kluwer; 1996:239–252.
95 Laufer G, Laczkovics A, Wollenek G, Buxbaum P, Seitelberger R, et al. The progression of mild acute cardiac rejection evaluated by risk factor analysis: the impact of maintenance steroids and serum creatinine. Transplantation. 1991;51(1):184–189.
96 Caves PK, Stinson EB, Billingham ME, Rider AK, Shumway NE. Diagnosis of human cardiac allograft rejection by serial cardiac biopsy. J Thorac Cardiovasc Surg. 1973;66(3):461–466.
97 Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. International Society for Heart Transplantation. J Heart Transplant. 1990;9(6):587–593.
98 Lloveras JJ, Escourrou G, Delisle MB, Fournial G, Cerene A, et al. Evolution of untreated mild rejection in heart transplant recipients. J Heart Lung Transplant. 1992;11(4 Pt 1):751–756.
99 Olsen SL, Wagoner LE, Hammond EH, Taylor DO, Yowell RL, et al. Vascular rejection in heart transplantation: clinical correlation, treatment options, and future considerations. J Heart Lung Transplant. 1993;12(2):S135–S142.
100 Partanen J, Nieminen MS, Krogerus L, Harjula AL, Mattila S. Heart transplant rejection treated with plasmapheresis. J Heart Lung Transplant. 1992;11(2 Pt 1):301–305.
101 Taylor DO. Cardiac transplantation: Drug regimens for the 21st century. Ann Thorac Surg. 2003;75(6 Suppl):S72–S78.
102 Wade CR, Reith KK, Sikora JH, Augustine SM. Postoperative nursing care of the cardiac transplant recipient. Crit Care Nurs Q. 2004;27(1):17–28.
103 Keogh A. Calcineurin inhibitors in heart transplantation. J Heart Lung Transplant. 2004;23(5 Suppl):S202–S206.
104 Morris PJ, Monaco AP. A meta-analysis from the Cochrane Library reviewing interleukin 2 receptor antagonists in renal transplantation. Transplantation. 2004;77(2):165.
105 Farmer DG, McDiarmid SV, Edelstein S, Renz JF, Hisatake G, et al. Induction therapy with interleukin-2 receptor antagonist after intestinal transplantation is associated with reduced acute cellular rejection and improved renal function. Transplantat Proc. 2004;36(2):331–332.
106 Carey JA, Frist WH. Use of polyclonal antilymphocytic preparations for prophylaxis in heart transplantation. J Heart Transplant. 1990;9(3 Pt 2):297–300.
107 Williams TJ, Snell GI. Lung transplantation. In: Albert RK, Spiro SG, Jett JR. Clinical respiratory medicine. Mosby; 2004:831–845.
108 Mason JW, Stinson EB, Hunt SA, Schroeder JS, Rider AK. Infections after cardiac transplantation: relation to rejection therapy. Ann Intern Med. 1976;85(1):69–72.
109 Miller LW, Naftel DC, Bourge RC, Kirklin JK, Brozena SC, et al. Infection after heart transplantation: a multiinstitutional study. Cardiac Transplant Research Database Group. J Heart Lung Transplant. 1994;13(3):381–392.
110 Hughes WT, Rivera GK, Schell MJ, Thornton D, Lott L. Successful intermittent chemoprophylaxis for Pneumocystis carinii pneumonitis. N Eng J Med. 1987;316(26):1627–1632.
111 Kocher AA, Bonaros N, Dunkler D, Ehrlich M, Schlechta B, et al. Long-term results of CMV hyperimmune globulin prophylaxis in 377 heart transplant recipients. J Heart Lung Transplant. 2003;22(3):250–257.
112 Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–956.
113 Couchoud C. Cytomegalovirus prophylaxis with antiviral agents for solid organ transplantation. Cochrane Database Syst Rev 2000; 2: CD001320.
114 Walsh TR, Guttendorf J, Dummer S, Hardesty RL, Armitage JM, et al. The value of protective isolation procedures in cardiac allograft recipients. Ann Thorac Surg. 1989;47(4):539–544.
115 Wade CR, Reith KK, Sikora JH, Augustine SM. Postoperative nursing care of the cardiac transplant recipient. Crit Care Nurs Q. 2004;27(1):17–28.
116 Rourke TK, Droogan MT, Ohler L. Heart transplantation: state of the art. AACN Clin Iss. 1999;10(2):185–201.
117 Bowden RA, Slichter SJ, Sayers M, Weisdorf D, Cays M, et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood. 1995;86(9):3598–3603.
118 Luckraz HM, Goddard M, Charman SC, Wallwork J, Parameshwar J, Large SR. Early mortality after cardiac transplantation: should we do better? J Heart Lung Transplant. 2005;24(4):401–405.
119 Cooper DKC, Lidsky NM. Immediate postoperative care and potential complications. In: Cooper DKC, Miller LW, Patterson GA. The transplantation and replacement of thoracic organs. 2nd edn. London: Kluwer; 1996:221–227.
120 Taylor DO. Cardiac transplantation: drug regimens for the 21st century. Ann Thorac Surg. 2003;75(6 Suppl):S72–S78.
121 McCrystal GD, Pepe S, Esmore DS, Rosenfeldt FL. The challenge of improving donor heart preservation. Heart Lung Circ. 2004;13(1):74–83.
122 Rosenfeldt FL, McCrystal G, Pepe S, Esmore D. Myocyte or heart preservation: towards optimising donor heart quality [Conference Abstracts]. Cambridge, England; 2002.
123 Esmore DS, Rosenfeldt FL, Mack JA, Waters KN, Bergin P. Long ischaemic time allografts (>6 hr) further expand the transplant donor pool. Washington: ISHLT Conference Abstracts; 2002.
124 Kieler-Jensen N, Lundin S, Ricksten SE. Vasodilator therapy after heart transplantation: effects of inhaled nitric oxide and intravenous prostacyclin, prostaglandin E1, and sodium nitroprusside. J Heart Lung Transplant. 1995;14(3):436–443.
125 Kristof AS, Magder S. Low systemic vascular resistance state in patients undergoing cardiopulmonary bypass. Crit Care Med. 1999;27(6):1121–1127.
126 Myles PS, Leong CK, Currey J. Endogenous nitric oxide and low systemic vascular resistance after cardiopulmonary bypass. J Cardiothorac Vasc Anaesth. 1997;11(5):571–574.
127 Landry DW, Levin HR, Gallant EM, Ashton RC, Jr., Seo S, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122–1125.
128 Argenziano M, Choudhri AF, Oz MC, Rose EA, Smith CR, Landry DW. A prospective randomized trial of arginine vasopressin in the treatment of vasodilatory shock after left ventricular assist device placement. Circulation. 1997;96(9 Suppl):286–290.
129 Ardehali A, Hughes K, Sadeghi A, Esmailian F, Marelli D, et al. Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation. 2001;72(4):638–641.
130 Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Eng J Med. 1993;328(6):399–405.
131 Armitage JM, Hardesty RL, Griffith BP. Prostaglandin E1: an effective treatment of right heart failure after orthotopic heart transplantation. J Heart Lung Transplant. 1987;6(6):348–351.
132 Wang SS, Ko WJ, Chen YS, Hsu RB, Chou NK, Chu SH. Mechanical bridge with extracorporeal membrane oxygenation and ventricular assist device to heart transplantation. Artif Organs. 2001;25(8):599–602.
133 Ellenbogen KA, Thames MD, DiMarco JP, Sheehan H, Lerman BB. Electrophysiological effects of adenosine in the transplanted human heart: evidence of supersensitivity. Circulation. 1990;81(3):821–828.
134 Macdonald P, Hackworthy R, Keogh A, Sivathasan C, Chang V, Spratt P. Atrial overdrive pacing for reversion of atrial flutter after heart transplantation. J Heart Lung Transplant. 1991;10(5 Pt 1):731–737.
135 Mackintosh AF, Carmichael DJ, Wren C, Cory-Pearce R, English TA. Sinus node function in first three weeks after cardiac transplantation. Br Heart J. 1982;48(6):584–588.
136 Stark RP, McGinn AL, Wilson RF. Chest pain in cardiac-transplant recipients: evidence of sensory reinnervation after cardiac transplantation. N Eng J Med. 1991;324(25):1791–1794.
137 Parry A, Roberts M, Parameshwar J, Wallwork J, Schofield P, Large S. The management of post-cardiac transplantation coronary artery disease. Eur J Cardiothorac Surg. 1996;10(7):528–532.
138 Shiba N, Chan MC, Kwok BW, Valantine HA, Robbins RC, Hunt SA. Analysis of survivors more than 10 years after heart transplantation in the cyclosporine era: Stanford experience. J Heart Lung Transplant. 2004;23(2):155–164.
139 Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23(5 Suppl):S187–S193.
140 Kobashigawa J. What is the optimal prophylaxis for treatment of cardiac allograft vasculopathy? Curr Control Trials Cardiovasc Med. 2000;1(3):166.
141 Rose EA, Pepino P, Barr ML, Smith CR, Ratner AJ, et al. Relation of HLA antibodies and graft atherosclerosis in human cardiac allograft recipients. J Heart Lung Transplant. 1992;11(3 Pt 2):S120–S123.
142 Johnson DE, Alderman EL, Schroeder JS, Gao SZ, Hunt S, et al. Transplant coronary artery disease: histopathologic correlations with angiographic morphology. J Am Coll Cardiol. 1991;17(2):449–457.
143 Keogh A, Richardson M, Ruygrok P, Spratt P, Galbraith A, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110(17):2694–2700.
144 Krikorian JG, Anderson JL, Bieber CP, Penn I, Stinson EB. Malignant neoplasms following cardiac transplantation. JAMA. 1978;240(7):639–643.
145 Ong CS, Keogh AM, Kossard S, Macdonald PS, Spratt PM. Skin cancer in Australian heart transplant recipients. J Am Acad Dermatol. 1999;40(1):27–34.
146 Veness MJ, Quinn DI, Ong CS, Keogh AM, Macdonald PS, et al. Aggressive cutaneous malignancies following cardiothoracic transplantation: the Australian experience. Cancer. 1999;85(8):1758–1764.
147 Armitage JM, Kormos RL, Stuart RS, Fricker FJ, Griffith BP, et al. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10(6):877–886.
148 Cole WH. The increase in immunosuppression and its role in the development of malignant lesions. J Surg Oncol. 1985;30(3):139–144.
149 Penn I, First MR. Development and incidence of cancer following cyclosporine therapy. Transplant Proc. 1986;18(2 Suppl 1):210–215.
150 Penn I. Cancers following cyclosporine therapy. Transplantation. 1987;43(1):32–35.
151 Greenberg A, Thompson ME, Griffith BJ, Hardesty RL, Kormos RL, et al. Cyclosporine nephrotoxicity in cardiac allograft patients – a seven-year follow-up. Transplantation. 1990;50(4):589–593.
152 Eisen HJ. Hypertension in heart transplant recipients: more than just cyclosporine. J Am Coll Cardiol. 2003;41(3):433–434.
153 Ventura HO, Malik FS, Mehra MR, Stapleton DD, Smart FW. Mechanisms of hypertension in cardiac transplantation and the role of cyclosporine. Curr Opin Cardiol. 1997;12(4):375–381.