89 Cardiac Surgery
Indications and Complications
 Surgical Indications for Coronary Artery Diseases
Surgical Indications for Coronary Artery Diseases
In the early 1990s, three large multicenter randomized trials were undertaken in Europe and the United States. The Veterans Administration Cooperative Study, the European Coronary Surgery Study, and the Coronary Artery Surgery Study indicated that patients who underwent coronary artery bypass grafting (CABG) always had extended survival compared with medically treated patients.1 Since then, medical treatment has evolved, with the advent of plaque stabilizers and percutaneous coronary angioplasty (PTCA). Nowadays, referring a patient to medical treatment or PTCA or surgery is an expert decision based on appropriateness criteria2 and often discussed on multidisciplinary rounds. In patients with myocardial ischemia, indications for surgery will be based on symptoms (Canadian Cardiovascular Society classification [CCS]), medical history (left ventricular [LV] function, diabetes), and sets of lesions defined by the anatomic localization of the coronary artery stenosis on a coronarography and best defined by the Syntax score.3
In acute coronary syndromes, most clinical scenarios are amenable to revascularization, except for ST-segment elevation myocardial infarction (STEMI) with onset of symptoms later than 12 hours. Even patients in shock will benefit from revascularization compared with medical treatment.4,5 In less acute ischemia, the indication for revascularization will depend on symptoms classified according to the CCS. Most asymptomatic patients will benefit from medical treatment, whereas most symptomatic patients will benefit from invasive treatment. Surgery is preferred over PTCA in three situations:
Use of a saphenous graft has been supplanted by total arterial revascularization.6,7,8 Although surgery is certainly the best method to restore coronary flow, it is also the most invasive one, with attendant complications. Less invasive CABG procedures may broaden surgical indications by reducing morbidity and mortality. Efforts have been made to reduce handling of the heart to cannulate, avoid cardiopulmonary bypass (CPB), and avoid sternal splitting. Current efforts are made in various directions.
While initially very promising, off-pump CABG performed via sternotomy on a beating heart—avoiding CPB and heart handling—has somehow failed to show real advantages, mainly because it is more technically demanding, and studies may have suffered from performance biases.9 Minimally invasive direct coronary artery bypass (MIDCAB) performed via a small left thoracotomy without CPB is widely accepted but limited to bypass of one artery: the left anterior descending (LAD) artery. CABG with femorofemoral CPB or off-pump techniques using thoracoscopic instruments and the support of a robot (da Vinci system) are under investigation worldwide and need large-scale validation but are certainly part of the armamentarium of tomorrow.10 These newer techniques are also combined with PTCA in hybrid procedures, narrowing the gap between cardiology and cardiac surgery.11
 Surgical Indications for Aortic Valve Surgery
Surgical Indications for Aortic Valve Surgery
Aortic Stenosis
Echocardiography is the most efficient technique to evaluate the degree of stenosis, LV hypertrophy, and LV function in patients with aortic valve stenosis.12 The American College of Cardiology/American Heart Association Task Force on Practice Guidelines has graded the degree of aortic stenosis as mild (effective valve area >1.5 cm2), moderate (area >1 to 1.5 cm2), or severe (area ≤1 cm2).13 When stenosis is severe and cardiac output is normal, the mean transvalvular pressure gradient is generally greater than 50 mm Hg. Symptomatic patients (dyspnea, angina, or palpitations) with severe stenosis are candidates for surgery (class 1 recommendation), as are asymptomatic patients with reduced ventricular function (left ventricular ejection fraction [LVEF] <50%) or patients undergoing any other cardiac surgery (CABG, mitral valve, or thoracic aorta). When cardiac output is reduced, transvalvular gradient is reduced (low flow/low output), and estimation of the severity of the stenosis may require advanced diagnostic tools such as stress test, echocardiography, or pressure measurement in the cath lab. An accurate estimation of the degree of stenosis is essential in those patients with low cardiac output who, despite being at high risk for surgery, do better than with medical treatment if correctly diagnosed.14
Management of patients with coronary artery disease who will have CABG and are incidentally diagnosed with mild to moderate aortic stenosis during workup is controversial. For asymptomatic patients with mild aortic stenosis (mean gradient between 30 and 50 mm Hg) who require CABG, it may be reasonable to replace the valve (class IIa recommendation). For patients with lower mean gradient, leaving the native valve is advised unless there is a risk of rapid progression, such as important calcification (class IIb).15 In very high risk patients, transcatheter aortic valve implantation (TAVI) is presently in its evaluation phase.16
Aortic Regurgitation
Chronic aortic regurgitation is usually well tolerated, and pure regurgitation is not considered for surgery unless severe (i.e., regurgitant volume >60 mL per beat or regurgitant orifice >0.3 cm2) in a symptomatic patient at rest (class I) or on exercise testing (class I) or in an asymptomatic patient with LV dysfunction (LVEF <50%) (class I) or with LV enlargement (end-systolic diameter >55 mm or end-diastolic diameter >75 mm) (class IIa).13 Symptomatic patients with mild aortic regurgitation should be investigated for other causes (ischemic cardiomyopathy). Regurgitation due to cusp lesions such as calcifications or destruction due to endocarditis are indications for valve replacement, except perhaps in very experienced hands in which repair is sometimes performed. Regurgitation due to annular enlargement with none or very little cusp lesion is now usually repaired with good results.17,18 In the latter, annular enlargement is often concomitant with ascending aortic enlargement, and the aorta is replaced by a valve conduit if repair is not feasible or by a straight Dacron tube, with the native valve resuspended.19
 Surgical Indications for Mitral Valve Surgery
Surgical Indications for Mitral Valve Surgery
Indications for mitral valve surgery have changed with the extension of mitral valve repair. With a better understanding of the specific anatomic lesions of the mitral valve associated with improvements in the surgical techniques, successful mitral repair can be achieved in specific ischemic and nonischemic mitral regurgitation.13
Mitral Regurgitation
Mitral regurgitation is responsible for pulmonary hypertension, left atrial enlargement with atrial fibrillation, and excessive workload on the heart, leading to dyspnea. Much work has been done on the mechanisms and causes of mitral regurgitation, pioneered by Carpentier and colleagues in the early 1980s; advances include the development of repair techniques other than systematic valve replacement. In experienced hands, more than 90% of regurgitant valves are repaired, avoiding problems associated with prosthetic valves (degeneration, need for anticoagulation, prosthetic valve infection). The lower morbidity related to valve repair has broadened the indication for mitral valve surgery to asymptomatic patients with no ventricular dysfunction and no pulmonary hypertension or atrial fibrillation, if the regurgitant surface is more than 40 mm. In those patients, repair is mandatory, whereas in patients with any of the aforementioned complications of regurgitation, replacement is an option.20,21 Recently, minimally invasive approaches through a 5-cm thoracotomy have been successfully applied to mitral repair and are gaining wide acceptance.22,23
Most mitral valves successfully repaired suffer from structural abnormalities and are identified as organic mitral regurgitation. Functional mitral regurgitation is the term used when the valve has no anatomic defect but is incompetent secondary to LV dysfunction, annulus dilation, or papillary muscle dysfunction. In some cases, CABG alone may improve LV function and reduce mitral regurgitation. Some advocate the use of ring annuloplasty or ventricular reduction surgery, but results are less convincing than in structural disorders.24
In long-standing mitral regurgitation, chronic right ventricular overload causes tricuspid regurgitation and atrial enlargement, promoting atrial fibrillation. Tricuspid regurgitation and atrial fibrillation also have to be assessed during the intervention, with a tricuspid annuloplasty and lesions made to the atria to stop reentrant circuits causing the arrhythmia.25,26
Ascending Aorta
Dilation of the ascending aorta is associated with hypertension, atherosclerotic disease, and structural (bicuspid aortic diseases)27 and genetic factors that arise with entities such as Marfan or Ehlers-Danlos syndromes. When reaching threshold values28 or when rapidly enlarging, dilated ascending aortas present a risk of rupture and dissection, prompt surgery is indicated.29 In patients with structural arterial wall abnormalities, surgery is warranted when the largest diameter is 45 mm, while in general population 55 mm is the cutoff value for surgery.30
Axillary cannulation and selective cerebral perfusion have permitted more thorough repair of aneurysm and dissection of the ascending aorta, prolonging in the aortic arch. Actual development is directed towards one-step treatment of the entire thoracic aorta.31
 Complications After Cardiac Surgery
Complications After Cardiac Surgery
Bleeding and Cardiac Tamponade
Hemostasis is deeply altered after cardiac surgery under CPB.32 Problems include decreased platelet numbers and function and activation of the coagulation and fibrinolytic cascade. All these factors, associated with cytokine activation and kallikrein stimulation of neutrophils, lead to a propensity for patients to bleed after the procedure.
Besides careful surgical techniques, diffuse bleeding can be prevented.33 Patients should be rapidly rewarmed at 37°C, since hypothermia inhibits coagulation and alters platelet function. Arterial hypertension should be aggressively treated in the first 24 hours with short-acting drugs. Even if transfusion affects long-term outcome,34–36 one should not be afraid to transfuse blood components: packed red blood cells, platelets, and plasma are to be given in a bleeding patient even before coagulation results are available. Correction of fluid deficits with crystalloid or colloid infusion induces some degree of hemodilution, contributing to altered hemostasis. If the patient is bleeding, correcting hemostasis according to lab results actually corrects a past situation, so blood components must be given on an empirical basis. When the coagulation tests are available, specific measures are taken; prolonged partial thromboplastin time (PTT) should be treated with a protamine supplement first, before fresh frozen plasma (FFP) is considered. Whereas prolonged prothrombin time is treated with FFP and cryoprecipitate, low platelet count should be corrected by platelet transfusion. A normal platelet count does not exclude platelet dysfunction, so a platelet transfusion may be indicated even in the presence of a normal platelet count if the patient had been treated by antiplatelet agents, is uremic, or is suspected of von Willebrand disease. In the latter patients, the use of desmopressin is warranted. The use of aprotinin, once commonly given to reduce bleeding in cardiac surgery, has been abandoned in view of its serious side effects.
Myocardial Dysfunction After Cardiac Surgery
Most cardiac interventions are done under cardiac arrest; the heart is isolated from the circulation and hence not perfused for some time, causing ischemia-reperfusion injury. Schematically, there is an overload of intracellular calcium during ischemia and generation of reactive oxygen species during reperfusion. This cellular environment is responsible for various protein activation, leading to depressed cellular contractility (myocardial stunning), apoptosis, or cell necrosis. Various forms of myocardial protection have been developed to prevent myocardial injury, including intermittent cross-clamping, cold crystalloid, or cold or warm blood cardioplegia. While cold blood cardioplegia is the most used technique worldwide, it should be emphasized that there is no definitive evidence favoring one strategy over another.37,38 Failing to protect the myocardium during surgery leads to 2% to 7% of diffuse ventricular failure. Although most patients will respond to inotropic support and recover global function after a few hours or a few days—depending on the extent of injury—patients with extensive apoptosis and necrosis evidenced as cardiac marker elevation will have a statistical survival impairment. Patients with preoperative cardiac dysfunction and diffuse coronary disease are more at risk for myocardial protection deficit. Segmental myocardial zones might be electively damaged in incomplete revascularization, technical failure to complete anastomoses, or distal disease impeding delivery of the cardioplegic solution. STEMI and NSTEMI may occur, requiring specific treatment, possibly including the need for coronary angiogram. Electrocardiographic (ECG) interpretation is difficult in the perioperative period, and biomarker assays are delayed.39 Liberal use of echocardiography is advised to discern segmental ischemia from diffuse dysfunction; any other causes of low cardiac output will be diagnosed along.
Treatment involves preload, afterload, and rhythm optimization and administration of inotropic agents like dobutamine and levosimendan.40 Should all these measures fail, the use of mechanical devices to support the circulation is indicated. All devices have advantages and risks. The first device generally considered is the intraaortic balloon pump (IABP). Contraindications include aortic regurgitation, dissecting thoracic aortic aneurysm, and synthetic thoracic aortic graft. At best, cardiac output may be increased by 20% by an IABP, depending on the extent of myocardial injury and preexisting myocardial function. If the IABP fails to increase cardiac output to a sufficient level, one should consider the insertion of an extracorporeal membrane oxygenation41,42 and/or a ventricular assist device.43,44 These supports generally require anticoagulation to avoid embolic complications and may induce severe hemorrhagic complications.
Right Ventricular Failure
In the absence of pulmonary hypertension, the right ventricle plays a marginal role at rest. This is illustrated by the Fontan operation (bypassing the right ventricle) as a successful operation in congenital surgery. Nevertheless, in adult cardiac surgery, pulmonary hypertension is common and can be due to intrinsic disease of the pulmonary vasculature (chronic obstructive pulmonary disease [COPD], etc.) or secondary to left-sided heart disease45; hence, patients with pulmonary hypertension, even mild forms, need acceptable right ventricular function to overcome this increased afterload. The right ventricle is more vulnerable to the aggressions of surgery because of less myocardial protection (anatomically less topical cooling, less retroplegic protection).46,47 Isolated right ventricular failure is observed, and treatment must be tailored: preload optimization (fluids should be administered to bring the central venous pressure up to 15-20 mm Hg); AV conduction through dual-chamber pacing or cardioversion; and postcharge control through avoiding hypoxemia, hypercarbia, acidosis, excessive positive end-expiratory pressure, or inspiratory plateau pressures over 30 mm Hg. One should consider the administration of inhaled nitric oxide (iNO) or prostanoids and the administration of inotropes like dobutamine. Right ventricular failure might also benefit from right ventricular assist devices.
Cardiac Arrhythmias
Ventricular epicardial pacing wires and often atrial wires are placed during the operation and left for up to 10 postoperative days to help in the treatment of arrhythmia through external pacing. Some patients with cardiac asynchrony and poor ventricles (LVEF < 30%) may benefit from biventricular pacing.48
Atrial Arrhythmias
Atrial fibrillation: after open heart procedures, up to 40% of patients may develop atrial fibrillation. Flutter is generally more difficult to treat than fibrillation. Age, previous history of atrial fibrillation, mitral valvular disease, increased left atrial size, right coronary disease, and previous cardiac surgery are risk factors for postoperative atrial fibrillation. Postoperative atrial fibrillation is related to an increase in in-hospital stroke (3.3% versus 1.4% with sinus rhythm) and an increase in long-term mortality in the CABG population, but these observations could be related to underlying comorbidities.49 Nevertheless, such observations have led to recommendations for prophylactic therapy before surgery or as soon as possible after surgery with beta-blockers in most patients. If beta-blockers are contraindicated, amiodarone can be used in high-risk patients (especially after mitral valve surgery and in those with a history of atrial fibrillation).50 Although prophylactic therapy decreases the incidence of atrial fibrillation by up to 60%, about one patient out of five will still develop atrial arrhythmias.51 If hemodynamic instability occurs, aggressive treatment is warranted and mostly achieved through electrical cardioversion with or without pharmacologic support (with ibutilide or amiodarone). If this therapy is ineffective, intravenous esmolol can be given to control heart rate.
In stable patients, spontaneous reversion to sinus rhythm is observed in 80% of patients and 90% by 8 weeks. One treatment option is rate control with beta-blockers and anticoagulation; if atrial fibrillation persists after 48 hours,52 rhythm control might be preferred when anticoagulation is to be avoided. Class Ia, (quinidine, procainamide) class Ic (propafenone, flecainide), and class III (ibutilide, dofetilide, amiodarone) are equivalent in reversing atrial fibrillation, but class Ia and Ic might increase the atrioventricular (AV) conduction rate transiently, inducing a badly tolerated rapid ventricular response before conversion to sinus rhythm occurs, hence class III should be preferred (except sotalol, considered less effective and not recommended).
A bolus of 5 mg/kg of amiodarone is given initially over 20 minutes, followed by 15 mg/kg during the first 24 hours. Oral therapy is continued for 3 months.53
Mediastinitis and Sternal Dehiscence
Wound complications and infections are uncommon in cardiac surgery and generally include sternal dehiscence and mediastinitis.54 Deep sternal wound infection occurs in 1% to 4% of patients after cardiac surgery and has an overall mortality of around 25%. Risk factors of mediastinitis are imperfect aseptic technique, prolonged operative time, harvesting both internal mammary arteries, undrained retrosternal hematoma, insecure sternal closure, obesity, diabetes mellitus, COPD, prolonged mechanical ventilation, long-term corticosteroid treatment, and male gender.55 Early diagnosis is one of the cornerstones in the management of mediastinitis. The gold-standard treatment in early diagnosed mediastinitis includes early radical débridement to remove all the infected tissue, and closed drainage techniques. Severe mediastinitis necessitates complete sternal resection and associated techniques using omental or bilateral pectoralis major flap transposition to achieve chest stabilization and restore pulmonary function.
Phrenic Nerve Injury and Paralysis
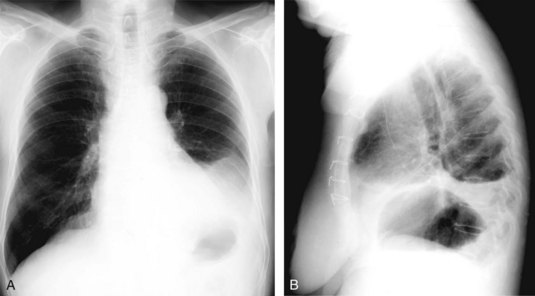
Phrenic nerve paralysis may enhance the risk of postoperative respiratory dysfunction (Figure 89-1). Consequences of postoperative phrenic nerve palsy range from asymptomatic radiographic abnormality, to severe respiratory failure requiring prolonged mechanical ventilation, to other associated morbidities and even mortality.
Transient or definitive phrenic nerve injury may be the result of internal mammary artery pedicle mobilization, ductus arteriosus closure, and aortic coarctation surgery. Reoperations in cardiac surgery enhance the risk of phrenic nerve injury, leading even to double phrenic nerve injury.56
Aortic Dissection After Cardiac Surgery
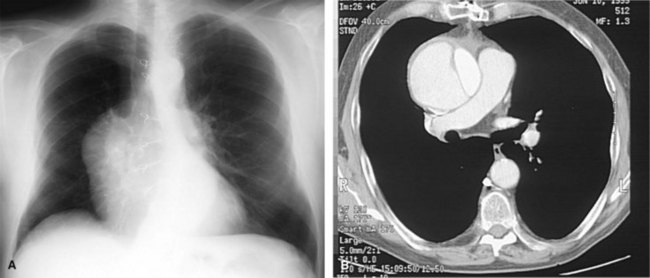
Acute aortic dissection after cardiac surgery is a feared complication in which the blood leaves the normal aortic channel, the true lumen, and dissects the media to produce a false lumen (Figure 89-2). Cardiac surgery also may lead to aortic dissection. Aorta cannulation or partial clamping in the presence of excessive aortic pressure may induce shear stress and subsequent intimal tears.57
Treatment
After diagnosis of an acute postoperative dissection, an aggressive surgical approach is mandatory. Surgery is performed to prevent death from hemorrhage and to reestablish blood flow in nonperfused organs. Limited ascending aortic replacement, associated with intimal tear resection, if any, is the standard procedure for a Stanford A dissection.29
Organ Dysfunction After Cardiac Surgery
Pulmonary Dysfunction
Risk factors for acute respiratory failure after cardiac surgery58 include older age (>60 years), pulmonary hypertension, COPD, hemodynamic pulmonary edema due to elevated left atrial pressure, prolonged mechanical ventilation, and phrenic nerve paralysis.59 Pulmonary dysfunction may lead to acute respiratory distress syndrome (ARDS). Mild pulmonary dysfunction generally resolves slowly after the patient is extubated and can be treated with ambulation and breathing exercises, but residual dysfunction may persist 10 days after operation. More severe cases are treated according to the underlying disease (infection, left heart failure, etc.).60
Neurologic Complications
After cardiac surgery under CPB, neurologic complications may be attributed to hypoxia, metabolic abnormalities, emboli, or hemorrhage. One may identity two types of complications, occurring with the same frequency61: a type 1 complication (3%) is a major focal deficit, stupor, or coma; and a type 2 complication (3%) is intellectual dysfunction. Cautious surgical technique to avoid microemboli shedding, avoidance of perioperative hypotension, placing a sterile ultrasound probe on the aorta to guide the cannulation site (epiaortic ultrasound), and preoperative carotid ultrasound (to screen for patients who could benefit from simultaneous carotid endarterectomy)62 can be useful to reduce the incidence of neurologic complications.
Renal Dysfunction
Up to 10 % of patients who undergo cardiac surgery with CPB develop renal dysfunction in the postoperative period,63 as defined by increases in serum creatinine levels above 2 mg/dL; 20% of these patients require dialysis. Overall mortality in those is 20%, and it may increase to 75% in patients who require dialysis. Predictive factors of renal dysfunction include advanced age, history of congestive heart failure, prior bypass surgery, type 1 diabetes, prior renal disease, and preoperative advanced renal dysfunction.64 The association between preoperative renal dysfunction and adverse events after cardiac surgery has been reported to be stronger if renal dysfunction is defined using creatinine clearance rather than the plasma creatinine concentration, particularly in patients with normal plasma creatinine levels.65
Key Points
Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875-883.
David TE, Feindel CM, Bos J. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J Thorac Cardiovasc Surg. 1995;109:345-351. discussion 351-2
Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877-S1880.
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-972.
1 Yusuf S, Zucker D, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563-570.
2 Patel MR, Dehmer GJ, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: A Report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53(6):530-553.
3 Serruys PW, Morice M-C, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972.
4 Hochman JS, Sleeper LA, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N. Engl. J. Med. 1999;341(9):625-634.
5 Mehta RH, Grab JD, et al. Clinical characteristics and in-hospital outcomes of patients with cardiogenic shock undergoing coronary artery bypass surgery: insights from the Society of Thoracic Surgeons National Cardiac Database. Circulation. 2008;117(7):876-885.
6 Rankin JS, Tuttle RH, et al. Techniques and benefits of multiple internal mammary artery bypass at 20 years of follow-up. Ann Thorac Surg. 2007;83(3):1008-1015.
7 Veeger NJ, Panday GF, et al. Excellent long-term clinical outcome after coronary artery bypass surgery using three pedicled arterial grafts in patients with three-vessel disease. Ann Thorac Surg. 2008;85(2):508-512.
8 Nasso G, Coppola R, et al. Arterial revascularization in primary coronary artery bypass grafting: Direct comparison of 4 strategies–results of the Stand-in-Y Mammary Study. J Thorac Cardiovasc Surg. 2009;137(5):1093-1100.
9 Shroyer AL, Grover FL, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361(19):1827-1837.
10 Srivastava S, Gadasalli S, et al. Beating heart totally endoscopic coronary artery bypass. Ann Thorac Surg. 2010;89(6):1873-1880.
11 Katz MR, Van Praet F, et al. Integrated coronary revascularization: percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation. 2006;114(1 Suppl):I473-I476.
12 Rosenhek R, Zilberszac R, et al. Natural History of Very Severe Aortic Stenosis. Circulation. 2010;121(1):151-156.
13 Bonow RO, Carabello BA, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(13):e1-142.
14 Levy F, Laurent M, et al. Aortic valve replacement for low-flow/low-gradient aortic stenosis: operative Risk Stratification and Long-Term Outcome: A European Multicenter Study. J Am Coll Cardiol. 2008;51(15):1466-1472.
15 Malaisrie SC, McCarthy PM, et al. Contemporary perioperative results of isolated aortic valve replacement for aortic stenosis. Ann Thorac Surg. 2010;89(3):751-756.
16 Yan TD, Cao C, et al. Transcatheter aortic valve implantation for high-risk patients with severe aortic stenosis: A systematic review. J Thorac Cardiovasc Surg. 2010;139(6):1519-1528.
17 David TE, Feindel CM, et al. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J Thorac Cardiovasc Surg. 1995;109(2):345-352.
18 Tirone ED, Christopher MF, et al. Long-term results of aortic valve-sparing operations for aortic root aneurysm. J Thorac Cardiovasc Surg. 2006;132:347-354.
19 le Polain de Waroux J-B, Pouleur A-C, et al. Functional anatomy of aortic regurgitation: accuracy, Prediction of Surgical Repairability, and Outcome Implications of Transesophageal Echocardiography. Circulation. 2007;116(11_suppl):I-264-9.
20 Enriquez-Sarano M, Avierinos J-F, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352(9):875-883.
21 Kang D-H, Kim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119(6):797-804.
22 Casselman FP, La Meir M, et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation. 2007;116(11 Suppl):I270-I275.
23 Svensson LG, Atik FA, et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J Thorac Cardiovasc Surg. 2010;139(4):926-932.
24 Braun J, van de Veire NR, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. 2008;85(2):430-436. discussion 436-7
25 Dreyfus GD, Corbi PJ, et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79(1):127-132.
26 Jose LN, Edward RN, et al. Surgical management of secondary tricuspid valve regurgitation: Annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg. 2010;139(6):1473-1482. e5
27 Tadros TM, Klein MD, et al. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119(6):880-890.
28 Wolak A, Gransar H, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. J Am Coll Cardiol Imaging. 2008;1(2):200-209.
29 American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease: Executive Summary. et al. J Am Coll Cardiol. 2010;55(14):1509-1544.
30 Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74(5):S1877-S1880.
31 Sun L-Z, Qi R-D, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: Experience with 107 patients. J Thorac Cardiovasc Surg. 2009;138(6):1358-1362.
32 Despotis GJ, Skubas NJ, et al. Optimal management of bleeding and transfusion in patients undergoing cardiac surgery. Semin Thorac Cardiovasc Surg. 1999;11(2):84-104.
33 Brown JR, Birkmeyer NJO, et al. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic Agents in cardiac surgery. Circulation. 2007;115(22):2801-2813.
34 Spiess BD. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8:267-281.
35 Koch CG, Li L, et al. Transfusion in Coronary Artery Bypass Grafting is Associated with Reduced Long-Term Survival. Ann Thorac Surg. 2006;81(5):1650-1657.
36 van Straten AHM, Bekker MWA, et al. Transfusion of red blood cells: the impact on short-term and long-term survival after coronary artery bypass grafting, a ten-year follow-up. Interact Cardiovasc Thorac Surg. 2010;10(1):37-42.
37 Flameng WJ, Herijgers P, et al. Continuous retrograde blood cardioplegia is associated with lower hospital mortality after heart valve surgery. J Thorac Cardiovasc Surg. 2003;125(1):121-125.
38 Braathen B, Tonnessen T. Cold blood cardioplegia reduces the increase in cardiac enzyme levels compared with cold crystalloid cardioplegia in patients undergoing aortic valve replacement for isolated aortic stenosis. J Thorac Cardiovasc Surg. 2010;139(4):874-880.
39 Steuer J, Horte LG, et al. Impact of perioperative myocardial injury on early and long-term outcome after coronary artery bypass grafting. Eur Heart J. 2002;23(15):1219-1227.
40 Mebazaa A, Pitsis A, et al. Clinical review: practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit Care. 2010;14(2):201.
41 Ko WJ, Lin CY, et al. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg. 2002;73(2):538-545.
42 Combes A, Leprince P, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404-1411. 10.1097/CCM.0b013e31816f7cf7
43 Hernandez AF, Grab JD, et al. A Decade of short-term outcomes in post cardiac surgery ventricular assist device implantation: data from the Society of Thoracic Surgeons’ National Cardiac Database. Circulation. 2007;116(6):606-612.
44 Haft JW, Pagani FD, et al. Short- and long-term survival of patients transferred to a tertiary care center on temporary extracorporeal circulatory support. Ann Thorac Surg. 2009;88(3):711-718.
45 Bogaard HJ, Abe K, et al. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135(3):794-804.
46 Haddad F, Ashley E, et al. New insights for the diagnosis and management of right ventricular failure, from molecular imaging to targeted right ventricular therapy. Curr Opin Cardiol. 2010;25(2):131-140.
47 Zong P, Tune JD, et al. Mechanisms of oxygen demand/supply balance in the right ventricle. Exp Biol Med (Maywood). 2005;230(8):507-519.
48 Eberhardt F, Heringlake M, et al. The effect of biventricular pacing after coronary artery bypass grafting: A prospective randomized trial of different pacing modes in patients with reduced left ventricular function. J Thorac Cardiovasc Surg. 2009;137(6):1461-1467.
49 Mathew JP, Fontes ML, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720-1729.
50 Echahidi N, Pibarot P, et al. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51(8):793-801.
51 Crystal E, Connolly SJ, et al. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation. 2002;106(1):75-80.
52 Kollar A, Lick SD, et al. Relationship of atrial fibrillation and stroke after coronary artery bypass graft surgery: When is Anticoagulation Indicated? Ann Thorac Surg. 2006;82(2):515-523.
53 Fuster V, Ryden LE, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation–Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2006;48(4):854-906.
54 El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg. 1996;61(3):1030-1036.
55 Risnes I, Abdelnoor M, et al. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg. 2010;89(5):1502-1509.
56 Tripp HF, Bolton JW. Phrenic nerve injury following cardiac surgery: a review. J Card Surg. 1998;13(3):218-223.
57 Jonker FHW, Schlosser FJV, et al. Management of type A aortic dissections: a meta-analysis of the literature. Ann Thorac Surg. 2010;89(6):2061-2066.
58 Filsoufi F, Rahmanian PB, et al. Logistic risk model predicting postoperative respiratory failure in patients undergoing valve surgery. Eur J Cardiothorac Surg. 2008;34(5):953-959.
59 Canver CC, Chanda J. Intraoperative and postoperative risk factors for respiratory failure after coronary bypass. Ann Thorac Surg. 2003;75(3):853-857.
60 Creagh-Brown BC, Griffiths MJ, et al. Bench-to-bedside review: Inhaled nitric oxide therapy in adults. Crit Care. 2009;13(3):221.
61 Roach GW, Kanchuger M, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335(25):1857-1863.
62 Akins CW, Hilgenberg AD, et al. Late results of combined carotid and coronary surgery using actual versus actuarial methodology. Ann Thorac Surg. 2005;80(6):2091-2097.
63 Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19-32.
64 Karkouti K, Wijeysundera DN, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119(4):495-502.
65 Wang F, Dupuis JY, et al. An analysis of the association between preoperative renal dysfunction and outcome in cardiac surgery: estimated creatinine clearance or plasma creatinine level as measures of renal function. Chest. 2003;124(5):1852-1862.