Chapter 86 Cardiac Resynchronization Therapy for Congestive Heart Failure
Physiological Basis, Technology, Indications, and Management
Over the past decade, treatment of heart failure has markedly improved with progress in pharmacologic treatment modalities.1–5 Rates of death from pump failure and sudden cardiac death (SCD) caused by ventricular tachyarrhythmic events have significantly declined.5,6 Hospitalizations for severe symptoms of heart failure have decreased after the use of angiotensin-converting enzyme inhibitors, β-blockers, diuretics, and spironolactone became more frequent. Heart transplantation is considered the therapy of choice for end-stage heart failure, but the limited availability of donor organs and the unresolved issue of tissue rejection after transplantation have stimulated research in other nonpharmacologic approaches to symptomatic heart failure, such as multi-site cardiac pacing and left ventricular assist device therapy.
Electrical Dyssynchrony and Heart Failure
A common pathophysiological finding in the failing heart is a delay in the spread of ventricular activation. This can be caused by structural abnormalities of the myocardium leading to asynchronous ventricular contraction, referred to as cardiac dyssynchrony.6 Cardiac dyssynchrony can result in inefficient ventricular performance (discussed later) and may be prevalent in 25% to more than 60% of patients with congestive heart failure.7 These structural changes can also provide an electrophysiological substrate that can support potentially life-threatening ventricular tachyarrhythmias. Ventricular conduction delay has been shown to be an independent risk marker for the development of heart failure and increased mortality rates.8–10
Pathophysiological Concepts Underlying Resynchronization Therapy
Electrical and Mechanical Abnormalities in Heart Failure
Abnormalities of electrical function in heart failure can occur singly or in combination in either atrioventricular (AV) conduction or interventricular or intraventricular conduction. Prolongation of the A-V interval is associated with impaired atrial contribution to ventricular filling and reduced diastolic ventricular filling. Intraventricular as well as interventricular conduction delays prolong the pre-ejection time and reduce the global and regional ventricular ejection fraction (EF) because of dyssynchronous mechanical contraction and relaxation patterns. This mechanical dysfunction interferes with mitral valve function and permits mitral regurgitation.11–14 One electrical indicator for delayed asynchronous ventricular contraction is the presence of a left bundle branch block (LBBB) pattern on the surface electrocardiogram (ECG). Ventricular conduction abnormalities, especially LBBB, may be responsible for the unequal regional distribution of ventricular work and wall stress.15–17 At the beginning of ventricular systole, the region of earliest ventricular activation (usually the interventricular septum) contracts against minimal workload because the remaining ventricular myocardium (usually the lateral and posterolateral left ventricular regions) is still in the relaxation period or in a nonactivated phase. The regions of early ventricular activation waste contraction energy because no effective intraventricular pressure can develop. In contrast, the delayed depolarized regions, that is, the lateral and posterolateral ventricular regions, must contract against a pre-existing stiffened portion of the ventricular wall (the septum). This generates increased wall stress with increased cardiac work. These changes in regional wall stress can contribute to myocyte damage, production of fibrous tissue, and development of regional hypertrophy and may induce regional apoptosis. The mechanical contractile dysfunction caused by asynchrony or dyssynchrony can be assessed by standard echocardiographic indexes, but greater precision is obtained with tissue Doppler imaging and magnetic resonance imaging.12,15–17
Mechanical synchrony between the atrium and the ventricle can also be disturbed when AV conduction is pathologically prolonged. A prolonged electromechanical A-V interval can be assessed by measuring the onset of the P wave of the surface ECG to the aortic valve closure. The mechanical A-V interval is always longer than the electrical A-V interval. This interval can be prolonged even in the absence of a prolonged P-R interval on the surface ECG. Prolongation of AV conduction reduces the active ventricular filling phase and shortens the passive diastolic filling, creating a ventriculoatrial (VA) gradient causing presystolic mitral regurgitation.13,14 A prolonged mechanical A-V interval is frequently found in patients with heart failure, even with an almost normal electrical A-V interval.18
Cardiac Resynchronization Using Pacing Techniques
LBBB causes delayed electrical activation and mechanical contraction of the lateral left ventricular wall, whereas the ventricular septum itself exhibits a paradoxic movement.19–22 Pacing electrode placement becomes a critical component of the extent of effective resynchronization achieved by CRT. For example, pre-excitation of the left lateral ventricular wall with atrio–bi-ventricular pacing in hearts with LBBB resynchronizes the ventricular contraction pattern by bypassing the conduction delay, resulting in improved ventricular contraction pattern and performance.20–23 It is conceivable that differing patterns of intraventricular conduction delay may also produce regional wall motion abnormalities that could be addressed with CRT. Optimizing AV synchrony is a critical element in obtaining good hemodynamic outcomes in these patients.
Atrioventricular Synchrony During Physiological Pacing
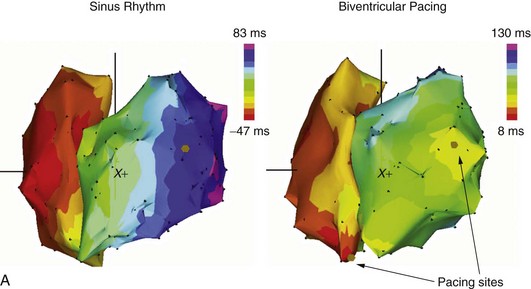
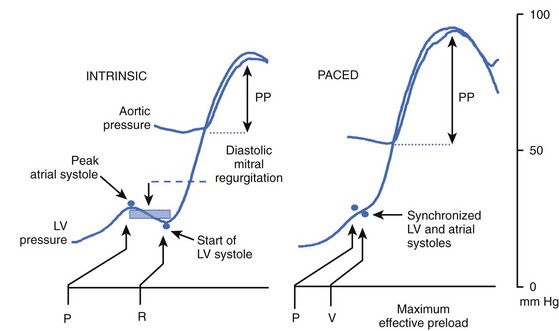
Programming the AV delay appropriately is essential for the improvement of hemodynamic performance with CRT. A very long AV delay will not support ventricular resynchronization because the atrial electrical impulse will follow the same route as during sinus rhythm without pacing. A very short AV delay, in contrast, will cause early depolarization at the site of left ventricular stimulation, leaving the ventricle partially or totally refractory by the time the regularly conducted impulse reaches this region. An AV delay between these two extremes causes a collision of two activation wavefronts: one coming from the regular His-Purkinje system and the other from the pre-excited ventricular activation. The region of collision depends on individual intraventricular and interventricular conduction properties and the left ventricular pacing site (Figure 86-1). Therefore appropriate timing with respect to the AV delay, as well as the left lateral ventricular stimulus, is crucial for achieving the hemodynamic benefits of the resynchronized ventricular contraction pattern in a failing heart.24,25 A nonphysiological short electrical A-V interval must be used with AV sequential bi-ventricular pacing to avoid AV asynchrony and to reduce presystolic mitral regurgitation. The discrepancy between electrical and mechanical AV sequences is most likely caused by a prolonged intraventricular conduction time. In patients with LBBB, the onset of the electrical depolarization of the left ventricular free wall is significantly delayed. This causes delayed mechanical onset of the left ventricular systole. Consequently, AV sequential pacing with a shortened AV delay is able to restore an adequate mechanical AV synchrony.24,25 Maximal hemodynamic benefit is achieved when the peak of the atrial pressure curve coincides with the onset of the mechanical ventricular systole (Figure 86-2).
Effect of Bundle Branch Block and Pacing Electrode Location on Efficacy of Resynchronization Therapy
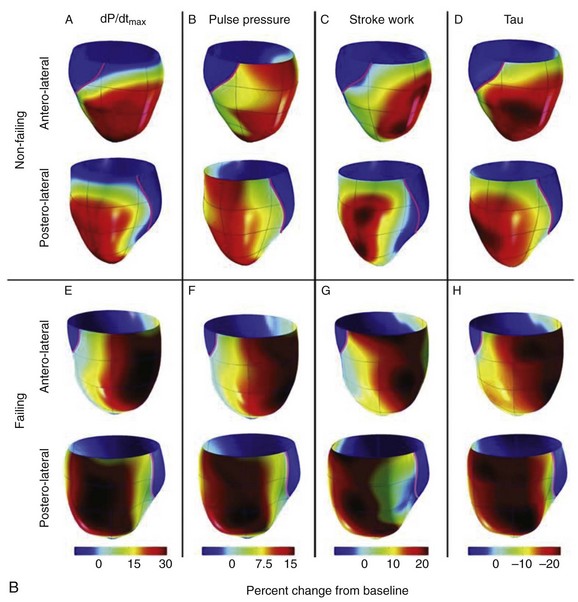
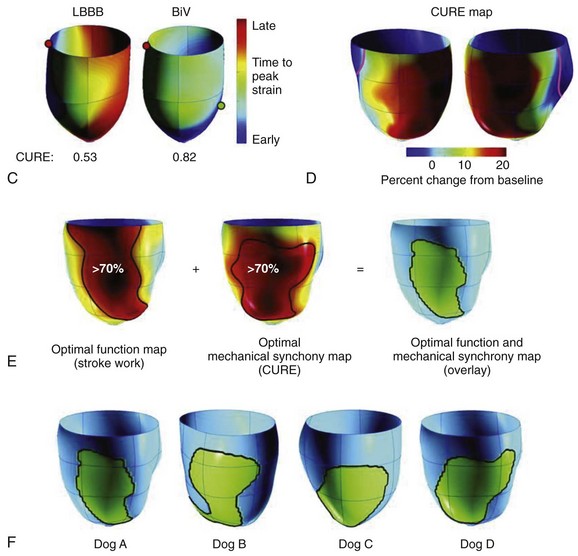
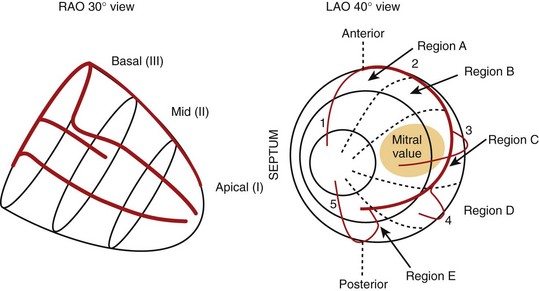
In early experimental studies, Verbeek et al demonstrated that the induction of LBBB produced mechanical dyssynchrony, which could be improved by bi-ventricular pacing.26 In normal or rapid pacing–induced failing canine hearts, Helms et al performed either left ventricular pacing or left bundle ablation and CRT using a fixed right ventricular pacing site and randomly selected left ventricular pacing sites covering the entire free wall (see Figure 86-1, B).27 Cardiac stroke volume was measured with a conductance catheter, and mechanical synchrony was evaluated by using magnetic resonance imaging tagging. In this model, right bundle branch block (RBBB) was associated with a lesser degree of cardiac dyssynchrony compared with LBBB. Three-dimensional maps showed that optimal CRT was achieved from lateral left ventricular wall sites, which were slightly more anterior than posterior and more apical than basal (see Figure 86-1).27 Left ventricular sites yielding a 70% or greater increase in dP/dTmax covered approximately 43% of the left ventricular free wall. In this model, their distribution and size were similar in both normal and failing hearts. Controversy has swirled around the benefits of left ventricular pacing alone versus bi-ventricular pacing in LBBB. In some clinical studies, the concept of tailored CRT has been advanced, but better imaging approaches such as three-dimensional echocardiography, intracardiac echocardiography, or other methods may be needed in clinical practice to prove this concept.28–30
Effect of Resynchronization Therapy on Mitral Regurgitation
Patients with symptoms of heart failure and a dilated left ventricle (LV) often demonstrate moderate or even severe mitral regurgitation. This is often seen in patients with LBBB. The delay in regional ventricular activation caused by intercellular fibrosis enhances mechanical asynchrony between different ventricular regions and can involve both papillary muscles.25 Geometric distortion in the dilated LV and delayed left ventricular free wall activation further decrease the timely closure of mitral leaflets along with an increased tethering of the mitral apparatus.23,25
Pacing from the left lateral wall, especially from the proximity of the posterior papillary muscle, diminishes conduction delay and decreases mitral regurgitation. Mean capillary wedge pressure drops significantly with left ventricular free wall pacing, whereas systolic blood pressure rises. The altered ventricular geometry of the dilated failing ventricle results in incomplete closure of the mitral valve at the onset of ventricular contraction causing early systolic mitral regurgitation. Therefore, shortening of the A-V interval during AV sequential pacing, along with left ventricular pre-excitation by left ventricular pacing, diminishes or even abolishes mitral regurgitation.31
Clinical Results of Cardiac Resynchronization Therapy
Short-Term Results
Acute hemodynamic testing has demonstrated that the type of intraventricular conduction block and the pacing site location are the primary determinants of hemodynamic benefits. In addition, short-term data suggest a dichotomous behavior in patients who present with a QRS duration between 120 and 150 ms.23 Patients with a QRS duration of greater than 150 ms showed the largest hemodynamic benefit. The Pacing Therapies for Congestive Heart Failure (PATH-CHF) trial results and data from Kass and colleagues suggest that patients with LBBB and diffuse intraventricular conduction delay tend to benefit more from bi-ventricular pacing than from right ventricular pacing.21,23 Atrial synchronous left or bi-ventricular stimulation at a nominal AV delay is significantly more beneficial than is right ventricular pacing alone. Parameters of acute systolic function significantly improved during pacing of the left ventricular free wall alone or synchronously with the right ventricle (RV) but not by pacing the right ventricular apex or septum alone. Pressure-volume loops of the LV show that in patients with LBBB, left ventricular pacing but not right ventricular pacing, increased the stroke volume while minimally affecting the end-diastolic volume. Pulmonary capillary wedge pressure dropped significantly with left ventricular pacing and bi-ventricular pacing but not with right ventricular pacing alone. Resynchronization of ventricular contraction improves mechanical performance with a net decrease in myocardial energy consumption.22 Acute benefits of resynchronization therapy depend on the A-V interval for each ventricular pacing site, with the shortest and longest AV delays being suboptimal.23 In general, a range of AV delay around 100 ms produced the most beneficial hemodynamic effect. However, a large variability was present in the optimal AV delay during sequential right ventricular pacing, ranging from 50 to 120 ms, and during bi-ventricular stimulation, ranging from 100 to 150 ms.32
Intermediate-Term Results
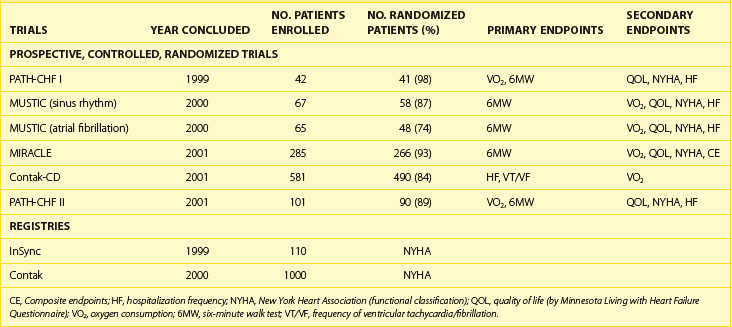
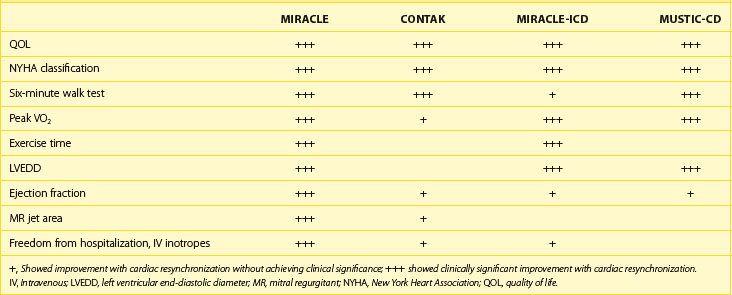
In the short term, hemodynamic results of bi-ventricular or left ventricular pacing alone clearly demonstrate hemodynamic improvement, but it is necessary to show that this translates into symptomatic improvement and eventually survival benefit.33–40 Data from several prospective randomized studies on CRT pacing (CRT-P) and on CRT with defibrillator therapy (CRT-D) (Table 86-1) and large patient registries have been very encouraging in the intermediate term. The majority of patients enrolled in these randomized studies had severely impaired functional capacity (New York Heart Association [NYHA] heart failure classes III to IV), left ventricular systolic dysfunction (EF <35%), a wide QRS complex (>120 ms) and, in most cases, LBBB. None of the patients had conventional indications for pacing therapy. The Multi-site Stimulation in Cardiomyopathy (MUSTIC) study, the Contak-CD trial, and the Multicenter InSync Randomized Chronic Evaluation (MIRACLE) study exclusively assessed the role of bi-ventricular stimulation in patients with heart failure. The PATH-CHF I study was performed to address the question of whether acutely optimized atrial-synchronous ventricular stimulation (right ventricular, left ventricular, or bi-ventricular stimulation based on acute hemodynamic evaluation) reduces heart failure in patients with intraventricular conduction defects. In these studies, CRT increased exercise tolerance, improved quality of life, and reduced hospitalization (Table 86-2).23,25,33–35 Oxygen consumption at maximal exercise capacity increased, on average, from 11 to 12 mL/kg/min before pacing to 15 to 16 mL/kg/min after 3 to 6 months of pacing (see Table 86-2). The 6-minute walk test, a generally accepted parameter of physical exercise capacity, increased on average by 10% to 15%, and patients showed a positive improvement in quality of life. Nearly two thirds of patients who underwent bi-ventricular therapy improved to NYHA class I or II from class III or IV. Patients in whom the CRT device was turned on were hospitalized less frequently and needed fewer days in the hospital for worsening of heart failure.
Table 86-2 Summary of Early North American Long-Term Clinical Studies Evaluating Cardiac Resynchronization
Objective assessment of autonomic and neurohormonal systems has shown the beneficial effects of CRT. Changes in heart rate variability (HRV) and resting heart rate reflect changes of the autonomic nervous system. The PATH-CHF I study showed that resting heart rate was significantly reduced after 3 months of pacing. HRV increased during CRT, whereas during the CRT-off phase, an almost complete reversion to baseline values was observed. In the Vigor in Congestive Heart Failure (VIGOR-CHF) study, a significant reduction of the norepinephrine plasma level after 16 weeks of continuous bi-ventricular stimulation was seen, which also confirmed the positive effect of CRT on neurohumoral activation.36
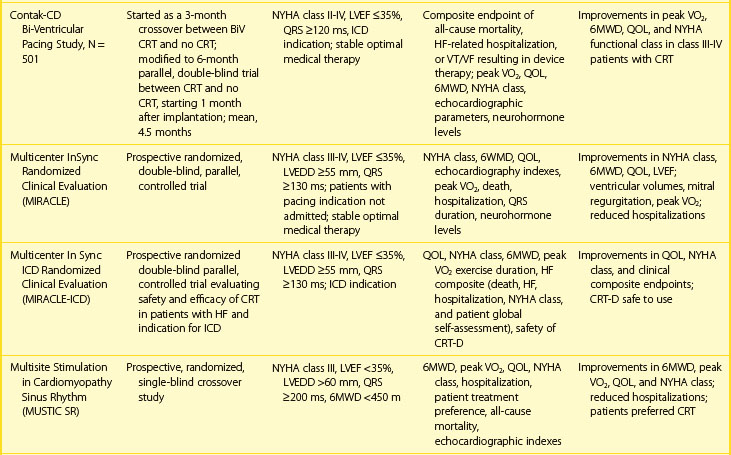
The Contak-CD study, which is considered one of the first-generation CRT-D studies, differed from many other studies of CRT by including patients with NYHA class I indication for an implantable cardioverte-defibrillator (ICD).34 A reduction of 21% occurred in the overall combined endpoint in Contak-CD, which was not statistically significant (P = .17). This may be attributed to the fact that the relatively large proportion of patients in NYHA class II enrolled in Contak-CD did not show a mortality benefit from CRT-D. Nevertheless, CRT-D in the Contak-CD trial was associated with fewer deaths (23% relative risk [RR] reduction), a lower hospitalization rate (13% RR reduction), and a smaller proportion of patients with worsening heart failure (26% RR reduction). Ventricular tachyarrhythmias were only modestly reduced in some studies of patients receiving CRT.34,40 All patients showed a significant increase in peak oxygen consumption. Patients in an advanced functional class (NYHA class III or IV) showed double the average increase of oxygen consumption. Longer term outcomes of CRT in the ICD subpopulations are available from the recently completed Multicenter Automatic Defibrillator Implantation Trial (MADIT)-CRT and Resynchronization/Defibrillation for Ambulatory Heart Failure Trial (RAFT) studies (discussed later).37,38
Although the effect of CRT on the diseased myocardial structure is still not completely understood, it is now well accepted that CRT can lead to reverse remodeling with a significant reduction of left ventricular diameters within 6 to 12 months. CRT is able to reduce abnormal myocardial strain distribution and induce reverse remodeling with a significant decrease of left ventricular volume within the first 6 months after initiation of CRT.39 These effects may be attributed to a direct reduction of regional wall stress or a reduction of increased oxygen demand of the asynchronously contracting ventricles. It is possible, however, that at a critical size of the left ventricular end-diastolic or end-systolic volume, reverse remodeling cannot be achieved. A beneficial effect is also achieved with a decrease in mitral regurgitation.
Many patients with heart failure are not candidates for CRT; the current indications for CRT are limited to patients who fulfill the disease and selection criteria outlined in the practice guidelines, which are largely based on clinical trials. The type of conduction delays, typically RBBB or LBBB or nonspecific intraventricular conduction delays, may play an important role in predicting a beneficial effect of pacing. Although morphologic ECG features may be similar in patients with either RBBB or LBBB, left ventricular electrical activation sequences may differ. The precise spread of activation may only be assessed with detailed high resolution invasive electroanatomic mapping.41 This, however, is difficult to perform in daily practice. In some cases, it may, indeed, help to select the optimal left ventricular pacing site by accurately detecting the region(s) of delayed left ventricular activation that may be optimal left ventricular pacing sites for CRT. Another approach that has been recently proposed uses intracardiac echocardiography intraoperatively to guide left ventricular pacing to maximize acute improvement in left ventricular EF (LVEF) with varying left ventricular lead position and AV interval programming. Reduction in nonresponder rates has been suggested with this approach, but a large clinical trial is awaited.30
Long-Term Clinical Outcomes of Cardiac Resynchronization Therapy
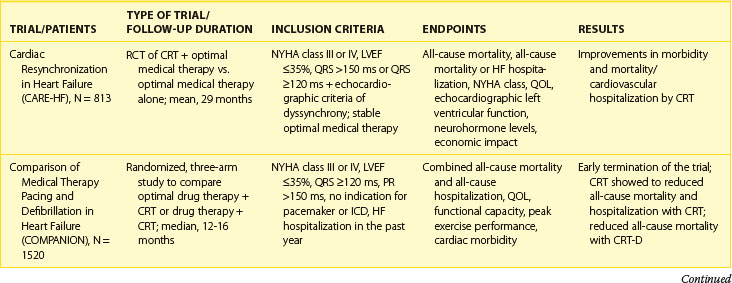
Second-generation prospective, randomized, controlled studies have examined the effect of CRT and CRT-D on clinical outcomes, morbidity, and disease progression.42–45 Several have examined the effect of CRT-P (exclusively bi-ventricular pacing) and CRT-D (bi-ventricular pacing with ICD) therapy on outcome and morbidity. The Comparison of Medical Therapy, Pacing and Defibrillation in Chronic Heart Failure (COMPANION) trial was a multi-center trial evaluating the effect of CRT on mortality, morbidity, and exercise performance in symptomatic heart failure patients without ICD indications.43 The study randomized patients in NYHA class III or IV with an LVEF of less than 35% and a prolonged QRS duration (>120 ms) and left ventricular dilation (left ventricular end-diastolic diameter >60 mm). This trial showed that CRT reduced the composite endpoint of death or hospitalization for major cardiovascular event by 12%. However, significant mortality reduction was achieved only in the CRT-ICD arm. Similarly, Cardiac Resynchronization in Heart Failure (CARE-HF) was a mortality and morbidity trial that included patients with NYHA class III or IV heart failure, an LVEF of less than 35%, and a prolonged QRS duration (>150 ms or >120 ms with echocardiographic criteria of dyssynchrony on optimal medical therapy).43 CRT plus optimal pharmacologic treatment was compared with pharmacologic treatment alone. Over a mean follow-up of 29 months, the CARE-HF study showed significant reduction of 37% for the composite endpoint of death or hospitalization for a major cardiovascular event, and a 46% reduction in SCD. CRT-P also reduced mortality rate from 30% in the medical therapy group to 20% in the CRT-P group (hazard ratio [HR], 0.64; P < .002). Reductions in mortality rate from heart failure (HR, 0.55; P = .003) and SCD (HR, 0.54; P < .006) were seen compared with medical therapy. Compared with the control group, the CRT group showed significant improvements in indexes of left ventricular function, symptoms, and quality of life. Longer term follow-up in this study has confirmed long-term benefits in both mortality and morbidity.44
The impact of CRT on ventricular remodeling and function was assessed in the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study.39,45 At 12 months, the clinical composite response endpoint, which measured disease progression, rose by 16% with CRT-on compared with 21% in CRT-off (P = .10). However, the patients assigned to CRT-on experienced a greater improvement in left ventricular end-systolic volume index (−18.4 + 29.5 mL/m2 vs. −1.3 + 23.4 mL/m2; P < .0001) and other measures of left ventricular remodeling. Time to first heart failure hospitalization was significantly delayed in CRT-on (HR, 0.47; P = .03).
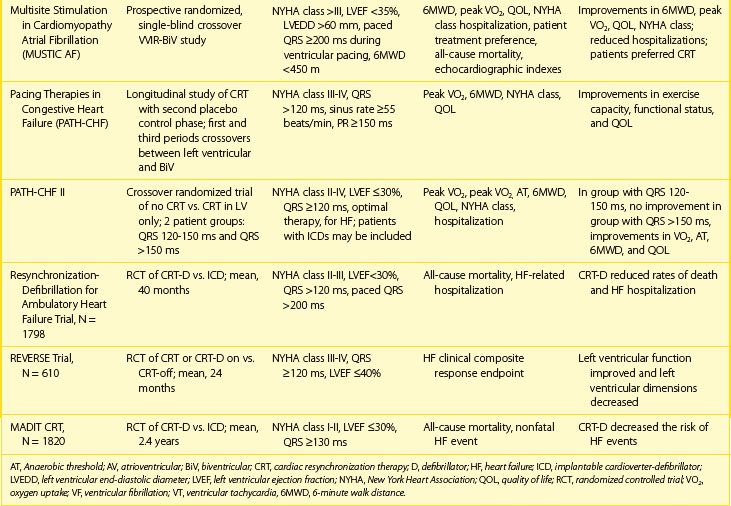
The MADIT-CRT study evaluated the additional beneficial effect of CRT on the occurrence of heart failure or death in patients with moderate to severe left ventricular systolic dysfunction, with minimal or no symptoms of heart failure and a QRS width of 130 ms (Table 86-3).37 Patients with CRT-D were compared with those receiving ICD-only therapy. CRT provided an additional 34% risk reduction in the primary endpoint after a mean follow-up of 2.4 years. The benefit of CRT was mainly attributable to a 41% reduction of heart failure. Patients with LBBB and women showed the most benefit, whereas patients with RBBB and intraventricular conduction delay demonstrated no benefit. Patients with ischemic and nonischemic cardiomyopathy derived similar benefits. Evidence of left ventricular reverse remodeling was evident as marked reduction in left ventricular volumes and an increase in LVEF with CRT-D. Overall mortality rate was not significantly reduced, which may be a result of follow-up duration. Similar findings were observed in RAFT. (see Table 86-3).38 This trial had a longer follow-up of the enrolled patients and reported a reduction of overall mortality rate. These trials suggest a potential for an early role of CRT in the prevention of progression of heart failure.
Cardiac Resynchronization Therapy with Defibrillator Therapy
Controversy has swirled on the need for defibrillation therapy in patients receiving CRT therapy. Death in patients with heart failure can result from a variety of causes other than mechanical pump failure. SCD in heart failure is a catastrophic event. The incidence increases from 2% to 6% per year in patients with NYHA class II symptoms and up to 24% per year for patients with class III or IV symptoms.46 Mechanisms of SCD can include ventricular tachycardia,ventricular fibrillation, bradycardia- or tachycardia-dependent polymorphic ventricular tachycardia, primary bradyarrhythmias, and conduction disturbances resulting in asystolic cardiac arrest and electrical mechanical dissociation or pulseless electrical activity. Early ICD trials did not systematically select patients with advanced heart failure despite observational data and clinical trial subgroup analyses that suggested major benefits accruing with appropriate ICD use in patients with severe left ventricular dysfunction.47–52 In the Antiarrhythmics Versus Implantable Defibrillators (AVID) study, ICD survival benefit was restricted to patients with an EF below 35%.50 The survival benefit in the MADIT I study was almost entirely confined to the ICD group with an EF below 26%.51 The Canadian Implantable Defibrillator Study (CIDS) showed the greatest benefit was derived by patients in the highest risk quartiles, that is, those with a low EF and a poorer NYHA functional classification.52
In early CRT studies, SCD rates ranged from 33% to 47%. In examining modes of death in the CRT-only group in the CARE-HF study, SCD was observed in 32 of 101 deaths during the extended follow-up, and a certain proportion of these deaths would be deemed preventable by ICD therapy.43,44 The Multicenter Longitudinal Observational Study (MILOS) group reported their analysis of the long-term outcomes of 1303 patients treated with CRT alone, CRT-P, or CRT-D.53 The cumulative event-free survival rates were 92% and 56% at 1 and 5 years, respectively; and the cumulative incidence rates of death from heart failure and SCD were 25.1% and 9.5%, respectively. CRT-D was associated with a 20% decrease in mortality rate, and its protective effect against SCD was highly significant (P < .002).
Dual-chamber ICD devices have traditionally been used in these patients, but some pacing features may be deleterious. Data from the Dual Chamber and VVI Implantable Defibrillator (DAVID) study suggest that adverse physiological effects and outcomes result from chronic right ventricular apical pacing.54 CRT can be considered when intraventricular conduction disturbances, as previously discussed, are present. However, the use of CRT in ICD devices is primarily based on the assumption that prevention of SCD in heart failure populations will provide survival benefits not seen with pacing alone. Table 86-3 summarizes the experience with CRT-D therapy in multi-center clinical trials in this population. Initial experience with an ICD incorporating ventricular resynchronization therapy was assessed in a prospective study using the InSync model 7272 ICD (Medtronic Inc., Minneapolis, MN).55 Significant improvement of heart failure symptoms and left ventricular dimensions were seen in these patients, particularly those in NYHA classes III and IV. Patients showed improvement in the 6-minute walk test at 3 and 6 months. All ventricular tachyarrhythmias were correctly identified, and double counting of sensed QRS events did not occur. In the Contak-CD trial, patients with class I indication for ICD therapy and NYHA class II or more heart failure had a 21% reduction of overall mortality rate (nonsignificant).
Table 86-3 summarizes the long-term outcomes of major CRT trials. In the COMPANION study, CRT alone improved NYHA class and quality of life and reduced hospitalizations for heart failure. However, significant mortality rate reduction was achieved only in the CRT-ICD arm. These data are consistent with the original pacing trials in this population, which suggested that SCD can limit the benefits achieved with CRT and challenges the notion that CRT, per se, reduced SCD. However, the delayed separation (after 9 months) of mortality curves (P = .12) between the pacing and medical therapy arms in this study raises the possibility of ventricular remodeling trending to improving survival. This is supported by long-term follow-up in CARE-HF. Over a mean follow-up of 29 months, CRT reduced mortality rates from 30% in the medical therapy group to 20% in the CRT group (HR, 0.64; P < .002), with a reduction in SCD rates (HR, 0.54; P = .005).44
Indications for Cardiac Resynchronization Therapy
CRT was originally recommended as heart failure therapy to reduce mortality and morbidity in patients with class III and IV heart failure with markers of ventricular dyssynchrony. For clinical practice purposes, early patient selection criteria are enumerated in Box 86-1. It is important to state that CRT is adjunctive therapy to medical therapy for heart failure and requires careful monitored prescription to achieve benefit. CRT is not a “stand alone” therapy or a “replacement” therapy for medical therapy in patients with heart failure. CRT should always be an additional step in therapy when drug therapy is unable to relieve symptoms or improve quality of life. Medical therapy of heart failure, as it is currently recommended in various guidelines, should be thoroughly tried before CRT is initiated. A careful titration—lasting over months—of angiotensin-converting enzyme inhibitors, β-blocking agents, and diuretic compounds, including aldosterone antagonists, is mandatory and should be continued after initiating CRT. The currently accepted American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines for CRT device implantations in patients with heart failure are given in Box 86-2.56
Box 86-1 Patient Selection for Resynchronization Therapy in 2004
Box 86-2 Indications for Cardiac Resynchronization Therapy
Class IIa
The recently updated European Society of Cardiology (ESC) guidelines now recommend the use of CRT-D in patients with NYHA class II heart failure, in sinus rhythm, and with QRS duration greater than 150 ms and an LVEF less than 35% to reduce morbidity and prevent disease progression.57 In patients with atrial fibrillation (AF), CRT should be considered to reduce morbidity in those with NYHA class III or IV heart failure, an LVEF less than 35% on optimal medical therapy, and QRS duration greater than 130 ms, and in those with a slow ventricular rate or in whom AV junctional ablation has been performed to create pacemaker dependency. In patients with indications for pacemaker therapy, CRT is recommended to reduce morbidity in those with class III or IV heart failure, an LVEF less than 35%, and a QRS duration greater than120 ms. It may be considered in patients without a prolonged QRS interval (class IIa indication, but level C evidence).
Cardiac Resynchronization Therapy in Atrial Fibrillation Populations with Heart Failure
The complex association between AF and heart failure, with relationships to both cause and effect, makes this a challenging patient population for CRT therapy. Right ventricular pacing can be deleterious in the AF population, as also seen in the heart failure population, with an increase in AF recurrences as well as in atrial and ventricular dilatation.58,59 AF prevents normal sequential AV relationships, and rapid intrinsic AV nodal conduction can result in inconsistent delivery of CRT therapy. Thus the true benefits of CRT may not be evident or appreciated in patients with AF. However, the benefits of CRT in this population have been achieved in several studies, as suggested by a recent metanalysis.60–63 One consistent feature of these studies is achieving a high percentage of bi-ventricular pacing with rate control. Reversion to sinus rhythm or rhythm control with atrial pacing and antiarrhythmic therapy should also be attempted. In MUSTIC-AF, a single-blind, randomized, controlled, crossover study, significant improvement was observed in the 6-minute walk distance (9.3%) and peak oxygen uptake (13%) in patients treated with bi-ventricular pacing compared with those treated with conventional single-chamber ventricular demand (VVIR) pacing. Furthermore, hospitalization decreased by 70% and 85%, respectively, in the two groups during the bi-ventricular pacing period, with patients preferring this mode based on symptoms.60 AV junction ablation may provide control of ventricular rate for CRT. In clinical trials, the strategy of AV nodal ablation and CRT provided greater benefit in patients with depressed left ventricular function and prevented further deterioration of left ventricular systolic function compared with conventional pacing.61 AV nodal ablation was independently associated with survival benefit from death (HR, 0.13; 95% confidence interval [CI], 0.03 to 0.58; P = .007) and from combined death, heart transplantation, and left ventricular assisted device (HR, 0.19; 95% CI, 0.06 to 0.62; P = .006) after CRT.62 The 2010 ESC update and ACC/AHA/HRS guidelines have included CRT therapy for patients with AF and class III or IV refractory heart failure and interventricular conduction delay greater than 130 ms.57
Cardiac Resynchronization Therapy in Recipients of Implantable Cardioverter Defibrillators
Prior and current guidelines for ICD implantation in patients with severely depressed ventricular function are also applicable to candidates for CRT.64,65 Standard ICD indications (secondary or primary prevention of SCD) can also be applied once the patient has become a candidate for CRT. The incremental benefits of CRT-D therapy in candidates for each therapy have been individually debated. Some large clinical trials have looked at these issues. Most recently, the need for early CRT therapy in patients with ICDs has been evaluated by the MADIT-CRT and RAFT studies. Both studies provide evidence of favorable effects of CRT in this population on heart failure events, left ventricular function and, in the RAFT trial, mortality benefits. These benefits were most evident in patients with a QRS duration of greater than 150 ms regardless of the etiology of the cardiomyopathy.
Cardiac Resynchronization Therapy in Patients with Heart Failure and Normal QRS Complex
CRT has now been applied in patients with mechanical dyssynchrony without ventricular conduction delay.66 Evidence of a consistent beneficial role of CRT in patients with narrow QRS complexes is not evident, despite the observation that some of these patients can have mechanical dyssynchrony. Several small trials and a meta-analysis of some of these trials suggested that CRT resulted in significant improvement in the parameters of NYHA heart failure class, LVEF, and 6-minute walk distance in patients with heart failure, mechanical dyssynchrony, and narrow QRS complexes.67–72
However, the Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS (ReThinQ study) did not substantiate the role of CRT in this population.73 In this large randomized, controlled study, patients who had a standard indication for ICD (ischemic or nonischemic cardiomyopathy with LVEF <35%), NYHA class III symptoms, a QRS duration of less than 130 ms, and evidence of mechanical dyssynchrony measured on echocardiography were enrolled. No improvement was observed with CRT in the endpoints of improvement in peak oxygen consumption, Minnesota Living with Heart Failure Questionnaire score, 6-minute walk test distance, left ventricular volumes, or LVEF at 6 months.
Cardiac Resynchronization Therapy in Patients with Right Bundle Branch Block
CRT therapy has been attempted in patients with RBBB. Detailed high-resolution invasive electroanatomic mapping of RV and LV activation patterns in the presence of RBBB and LBBB has shown a similar degree of left ventricular activation delay in patients with heart failure and LBBB or RBBB.41 Patients with RBBB have a larger degree of right-sided conduction delay compared with patients with LBBB. Based on this premise, CRT has been advocated in patients with RBBB and heart failure.74–76 Earlier data from a single center indicated that only patients with RBBB associated with echocardiographically detected left-sided intraventricular dyssynchrony of a major degree may respond to CRT. In the MIRACLE study, CRT showed functional benefits in patients with RBBB, most of whom also had left anterior hemi-block. A pooled analysis from the MIRACLE and Contak-CD trials demonstrated significant benefit of CRT in patients with RBBB with respect to NYHA class at 6 months. However, concern regarding inconsistent response to CRT in these patients remains and is an area of active investigation.
Technical Aspects of Cardiac Resynchronization Therapy
Implant Technique
The implantation technique for CRT devices does not differ from the currently used technique for standard pacemakers or ICDs (see Videos 86-1 to 86-6 on the Expert Consult site accompanying this text). The right or left subclavian or cephalic vein approach is used for the insertion of a conventional right atrial and right ventricular lead. An additional lead is required for pacing of the LV. Initially, the left ventricular lead was positioned on the epicardial surface of the left ventricular wall via a small left lateral thoracotomy. Alternative percutaneous techniques have been developed to insert the lead via the coronary sinus into the coronary veins. The left lateral wall is currently stimulated epicardially from the coronary vein. The anatomy of the coronary vein is therefore crucial for correct positioning of the left ventricular lead to achieve the most beneficial effect of resynchronization. The sequence of insertion of the three leads is not standardized and is based on personal experience. However, as most patients requiring CRT devices have significant His-Purkinje dysfunction, including LBBB, they are at a risk of developing complete heart block with catheter and sheath manipulation. In general, we prefer, and recommend, insertion of the active fixation pacing lead with or without defibrillation electrodes in the RV as the initial lead. For placement of the left ventricular lead, a preshaped long introducer or guiding catheter, which stabilizes the lead while introducing it into the coronary sinus, is necessary.28,32,77–79 Severe dilation of the atrial or ventricular chambers may modify the usual position of the coronary sinus os and its anatomic course, so extensive and prolonged manipulation of the guiding catheter or different approaches may be required.
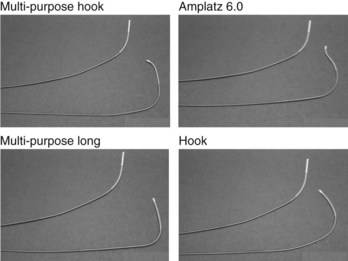
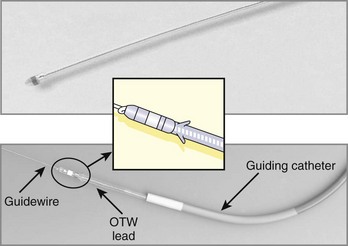
We preload the guiding catheter with a flexible 0.038-inch coated guidewire (e.g., Terumo guidewire), which is used for exploring the inferoseptal portion of the right atrium and facilitates the atraumatic insertion of a large (8 to 10 Fr) guiding catheter into the coronary sinus. A series of guiding catheters with different shapes is available to adapt to the anatomic variation of the coronary sinus (Figure 86-3). Other investigators preload the guiding catheter with a steerable catheter used for electrophysiological study (EPS). These catheters have deflecting tips to shape different curves and have been shown to reduce the time to coronary sinus cannulation.80
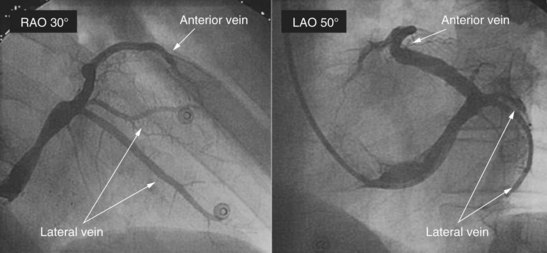
Major obstacles for cannulating the coronary sinus may be a severely dilated right atrium, a thick lamina cribrosa, or a large eustachian valve. We have occasionally seen narrowing of the body of the coronary sinus, usually in patients after previous open heart surgery (e.g., mitral valve surgery). Once the guiding catheter is inserted, occlusive angiography by an inflated large balloon catheter is highly recommended for better evaluation of the coronary vein anatomy (Figure 86-4; see Videos 86-1 and 86-2). Particular attention should be paid when performing balloon occlusion retrograde angiography of the coronary sinus and coronary vein. Inappropriate sizing of the balloon or inflation of the balloon at the most proximal portion of a side vein can produce endothelial damage or extensive intimal lesions, or even rupture of the coronary vein. These dramatic complications are rare—in the range of 1% to 3%. Radiographic examination from at least two different plane views should be performed (right anterior oblique 30 degrees and left anterior oblique 30 to 40 degrees; see Videos 86-3 to 86-6). Additional radiographic examinations (right anterior oblique 25 degrees, caudal 25 degrees, or anteroposterior view) are suggested when a tortuous or small lateral coronary vein (<2.5 mm in diameter) is noted (Figure 86-5). If the occlusive balloon is placed more distally in the body of the coronary sinus, it may be necessary to run longer cine loops to adequately visualize the branches (e.g., the middle cardiac and interventricular veins) that drain close to the os. In any instance, multiple views are needed to obtain anatomic delineation with standard techniques.
Alternative methods for performing coronary venography include high-speed rotational angiography. Using one of these methods, a strategy for left ventricular electrode placement should be developed and implemented at the procedure. The ESC has developed a classification for lead placement (Figure 86-6).28 This classification identifies the location of the left ventricular lead and provides a basis for standardized reporting. Careful examination of coronary vein anatomy often reveals one or more large left ventricular lateral or posterolateral veins. These are the target veins for permanent lead implantation. Investigative interest in left ventricular endocardial pacing is currently growing.81,82 Hemodynamic advantages have been demonstrated acutely compared with epicardial left ventricular pacing in humans.81 Improved systolic performance and cardiac output have been noted, and efforts at long-term lead placement are now being reported. However, this technique remains in development for general clinical use at this time.
Lead Technology
Early attempts to pace from the coronary veins were done by using either standard endocardial leads, which were modified for coronary venous placement by removing the tines or used for other coronary sinus techniques.31,83,84 It was soon realized that specifically designed pacing catheters were necessary to better navigate the coronary sinus and to insert leads into the coronary veins long term. To achieve this, innovative coronary venous lead systems that could incorporate components, accessories, and elements patterned after angioplasty devices were designed (Figure 86-7).
Extensive experience has now accumulated with over-the-wire lead systems, which are commercially available from most major manufacturing companies (Medtronic, Minneapolis, MN; Boston Scientific, Natick, MA; St Jude Medical, St Paul, MN; and Biotronik, Erlangen, Germany). Successful placement of the coronary vein lead is now possible in the majority of patients within a reasonable time. The over-the-wire design allows a 95% implantation success rate, even when particularly challenging vein anatomy is present (e.g., stenosis of target vein requiring coronary venoplasty) or when repositioning in a second vein is needed.83,84 If attempts to place a left ventricular lead through the coronary sinus fail, other approaches can be considered. A surgical approach, either minimally invasive or robotically assisted, is usually considered, but in patients who are not candidates for such intervention, a left ventricular endocardial lead placement with a trans-septal approach may be considered.81,82 In nonresponders to CRT therapy, a second left ventricular lead has been implanted with some success.85,86 Left ventricular lead dislodgement is an important cause of failed CRT. This may be related to technical factors (e.g., vein-lead size mismatch) or excess lead body slack in the right atrium. It is best detected by a change in the paced QRS morphology and emphasizes the need for a 12-lead ECG during follow-up visits. Lead impedance changes or threshold rise may also herald dislodgement. Programming to retain capture can be attempted if a significant margin of output and threshold for phrenic nerve stimulation are present. Lead repositioning can be attempted, as can the use of a different lead, perhaps with better sizing, or a different vein can be considered, as troubleshooting options.
Monitoring of Hemodynamic Parameters and Heart Rate
Hemodynamic parameters can be either directly or indirectly monitored via the implanted devices. Specific sensors have been placed on right ventricular leads to detect the maximal positive dP/dT.87 The accuracy of monitoring long-term hemodynamic changes in patients with heart failure has been evaluated in observational studies and clinical trials.88,89 Hemodynamic changes can also be indirectly assessed by monitoring heart rate and HRV. Heart rate at rest and during exercise as well as HRV depend on a variety of autonomic mechanisms such as baro-receptor reflex, autonomic feedback, and contractility. Changes in heart rate or HRV can indirectly track spontaneous hemodynamic changes as well as those induced by CRT.
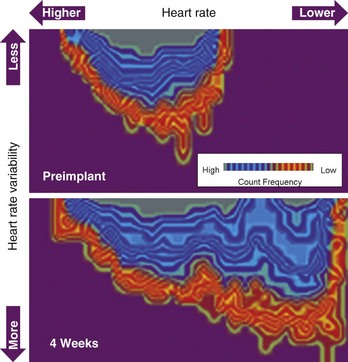
Patients with heart failure present with a significantly reduced HRV and high resting heart rate. Reduced standard deviation measured over the standard deviation of normal-to-normal intervals index has been shown to be associated with left ventricular dysfunction and increased mortality. Because pacemakers and ICDs can measure the intrinsic heart rate and perform beat-to-beat analysis, it is possible to monitor the daily, weekly, or monthly changes of mean heart rate, minimal heart rate, and HRV. A combined plot includes heart rate, HRV, and frequency count, and provides more information than each parameter alone. The automatic, permanent, long-term monitoring capability of one or all of these parameters is helpful in assessing the efficacy of certain medications and for evaluating the therapeutic influence of changes in pacing device programming. It is conceivable that in the future these parameters will allow us to automatically titrate parameters such as AV delay and pacing site and mode that determine the efficacy of CRT. One available device for CRT incorporates three-dimensional plots of heart rate, HRV, and frequency count. Initial experience showed significant changes during the first 12 weeks after the implantation compared with the preimplantation status (Figure 86-8). Whether changes in HRV really reflect improvement in patients with heart failure and whether they are associated with lower overall mortality rates remain to be determined.
Pulse Generators in Cardiac Resynchronization Therapy
Programmable features such as negative hysteresis allow the device to shorten the paced AV delay upon detection of conducted rather than bi-ventricular paced beats and enhance CRT pacing. Similarly, new pacing algorithms such as ventricular rate regularization (VRR) allow the device to override intrinsic rhythm through faster but regular ventricular pacing to enable continuous and uninterrupted bi-ventricular pacing during AF.90,91
Follow-up and Programming Issues
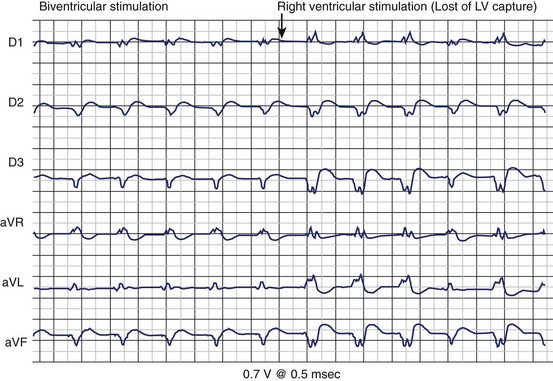
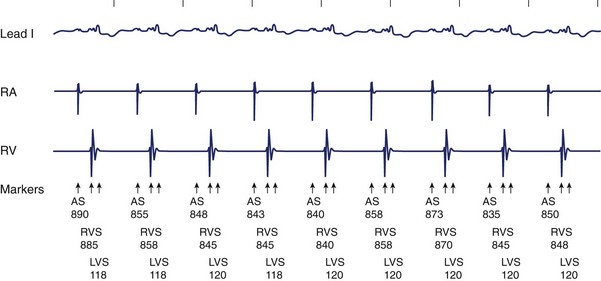
The follow-up of patients with a CRT device is more complex and time intensive than a standard pacemaker or ICD check-up.92 The follow-up visit of the patient with heart failure includes testing of each of the three leads, careful evaluation of the AV delay, guarantee of a high percentage of CRT therapy delivery, and assessment of the maximal tracking rate. Correlation with patient symptoms, heart failure status, and device-monitored event data is important to a comprehensive follow-up. The use of new hybrid CRT-D devices initially posed special technologic challenges. In the first generation of CRT devices (Contak-TR, Guidant Corporation, Minneapolis, MN; and InSync, Medtronic), the two ventricular outputs were electrically connected (i.e., parallel pacing of the RV and LV was present). All electrical parameters (i.e., pacing threshold and R-wave sensing) were the algebraic sum of each of the two ventricles. Pacing threshold was indirectly measured by monitoring the ECG signals and changes in the axis orientation as well as the QRS duration (Figure 86-9). Because the LV often presents with a higher pacing threshold, a sudden change of the axis orientation from a superior rightward orientation to a superior leftward rotation with only right ventricular pacing can be noted (see Figure 86-9). A change in the duration of the QRS complex is easy to recognize. Programming the pacing output to at least twice pacing threshold is strongly recommended because a rise of left ventricular pacing threshold is not unusual during the first few weeks after implantation. The most recent generation of devices for CRT has independently programmable ventricular and atrial channels so that most of these technical issues have been solved (Figure 86-10). The new-generation devices yield separate output programming for each of the three leads and also automatically perform continuous electrical measurements. A high percentage of CRT therapy requires greater than 85% left ventricular pacing, biventricular pacing, or both. This information is generally available in device datalogs.
Atrioventricular and Interventricular Delay Optimization
Echocardiographic measurements of the most suitable AV delay are usually performed. Preload optimization methods, such as evaluation of the relationship of the E and A waves with respect to ventricular systolic contraction, may not be necessarily as precise as once thought. Improvement of systolic pump function in patients receiving a CRT device is both preload (AV optimization) and non-preload dependent (improvement of ventricular synchrony), the echocardiographic optimization of the AV delay may lead to the selection of an AV delay that is longer than the AV delay, resulting in the maximal pulse pressure that can be achieved. Continuous adjustment of the AV delay is rarely required over the follow-up for patients with QRS duration greater than 150 ms. This is caused by the flat increase of pulse pressure while changing the AV delay within the range of 70 to 150 ms. Minor adjustment, however, may not be relevant in this specific subgroup of patients, and A-V interval optimization may be performed by echocardiographic methods of preload (E and A waves). In contrast, in patients with a narrow QRS complex (120 to 150 ms), the hemodynamically determined optimal AV delay is very close to the intrinsic value. For this reason, optimization methods that use preload-dependent parameters only may not be effective. It may be more suitable to optimize the degree of ventricular resynchronization (A-V and V-V intervals) by following changes in either left ventricular volumes and EF or tissue Doppler imaging of ventricular wall motion synchrony, rather than measuring preload.28,30 Recently, intracardiac echocardiography has been used to guide this with the use of global LVEF measured on two-dimensional imaging.30 Three-dimensional echocardiographic assessment of ventricular stroke volume and chamber volumes may hold promise in this regard.
Evaluation of the Maximal Tracking Rate of Physical Activity
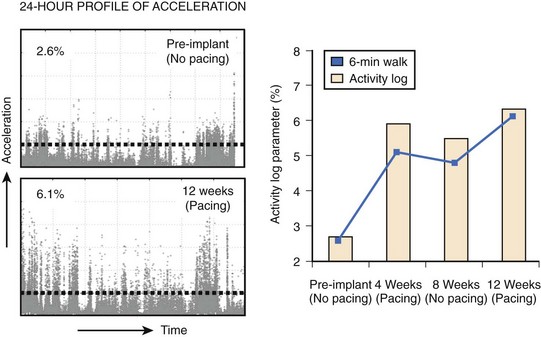
Patients with heart failure have a significantly reduced exercise capacity because of limited pump efficacy, impaired systemic hemodynamics, altered muscular activity, and alteration of metabolic demand. The 6-minute walk test has been used to assess the submaximal exercise capacity of patients with heart failure.93 Although the walk distance in patients with heart failure has been shown to predict overall mortality and the need for hospitalization, the walking distance can be monitored only periodically and may be influenced by several factors, including patient motivation, coaching, familiarity with the test, and physical limitations.94 Variabilities in the walking distance between two subsequent tests are not unusual in patients with heart failure.95 A more objective measurement of the functional capacity that is continuous, unbiased, and based on activities of daily living will be necessary in the future. In this regard, continuous recording of accelerometer signals, reflecting the patient’s physical activity, is already used in pacemakers and ICDs. Experience with recording of patient activity tracked by accelerometer has shown that activity monitored by accelerometers or activity log index (ALI) is highly sensitive and specific in detecting the patient’s physical activity.96 Studies have demonstrated that the ALI increased substantially when CRT was applied in a blinded fashion. The magnitudes of ALI change significantly and correlate with the change in the walking distance measured with 6-minute walk testing (Figure 86-11).
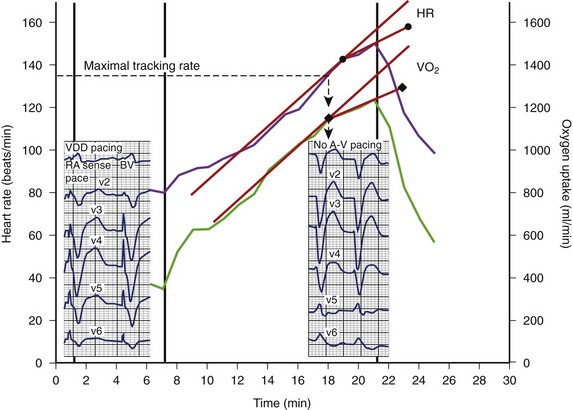
To provide continuous resynchronization therapy at rest as well as during exercise, careful assessment of heart rate during physical activity above the anaerobic threshold is important during the follow-up of patients with CRT. We perform a symptom-limited exercise testing before and 1 month after the implantation of a CRT device in all patients. Almost all patients with severe heart failure demonstrated a high resting heart rate and chronotropic incompetence, with a markedly reduced heart rate reserve during exercise. CRT increases both maximal and submaximal exercise capacities and improves ventilation efficiency and heart rate adaptation during physical activity. When the patient’s heart rate overrides the maximal tracking rate of the device, an immediate loss of pre-excitation of the left ventricular lateral wall, together with a drop in stroke volume, can be measured (Figure 86-12). A loss of CRT leads to dyspnea and fatigue. Thus sudden loss of CRT during exercise may prevent the patient from otherwise beneficial physical activity.
Assessment of Device Datalogs
Current-generation devices provide a large body of data that should be interrogated and assessed at follow-up. In the recently completed Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure (PARTNERS-HF) study, routine device diagnostics were evaluated for their value in prevention of heart failure hospitalizations.97 A combined diagnostic algorithm based on eight elements (trans-thoracic impedance, activity status, AF, ventricular rate during AF, heart rate, HRV, CRT, and shock delivery) was used. Development of abnormalities in two or more of these parameters was highly predictive of subsequent heart failure hospitalizations (HR, 4.8). Increasing patient inactivity, impedance changes beyond prespecified threshold values, and reduced HRV were important trends in predicting events. More frequent assessment (every 15 days) was more effective than longer periods (30- or 90-day intervals).
Future Developments
Evidence of long-term pacing for bradycardia at the right ventricular apex being associated with structural changes of the heart, and in some patients probably with the induction of heart failure, is growing. Interest in the hemodynamic effect of the “abnormal” sequence of activation induced by ventricular pacing is increasing as well.98 Animal studies have shown that long-term changes during continuous right ventricular pacing include asymmetric ventricular hypertrophy and dilation, myocardial fiber disarray, increased myocardial catecholamine concentration, and impaired perfusion.99 The long-term effect of such a heterogeneous distribution of stretch and workload may be responsible for triggering maladaptation mechanisms that might lead to decreases in pump function, pump failure, or progression of pre-existing heart failure.100 CRT can potentially reduce, or perhaps normalize, the spatio-temporal distribution of activation and restore the mechanical synchrony of contraction. Recent animal studies have shown that bi-ventricular stimulation, compared with right ventricular pacing, reduced the temporal asynchrony and the spatio-temporal asynchrony of the left ventricular midwall contraction.99,101 Ventricular function is restored to baseline levels after induction of LBBB by radiofrequency ablation of the left bundle.102 These data, however, have been generated in healthy canine hearts, which may differ from a diseased human heart. Whether patients in need of standard pacemaker therapy should undergo conventional, biventricular, or left ventricular pacing is an issue that remains to be resolved and deserves further research. Early clinical trials have been suggestive but not conclusive.103,104
Important and challenging questions concerning optimizing CRT therapy remain. One of the most important issues is whether electrocardiographic evidence of LBBB is an ideal selection criterion for patients with heart failure who are candidates for CRT.105 Indeed, increasing evidence points to the presence of mechanical evidence of dyssynchrony as the major basis for CRT intervention, although current indexes may not be ideal.106 This will need serious study in the future. The optimal site of CRT electrodes will then become open to examination. CRT could therefore be applied in two or more sites in either ventricle. In fact, newer techniques with minimally invasive and robotic lead epicardial placement can make this a reality and are important avenues of future research.107 Future studies are likely to address the impact of disease progression, optimal timing of intervention, new approaches to CRT, and widening patient populations. CRT has become an important modality in the treatment of heart failure syndromes and its application will continue to increase in the foreseeable future.
Key References
Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845-1853.
Auricchio A, Stellbrink C, Butter C, et al. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003;42:2109-2116.
Auricchio A, Stellbrink C, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay, for the the Pacing Therapies in Congestive Heart Failure Study Group. J Am Coll Cardiol. 2002;39:2026-2033.
Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes, for the RethinQ Study Investigators. N Engl J Med. 2007;357:2461-2671.
Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure, for the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. N Engl J Med. 2004;350(21):2140-2150.
Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873-880.
Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608-2616.
Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization therapy on morbidity and mortality in heart failure. N Eng J Med. 2005;352:1539-1549.
Daubert CJ, Ritter P, LeBreton H, et al. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. PACE. 1998;21:239-245.
Dickstein K, Vardas PE, Auricchio A, et al. 2010 focused update of ESC guidelines on device therapy in heart failure: An update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy, for the ESC Committee for Practice Guidelines (CPG). Eur Heart J. 2010;31(21):2677-2687.
Domanski MJ, Saksena S, Epstein AE, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. AVID investigators. J Am Coll Cardiol. 1999;34:1090-1095.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2008;117:e350-e408.
Garrigue S, Reuter S, Labeque JN, et al. Usefulness of biventricular pacing in patients with congestive heart failure and right bundle branch block. Am J Cardiol. 2001;88(12):1436-1441.
Gorcsan JIII, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191-213.
Helm RH, Byrne M, Helm PA, et al. Three-dimensional mapping of optimal left ventricular pacing site for cardiac resynchronization. Circulation. 2007;115(8):953-961.
Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454-1459.
Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119(14):e391-e479.
Jung W, Rillig A, Birkemeyer R, et al. Advances in remote monitoring of implantable pacemakers, cardioverter defibrillators and cardiac resynchronization therapy systems. J Interv Card Electrophysiol. 2008;23(1):73-85.
Kolettis TM, Saksena S, Mathew P, et al. Right and left ventricular hemodynamic performance during sustained ventricular tachycardia. Am J Cardiol. 1997;79(3):323-327.
Lau CP, Barold S, Tse HF, et al. Advances in devices for cardiac resynchronization in heart failure. J Interv Card Electrophysiol. 2003;9:167-181.
Lau CP, Jiang ZY, Tang MO. Efficacy of ventricular rate stabilization by right ventricular pacing during atrial fibrillation. Pacing Clin Electrophysiol. 1998;21:542-548.
Lau EW. A streamlined technique of trans-septal endocardial left ventricular lead placement. J Interv Card Electrophysiol. 2009;26(1):73-81.
Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in asymptomatic and mildly symptomatic heart failure. J Am Coll Cardiol. 2008;52:1834-1843.
Moss A, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.
Saksena S. Bundle branch block and cardiac resynchronization therapy: Do we need to look further before we leap? J Interv Card Electrophysiol. 2003;8:163-164.
Stevenson LW, Zile M, Bennett TD, et al. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 2010;3(5):580-587.
Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure, for the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. N Engl J Med. 2010;363:2385-2395.
Upadhyay GA, Choudhry NK, Auricchio A, et al. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008;52(15):1239-1246.
Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force For cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology. Eur Heart J. 2007;28:2256-2295.
Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: Results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study, for the PARTNERS Study Investigators. J Am Coll Cardiol. 2010;55:1803-1810.
Wilkoff BL, Cook JR, Epstein AE. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115-3131.
Yu CM, Chan JY, Zhang Q, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123-2134.
1 Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119(14):e391-e479.
2 Howlett JG, McKelvie RS, Costigan J, et al. The 2010 Canadian Cardiovascular Society guidelines for the diagnosis and management of heart failure update: Heart failure in ethnic minority populations, heart failure and pregnancy, disease management, and quality improvement/assurance programs, for the Canadian Cardiovascular Society. Can J Cardiol. 2010;26(4):185-202.
3 Heart Failure Society of AmericaLindenfeld J, Albert NM, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1-e194.
4 Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303-310.
5 Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol. 1983;2:755-763.
6 Herman MV, Heinle RA, Klein MD, Gorlin R. Localized disorders in myocardial contraction: Asynergy and its role in congestive heart failure. N Engl J Med. 1967;277:222-232.
7 Kass DA. Pathobiology of cardiac dyssynchrony and resynchronization. Heart Rhythm. 2009;6:1660-1665.
8 Xiao HB, Roy C, Fujimoto S, Gibson DG. Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol. 1996;53:163-170.
9 Aaronson KD, Schwartz S, Chen T-M, et al. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660-2667.
10 Shamim W, Francis DP, Yousufuddin M, et al. Intraventricular conduction delay: A prognostic marker in chronic heart failure. Int J Cardiol. 1999;70:171-178.
11 Grines CL, Bashore TM, et al. Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation. 1989;79:845-853.
12 Xiao HB, Brecker SJD, Gibson DG. Differing effects of right ventricular pacing and left bundle branch block on left ventricular function. Br Heart J. 1993;69:166-173.
13 Freedman RA, Yock PG, Echt DS, Popp RL. Effect of variation in PQ interval on pattern of atrioventricular valve motion and flow in patients with normal ventricular function. J Am Coll Cardiol. 1986;7:595-602.
14 Ishikawa T, Sumita S, Kimura K, et al. Critical PQ interval for the appearance of diastolic mitral regurgitation and optimal PQ interval in patients implanted with DDD pacemakers. PACE Pacing Clin Electrophysiol. 1994;17:1989-1994.
15 Curry CW, Nelson GS, Wyman BT, et al. Mechanical dyssynchrony in dilated cardiomyopathy with intraventricular conduction delay as depicted by 3D tagged magnetic resonance imaging. Circulation. 2000;101:E2.
16 Gorcsan JIII, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: Recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191-213.
17 Abraham T, Kass D, Tonti G, et al. Imaging cardiac resynchronization therapy. J Am Coll Cardiol Imag. 2009;2:486-497.
18 Auricchio A, Salo RW. Acute hemodynamic improvements by pacing in patients with severe congestive heart failure. Pacing Clin Electrophysiol. 1997;20:313-324.
19 Xiao HB, Roy C, Gibson DG. Nature of ventricular activation in patients with dilated cardiomyopathy: Evidence for bilateral bundle branch block. Br Heart J. 1994;72:167-174.
20 Saxon LA, Kerwin WF, Cahalan MK, et al. Acute effects of intraoperative multisite ventricular pacing on left ventricular function and activation contraction sequence in patients with depressed ventricular function. J Cardiovasc Electrophysiol. 1998;9:13-21.
21 Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics form acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567-1573.
22 Nelson GS, Berger RD, Fetics BJ, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053-3059.
23 Auricchio A, Stellbrink C, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay, for the The Pacing Therapies in Congestive Heart Failure Study Group. J Am Coll Cardiol. 2002;39:2026-2033.
24 Auricchio A, Stellbrink C, Sack S, et al. The PATH-CHF Study Investigators. The Pacing Therapies for Congestive Heart Failure (PATH-CHF) Study: Rationale, design and end-points of a prospective randomized multicenter study. Am J Cardiol. 1999;83:130D-135D.
25 Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873-880.
26 Verbeek XA, Vernooy K, Peschar M, et al. Intra-ventricular resynchronization for optimal left ventricular function during pacing in experimental left bundle branch block. J Am Coll Cardiol. 2003;42(3):558-567.
27 Helm RH, Byrne M, Helm PA, et al. Three-dimensional mapping of optimal left ventricular pacing site for cardiac resynchronization. Circulation. 2007;115(8):953-961.
28 Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology. Eur Heart J. 2007;28:2256-2295.
29 Auger D, Ducharme A, Harel F, et al. Patient assessment for cardiac resynchronization therapy: Past, present and future of imaging techniques. Can J Cardiol. 2010;26(1):27-34.
30 Saksena S, Simon AM, Mathew P, Nagarakanti R. Intracardiac echocardiography-guided cardiac resynchronization therapy: Technique and clinical application. Pacing Clin Electrophysiol. 2009;32(8):1030-1039.
31 Lau CP, Barold S, Tse HF, et al. Advances in devices for cardiac resynchronization in heart failure. J Interv Card Electrophysiol. 2003;9:167-181.
32 Auricchio A, Klein H, Tockman B, et al. Transvenous biventricular pacing for heart failure: Can the obstacles be overcome? Am J Cardiol. 1999;83:136D-142D.
33 Auricchio A, Stellbrink C, Sack S, et al. Long-term effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay, for the The Pacing Therapies in Congestive Heart Failure Study Group. J Am Coll Cardiol. 2002;39:2026-2033.
34 Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454-1459.
35 Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845-1853.
36 Saxon LA, DeMarco T, Chatterjee K, Boehmer J. The magnitude of sympathoneural activation in advanced heart failure is altered with chronic biventricular pacing [abstract], for the The VIGOR-CHF Investigators. PACE Pacing Clin Electrophysiol. 1998;21:914.
37 Moss A, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.
38 Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure, for the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. N Engl J Med. 2010;363:2385-2395.
39 Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in asymptomatic and mildly symptomatic heart failure. J Am Coll Cardiol. 2008;52:1834-1843.
40 Saksena S. Implantable defibrillators in the third millennium: Increasingly relegated to a standby role? J Am Coll Cardiol. 2000;36(3):828-831.
41 Fantoni C, Kawabata M, Massaro R, et al. Right and left ventricular activation sequence in patients with heart failure and right bundle branch block: A detailed analysis using three-dimensional non-fluoroscopic electroanatomic mapping system. J Cardiovasc Electrophysiol. 2005;16:112-119.
42 Cleland JG, Daubert JC, Erdmann E, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase. Eur Heart J. 2006;27(16):1928-1932.
43 Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure, for the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. N Engl J Med. 2004;350(21):2140-2150.
44 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization therapy on morbidity and mortality in heart failure. N Eng J Med. 2005;352:1539-1549.
45 Daubert C, Gold MR, Abraham WT, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: Insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial, for the REVERSE Study Group. J Am Coll Cardiol. 2009;54(20):1837-1846.
46 Goldman S, Johnson G, Cohn J, et al. Mechanisms of death in heart failure. Circulation. 1993;87:V124-V131.
47 Tchou PJ, Kadri N, Anderson J, et al. Automatic implantable cardioverter defibrillators and survival in patients with left ventricular dysfunction and malignant ventricular arrhythmias. Ann Intern Med. 1988;109:529-534.
48 Axtell K, Tchou P, Akhtar M. Survival in patients with depressed left ventricular function treated by implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 1991;14:291-296.
49 Mehta D, Saksena S, Krol RB, et al. Device use patterns and clinical outcome of implantable cardioverter defibrillator patients with moderate and severe impairment of left ventricular function. Pacing Clin Electrophysiol. 1993;16:179-185.
50 Domanski MJ, Saksena S, Epstein AE, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. AVID investigators. J Am Coll Cardiol. 1999;34:1090-1095.
51 Moss AJ. Implantable cardioverter defibrillator therapy: The sickest patients benefit the most. Circulation. 2000;101:1638-1640.
52 Sheldon R, Connoly S, Krahn AA, et al. Identification of patients most likely to benefit from implantable cardioverter-defibrillator therapy: The Canadian Implantable Defibrillator Study. Circulation. 2000;101:1660-1664.
53 Gasparini M, Auricchio A, Metra M, et al. Long-term survival in patients undergoing cardiac resynchronization therapy: The importance of performing atrio-ventricular junction ablation in patients with permanent atrial fibrillation, for the Multicentre Longitudinal Observational Study (MILOS) Group. Eur Heart J. 2008;29(13):1644-1652.
54 Wilkoff BL, Cook JR, Epstein AE. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115-3131.
55 Gras D, Leclercq C, Anthony SL, et al. Cardiac Resynchronization therapy in advanced heart failure: The Multicenter InSync Clinical Study. Eur J Heart Fail. 2002;4:311-320.
56 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2008;117:e350-e408.
57 Dickstein K, Vardas PE, Auricchio A, et al. 2010 focused update of ESC guidelines on device therapy in heart failure: An update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy, for the ESC Committee for Practice Guidelines (CPG). Eur Heart J. 2010;31(21):2677-2687.
58 Saksena S, Prakash A, Ziegler P, et al. Improved suppression of recurrent atrial fibrillation with dual-site right atrial pacing and antiarrhythmic drug therapy, for the DAPPAF Investigators. J Am Coll Cardiol. 2002;40(6):1140-1150.
59 Prakash A, Saksena S, Ziegler P, et al. Dual site atrial pacing for prevention of atrial fibrillation (DAPPAF) trial: Echocardiographic evaluation of atrial and ventricular function during a randomized trial of support: High right atrial and dual site right atrial pacing, for the DAPPAF Investigators. Pacing Clin Electrophysiol. 2001;24:579.
60 Leclercq C, Walker S, Linde C, et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J. 2002;23:1780-1787.
61 Doshi RN, Daoud EG, Fellows C, et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (The PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160-1165.
62 Dong K, Shen WK, Powell BD, et al. Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation and receiving cardiac resynchronization therapy. Heart Rhythm. 2009;7(9):1240-1245.
63 Upadhyay GA, Choudhry NK, Auricchio A, et al. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008;52(15):1239-1246.
64 Gregaratos G, Cheitlin MD, Conill A, et al. ACC, AHA guidelines for implantation of cardiac pacemakers and antiarrhythmia devices. J Am Coll Cardiol. 1998;31:1175-1209.
65 Gregoratos G, Abrams J, Epstein AE, et al. Guideline update for implantation of cardiac pacemakers and antiarrhythmia devices—summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC, AHA, NASPE Committee to Update the 1998 Pacemaker Guidelines. J Am Coll Cardiol. 2002;40:1703-1719.
66 Auricchio A, Stellbrink C, Butter C, et al. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003;42:2109-2116.
67 Achilli A, Sassara M, Ficili S, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42:2117-2124.
68 Yu CM, Chan YS, Zhang Q, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48:2251-2257.
69 Bleeker GB, Holman ER, Steendijk P, et al. Cardiac resynchronization therapy in patients with a narrow QRS complex. J Am Coll Cardiol. 2006;48:2243-2250.
70 Turner MS, Bleasdale RA, Vinereanu D, et al. Electrical and mechanical components of dyssynchrony in heart failure patients with normal QRS duration and left bundle-branch block: Impact of left and biventricular pacing. Circulation. 2004;109:2544-2549.
71 Gasparini M, Mantica M, Galimberti P, et al. Beneficial effects of biventricular pacing in patients with a “narrow” QRS. Pacing Clin Electrophysiol. 2005;28:357-360.
72 Jeevanantham V, Zareba W, Navaneethan S, et al. Meta-analysis on effects of cardiac resynchronization therapy in heart failure patients with narrow QRS complex. Cardiol J. 2008;15(3):230-236.
73 Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes, for the RethinQ Study Investigators. N Engl J Med. 2007;357:2461-2671.
74 Garrigue S, Reuter S, Labeque JN, et al. Usefulness of biventricular pacing in patients with congestive heart failure and right bundle branch block. Am J Cardiol. 2001;88(12):1436-1441.
75 Aranda JMJr, Conti JB, Johnson JW, et al. Cardiac resynchronization therapy in patients with heart failure and conduction abnormalities other than left bundlebranch block: Analysis of the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Clin Cardiol. 2004;27:678-682.
76 Egoavil CA, Ho RT, Greenspon AJ, Pavri BB. Cardiac resynchronization therapy in patients with right bundle branch block: Analysis of pooled data from the MIRACLE and Contak CD trials. Heart Rhythm. 2005;2:611-615.
77 Daubert CJ, Ritter P, LeBreton H, et al. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. PACE. 1998;21:239-245.
78 Lau CP, Barold S, Tse HF, et al. Advances in devices for cardiac resynchronization in heart failure. J Interv Card Electrophysiol. 2003;9:167-181.
79 Vogt J, Schwarz T, Gras D, et al. The use of telescoping guide catheters for coronary sinus cannulation and sub-selecting tributaries in left ventricular lead placement. J Interv Card Electrophysiol. 2007;19(1):61-68.
80 De Martino G, Sanna T, Dello Russo A, et al. A randomized comparison of alternative techniques to achieve coronary sinus cannulation during biventricular implantation procedures. J Interv Card Electrophysiol. 2004;10(3):227-230.
81 Lau EW. A streamlined technique of trans-septal endocardial left ventricular lead placement. J Interv Card Electrophysiol. 2009;26(1):73-81.
82 Spragg DD, Dong J, Fetics BJ, et al. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2010;56(10):774-781.
83 Hansky B, Lamp B, Minami K, et al. Coronary vein balloon angioplasty for left ventricular pacemaker lead implantation. J Am Coll Cardiol. 2002;40:2144-2149.
84 Kowalski O, Lenarczyk R, Prokopczuk J, et al. Effect of percutaneous interventions within the coronary sinus on the success rate of the implantations of resynchronization pacemakers. Pacing Clin Electrophysiol. 2006;29(10):1075-1080.
85 Lenarczyk R, Kowalski O, Pruszkowska-Skrzep P, et al. Triple site biventricular pacing in a patient with congestive heart failure and severe mechanical dyssynchrony. J Interv Card Electrophysiol. 2007;18(2):187-190.
86 Leclercq C, Gadler F, Kranig W, et al. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure, for the TRIP-HF (Triple Resynchronization In Paced Heart Failure Patients) Study Group. J Am Coll Cardiol. 2008;51(15):1455-1462.
87 Bennett T, Kjellstrom B, Taepke R, Ryden L. Development of implantable devices for continuous ambulatory monitoring of central hemodynamic values in heart failure patients. Pacing Clin Electrophysiol. 2005;28(6):573-584.
88 Kolettis TM, Saksena S, Mathew P, et al. Right and left ventricular hemodynamic performance during sustained ventricular tachycardia. Am J Cardiol. 1997;79(3):323-327.
89 Stevenson LW, Zile M, Bennett TD, et al. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 2010;3(5):580-587.
90 Lau CP, Jiang ZY, Tang MO. Efficacy of ventricular rate stabilization by right ventricular pacing during atrial fibrillation. Pacing Clin Electrophysiol. 1998;21:542-548.
91 Simpson CS, Yee R, Lee JK, et al. Safety and feasibility of a novel rate-smoothed ventricular pacing algorithm for atrial fibrillation. Am Heart J. 2001;142:294-300.
92 Jung W, Rillig A, Birkemeyer R, et al. Advances in remote monitoring of implantable pacemakers, cardioverter defibrillators and cardiac resynchronization therapy systems. J Interv Card Electrophysiol. 2008;23(1):73-85.
93 Cahalin LP, Mathier MA, Semigran MJ, et al. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325-332.
94 Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702-1707.
95 Pinna D, Opasich C, Mazza A, et al. Reproducibility of the six-minute walking test in chronic heart failure patients. Stat Med. 2000;19:3087-3094.
96 Kadhiresan VA, Pastore J, Auricchio A, et al. The PATH-CHF Study Group: A novel method—the activity log index—for monitoring physical activity of patients with heart failure. Am J Cardiol. 2002;89:1435-1437.
97 Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: Results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study, for the PARTNERS Study Investigators. J Am Coll Cardiol. 2010;55:1803-1810.
98 Badke FR, Boinay P, Covell JW. Effects of ventricular pacing on regional left ventricular performance in the dog. Heart Circ Physiol. 1980;7:H858-H867.
99 Breithardt OA, Stellbrink C, Herbots L, et al. Cardiac resynchronization therapy can reverse abnormal myocardial strain distribution in patients with heart failure and left bundle branch block. J Am Coll Cardiol. 2003;42:486-494.
100 Leclercq C, Faris O, Halperin H, et al. Regional disparity of calcium handling and stress protein expression in failing hearts with dyssynchronous contraction. Circulation. 2001;104:II-128.
101 Prinzen FW, Van Oosterhout MF, Vanagt WY, et al. Optimization of ventricular function by improving the activation sequence during ventricular pacing. Pacing Clin Electrophysiol. 1998;21:2256-2260.
102 Prinzen FW, Hunter WC, Wyman BT, et al. Mapping of regional myocardial strain and work during ventricular pacing: Experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735-1742.
103 Kindermann M, Hennen B, Jung J, et al. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: The Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol. 2006;47(10):1927-1937.
104 Yu CM, Chan JY, Zhang Q, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123-2134.
105 Saksena S. Bundle branch block and cardiac resynchronization therapy: Do we need to look further before we leap? J Interv Card Electrophysiol. 2003;8:163-164.
106 Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608-2616.
107 Jansens JL, Jottrand M, Preumont N, et al. Robotic-enhanced biventricular resynchronization: An alternative to endovenous cardiac resynchronization therapy in chronic heart failure. Ann Thorac Surg. 2003;76:413-417.