25 Cardiac Pacing and Defibrillation
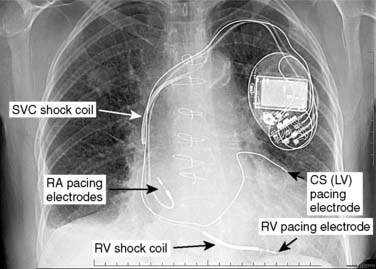
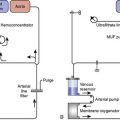
Battery-operated, implantable pacing devices were first introduced in 1958, just 4 years after the invention of the transistor. The complexity, calculation, and data storage abilities of these devices have grown in a manner similar to that seen within the computer industry. The natural progression of pacemaker developments led to the invention of the implantable cardioverter-defibrillator (ICD) around 1980. As this technology has advanced, the lines between these devices have become less clear. For example, every ICD currently implanted has antibradycardia pacing capability; and patients, news media, and even physicians often misidentify an implanted defibrillator as a pacemaker. The consequence of mistaking an ICD for a conventional pacemaker can lead to patient harm, either because of electromagnetic interference (EMI) issues resulting in inappropriate ICD therapy, or the unintentional disabling of ICD therapies in some ICDs that can be permanently disabled by magnet placement.1 Figure 25-1 shows a three-lead defibrillation system and identifies the right ventricular (RV) shock coil, which differentiates an ICD system from a conventional pacemaking system. The complexity of cardiac pulse generators and the multitude of programmable parameters limit the number of sweeping generalizations that can be made about the perioperative care of the patient with an implanted pulse generator. Population aging, continued enhancements in implantable technology, and new indications for implantation will lead to growing numbers of patients with these devices in the new millennium. Both the American College of Cardiology and the North American Society for Pacing and Electrophysiology/The Heart Rhythm Society (HRS-NASPE)* have taken note of these issues, and guidelines have been published regarding the care of the perioperative patient with a device.2,3 Pinski and Trohman4,5 also have reviewed this subject, and they have published similar recommendations. Additional reviews have been published, and the American Society of Anesthesiologists has issued a practice advisory.6–8
Pacemakers
Since 1958, more than 26 companies have produced more than 2000 pacemaker models. Determining the actual number of implants and prevalence of devices is difficult. A variety of economic and market reports suggest that more than 300,000 adults and children in the United States underwent pacemaker placement (new or revision) in 2009. It is likely that more than 3 million patients have pacemakers today. Many factors lead to confusion regarding the behavior of a device and the perioperative care of a patient with a device, especially because case reports, textbooks, and literature reviews have not kept pace with technologic developments, and many of these reviews contain incorrect statements.9,10 In addition, sometimes the preoperative consultation process leads to improper advice as well.11 Most patients with a pacemaker have significant comorbid disease. The care of these patients requires attention to both their medical and psychological problems. In addition, an understanding of pulse generators and their likely idiosyncrasies in the operating or procedure room is needed. Whether the patient with a pacemaker is at increased perioperative risk remains unknown, but two reports suggest that these patients deserve extra perioperative attention. In 1995, Badrinath et al12 retrospectively reviewed ophthalmic surgery cases in one hospital in Madras, India, from 1979 through 1988 (14,787 cases), and wrote that the presence of a pacemaker significantly increased the probability of a mortal event within 6 weeks after surgery, regardless of the anesthetic technique. In 2007, Pili-Floury et al13 reported a prospective study of 65 consecutive patients undergoing any anesthetic for any invasive noncardiac procedure unrelated to their device; they found seven (11%) postoperative myocardial infarctions, two (3%) patients experienced development of left ventricular failure, and two (3%) patients died of cardiac causes during their hospitalization.
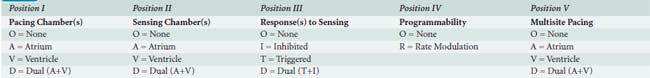
No discussion of pacemakers can take place without an understanding of the generic pacemaker code (NBG; Table 25-1), which has been published by the North American Society of Pacing and Electrophysiology (HRS-NASPE) and British Pacing and Electrophysiology Group. This code, initially published in 1983, was revised in 2002.14 The code describes the basic behavior of the pacing device. Pacemakers also come with a variety of terms generally unfamiliar to the anesthesiologist, many of which are shown in the Glossary at the end of this chapter.
 Pacemaker Indications
Pacemaker Indications
Indications for permanent pacing are shown in Box 25-1 and are reviewed in detail elsewhere.15 Devices have also been approved by the U.S. Food and Drug Administration (FDA) for three-chamber pacing (right atrium, both ventricles) to treat dilated cardiomyopathy (DCM16,17; also called biventricular pacing [BiV] or cardiac resynchronization therapy [CRT]). Also, specially programmed devices are used to treat hypertrophic obstructive cardiomyopathy in both adults and children.18,19 BiV and hypertrophic obstructive cardiomyopathy indications require careful attention to pacemaker programming because effective pacing in these patients often requires a pacing rate greater than native sinus or junctional escape rate (often accomplished with drugs) and an atrioventricular delay shorter than the native P-R interval so that the ventricle is paced 100% of the time.20 Inhibition or loss of pacing (i.e., from native conduction, atrial irregularity, ventricular irregularity, development of junctional rhythm, or EMI) can lead to deteriorating hemodynamics in these patients. BiV can lengthen the Q-T interval in some patients, producing torsade de pointes.21 Multisite atrial pacing to prevent or treat atrial arrhythmias remains in clinical trial.22,23
 Pacemaker Magnets
Pacemaker Magnets
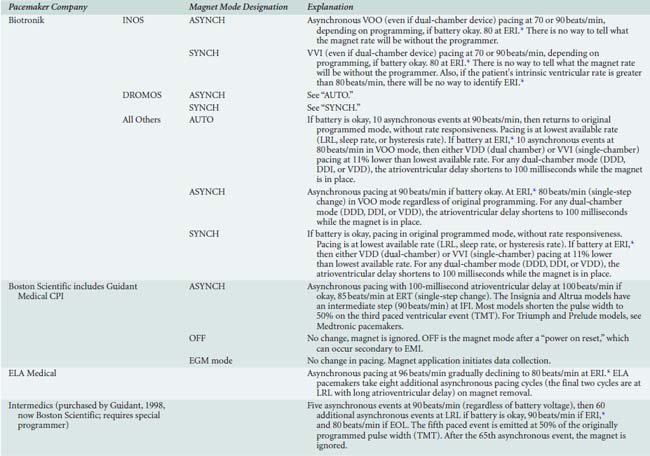
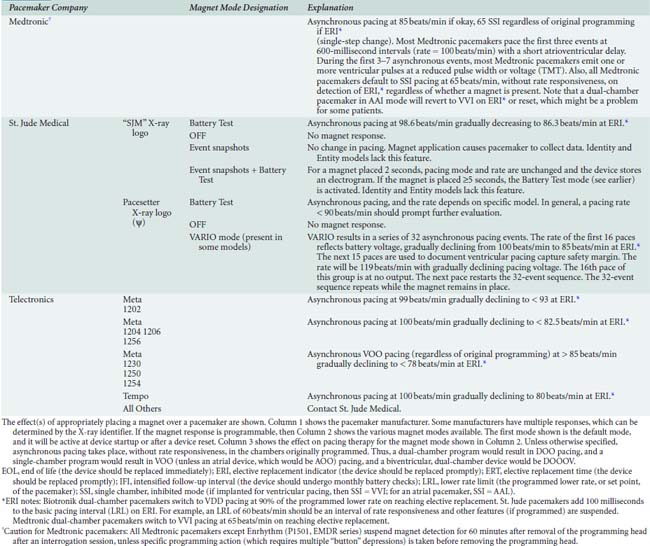
Placement of a magnet over a generator might produce no change in pacing because not all pacemakers switch to a continuous asynchronous mode when a magnet is placed. Also, not all models from a given company behave the same way. Although more than 90% of pacemakers have “high-rate (80 to 100 beats/min)” asynchronous pacing with magnet application, some switch to asynchronous pacing at program rate, and some respond with only a brief (60 to 100 beats) asynchronous pacing event. Possible effect(s) of magnet placement are shown in Box 25-2.24–26 In many devices, magnet behavior can be altered via programming. Also, any pacemaker from Boston Scientific (Natick, MA)* will ignore magnet placement after any electrical reset, which is a possibility in the presence of strong EMI. Appendix 25-1 lists pacemakers by manufacturers and has a complete listing of all magnet behaviors.
BOX 25-2. Pacemaker Magnet Behavior
For all generators, calling the manufacturer remains the most reliable method for determining magnet response and using this response to predict remaining battery life. A list of telephone numbers is shown in Appendix 25-2 at the end of this chapter.
 Preanesthetic Evaluation and Pacemaker Reprogramming
Preanesthetic Evaluation and Pacemaker Reprogramming
Preoperative management of the patient with a pacemaker includes evaluation and optimization of coexisting disease(s). No special laboratory tests or radiographs (chest films are remarkably insensitive for determination of lead problems) are needed for the patient with a pacemaker. Such testing should be dictated by the patient’s underlying disease(s), medication(s), and planned intervention. For programmable devices, interrogation with a programmer remains the only reliable method for evaluating lead performance and obtaining current program information. A chest film might be useful to document the position of the coronary sinus lead in a patient with a BiV pacemaker or defibrillator, especially if central venous catheter placement is planned, because spontaneous coronary sinus lead dislodgement was found in more than 11% of patients in early studies.27,28 A chest radiograph is certainly indicated for the patient with a device problem discovered during his or her pacemaker evaluation.
The prudent anesthesiologist will review the patient’s pacemaker history and follow-up schedule. Under the name NASPE, the HRS has published a consensus statement suggesting that pacemakers should be evaluated routinely with telephone checks for battery condition at least every 3 months. NASPE also recommends a comprehensive evaluation (interrogation) at least once per year. There are additional checks for devices implanted fewer than 6 or greater than 48 (dual chamber) or 72 (single chamber) months.29 In abstract form, Rozner et al30 reported a two-year retrospective review of follow-up intervals in patients who presented for an anesthetic, finding that more than 32% of 172 patients presenting for an anesthetic at their hospital did not meet the HRS-NASPE guideline for comprehensive evaluation. They also reported that 5% of the patients presented for their anesthetic with a pacemaker in need of replacement for battery depletion, and nearly 10% of patients had less than optimal pacing settings.30 Note that a recent preoperative interrogation remains a part of the American College of Cardiology guidelines.2
Important features of the preanesthetic device evaluation are shown in Box 25-3. Determining pacing dependency might require temporary reprogramming to a VVI mode with a low rate. In patients from countries where pacemakers might be reused,31,32 battery performance might not be related to length of implantation in the current patient. Clinicians also should note that in a registry of 345 pacemaker generator failures, 7% of failures were not related to battery depletion.33
BOX 25-3. Preanesthetic Pulse Generator (Pacemaker, Implanted Cardioverter-Defibrillator) Evaluation
Appropriate reprogramming (Box 25-4) might be the safest way to avoid intraoperative problems, especially if monopolar “Bovie” electrocautery will be used. For lithotripsy, consideration should be given to programming the pacing function from an atrial-paced mode, as some lithotriptors are designed to fire on the R wave, and the atrial pacing stimulus could be misinterpreted as the contraction of the ventricle.34
BOX 25-4. Pacemaker Reprogramming Probably Needed
Most cardiac rhythm management device manufacturers stand ready to assist with this task (see Appendix 25-2 for company telephone numbers). Reprogramming a pacemaker to asynchronous pacing at a rate greater than the patient’s underlying rate usually ensures that no oversensing or undersensing during EMI will take place, thus protecting the patient. Reprogramming a device will not protect it from internal damage or reset caused by EMI.
Experts do not agree on the appropriate reprogramming for the pacing-dependent patient. Setting a device to asynchronous mode to prevent inappropriate oversensing and ventricular output suppression can cause the pacemaker to ignore premature atrial or ventricular systoles, which could have the potential to create a malignant rhythm in the patient with significant structural compromise of the myocardium.35 Reviews by Stone and McPherson,7 as well as Rozner,36 and several case reports37,38 demonstrate inappropriate R-on-T pacing with the development of a malignant ventricular rhythm. Hayes and Strathmore39 suggest the VVT mode for the pacing-dependent patient because EMI will generally increase the pacing rate rather than inhibit the pacing output. However, they do not consider the upper pacing rate for this mode. Although some pacemakers limit VVT pacing rates to the maximum tracking rate (i.e., Boston Scientific), others will pace to the lower of the runaway pacing rate (typically around 200 beats/min) or the minimum V-V interval defined by the ventricular refractory period (i.e., Medtronic Corporation, Minneapolis, MN), which is typically 200 milliseconds (representing 300 beats/min). There are two other caveats for this mode: for the patient with a dual-chamber device and in need of atrioventricular synchrony to sustain cardiac output, hemodynamics might be compromised during VVT operation because ventricular pacing will take place without regard to atrial activity. In addition, in the VVT mode without rate-smoothing enabled, considerable increases and decreases in paced rate could result during EMI. If VVT reprogramming is to be considered, the manufacturer should be contacted regarding programming for the upper rate.
In general, rate responsiveness and other “enhancements” (hysteresis, sleep rate, atrioventricular search, etc.) should be disabled by programming because many of these can mimic pacing system malfunction (see Figure 25-2).40–42 Note that for many CPI Boston Scientific devices, the physician’s manual recommends increasing the pacing voltage to “5 volts or higher” in any case in which the monopolar electrosurgical unit (ESU) will be used. In 1986, Levine et al43 noted an increase in the amount of energy required to pace the ventricle (i.e., a pacing threshold increase) in the setting of intrathoracic surgery and mono-polar ESU use. Both Pili-Floury et al13 and Rozner et al30 have reported increases in atrial (Rozner only) and ventricular (both reports) pacing thresholds after operations involving pacemaker (but not ICD) cases in which the monopolar ESU was used, large volume and blood shifts were observed, or both. Although many of the operations were thoracic explorations, no pacing threshold changes were noted for these cases. No cardiopulmonary bypass cases were in these cohorts.
Special attention must be given to any device with a minute ventilation (bioimpedance) sensor (Box 25-5), because inappropriate tachycardia has been observed secondary to mechanical ventilation,44,45 monopolar “Bovie” ESU,44,46,47 and connection to an electrocardiographic (ECG) monitor with respiratory rate monitoring.48,49
BOX 25-5. Pacemakers with Minute Ventilation (Bioimpedance) Sensors
 Intraoperative (or Procedure) Management
Intraoperative (or Procedure) Management
No special monitoring or anesthetic technique is required for the patient with a pacemaker. However, ECG monitoring of the patient must include the ability to detect pacemaker discharges. Often, noise filtering on the ECG monitor must be changed to permit demonstration of the pacemaker pulse, and devices such as a nerve stimulator can interfere with detection and display of the pacemaker pulses.50
With respect to anesthetic technique, no studies have championed one over another. Nevertheless, a number of reports of prolongation of the QT interval with the use of isoflurane or sevoflurane have been published. Halothane appears to reduce this interval.51–55 No interactions have been reported for enflurane or desflurane.
Monopolar “Bovie” electrocautery (ESU) use remains the principal intraoperative issue for the patient with a pacemaker. Between 1984 and 1997, the FDA was notified of 456 adverse events with pulse generators, 255 from electrocautery, and a “significant number” of device failures.56 Monopolar ESU is more likely to cause problems than bipolar ESU, and patients with unipolar electrode configuration are more sensitive to EMI than those with bipolar configurations. Coagulation ESU will likely cause more problems than nonblended “cutting” ESU.57 Magnet placement during electrocautery might allow reprogramming of an older (pre-1990) generator; however, newer generators are relatively immune to such effects. In fact, most devices from Boston Scientific, as well as St. Jude, cannot be reprogrammed in the presence of a magnet. Note, however, that strong EMI can produce an electrical reset or a detection of battery depletion, which might change the programming mode or rate, or both. If monopolar electrocautery is to be used, then the current return pad should be placed to ensure that the electrocautery current path does not cross the pacemaking system. For cases such as head and neck surgery, the pad might be best placed on the shoulder contralateral to the implanted device. For breast and axillary cases, the pad might need to be placed on the ipsilateral arm with the wire prepped into the field by sterile plastic cover. Procedures with special pacing ramifications are shown in Box 25-6.
BOX 25-6. Special Procedures in Patients with Implantable Generators
The use of an ultrasonic cutting device, commonly called a harmonic scalpel, has been championed to prevent EMI while providing the surgeon with the ability to both cut and coagulate tissue. A number of case reports demonstrate successful surgery without EMI issues in these patients.58–61
At this time, MRI deserves special mention. In general, MRI has been contraindicated in pacemaker and ICD patients.62,63 However, a landmark article showing that MRI could be conducted safely in some patients has led to performance of MRI evaluations in these patients.64 Nevertheless, not all MRI sequences and energy levels have been studied, and judicious monitoring and caution are advised.65,66 Medtronic Corporation is testing a pacing generator and lead system called Enrhythm MRI SureScan (current model is EMDR01), which has special programming modes and leads for MRI scanning.67 It is already approved in several European countries.
 Pacemaker Failure
Pacemaker Failure
Pacemaker failure has three causes: (1) failure of capture, (2) lead failure, or (3) generator failure. Failure of capture because of a defect at the level of the myocardium (i.e., the generator continues to fire but no myocardial depolarization takes place) remains the most difficult problem to treat. Myocardial changes that result in noncapture include myocardial ischemia/infarction, acid-base disturbance, electrolyte abnormalities, or abnormal levels of antiarrhythmic drug(s). Note that temporary pacing (transvenous, transcutaneous, transthoracic, or transesophageal) might inhibit pacemaker output at voltages that will not produce myocardial capture.68 Sympathomimetic drugs generally lower pacing threshold. Outright generator or lead failure is rare.
 Temporary Pacemakers
Temporary Pacemakers
Several techniques are available to the anesthesiologist to establish reliable temporary pacing during the perioperative period or in the intensive care unit.69 Cardiovascular anesthesiologists are more likely than the generalists to routinely use temporary transvenous or epicardial pacing in their practices. Temporary cardiac pacing can serve as definitive therapy for transient bradyarrhythmias or as a bridge to permanent generator placement.
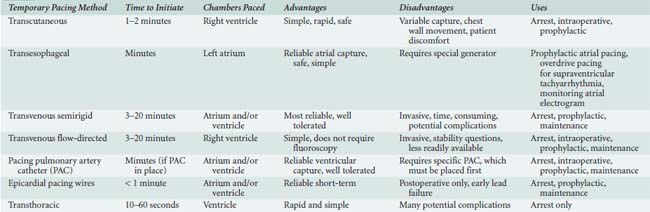
The various forms of temporary pacing include many transvenous catheter systems, transcutaneous pads, transthoracic wires, and esophageal pacing techniques. This section reviews the indications for temporary cardiac pacing and discusses the techniques available to the anesthesiologist. Many of the references in this section are older because temporary pacing is a well-established technique and not many advances have taken place since the early 1990s. Table 25-2 summarizes these techniques.
Indications for Temporary Pacing
Temporary pacemakers are commonly used after cardiac surgery,70 in the treatment of drug toxicity resulting in arrhythmias, with certain arrhythmias complicating myocardial infarction, and for intraoperative bradycardia caused by β-blocker use. On occasion, the placement of a temporary pacing system can assist in the hemodynamic management in the perioperative period. Abnormal electrolytes, preoperative β-blocker use, and many intraoperative drugs have the potential to aggravate bradycardia and bradycardia-dependent arrhythmias.71 Because drugs used to treat bradyarrhythmias have a number of important disadvantages compared with temporary pacing, hemodynamically unstable perioperative bradyarrhythmias should be considered an indication for temporary pacing (Table 25-3). If the patient already has epicardial wires or a pacing catheter or wires, or transesophageal pacing is feasible, pacing is preferred to pharmacologic therapy. However, transcutaneous and ventricular-only transvenous pacing, even if feasible, may exacerbate hemodynamic problems in patients with heart disease because these pacing modalities do not preserve atrioventricular synchrony (i.e., produce ventricular or global activation).
| Patient Condition | Event Requiring Temporary Pacing |
|---|---|
| Acute myocardial infarction | Symptomatic bradycardia, medically refractory New bundle branch block with transient complete heart block Complete heart block Postoperative complete heart block Symptomatic congenital heart block Mobitz II with anterior myocardial infarction New bifascicular block Bilateral bundle branch block and first-degree atrioventricular block Symptomatic alternating Wenckebach block Symptomatic alternating bundle branch block |
| Tachycardia treatment or prevention | Bradycardia-dependent VT Torsade de pointes Long QT syndrome Treatment of recurrent SVT or VT |
| Prophylactic | Pulmonary artery catheter placement with left bundle branch block (controversial) New atrioventricular block or bundle branch block in acute endocarditis Cardioversion with sick sinus syndrome Postdefibrillation bradycardia Counteract perioperative pharmacologic treatment causing hemodynamically significant bradycardia AF prophylaxis postcardiac surgery Postorthotopic heart transplantation |
AF, atrial fibrillation; SVT, supraventricular tachycardias, VT, ventricular tachycardia.
Temporary pacing is also indicated if a patient with a myocardial infarction complicated by second- or third-degree heart block is scheduled for emergency surgery. Bifascicular block in an asymptomatic patient is not reason enough for temporary pacing before surgery.72 Bellocci et al73 reported no occurrence of complete heart block in 98 patients with preoperative bifascicular block undergoing general anesthesia, despite 14% having prolonged conduction through their His-Purkinje system. The development of new bifascicular block immediately after surgery, though, suggests perioperative myocardial ischemia or infarction, and temporary pacing might be required. Surgical resection of neck and carotid sinus tumors may cause bradyarrhythmias requiring temporary cardiac pacing during surgical manipulation. Neurosurgical procedures involving the brainstem also may be associated with significant bradycardia.
Temporary antitachycardia pacing is most commonly used after cardiac surgery.74 With increased availability of effective noninvasive pacing technology, antitachycardia pacing might be offered to other perioperative patients as well. Even when used properly, these techniques can induce more dangerous arrhythmias, and proper resuscitation equipment should be available.
Relative contraindications to transvenous ventricular pacing include digitalis toxicity with ventricular tachycardia (VT), tricuspid valve prostheses, or the presence of a coagulopathy. Pacing in the setting of severe hypothermia might induce ventricular fibrillation (VF) or alter the normal compensatory physiologic mechanisms to the hypothermia, although one prospective study in dogs using transcutaneous pacing suggests that pacing decreases rewarming time.75 Atrial fibrillation, multifocal atrial tachycardia, and significant atrioventricular conduction system disease are contraindications to transvenous atrial pacing.
Transvenous Temporary Pacing
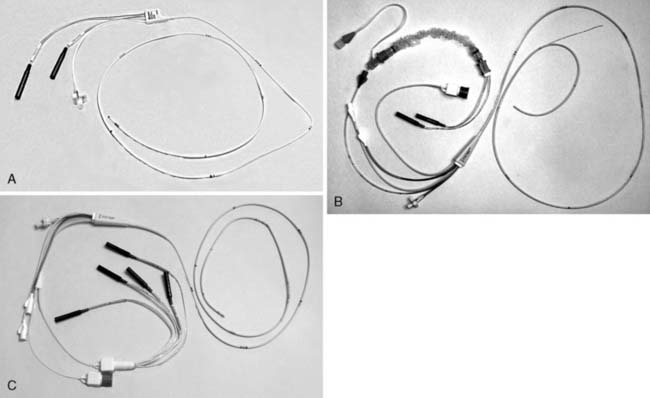
Transvenous cardiac pacing provides the most reliable means of temporary pacing. Temporary transvenous pacing is dependable and well tolerated by patients. With a device that can provide both atrial and ventricular pacing, transvenous pacing can maintain atrioventricular synchrony and improve cardiac output. Disadvantages include the need for practitioner experience, time to appropriately place the wire(s) to provide capture, the potential complications of line placement and manipulation, and the need for fluoroscopy in many cases. Three different types of typical transvenous leads are shown in Figure 25-3. (See Videos on the website.)
Rapid catheter positioning is most easily obtained by using the right internal jugular vein, even without fluoroscopy,76 although a prudent practitioner might want to clearly document the final position(s) of the catheters. The left subclavian vein is also easily utilized in emergent situations. Other sites are often impassable without fluoroscopy. In addition, brachial and femoral venous routes can increase the frequency of lead dislodgments during motion of the extremities, especially during patient transport.
Once central access is obtained, the lead is guided into position using hemodynamic data (not possible with the simple bipolar lead catheter) or by fluoroscopic guidance. Electrocardiographic guidance is less desirable. The right atrial appendage and RV apex provide the most stable catheter positions. Techniques for placement into these positions are part of cardiology training and likely are foreign to most anesthesiologists. When fluoroscopy is unavailable or in emergency situations, a flow-directed catheter can be attempted using pressure or ECG guidance. Once the right ventricle is entered, the balloon is deflated, if used, and the catheter gently advanced until electrical capture is noted. Flow-directed catheters and a right internal jugular approach afford the shortest insertion times.77 The reported incidence of successful capture in urgent situations without fluoroscopy ranges from 30% to 90%.76,78,79
Once catheters are positioned, pacing is initiated using the distal electrode as the cathode and the proximal electrode as the anode. Ideally, the capture thresholds should be less than 1 mA and generator output should be maintained at three times threshold as a safety margin. In dual-chamber pacing, atrioventricular delays of between 100 and 200 milliseconds are used. Many patients are sensitive to this parameter. Cardiac output optimization with echocardiography and/or mixed venous oxygen saturation can be used to maximize hemodynamics when adjusting atrioventricular delay.80 Atrioventricular sequential pacing is clearly beneficial in many patients,80–84 but it should be remembered that emergency pacing starts with ventricular capture alone. There is a potential risk of interference of external pacemaker generators by walkie-talkies and digital cellular phones.85,86 Clinicians should also be aware of all complications related to transvenous lead placement.87
 Pacing Pulmonary Artery Catheters
Pacing Pulmonary Artery Catheters
The pulmonary artery atrioventricular pacing TD catheter (see Figure 25-3C) was described by Zaidan in 1983.88 It allows for atrioventricular sequential pacing via electrodes attached to the outside of the catheter, as well as routine PAC functions. Combination of the two functions into one catheter eliminates the need for separate insertion of temporary transvenous pacing electrodes. However, several potential disadvantages exist with this catheter including: (1) varying success in initiating and maintaining capture,88 (2) external electrode displacement from the catheter,89 and (3) relatively high cost as compared with standard PACs. The Paceport PAC (see Figure 25-3B) provides ventricular pacing with a separate bipolar pacing lead (Chandler probe), which allows for more stable ventricular pacing, as well as hemodynamic measurements.90 This catheter has been used for successful resuscitation after cardiac arrest during closed-chest cardiac massage when attempts to capture with transcutaneous and transvenous flow-directed bipolar pacing catheters had failed. However, this unit does not provide the potential advantages associated with atrial pacing capability. The newer pulmonary artery A-V Paceport adds a sixth lumen to the older Paceport to allow placement of an atrial J-wire, flexible tip bipolar pacing lead. Both of these Paceport catheters are placed by transducing the RV port to assure correct positioning of the port 1 to 2 cm distal to the tricuspid valve. This position usually guides the ventricular wire (Chandler probe) to the apex where adequate capture should occur with minimal current requirements. Although ventricular capture is easily obtained, atrial capture can be more difficult and less reliable.80 This catheter has been used successfully after cardiac surgery.80,91 The atrial wire can be used to diagnose supraventricular tachyarrhythmias (SVTs) by atrial electrograms and to overdrive atrial flutter and reentrant SVTs.92
 Transcutaneous Pacing
Transcutaneous Pacing
Transcutaneous pacing, first described by Zoll,93 is readily available and can be implemented rapidly in emergency situations. Capture rate is variable and the technique may cause pain in awake patients, but usually it is tolerated until temporary transvenous pacing can be instituted. It may be effective even when endocardial pacing fails.94 It is now considered by many to be the method of choice for prophylactic and emergent applications.95
The large patches typically are placed anteriorly (negative electrode or cathode) over the palpable cardiac apex (or V3 lead location) and posteriorly (positive electrode or anode) at the inferior aspect of the scapula. The anode also has been placed on the anterior right chest with success in healthy volunteers.96 The skin should be cleaned with alcohol (but not abraded) to reduce capture threshold and improve patient comfort. Abraded skin can cause more discomfort. Typical thresholds are 20 to 120 mA, but pacing may require up to 200 mA at long pulse durations of 20 to 40 milliseconds.97 Transcutaneous pacing appears to capture the right ventricle, followed by near-simultaneous activation of the entire left ventricle. The hemodynamic response is similar to that of RV endocardial pacing. Both methods can cause reductions in left ventricular systolic pressure, a decrease in stroke volume, and an increase in right-sided pressures because of atrioventricular dyssynchrony. Capture should be confirmed by palpation or display of a peripheral pulse. Maintenance current is set 5 to 10 mA above threshold as tolerated by the patient. Success rates appear to be greatest when the system is used prophylactically or early after arrest, upward of 90%.98,99 When used in emergent situations, successful capture rates are usually lower but range from 10% to 93%.100–102 A 3-year study of out-of-hospital asystolic arrest showed no difference in survival for the group that received early transcutaneous pacing compared with the group that received basic CPR without pacing.103 Similarly, early use during in-hospital arrests may not alter long-term survival.104 This technique also has been used to terminate VT, atrioventricular nodal reentrant tachycardia, and atrioventricular reciprocating tachycardia.102,105
Coughing and discomfort from cutaneous stimulation are the most frequent problems. The technique poses no electrical threat to medical personnel, and complications are rare. There have been no reports of significant damage to myocardium, skeletal muscle, skin, or lungs in humans despite continuous pacing for up to 108 hours and intermittent pacing for up to 17 days.93,98,106 Several commercially available defibrillators include transcutaneous pacing generators as standard equipment.
 Esophageal Pacing
Esophageal Pacing
The newest technique available to anesthesiologists is esophageal pacing, and it has been shown to be quite reliable,107–109 even in children.110 Significant bradycardia, secondary to underlying sinus node pathology or pharmacologic effects, can occur during anesthesia. The response to pharmacologic therapy for significant bradycardia with vagolytic drugs can be unpredictable and difficult to sustain accurately.111 Chronotropic drugs may have little effect, or they can produce tachyarrhythmias or myocardial ischemia, or both. Esophageal pacing is relatively noninvasive and well tolerated, even in the majority of awake patients, and it appears to be devoid of serious complications. This modality is useful for heart rate support of cardiac output, overdrive suppression of reentrant SVT, and for diagnostic atrial electrograms. Ventricular capture must be excluded before attempts at rapid atrial pacing for overdrive suppression to prevent potential VT or VF. Some surgical positions (e.g., three-quarter prone) can increase the chance of unintentional ventricular capture.112
Problems with esophageal pacing include (1) the necessity for special generators that must provide 20 to 30 mA of current with wide pulse widths of 10 to 20 milliseconds and (2) the ability to pace only the left atrium reliably and not the left ventricle, which can be a significant problem in emergency situations.107 In comparison, typical temporary generators designed for endocardial pacing have a maximum output of 20 mA with pulse width durations of only 1 to 2 milliseconds.
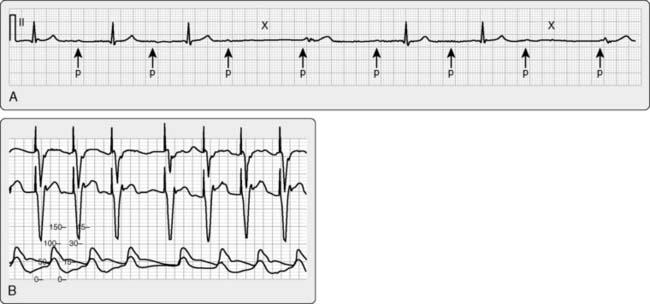
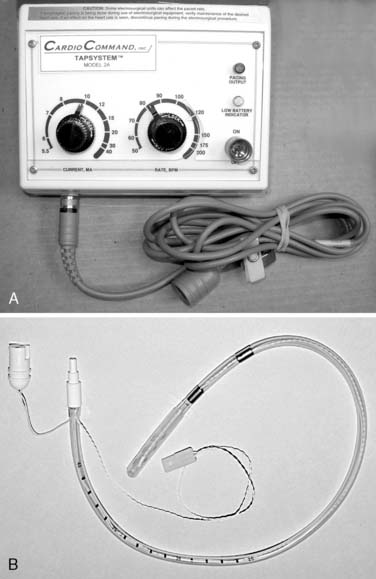
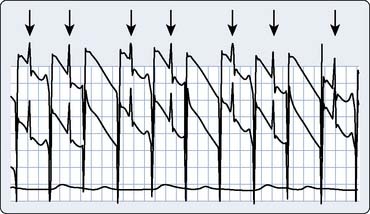
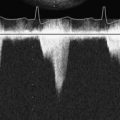
A typical transesophageal pacing generator and lead are shown in Figure 25-4. As noted in Figure 25-4, the pacing stimulus is delivered in asynchronous atrial-only mode through a modified esophageal stethoscope. Pacing is initiated by connecting the system and placing the esophageal stethoscope to a depth of 30 to 40 cm from the teeth. Capture should be confirmed using the peripheral pulse (i.e., from the pulse oximeter plethysmogram or an invasive hemodynamic monitor) because the pacing stimulus often is large relative to the QRS and frequently fools the ECG counting algorithm on the monitor (Figure 25-5). Atrial capture is obtained in virtually all patients using outputs of 8 to 20 mA, and the output should be set to two to three times the threshold for capture. Thresholds are not influenced by weight, age, atrial size, or previous cardiac surgery.109 Because there is no sensing element involved, esophageal pacing is AOO mode pacing.
Transesophageal ventricular pacing is generally unreliable, yet the optimal site appears to be 2 to 4 cm distal to the atrial site.113 The esophageal stethoscope also can be used (with a special adapter) to record the intra-atrial electrogram.
No long-term complications with this modality have been described. Induction of ventricular tachyarrhythmias during rapid atrial pacing has been noted. No significant esophageal trauma has been reported despite long-term use of up to 60 hours.114 Phrenic nerve stimulation has been described.108
 Postanesthesia Pacemaker Evaluation
Postanesthesia Pacemaker Evaluation
Any pacemaker that was reprogrammed for the perioperative period should be re-evaluated and programmed appropriately. For nonreprogrammed devices, most manufacturers recommend interrogation to ensure proper functioning and remaining battery life if any monopolar electrosurgery was used. In their retrospective review, Trankina et al115 reported 6% of 169 patients showed a problem during postoperative checks of pacemakers. Senthuran et al116 suggested that failure to perform a postoperative pacemaker check led to an unexpected postoperative death in Great Britain, and both Pili-Floury et al13 and Rozner et al30 reported perioperative pacing issues that could be found and mitigated at the postoperative check. American College of Cardiology guidelines recommend a postprocedure interrogation.2
Implantable cardioverter-defibrillators
The development of an implantable, battery-powered device able to deliver sufficient energy to terminate VT or VF has represented a major medical breakthrough for patients with a history of ventricular tachyarrhythmias. These devices prevent death in the setting of malignant ventricular tachyarrhythmias,117–119 and they clearly remain superior to antiarrhythmic drug therapy.120,121 Initially approved by the FDA in 1985, the implantation rate currently exceeds 10,000 ICDs per month in the United States.122 Industry sources report that more than 300,000 patients have these devices today.
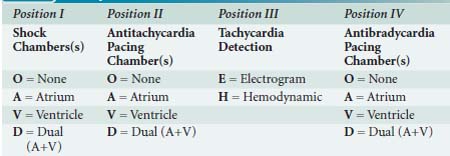
A significant number of technologic advances have been applied since the first ICD was placed, including considerable miniaturization (pectoral pocket placement with transvenous leads is the norm), as well as battery improvements that now permit permanent pacing with these devices. Thus, clinicians could easily confuse a pectoral ICD with a pacemaker. Like pacemakers, ICDs have a generic code to indicate lead placement and function (see Table 25-4).123 The most robust form of identification, called the label form, expands the fourth character into its component generic pacemaker code (NBG; see Table 25-1).
TABLE 25-4 North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group Generic Defibrillator Code (NBD)
IST occurs in 20% to 40% of ICD patients, with shocks for rhythm other than VT or VF.124–126 Atrial fibrillation with rapid ventricular response and supraventricular tachycardia remain the most common cause of IST.127 Whether inappropriate shocks injure patients remains a subject of considerable debate, but a significant number of patients who receive an inappropriate shock will demonstrate increased troponin levels in the absence of an ischemic event,128 and a death has been reported.129 Also, IST predicts increased mortality,130 and statin therapy might reduce the incidence of IST through a reduction in atrial fibrillation.131 Dual-chamber ICD technology might reduce IST from atrial fibrillation as well. Programmable features in current ICDs to differentiate VT from a tachycardia of supraventricular origin (SVT) include132:
 Implantable Cardioverter-Defibrillator Indications
Implantable Cardioverter-Defibrillator Indications
Initially, ICDs were placed for hemodynamically significant VT or VF. Newer indications associated with sudden death include long QT syndrome, Brugada syndrome (right bundle branch block, ST-segment elevation in leads V1-V3), and arrhythmogenic RV dysplasia.133 Recent studies suggest that ICDs can be used for primary prevention of sudden death (i.e., before the first episode of VT or VF) in young patients with hypertrophic cardiomyopathy,134 and data from the second Multicenter Automatic Defibrillator Intervention Trial (MADIT II) suggest that any post-MI patient with ejection fraction (EF) less than 30% should undergo prophylactic implantation of an ICD.135 Currently, however, the Centers for Medicare and Medicaid (CMS) requires a prolonged QRS interval (to > 120 milliseconds) to qualify for ICD placement in this group.
Several newer trials have included patients with nonischemic cardiomyopathy as well. Data from the Sudden Cardiac Death–Heart Failure Trial (SCD-HeFT),121 as well as the Defibrillators In Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE)136 study, now suggest that ICD placement will lower mortality in any patient with EF less than 35% regardless of the cause of the cardiomyopathy. The DEFINITE results are important because these patients were randomized only after initiation of β-blockade and angiotensin-converting enzyme inhibitor therapy, which form the backbone of medical therapy for cardiomyopathy.
Three-chamber (leads placed in atrium, right ventricle, and coronary sinus) ICDs (see Figure 25-1) for CRT (also called biventricular pacing [BiV]) have been FDA approved for patients with DCM and prolonged QRS intervals. Two-chamber (leads placed in atrium and right ventricle) ICDs are in clinical trial for patients with hypertrophic obstructive cardiomyopathy who have experienced VT or VF. Box 25-7 reviews ICD indications.
Dilated Cardiomyopathy
With the advent of CRT pacing (CRT-P) for the patient with DCM and prolonged QRS interval,137 and the approval of ICDs with CRT capability (CRT-D), the presence of a defibrillator with BiV pacing will become more common. Currently, about 550,000 new diagnoses of congestive heart failure are made annually in the United States,138 and the prevalence of this disease includes 5.7 million patients.139 Significant risk factors for the development of congestive heart failure include both ischemic heart disease and hypertension.140 These data, combined with the recent results from SCD-HeFT121 and MADIT II trials (ICD is indicated in any patient with cardiomyopathy and EF < 30–35%),135 suggest that the number of patients eligible to receive a defibrillator to include CRT-P will increase dramatically. Whether any country’s economy can absorb this economic burden remains to be seen. Currently, CRT-P improves functional status and quality of life141 and reduces congestive heart failure events,142 primarily by decreasing the dyssynchrony between the two ventricles in the dilated heart (see Videos on the website.) whether the CRT device includes ICD capability (CRT-D) or not (CRT-P). In addition, CRT-D has been shown to reduce mortality in some but not all studies.17 However, about 30% of patients who undergo CRT implantation achieve no additional benefit from the multichamber pacing.143
 Implantable Cardioverter-Defibrillator Magnets
Implantable Cardioverter-Defibrillator Magnets
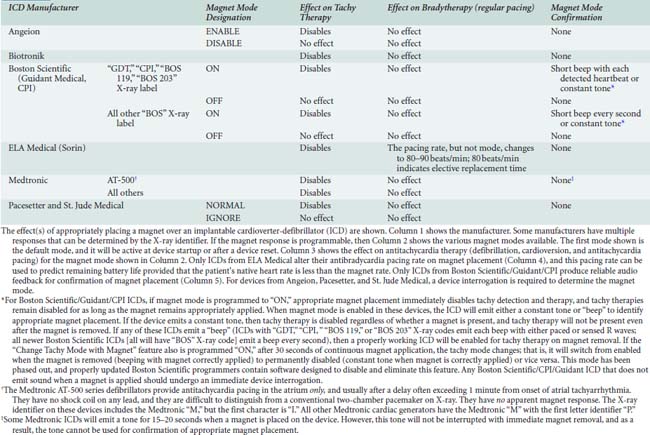
Like pacemakers, magnet behavior in some ICDs can be altered by programming. Most devices will suspend tachyarrhythmia detection (and therefore therapy) when a magnet is appropriately placed to activate the magnet sensor. Some devices from Angeion, Boston Scientific, Pacesetter, and St. Jude Medical can be programmed to ignore magnet placement. Antitachycardia therapy in some CPI devices can be permanently disabled by magnet placement for 30 seconds,1 although recent upgrades to the Boston Scientific programmer have eliminated this setting after a programming session in most of their Guidant ICDs. In general, magnet application will not affect antibradycardia pacing rate (except ELA* [Milano, Italy; U.S. Headquarters: Arvado, CO]) or pacing mode (except pacing is disabled in Telectronics Guardian 4202/4203*). Interrogating the device and calling the manufacturer remain the most reliable methods for determining magnet response. Magnet effects on ICDs are shown in Appendix 25-3.
 Preanesthetic Evaluation and Implantable Cardioverter-Defibrillator Reprogramming
Preanesthetic Evaluation and Implantable Cardioverter-Defibrillator Reprogramming
In general, ALL ICDs should have their antitachycardia therapy disabled before the commencement of any procedure (see American College of Cardiology guidelines2), although such action might be unnecessary in a setting without EMI or placement of a metal guidewire into the chest.144 The comments in the pacing section apply here for any ICD with antibradycardia pacing. Guidelines from HRS-NASPE suggest that every patient with an ICD have an in-office comprehensive evaluation every 1 to 4 months.145 Devices with CRT-P must have a sufficiently short atrioventricular delay for sensed events to ensure that all ventricular activity is paced. Failure of ventricular pacing (either right or left) because of native atrioventricular conduction or threshold issues has been associated with inappropriate antitachycardia therapy (i.e., shock).146
 Intraoperative (or Procedure) Management
Intraoperative (or Procedure) Management
In addition, no special anesthetic techniques have been championed for the patient with an ICD. Most of these patients will have severely depressed systolic function, dilated ventricular cavities, and significant valvular regurgitation, and the choice of anesthetic technique should be dictated by the underlying physiologic derangements that are present. Conflicting data have been published regarding the choice of anesthetic agent(s) and changes to defibrillation threshold (DFT). In 1992, Gill et al147 examined DFT in dogs and concluded that neither halothane nor isoflurane changed DFT in open-chest defibrillation compared with a pentobarbital infusion. However, Weinbroum et al148 evaluated DFTs in humans during ICD implant and found that halothane, isoflurane, and fentanyl increased DFT. Even with these increases, though, the increased DFTs found were still substantially lower than the maximum energy generally available in ICDs, and these increases would not have been noted under usual ICD testing conditions. As noted earlier, both isoflurane and sevoflurane have been reported to lengthen the QT interval, which could increase the risk for torsades de pointe in certain patients.
Caution should be observed when placing a central venous catheter in any patient with an ICD. In the patient with an integrated bipolar ventricular sensing configuration, an inappropriate 30-joule shock was delivered because of noise artifact created by the guidewire hitting the ventricular shock coil (which was serving as the heart rate sensor). The output of the ICD was short-circuited because of the presence of a shock coil in the superior vena cava, and the ICD was unknowingly rendered ineffective. Only after failure to deliver subsequent therapy was this problem noted, and the patient subsequently expired.149 It is important to note that some ICDs are configured to the “integrated bipolar sensing configuration” even in the presence of a true bipolar RV lead.
 Postanesthesia Implantable Cardioverter-Defibrillator Evaluation
Postanesthesia Implantable Cardioverter-Defibrillator Evaluation
The ICD must be reinterrogated and re-enabled. All events should be reviewed, and counters should be cleared because the next device evaluator might not receive information about the EMI experience of the patient and make erroneous conclusions regarding the patient’s arrhythmia events. One patient death has been reported to the FDA because of failure to reactivate tachyarrhythmia therapy in an ICD patient after a cardiac ablation procedure.150
 Glossary
Glossary
Atrioventricular Delay The time that a dual-chamber system waits after detecting (or initiating) an atrial event before pacing the ventricle. Some generators shorten this time as heart rate increases (termed rate-adaptive atrioventricular delay or dynamic atrioventricular delay). Some generators can be programmed to extend the atrioventricular delay to search for intrinsic conduction (“search atrioventricular delay”). Some generators will prolong an atrioventricular delay after any atrial event in which the last ventricular event was intrinsic (“atrioventricular delay hysteresis”). In a patient with a conducting atrioventricular node, the sensed A-V delay will be slightly longer than the “P-R” interval on the surface electrocardiogram (see “Fusion Beat” and “Pseudofusion Beat”) because the ventricular sensing element is attached to the apex of the right ventricle and detects the depolarization only after right ventricular activation.
Bipolar Lead An electrode with two conductors. Bipolar sensing is more resistant to oversensing from muscle artifact or stray electromagnetic fields. Some pacing generators can be programmed to unipolar mode even in the presence of bipolar electrodes.
Dynamic Atrioventricular Delay see Atrioventricular Delay
EGM Mode Passive acquisition and internal storage of electrocardiographic data for diagnostic purposes while pacing (or monitoring) with programmed parameters.
Fusion Beat (PFB) A pacemaker spike delivered shortly before a native depolarization of the ventricle that alters the morphology of the QRS, often misdiagnosed as undersensing. It is caused by the position of the sensing electrode relative to the depolarizing wavefront. Confirmation of appropriate sensing behavior can be made by lengthening the sensing interval (i.e., lengthening the atrioventricular delay). Fusion beats suggest ventricular capture.
Generator The device with a power source and circuitry to produce an electrical impulse designed to be conducted to the heart. Typically, pacing generators are placed in a pectoral pocket and leads are inserted into the right atrium, right ventricle, or both. Since 1995, though, implantable cardioverter-defibrillators (ICDs) also have been approved for pectoral pocket placement.
Hysteresis If present, the amount by which the patient’s intrinsic rate must decline below the programmed rate before the generator begins pacing. Some pacers periodically decrease the pacing rate to search for resumption of intrinsic activity (called search hysteresis). These functions, when present, can mimic pacemaker malfunction.
ICD Mode The designation of chamber(s) shocked, chamber(s) paced for antitachycardia pacing, method of tachycardia detection, and chambers paced for antitachycardia therapy. Table 25-4 shows the North American Society for Pacing and Electrophysiology/British Pacing and Electrophysiology Group (NASPE/BPEG) generic implantable cardioverter-defibrillator (ICD) code.
Managed Ventricular Pacing Some evidence exists that right ventricular (RV) pacing increases mortality in patients with intact atrioventricular nodal conduction. As a result, several companies have algorithms to reduce the incidence of RV pacing. Pacing modes called Managed Ventricular Pacing (Medtronic) or AAI Safe-R (ELA Medical) can permit an occasional dropped QRS (more likely with MVP than AAI Safe-R). However, no pacing device should allow two consecutive dropped QRS events. After several beats with a dropped QRS, however, these devices begin pacing in a true DDD mode for several cardiac cycles. These dropped QRS events can mimic pacing system malfunction (pseudomalfunction).
Oversensing Detection of undesired signals that are interpreted as cardiac activity. Oversensing can lead to pacemaker-driven tachycardia (pacing device, DDD mode with atrial oversensing and ventricular tracking); ventricular pause (pacing device with electrosurgically induced ventricular oversensing, leading the pacer to “detect” ventricular activity); or inappropriate shock (defibrillator, event oversensing).
Pacing Mode The designation of chamber(s) paced, chamber(s) sensed, sensing response, rate responsiveness, and antitachyarrhythmia function for a pacemaker system. Table 25-1 shows the North American Society for Pacing and Electrophysiology/British Pacing and Electrophysiology Group (NASPE/BPEG) generic pacemaker code.
Postventricular Atrial Blanking Period (PVAB) Present only in a dual-chamber pacemaker, the PVAB is the period immediately after any ventricular event during which atrial events will not be detected by the atrial sensing circuitry. In general, PVAB is used to determine where, in the postventricular period, atrial event counting should resume for mode switch determination. PVAB is the early part of postventricular atrial refractory period.
Postventricular Atrial Refractory Period (PVARP) Present only in a dual-chamber pacemaker, the PVARP is the period immediately after any ventricular event during which atrial events are ignored (for the purpose of pacing the ventricle). In some devices, atrial events during PVARP (but after the expiration of the postventricular atrial blanking period timer) will be counted for atrial rate determinations leading to possible mode switch. The duration of PVARP added to the atrioventricular delay (called total atrial refractory period [TARP]) determines the 2:1 block rate of pacing. Some devices allow the PVARP to vary based on the heart rate.
Programmed Rate (also Automatic Rate) The lowest sustained regular rate at which the generator will pace. Typically, the device begins pacing when the patient’s intrinsic rate declines below this value.
Pseudofusion Beat (PFB) A pacemaker spike delivered shortly after a native depolarization without alteration of the QRS morphology. PFBs are often misdiagnosed as undersensing, and they result from the position of the sensing electrode relative to the depolarizing wavefront (see “Fusion Beat”). Confirmation of appropriate sensing behavior can be made by lengthening the sensing interval (i.e., decreasing the program rate [atrial FB] or lengthening the atrioventricular delay [ventricular PFB]). PFB cannot be used to confirm electronic capture.
Rate Enhancements The features such as rate adaptive atrioventricular delay (shortens the atrioventricular delay with increasing heart rate); atrioventricular search hysteresis (lengthens/shortens the atrioventricular delay to produce intrinsic atrioventricular conduction); AF suppression (also called dynamic atrial overdrive; increases the lower rate on appearance of native atrial depolarization to create nearly constant atrial pacing but at a rate only slightly higher than the patient’s intrinsic rate); rate smoothing (limits changes in ventricular paced rates because of changes in atrial rates; increasing and decreasing rate limits can be programmed); sleep rate (see later); ventricular rate regulation (similar to rate smoothing but used to prevent atrial fibrillation); and hysteresis (see earlier). Each of these enhancements can produce pacing/nonpacing that can mimic pacemaker dysfunction, and these enhancements should be programmed “OFF” before any anesthetic.
Rate Modulation The ability of the generator to sense the need to increase heart rate. Mechanisms include (1) a mechanical sensor in the generator to detect motion or vibration; (2) electronic detection of Q-T interval (shortens during exercise) or transthoracic impedance to measure changes in respiration; or (3) sensor(s) for central venous blood temperature or oxygen saturation. Some generators now incorporate multiple sensors.
Runaway Pacing Rate The highest pacing rate (typically around 200 beats/min) that could occur in the setting of multiple internal component failures in a cardiac generator.
Sleep Rate (also Circadian Rate) The rate (lower than the programmed rate) at which the pacing generator will pace during programmed “nighttime” hours.
Total Atrial Refractory Period (TARP) Present only in dual-chamber pacing devices, the TARP refers to the sum of the postventricular atrial refractory period (PVARP) and the atrioventricular delay, and it determines the point at which the pacing device will pace the ventricle every other atrial event. This 2:1 block rate can be calculated by dividing 60,000 (msec/min) by the TARP (measured in milliseconds). This 2:1 block results from the ignoring of the atrial event during the PVARP, so these 2:1 blocks appear only when ventricular pacing is needed in a patient and the 2:1 block rate is lower than the maximum tracking rate. In some pacing devices, the dynamic atrioventricular delay will make the calculation of TARP dependent on atrial rate, and many of the programmers will report the final 2:1 block rate for any given combination of programmed parameters.
Undersensing Failure to detect a desired event.
Unipolar Lead An electrode with only one conductor. Some devices with bipolar leads are programmed to the unipolar lead mode. Systems with unipolar leads produce larger spikes on the electrocardiogram than bipolar leads. Systems with unipolar leads utilize the generator case as the second conductor.
Upper Sensor Rate (USR; also Upper Activity Rate [UAR]) The maximum rate to which a rate-modulated pacemaker can drive the heart. USR is not affected by UTR because when USR becomes active, the pacemaker is pacing the atrium.
Upper Tracking Rate (UTR; also called Upper Rate Limit [URL]) Pacemakers programmed to VDDxx or DDDxx mode cause the ventricles to track atrial activity. Should a patient develop an atrial tachyarrhythmia, such as a supraventricular tachycardia, atrial fibrillation, or atrial flutter, the generator acts to limit ventricular pacing. When the atrial rate exceeds the UTR, the generator can change mode (i.e., switch to DDI) or introduce second-degree A-V block. Second-degree blocks can be Mobitz type I (Wenckebach) or Mobitz type II, depending on a variety of programmed settings within the pacemaker.
Ventricular Refractory Period (VRP) The period immediately after any ventricular event during which the pacing device will not respond to a sensed event on the ventricular channel. Depending on the manufacturer and programming, though, events sensed during VRP might be counted for determining a high-rate ventricular condition.
1 Rasmussen M.J, Friedman P.A., Hammill S.C., et al. Unintentional deactivation of implantable cardioverter-defibrillators in health care settings. Mayo Clin Proc. 2002;77:855-859.
2 Fleisher L.A., Beckman J.A., Brown K.A., et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the 2002 guidelines on perioperative cardiovascular evaluation for noncardiac surgery). Circulation. 2007;116:e418-e500.
3 Goldschlager N., Epstein A., Friedman P., et al. Environmental and drug effects on patients with pacemakers and implantable cardioverter/defibrillators: A practical guide to patient treatment. Arch Intern Med. 2001;161:649-655.
4 Pinski S.L., Trohman R.G. Interference in implanted cardiac devices, part I. Pacing Clin Electrophysiol. 2002;25:1367-1381.
5 Pinski S.L., Trohman R.G. Interference in implanted cardiac devices, part II. Pacing Clin Electrophysiol. 2002;25:1496-1509.
6 Practice advisory for the perioperative management of patients with cardiac rhythm management devices: Pacemakers and implantable cardioverter-defibrillators: A report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Rhythm Management Devices. Anesthesiology. 2005;103:186-198.
7 Stone K.R., McPherson C.A. Assessment and management of patients with pacemakers and implantable cardioverter defibrillators. Crit Care Med. 2004;32:S155-S165.
8 Salukhe T.V., Dob D., Sutton R. Pacemakers and defibrillators: Anaesthetic implications. Br J Anaesth. 2004;93:95-104.
9 Rozner M.A. Corrections to electrosurgery in patients with cardiac pacemakers or implanted cardioverter defibrillators. Ann Plast Surg. 2007;58:226-227.
10 Rozner M.A. Pacemakers and implantable cardioverter defibrillators. Crit Care Med. 2004;32:1809-1812.
11 Rozner M.A. Pacemaker misinformation in the perioperative period: Programming around the problem. Anesth Analg. 2004;99:1582-1584.
12 Badrinath S.S., Bhaskaran S., Sundararaj I., et al. Mortality and morbidity associated with ophthalmic surgery. Ophthalmic Surg Lasers. 1995;26:535-541.
13 Pili-Floury S., Farah E., Samain E., et al. Perioperative outcome of pacemaker patients undergoing non-cardiac surgery. Eur J Anaesthesiol. 2008;25:514-516.
14 Bernstein A.D., Daubert J.C., Fletcher R.D., et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing Clin Electrophysiol. 2002;25:260-264.
15 Atlee J., Bernstein A. Cardiac rhythm management devices part I. Anesthesiology. 2001;95:1265-1280.
16 Auricchio A., Stellbrink C., Sack S., et al. The pacing therapies for congestive heart failure (PATH-CHF) study: Rationale, design, and endpoints of a prospective randomized multicenter study. Am J Cardiol. 1999;83:130D-135D.
17 Bristow M.R., Saxon L.A., Boehmer J., et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
18 Hayes D.L. Evolving indications for permanent pacing. Am J Cardiol. 1999;83:161D-165D.
19 Bevilacqua L., Hordof A. Cardiac pacing in children. Curr Opin Cardiol. 1998;13:48-55.
20 Gras D., Mabo P., Tang T., et al. Multisite pacing as a supplemental treatment of congestive heart failure: Preliminary results of the Medtronic Inc. InSync Study. Pacing Clin Electrophysiol. 1998;21:2249-2255.
21 Medina-Ravell V.A., Lankipalli R.S., Yan G.X., et al. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: Does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation. 2003;107:740-746.
22 Delfaut P., Saksena S. Electrophysiologic assessment in selecting patients for multisite atrial pacing. J Interv Card Electrophysiol. 2000;4(Suppl 1):81-85.
23 Prakash A., Saksena S., Ziegler P.D., et al. Dual site right atrial pacing can improve the impact of standard dual chamber pacing on atrial and ventricular mechanical function in patients with symptomatic atrial fibrillation: Further observations from the dual site atrial pacing for prevention of atrial fibrillation trial. J Interv Card Electrophysiol. 2005;12:177-187.
24 Rozner M.A., Nishman R.J. Pacemaker-driven tachycardia revisited. Anesth Analg. 1999;88:965.
25 Purday J.P., Towey R.M. Apparent pacemaker failure caused by activation of ventricular threshold test by a magnetic instrument mat during general anaesthesia. Br J Anaesth. 1992;69:645-646.
26 Bourke M.E. The patient with a pacemaker or related device. Can J Anaesth. 1996;43:24-41.
27 Valls-Bertault V., Mansourati J., Gilard M., et al. Adverse events with transvenous left ventricular pacing in patients with severe heart failure: Early experience from a single centre. Europace. 2001;3:60-63.
28 Alonso C., Leclercq C., d’Allonnes F.R., et al. Six year experience of transvenous left ventricular lead implantation for permanent biventricular pacing in patients with advanced heart failure: Technical aspects. Heart. 2001;86:405-410.
29 Bernstein A.D., Irwin M.E., Parsonnet V., et al. Report of the NASPE Policy Conference on antibradycardia pacemaker follow-up: Effectiveness, needs, and resources. North American Society of Pacing and Electrophysiology. Pacing Clin Electrophysiol. 1994;17:1714-1729.
30 Rozner M.A., Roberson J.C., Nguyen A.D. Unexpected high incidence of serious pacemaker problems detected by pre-and postoperative interrogations: A two-year experience. J Am Coll Cardiol. 2004;43:113A.
31 Sethi K.K., Bhargava M., Pandit N., et al. Experience with recycled cardiac pacemakers. Indian Heart J. 1992;44:91-93.
32 Panja M., Sarkar C.N., Kumar S., et al. Reuse of pacemaker. Indian Heart J. 1996;48:677-680.
33 Hauser R., Hayes D., Parsonnet V., et al. Feasibility and initial results of an Internet-based pacemaker and ICD pulse generator and lead registry. Pacing Clin Electrophysiol. 2001;24:82-87.
34 Kato Y., Hou K., Hori J., et al. Extracorporeal shock wave lithotripsy for ureteral stone in patient with implanted cardiac pacemaker: A case report. Nippon Hinyokika Gakkai Zasshi. 2003;94:626-629.
35 Preisman S., Cheng D.C. Life-threatening ventricular dysrhythmias with inadvertent asynchronous temporary pacing after cardiac surgery. Anesthesiology. 1999;91:880-883.
36 Rozner M.A. Implantable cardiac pulse generators: Pacemakers and cardioverter-defibrillators. In Miller R.D., Fleisher L., Johns R., Savarese J., editors: Anesthesia, ed 6, New York: Churchill Livingstone, 2004.
37 Ren X., Hongo R.H. Polymorphic ventricular tachycardia from R-on-T pacing. J Am Coll Cardiol. 2009;53:218.
38 Vogelgesang D., Vogelgesang S. Pacemaker-induced ventricular tachycardia. Europace. 2008;10:46-47.
39 Hayes D.L., Strathmore N.F. Electromagnetic interference with implantable devices. In: Ellenbogen K.A., Kay G.N., Wilkoff B.L., editors. Clinical cardiac pacing and defibrillation. ed 2. Philadelphia: WB Saunders Company; 2000:939-952.
40 Augoustides J.G., Fleisher L.A. The future for B-type natriuretic peptide in preoperative assessment. Anesthesiology. 2008;108:332-333.
41 Andersen C., Madsen G.M. Rate-responsive pacemakers and anaesthesia. A consideration of possible implications. Anaesthesia. 1990;45:472-476.
42 Levine P.A. Response to “rate-adaptive cardiac pacing: implications of environmental noise during craniotomy”. Anesthesiology. 1997;87:1261.
43 Levine P.A., Balady G.J., Lazar H.L., et al. Electrocautery and pacemakers: Management of the paced patient subject to electrocautery. Ann Thorac Surg. 1986;41:313-317.
44 Madsen G.M., Andersen C. Pacemaker-induced tachycardia during general anaesthesia: A case report. Br J Anaesth. 1989;63:360-361.
45 von Knobelsdorff G., Goerig M., Nagele H., et al. Interaction of frequency-adaptive pacemakers and anesthetic management. Discussion of current literature and two case reports. Anaesthesist. 1996;45:856-860.
46 Van Hemel N.M., Hamerlijnck R.P., Pronk K.J., et al. Upper limit ventricular stimulation in respiratory rate responsive pacing due to electrocautery. Pacing Clin Electrophysiol. 1989;12:1720-1723.
47 Wong D.T., Middleton W. Electrocautery-induced tachycardia in a rate-responsive pacemaker. Anesthesiology. 2001;94:710-711.
48 Wallden J., Gupta A., Carlsen H.O. Supraventricular tachycardia induced by Datex patient monitoring system. Anesth Analg. 1998;86:1339.
49 Center for Devices and Radiologic Health, Interaction between minute ventilation rate-adaptive pacemakers and cardiac monitoring and diagnostic equipment; 2009; Published October 14, 1998. Available at: http://www.fda.gov/cdrh/safety/minutevent.html. Accessed April 8
50 Rozner M.A. Peripheral nerve stimulators can inhibit monitor display of pacemaker pulses. J Clin Anesth. 2004;16:117-120.
51 Michaloudis D., Fraidakis O., Lefaki T., et al. Anaesthesia and the QT interval in humans. The effects of isoflurane and halothane. Anaesthesia. 1996;51:219-224.
52 Michaloudis D., Fraidakis O., Petrou A., et al. Anaesthesia and the QT interval. Effects of isoflurane and halothane in unpremedicated children. Anaesthesia. 1998;53:435-439.
53 Paventi S., Santevecchi A., Ranieri R. Effects of sevoflurane versus propofol on QT interval. Minerva Anestesiol. 2001;67:637-640.
54 Gallagher J.D., Weindling S.N., Anderson G., et al. Effects of sevoflurane on QT interval in a patient with congenital long QT syndrome. Anesthesiology. 1998;89:1569-1573.
55 Michaloudis D., Fraidakis O., Lefaki T., et al. Anaesthesia and the QT interval in humans: Effects of halothane and isoflurane in premedicated children. Eur J Anaesthesiol. 1998;15:623-628.
56 Pressly N., Review of MDR Reports reinforces concern about EMI; 1997; FDA User Facility Reporting #20 Published 1997. Available at: http://www.fda.gov/cdrh/fuse20.pdf Accessed April 8, 2009
57 Rozner M.A. Review of electrical interference in implanted cardiac devices. Pacing Clin Electrophysiol. 2003;26:923-925.
58 Nandalan S.P., Vanner R.G. Use of the harmonic scalpel in a patient with a permanent pacemaker. Anaesthesia. 2004;59:621.
59 Epstein M.R., Mayer J.E.Jr, Duncan B.W. Use of an ultrasonic scalpel as an alternative to electrocautery in patients with pacemakers. Ann Thorac Surg. 1998;65:1802-1804.
60 Ozeren M., Dogan O.V., Duzgun C., et al. Use of an ultrasonic scalpel in the open-heart reoperation of a patient with pacemaker. Eur J Cardiothorac Surg. 2002;21:761-762.
61 Erdman S., Levinsky L., Strasberg B., et al. Use of the Shaw Scalpel in pacemaker operations. J Thorac Cardiovasc Surg. 1985;89:304-307.
62 Gimbel J.R., Johnson D., Levine P.A., et al. Safe performance of magnetic resonance imaging on five patients with permanent cardiac pacemakers. Pacing Clin Electrophysiol. 1996;19:913-919.
63 Gimbel J.R., Kanal E. Can patients with implantable pacemakers safely undergo magnetic resonance imaging? J Am Coll Cardiol. 2004;43:1325-1327.
64 Martin E.T., Coman J.A., Shellock F.G., et al. Magnetic resonance imaging and cardiac pacemaker safety at 1.5-Tesla. J Am Coll Cardiol. 2004;43:1315-1324.
65 Rozner M.A., Burton A.W., Kumar A.J. Pacemaker complication during MRI. J Am Coll Cardiol. 2005;45:161-162.
66 Gimbel J.R., Wilkoff B.L., Kanal E., et al. Safe, sensible, sagacious: Responsible scanning of pacemaker patients. Eur Heart J. 2005;26:1683-1684.
67 Mitka M. Researchers seek MRI-safe pacemakers. JAMA. 2009;301:476.
68 Mychaskiw G., Eichhorn J.H. Interaction of an implanted pacemaker with a transesophageal atrial pacemaker: Report of a case. J Clin Anesth. 1999;11:669-671.
69 Kaushik V., Leon A.R., Forrester J.S.Jr, et al. Bradyarrhythmias, temporary and permanent pacing. Crit Care Med. 2000;28:N121-N128.
70 Kashima I., Shin H., Yozu R., et al. Optimal positioning of temporary epicardial atrial pacing leads after cardiac surgery. Jpn J Thorac Cardiovasc Surg. 2001;49:307-310.
71 Atlee J.L.III, Pattison C.Z., Mathews E.L., et al. Evaluation of transesophageal atrial pacing stethoscope in adult surgical patients under general anesthesia. Pacing Clin Electrophysiol. 1992;15:1515-1525.
72 Zaidan J.R. Pacemakers. Anesthesiology. 1984;60:319-334.
73 Bellocci F., Santarelli P., Di Gennaro M., et al. The risk of cardiac complications in surgical patients with bifascicular block. A clinical and electrophysiologic study in 98 patients. Chest. 1980;77:343-348.
74 Del Nido P., Goldman B.S. Temporary epicardial pacing after open heart surgery: Complications and prevention. J Card Surg. 1989;4:99-103.
75 Dixon R.G., Dougherty J.M., White L.J., et al. Transcutaneous pacing in a hypothermic-dog model. Ann Emerg Med. 1997;29:602-606.
76 Syverud S.A., Dalsey W.C., Hedges J.R., et al. Radiologic assessment of transvenous pacemaker placement during CPR. Ann Emerg Med. 1986;15:131-137.
77 Lang R., David D., Klein H.O., et al. The use of the balloon-tipped floating catheter in temporary transvenous cardiac pacing. Pacing Clin Electrophysiol. 1981;4:491-496.
78 Hazard P.B., Benton C., Milnor J.P. Transvenous cardiac pacing in cardiopulmonary resuscitation. Crit Care Med. 1981;9:666-668.
79 Phillips S.J., Butner A.N. Percutaneous transvenous cardiac pacing initiated at beside: Results in 40 cases. J Thorac Cardiovasc Surg. 1970;59:855-858.
80 Trankina M.F., White R.D. Perioperative cardiac pacing using an atrioventricular pacing pulmonary artery catheter. J Cardiothorac Anesth. 1989;3:154-162.
81 Befeler B., Hildner F.J., Javier R.P., et al. Cardiovascular dynamics during coronary sinus, right atrial, and right ventricular pacing. Am Heart J. 1971;81:372-380.
82 Benchimol A., Ellis J.G., Dimond E.G. Hemodynamic consequences of atrial and ventricular pacing in patients with normal and abnormal hearts. Effect of exercise at a fixed atrial and ventricular rate. Am J Med. 1965;39:911-922.
83 Hartzler G.O., Maloney J.D., Curtis J.J., et al. Hemodynamic benefits of atrioventricular sequential pacing after cardiac surgery. Am J Cardiol. 1977;40:232-236.
84 Curtis J., Walls J., Boley T., et al. Influence of atrioventricular synchrony on hemodynamics in patients with normal and low ejection fractions following open heart surgery. Am Surg. 1986;52:93-96.
85 Trigano A.J., Azoulay A., Rochdi M., et al. Electromagnetic interference of external pacemakers by walkie-talkies and digital cellular phones: Experimental study. Pacing Clin Electrophysiol. 1999;22:588-593.
86 Betts T.R., Simpson I.A. Inhibition of temporary pacing by a mobile phone. Heart. 2002;87:130.
87 Cooper J.P., Swanton R.H. Complications of transvenous temporary pacemaker insertion. Br J Hosp Med. 1995;53:155-161.
88 Zaidan J.R., Freniere S. Use of a pacing pulmonary artery catheter during cardiac surgery. Ann Thorac Surg. 1983;35:633-636.
89 Heiselman D.E., Maxwell J.S., Petno V. Electrode displacement from a multipurpose Swan-Ganz catheter. Pacing Clin Electrophysiol. 1986;9:134-136.
90 Colardyn F., Vandenbogaerde J., De Niel C., et al. Ventricular pacing via a Swan Ganz catheter: A new mode of pacemaker therapy. Acta Cardiol. 1986;41:23-29.
91 Lumb P.D. Atrioventricular sequential pacing with transluminal atrial and ventricular pacing probes inserted via a pulmonary artery catheter: A preliminary comparison with epicardial wires. J Clin Anesth. 1989;1:292-296.
92 Trankina M.F. Pacemakers and automatic implantable cardiac defibrillators. Semin Anesth. 1993;12:165-167.
93 ZOLL P.M. Resuscitation of the heart in ventricular standstill by external electric stimulation. N Engl J Med. 1952;247:768-771.
94 Estes N.A.III, Deering T.F., Manolis A.S., et al. External cardiac programmed stimulation for noninvasive termination of sustained supraventricular and ventricular tachycardia. Am J Cardiol. 1989;63:177-183.
95 Zoll P.M. Noninvasive cardiac stimulation revisited. Pacing Clin Electrophysiol. 1990;13:2014-2016.
96 Falk R.H., Ngai S.T. External cardiac pacing: Influence of electrode placement on pacing threshold. Crit Care Med. 1986;14:931-932.
97 Gauss A., Hubner C., Meierhenrich R., et al. Perioperative transcutaneous pacemaker in patients with chronic bifascicular block or left bundle branch block and additional first-degree atrioventricular block. Acta Anaesthesiol Scand. 1999;43:731-736.
98 Zoll P.M., Zoll R.H., Falk R.H., et al. External noninvasive temporary cardiac pacing: Clinical trials. Circulation. 1985;71:937-944.
99 Madsen J.K., Meibom J., Videbak R., et al. Transcutaneous pacing: Experience with the Zoll noninvasive temporary pacemaker. Am Heart J. 1988;116:7-10.
100 Falk R.H., Ngai S.T., Kumaki D.J., et al. Cardiac activation during external cardiac pacing. Pacing Clin Electrophysiol. 1987;10:503-506.
101 Kelly J.S., Royster R.L. Noninvasive transcutaneous cardiac pacing. Anesth Analg. 1989;69:229-238.
102 Altamura G., Bianconi L., Boccadamo R., et al. Treatment of ventricular and supraventricular tachyarrhythmias by transcutaneous cardiac pacing. Pacing Clin Electrophysiol. 1989;12:331-338.
103 Cummins R.O., Graves J.R., Larsen M.P., et al. Out-of-hospital transcutaneous pacing by emergency medical technicians in patients with asystolic cardiac arrest. N Engl J Med. 1993;328:1377-1382.
104 Knowlton A.A., Falk R.H. External cardiac pacing during in-hospital cardiac arrest. Am J Cardiol. 1986;57:1295-1298.
105 Altamura G., Bianconi L., Toscano S., et al. Transcutaneous cardiac pacing for termination of tachyarrhythmias. Pacing Clin Electrophysiol. 1990;13:2026-2030.
106 Luck J.C., Markel M.L. Clinical applications of external pacing: A renaissance? Pacing Clin Electrophysiol. 1991;14:1299-1316.
107 Pattison C.Z., Atlee J.L.III, Mathews E.L., et al. Atrial pacing thresholds measured in anesthetized patients with the use of an esophageal stethoscope modified for pacing. Anesthesiology. 1991;74:854-859.
108 Backofen J.E., Schauble J.F., Rogers M.C. Transesophageal pacing for bradycardia. Anesthesiology. 1984;61:777-779.
109 Atlee J.L.III, Pattison C.Z., Mathews E.L., et al. Transesophageal atrial pacing for intraoperative sinus bradycardia or AV junctional rhythm: Feasibility as prophylaxis in 200 anesthetized adults and hemodynamic effects of treatment. J Cardiothorac Vasc Anesth. 1993;7:436-441.
110 Yamanaka A., Kitahata H., Tanaka K., et al. Intraoperative transesophageal ventricular pacing in pediatric patients. J Cardiothorac Vasc Anesth. 2008;22:92-94.
111 Smith I., Monk T.G., White P.F. Comparison of transesophageal atrial pacing with anticholinergic drugs for the treatment of intraoperative bradycardia. Anesth Analg. 1994;78:245-252.
112 Trankina M.F., Black S., Mahla M.E. Cardiac pacing using a pacing esophageal stethoscope in patients undergoing posterior fossa craniotomy in the three quarter prone position. J Neurosurg Anesth. 1994;6:340.
113 Roth J.V., Brody J.D., Denham E.J. Positioning the pacing esophageal stethoscope for transesophageal atrial pacing without P-wave recording: Implications for transesophageal ventricular pacing. Anesth Analg. 1996;83:48-54.
114 Burack B., Furman S. Transesophageal cardiac pacing. Am J Cardiol. 1969;23:469-472.
115 Trankina M.F., Black S., Gibby G. Pacemakers: Perioperative evaluation, management and complications. Anesthesiology. 2000;93:A1193.
116 Senthuran S., Toff W.D., Vuylsteke A., et al. Editorial III—Implanted cardiac pacemakers and defibrillators in anaesthetic practice. Br J Anaesth. 2002;88:627-631.
117 Hernandez A.F., Fonarow G.C., Hammill B.G., et al. Clinical effectiveness of implantable cardioverter-defibrillators among medicare beneficiaries with heart failure. Circ Heart Fail. 2010;3:7-13.
118 Moss A.J., Hall W.J., Cannom D.S., et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.
119 A.V.I.D. Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576-1583.
120 Buxton A.E., Lee K.L., Fisher J.D., et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
121 Bardy G.H., Lee K.L., Mark D.B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
122 Hammill S.C., Kremers M.S., Kadish A.H., et al. Review of the ICD Registry’s third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6:1397-1401.
123 Bernstein A.D., Camm A.J., Fisher J.D., et al. North American Society of Pacing and Electrophysiology policy statement. The NASPE/BPEG defibrillator code. Pacing Clin Electrophysiol. 1993;16:1776-1780.
124 Poole J.E., Johnson G.W., Hellkamp A.S., et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009-1017.
125 Mishkin J.D., Saxonhouse S.J., Woo G.W., et al. Appropriate evaluation and treatment of heart failure patients after implantable cardioverter-defibrillator discharge: Time to go beyond the initial shock. J Am Coll Cardiol. 2009;54:1993-2000.
126 Begley D.A., Mohiddin S.A., Tripodi D., et al. Efficacy of implantable cardioverter defibrillator therapy for primary and secondary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 2003;26:1887-1896.
127 Rinaldi C.A., Simon R.D., Baszko A., et al. A 17 year experience of inappropriate shock therapy in patients with implantable cardioverter-defibrillators: Are we getting any better? Heart. 2004;90:330-331.
128 Hasdemir C., Shah N., Rao A.P., et al. Analysis of troponin I levels after spontaneous implantable cardioverter defibrillator shocks. J Cardiovasc Electrophysiol. 2002;13:144-150.
129 Veltmann C., Borggrefe M., Schimpf R., et al. Fatal inappropriate ICD shock. J Cardiovasc Electrophysiol. 2007;18:326-328.
130 Daubert J.P., Zareba W., Cannom D.S., et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: Frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357-1365.
131 Bhavnani S.P., Coleman C.I., White C.M., et al. Association between statin therapy and reductions in atrial fibrillation or flutter and inappropriate shock therapy. Europace. 2008;10:854-859.
132 Swerdlow C.D. Supraventricular tachycardia-ventricular tachycardia discrimination algorithms in implantable cardioverter defibrillators: State-of-the-art review. J Cardiovasc Electrophysiol. 2001;12:606-612.
133 Brugada P., Geelen P. Some electrocardiographic patterns predicting sudden cardiac death that every doctor should recognize. Acta Cardiol. 1997;52:473-484.
134 Maron B.J., Shen W.K., Link M.S., et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365-373.
135 Moss A., Zareba W., Hall W., et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883.
136 Kadish A., Dyer A., Daubert J.P., et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158.
137 Peters R.W., Gold M.R. Pacing for patients with congestive heart failure and dilated cardiomyopathy. Cardiol Clin. 2000;18:55-66.
138 Hunt S.A., Abraham W.T., Chin M.H., et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
139 Adams K.F., Lindenfeld J., Arnold J.M.O., et al. HFSA 2006 Comprehensive heart failure practice guideline. J Card Fail. 2006;12:e1-e119.
140 Lloyd-Jones D.M., Larson M.G., Leip E.P., et al. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation. 2002;106:3068-3072.
141 Gras D., Leclercq C., Tang A.S., et al. Cardiac resynchronization therapy in advanced heart failure the multicenter InSync clinical study. Eur J Heart Fail. 2002;4:311-320.
142 Moss A.J., Hall W.J., Cannom D.S., et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.
143 Mullens W., Grimm R.A., Verga T., et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765-773.
144 Rozner M.A. Management of implanted cardiac defibrillators during eye surgery. Anesth Analg. 2008;106:671-672.
145 Winters S.L., Packer D.L., Marchlinski F.E., et al. Consensus statement on indications, guidelines for use, and recommendations for follow-up of implantable cardioverter defibrillators. North American Society of Electrophysiology and Pacing. Pacing Clin Electrophysiol. 2001;24:262-269.
146 Garcia-Moran E., Mont L., Brugada J. Inappropriate tachycardia detection by a biventricular implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2002;25:123-124.
147 Gill R.M., Sweeney R.J., Reid P.R. The defibrillation threshold: A comparison of anesthetics and measurement methods. Pacing Clin Electrophysiol. 1993;16:708-714.
148 Weinbroum A.A., Glick A., Copperman Y., et al. Halothane, isoflurane, and fentanyl increase the minimally effective defibrillation threshold of an implantable cardioverter defibrillator: First report in humans. Anesth Analg. 2002;95:1147-1153.
149 Varma N., Cunningham D., Falk R. Central venous access resulting in selective failure of ICD defibrillation capacity. Pacing Clin Electrophysiol. 2001;24:394-395.
150 U.S. Food and Drug Administration, MAUDE adverse event report—ICD not re-enabled; 2010; Published 2008. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Detail.cfm?MDRFOI__ID=868724 Accessed January 22
151 Schwartzenburg C.F., Wass C.T., Strickland R.A., et al. Rate-adaptive cardiac pacing: Implications of environmental noise during craniotomy. Anesthesiology. 1997;87:1252-1254.
152 Aldrete J.A., Brown C., Daily J., et al. Pacemaker malfunction due to microcurrent injection from a bioimpedance noninvasive cardiac output monitor. J Clin Monit. 1995;11:131-133.
153 Rozner M.A., Nishman R.J. Electrocautery-induced pacemaker tachycardia: Why does this error continue? Anesthesiology. 2002;96:773-774.
154 Alexopoulos G.S., Frances R.J. ECT and cardiac patients with pacemakers. Am J Psychiatry. 1980;137:1111-1112.
155 Philbin D.M., Marieb M.A., Aithal K.H., et al. Inappropriate shocks delivered by an ICD as a result of sensed potentials from a transcutaneous electronic nerve stimulation unit. Pacing Clin Electrophysiol. 1998;21:2010-2011.
156 Vlay S.C. Electromagnetic interference and ICD discharge related to chiropractic treatment. Pacing Clin Electrophysiol. 1998;21:2009.
* The North American Society of Pacing and Electrophysiology changed their name in 2004 to the Heart Rhythm Society. The abbreviation HRS-NASPE is used in this chapter to avoid confusion.
* Boston Scientific purchased the Guidant Medical Corporation in 2005, thus becoming the owner of the Guidant and CPI trademarks.
* These two device families were implanted during clinical trials only. Thus, the likelihood of encountering these devices is low; they are included for completeness.