Cardiac Impulse Is Initiated by a Coupled System of Membrane Ion Channels and Ca2+ Cycling Proteins
Realization of Importance of Ca2+ Signaling for Cardiac Pacemaker Function
Ca2+-Clocks in Pacemaker Cells
Crosstalk of Ryr and NCX to Transfer Intracellular Ca2+ Signals to M-Clock
The Essence of Pacemaker Function Is a Coupled Function of an Intracellular Ca2+-Clock and Membrane Ion Channel Clock
Autonomic Receptor Modulation of the Coupled-Clock System
Mitochondrial Function in SANC
An Additional Level of Complexity of Heart Pacemaker Function Arises Within the SAN Tissue
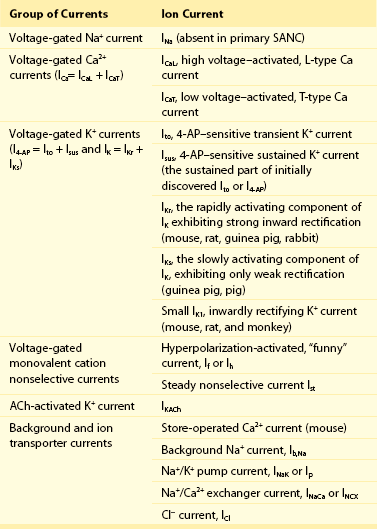
The present perspective views SANC, per se, as a system comprising several levels of complexity and integrated components. Sarcolemmal ion currents (Figure 25-1 and Table 25-1) have been extensively studied in the past (see review1), and the ensemble of these currents re-created in silico from experimental voltage-clamp data can generate spontaneous APs.2 The subsystem of sarcolemmal molecules forming a voltage membrane oscillator was conceptualized as a membrane clock3 (M-clock, for short) and has been the predominant feature in at least 12 SANC numerical models.2 During rhythmic spontaneous AP firing by SANC, the M-clock is dynamically coupled to Ca2+ cycling (see Figure 25-1, gray area) via multiple voltage-, Ca2+-, cyclic adenosine monophosphate (cAMP)-, and phosphorylation-dependent mechanisms (coupling factors).4 SANC sarcoplasmic reticulum (SR), however, in isolation from the M-clock can generate its own spontaneous local (submembrane) rhythmic Ca2+ signals. Thus, similar to the M-clock, the SR is intrinsically also an almost perfectly periodic oscillator that can be envisioned as a Ca2+-clock.3 During spontaneous AP firing by SANC, the M- and Ca2+-clocks do not exist as separate entities: They mutually entrain each other via aforementioned coupling factors. These coupled oscillators that differ in nature thus represent heterogeneous redundancy within SANC that confers a physiological robust, yet extremely flexible, united pacemaker cell system.4,5
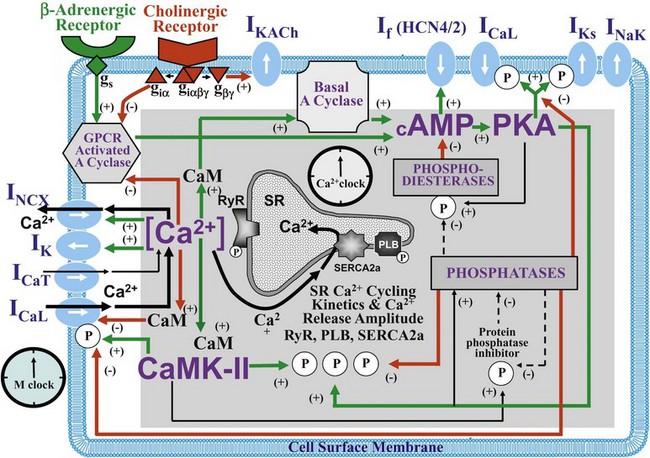
Figure 25-1 Schematic illustration of interactions of identified molecules comprising the fully coupled-pacemaker cell clock. Note that common regulatory factors (purple large lettering) govern the function of both the SR Ca2+-clock (gray intracellular area) and the M-clock (light-blue cell membrane area with blue labels depicting electrogenic proteins). These common factors act as nodes within the system to couple the function of the activities of both clocks. The system is balanced as illustrated by traffic light–like colors: Green arrows designate signaling driving AP firing, but red arrows show suppression, maintaining a given steady state level of cAMP and protein phosphorylation. G protein–coupled receptors (green and red shapes within the membrane) modulate both Ca2+-clock and M-clock function via those same crucial signaling nodes of the system. (Modified from Lakatta EG, Maltsev VA, Vinogradova TM: A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res 106:659–673, 2010.)
Realization of Importance of Ca2+ Signaling for Cardiac Pacemaker Function
In the late 1970s to the early 1980s, as the importance of an intracellular Ca2+ transient (as the trigger for ventricular myocyte contraction) came into focus, the idea that an intracellular Ca2+ oscillator could also drive membrane excitations in Purkinje fibers was suggested.6 But because oscillatory current in Purkinje fibers became manifest only in a Ca2+ overload state, such current fluctuations were interpreted in the context of “abnormal automaticity” (i.e., they were attributed to a disturbance in normal cardiac function7).
In the late 1980s, afterdepolarizations and aftercontractions were recorded in Purkinje fibers under normal Ca2+ loading conditions,8 and these fibers demonstrated localized, spontaneous myofilament motion caused by spontaneous local Ca2+ oscillations9 in the absence of Ca2+ overload. A cytosolic Ca2+ transient is evoked by each spontaneous SANC AP (see Figure 25-2, D), and that Ca2+ influx via L-type Ca2+ channels (LCCh) (see Figure 25-1) affects SR Ca2+ loading. Chelation of intracellular Ca2+ in SANC10–12 markedly slowed or abolished spontaneous AP firing. The importance of SR Ca2+ release and Na+/Ca2+ exchanger (NCX) current (INCX) for pacemaker cell pacemaker function was also demonstrated directly by effects of ryanodine on Ca2+ cycling.13,14 Ryanodine (by disabling the ryanodine receptor [RyR] and the SR release channel [Figure 25-2], and by depleting SR Ca2+ content] had a profound negative chronotropic effect on the automaticity of subsidiary atrial pacemakers15 and in SANC.13,14 Additional voltage-clamp experiments in perforated patch configuration in isolated cat atrial latent pacemaker cells demonstrated the occurrence of an inward current during late DD that was sensitive to ryanodine, and presumably was linked to INCX activated by SR Ca2+ release.16
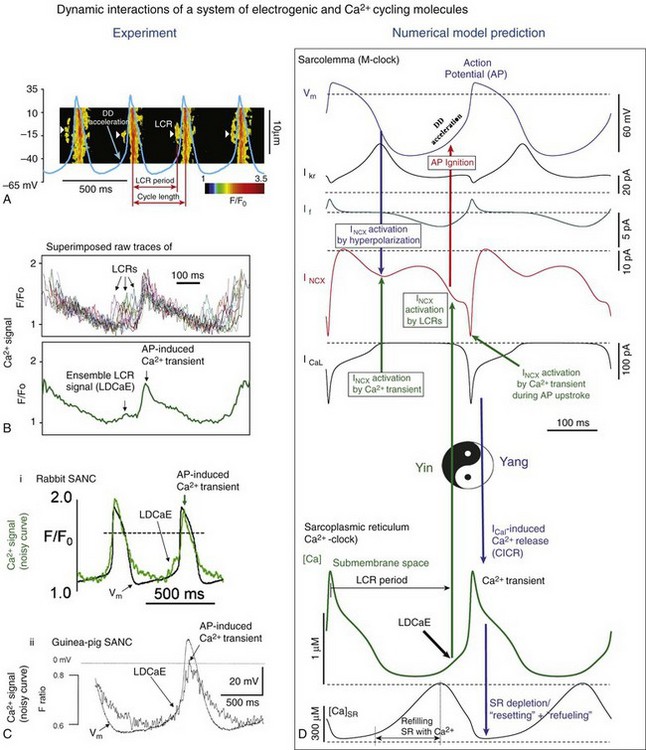
Figure 25-2 A, Line-scan image of LCRs (white arrowheads) with superimposed spontaneous APs recorded in rabbit SANC. B, Upper panel, LCRs imaged by confocal microscopy; lower panel, Temporal average of LCRs creates ensemble LCR signal or late diastolic Ca2+ elevation (LDCaE) that precedes AP-induced Ca2+ transient.29 C, LDCaE in single SANC of rabbit57 and guinea pig.20 D, Novel numerical model predicts “yin-yang” types of interactions within the coupled-clock system of SANC (see text for details). D, Diastolic depolarization; SR, sarcoplasmic reticulum. (Modified from Maltsev VA, Lakatta EG: Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol 296:H594–H615, 2009.)
Ca2+-Clocks in Pacemaker Cells
Confocal imaging of Ca2+ in mammalian SANC and atrial subsidiary pacemaker cells combined with noninvasive perforated patch-clamp electrophysiology17,18 and imaging of toad sinus venosus cells19 has documented the occurrence of spontaneous, localized, diastolic Ca2+ release in pacemaker cells in the absence of Ca2+ overload. These local Ca2+ releases (LCRs) during DD are initiated beneath the cell surface membrane via spontaneous activation of RyR (Figure 25-2A,B). SANC exhibit robust SERCA2 and RyR immunolabeling,20,21 and although SERCA2 immunolabeling in SANC occurs diffusely throughout the cytoplasm and in perinuclear area, RyR immunolabeling is most intense in the subsarcolemmal space.20,21 LCRs appear as 4- to 10-µm Ca2+ wavelets in confocal line-scan images of spontaneously firing rabbit SANC and emerge following dissipation of the global systolic transient effected by the previous AP; they crescendo during DD, peaking during late DD, as they merge into the global cytosolic Ca2+ transient triggered by the next AP (see Figure 25-2A).18,22 LCRs and the ensemble LCR signal (i.e., integral of all LCRs), that is, late diastolic Ca2+ elevations (LDCaE), have now been documented in numerous species (see review4) (see Figure 25-2, B-D).
LCR occurrence does not require triggering by depolarization of the surface membrane: Persistent rhythmic oscillatory membrane currents can be activated by rhythmic LCRs during voltage-clamp (at potentials that prevent cell Ca2+ depletion, e.g., −10 mV). Both persistent LCRs and the currents activate simultaneous fluctuations of the same frequency,22,23 and both are abolished by ryanodine. Sustained LCR activity is also observed in chemically “skinned” SANC (i.e., having a detergent-permeabilized cell surface membrane) bathed in a physiological [Ca2+] of 100 nM.22,23 Because LCRs are generated as rhythmic events at rates of 1 to 5 Hz (i.e., encompassing those of spontaneous AP firing in SANC), SR was conceptualized as an intracellular “Ca2+-clock.”3 The ability of SANC SR to generate sustained intracellular Ca2+ oscillations under normal physiological conditions within this broad frequency range has also been demonstrated in numerical model simulations that have embraced the occurrence of experimentally determined LCRs.5
RyR2 knockout not only causes failure of spontaneous Ca2+ releases in embryonic cardiac cells and suppresses spontaneous cell beating, it also leads to a marked depression of the obligatory developmental increase in heart rate and cardiac output that, in the absence of knockout, support continued cardiac embryonic differentiation.24 As in adult SANC, a crucial regulatory role of RyR2-mediated Ca2+ releases in pacemaker function is demonstrated in RyR2 knockout cells, as β-adrenergic receptor (β-AR) stimulation is ineffective in these cells.25 An essential role of RyR2 in pacemaker function has been recently demonstrated in tissue-specific RyR2 knockout mice with acute ≈50% loss of RyR2 protein in the heart, but not in other tissues.26 The RYR2 loss-of-function causes bradycardia and arrhythmia. Furthermore, cardiac RyR2 knockout mice exhibit some functional and structural hallmarks of heart failure, including sudden cardiac death.
Crosstalk of Ryanodine Receptor and Na+/ Ca2+ Exchanger to Transfer Intracellular Ca2+ Signals to M-Clock
NCX is not an ion channel, and its amplitude almost instantly follows changes in membrane potential or intracellular [Ca2+]. NCX function is both voltage- and Ca2+-dependent. When intracellular [Ca2+] is high as the result of AP-induced Ca2+ transient, AP repolarization activates NCX forward mode, generating inward current INCX by exchanging one Ca2+ (going out) to 3 Na+ (going into the cell). In contrast to hyperpolarization-activated “funny” current, If (see Table 25-1), INCX is activated earlier, as it almost instantly follows membrane hyperpolarization (see Figure 25-2, D). Furthermore while the non-selective If is decreasing and reversing during later DD, INCX is substantially increasing further by waxing diastolic Ca2+ release from the SR (see Figure 25-1 and Figure 25-2, D, described later in detail). Thus, INCX has been realized as a major transduction mechanism of intracellular Ca2+ signaling that crucially contributes to DD and normal automaticity of SANC. The NCX is one of the earliest functional genes that exist during early embryonic heart development,27 and NCX1 is required for normal pacemaker activity. Atrial-specific NCX knockout mice that lack NCX1 but have intact If, and express NCX1 in ventricular myocytes,28 live to adulthood, and exhibit a markedly reduced heart rate. However APs can be evoked under current-clamp conditions in SANC isolated from these mice, indicating that knockout cells retain electrical excitability.
At the resolution of the confocal microscope21 NCX and RyRs, molecules appear co-localized across the ≈12-nm subsarcolemmal gap between RyRs on the SR and NCX molecules on the sarcolemma. The crosstalk of LCRs and NCX activates a forward mode NCX operation that generates local inward INCX currents, which produce miniature membrane voltage fluctuations.18,29,30 The net late DD inward current initiated by the LCR ensemble (measured as ryanodine-sensitive current) in rabbit SANC varies from 0.3 pA/pF31 to 1.6 pA/pF,18 yielding a whole-cell INCX range from 9.6 to 51.2 pA for a 32-pF SANC (see Figure 25-2, D). Because an extremely small net ion current change (a few pA in rabbit SANC) has marked effects on membrane potential, an inward current of this magnitude is sufficient to broadly modulate the DD.5,30,31 For comparison, the peak of If achieved during DD in SANC ranges from 0.02 to 0.23 pA/pF as predicted by 12 different numerical models.2 LCR-activated inward INCX, together with other DD mechanisms (such as L-type Ca2+ current, ICaL), imparts the exponentially rising phase to the DD18,29,30 that initiates the subsequent AP (see Figure 25-2, A-C). Although voltage-activated L-type Ca2+ currents surely contribute to late DD, and L-type Ca2+ currents are required for AP initiation, failure to generate diastolic INCX signals results in pacemaker failure when ICaL density is normal (e.g., when RyRs are disabled ryanodine or during short-term NCX blockade18). Thus available experimental and numerical modeling data clearly indicate an important role of Ca2+ cycling molecules and their “crosstalk” in normal physiological function.
The Essence of Pacemaker Function Is a Coupled Function of an Intracellular Ca2+-Clock and Membrane Ion Channel Clock
The LCR Period Reports the Functional State of the Coupled System (Not That of the Ca2+-Clock Per Se)
The LCR period is the delay between the AP-triggered global cytosolic Ca2+ transient and LCR emergence during DD (Figure 25-2, A). It reports the time at which AP ignition is prompted via INCX activation, and it closely predicts the AP cycle length in numerous experimental perturbations of SANC function (Figure 25-3, B). Over the wide range of conditions and widely varying AP firing rates from about 1 to 4 Hz, the relationship of LCR periods to the spontaneous cycle lengths of SANC is nearly identical, with a slope being close to 1 and an offset “ignition” time of 10 to 100 ms (review4). The LCR period and the cycle length remain strongly coupled, not only in the steady state beating, but also during stringent transitions, such as the transient state after removal of voltage-clamp at the maximum diastolic potential (MDP),22 and during intrinsic beat-to-beat variations in AP cycle length.32
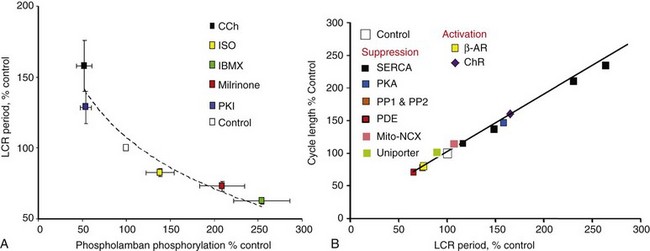
Figure 25-3 A, PKA-dependent changes in phosphorylation of phospholamban predict changes in the SANC LCR period. B, Changes in the steady state LCR period in response to numerous perturbations of the coupled-clock system predict concomitant changes in the steady state spontaneous AP cycle length. (Modified from Lakatta EG, Maltsev VA, Vinogradova TM: A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res 106:659-673, 2010. Updated with data from Vinogradova TM, Brochet DX, Sirenko S, et al: Sarcoplasmic reticulum Ca2+ pumping kinetics regulates timing of local Ca2+ releases and spontaneous beating rate of rabbit sinoatrial node pacemaker cells. Circ Res 107:767–775, 2010.)
Although LCRs are indeed generated by SR, the LCR period is not an exclusive property of the SR function per se, but rather is the result of the mutual entrainment of both Ca2+– and M- clocks regulating Ca2+ balance of the system (including Ca2+ available for pumping by the SR) via multiple Ca2+-, voltage-, and enzyme-dependent processes (described in detail later). Thus, the LCR period characterizes the integration and kinetics of the clock-system, rather than just the function of a Ca2+-clock alone. These ideas have recently evolved into a concept of a coupled-clock pacemaker system4,5 featuring a “yin-yang”–type coexistence via interdependence33 (see Figure 25-2, D).
Symmetrical Importance of Ca2+– and M-Clocks: Clocks Coupling Guarantees Each Other’s Timely Existence and Confers Robustness to SANC Normal Automaticity
Although spontaneous Ca2+ release is intrinsically stochastic, its potentially deleterious, unlimited regenerative nature is quenched by induction of an AP, thus shifting Ca2+ release properties from stochastic to periodic. The SR Ca2+ release periodicity is entrained by rhythmically occurring APs via rhythmically triggered depletion of the SR. While the M-clock–generated AP initially depresses SR Ca2+ release by effecting global Ca2+ SR depletion, this Ca2+ depletion simultaneously initiates the coupled-clock restitution process: Ca2+ influx via LCCh restores Ca2+ lost from the cell via NCX earlier in the prior cycle. At the same time, SR begins to refill Ca2+ (via sarco/endoplamic reticulum Ca2+-ATPase [SERCA] Ca2+pumping and, likely, inter-SR Ca2+ diffusion toward junctional SR) and then generates LCRs again when restitution is completed with the achievement of a threshold intra-SR [Ca2+] and removal of RyR inactivation, both of which are required for subsequent spontaneous RyR activation. The growth of submembrane LCRs activates INCX (i.e., the electrogenic component of the coupled system), which drives DD to reach a threshold of LCCh activation that continues to further accelerate DD, ultimately leading to the generation of rapid upstroke of the next AP. Of note, LCCh activation accelerates the DD of a given cycle not only directly via its inward current, but also indirectly via its Ca2+ influx (during DD, before the AP upstroke), resulting in Ca2+-induced Ca2+ release and, hence, in additional LCRs and greater NCX activation, creating (for a short time) a regenerative, positive-feedback loop. These chain reaction types of interactions (revealed by numerical modeling5; see Figure 25-2D) that become apparent during late DD may be construed as being akin to a “rocket launch” (i.e., likely important for robust late DD acceleration and spontaneous AP generation).
Beat-to-Beat Ca2+-Dependent Regulation of SAN Pacemaker Cell Rate and Rhythm
If the Ca2+– and M-clocks were indeed coupled, as has been described, then Ca2+ changes must regulate the AP firing rate on a beat-to-beat basis. Evidence for the role of Ca2+ in beat-to-beat regulation stems from experiments in rabbit SANC in which spontaneous AP firing is interrupted by a voltage-clamp to −65 mV.22 As voltage-clamp is applied, the ensemble LCR signal or LCR signal mass (a product of LCR amplitude, size, and duration) during the time interval, corresponding to the inter-AP interval (i.e., “would-be” cycle length), remains as during spontaneous AP firing but then wanes after a few “would-be” cycles as SR Ca2+ becomes depleted in the absence of regularly occurring APs. Upon removal of the voltage-clamp, spontaneous AP firing is restored, and this produces Ca2+ influx for pumping into the Ca2+-depleted SR. Beat-to-beat Ca2+-dependent regulation is revealed during the transition process, as SR Ca2+ load increases: The beat-to-beat increase in spontaneous AP firing rate closely follows the concomitant beat-to-beat increase in diastolic LCR activity (assessed as the LCR signal mass22).
A more direct type of evidence for beat-to-beat Ca2+ dependent regulation of SANC has been recently provided by acute photolysis of the intracellular caged Ca2+ compound.34 In response to loading of the caged Ca2+ buffer, the kinetics of Ca2+ removal from the cytosol is markedly slowed, the integral of Ca2+ release in the form of LCRs becomes substantially reduced, and LCRs become uncoupled from AP generation, which becomes markedly slowed and then dysrhythmic. When Ca2+ is acutely released from the caged compound by flash photolysis, intracellular Ca2+ dynamics are acutely restored, and rhythmic APs resume immediately at a normal rate. Thus, when Ca2+ is photo-released, a small increase in Ca2+ acutely affects SANC function: The initial effect of the flashes markedly accelerates DD and acutely reduces spontaneous cycle length and synchronous LCRs. After a few rhythmic cycles, however, these effects of the flash wane as interference with Ca2+ dynamics by the caged buffer is reestablished.
Another important piece of evidence for the beat-to-beat Ca2+-dependent regulation of SANC function is provided by two-dimensional (2D)-camera measurements of the whole-cell ensemble of LCRs simultaneously with perforated-patch AP recordings.32 These experiments demonstrate that the beat-to-beat variations in average LCR period (measured each cycle) of the whole-cell LCR ensemble predict variations in AP cycle length.
Basal Levels of cAMP and Protein Phosphorylation by PKA and CaMKII Drive the Coupled-Clock System
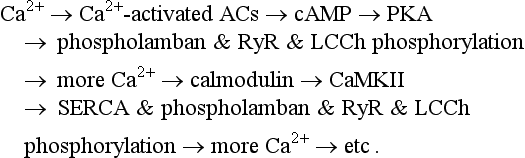
SANC have a high constitutive level of activation of adenylyl cyclases (ACs) that results in a high level of basal cAMP23 and PKA-dependent protein phosphorylation. Although SANC, like ventricular myocytes, express high levels of Ca2+-inhibited AC types 5 and 6, the discoveries of Ca2+-activated AC types (i.e., AC1 and AC8), in rabbit and guinea pig SANC35,36 and localization of the basal Ca2+-activated AC activity within lipid raft microdomains36 link Ca2+ to localized cAMP production (see Figure 25-1). Ca2+ binds to calmodulin to activate AC, leading to a high basal level of cAMP-mediated, PKA-dependent phosphorylation of surface membrane and intracellular proteins involved in cell Ca2+ balance and SR Ca2+ cycling23,36 (see Figure 25-1).
Confocal imaging of immunolabeled proteins demonstrates that active CaMKII is highly localized beneath the surface membrane.11 Thus, a geographical association has been noted between local CaMKII activity and AC activities and LCRs. CaMKII inhibition reduces ICaL in isolated SANC, thereby reducing the rate of AP firing.11 Increased CaMKII activity is also essential for the increase in SR Ca2+ release in SANC in response to β-AR stimulation.37 BayK 8644 (a LCCh agonist) requires CaMKII activation to increase SANC AP firing.38 CaMKII is activated in rabbit SANC during AP firing in the basal state (likely as the result of active basal intracellular Ca2+ cycling).
The likelihood for spontaneous SR Ca2+ release to occur increases as a function of the SR Ca2+ load, determined, in a physiological context, by the Ca2+ that is available for pumping into the SR and the phosphorylation status of the Ca2+ cycling proteins, including phospholamban (which regulates the activity of SR Ca2+ pumping protein SERCA), RyR (the SR release channel), and LCCh (see review39). SR Ca2+ refilling kinetics regulates the LCR period and the spontaneous beating rate of rabbit SANC.40 PKA- and CaMKII-dependent phosphorylation of SR cycling proteins and LCCh leads to synchronization of spontaneous RyR activation. The schematic in Figure 25-4A depicts the concept that the restitution process that determines the LCR period is regulated (1) by the kinetics of SR Ca2+ cycling (i.e., by the rate of Ca2+ pumping into the SR); and (2) by the threshold of SR Ca2+ load required for spontaneous RyR activation. This concept is also supported by numerical model simulations of the SANC coupled-clock system (Figure 25-4B).
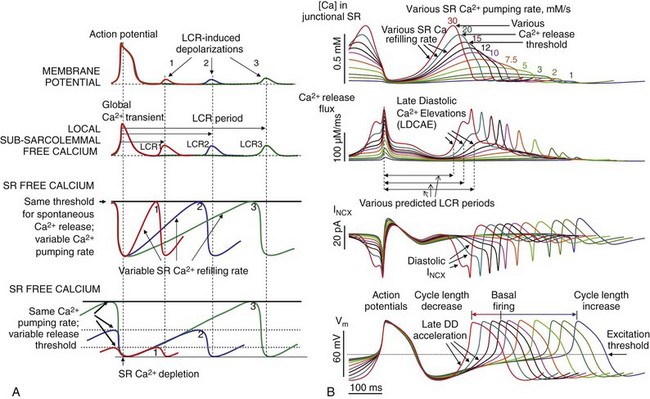
Figure 25-4 A, Schematic illustration of the concept of how the rate of SR Ca2+ refilling and the Ca2+ release threshold determine the LCR period, as well as the timing of the LCR-induced DD.4 B, A new numerical of the SANC model5 predicts the wide range of pacemaker rate modulation via variations in SR Ca2+ pumping rate (1 to 30 mM/s), mimicking various degrees of PKA-dependent phospholamban phosphorylation.
Experimental data clearly support the critical importance of Ca2+-dependent, basal protein phosphorylation for normal SANC automaticity. Reducing the phosphorylation state of these proteins in rabbit or guinea pig SANC by buffering intracellular [Ca2+] or directly inhibiting AC, or basal PKA or CaMKII activities (including by cholinergic receptor [ChR] stimulation, see below), has marked effects in slowing or even abolishing rhythmic APs (see review4). And vice versa—increasing PKA signaling via stimulation of β-ARs, or exposure to a membrane-permeable analog of cAMP, or inhibition of phosphodiesterase or phosphatase activity (see later) accelerates the AP firing rate. It is important to note that independent of the type of perturbation of protein phosphorylation, the LCR period always closely predicts the cycle length (see Figure 25-3B). Maneuvers that increase protein phosphorylation require an intact Ca2+-clock to effect an increase in the spontaneous AP firing rate.23,41 Normal automaticity of SANC, in fact, can be envisioned as a phenomenon that emerges from the phosphorylation state of the coupled-clock systems proteins; this synchronizes their functions to result in rhythmic spontaneous AP firing.
Mechanisms Intrinsic to the Coupled-Clock System That Restrain Its Basal AP Firing Rate
The coupled-pacemaker system dynamics are driven by a positive-feedback Ca2+ signaling, that is, Ca2+release begets more Ca2+ release via PKA and CaMKII pathways (see Figure 25-1, green arrows):
This positive-feedback signaling scheme obviously would drive the AP firing rate to its maximum. Thus, mechanisms to damp this signaling (see Figure 25-1, red arrows) must function continuously and concurrently to keep in check cell cAMP level and the phosphorylation of molecules that drive AP firing. Basal phosphodiesterase activity is one such restraining mechanism to reduce the high constitutive AC activity in SANC.31 Such a system of a high rate of cAMP production by Ca2+-activated ACs is balanced to a high rate of cAMP degradation by phosphodiesterase to still maintain high basal levels of cAMP and, hence, basal phosphorylation of the signaling proteins of both clocks. Basal activation of phosphoprotein phosphatases in SANC42 is another mechanism that limits PKA- and CaMKII-dependent phosphorylation. The net result of basal activation of phosphodiesterase and phosphoprotein phosphatases is that the basal LCR period and the AP cycle length are maintained near the midpoint of their functional ranges (see Figure 25-3A,B).
Thus, the robustness of the coupled-clock system is imparted by its numerous functional redundancies and by dual regulation of SR Ca2+cycling and membrane ion current generators by a common set of factors (coupling factors or nodes [see Figure 25-1, purple labels]) kept in check by phosphodiesterase and phosphatase activities, voltage-dependent negative feedback on ion fluxes, and calmodulin-dependent modulation of LCCh. These complex interactions (depicted in Figure 25-1) are the essence of the system’s ability to function as a clock (i.e., despite substantial transient cell Ca2+ changes during each AP cycle, the steady state average cell Ca2+ balance and levels of phosphorylation of M- and Ca2+-clock proteins remain stable), so that all events recur during each cycle in the same sequence and magnitude, ensuring a stable AP cycle length.
Autonomic Receptor Modulation of the Coupled-Clock System
Nature imparts flexibility to the coupled clock of the cardiac pacemaker to vary its AP firing rate over a wide physiological range (i.e., from 60 to 240 bpm in humans) via variation in GPCR signaling (see Figure 25-1, green and red shapes) that links both adrenergic and cholinergic receptors to the very same nodes of the coupled-clock system (see Figure 25-1, purple labels) that regulate the basal state LCR period4: Stimulation of sympathetic β-ARs in SANC, via Gs activation, increases the spontaneous AP firing rate via effects on the proteins of both clocks. It is well established that β-AR stimulation can modulate ion channels of the M-clock,1 specifically, IK and ICaL (via PKA-dependent phosphorylation) and If (via direct effects of cAMP; see Figure 25-1). However, β-AR stimulation, via changes in cAMP/PKA-dependent phosphorylation due to AC activation and phosphatase inhibition, can also alter SR Ca2+ efflux via RyR, as well as Ca2+ sequestration via SERCA.14,20,41 Thus, β-AR stimulation modulates cAMP and protein phosphorylation levels of both clocks, simultaneously increases Ca2+ within the system via more frequent and larger ICaL activation, and accelerates refilling of SR with Ca2+ to generate stronger LCRs with shorter LCR periods23,40,41 that herald stronger INCX, thus accelerating DD.43 CaMKII-dependent phosphorylation of phospholamban and RyR also increases in response to β-AR stimulation (likely as the result of changes in cell Ca2+ dynamics and concomitant calmodulin activation, effected primarily by PKA activation). Thus, CaMKII-dependent phosphorylation likely has an important role as a Ca2+-calmodulin–dependent amplifier of the initial effect of β-AR stimulation.
ChR act as a physiological “break” on the heart rate. ChR signaling activates Gi protein that couples to several downstream targets: (1) Gi-βγ activation of IKACh (see Table 25-1; see review1 for details), and (2) a Gi-αs–coupled reduction in AC activity that limits cAMP/protein kinase A (PKA)-dependent phosphorylation. The lack of effect of pertussis toxin (PTX), which prevents Gi activation, on basal AP firing rate indicates an absence of any significant basal Gi activity that relates to AP firing. In isolated, single rabbit SANC, the threshold for a cholinergic agonist, carbachol, to reduce the AP firing rate is ≈10 nM, and half maximal inhibition is achieved at 100 nM. IKACh activation becomes apparent at higher concentrations of carbachol (>30 nM).44 A reduction in AP firing rate in response to carbachol concentrations between 10 and 30 nM theoretically can be explained by a reduction in If via reduced cAMP, but stimulation remains intact in If-deficient mice. Alternative powerful, cAMP-dependent mechanisms include a concurrent reduction in PKA-dependent phosphorylation of Ca2+ cycling proteins (e.g., phospholamban, RyR)44,45 and likely the M-clock (e.g., LCCh and IK channels). Thus, in contrast to β-AR stimulation, ChR stimulation decreases the Ca2+ balance of the system, which leads to a reduction in AP firing rate. It is important to note that the transition to a new steady state of the reduced AP firing rate is a feed-forward process. Specifically, during this transition, each spontaneous cycle is affected by prior cycles featuring less frequent and smaller ICaL activation that further decelerates refilling of SR with Ca2+ to generate weaker LCRs with longer LCR periods,44,45 decreasing and delaying INCX activation and decelerating DD. Thus, the feed-forward mechanism of the AP firing rate reduction reinforces the initial effect of ChR stimulation on cell Ca2+ balance, which likely extends the regulation range and confers robustness to the ChR-mediated bradycardic effect.
The ideas of the Ca2+-clock and the coupled-clock system have served as the basis for a novel type of robust and flexible biological pacemakers.46–48 One way to actually mimic the clock coupling that naturally occurs in SANC is to express Ca2+-activated AC type 1 or 835,36 to activate proteins of both clocks via cAMP and cAMP-activated PKA-dependent phosphorylation.47,49 Another interesting approach is to reactivate within ventricular myocytes the genetic program of the SAN.48
Mitochondrial Function in SANC
Rabbit SANC specifically had been referred to as “empty” cells with sparse and scattered myofilaments and mitochondrial densities compared with atrial and ventricular cells. These conclusions, however, were based on rough-cut sections of the SAN visualized by electron microscopy requiring a thin tissue slice, in which mitochondria can be damaged, and on the fact that artifacts can be created, in part as the result of different cut angles, which can change the tissue morphology, giving rise to the impression of “empty” space. Mitochondrial density visualized in isolated intact rabbit SANC by specific mitochondrial labeling revealed the presence of abundant mitochondria and mitochondrial density in SANC that is similar to that in atrial and ventricular myocytes50; hence, mitochondria are an important part of the coupled-clock system (Figure 25-5).
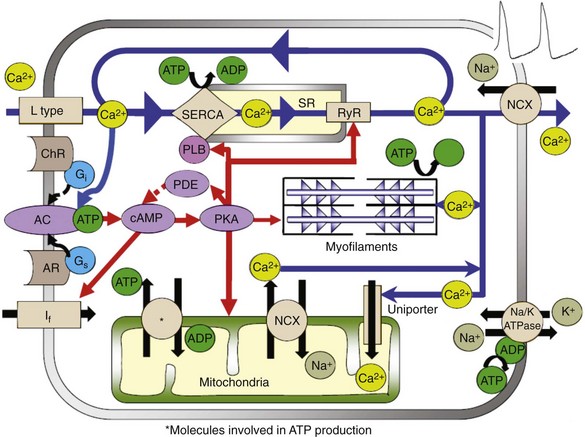
Figure 25-5 Illustration of the interplay of AC- and cAMP/PKA-dependent signaling to the myofilaments, SR Ca2+-cycling proteins, ion channels, and basal state ATP demand and supply in SANC.
The respiration or oxygen consumption rate in a physiologically coupled mitochondrial system reflects the rate of mitochondrial adenosine triphosphate (ATP) production. The respiration rate of SANC is comparable with that of unloaded, ventricular myocytes that are electrically stimulated at 3Hz (i.e., similar to spontaneous SANC AP firing rate).50 Therefore, the ability of the mitochondria to produce ATP in SANC is comparable with that of ventricular myocytes, but “the energy budget” is spent differently in the two cell types. The AP-initiated cytosolic Ca2+ transient delivers Ca2+ to the contractile proteins to induce myofilament force production and displacement, and myofilaments are the major ATP consumers in ventricular myocytes. But SANC myofilament density is relatively low compared with that of ventricular myocytes.51 In SANC, ATP consumption by spontaneous myofilament contraction is lower than in ventricular myocytes, and most ATP in SANC is consumed in the synthesis of cAMP and Ca2+-cycling maintenance of cell ionic homeostasis (see Figure 25-5). Short-term metabolic inhibition by cyanide, which inhibits cytochrome oxidase in the mitochondria, reduces the intracellular Ca2+ transient amplitude and the AP firing rate in primary pacemaker cells.50,52
Cytosolic Ca2+ enters mitochondria via the mitochondrial uniporter and is extruded by the mitochondrial NCX (see Figure 25-5). In ventricular myocytes, changes in mitochondrial Ca2+ alter the activity of several mitochondrial enzymes that take part in ATP production (see review cf53). In SANC, however, reducing Ca2+ influx into the mitochondria does not significantly alter the ATP level. These results strongly suggest that mitochondrial Ca2+ does not directly regulate ATP supply to demand. In contrast to ventricular myocytes, Ca2+ activation of cAMP/PKA signaling in SANC has a major role in linking basal ATP utilization and mitochondrial ATP production. A gradual reduction in cAMP/PKA signaling is accompanied by a gradual reduction in ATP and a reduction in AP firing rate, suggesting that the Ca2+-activated cAMP/PKA signaling cascade that drives spontaneous AP firing of SANC, as has been described, is a unique core feed-forward system that not only drives basal ATP consumption but also regulates ATP production. cAMP/PKA signaling can effect ATP production by phosphorylation of several mitochondrial proteins (e.g., voltage-dependent anion carrier [VDAC]) and complexes I through V (see review54). An increase in phosphorylation of these complexes in the electron transport chain by cAMP/PKA enhances the flux of protons and drives them to complex V to increase the rate of ATP production. Phosphorylation of VDAC can also increase the delivery rate of ATP from the mitochondria to the cytosol. Therefore, a decrease in basal cAMP/PKA signaling in SANC appears to signal to mitochondria that ATP production should be reduced.
ATP can be produced in the cytosol by glycolysis. In SANC, inhibition of glycolysis does not affect the basal spontaneous AP firing rate.50,55 Moreover, inhibition of glycolysis in SANC does not affect either ATP level or O2 consumption; therefore under basal conditions, glycolysis is not the major mechanism to produce ATP in SANC. However, during high demand, or hypoxic conditions (i.e., when the amount of oxygen to the cell is limited), glycolysis may have an important role in ATP production in SANC.
In ventricular myocytes, mitochondrial Ca2+ flux plays a fundamental role in buffering cytosolic Ca2+ and modulates the SR Ca2+ load in both normal and pathological conditions. Similar to ventricular myocytes, mitochondrial-SR Ca2+ crosstalk is present within SANC, and mitochondrial Ca2+ flux in SANC affects the SR Ca2+ load, thus indirectly affecting SR Ca2+ release.56An increase in mitochondrial Ca2+ attained by inhibition of mitochondrial NCX decreases the SR Ca2+ load and reduces the ensemble LCR Ca2+ signal. In contrast, a reduction in mitochondrial Ca2+ by inhibition of mitochondrial uniporter increases the SR Ca2+ load and increases the ensemble LCR Ca2+ signal. Basal coupled-clock system automaticity is modulated by changes in Ca2+ cycling into and out of mitochondria in SANC, impacting on cytosolic Ca2+, SR Ca2+ loading, and, indirectly, SR Ca2+ release characteristics. Changes in the spontaneous AP cycle length in response to changes in mitochondrial Ca2+ are predicted by concurrent changes in the periodicity of the spontaneous rhythmic LCR Ca2+ signal. Because basal Ca2+ is also linked to cAMP-PKA signaling within SANC that regulates ATP supply/demand matching, mitochondrial Ca2+-cytosolic Ca2+ crosstalk indirectly links to ATP supply and demand.
An Additional Level of Complexity of Heart Pacemaker Function Arises Within the SAN Tissue
In the final analysis, a requirement for the supremacy of the central SAN area in initiating the SAN impulse is that the mutually entrained rate of its cells, determined by the net balance of intrinsic mechanisms and their modulation by extrinsic factors, exceeds that of other neighborhoods to which the impulse spreads. A phase shift in mutually entrained APs, generated within a SAN neighborhood, must occur between neighborhoods to account for the often observed shift in the leading pacemaker site after interventions to alter intrinsic SANC properties (e.g., vagal or sympathetic stimulation [see review4]).
References
1. Mangoni, ME, Nargeot, J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008; 88:919–982.
2. Wilders, R. Computer modelling of the sinoatrial node. Med Biol Eng Comput. 2007; 45:189–207.
3. Maltsev, VA, Vinogradova, TM, Lakatta, EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006; 100:338–369.
4. Lakatta, EG, Maltsev, VA, Vinogradova, TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res. 2010; 106:659–673.
5. Maltsev, VA, Lakatta, EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009; 296:H594–H615.
6. Tsien, RW, Kass, RS, Weingart, R. Cellular and subcellular mechanisms of cardiac pacemaker oscillations. J Exp Biol. 1979; 81:205–215.
7. Kass, RS, Tsien, RW. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982; 38:259–269.
8. Lipsius, SL, Gibbons, WR. Membrane currents, contractions, and aftercontractions in cardiac Purkinje fibers. Am J Physiol. 1982; 243:H77–H86.
9. Lakatta, EG, Lappe, DL. Diastolic scattered light fluctuation, resting force and twitch force in mammalian cardiac muscle. J Physiol. 1981; 315:369–394.
10. Li, J, Qu, J, Nathan, RD. Ionic basis of ryanodine’s negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am J Physiol. 1997; 273:H2481–H2489.
11. Vinogradova, TM, Zhou, YY, Bogdanov, KY, et al. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ Res. 2000; 87:760–767.
12. Sanders, L, Rakovic, S, Lowe, M, et al. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J Physiol. 2006; 571:639–649.
13. Rigg, L, Terrar, DA. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol. 1996; 81:877–880.
14. Ju, YK, Allen, DG. How does beta-adrenergic stimulation increase the heart rate? The role of intracellular Ca2+ release in amphibian pacemaker cells. J Physiol. 1999; 516(Pt 3):793–804.
15. Rubenstein, DS, Lipsius, SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989; 64:648–657.
16. Zhou, Z, Lipsius, SL. Na+-Ca2+ exchange current in latent pacemaker cells isolated from cat right atrium. J Physiol. 1993; 466:263–285.
17. Huser, J, Blatter, LA, Lipsius, SL. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol. 2000; 524(Pt 2):415–422.
18. Bogdanov, KY, Vinogradova, TM, Lakatta, EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: Molecular partners in pacemaker regulation. Circ Res. 2001; 88:1254–1258.
19. Ju, YK, Allen, DG. The distribution of calcium in toad cardiac pacemaker cells during spontaneous firing. Pflugers Arch. 2000; 441:219–227.
20. Rigg, L, Heath, BM, Cui, Y, et al. Localisation and functional significance of ryanodine receptors during beta-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovasc Res. 2000; 48:254–264.
21. Lyashkov, AE, Juhaszova, M, Dobrzynski, H, et al. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res. 2007; 100:1723–1731.
22. Vinogradova, TM, Zhou, YY, Maltsev, V, et al. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004; 94:802–809.
23. Vinogradova, TM, Lyashkov, AE, Zhu, W, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006; 98:505–514.
24. Yang, HT, Tweedie, D, Wang, S, et al. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci U S A. 2002; 99:9225–9230.
25. Fu, JD, Li, J, Tweedie, D, et al. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB J. 2006; 20:181–183.
26. Bround, MJ, Asghari, P, Wambolt, R, et al. Cardiac ryanodine receptors control heart rate and rhythmicity in adult mice. Cardiovasc Res. 2012; 96:372–380.
27. Reppel, M, Sasse, P, Malan, D, et al. Functional expression of the Na+/Ca2+ exchanger in the embryonic mouse heart. J Mol Cell Cardiol. 2007; 42:121–132.
28. Groenke, S, Larson, DL, Nakano, H, et al. Atrial-specific NCX KO mice reveal dependence of sinoatrial node pacemaker activity on sodium-calcium exchange. Biophys J. 2012; 102:663a.
29. Maltsev, VA, Vinogradova, TM, Bogdanov, KY, et al. Diastolic calcium release controls the beating rate of rabbit sinoatrial node cells: Numerical modeling of the coupling process. Biophys J. 2004; 86:2596–2605.
30. Bogdanov, KY, Maltsev, VA, Vinogradova, TM, et al. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res. 2006; 99:979–987.
31. Vinogradova, TM, Sirenko, S, Lyashkov, AE, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008; 102:761–769.
32. Monfredi, OJ, Maltseva, LA, Boyett, MR, et al. Stochastic beat-to-beat variation in periodicity of local calcium releases predicts intrinsic cycle length variability in single sinoatrial node cells. Biophys J. 2011; 100:558a.
33. Maltsev, VA, Lakatta, EG. The funny current in the context of the coupled clock pacemaker cell system. Heart Rhythm. 2012; 9:302–307.
34. Yaniv, Y, Maltsev, VA, Escobar, AL, et al. Beat-to-beat Ca2+-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm. J Mol Cell Cardiol. 2011; 51:902–905.
35. Mattick, P, Parrington, J, Odia, E, et al. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the If pacemaker current. J Physiol. 2007; 582:1195–1203.
36. Younes, A, Lyashkov, AE, Graham, D, et al. Ca2+-stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008; 283:14461–14468.
37. Wu, Y, Gao, Z, Chen, B, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009; 106:5972–5977.
38. Gao, Z, Singh, MV, Hall, DD, et al. Catecholamine-independent heart rate increases require Ca2+/calmodulin-dependent protein kinase II. Circ Arrhythm Electrophysiol. 2011; 4:379–387.
39. Lakatta, EG. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc Res. 1992; 26:193–214.
40. Vinogradova, TM, Brochet, DX, Sirenko, S, et al. Sarcoplasmic reticulum Ca2+ pumping kinetics regulates timing of local Ca2+ releases and spontaneous beating rate of rabbit sinoatrial node pacemaker cells. Circ Res. 2010; 107:767–775.
41. Vinogradova, TM, Bogdanov, KY, Lakatta, EG. Beta-adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002; 90:73–79.
42. Zahanich, I, Li, Y, Lyashkov, AE, et al. Protein phosphatase 1 regulates normal automaticity of the heart’s pacemaker node cells by site-specific modulation of phospholamban phosphorylation that regulates spontaneous subsarcolemmal local Ca2+ releases. Circulation. 2010; 122:A21546.
43. Maltsev, VA, Lakatta, EG. A novel quantitative explanation for autonomic modulation of cardiac pacemaker cell automaticity via a dynamic system of sarcolemmal and intracellular proteins. Am J Physiol Heart Circ Physiol. 2010; 298:H2010–H2023.
44. Lyashkov, AE, Vinogradova, TM, Zahanich, I, et al. Cholinergic receptor signaling modulates spontaneous firing of sinoatrial nodal cells via integrated effects on PKA-dependent Ca2+ cycling and IKACh. Am J Physiol Heart Circ Physiol. 2009; 297:H949–H959.
45. van Borren, MM, Verkerk, AO, Wilders, R, et al. Effects of muscarinic receptor stimulation on Ca2+ transient, cAMP production and pacemaker frequency of rabbit sinoatrial node cells. Basic Res Cardiol. 2010; 105:73–87.
46. Zahanich, I, Sirenko, SG, Maltseva, LA, et al. Rhythmic beating of stem cell-derived cardiac cells requires dynamic coupling of electrophysiology and Ca cycling. J Mol Cell Cardiol. 2011; 50:66–76.
47. Boink, GJ, Nearing, BD, Shlapakova, IN, et al. Ca2+-stimulated adenylyl cyclase AC1 generates efficient biological pacing as single gene therapy and in combination with HCN2. Circulation. 2012; 126:528–536.
48. Kapoor, N, Liang, W, Marbán, E, Cho, HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013; 31:54–62.
49. Maltsev, VA, Lakatta, EG, Zahanich, I, Sirenko, SG. Engineered biological pacemakers (U. S. provisional patent application no. 61/180,491). Federal Register. 2009; 74:53268.
50. Yaniv, Y, Juhaszova, M, Lyashkov, AE, et al. Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand. J Mol Cell Cardiol. 2011; 51:740–748.
51. Boyett, MR, Honjo, H, Kodama, I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000; 47:658–687.
52. Ju, YK, Allen, DG. Early effects of metabolic inhibition on intracellular Ca2+ in toad pacemaker cells: Involvement of Ca2+ stores. Am J Physiol Heart Circ Physiol. 2003; 284:H1087–H1094.
53. Yaniv, Y, Juhaszova, M, Nuss, HB, et al. Matching ATP supply and demand in mammalian heart: In vivo, in vitro, and in silico perspectives. Ann N Y Acad Sci. 2010; 1188:133–142.
54. Covian, R, Balaban, RS. Cardiac mitochondrial matrix and respiratory complex protein phosphorylation. Am J Physiol Heart Circ Physiol. 2012; 303:H940–H966.
55. Senges, J, Brachmann, J, Pelzer, D, et al. Effect of glycolytic inhibitors on the sinoatrial node, atrium and atrioventricular node in the rabbit heart. J Mol Cell Cardiol. 1981; 13:811–821.
56. Yaniv, Y, Spurgeon, HA, Lyashkov, AE, et al. Crosstalk between mitochondrial and sarcoplasmic reticulum Ca2+ cycling modulates cardiac pacemaker cell automaticity. PLoS One. 2012; 7:e37582.
57. Lakatta, EG, Maltsev, VA, Bogdanov, KY, et al. Cyclic variation of intracellular calcium: A critical factor for cardiac pacemaker cell dominance. Circ Res. 2003; 92:e45–e50.