Chapter 4 Cardiac Embolism
Cardiac embolism represents one of the most common mechanisms of ischemic stroke. It is most common in the elderly as a consequence of the high incidence of atrial fibrillation, but it is also one of the most frequent causes of stroke in the young. Yet several factors often make the diagnosis of cardiac embolism complicated: (1) cardiac embolism can produce infarctions in any vascular distribution; (2) some of the potential sources of cardiac embolism are prevalent in the general population; (3) cardioembolic sources often coexist with atherosclerotic vascular lesions in the cerebral vasculature. Furthermore, even when the diagnosis of a cardioembolic stroke is typically followed by the initiation of anticoagulation for secondary prevention, this intervention has only been scientifically validated for atrial fibrillation and mechanical valve prosthesis. With these caveats in mind, Table 4-1 lists the potential causes of cardiac embolism divided according to the strength of the evidence supporting the pathophysiological association.
TABLE 4-1 Cardiac sources of embolism.
| Definite |
| Atrial fibrillation (chronic and paroxysmal)* |
| Mechanical valve prosthesis |
| Rheumatic valve disease |
| Infective endocarditis |
| Nonbacterial thrombotic endocarditis |
| Dilated cardiomyopathy with severely reduced left ventricular ejection fraction |
| Acute transmural myocardial infarction (especially of the anterior wall) |
| Mural thrombi |
| Apical aneurysm |
| Left atrial thrombus |
| Atrial myxoma |
| Probable |
| Patent foramen ovale with atrial septal aneurysm |
| Biological prosthetic valves (early after surgery) |
| Possible |
| Patent foramen ovale |
| Spontaneous echo contrast |
| Left atrial enlargement |
| Degenerative mitral or aortic valve disease |
| Valve strands |
| Mitral annular calcification |
| Valvular fibroelastoma |
| Nondilatated cardiomyopathies |
| Sick sinus syndrome |
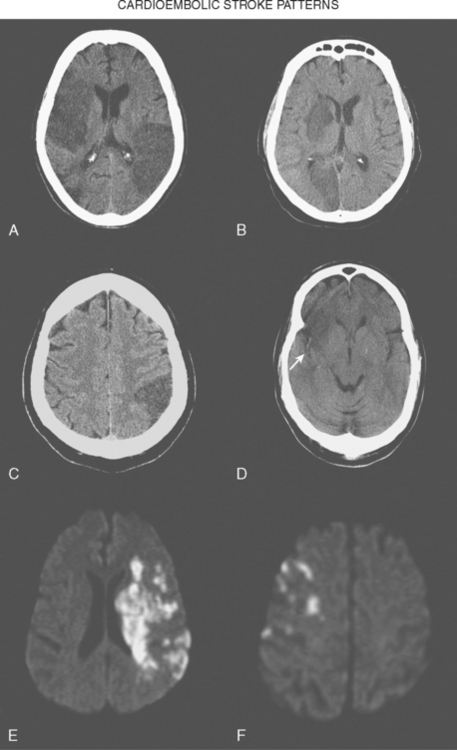
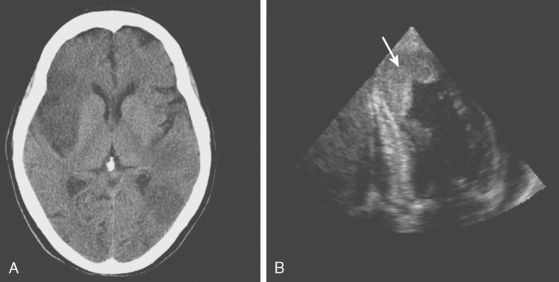
Brain infarctions caused by cardiac embolism tend to share similar radiological characteristics regardless of the type of cardiac disease responsible for the embolism. These general characteristics are listed in Table 4-2 and illustrated in Figure 4-1. The pattern of acute multiple bilateral infarctions on diffusion-weighted imaging (DWI), especially when involving the anterior and posterior circulation territories, is strongly associated with cardiac embolism.1,2 Infarctions with embolic appearance and acute multiple brain infarctions on DWI2–4 should raise suspicion of a cardiac source but may also be produced by artery-to-artery embolism. In fact, it is essentially impossible solely on the basis of brain imaging to distinguish cardioembolic infarctions from infarctions due to aortic embolism. It is important to keep in mind that cardioembolic strokes can have atypical presentations, such as relatively small subcortical infarctions.5 Thus the presence of radiological features highly suggestive of cardiac embolism should prompt comprehensive cardiac assessment, but their absence does not exclude the possibility of a cardioembolic mechanism.
TABLE 4-2 Radiological characteristics of cardioembolic strokes.
| Wedge-shaped infarctions based in the cortex |
| Concurrent acute bilateral infarctions |
| Concurrent acute infarctions in the anterior and posterior circulations |
| Multiple cortical infarctions in various vascular distributions (even if infarctions are of different ages) |
| Greater tendency to hemorrhagic transformation |
The advent of echocardiography, particularly transesophageal echocardiography (TEE), has provided a wealth of information on various cardiac abnormalities that may increase the risk of ischemic stroke. Transthoracic echocardiography (TTE) provides better visualization of the left ventricle and mitral valve, but it typically adds little to TEE in the evaluation of stroke patients. TEE offers better visualization of the left atrium, left atrial appendage, interatrial septum, aortic valve, and aortic arch. The superiority of TEE for the study of the cardiac structures more commonly associated with embolism makes it the preferred choice for the evaluation of cardiac embolism in combination with electrocardiography (to detect myocardial ischemia and arrhythmias) and Holter monitoring (to recognized paroxysmal arrhythmias not apparent on the electrocardiogram [ECG]).6 TTE is justified when myocardial ischemia, areas of ventricular hypokinesis/akinesis, or dilatated cardiomyopathy are suspected by the history or the initial ECG. Although we strongly advocate the use of TEE for the study of cardiac sources of embolism after a transient ischemic attack (TIA) or stroke, in this chapter we present several examples of diagnostic TTE, because it was the institutional practice at Jackson Memorial Hospital’University of Miami to perform a transthoracic study first.
ATRIAL FIBRILLATION
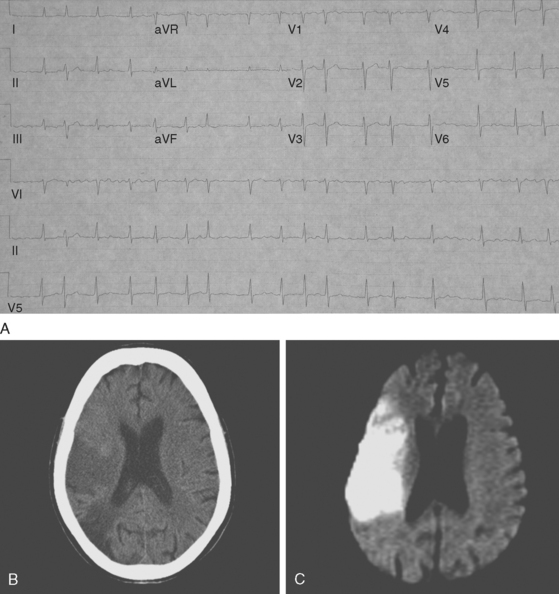
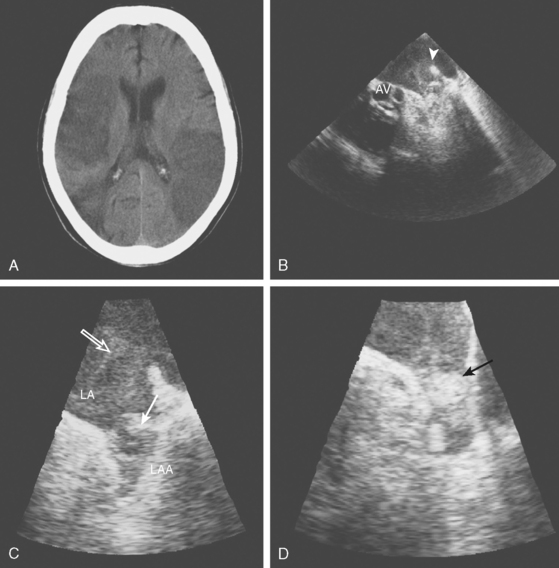
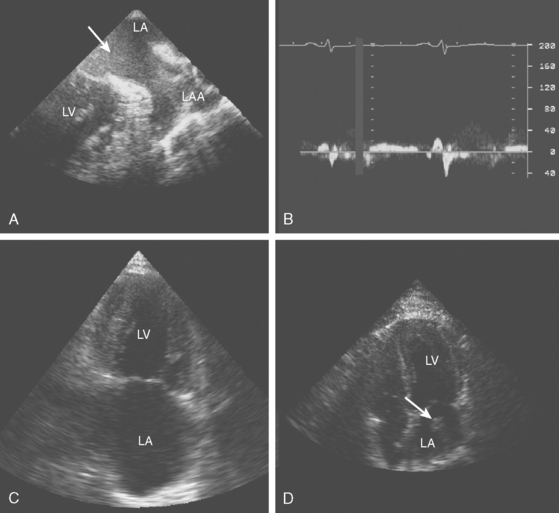
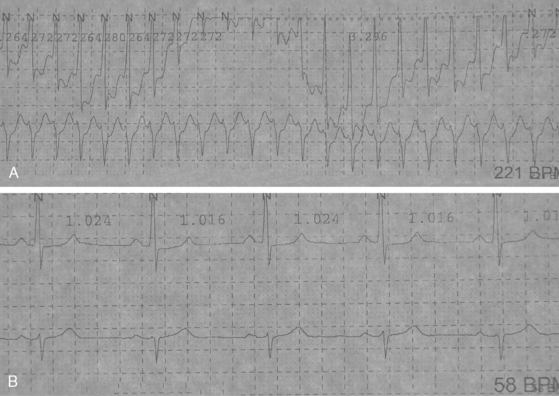
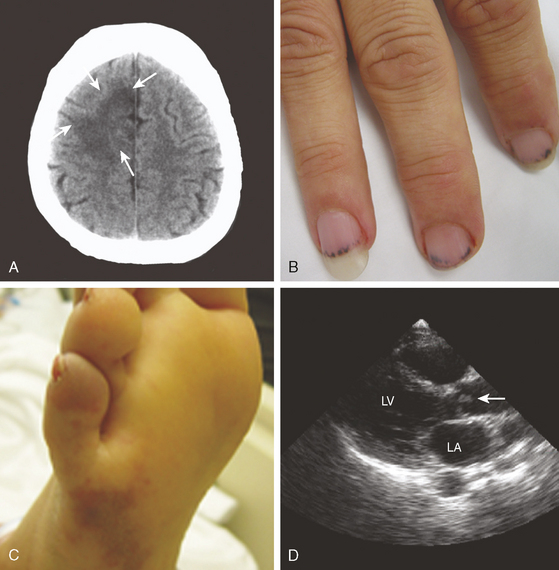
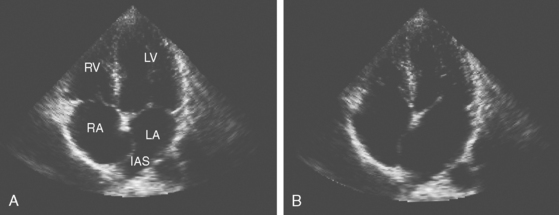
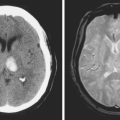
A 72-year-old man with history of hypertension, dyslipidemia, and coronary artery disease presented with sudden left-sided weakness. Examination revealed an irregularly irregular pulse, left visual field deficit, left hemiparesis, and left hemihypoesthesia with neglect. ECG confirmed the suspicion of atrial fibrillation, and brain imaging showed a right middle cerebral artery infarction (Figure 4-2). TEE was remarkable for left atrial enlargement but did not show any atrial thrombus or dense spontaneous echo contrast. The patient was initially treated with aspirin because of concerns about possible hemorrhagic transformation of the cerebral infarction. Beta-blockers were successful in maintaining the ventricular rate controlled. Warfarin was started 3 days after the stroke without complications. The patient evolved favorably and remained free of stroke recurrence 3 years later.
DILATED CARDIOMYOPATHY
MYOCARDIAL INFARCTION
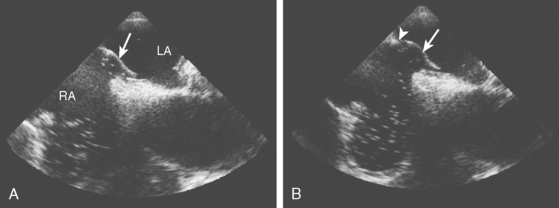
INFECTIVE ENDOCARDITIS
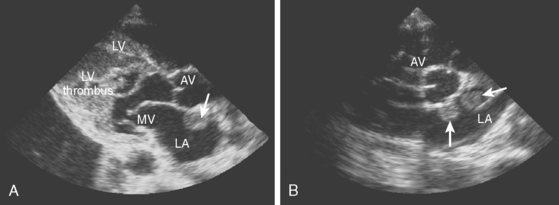
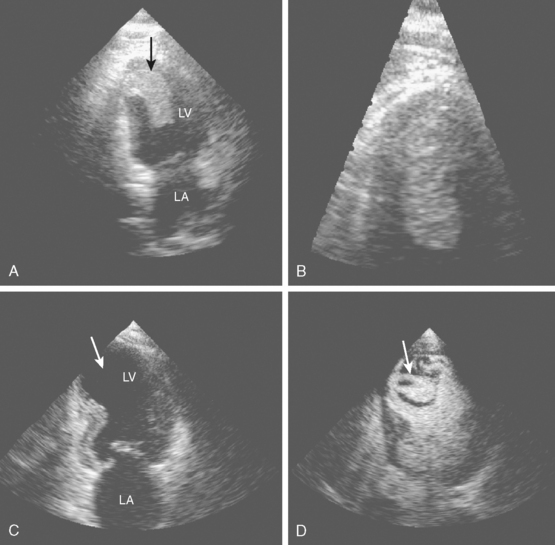
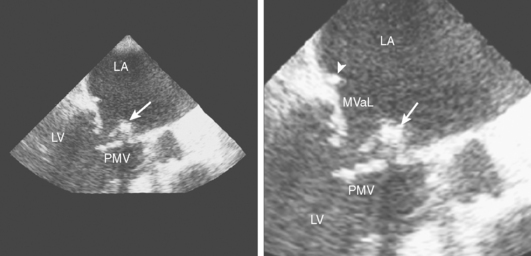
A 46-year-old man with recent community-acquired pneumonia and history of diabetes presented with worsening dyspnea. Examination revealed that the patient was confused and febrile, and careful cardiac auscultation denoted a new systolic murmur with characteristics suggestive of mitral regurgitation. Brain imaging showed scattered, small, acute ischemic infarctions in cortical locations. Echocardiography disclosed a vegetation attached to the mitral valve (Figure 4-10, F and G), and blood cultures grew Staphylococcus aureus. The patient was successfully treated with vancomycin and rifampin first and later switched to nafcillin with rifampin when susceptibilities proved that the organism was not methicillin-resistant. The patient did not have recurrent episodes of systemic or cerebral embolism, and repeat echocardiography 4 weeks later confirmed resolution of the vegetation.
PROSTHETIC VALVES
NONBACTERIAL THROMBOTIC ENDOCARDITIS
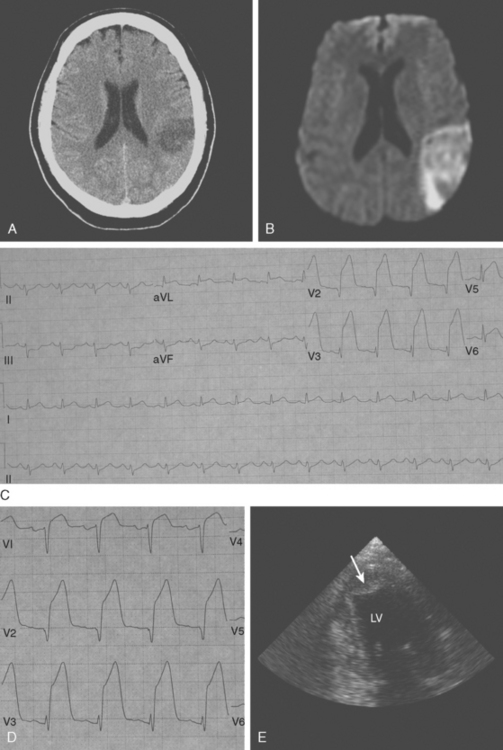
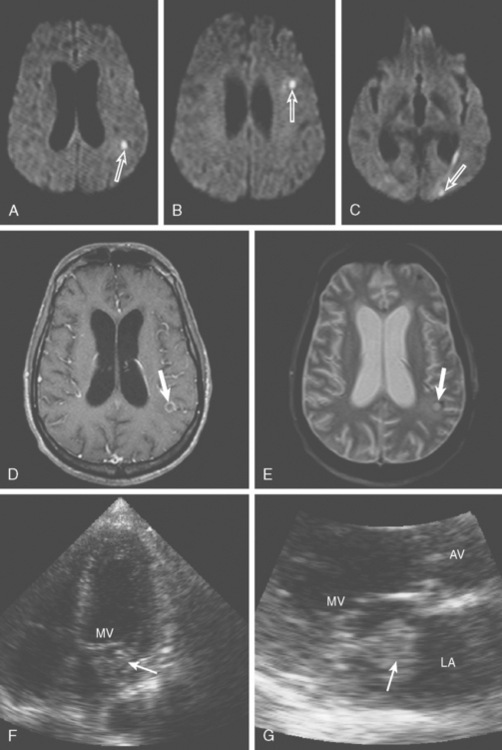
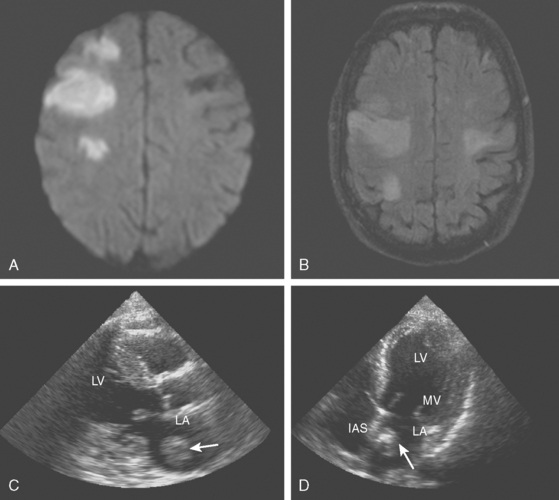
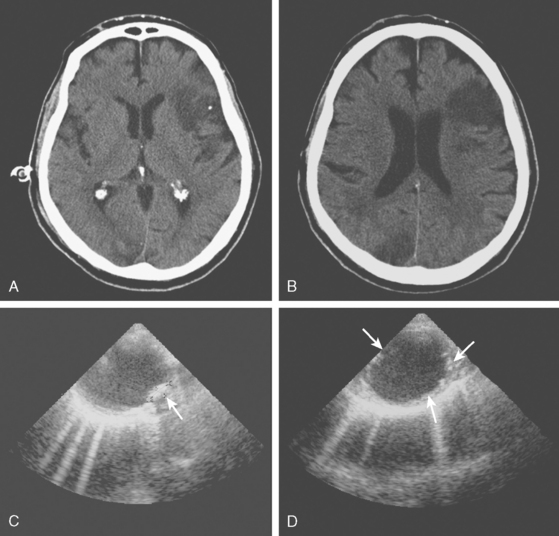
A 42-year-old woman without previous medical problems presented with acute left inferior quadrantanopsia, left hemiparesis, and diffuse petechiae in all limbs. Brain computed tomography (CT) showed high fronto-parietal hypodensities involving the cortex (Figure 4-13, A). Blood studies revealed thrombocytopenia (40,000 per mm3), leukocytosis (30,000 per mm3) and elevated lactate dehydrogenase (5060 U/L). An electrocardiogram disclosed inverted T waves in the inferior leads. Heparin and aspirin were started. Hours after admission, she developed excruciating left flank pain and became confused. Examination showed a pulseless and cold right foot. After four blood cultures were obtained, the patient was empirically started on antibiotics and high-dose dexamethasone. She improved over the following day, with less pain, improved foot temperature, and increasing platelet count. However, she remained confused and exhibited more ecchymotic lesions and subungual splinter hemorrhages (Figure 4-13, B and C). Chest CT revealed large mediastinal lymphadenopathy, and CT of abdomen showed multiple splenic and renal infarcts. Testing for cryoglobulins, antinuclear antibodies, antiphospholipid antibodies, complement levels, as well as serial blood cultures were negative. Transthoracic echocardiogram (TTE) (Figure 4-13, D) showed a large vegetation attached to the aortic valve, with moderate aortic regurgitation and moderate posterior and apical hypokinesis. The patient underwent aortic valve replacement, and pathological examination revealed marantic vegetations with myxoid degeneration of the removed valve. Stains and cultures of the vegetations were negative. After surgery, she developed bilateral leg ischemia, renal failure, and congestive heart failure due to global left ventricular hypokinesis documented by a new TTE that revealed acceptable function of the prosthetic aortic valve. She expired 7 days after the surgery. Necropsy findings included metastatic adenocarcinoma of unknown primary involving paratracheal nodes and pleura, bilateral kidney and splenic infarctions, congestive hepatomegaly, serosanguineous pleural effusions and ascites, large organizing right cerebral infarcts, and microscopic cerebellar infarcts. The prosthetic valve was covered by multiple vegetations that resulted in partial luminal occlusion.
MITRAL STENOSIS AND ANNULAR CALCIFICATION
ATRIAL MYXOMA
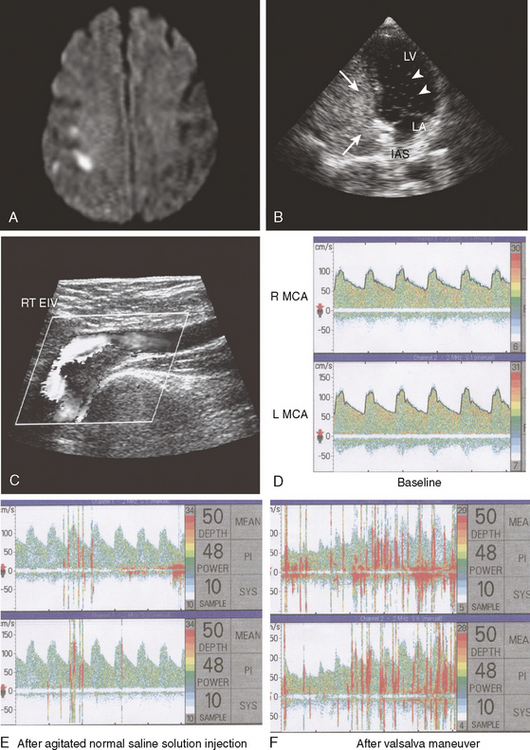
PATENT FORAMEN OVALE
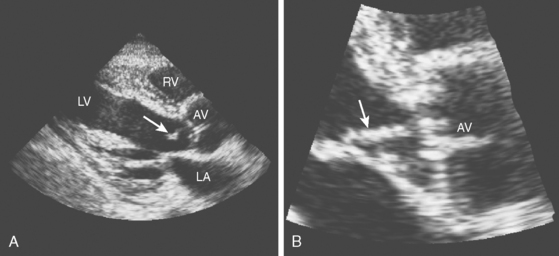
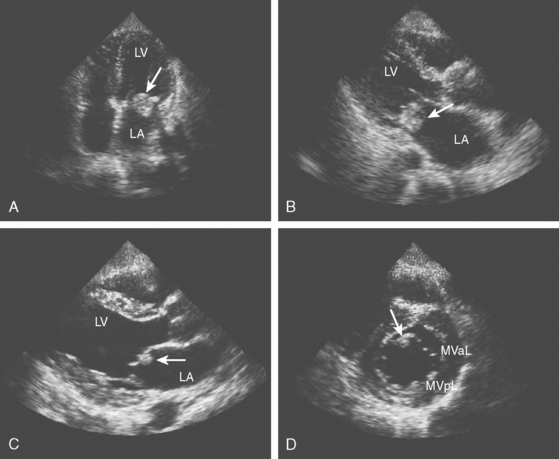
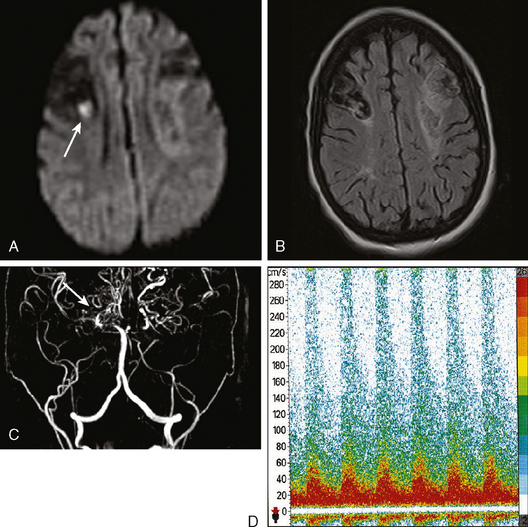
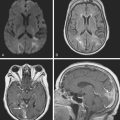
A 44-year-old woman presented with sudden left-sided weakness and paresthesias and problems with speech articulation. Her symptoms had started after she had tried to lift a heavy box from the floor. Her only risk factors for atherosclerosis were obesity and smoking. Her medical history was otherwise significant for a previous episode of deep venous thrombosis in one leg 3 years before, which was considered spontaneous and had been treated with nearly 6 months of anticoagulation. Brain imaging revealed small cortical infarctions in the posterior right frontal lobe (Figure 4-17, A). Carotid ultrasound was unrevealing, and ECG showed sinus rhythm. Transthoratic echocardiogram with bubble study disclosed a patent foramen ovale (PFO) (Figure 4-17, B). The right-to-left shunt at rest and exacerbated with Valsalva was subsequently confirmed by transcranial Doppler (Figure 4-17, D–F). Venous Doppler of the lower extremities showed an acute deep venous thrombosis in the right leg (Figure 4-17, C). Hypercoagulability workup was positive for factorLeiden mutation (heterozygous). The patient was treated with oral anticoagulation and had no stroke recurrences at the last follow-up 32 months later.
PROXIMAL AORTIC ATHEROSCLEROTIC PLAQUE
A 76-year-old man with history of hypertension, hyperlipidemia, smoking, coronary artery disease, peripheral vascular disease with claudication, and previous right carotid endarterectomy for severe, asymptomatic stenosis presented with acute right sided weakness. Examination confirmed the presence of right hemiparesis but also disclosed a left visual field deficit. CT scan of the brain showed cortical infarctions in the left frontal and right occipital lobes (Figure 4-20, A and B). Carotid duplex showed moderate stenosis on the left internal carotid origin and no significant restenosis on the right side. Electrocardiography and cardiac telemetry proved that the patient was in sinus rhythm. TEE showed extensive aortic arch atherosclerosis including a thick focal plaque with an area of small ulceration on its surface. Although there was no mobile component in the aortic plaque, the patient was treated with oral anticoagulation for 3 months along with low-dose aspirin, high-dose statin, and adjustment in the doses of his antihypertensive agents. The patient recovered favorably with help from rehabilitation services, and repeat TEE 3 months later showed reduction in the size of the larger aortic arch plaque. Warfarin was stopped, and he was continued on aspirin without stroke recurrence.
1 Wessels T, Wessels C, Ellsiepen A, Reuter I, Trittmacher S, Stolz E, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol. 2006;27:35-39.
2 Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol. 2003;60:1730-1734.
3 Roh JK, Kang DW, Lee SH, Yoon BW, Chang KH. Significance of acute multiple brain infarction on diffusion-weighted imaging. Stroke. 2000;31:688-694.
4 Baird AE, Lovblad KO, Schlaug G, Edelman RR, Warach S. Multiple acute stroke syndrome: marker of embolic disease? Neurology. 2000;54:674-678.
5 Seifert T, Enzinger C, Storch MK, Pichler G, Niederkorn K, Fazekas F. Acute small subcortical infarctions on diffusion weighted MRI: clinical presentation and aetiology. J Neurol Neurosurg Psychiatry. 2005;76:1520-1524.
6 Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke. 2006;37:859-864.
7 Douen AG, Pageau N, Medic S. Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6-fold in patients with acute stroke. Stroke. 2008;39:480-482.
8 Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35:1647-1651.
9 Liao J, Khalid Z, Scallan C, Morillo C, O’Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke. 2007;38:2935-2940.
10 Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835-841.
11 Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:6-12.
12 Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998;31:1622-1626.
13 The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med. 1998;128:639-647.
14 Strandberg M, Raatikainen MJ, Niemela M, Luotolahti M, Hartiala J, Airaksinen KE. Clinical practicality and predictive value of transesophageal echocardiography in early cardioversion of atrial fibrillation. Europace. 2006;8:408-412.
15 Hart RG, Sherman DG, Easton JD, Cairns JA. Prevention of stroke in patients with nonvalvular atrial fibrillation. Neurology. 1998;51:674-681.
16 Ostermayer SH, Reisman M, Kramer PH, Matthews RV, Gray WA, Block PC, et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005;46:9-14.
17 Koniaris LS, Goldhaber SZ. Anticoagulation in dilated cardiomyopathy. J Am Coll Cardiol. 1998;31:745-748.
18 Pullicino PM, Halperin JL, Thompson JL. Stroke in patients with heart failure and reduced left ventricular ejection fraction. Neurology. 2000;54:288-294.
19 Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336:251-257.
20 Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409-e449.
21 Ruttmann E, Willeit J, Ulmer H, Chevtchik O, Hofer D, Poewe W, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke. 2006;37:2094-2099.
22 Delahaye JP, Poncet P, Malquarti V, Beaune J, Gare JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J. 1990;11:1074-1078.
23 Anderson DJ, Goldstein LB, Wilkinson WE, Corey GR, Cabell CH, Sanders LL, et al. Stroke location, characterization, severity, and outcome in mitral vs aortic valve endocarditis. Neurology. 2003;61:1341-1346.
24 Hart RG, Foster JW, Luther MF, Kanter MC. Stroke in infective endocarditis. Stroke. 1990;21:695-700.
25 Salgado AV, Furlan AJ, Keys TF, Nichols TR, Beck GJ. Neurologic complications of endocarditis: a 12-year experience. Neurology. 1989;39:173-178.
26 Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke. 2002;33:1267-1273.
27 Paschalis C, Pugsley W, John R, Harrison MJ. Rate of cerebral embolic events in relation to antibiotic and anticoagulant therapy in patients with bacterial endocarditis. Eur Neurol. 1990;30:87-89.
28 Hart RG, Kagan-Hallet K, Joerns SE. Mechanisms of intracranial hemorrhage in infective endocarditis. Stroke. 1987;18:1048-1056.
29 Wijdicks EF, Schievink WI, Brown RD, Mullany CJ. The dilemma of discontinuation of anticoagulation therapy for patients with intracranial hemorrhage and mechanical heart valves. Neurosurgery. 1998;42:769-773.
30 Biller J, Challa VR, Toole JF, Howard VJ. Nonbacterial thrombotic endocarditis. A neurologic perspective of clinicopathologic correlations of 99 patients. Arch Neurol. 1982;39:95-98.
31 Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med. 1987;83:746-756.
32 Rabinstein AA, Giovanelli C, Romano JG, Koch S, Forteza AM, Ricci M. Surgical treatment of nonbacterial thrombotic endocarditis presenting with stroke. J Neurol. 2005;252:352-355.
33 Nair CK, Thomson W, Ryschon K, Cook C, Hee TT, Sketch MH. Long-term follow-up of patients with echocardiographically detected mitral anular calcium and comparison with age- and sex-matched control subjects. Am J Cardiol. 1989;63:465-470.
34 Ekinci EI, Donnan GA. Neurological manifestations of cardiac myxoma: a review of the literature and report of cases. Intern Med J. 2004;34:243-249.
35 Sabolek M, Bachus-Banaschak K, Bachus R, Arnold G, Storch A. Multiple cerebral aneurysms as delayed complication of left cardiac myxoma: a case report and review. Acta Neurol Scand. 2005;111:345-350.
36 Messe SR, Silverman IE, Kizer JR, Homma S, Zahn C, Gronseth G, et al. Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;62:1042-1050.
37 Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740-1746.
38 Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55:1172-1179.
39 Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262-2268.
40 Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O’Fallon WM, Pascoe RD, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc. 1999;74:862-869.
41 Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47:440-445.
42 Petty GW, Khandheria BK, Meissner I, Whisnant JP, Rocca WA, Christianson TJ, et al. Population-based study of the relationship between patent foramen ovale and cerebrovascular ischemic events. Mayo Clin Proc. 2006;81:602-608.
43 Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625-2631.
44 Jauss M, Wessels T, Trittmacher S, Allendorfer J, Kaps M. Embolic lesion pattern in stroke patients with patent foramen ovale compared with patients lacking an embolic source. Stroke. 2006;37:2159-2161.
45 Belvis R, Leta RG, Marti-Fabregas J, Cocho D, Carreras F, Pons-Llado G, et al. Almost perfect concordance between simultaneous transcranial Doppler and transesophageal echocardiography in the quantification of right-to-left shunts. J Neuroimaging. 2006;16:133-138.
46 Ziai WC, Oh S, Razumovsky AY, Wityk RJ. Quantitation of contrast TCD in patients with and without atrial septal aneurysm. J Neuroimaging. 2005;15:250-253.
47 Droste DW, Schmidt-Rimpler C, Wichter T, Dittrich R, Ritter M, Stypmann J, et al. Right-to-left-shunts detected by transesophageal echocardiography and transcranial Doppler sonography. Cerebrovasc Dis. 2004;17:191-196.
48 Droste DW, Kriete JU, Stypmann J, Castrucci M, Wichter T, Tietje R, et al. Contrast transcranial Doppler ultrasound in the detection of right-to-left shunts: comparison of different procedures and different contrast agents. Stroke. 1999;30:1827-1832.
49 Steiner MM, Di Tullio MR, Rundek T, Gan R, Chen X, Liguori C, et al. Patent foramen ovale size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;29:944-948.
50 De Castro S, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407-2413.
51 Bonati LH, Kessel-Schaefer A, Linka AZ, Buser P, Wetzel SG, Radue EW, et al. Diffusion-weighted imaging in stroke attributable to patent foramen ovale: significance of concomitant atrial septum aneurysm. Stroke. 2006;37:2030-2034.
52 Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474-1479.
53 Jones EF, Kalman JM, Calafiore P, Tonkin AM, Donnan GA. Proximal aortic atheroma. An independent risk factor for cerebral ischemia. Stroke. 1995;26:218-224.
54 Tunick PA, Rosenzweig BP, Katz ES, Freedberg RS, Perez JL, Kronzon I. High risk for vascular events in patients with protruding aortic atheromas: a prospective study. J Am Coll Cardiol. 1994;23:1085-1090.
55 The French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
56 Meissner I, Khandheria BK, Sheps SG, Schwartz GL, Wiebers DO, Whisnant JP, et al. Atherosclerosis of the aorta: risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiography study. J Am Coll Cardiol. 2004;44:1018-1024.
57 Petty GW, Khandheria BK, Meissner I, Whisnant JP, Rocca WA, Sicks JD, et al. Population-based study of the relationship between atherosclerotic aortic debris and cerebrovascular ischemic events. Mayo Clin Proc. 2006;81:609-614.
58 Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation. 2006;114:63-75.
59 Petty GW, Khandheria BK, Meissner I, Whisnant JP, Rocca WA, Sicks JD, et al. Population-based study of the relationship between atherosclerotic aortic debris and cerebrovascular ischemic events. Mayo Clin Proc. 2006;81:609-614.
60 Tunick PA, Nayar AC, Goodkin GM, Mirchandani S, Francescone S, Rosenzweig BP, et al. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol. 2002;90:1320-1325.
61 Lima JA, Desai MY, Steen H, Warren WP, Gautam S, Lai S. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation. 2004;110:2336-2341. 19
62 Ferrari E, Vidal R, Chevallier T, Baudouy M. Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants. J Am Coll Cardiol. 1999;33:1317-1322.
63 Davila-Roman VG, Phillips KJ, Daily BB, Davila RM, Kouchoukos NT, Barzilai B. Intraoperative transesophageal echocardiography and epiaortic ultrasound for assessment of atherosclerosis of the thoracic aorta. J Am Coll Cardiol. 1996;28:942-947.
64 Gold JP, Torres KE, Maldarelli W, Zhuravlev I, Condit D, Wasnick J. Improving outcomes in coronary surgery: the impact of echo-directed aortic cannulation and perioperative hemodynamic management in 500 patients. Ann Thorac Surg. 2004;78:1579-1585.
65 Ascione R, Ghosh A, Reeves BC, Arnold J, Potts M, Shah A, et al. Retinal and cerebral microembolization during coronary artery bypass surgery: a randomized, controlled trial. Circulation. 2005;112:3833-3838.
66 Stern A, Tunick PA, Culliford AT, Lachmann J, Baumann FG, Kanchuger MS, et al. Protruding aortic arch atheromas: risk of stroke during heart surgery with and without aortic arch endarterectomy. Am Heart J. 1999;138(4 Pt 1):746-752.