CHAPTER 5 Cardiac and vascular disorders
Cardiovascular assessment: general
Palpation
Pulse assessment to evaluate for decreased tissue perfusion:
• Pulse quality and regularity bilaterally (scale 0 to 4+)
• Edema (scale 0 to 4+): extremities, back, and sacrum
• Evaluate all peripheral pulses to assess for vascular disease.
• Heart sounds to evaluate for contributors to decreased cardiac output (note changes with body positioning and respirations):
Labwork
Blood studies can reveal causes of dysrhythmias or changes in pacing/conduction or HR changes:
• Electrolyte levels: ↑ or ↓ potassium or magnesium
• Complete blood counts: anemia, ↑ white blood cells (WBCs)
CARE PLANS FOR GENERALIZED CARDIOVASCULAR DYSFUNCTIONS
![]() related to decreased cardiac output
related to decreased cardiac output
1. Determine patient’s physical limitations.
2. Determine causes of fatigue and perceived causes of fatigue.
3. Monitor cardiorespiratory response to activity (tachycardia, other dysrhythmias, tachypnea, dyspnea, diaphoresis, pallor) and hemodynamic response (elevated pulmonary artery pressures [PAPs], central venous pressure [CVP], or no change/little increase in cardiac output) if a pulmonary artery catheter or bioimpedance device is in place.
4. Monitor for chest discomfort during activity.
5. Reduce all causes of discomfort, including those induced by the patient’s environment, such as uncomfortable room temperature or position, thirst/dry mouth, and wrinkled or damp bedding.
Self-care assistance: instrumental activities of daily living (iadls)
1. Determine need for assistance with IADLs including walking, cooking, shopping, housekeeping, transportation, and money management.
2. Provide for methods of contacting support of assistance people (such as lifeline services, emergency response services including readily accessible telephone numbers if patient’s area is not 911 accessible).
3. Determine financial resources and personal preferences for modifying their home to accommodate any disabilities.
related to altered cardiac pump function
1. Palpate and evaluate quality of peripheral pulses, for presence of edema, capillary refill, and skin color and temperature of extremities.
2. Monitor ECG continuously, noting HR and rhythm. Select the most diagnostic lead(s) for monitoring patient. Consider use of ST-segment monitoring if available.
3. Compare current ECG readings with past readings and report abnormal findings that create instability or have the potential to create instability.
4. Use a 12- or 15-lead ECG to diagnose heart rhythm changes, because one or two leads are often insufficient to fully diagnose ECG changes.
5. Provide antidysrhythmic medications as appropriate to abate heart rhythms that prompt hypotension.
6. Provide positive inotropic drugs as appropriate to help increase cardiac output to maintain stable BP.
7. Monitor effects of negative inotropic medications (e.g., beta blockers) carefully, as the decreased myocardial workload may prompt hypotension.
8. Evaluate chest pain for location, radiation, intensity, duration, and precipitating factors. Emphasize to patient the importance of reporting all instances of chest pain and pressure and arm, neck, and jaw pain.
9. Apply oxygen when chest pain is present, according to Advanced Cardiac Life Support (ACLS) guidelines.
10. Monitor pacemaker function as appropriate to insure device is sensing, pacing and capturing appropriately.
11. Auscultate heart tones; be alert for development of new S3 and S4, new “split” sounds, or pericardial friction rubs.
12. Auscultate lungs for rales, crackles, wheezes, rhonchi, pleural friction rubs, or other adventitious sounds indicative of fluid retention.
13. Monitor for diminished level of consciousness, which may signal cerebral perfusion is compromised secondary to decreased cardiac output.
14. Auscultate abdomen and monitor for decreased bowel sounds and/or abdominal distention, which may indicate abdominal perfusion is compromised.
15. Record intake and output, urine output, and daily weight and evaluate for fluid retention, which may indicate renal perfusion is compromised.
16. Note electrolyte values at least daily, monitoring closely for changes in potassium and magnesium, which may prompt dysrhythmias; increased blood urea nitrogen (BUN) or increased creatinine, which may indicate low CO is causing renal insufficiency; and hyperglycemia, which may indicate patient has underlying diabetes.
17. Monitor for increasing activity intolerance, dyspnea, excessive fatigue, and orthopnea, which may all indicate CO is lessening.
18. Keep head of the bed (HOB) elevated if patient is unable to breathe comfortably when flat in bed.
19. Insert urinary catheter if patient is unable to void without markedly increasing activity level, or anuria is noted, as appropriate.
1. Monitor values generated by pulmonary artery catheter to directly assess CO.
2. Assess for further decreases in CO reflected by elevated pulmonary artery occlusive/wedge pressure, elevated CVP, and elevated pulmonary vascular resistance (PVR).
3. Monitor for fluid overload by assessing for elevated systemic vascular resistance (SVR).
4. Monitor the effects of all medications on hemodynamic readings, including effects of positive or negative inotropic agents, antidysrhythmics, and vasodilating or vasoconstricting medications.
related to decreased perfusion to the lungs
Respiratory Status: Ventilation
1. Assess for patent airway; if snoring, crowing, or strained respirations are present, indicative of partial or full airway obstruction, open airway using chin-lift or jaw-thrust.
2. Insert oral or nasopharyngeal airway if patient cannot maintain patent airway; if severely distressed, patient may require endotracheal intubation.
3. Position patient to alleviate dyspnea and ensure maximal ventilation—generally in a sitting upright position unless severe hypotension is present.
4. Clear secretions from airway by having patient cough vigorously, or provide nasotracheal, oropharyngeal, or endotracheal tube suctioning as needed.
5. Have patient breathe slowly or manually ventilate with Ambu bag slowly and deeply between coughing or suctioning attempts.
6. Assist with use of incentive spirometer as appropriate.
7. Turn patient every 2 hours if immobile. Encourage patient to turn self, or get out of bed as much as tolerated if able.
8. Provide mucolytic and bronchodilating medications orally, intravenously (IV), or by inhaler, aerosol, or nebulizer as ordered to assist with thinning secretions and relaxing muscles in lower airways.
9. Provide chest physical therapy as appropriate, if other methods of secretion removal are ineffective.
1. Provide humidity in oxygen or bilevel positive airway pressure (BiPAP) device if used for longer than 12 hours to help thin secretions.
2. Administer supplemental oxygen using liter flow and device as ordered.
3. Restrict patient and visitors from smoking while oxygen is in use.
4. Document pulse oximetry with oxygen liter flow in place at time of reading as ordered. Oxygen is a drug; the dose of the drug must be associated with the oxygen saturation reading or the reading is meaningless.
5. Obtain arterial blood gases (ABGs) if patient experiences behavioral changes or respiratory distress to check for hypoxemia or hypercapnia.
6. Monitor for oxygen-induced hypoventilation, especially in patients with chronic obstructive pulmonary disease (COPD).
7. Monitor for changes in chest radiograph and breath sounds indicative of oxygen toxicity and absorption atelectasis in patients receiving higher concentrations of oxygen (greater than FIO2 45%) for longer than 24 hours. The higher the oxygen concentration, the greater is the chance of toxicity.
8. Monitor for skin breakdown where oxygen devices are in contact with skin, such as nares and around edges of mask devices.
9. Provide oxygen therapy during transportation and when patient gets out of bed.
10. If patient is unable to maintain SPO2 reading of greater than 88% off oxygen, consult with respiratory care practitioner and physician about the need for home oxygen therapy.
1. Monitor rate, rhythm, and depth of respirations.
2. Note chest movement for symmetry of chest expansion and signs of increased work of breathing such as use of accessory muscles or retraction of intercostal or supraclavicular muscles. Consider use of BiPAP for impending respiratory failure.
3. Ensure airway is not obstructed by tongue (snoring or choking-type respirations) and monitor breathing patterns. New patterns that impair ventilation should be managed as appropriate for setting.
4. Note that trachea remains midline, as deviation may indicate patient has a tension pneumothorax.
5. Auscultate breath sounds following administration of respiratory medications to assess for improvement.
6. Note changes in oxygen saturation (SaO2), pulse oximetry (SpO2), and end-tidal CO2 (ETCO2) and ABGs as appropriate.
7. Monitor for dyspnea and note causative activities or events.
8. If increased restlessness or unusual somnolence occur, evaluate patient for hypoxemia and hypercapnia as appropriate.
9. Monitor chest radiograph reports as new films become available.
Heart failure
Pathophysiology
HF is a syndrome stemming from impaired cardiac pump function, resulting in systemic perfusion that is inadequate to meet the body’s metabolic demands for energy production. The condition may be divided into systolic or diastolic HF. In systolic HF, there is reduced cardiac contractility, while in diastolic HF, there is impaired cardiac relaxation and abnormal ventricular filling. HF is the leading cause of death in the United States, affecting approximately 5 million patients. One in five patients dies within 1 year of diagnosis. The annual medical cost is over $30 billion. Although much progress has been made in the treatment, the annual mortality rate remains high (5% to 20%). The greatest number of patients die from New York Heart Association (NYHA) Class IV symptoms, including progressive pump failure and congestion. Over half die from sudden cardiac death. Many die from end-organ failure resulting from inadequate perfusion. The kidneys are especially vulnerable. Those with a poor cardiac prognosis typically manifest a higher NYHA HF class, high catecholamine and BNP levels, renal dysfunction, cachexia, valvular regurgitation, ventricular dysrhythmias, lower ejection fraction, hyponatremia, and left ventricular (LV) dilation. Patients with both systolic and diastolic LV dysfunction have a worse prognosis than do patients with either condition alone.
Biventricular failure
Patients who experience both LV and RV MI (a combination often seen with inferior wall MI) experience hemodynamics that are extremely complex to manage. The impaired right ventricle needs volume infusion to promote better expansion, or “more stretch,” of the ventricle, whereas the left ventricle may be unable to accommodate a normal or pre-MI volume and requires volume reduction. Deviation of the intraventricular septum associated with right-sided HF caused by distention of the ventricle can significantly reduce the size of the left ventricle. Ultimately, failure in either side of the heart will affect both sides, because the ventricles are interdependent.
Cardiovascular assessment: heart failure
Goal of system assessment
Evaluate for decreased CO and decreased tissue perfusion initially with General Assessment, p. 418. If patient has developed HF secondary to acute coronary syndrome, see Assessment in Acute Coronary Syndromes, p. 434.
History and risk factors
![]() History of HF, CAD, and MI; familial history of CAD; age greater than 65 years; cigarette smoking; alcohol use; hypercholesterolemia; hypertension; diabetes; obesity; dysrhythmias; weight gain; and decreasing activity tolerance. Fatigue may be the only presenting symptom. Other important data include understanding of and compliance with low-sodium diet, fluid restriction or medications, and a decreased exercise tolerance.
History of HF, CAD, and MI; familial history of CAD; age greater than 65 years; cigarette smoking; alcohol use; hypercholesterolemia; hypertension; diabetes; obesity; dysrhythmias; weight gain; and decreasing activity tolerance. Fatigue may be the only presenting symptom. Other important data include understanding of and compliance with low-sodium diet, fluid restriction or medications, and a decreased exercise tolerance.
| Left-Sided Heart Failure Pulmonary Edema and Congestion |
Right-Sided Heart Failure Cor Pulmonale and Systemic Congestion |
Biventricular Failure Pulmonary and Systemic Congestion |
| Anxiety, air hunger, tachypnea, nocturnal dyspnea, dyspnea on exertion (DOE), orthopnea, moist cough with frothy sputum, tachycardia, diaphoresis, cyanosis or pallor, insomnia, palpitations, weakness, fatigue, anorexia, and changes in mentation | Fluid retention, peripheral edema, weight gain, decreased urinary output, abdominal tenderness, nausea, vomiting, constipation, and anorexia. Because the edema of heart failure is dependent, patients on bed rest may have edema of the feet, ankles, legs, hands, and/or sacrum. | All signs of both right- and left-sided heart failure, as stated, along with possible signs of cardiogenic shock in acutely ill patients: peripheral cyanosis, fatigue, decreased tissue perfusion, decrease in metabolism, and low urinary output |
| Physical Assessment | ||
| Decreased BP, orthostasis (drop in BP with sitting or standing), tachycardia, dysrhythmias, tachypnea, crackles or bibasilar (or dependent) rales, S3, or summation gallop | Hepatomegaly, splenomegaly, dependent pitting edema, jugular venous distention, positive hepatojugular reflex, and ascites | Hypotension, tachycardia, tachypnea, pulmonary edema, dependent pitting edema, hepatosplenomegaly, distended neck veins, pallor, and cyanosis |
| Monitoring | ||
| Decreased CO/CI, SpO2 and SVO2; elevated PAP, PAWP, SVR; dysrhythmias | Dysrhythmias, elevated RAP and CVP, precipitous drop in SVO2 with minimal activity, and possibly decreased CO/CI, caused by failure of right ventricle to pump adequate blood through the pulmonary vasculature to maintain adequate left ventricular filling volumes for normal cardiac output | Elevated PAP, PAWP, SVR, pulmonary vascular resistance (PVR), RAP, and CVP, decreased CO/CI, dysrhythmias, and decreasing SpO2 and SVO2, despite increasing administered oxygen |
Diagnostic Tests for Acute Heart Failure
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Noninvasive Cardiology | ||
| Electrocardiogram 12-, 15-, or18-lead ECG |
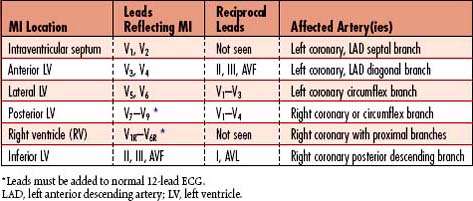
Assess for ischemic heart disease and acute or older myocardial infarction (MI); may reveal atrial and/or ventricular hypertrophy, dysrhythmias such as atrial fibrillation, which may precipitate heart failure by decreasing cardiac output, and dysrhythmias associated with electrolyte imbalance. | Presence of ST-segment depression or T wave inversion (myocardial ischemia), or pathologic Q waves (resolved MI) in 2 contiguous or related leads Contiguous leads indicative of location of ischemia or old MI: V1 and V2: Intraventricular septum V3 and V4: Anterior wall of left ventricle V5 and V6: Lateral wall of left ventricle V7–V9: Posterior wall of left ventricle II, III, AVF: Inferior wall of left ventricle V1, V1R–-V6R: Right ventricle |
| Blood Studies | ||
| Digitalis levels | Digitalis levels are often difficult to manage in heart failure patients, so levels should be done daily if the dosage is being altered. | Chronic heart failure predisposes the patient to digitalis toxicity because of the low cardiac output state, which also causes decreased renal excretion of the drug. |
| Complete blood count (CBC) Hemoglobin (Hgb) Hematocrit (Hct) RBC count (RBCs) WBC count (WBCs) |
Assess for anemia, inflammation, and infection; assists with differential diagnosis of chest discomfort and fluid balance. | May reveal decreased Hgb and Hct levels in the presence of anemia or dilution. |
| Electrolytes Potassium (K+) Magnesium (Mg2+) Calcium (Ca2+) Sodium (Na+) |
Assess for possible causes of dysrhythmias and/or heart failure. | Abnormal levels of K+, Mg2+, or Ca2+ may cause dysrhythmias; elevation of Na+ may indicate dehydration (blood is more coagulable); may reveal hyponatremia (dilutional); and may reveal hypokalemia, which can result from use of diuretics, or hyperkalemia, if glomerular filtration is decreased. Hyperkalemia can also be a side effect of angiotensin-converting enzyme inhibitors (ACEIs) and potassium-sparing diuretics. |
| Coagulation profile Prothrombin time (PT) with international normalized ratio (INR) Partial thromboplastin time (PTT) Fibrinogen D-dimer |
Assess for efficacy of anticoagulation in heart failure patients receiving warfarin therapy; also helps to evaluate for the presence of cardiogenic shock or hypoperfusion. | Decreased PT with low INR promotes clotting and reflects inadequate anticoagulation; elevation promotes bleeding; elevated fibrinogen and D-dimer reflects abnormal clotting is present. |
| B-type natriuretic peptide (BNP) | BNP, a hormone secreted by the ventricles, can be useful in distinguishing dyspnea due to heart failure from dyspnea due to pulmonary causes and in monitoring response to therapy. | Levels >100 pg/ml support the diagnosis of heart failure. However, though the BNP level decreases with effective therapy, it may remain chronically >100, even when the patient is no longer symptomatic. |
| Arterial blood gas (ABG) analysis | Assesses for changes in pH and problems with oxygenation | May reveal hypoxemia caused by the decreased oxygen available from fluid-filled alveoli. Decreased pH may be present reflecting hypoperfusion at the cellular level resulting in lactic acidosis. Lactate level may be done in addition to the ABG to assess if shock is ensuing. If the lactate level is more than 4, the patient may be in cardiogenic shock. |
| Hepatic enzymes and serum bilirubin levels | Serum glutamate oxaloacetate transaminase/aspartate aminotransferase (SGOT/AST), serum glutamate pyruvate transaminase/alanine aminotransferase (SGPT/ALT), and serum bilirubin levels may be elevated because of hepatic venous congestion. | Elevation reflects vascular congestion resulting from heart failure that has caused decreased forward blood flow from the liver to the heart. The liver becomes engorged with blood, which results in increased hepatic enzymes and bilirubin. |
| Blood urea nitrogen (BUN) and creatinine levels | Rising BUN and creatinine indicate undesirable renal response to diuretic therapy. | Elevation places patients at higher risk for renal failure secondary to heart disease. |
| Radiology | ||
| Chest radiograph (CXR) | Assesses size of heart, thoracic cage (for fractures), thoracic aorta (for aneurysm) and lungs (pneumonia, pneumothorax); assists with differential diagnosis of chest discomfort and activity intolerance | May reveal pulmonary edema, increased interstitial density, infiltrates, engorged pulmonary vasculature, and cardiomegaly Note: Portable CXR should be done with patient centered on the plate and with head of bed elevated whenever possible. |
| Cardiac magnetic resonance imaging (MRI) | Assesses ventricular size, morphology, function, status of cardiac valves, and circulation | Enlarged heart, remodeled heart, incompetent of stenotic heart valves, narrowed or occluded coronary arteries, which may be the cause of heart failure. |
| Cardiac computed tomography (CT scan) | Assesses ventricular size, morphology, function, status of cardiac valves, and circulation | Enlarged heart, remodeled heart, incompetent of stenotic heart valves, narrowed or occluded coronary arteries; technology is improving in accuracy; may eventually reduce the need for cardiac catheterization. |
| Cardiac ultrasound echocardiography (echo) | Assess for mechanical and structural abnormalities related to effective pumping of blood from both sides of the heart. | May reveal a reduced ejection fraction (ejection fraction <40%), ventricular wall motion disorders, valvular dysfunction, cardiac chamber enlargement, pulmonary hypertension, or other cardiac dysfunction |
| Transesophageal echo | Assess for mechanical and structural abnormalities related to effective pumping of blood from both sides of the heart using a transducer attached to an endoscope. | Same as for echo but can provide enhanced views, particularly of the posterior wall of the heart |
| Cardiac positron emission tomography (PET scan) | Isotopes are used to assess if viable cardiac tissue is present. | Viable tissue has increased uptake of the glucose tracer and decreased uptake of the blood flow tracer (ammonia). |
| Invasive Cardiology | ||
| Coronary angiography/cardiac catheterization | Assesses for presence and extent of CAD, left ventricular function, and valvular disease using a radiopaque catheter inserted through a peripheral vessel and advanced into the heart and coronary arteries | Treatable coronary artery blockages are a major cause of new-onset HF. Low ejection fraction indicates heart failure, stenotic or incompetent heart valves can decrease CO, narrowed or occluded coronary arteries cause chest pain, abnormal pressures in the main coronary arteries indicate impaired circulation, elevated pressures inside the chambers of the heart indicate heart failure, abnormal ventricular wall motion decreases CO, and elevated pulmonary artery pressures indicate heart failure. |
Collaborative management
Care priorities
1. Treat the underlying cause and precipitating factors.
• Diseases/conditions causing left-sided HF: Atherosclerotic heart disease, acute MI (AMI), dysrhythmias, cardiomyopathy, increased circulating volume, systemic hypertension, aortic stenosis, aortic regurgitation, mitral regurgitation, coarctation of the aorta, atrial septal defect, ventricular septal defect, cardiac tamponade, and constrictive pericarditis
• Diseases/conditions causing right-sided HF: Left-sided HF, pulmonary hypertension, atherosclerotic heart disease, AMI, dysrhythmias, pulmonary embolism, fluid overload or excess sodium intake, COPD, mitral stenosis, pulmonary stenosis, and myocardial contusion
• Diseases/conditions causing biventricular failure: Any combination of the diseases that cause either right- or left-sided HF
2. Provide oxygen therapy and support ventilation.
Supplemental oxygen is required to optimize the patient’s oxygen saturation.
![]() Pulse oximetry is done in combination with respiratory assessment, as use of pulse oximetry alone is an inaccurate reflection of efficacy of oxygenation at the cellular level. If patient is tachypneic with increased work of breathing, noninvasive positive pressure ventilation (NiPPV, NPPV, BiPAP) may be used to reduce the work of breathing, and thus relieve additional stress associated with HF (see Acute Respiratory Failure, p. 383, for additional information regarding NiPPV, mechanical ventilation, and oxygen therapy).
Pulse oximetry is done in combination with respiratory assessment, as use of pulse oximetry alone is an inaccurate reflection of efficacy of oxygenation at the cellular level. If patient is tachypneic with increased work of breathing, noninvasive positive pressure ventilation (NiPPV, NPPV, BiPAP) may be used to reduce the work of breathing, and thus relieve additional stress associated with HF (see Acute Respiratory Failure, p. 383, for additional information regarding NiPPV, mechanical ventilation, and oxygen therapy).
• Diuretics: Reduce blood volume and decrease preload. Should be used in conjunction with an ACEI or ARB. A loop diuretic is generally used initially, while a thiazide diuretic is added for patients refractory to the loop diuretic (diuretic resistance or possibly, cardiorenal syndrome) (Table 5-1). Diuretics effectively manage respiratory distress but have not been shown to improve survival. Diuretics may cause azotemia, hypokalemia, metabolic alkalosis, and elevation of neurohormone (e.g., BNP) levels.
• Morphine: Induce vasodilation and decrease venous return, preload, sympathetic tone, anxiety, myocardial oxygen consumption, and pain.
• Inodilators (milrinone and inamrinone): Phosphodiesterase-inhibiting drugs increase contractility of the heart and lower SVR through vasodilation. This allows the failing heart to pump against less pressure (reduced afterload), resulting in increased CO. Milrinone is used for hypotensive patients with low-CO HF and pulmonary hypertension. It is a more potent pulmonary vasodilator than dobutamine. Milrinone is superior to dobutamine for patients on chronic oral beta blocker therapy who develop acute hypotensive HF.
• ![]() Inotropic agents: Administer digitalis to slow HR, giving the ventricles more time to fill, and to strengthen contractions; administer dopamine or dobutamine to support BP and enhance contractility (see Appendix 6). Digoxin is excreted by the kidneys, so the dose is reduced for those with renal failure. Digoxin may be prescribed for patients with LVSD who remain symptomatic on standard therapy, especially if they develop atrial fibrillation. Dobutamine enhances contractility by directly stimulating cardiac beta1 receptors. IV dobutamine infusions are sometimes used for patients with acute hypotensive HF or shock. The dose of dobutamine should always be titrated to the lowest dose that maintains hemodynamic stability, to minimize adverse events. As with many inotropes, long-term infusions of dobutamine may increase mortality due to lethal dysrhythmias. Chronic dobutamine infusions should be reserved as part of palliative symptom relief and for those who have an implantable cardioverter-defibrillator (ICD) while awaiting heart transplantation. Intermittent outpatient infusions of dobutamine are no longer recommended for routine management of HF. Dopamine and dobutamine are both associated with tachycardia, which can reduce ventricular filling time.
Inotropic agents: Administer digitalis to slow HR, giving the ventricles more time to fill, and to strengthen contractions; administer dopamine or dobutamine to support BP and enhance contractility (see Appendix 6). Digoxin is excreted by the kidneys, so the dose is reduced for those with renal failure. Digoxin may be prescribed for patients with LVSD who remain symptomatic on standard therapy, especially if they develop atrial fibrillation. Dobutamine enhances contractility by directly stimulating cardiac beta1 receptors. IV dobutamine infusions are sometimes used for patients with acute hypotensive HF or shock. The dose of dobutamine should always be titrated to the lowest dose that maintains hemodynamic stability, to minimize adverse events. As with many inotropes, long-term infusions of dobutamine may increase mortality due to lethal dysrhythmias. Chronic dobutamine infusions should be reserved as part of palliative symptom relief and for those who have an implantable cardioverter-defibrillator (ICD) while awaiting heart transplantation. Intermittent outpatient infusions of dobutamine are no longer recommended for routine management of HF. Dopamine and dobutamine are both associated with tachycardia, which can reduce ventricular filling time.
• Aldosterone antagonists: Spironolactone and eplerenone have been approved for patients with HF. Aldosterone inhibition reduces sodium and water retention, endothelial dysfunction, and myocardial fibrosis but may cause hyperkalemia. Serum potassium levels must be closely monitored. These drugs should not be used in patients with a creatinine level higher than 2.5 mg/dl. Data are inconclusive for patients with mild HF. Adding an aldosterone antagonist is reasonable for those with moderately severe to severe symptoms of HF and reduced CO who agree to have both renal function and potassium concentration closely monitored. The RALES trial reported a 30% reduction in mortality and hospitalizations when spironolactone was added to standard therapy for patients with advanced HF. The EPHESUS trial reported a 15% reduction in the risk of death and hospitalization in patients receiving eplerenone with low CO HF with an ejection fraction less than 40% after an MI.
• Vasodilators: Nitrates (oral, topical, or IV) to dilate venous or capacitance vessels, thereby reducing preload and cardiac and pulmonary congestion. Hydralazine will dilate the resistant vessels and reduce afterload, thus increasing forward flow. The combination of hydralazine and nitrate is inferior to an ACEI in improving survival but better than the ACEI in improving hemodynamics. Nitroprusside is used when oral agents, hydralazine, or nitrates are ineffective. Prior to discontinuing nitroprusside infusions, patients should be converted to oral vasodilators (e.g., ACEIs, ARBs, or hydralazine and a nitrate.) Nitroprusside is ideally used only for a short time in patients with advanced renal disease to avoid thiocyanate toxicity, an accumulation of this byproduct of the hepatic metabolism of nitroprusside. Thiocyanate is renally excreted and may not be excreted well in those with severe azotemia or kidney failure. Nitroprusside should also be avoided in patients with acute coronary syndrome because it may cause coronary steal syndrome, which shunts blood away from the ischemic myocardium to better-perfused muscle.
• Cardiac neurohormones: Infusion of BNP/nesiritide is used for patients with cardiorenal syndrome, which is renal insufficiency resulting from reduced renal perfusion due to HF. Nesiritide increases CO by inducing vasodilation without increasing HR or oxygen consumption. The drug helps to regulate vasoconstrictive and sodium-retaining effects of other neurohormones. Nesiritide is administered to patients with acutely decompensated HF as a weight-based bolus followed by continuous IV infusion. It may be initiated in the emergency department and does not require hemodynamic monitoring or frequent titration. Drug tolerance and dysrhythmias are unlikely.
• ![]() ACEIs (benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, trandolapril): ACEIs affect the renin-angiotensin system by inhibiting the conversion of circulating angiotensin I into angiotensin II. They reduce remodeling, and both preload and afterload, to decrease the work of the ventricles while resulting in increased CO and systemic perfusion/oxygenation. Vasodilation and neurohormonal modulation with ACEIs reduce mortality and HF symptoms while improving exercise tolerance and LV ejection fraction. Emergency department visits and hospitalizations are also decreased. All patients with LVSD should be treated with an ACEI unless they have a contraindication or intolerance. ACEIs should be used in combination with beta blockers in most HF patients, particularly those with a prior MI, regardless of CO or ejection fraction. These drugs help prevent HF in patients at high risk with atherosclerosis, diabetes mellitus, or hypertension with other cardiovascular risk factors. ACEI dose should be titrated to the maximum tolerated; however, 10% to 20% of patients are ACEI intolerant. The most troubling side effect from ACEIs is cough, which may prompt a change to an angiotensin II receptor blocker (ARB) or a combination of hydralazine and a nitrate.
ACEIs (benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, trandolapril): ACEIs affect the renin-angiotensin system by inhibiting the conversion of circulating angiotensin I into angiotensin II. They reduce remodeling, and both preload and afterload, to decrease the work of the ventricles while resulting in increased CO and systemic perfusion/oxygenation. Vasodilation and neurohormonal modulation with ACEIs reduce mortality and HF symptoms while improving exercise tolerance and LV ejection fraction. Emergency department visits and hospitalizations are also decreased. All patients with LVSD should be treated with an ACEI unless they have a contraindication or intolerance. ACEIs should be used in combination with beta blockers in most HF patients, particularly those with a prior MI, regardless of CO or ejection fraction. These drugs help prevent HF in patients at high risk with atherosclerosis, diabetes mellitus, or hypertension with other cardiovascular risk factors. ACEI dose should be titrated to the maximum tolerated; however, 10% to 20% of patients are ACEI intolerant. The most troubling side effect from ACEIs is cough, which may prompt a change to an angiotensin II receptor blocker (ARB) or a combination of hydralazine and a nitrate.
| Type of Diuretic | Generic Name and Initial Dose | Usage Information |
| Loop | Furosemide (Lasix) 20 mg | Given PO or IV; PO dosage is doubled for the equivalent effect of IV dosing. |
| Bumetanide (Bumex) 0.5 mg | PO and IV dosing result in the same effects from the same dosage. | |
| Torsemide (Demadex) 10–20 mg | Given PO or IV. Has strongest PO effects of all loop diuretics. | |
| Ethacrynic Acid (Edecrin) 50 mg | Given IV to patients who are allergic to furosemide, or other loop diuretics | |
| Thiazide | Hydrochlorothiazide (HCTZ) 12.5 mg | Given PO mainly to manage hypertension; can easily lead to hypokalemia, hyponatremia, and dehydration |
| Metolazone (Zaroxolyn) 2.5 mg | Given PO; should be given 30 minutes before furosemide if used together; has high incidence of hypokalemia |
• AT1 receptor antagonist (candesartan, eposartan, irbesartan, olmesartan, losartan, telmisartan): Have effects similar to ACEIs but have not proved to reduce mortality. AT1 receptor-blocking agents are used in patients who are ACEI intolerant. These drugs were not found to be superior to ACEIs in improving mortality, but they generally have fewer side effects. ARBs are recommended as second-line therapy in patients who are intolerant to ACEIs because of cough or angioedema. ARBs help to prevent HF in high-risk patients with atherosclerosis, diabetes mellitus, and hypertension. ARBs should not be substituted for ACEIs in patients with hyperkalemia or renal dysfunction, as they are associated with similar complications.
• ![]() Beta adrenergic blocking agents (acebutolol, atenolol, betaxolol, bisoprolol, carteolol, carvedilol, esmolol, labetalol, metoprolol, nadolol, oxprenolol, propanolol, penbutolol, sotalol, pindolol, timolol): Not all beta adrenergic blockers are approved for use in managing HF. Only three (carvedilol, metoprolol succinate [Toprol XL], and bisoprolol) have improved survival. All stable patients with current or prior symptoms of HF and reduced ejection fraction should receive a beta blocker unless contraindicated. These drugs block the effects of circulating catecholamines released during HF. Catecholamines cause peripheral vasoconstriction, increased resistance to ventricular ejection, increased HR, and increased myocardial oxygen consumption and may precipitate myocardial ischemia and ventricular dysrhythmias. Beta blockers reduce contractility, resulting in decreased myocardial oxygen consumption and demand. Historically, reduction in contractility was the main reason many physicians hesitated to prescribe beta blockers to HF patients. Diabetes mellitus, COPD, and peripheral arterial disease do not contraindicate the use of beta blockers; however, patients with severe bronchospasm and hypotension may not tolerate these drugs. The combination of ACEI, diuretics, and beta blockers administered together may cause hypotension. Spacing the drug administration times by at least 2 hours usually relieves this effect.
Beta adrenergic blocking agents (acebutolol, atenolol, betaxolol, bisoprolol, carteolol, carvedilol, esmolol, labetalol, metoprolol, nadolol, oxprenolol, propanolol, penbutolol, sotalol, pindolol, timolol): Not all beta adrenergic blockers are approved for use in managing HF. Only three (carvedilol, metoprolol succinate [Toprol XL], and bisoprolol) have improved survival. All stable patients with current or prior symptoms of HF and reduced ejection fraction should receive a beta blocker unless contraindicated. These drugs block the effects of circulating catecholamines released during HF. Catecholamines cause peripheral vasoconstriction, increased resistance to ventricular ejection, increased HR, and increased myocardial oxygen consumption and may precipitate myocardial ischemia and ventricular dysrhythmias. Beta blockers reduce contractility, resulting in decreased myocardial oxygen consumption and demand. Historically, reduction in contractility was the main reason many physicians hesitated to prescribe beta blockers to HF patients. Diabetes mellitus, COPD, and peripheral arterial disease do not contraindicate the use of beta blockers; however, patients with severe bronchospasm and hypotension may not tolerate these drugs. The combination of ACEI, diuretics, and beta blockers administered together may cause hypotension. Spacing the drug administration times by at least 2 hours usually relieves this effect.
4. Manage acute pulmonary edema; include the following immediate interventions.
• Monitoring for signs and symptoms of acute respiratory failure
• Titrating supplemental oxygen to maintain adequate oxygenation
• Providing NiPPV for patients with increased work of breathing
• Elevating HOB as needed to promote oxygenation
• If NiPPV is unsuccessful, consider endotracheal (ET) intubation with mechanical ventilation (see Acute Respiratory Failure, p. 383).
• ![]() Diuretic therapy: In severely ill patients, furosemide or bumetanide may be used as continuous IV infusion to assist with constant fluid removal. Patients with renal impairment/failure may require infusions of appropriate diuretics or ultrafiltration if other efforts to remove fluid fail.
Diuretic therapy: In severely ill patients, furosemide or bumetanide may be used as continuous IV infusion to assist with constant fluid removal. Patients with renal impairment/failure may require infusions of appropriate diuretics or ultrafiltration if other efforts to remove fluid fail.
• Pharmacologic therapy, including continuous IV infusions of inotropic agents, vasodilators, beta blockers, and IV morphine. If cardiogenic shock ensues, vasopressors and intra-aortic balloon pumping (IABP) may also be necessary. If the person has evidence of renal insufficiency or failure, ACEI dosage may be reduced or the drug discontinued.
5. Initiate a low-calorie (if weight control is necessary) and low-sodium diet.
• Extra salt and water are held in the circulatory system, causing increased strain on the heart. Limiting sodium (Table 5-2) will reduce the amount of fluid retained by the body. In addition, fluids may be limited to 1500 to 2000 ml/day.
| Foods High in Sodium* | Foods Low in Sodium |
|---|---|
| Beans and frankfurters | Bread |
| Bouillon cubes | Cereal (dry or hot); read labels |
| Canned or packaged soups | Fresh fish, chicken, turkey, veal, beef, and lamb |
| Canned, smoked, or salted meats; salted fish | |
| Dill pickles | Fresh fruits and vegetables |
| Fried chicken dinners and other fast foods | Fresh or dried herbs |
| Monosodium glutamate (e.g., Accent) | Gelatin desserts |
| Olives | Oil, salt-free margarine |
| Packaged snack foods | Peanut butter |
| Pancake or waffle mix | Tabasco sauce |
| Processed cheese | Low-salt tuna packed in water |
| Seasoned salts (e.g., celery, onion, garlic) | |
| Sauerkraut | |
| Soy sauce | |
| Vegetables in brine or cans | |
| Additional suggestions | |
| Do not add table salt to foods. | Do not buy convenience foods; remember that fresh is best. |
| Season with fresh or dried herbs. | Read all labels for salt, sodium, or sodium chloride content. |
| Avoid salts or powders that contain salt. | |
* Many of these foods now are available in low-salt or salt-free versions.
6. Initiate device or electronic therapy.
• Cardiac resynchronization therapy: Consider biventricular pacing, wherein a third electrode is implanted in a left cardiac vein via the coronary sinus so that the right and left ventricles are activated simultaneously. Relief of symptoms is achieved in approximately 70% of patients because of improved ventricular contraction and reduction of mitral regurgitation. Multiple clinical trials have shown the benefit of cardiac resynchronization therapy (CRT) for those with severe symptomatic HF with a wide QRS complex.
• ICD: Approximately 50% of patients with HF die of sudden death. Implanting an ICD may improve survival in some of these patients. ICD therapy has been superior to antiarrhythmic drug therapy in preventing sudden death. Cardiac resynchronization therapy can be combined with an ICD as a single device if the patient meets the requirements for both therapies.
• LV assist devices (LVADs): Some patients with cardiogenic shock unresponsive to intra-aortic balloon counterpulsation and IV inotrope therapy may be referred for mechanical circulatory support. At present, LVADs are most often used as a bridge to cardiac transplantation in patients who are appropriate candidates. The inflow cannula of an LVAD is connected to the apex of the left ventricle. Blood is pumped by the device via the outflow cannula to the aorta. Complications include stroke, infection, coagulopathy with bleeding, multiple organ dysfunction syndrome (MODS), and prosthetic valve insufficiency. LVADs have been used as permanent implants (destination therapy), and advances in technology have allowed for more widespread implementation.
• Ventricular reconstruction surgery: Ventricular remodeling surgery, or a Dor procedure, is used to manage HF secondary to ischemic cardiomyopathy. There are several components to the procedure including coronary artery bypass grafting (CABG), mitral and tricuspid valve repair, resection of LV scar or aneurysm, reshaping the left ventricle from the remodeled spherical shape back to an elliptical shape, and epicardial LV pacing lead placement. Candidates for this procedure have CAD, severe ischemia or hibernating myocardium, LV dysfunction with akinetic or dyskinetic ventricular segments, and mitral or tricuspid regurgitation.
• Cardiac transplantation: Replacing the heart is a procedure of limited availability done on patients with little disease outside of end-stage HF with severely functional impairment despite optimal medical management. Patients are not transplant candidates if they have significant comorbidities, pulmonary hypertension, active infection, significant psychosocial issues, or history of medical noncompliance. Survival following a heart transplant is about 85% at 1 year. Survival declines by an additional 4% annually. Complications include rejection of the transplanted heart, infection, transplant-related CAD, and malignancy. Following cardiac transplantation, patients must maintain lifelong immunosuppression to prevent rejection, which places them at high risk for opportunistic infections and malignancies.
CARE PLANS FOR HEART FAILURE
![]() related to compromised regulatory mechanism secondary to decreased cardiac output
related to compromised regulatory mechanism secondary to decreased cardiac output
Fluid Overload Severity; Fluid Balance; Electrolyte and Acid-Base Balance
1. Auscultate lung fields for presence of crackles and rhonchi or other adventitious sounds.
2. Monitor input and output (I&O) closely. Report positive fluid state or decrease in urine output to less than 0.5 ml/kg/hr.
3. Weigh patient daily; report increases in weight. An acute gain in weight of 1 kg can signal a 1-L gain in fluid.
4. Note changes from baseline assessment to detect worsening of HF, such as increased pedal edema, increased jugular venous distention, development of S3 heart sound or new murmur, and dysrhythmias.
5. Monitor hemodynamic status every 1 to 2 hours and on an as-needed basis. Note response to drug therapy as well as indicators of the need for more aggressive therapy, including increasing PAWP and SVR and decreasing CO.
6. Administer diuretics, positive inotropes, inodilators, beta blockers, and vasodilators as prescribed. (See Appendix 6 for more information about inotropic and vasoactive drugs.)
7. Watch for signs and symptoms of renal insufficiency.
8. Limit oral fluids as prescribed, and offer patient ice chips or frozen juice pops to decrease thirst and relieve discomfort of dry mouth.
Cardiac Pump Effectiveness; Circulation Status
Cardiac care: acute hemodynamic regulation
1. Monitor cardiac rhythm and rate continuously.
2. Monitor CO, CI, pulmonary and systemic vascular pressures, and other hemodynamic values at least hourly, as appropriate. Implement continuous CO and SvO2 monitoring if available.
3. Monitor neurologic status to assess for adequate cerebral perfusion.
4. Monitor renal function (BUN and creatinine) daily, as appropriate.
5. Monitor liver studies (SGOT/AST, SGPT/ALT, and/or bilirubin), as appropriate.
6. Monitor the other determinants of oxygen delivery, including level of Hgb and oxygen saturation.
7. Refrain from taking rectal temperatures, to prevent bradycardias.
8. Control tachycardia as soon as possible with beta blockers or other appropriate measures as determined by the physician and ACLS guidelines.
9. Obtain 12/15/18-lead ECG to assess new dysrhythmias or profound instability.
10. IABP may be necessary; prepare needed equipment for insertion of the balloon catheter and implementation of pumping.
11. If patient has atrial fibrillation, ensure that patient has been receiving appropriate anticoagulants or antiplatelet agents to prevent thrombus formation.
Respiratory Status: Gas Exchange; Mechanical Ventilation Response: Adult
1. Monitor respiratory rate, rhythm, and character every 1 to 2 hours. Be alert to RR greater than 20 breaths/min, irregular rhythm, use of accessory muscles of respiration, or cough.
2. Auscultate breath sounds, noting presence of crackles, wheezes, and other adventitious sounds.
3. Provide supplemental oxygen as prescribed and titrate to SpO2.
4. Monitor SpO2 for decreases to less than 92%.
5. Assess ABG findings; note changes in response to oxygen supplementation or treatment of altered hemodynamics.
6. Suction patient’s secretions as needed.
7. Establish a protocol for deep breathing, coughing, and turning every 2 hours.
8. Place patient in semi-Fowler’s or high Fowler’s position to maximize chest excursion.
9. If mechanical ventilation is necessary, monitor ventilator settings, ET tube function and position, and respiratory status.
![]() Activity Tolerance; Energy Conservation
Activity Tolerance; Energy Conservation
1. ![]() Maintain prescribed activity level, and teach patient the rationale for activity limitation.
Maintain prescribed activity level, and teach patient the rationale for activity limitation.
2. Organize nursing care so that periods of activity are interspersed with extended periods of uninterrupted rest.
3. To help prevent complications of immobility, assist patient with active/passive range-of-motion (ROM) exercises, as appropriate. Encourage patient to do as much as possible within prescribed activity allowances.
4. Note patient’s physiologic response to activity, including BP, HR, RR, and heart rhythm. Signs of activity intolerance include chest pain, increasing SOB, excessive fatigue, increased dysrhythmias, palpitations, HR response greater than 120 bpm, SBP greater than 20 mm Hg from baseline or greater than 160 mm Hg, and ST-segment changes. If activity intolerance is noted, instruct patient to stop the activity and rest.
5. Administer medications as prescribed, and note their effect on patient’s activity tolerance.
6. As needed to help prevent muscle loss and wasting, refer patient to physical therapy department.
![]() Activity Therapy; Energy Management; Teaching: Prescribed Activity/Exercise; Dysrhythmia Management; Pain Management; Medication Management
Activity Therapy; Energy Management; Teaching: Prescribed Activity/Exercise; Dysrhythmia Management; Pain Management; Medication Management
Knowledge: Cardiac Disease Management
1. Teach patient the physiologic process of HF, discussing in terms appropriate to the patient how fluid volume increases because of poor heart function.
2. Teach the patient about the adverse effects of smoking and how smoking cessation may benefit him or her. Provide information about smoking cessation classes and nicotine patches and medications prescribed to help people stop smoking, such as varenicline and bupropion.
3. Teach patient about the importance of a low-sodium diet to help reduce volume overload. Provide patient with a list of foods that are high and low in sodium. Teach patient how to read and evaluate food labels.
4. Teach patient the signs and symptoms of fluid volume excess that necessitate medical attention: irregular or slow pulse, increased SOB, orthopnea, decreased exercise tolerance, and steady weight gain (≥1 kg/day for 2 successive days).
5. Advise patient about the need to keep a journal of daily weight. Explain that an increase of ≥1 kg/day on 2 successive days of normal eating necessitates notification of physician.
6. If patient is taking digitalis, teach the technique for measuring pulse rate. Provide parameters for withholding digitalis (usually for pulse rate less than 60/min) and notifying the physician.
7. Teach patient how to manage any advanced therapy that is used, biventricular pacemaker or internal cardiac defibrillator used for CRT, ventricular assist device (VAD), or heart transplant.
8. ![]() Instruct patient regarding the prescribed activity progression after hospital discharge, signs of activity intolerance that signal the need for rest, and use of prophylactic nitroglycerin (NTG) to reduce congestion of the heart and lungs. General activity guidelines are as follows:
Instruct patient regarding the prescribed activity progression after hospital discharge, signs of activity intolerance that signal the need for rest, and use of prophylactic nitroglycerin (NTG) to reduce congestion of the heart and lungs. General activity guidelines are as follows:
| Week | Distance Walked | Time |
|---|---|---|
| 1–2 | ¼ mi | Leisurely; twice daily |
| 2–3 | ½ mi | 15 min |
| 3–4 | 1 mi | 30 min |
| 4–5 | 1½ mi | 30 min |
| 5–6 | 2 mi | 40 min |
Acute coronary syndromes
Cardiovascular assessment: acute coronary syndrome
History and risk factors
• ![]() Family history of CAD, age greater than 70 years, male sex, postmenopausal females, cigarette smoking, hypercholesterolemia, hyperlipidemia, hypertension, hyperglycemia, increased waist circumference, diabetes, obesity, increased stress, sedentary lifestyle, noncompliance with medication management (beta adrenergic blockers, diuretics, ACEIs, ARBs, nitrates, aspirin or other platelet inhibitors)
Family history of CAD, age greater than 70 years, male sex, postmenopausal females, cigarette smoking, hypercholesterolemia, hyperlipidemia, hypertension, hyperglycemia, increased waist circumference, diabetes, obesity, increased stress, sedentary lifestyle, noncompliance with medication management (beta adrenergic blockers, diuretics, ACEIs, ARBs, nitrates, aspirin or other platelet inhibitors)
Chest pain: angina
• May result from exertion or emotional stress
• Onset can be abrupt or gradual
• Stable angina: Gradually increases in severity during episodes over several months; does not occur with rest; subsides gradually with rest
• Unstable angina: Pain that has changed significantly from past patterns and can occur at rest; includes Wellens syndrome (left anterior descending coronary artery lesion), rest angina, preinfarction (crescendo) angina (may cause slight ST elevation and increased troponin level), Prinzmetal’s angina (from coronary artery vasospasms at rest), and new-onset angina
• Most common feelings: Substernal pressure, chest tightness, heaviness or squeezing in the chest
• Extreme pain: Crushing substernal chest pain radiating down the left arm, or up to the jaw with shortness of breath
• Variations: Jaw or arm pain only, right- or left-sided chest discomfort, pain in the teeth, nausea, heartburn, syncope
• ![]() No pain: Does not rule out ischemia; common in elders, women, and diabetics, who may feel extreme, sudden onset fatigue rather than pain
No pain: Does not rule out ischemia; common in elders, women, and diabetics, who may feel extreme, sudden onset fatigue rather than pain
Chest pain: acute myocardial infarction
• Onset can be abrupt or gradual.
• Lasts for longer than 30 minutes
• Most common feelings: Continuous substernal pressure, chest tightness, heaviness or squeezing in the chest
• Extreme pain: Continuous crushing substernal chest pain radiating down the left arm or up to the jaw, nausea, vomiting, SOB, orthopnea, anxiety, apprehension, diaphoresis, cyanosis, syncope, strokelike symptoms
• Variations: Jaw or arm pain only, right- or left-sided chest discomfort, pain in the teeth, heartburn
• ![]() No pain: Does not rule out infarction; 25% of MIs are “silent” or without pain; common in older adults, women, and diabetic persons, who may feel extreme, sudden-onset fatigue rather than pain
No pain: Does not rule out infarction; 25% of MIs are “silent” or without pain; common in older adults, women, and diabetic persons, who may feel extreme, sudden-onset fatigue rather than pain
12-lead electrocardiogram: angina and acute myocardial infarction
• Compare current ECG with past ECG.
• Angina: Review for ST-segment depression in at least two contiguous leads, which indicates myocardial ischemia.
• Acute MI: Review for ST-segment elevation in at least two contiguous leads, which indicates active myocardial damage (acute infarction); may or may not form Q waves.
• Evaluate pacing and conduction: Rhythm regularity, rather, and conduction velocity (PR, QRS, QT intervals)
• Dysrhythmias: New bundle branch block (especially left) is diagnostic for MI; sinus bradycardia, atrioventricular (AV) heart blocks, and ventricular ectopy may also be present.
Vital signs
• Possible fever in patients with AMI
• BP may increase or decrease, depending on sympathetic nervous system (SNS) response to change in CO.
• HR may increase or decrease depending on SNS response and ensuing damage to the conduction pathway, hypoxia; SNS response is blunted by beta adrenergic blocking agents.
Labwork
Blood studies can reveal causes of dysrhythmias or changes in pacing/conduction or HR changes:
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Electrocardiogram (ECG) 12-, 15-, and 18-lead ECG: must be obtained during an episode of chest pain for full benefit of help with diagnosis; should be done in a series to view evolving changes; may not reveal changes if not during an episode of chest pain |
Assess for ischemic heart disease and acute or older myocardial infarction (MI); helps identify ST-segment elevation MI (STEMI) versus non–ST-segment elevation MI (NSTEMI); frames need for antiplatelet drugs versus thrombin inhibitors versus thrombolytic drugs or angioplasty (for STEMI). | Presence of ST segment depression or T wave inversion (myocardial ischemia), ST elevation (acute MI), new bundle branch block (especially left BBB) or pathologic Q waves (resolving/resolved MI) in 2 contiguous or related leads Contiguous leads indicative of location of ischemia or infarction: V1 and V2: Intraventricular septum V3 and V4: Anterior wall of left ventricle V5 and V6: Lateral wall of left ventricle V7–V9: Posterior wall of left ventricle II, III, AVF: Inferior wall of left ventricle V1, V1R–V6R: Right ventricle |
| Stress tests Stress test on a treadmill with or without thallium Thallium stress test using medications |
Assess for cardiac ischemia by monitoring ECG changes and chest pain during exercise on a treadmill. Thallium scan is done following the exercise to further assess for ischemic areas. Stress can also be induced using drugs that increase cardiac workload instead of treadmill exercise. | ST depression on ECG, chest pain during exercise; thallium does not accumulate normally in ischemic areas (cold spots) of the heart; for MI patients, generally used after the acute phase of MI |
| Cardiac radionuclide imaging Technetium-99m with pyrophosphate Technetium-99m with sestamibi |
Assess myocardial perfusion to determine areas of infarction and ischemia. | Infarcted areas of myocardium appear as “hot spots” on the scan up to 10 days after MI. |
| Blood Studies | ||
| Serial cardiac enzymes Myoglobin CK-MB isoform CK-MB Troponin I Troponin T |
Assess for enzyme changes indicative of myocardial tissue damage; diagnostic for MI; should be done at least every 8 hours during the first 24 hours following severe chest pain. | Elevated enzymes reflect muscle damage; if CK-MB and troponins are elevated, MI has occurred. Myoglobin: Elevation begins in 1–3 hours, peaks 6–7 hours, subsides 24 hours CK-MB isoform: Elevation begins in 2–6 hours, peaks 18 hours, subsides (varies) CK-MB: Elevation begins in 3–12 hours, peaks 24 hours, subsides in 48–72 hours Troponin I: Elevation begins in 3–12 hours, peaks 24 hours, subsides 5–10 days Troponin T: Elevation begins in 3–12 hours, peaks 12–48 hours, subsides 5–10 days Ratio of the MB2 (cardiac) to MB1 (all muscle) types of CK-MB is also diagnostic for MI if the ratio becomes >2.5. Normal levels may vary from one institution or laboratory instrument to another. If MI is strongly suspected and CK total and MB are within normal limits (WNL), testing for troponin will provide the best diagnostic information. |
| Complete blood count (CBC) Hemoglobin (Hgb) Hematocrit (Hct) RBC count (RBCs) WBC count (WBCs) |
Assess for anemia, inflammation and infection; assists with differential diagnosis of chest pain. | Decreased RBCs, Hgb, or Hct reflects anemia, which exacerbates chest pain; MI may increase WBCs. |
| Electrolytes Potassium (K+) Magnesium (Mg2+) Calcium (Ca2+) Sodium (Na+) |
Assess for possible causes of dysrhythmias and/or heart failure. | Decrease in K+, Mg2+, or Ca2+ may cause dysrhythmias; elevation of Na+ may indicate dehydration (blood is more coagulable); low Na+ may indicate fluid retention and/or heart failure. |
| Coagulation profile Prothrombin time (PT) with international normalized ratio (INR) Partial thromboplastin time (PTT) Fibrinogen D-dimer |
Assess for causes of bleeding, clotting, and disseminated intravascular coagulation (DIC) indicative of abnormal clotting present in shock or ensuing shock. | Decreased PT with low INR promotes clotting; elevation promotes bleeding; elevated fibrinogen and D-dimer reflect abnormal clotting is present. |
| B-type natriuretic peptide (BNP) | Assess for heart failure. | Elevation indicates heart failure is present. |
| Lipid profile and lipoprotein-cholesterol fractionation Total cholesterol High-density lipoprotein (HDL) cholesterol Low-density lipoprotein (LDL) cholesterol Very-low-density lipoprotein (VLDL) cholesterol Triglycerides |
Assess for causes of arterial plaque formation contributing to coronary artery disease (CAD). Total cholesterol measures circulating levels of free cholesterol and cholesterol esters. Triglycerides assesses storage form of lipids. |
Elevation of total cholesterol, LDL, VLDL, and triglycerides indicates a greater potential for developing CAD; elevated HDL lowers probability of CAD. Concentrations vary with age. Total cholesterol: Many physicians prefer patients to have a total cholesterol level of <200 mg/dl, but if fractionation is used, other risk factors are considered prior to recommending patients lower their cholesterol level if >200 mg/dl. |
| C-reactive protein (CRP) | Assess for inflammation of coronary plaque. | Elevation places patients at higher risk for acute MI. |
| Homocysteine | Assess for potential of accelerated plaque formation. | Elevation places patients at higher risk for acute MI. |
| Radiology | ||
| Chest radiograph (CXR) | Assess size of heart, thoracic cage (for fractures), thoracic aorta (for aneurysm) and lungs (pneumonia, pneumothorax); assists with differential diagnosis of chest pain. | Cardiac enlargement, increased vascular markings and bilateral infiltrates reflect heart failure (pulmonary edema). Note: Portable CXR should be done with patient centered on the plate and with head of bed elevated whenever. |
| Magnetic resonance imaging (MRI) Cardiac MRI |
Assesses ventricular size, morphology, function, status of cardiac valves and circulation | Enlarged heart, remodeled heart, incompetent of stenotic heart valves, narrowed or occluded coronary arteries |
| Computed tomography (CT) Cardiac CT scan |
Assesses ventricular size, morphology, function, status of cardiac valves and circulation | Enlarged heart, remodeled heart, incompetent of stenotic heart valves, narrowed or occluded coronary arteries; technology is improving in accuracy; may eventually reduce the need for cardiac catheterization |
| Ultrasound echocardiography (echo) |
Assess for mechanical and structural abnormalities related to effective pumping of blood from both sides of the heart. | Abnormal ventricular wall movement or motion, low ejection fraction, incompetent or stenosed heart valves, abnormal intracardiac chamber pressures |
| Transesophageal echo | Assess for mechanical and structural abnormalities related to effective pumping of blood from both sides of the heart using a transducer attached to an endoscope. | Same as above but can provide enhanced views, particularly of the posterior wall of the heart |
| Positron emission tomography (PET) PET scan: cardiac |
Isotopes are used to assess if viable cardiac tissue is present. | Viable tissue has increased uptake of the glucose tracer and decreased uptake of the blood flow tracer (ammonia). |
| Indium-111 anti-myosin imaging Indium scan: cardiac |
Anti-myosin antibodies are injected along with radioactive indium-111 to visualize damaged areas | Antibodies are taken up by damaged myocardial cells as white blood cells rush to the area as part of the inflammatory process. |
| Invasive Cardiology | ||
| Coronary angiography cardiac catheterization |
Assesses for presence and extent of CAD, left ventricular function, and valvular disease using a radiopaque catheter inserted through a peripheral vessel and advanced into the heart and coronary arteries; allows direct injection of thrombolytic drugs | Low ejection fraction indicates heart failure, stenotic or incompetent heart valves can decrease CO, narrowed or occluded coronary arteries cause chest pain, abnormal pressures in the main coronary arteries indicate impaired circulation, elevated pressures inside the chambers of the heart indicate heart failure, abnormal ventricular wall motion decreases CO, and elevated pulmonary artery pressures indicate heart failure. Test is used to prescribe the most appropriate treatment: percutaneous coronary intervention (PCI) or cardiac surgery. |
Significant electrocardiogram changes
St-segment changes and new bundle branch block:
The presence or absence of ST-segment elevation is used to risk stratify patients and determine the best treatment plan. ST segments are elevated in the leads “over” or facing the infarcted area. Reciprocal changes (ST-segment depressions) will be found in leads 180 degrees from the area of infarction. New bundle branch block, especially left bundle branch block, coupled with other findings, may also indicate MI is present. Patients with STEMI are candidates for emergency reperfusion strategy: either thrombolytics within 30 minutes of arrival or percutaneous coronary intervention (PCI) within 90 minutes of arrival.
Within the initial hour of infarction, tall, peaked, “hyperacute” upright T waves may be seen in leads over the infarct. Within several hours to days, the T wave becomes inverted. Gradually over time, the ST segment becomes isoelectric and the T wave may remain inverted. T-wave changes may last for weeks and return to normal or remain inverted for the rest of the patient’s life. T-wave changes reflective of posterior and RV MI are not clearly seen in the 12-lead ECG. Use of 15- or 18-lead ECGs should provide clearer information about these areas (Table 5-4).
Collaborative management
ACUTE MYOCARDIAL INFARCTION HOSPITAL QUALITY ALLIANCE INDICATORS
| In December 2002, the American Hospital Association (AHA), Federation of American Hospitals (FAH), and Association of American Medical Colleges (AAMC) launched the Hospital Quality Alliance (HQA), an initiative to provide the public with specific reported information about hospital performance. This national public-private collaboration encourages hospitals to voluntarily collect and report quality performance information. The Centers for Medicare and Medicaid Services (CMS) along with The Joint Commission participate in the HQA. Hospitals are expected to track and analyze their performance ratings and use the information to improve quality. The table reflects HQA measures considered essential when caring for patients following an acute MI. All indicators are evidence-based actions that should be included in the plan of care. The measurement describes the details of each indicator. Evidence of performance is derived from review of each patient’s medical record following hospital discharge. | |
| Indicators | Measure |
| Aspirin at arrival | Acute myocardial infarction (AMI) patients without aspirin contraindications who received aspirin within 24 hours before or after hospital arrival |
| Aspirin at discharge | AMI patients without aspirin contraindications who were prescribed aspirin at hospital discharge |
| Angiotensin-converting enzyme (ACE) inhibitor or ARB for left ventricular systolic dysfunction | AMI patients with left ventricular systolic dysfunction (LVSD) and without both ACE inhibitor and angiotensin receptor blocker (ARB) contraindications who were prescribed an ACE inhibitor or an ARB at hospital discharge |
| Beta-blocker at discharge | AMI patients without beta-blocker contraindications who were prescribed a beta-blocker at hospital discharge |
| Fibrinolytic agent received within 30 minutes of hospital arrival | AMI patients receiving thrombolytic therapy during hospital stay with a time from hospital arrival to thrombolysis of 30 minutes or less |
| Percutaneous coronary intervention (PCI) received within 90 minutes of hospital arrival | AMI patients receiving a PCI during the hospital stay with a time from hospital arrival to PCI of 120 minutes or less |
| Smoking cessation advice/counseling | AMI patients with a history of smoking cigarettes, who are given smoking cessation counseling during a hospital stay |
| 30-day risk adjusted heart attack mortality | The measures comply with standards for publicly reported outcomes models that have been endorsed by the American Heart Association and the American College of Cardiology. These measures have been published in peer review literature and approved by the rigorous process of the National Quality Forum. |
Care priorities for all acute coronary syndromes
1. Relief of acute ischemic pain:
• Oxygen: Usually 2 to 4 L/min by nasal cannula, or mode and rate as directed by ABG values, to promote both myocardial and generalized increases in oxygenation. As oxygen delivery to the heart is enhanced, pain can be relieved. If the patient deteriorates, other methods of oxygen delivery may be implemented (e.g., nonrebreather mask with reservoir and mechanical ventilation for those who deteriorate markedly).
• Aspirin: 160 to 320 mg ideally chewed should be given immediately if the patient has not been given aspirin prior to arrival at the hospital.
• Oral, sublingual, and other forms of nitrates/NTG: Can be used for short-term therapy or longer-lasting prophylactic effects. These non-IV medications are used for management of myocardial ischemia or angina pectoris rather than for MI.
• IV nitrates/NTG: For unstable angina or evolving MI, it is titrated until relief is obtained, generally up to 200 mcg/min as long as the patient maintains a systolic BP (SBP) of at least 80 mm Hg.
• IV or oral immediate-release morphine sulfate: Given in small increments (e.g., 2 mg) until relief is obtained. This medication is usually not necessary unless an MI is occurring. Low BP may contraindicate administration.
2. Prevention of coronary artery clot formation:
• Antithrombin therapy: Heparin (unfractionated) or low-molecular-weight heparin (fractionated) infusion is sometimes implemented to prevent clot extension and/or formation, particularly if significant ST depression (greater than 1 mm) is noted or troponins are slightly positive. Dosage should be weight based and follow a titration protocol based on ongoing studies of PTT/aPTT (partial thromboplastin time/activated partial thromboplastin time). If patients experience a drop in platelets with heparins, a direct thrombin inhibitor (i.e., Argatroban) may be used.
• Antiplatelet therapy: Infusion of GP IIb/IIIa inhibitors (e.g., abciximab, eptifibatide, tirofiban) is implemented more regularly since receiving support in the AHA/ACC 2000 ACLS standards of emergency cardiac care. In patients with marked ST-segment depression (greater than 1 mm) or progressively unstable angina, antiplatelet therapy may halt the vessel occlusion process by interrupting platelet aggregation. Clots are unable to form without the “white clot scaffolding” provided by platelet aggregation. Aspirin is an antiplatelet drug.
• Direct thrombin inhibitors: A new class of anticoagulants that bind directly to thrombin and block its interaction with its substrates, DTIs act independently of antithrombin, so they can inhibit thrombin bound to fibrin or fibrin degradation products. Bivalirudin is commonly used during PCI because its duration of action is short. Coagulation times return to baseline approximately 1 hour following cessation of administration.
3. Reduction of myocardial workload and myocardial oxygen consumption:
• Limit activities: Restrictions based on patient’s activity tolerance. Bed rest with bedside commode privileges generally is recommended for patients with AMI for up to 12 hours after symptom onset. Longer periods of bedrest can promote development of orthostatic intolerance, which is prevented by elevation of the HOB, dangling the lower extremities, and other low-exertion activities. Patients should be instructed to avoid the Valsalva maneuver when toileting, because it may predispose them to ventricular dysrhythmias.
Additional treatments
1. Management of unstable ami with st-segment elevation (stemi):
• Hemodynamic monitoring: Used in a patient with a complicated MI resulting in ventricular failure with threat of cardiogenic shock. Pulmonary artery (PA) and capillary pressures are measured, along with CO and SVR. Unstable patients may manifest increased PAP, increased PAWP, decreased CO, and increased SVR.
2. Acute stemi: pci procedures:
• Percutaneous transluminal coronary angioplasty (PTCA): The original PCI performed for improving blood flow through stenotic coronary arteries. A balloon-tipped catheter is inserted into the coronary arterial lesion, and the balloon is inflated to compress the plaque material against the vessel wall, thereby opening the narrowed lumen. PTCA is performed on individuals with AMI, postinfarction angina, postbypass angina, and chronic stable angina. The ideal candidate has single-vessel disease with a discrete, proximal, noncalcified lesion. As technology has improved, patients with more complex conditions have become routine candidates for the procedure if performed by an experienced invasive cardiologist. During the procedure,the patient is sedated lightly and is given a local anesthetic at the insertion site—usually the femoral artery. ECG electrodes are placed on the chest. An introducer sheath is inserted into the femoral artery, a guide wire is passed into the aorta and coronary artery, and the balloon catheter is passed over the guide wire to the stenotic site. The patient may be asked to take deep breaths and cough to facilitate passage of the catheter. Heparin, GP IIb/IIIa inhibitor, and/or a direct thrombin inhibitor, such as bivalirudin, is administered to prevent clot formation. The balloon is inflated repeatedly for 60 to 90 seconds at a pressure of 4 to 15 atmosphere (atm). Subsequently, radiopaque dye is injected to determine whether the stenosis has been reduced to less than 50% of the vessel diameter, which is the goal of the procedure. The femoral artery site may be closed using a device or the introducer sheath left in the femoral artery until the effect of anticoagulant medications has diminished.
• Complications after PTCA: These include acute coronary artery occlusion, coronary artery dissection, reocclusion in AMI patients, AMI, coronary artery spasm, bleeding, circulatory insufficiency, renal hypersensitivity to contrast material, hypokalemia, vasovagal reaction, dysrhythmias, and hypotension. Restenosis can occur 6 weeks to 6 months after PCI, although the patient may not experience angina.
• Coronary artery atherectomy: A PCI that removes atherosclerotic plaque from coronary arteries using a special catheter equipped with a cutting device that shaves the lesion. Fragments from the technique are collected into the “nose cone” of the device (directional atherectomy); pulverized and dispersed into the circulation (rotational or “rotoblade” atherectomy); or aspirated (transluminal extraction catheterization [TEC]). May be used for patients with myocardial ischemia and during or after an AMI.
• Intracoronary stent procedure: A PCI wherein endovascular stents (metal-mesh tubes) are used to keep arteries open. A variety of designs, materials, and deployment procedures are available. Newer “drug-eluting” stents are coated with drugs to help prevent restenosis of the affected artery. Balloon-expanded stents are most commonly used in the United States and are inserted during PCI. May be used for stenosed coronary arteries or to reopen stenotic CABG replacement vessels (grafts).
• Laser coronary angioplasty: A PCI that enables debulking of distal coronary lesions in tortuous arteries to allow for reperfusion. The laser is a part of the coronary artery catheter (similar to the device used in PTCA) and ablates only the tissue it contacts.
3. Surgical revascularization:
• Surgical revascularization procedures are seldom used as the primary management strategy for AMI. Several approaches are available for myocardial revascularization, including CABG via median sternotomy or minimally invasive technique. Patients with multivessel or diffuse CAD are the most appropriate candidates for these procedures. Surgical indications include (1) stable angina with 50% stenosis of the left main coronary artery, (2) stable angina with three-vessel CAD, (3) unstable angina with three-vessel disease or severe two-vessel disease, (4) recent MI, (5) ischemic HF with cardiogenic shock, and (6) signs of ischemia or impending MI after angiography procedure. Robotics have been used recently to assist the surgeon with the procedure. Cardiac surgery should be readily available for patients who experience complications undergoing any diagnostic or treatment procedures in the cardiac catheterization laboratory.
4. Acute stemi: thrombolytic therapy:
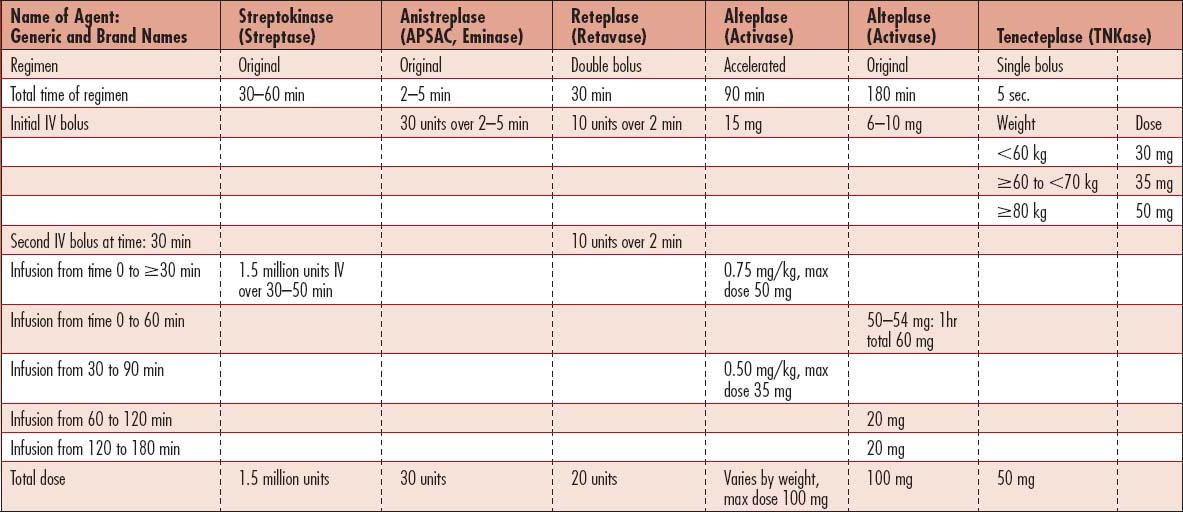
• Thrombolytic therapy (lysis of coronary arterial clot): Used for reperfusion of the occluded coronary vessel(s) that causes AMI. Drugs include tenecteplase (TNK-TPA), alteplase (rtPA, Activase), reteplase (Retavase), streptokinase, urokinase, and anisoylated plasminogen streptokinase activator complex (APSAC, Eminase) (Box 5-1 and Table 5-5). Thrombolytic therapy is an AHA/ACC Class I intervention for patients with ST-segment elevation in two or more contiguous leads, bundle branch block (obscuring ST-segment analysis), and history suggestive of AMI who present within 12 hours of symptom onset and are less than 75 years of age. Patients must be carefully screened for risk of bleeding before administration of these IV medications (Box 5-2). Time from the entry of the patient into the emergency department of the hospital to treatment with thrombolytics should be within 30 minutes of arrival. Thrombolytics are also used for direct injection into the coronary arteries as part of coronary angiography during cardiac catheterization and angioplasty.
Box 5-1 THROMBOLYTICS
First-generation thrombolytics
Streptokinase: An enzyme derived from group C beta-hemolytic streptococci. Because it is an antigen, patients who have had previous exposure to streptococcal organisms may have antibodies against streptokinase. Therefore steroids or antihistamines are administered before streptokinase therapy to prevent a hypersensitivity reaction.
Anistreplase: A plasminogen activator that induces clot lysis with fewer systemic lytic effects than does streptokinase. Allergic and anaphylactic reactions are possible.
Second-generation thrombolytics
Alteplase: A recombinant tissue plasminogen activator (rtPA) with the same amino acid sequence as endogenous tissue plasminogen activator (tPA). Has a shorter half-life than do the other agents, less than 5 minutes. Several dosage regimens are approved for coronary thrombolysis.
Reteplase: A recombinant deletion mutein of tPA. Catalyzes cleavage of endogenous plasminogen to generate plasmin. It is not clot-specific, so fibrinogen levels fall to lower levels than those seen with rtPA (alteplase), with a return to baseline value within 48 hours after infusion. The half-life is 13 to 16 minutes, and the drug is cleared by the liver and kidneys. Dosage: given as two boluses 30 minutes apart, each over 2 minutes.
Tenecteplase: A modified form of human tPA that binds to fibrin and converts plasminogen to plasmin. In the presence of fibrin, conversion of plasminogen to plasmin is increased relative to its conversion in the absence of fibrin. This fibrin-specificity decreases systemic activation of plasminogen and the resulting degradation of circulating fibrinogen compared to a molecule lacking this property. Following administration of tenecteplase, there are decreases in circulating fibrinogen and plasminogen. Tenecteplase is given as a single 5-second bolus and is dosed by weight.
Box 5-2 Contraindications and Warnings for Thrombolytic Therapy
Warnings (risks may be increased and should be weighed against anticipated benefits)
• Recent major surgery (e.g., coronary artery bypass graft, obstetric delivery, organ biopsy)
• Previous puncture of noncompressible vessels
• Recent gastrointestinal or genitourinary bleeding
• Hypertension (systolic BP ≥180 mm Hg and/or diastolic BP ≥110 mm Hg)
• High likelihood of left-sided heart thrombus (e.g., mitral stenosis with atrial fibrillation)
• Subacute bacterial endocarditis
• Hemostatic defects including those secondary to severe hepatic or renal disease
• Severe hepatic or renal dysfunction
• Diabetic hemorrhagic retinopathy or other hemorrhagic ophthalmic conditions
• Septic thrombophlebitis or occluded arteriovenous cannula at a seriously infected site
• Currently receiving anticoagulants (e.g., warfarin sodium)
• Any other condition in which bleeding constitutes a significant hazard or would be particularly difficult to manage because of its location
CARE PLANS FOR ACUTE CORONARY SYNDROMES
Energy Conservation, Instrumental Activities of Daily Living (IADL)
1. Assist patient with identifying activities that precipitate chest pain, and teach patient to use NTG prophylactically before the activity.
2. Assist patient as needed in progressive activity program, beginning with level I and progressing to level IV, as tolerated (Table 5-6).
3. Assess patient’s response to activity progression. Be alert to presence of chest pain, SOB, excessive fatigue, and dysrhythmias. Monitor for a decrease in BP greater than 20 mm Hg and an increase in HR to greater than 120 bpm (greater than 20 bpm above resting HR in patients receiving beta-blocker therapy).
4. Teach patient about measures that prevent complications of decreased mobility, such as active ROM exercises. (For additional details, see “Prolonged Immobility,” p. 149.)
Table 5-6 ACTIVITY LEVEL PROGRESSION FOR HOSPITALIZED PATIENTS*
| Level | Activity | |
| I | Bed rest | Flexion and extension of extremities 4 times daily, 15 times each extremity; deep breathing 4 times daily, 15 breaths; position change from side to side every 2 hr |
| II | OOB to chair | As tolerated, 3 times daily for 20–30 min |
| III | Ambulate in room | As tolerated, 3 times daily for 20–30 min |
| IV | Ambulate in hall | Initially, 50–200 ft twice daily; progressing to 50–200 ft 4 times daily |
BP, blood pressure; HR, heart rate; OOB, out of bed.
* Signs of activity intolerance: Decrease in BP <20 mm Hg; increase in HR to >120 beats/min (or >20 beats/min above resting HR in patients receiving beta-blocker therapy).
Risk for deficient fluid volume
related to increased risk of bleeding
1. Monitor dosing of all drugs known to increase bleeding, including aspirin and other antiplatelet drugs, GP IIb/IIIa inhibitors, direct thrombin inhibitors, and thrombolytics.
2. Monitor Hgb and Hct closely. Report Hgb drop greater than 2 g to physician immediately.
3. Monitor vital signs closely. Drop in BP and increase in HR may indicate developing shock, which may be cardiogenic or indicate hidden bleeding.
4. After PCI via femoral sheath, keep HOB less than 30 degrees and affected leg straight as ordered.
5. After PCI, monitor vital signs, sheath site, and distal pulses every 15 minutes until sheath is removed, then every 15 minutes × 2, then every 30 minutes × 2, then every 4 hours.
6. Carefully follow physician’s orders for sheath removal after PCI.
7. If bleeding occurs at the insertion site, position the patient flat and apply pressure until hemostasis is achieved and obtain stat CBC. Notify physician or midlevel practitioner of drop in Hgb.
8. Instruct the patient to report any discomfort. Groin pain may indicate pseudoaneurysm; back pain may indicate retroperitoneal bleed.
![]() Bleeding Reduction; Surveillance; Hemorrhage Control; Neurologic Monitoring
Bleeding Reduction; Surveillance; Hemorrhage Control; Neurologic Monitoring
Decreased co (or risk for same)
1. Monitor ECG continuously for evidence of dysrhythmias. Consult physician or midlevel practitioner for significant dysrhythmias or new or worsening ST-segment elevation.
2. With any dysrhythmia, check vital signs and note accompanying signs and symptoms such as dizziness, lightheadedness, syncope, and palpitations.
3. Ensure availability of emergency drugs and equipment: atropine, isoproterenol, epinephrine, amiodarone, lidocaine (use cautiously with AMI), defibrillator-cardioverter, and external and transvenous pacemaker.
4. Evaluate patient’s response to medications and emergency treatment.
5. Monitor patient for signs of reocclusion: chest pain, nausea, diaphoresis, and dysrhythmias.
6. Consult physician for any signs of reocclusion.
7. Obtain 12/15/18-lead ECG if reocclusion is suspected.
8. Anticipate and prepare patient for cardiac catheterization, PCI with stent, or repeated thrombolytic therapy.
9. Monitor for signs of bleeding and bleeding complications: bleeding at sheath site or at IV sites, back pain (may indicate retroperitoneal bleed), signs and symptoms of cerebrovascular accident (CVA)/stroke (see Stroke: Acute Ischemic and Hemorrhagic, p. 674).
![]() Hemodynamic Regulation; Circulatory Care; Dysrhythmia Management; Cardiac Care: Acute
Hemodynamic Regulation; Circulatory Care; Dysrhythmia Management; Cardiac Care: Acute
1. Before treatment, question patient about history of previous streptokinase therapy or streptococcal infection. Consult physician or midlevel practitioner for positive findings.
2. Administer prophylactic hydrocortisone as prescribed.
3. Monitor patient during and for 48 to 72 hours after infusion for indicators of allergy: hypotension (brief or sustained), urticaria, fever, itching, flushing, nausea, headache, muscular pain, bronchospasm, abdominal pain, dyspnea, or tachycardia. These indicators can appear immediately after or as long as several days after streptokinase therapy.
4. If hypotension develops, increase rate of IV infusion/administer volume replacement as prescribed. Prepare for vasopressor administration if there is no response to volume replacement.
5. Treat allergic response with diphenhydramine or other antihistamine as prescribed.
![]() related to cardiac dysfunction following acute coronary event
related to cardiac dysfunction following acute coronary event
1. See Care Plans for Generalized Cardiovascular Dysfunctions, p. 419.
2. Monitor for ST-segment elevation indicative of myocardial injury, ST depression indicative of myocardial ischemia, new left bundle branch block, and Q waves indicative of MI. All ECG changes must be present in at least two contiguous (related) leads.
3. Collaborate with physician if decreased CO does not respond to acute cardiac care interventions.
4. Maintain fluid balance carefully, with close assessment for need for fluids versus diuretics. Patients with right HF may require support with IV fluids, while those with left HF may respond better to diuretics.
5. Provide patient with platelet inhibiting (e.g., clopidogrel, aspirin, abciximab) and thrombin-inhibiting medications (e.g., heparin, enoxaparin, fondaparinux, bivalirudin) as appropriate, according to AHA guidelines for STEMI or NSTEMI.
1. Monitor for unstable BP and use hemodynamic readings to help assess need for titration of vasoactive, inotropic, and antidysrhythmic medications.
2. Collaborate with physician to assess if patients with severe bundle branch block might benefit from biventricular cardiac pacing to improve synchrony of ventricular contraction.
3. Assess effects of fluid challenges on CVP and pulmonary artery occlusive pressure (PAOP); especially in patients with RV infarction.
4. Elevate legs rather than placing patients in Trendelenberg position to help augment venous return to increase BP.
Circulatory care: mechanical assist device
1. Assess peripheral circulation meticulously when intra-aortic balloon catheter is in place.
2. Evaluate impact of changes in balloon counterpulsation settings on CO, CI, all hemodynamic pressures, and BP.
3. Examine all cannulas for kinking or disconnection if patient becomes suddenly unstable without other notable cause.
4. Use strict aseptic technique when changing device-related dressings.
5. Administer anticoagulants or thrombolytics as appropriate.
6. Collaborate with physician regarding need for further interventions if patient remains unstable despite mechanical assist device and vasoactive, inotropic, and antidysrhythmic medications.
Deficient knowledge: coronary artery disease process and its lifestyle implications
related to the need to help prevent further incidence of heart disease
Teaching: cardiac disease process
1. Teach patient about ischemia and its resultant chest pain, referred to as angina pectoris.
2. Discuss the pathophysiologic process underlying patient’s angina, using drawings or heart models as indicated.
3. Assist patient in identifying his or her own risk factors (e.g., cigarette smoking, high-stress lifestyle, hyperglycemia, high-fat diet).
4. Teach patient about risk factor modification:
![]() Teaching: Individual; Teaching: Disease Process, Thrombolysis
Teaching: Individual; Teaching: Disease Process, Thrombolysis
![]() related to risk of bleeding/hemorrhage secondary to nonspecific thrombolytic effects of therapy
related to risk of bleeding/hemorrhage secondary to nonspecific thrombolytic effects of therapy
1. When patient is admitted, obtain a thorough history, assessing for the following:
2. Monitor clotting studies per agency protocol. Regulate heparin drip to maintain PTT at 1½ to 2 times control levels or according to protocol. Never discontinue heparin without consulting with a physician or midlevel practitioner.
3. Apply pressure dressing over puncture sites. If cardiac catheterization was performed, inspect site at frequent intervals for evidence of hematoma formation. Immobilize extremity for 6 to 8 hours after catheterization procedure.
4. Avoid unnecessary venipunctures, IM injections, or arterial puncture. Obtain laboratory specimens from heparin-lock device.
5. Monitor patient for indicators of internal bleeding: back pain, abdominal pain, decreased BP, pallor, and bloody stool or urine. Report significant findings to physician or midlevel practitioner.
6. Monitor patient for signs of intracranial bleeding every 2 hours: change in LOC, headache, dizziness, vomiting, and confusion.
7. Test all stools, urine, and emesis for occult blood.
8. Use care with oral hygiene and when shaving patient. For more information about safety precautions, see Pulmonary Embolus, p. 396.
Teaching: diet and prescribed medications
1. Diet low in cholesterol and saturated fat: Provide sample diet plan for meals that are low in cholesterol and saturated fat. Teach patient about foods that are high and those that are low in cholesterol and saturated fat. Stress the importance of reading food labels.
2. Blood pressure control: If patient was found to have hypertension associated with MI, the patient should be taught the importance of taking appropriate medications to control BP and to follow recommended dietary guidelines to minimize sodium intake. Sodium promotes water retention, which can increase the BP. Higher systemic BP increases the workload of the heart, demanding that more myocardial oxygen be consumed.
2. Take 1 NTG; wait 5 minutes. If pain is not relieved, take a second NTG; wait 5 minutes. If pain is not relieved, take a third NTG.
3. Lie down if headache occurs. The vasodilation effect of NTG causes a decrease in BP, which may result in orthostatic hypotension and transient headache.
4. If the pain is not relieved after 3 NTGs taken over a 15-minute period, dial 911 or the local emergency number.
5. Explain to the patient that it is no more beneficial to be in the emergency department than it is to be at home during episodes of chest pain caused by angina and therefore traveling to a hospital at the first sign of chest pain usually is unnecessary.
6. Review activity limitations and prescribed progressions (see Tables 5-3 and 5-6). Provide the following information:
7. Avoid exercising outdoors in very cold, hot, or humid weather. Extreme weather places an additional stress on the heart. If you do exercise in extremes of weather, decrease the pace and monitor your response carefully.
8. Pulse monitoring: Teach patient how to take pulse, including parameters for target HRs and limits.
1. Rest is beneficial before engaging in intercourse.
2. Find a position that is comfortable for you and your partner. Assuming a different position that is uncomfortable to both may increase the workload of the heart.
3. Medications such as NTG may be taken prophylactically by the patient before intercourse to prevent chest pain.
4. Postpone intercourse for 1 to 2 hours after eating a heavy meal.
5. Report the following symptoms to your physician if they are experienced after sexual relations: SOB, increased HR that persists for longer than 15 minutes, unrelieved chest pain.
Related to biophysiologic injury related to decreased oxygen supply to the myocardium
1. Assess and document the character of the patient’s chest pain, including location, duration, quality, intensity, precipitating and alleviating factors, presence or absence of radiation, and associated symptoms. Devise a pain scale with patient, rating discomfort from 0 (no pain) to 10 or any system that assists in objectively reporting pain level.
2. Measure BP and HR with each episode of chest pain. BP and HR may increase because of sympathetic stimulation as a result of pain. If the chest pain is caused by ischemia, the heart muscle may not be functioning normally and CO may decrease, resulting in a low BP. In addition, dysrhythmias such as bradycardia and ventricular ectopy may be noted with ischemia. If BP is low, it may not be advisable to administer nitrates and morphine, which can further reduce BP, adding to myocardial ischemia.
3. After each titration of IV NTG, evaluate patient’s BP and the effects of therapy in relieving patient’s chest pain. If slight hypotension occurs (80 to 90 mm Hg systolic), reduce the flow rate to one-half or less of the infusing dose. If severe hypotension (less than 80 mm Hg SBP) occurs, stop the infusion and contact the physician for further directions. In either situation, the physician may prescribe a normal saline infusion or low-dose positive inotropic agent (e.g., dopamine, dobutamine) to enhance cardiac contractility.
4. Monitor for side effects of NTG, including headache, hypotension, syncope, facial flushing, and nausea. If side effects occur, place patient in a supine position and consult physician for further interventions.
5. Administer heparin, GP IIb/IIIa inhibitors (abciximab, tirofiban, eptifibatide), and aspirin as prescribed. Heparin infusion usually should be administered using a weight-based protocol, which is titrated according to PTT results. These patients are predisposed to bleeding and may need to be placed on bleeding precautions.
6. Maintain a quiet environment and group patient care activities to allow for periods of uninterrupted rest. Consider healing touch therapy, relaxation exercises, or music therapy.
7. Position patient according to his or her comfort level.
8. Provide care calmly and efficiently; reassure patient during chest pain episodes.
9. Ensure that activity restrictions and bedrest are maintained; teach patient about activity limitation and its rationale—to minimize oxygen requirements and thus decrease chest pain. Reassure patient activities are allowed based on individual response. Often, patients may be afraid to engage in activities for fear of further deterioration.
10. Instruct patient to report any further episodes of chest pain.
1. Obtain a 12/15/18-lead ECG during patient’s episode of chest pain. During angina, ischemia usually is demonstrated on the ECG by ST-segment depression and T-wave inversion.
2. Administer nitrates as prescribed, titrating IV NTG so that chest pain is relieved yet SBP remains greater than 90 mm Hg. NTG drip is usually 50 mg NTG in 250 ml D5W. Begin with 6 ml/hr, which is 5 mcg/min. Titrate by increments of 10 to 20 ml every 5 minutes (or 10 to 20 mcg/min every 5 minutes) up to a maximum dosage determined by agency protocol, physician, or midlevel practitioner.
3. As prescribed, administer beta-blockers and possibly calcium channel blockers, which relieve chest pain by (1) diminishing coronary artery spasm, causing coronary and peripheral vasodilation, and (2) decreasing myocardial contractility and oxygen demand. Monitor for side effects, including bradycardia and hypotension. Be alert to indicators of HF, including fatigue, SOB, weight gain, and edema, and to indicators of heart block, such as syncope and dizziness.
4. Administer oxygen per nasal cannula at 2 to 4 L/min, as prescribed.
![]() Analgesic Administration; Cardiac Care: Acute; Hemodynamic Regulation
Analgesic Administration; Cardiac Care: Acute; Hemodynamic Regulation
CARE PLANS FOR PATIENTS UNDERGOING PERCUTANEOUS CORONARY INTERVENTION
related to angioplasty procedure and postprocedure care
Knowledge: Treatment Procedure(s); Knowledge: Medications; Knowledge: Disease Process
1. Use of local anesthesia and sedation during procedure
2. Insertion site of catheter: groin or arm
3. Sensations that may occur: mild chest discomfort, a feeling of heat as the dye is injected
4. Use of fluoroscopy during procedure. Determine patient’s history of sensitivity to contrast material.
5. Ongoing observations made by nurse after procedure: BP, HR, ECG, leg or arm pulses, blood tests
6. Importance of lying flat in bed for 6 to 12 hours after procedure unless a vascular closure device was used. Patients are able to get out of bed sooner when a closure strategy is used.
7. Necessity for nursing assistance with eating, drinking, and toileting needs after procedure
8. Need for increased fluid intake after procedure to flush dye from system
9. If patient and significant others express or exhibit evidence of anxiety regarding the procedure, try to arrange for them to meet with another patient who has had a successful angioplasty.
Teaching: prescribed medication
1. Importance of taking antiplatelet drugs to prevent restenosis
2. Importance of taking statins and other antilipid drugs to prevent progression of CAD
3. Avoidance of strenuous activity during first few weeks at home
4. Follow-up visit with cardiologist 1 week after hospital discharge
5. Signs and symptoms to report to health care professional (e.g., GI upset, repeat of angina, fainting)
![]() Teaching: Procedure/Treatment; Anxiety Reduction; Support Group
Teaching: Procedure/Treatment; Anxiety Reduction; Support Group
Additional nursing diagnoses
Also see nursing diagnoses and interventions as appropriate in Nutritional Support (p. 117), Mechanical Ventilation (p. 99), Hemodynamic Monitoring (p. 75), Prolonged Immobility (p. 149), Emotional and Spiritual Support of the Patient and Significant Others (p. 200), Acute Cardiac Tamponade (p. 257), Heart Failure (p. 421), and Dysrhythmias and Conduction Disturbances (p. 492).
Acute infective endocarditis
Pathophysiology
![]() Portals of entry for the infecting organism include the mouth and GI tract, upper airway, skin, and external genitourinary (GU) tract. All heart valves are at risk for infection, but the aortic and mitral valves are more commonly affected than the right-sided pulmonic and tricuspid valves. IV drug abuse increases the possibility of tricuspid IE. Once the infection process begins, valvular dysfunction, manifested by insufficiency with regurgitant blood flow, can occur, ultimately resulting in a decrease in CO. The vegetation may enlarge and obstruct the valve orifice, further reducing CO. The vegetation may break apart and embolize to vital organs. In severe cases, the affected valve may necrose, develop an aneurysm, and rupture or the infection may extend through the myocardium and epicardium to cause a pericarditis (see Acute Pericarditis, p. 461). If the conduction system is affected by the spreading infection, bundle branch block may occur. The chordae tendineae can become infected and rupture, resulting in severe acute mitral or tricuspid regurgitation. Complications of IE occur suddenly, with a dramatic change in the clinical picture. Mortality rates between 20% and 50% have been reported. The infection recurrence rate is 10% to 20%. The incidence of IE is higher in patients over age 50 years, is more common in men than in women, and is uncommon in children.
Portals of entry for the infecting organism include the mouth and GI tract, upper airway, skin, and external genitourinary (GU) tract. All heart valves are at risk for infection, but the aortic and mitral valves are more commonly affected than the right-sided pulmonic and tricuspid valves. IV drug abuse increases the possibility of tricuspid IE. Once the infection process begins, valvular dysfunction, manifested by insufficiency with regurgitant blood flow, can occur, ultimately resulting in a decrease in CO. The vegetation may enlarge and obstruct the valve orifice, further reducing CO. The vegetation may break apart and embolize to vital organs. In severe cases, the affected valve may necrose, develop an aneurysm, and rupture or the infection may extend through the myocardium and epicardium to cause a pericarditis (see Acute Pericarditis, p. 461). If the conduction system is affected by the spreading infection, bundle branch block may occur. The chordae tendineae can become infected and rupture, resulting in severe acute mitral or tricuspid regurgitation. Complications of IE occur suddenly, with a dramatic change in the clinical picture. Mortality rates between 20% and 50% have been reported. The infection recurrence rate is 10% to 20%. The incidence of IE is higher in patients over age 50 years, is more common in men than in women, and is uncommon in children.
Assessment
Observation
• If right-sided HF is present, skin and sclera may be jaundiced and edematous, with neck vein distention, a positive hepatojugular reflex, and ascites.
• Late assessment findings include anemia, petechiae, and clubbing of the fingers.
• Splenomegaly occurs by 10 days due to the activation of the reticuloendothelial system. If marked, a splenic infarct may have occurred.
• Acute infective stage: Diaphoresis, fatigue, anorexia, joint pain, weight loss, and abdominal pain
• Splinter hemorrhages: Small red streaks on the distal third of the fingernails or toenails
• Janeway lesions: Painless, small, hemorrhagic lesions found on the fingers, toes, nose, or earlobes, probably occurring as the result of immune complex deposition with inflammation
• Osler nodes: Painful, red, subcutaneous nodules found on the pads of the fingers or on the feet, probably occurring as a result of emboli producing small areas of gangrene or vasculitis
• Roth spots: Retinal hemorrhages with pale centers seen on fundoscopic examination
12-lead electrocardiogram
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Noninvasive Cardiology | ||
| 12-lead electrocardiogram (ECG) | Frequently performed to determine if ischemia or conduction system defects are present | Heart block may manifest if the AV node or bundle of His is affected by the infection. Atrial and/or ventricular enlargement may be seen from the prolonged effects of valve disease. Chambers may be enlarged or muscle walls thickened (see Table 5-7). Atrial dysrhythmias including premature atrial contractions (PACs), paroxysmal atrial tachycardia (PAT), and atrial fibrillation (AF) are frequently seen as chambers enlarge from volume overload (see Table 5-8). |
| Blood Studies | ||
| Serial cardiac enzymes Myoglobin CK-MB isoform CK-MB Troponin I Troponin T |
Assess for enzyme changes indicative of myocardial tissue damage; diagnostic for MI; rule out MI. | Elevated if MI occurs from embolization of vegetations into the coronary arteries |
| Complete blood count (CBC) RBC count (RBCs) WBC count (WBCs) |
Assess for signs of infection. | Increased WBCs and eosinophils, with reduced RBCs/possible anemia |
| Electrolytes Potassium (K+) Magnesium (Mg2+) Calcium (Ca2+) Sodium (Na+) |
Assess for electrolyte imbalance, which helps with differential diagnosis. | May be normal. Potassium or magnesium is sometimes increased or decreased. |
| ABGs | Determine effectiveness of oxygenation. | Indicative of cardiac and pulmonary status (see Acid-Base Imbalances, p. 1) |
| Coagulation profile Prothrombin time (PT) with international normalized ratio (INR) Partial thromboplastin time (PTT) Fibrinogen D-dimer |
Assess for causes of bleeding, clotting, and disseminated intravascular coagulation (DIC) indicative of abnormal clotting present in shock or ensuing shock. | Decreased PT with low INR promotes clotting; elevation promotes bleeding; elevated fibrinogen and D-dimer reflects abnormal clotting is present. In the presence of effusions, anticoagulants are contraindicated because of the high risk of cardiac tamponade, which can result from bleeding into the pericardium. |
| Blood cultures For low suspicion of IE: 3–6 sets of aerobic and anaerobic blood cultures should be drawn from different venipuncture sites over 24 hours. |
Provides definitive diagnosis of the infecting organism. Antibiotics are prescribed based on organism sensitivity. Drawn 1 hour apart over 3 hours. If suspicion is high, cultures should be drawn within 1–2 hours and empiric antibiotic treatment begun. Cultures can be negative when IE is present as a result of slow-growing organisms, prior antibiotic use, failure to obtain adequate number of specimens, or the organism’s failure to grow in standard culture media. | The most common bacteria found in native (the patient’s) valve IE are Streptococcus viridans (60%), Staphylococcus aureus (25%), and the HACEK group (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella spp.). Manipulations of the gastrointestinal (GI) or genitourinary (GU) tract may result in IE from Enterococcus faecalis. Early prosthetic valve infections are caused by Staphylococcus epidermidis (33%), gram-negative bacteria (19%), and S. aureus (17%). Candida is the most common fungal source of IE, accounting for 8% of prosthetic valve endocarditis and 1% of native valve endocarditis. |
| Additional studies Rheumatoid factor Erythrocyte sedimentation rate (ESR) IE gamma globulins B-type natriuretic peptide (BNP) |
To identify the causes of IE and associated diseases | Rheumatoid factor and ESR are elevated. IE gamma globulins may be present. BNP will be elevated if heart failure is present. |
| Radiology | ||
| Chest radiograph (CXR) | Assess size of heart, mediastinum, thoracic cage (for fractures), thoracic aorta (for aneurysm) and lungs (pneumonia, pneumothorax); assists with differential diagnosis of chest pain. | Cardiac enlargement may reflect widening mediastinum and tamponade. |
| Computed tomography (CT) Cardiac CT scan |
Assesses ventricular size, morphology, function, status of cardiac valves, and circulation. May be done to assess for embolization to other organs. | |
| Ultrasound echocardiography (echo) Transesophageal echo |
Reveals valvular involvement and vegetation size and defines severity of valvular dysfunction. M-mode, two-dimensional, Doppler, and transesophageal echocardiograms (TEEs) are used. TEE is the preferred test. | Detects vegetation, especially with prosthetic valves. Obtained within 2 hours of acute presentation, the test is 90% specific and sensitive. Preexisting IE may be indistinguishable from new vegetations. |
| Arteriograms: renal, mesenteric, and peripheral | Assess for embolization. | If embolization is present, affected organs are compromised and may fail. |
Collaborative management
Care priorities
1. Prevent infective endocarditis in patients undergoing invasive procedures:
Table 5-8 ECG CHANGES FREQUENTLY FOUND WITH VENTRICULAR AND ATRIAL HYPERTROPHY
| Chamber | ECG Change |
|---|---|
| Left ventricular enlargement | “R” voltage increases in V4–6; “S” voltage increases (deeper inflection) in V1–2; the sum of “S” in V1 or V2 and “R” in V5 or V6 will be more than 35 mm, or “R” in any V lead will be more than 25 mm |
| Left atrial enlargement | “P mitrale” in leads II, III, aVF, and V1; P wave is m-shaped with a duration more than 0.1 sec |
| Right ventricular enlargement | “R” voltage increases in V1 or V2; “S” voltage increases in V5, V6; sum of “R” in V1 or V2 and “S” in V5 or V6 will be more than 35 mm |
| Right atrial enlargement | “P pulmonale” in leads II, III, aVF, and V1; P wave is 2.5 mm voltage and 0.1 sec duration |
• High risk: Includes those with bioprosthetic valve replacement, valvular repair, complex cyanotic congenital repairs, systemic or pulmonary conduits, and previous IE
• Moderate risk: Includes those with other noncyanotic congenital defects, valvular dysfunction, hypertrophic cardiomyopathy, and mitral valve prolapse with evidence of regurgitant blood flow or thickened valve leaflets
• Treatment of streptococcal infected endocarditis: Patients should be treated for at least 2 weeks in the hospital and observed for cardiac and noncardiac complications. Patients may be a candidate for outpatient and home parenteral antibiotic therapy.
• Treatment of coagulase-negative species causing prosthetic valve endocarditis within the first year after valve replacement: Patients are usually methicillin-resistant. They should be given. A combination of vancomycin and refampicin for at least 6 weeks with the addition of gentamicin for the initial 2 weeks.
2. Treat infection and prevent further complications, such as emboli, hf, or cardiogenic shock:
• Antibiotics: Patients usually require 4 to 6 weeks of IV antibiotics. The first 2 weeks may be initiated during hospitalization and the remaining therapy done on outpatient status. The vegetation must be sterilized, abscesses treated, and spread of infection prevented. Initial antibiotic selection is empirical followed by therapy based on the results of the blood/tissue culture and sensitivity studies.
• Fluid and sodium restriction: Used for optimal fluid balance with reduced heart function or HF. Specific restrictions must be individualized and based on severity of symptoms.
• Bedrest: May be used initially, with activity as tolerated for the remainder of treatment
• Diet: High in protein and calories to prevent cardiac cachexia and support the immune system
• Watch for signs of embolization during the first 3 months of treatment: Monitor renal status, vital signs, and oxygenation.
• Oxygen therapy with pulse oximetry (SpO2): Oxygen (FIO2) to maintain PaO2 at 80 mm Hg or higher and pulse oximetry to monitor oxygen saturation continuously or intermittently to keep SpO2 at 95% or higher
• Diuretics: May be used to decrease symptoms of HF by reducing intravascular volume
• Positive inotropic agents (e.g., digoxin, dobutamine, milrinone, inamrinone): Used to increase contractility and CO
• Vasodilators: nitroprusside, nitroglycerin: Reduce cardiac work and improve coronary arterial perfusion. Both preload and afterload (end-diastolic ventricular volume and pressure) may be reduced to help relieve symptoms of HF. Aggressive vasodilation is not well tolerated by all patients
• Sedation: May be necessary to allay anxiety and to reduce myocardial oxygen consumption
5. Consider surgical valve replacement:
Required when HF worsens or if the infection fails to respond to antibiotics (see Valvular Heart Disease, p. 566). An abscess or infected tissue may be surgically removed if there is no response to long-term antibiotics. If the patient is hemodynamically stable, surgery may not be needed. A surgeon is usually consulted in case of an HF emergency.
• Indications for urgent native valve replacement:
• Indications for replacement of prosthetic valves: Complications that may prompt the need to replace a prosthetic valve include primary valve failure, prosthetic valve endocarditis, prosthetic valve thrombosis, thromboembolism, and mechanical hemolytic anemia.
Prosthetic valve endocarditis (PVE): The hallmark sign of PVE in mechanical valves is ring abscesses. Ring abscess may lead to valve dehiscence, perivalvular leakage, and formation of myocardial abscesses. Extension to the conduction system may prompt a new atrioventricular block. Valve stenosis and purulent pericarditis are seen less often. Valve stenosis is more common with bioprosthetic valves than mechanical valves. Bioprosthetic valve PVE results in leaflet tears or perforations. Ring abscesses, purulent pericarditis, and myocardial abscesses are seen less often in bioprosthetic valve PVE.
Early PVE: Occurs within 60 days of valve insertion and is usually the result of perioperative contamination
Late PVE: Occurs 60 days or later after insertion and is usually the result of transient bacteremia from dental or GU sources, GI manipulation, or IV drug abuse
CARE PLANS FOR ACUTE INFECTIVE ENDOCARDITIS
related to altered preload, afterload, or contractility secondary to valvular dysfunction
Cardiac Pump Effectiveness; Circulation Status
1. Assess heart sounds every 2 to 4 hours. A change in the characteristics of a heart murmur may signal progression of valvular dysfunction, which can occur with insufficiency, stenosis, dislodgment of vegetation, or unseating of a prosthetic valve.
2. Assess heart sounds. A new S3 or S4 sound may signal HF.
3. Monitor heart rhythm continuously. Report dysrhythmias, which may indicate the spread of infection to the conduction system or atrial volume overload.
4. Monitor for signs of left-sided HF: crackles, S3 or S4 sounds, dyspnea, tachypnea, digital clubbing, decreased BP, increased pulse pressure, increased serum BNP levels, increased LVEDP, and decreased CO.
5. Monitor for signs of right-sided HF: increased CVP, distended neck veins, positive hepatojugular reflex, edema, jaundice, increased serum BNP levels, and ascites.
6. Monitor I&O hourly, and measure weight daily. Use the same scale and amount of clothing, and weigh patient at the same time of day for accuracy. Consult physician if patient’s weight increases by more than 1 kg per day.
7. If patient’s CVP is high, decrease preload by limiting fluid and sodium intake and administer diuretics and venous dilators (e.g., NTG) as prescribed.
8. If patient’s MAP is high, decrease afterload with prescribed arterial dilators (e.g., nitroprusside).
9. For low CVP or BP, consult with physician or midlevel practitioner. Vasopressors may be prescribed. Patient may be developing sepsis, so monitor carefully.
10. If diastolic BP is low, coronary artery perfusion may be reduced. Prevent further reductions by avoiding administration of morphine sulfate or rapid warming of hypothermic patients. Increase contractility with inotropic drugs, as prescribed.
11. Provide activities as tolerated. Intolerance indicates ineffective oxygenation.
12. Help patient reduce stress and myocardial oxygen consumption by teaching stress-reduction techniques such as imagery, meditation, or progressive muscle relaxation. For description of a relaxation technique, see Appendix 7
13. Provide sedation as needed.
14. Prevent orthostatic hypotension by changing patient’s position slowly. See Cardiogenic Shock, p. 472, for a discussion of preload and afterload medications.
Respiratory Status: Gas Exchange; Tissue Perfusion: Pulmonary
1. Assess rate, effort, and depth of respirations. RR increases in response to inadequate oxygenation. Tachypnea may indicate pulmonary congestion.
2. Assess color of skin and mucous membranes. Pallor signals impaired oxygenation.
3. Auscultate lungs every 2 hours. Report crackles, rhonchi, and wheezing.
4. If hemodynamic monitoring with oximetry is used, assess SvO2. It may fall because increased metabolic demands have increased oxygen uptake, or because the patient has increased extraction as a result of reduced perfusion/oxygen delivery. SvO2 values may fall before the patient is symptomatic; they correlate with CO.
5. Monitor ABG values for evidence of hypoxemia (PaO2 less than 80 mm Hg), respiratory acidosis (PaCO2 greater than 45 mm Hg, pH less than 7.35), or respiratory alkalosis (PaCO2 less than 35 mm Hg, pH greater than 7.45) from tachypnea. Either may indicate impending respiratory failure.
6. Deliver oxygen as prescribed. Observe respiratory rate of COPD patients if oxygen is increased to avoid hypoventilation and/or respiratory arrest.
7. Assess arterial oxygen saturation with pulse oximetry. Normal oxygen saturation is 95%–99%. Levels of 90%–95% necessitate frequent assessment. Levels less than 90% require aggressive interventions to increase oxygen saturation. Consider increasing FIO2, decreasing preload, and taking measures to improve ventilation.
8. Place patient in high Fowler’s position to facilitate gas exchange as tolerated.
9. Have patient cough, deep breathe, and use incentive spirometry to prevent atelectasis.
![]() Invasive Hemodynamic Monitoring; Oxygen Therapy; Respiratory Monitoring
Invasive Hemodynamic Monitoring; Oxygen Therapy; Respiratory Monitoring
1. Use strict aseptic technique to care for all invasive monitoring device insertion sites and IV lines. Rotate central lines per hospital protocol. Discuss feasibility of a tunneled catheter or peripherally inserted central catheter (PICC) line with physician.
2. Change tubing, containers, and peripheral insertion sites per agency protocol. Inspect all catheter insertion sites daily for redness, drainage, or other evidence of infection. Rotate site immediately if infection is suspected.
3. Provide mouth care at least every 4 hours to minimize fungal and other infections. Women may require antifungal medications to manage vaginal yeast infections.
4. Provide perineal care with soap and water for patients with indwelling urinary catheters. Inspect urine for evidence of infection, such as casts, cloudiness, or foul odor. Be alert to patient complaints of burning with urination after catheter is removed.
5. Monitor temperature, WBC count, and HR. Increases may be signs of infection.
6. Calculate SVR with CO measurements. Symptoms of septic shock include increased CO, decreased SVR, and increased SvO2 during the early stages.
7. Teach patient and significant others the importance of reporting signs and symptoms of recurring infections (e.g., fever, malaise, flushing, anorexia) or HF (e.g., dyspnea, tachypnea, tachycardia, weight gain, peripheral edema).
8. Stress the importance of prophylactic antibiotics before invasive procedures such as dental examinations or surgery. The AHA publishes general guidelines for prophylactic antibiotic treatment to prevent IE.
![]() related to interrupted arterial blood flow secondary to emboli caused by vegetations
related to interrupted arterial blood flow secondary to emboli caused by vegetations
1. Monitor I&O at frequent intervals. Be alert to urinary output less than 0.5 ml/kg/hr for 2 consecutive hours. Report oliguria, as it may signal impending acute renal failure.
2. Monitor bowel sounds every 2 hours. Report hypoactive or absent bowel sounds. Patients are at risk for decreased mesenteric perfusion and mesenteric or bowel infarction.
3. Assess peripheral pulses, color, and temperature of extremities. Weak pulses (2+ or less on a 0 to 4+ scale) with pale, cool limbs/hands/feet may denote peripheral embolization.
4. Monitor patient for confusion and changes in sensorimotor capabilities or cognition, which may signal cerebral emboli.
5. Assess for chest pain, decreased BP, SOB, ischemic or injury pattern on 12-lead ECG, or elevated cardiac enzyme levels indicative of MI caused by vegetation emboli that have migrated to the coronary arteries (see Acute Coronary Syndromes, p. 434).
6. Assess for and report appearance of splinter hemorrhages, Osler’s nodes, Janeway’s lesions, and Roth’s spots (see Assessment, p. 418).
Additional nursing diagnoses
As appropriate, also see nursing diagnoses and interventions in Nutritional Support (p. 117), Hemodynamic Monitoring (p. 75), Prolonged Immobility (p. 149), Emotional and Spiritual Support of the Patient and Significant Others (p. 200), Acute Cardiac Tamponade (p. 257), Heart Failure (p. 421), and Cardiogenic Shock (p. 472).
Acute pericarditis
Pathophysiology
Pericarditis is the general term for an inflammatory process involving the pericardium and the epicardial surface of the heart. Inflammation can occur as the result of an AMI, an infection, chronic renal failure, or an immunologic, chemical, or mechanical event (Box 5-3). Often, early pericarditis manifests as a dry irritation, whereas late pericarditis (after 6 weeks) involves pericardial effusions that can lead to cardiac tamponade if severe. Pericarditis is often seen in the critical care unit as a secondary finding following coronary revascularization surgery or valve replacement or associated with chronic renal failure. A thorough assessment and recognition are essential for appropriate treatment, as symptoms can be masked by the primary condition. For example, patients are sometimes transferred into a critical care unit with acute cardiac decompensation resembling acute coronary syndrome, when in reality, the condition is caused by pericardial effusions.
Box 5-3 CAUSES OF PERICARDITIS
The initial pathophysiologic findings of pericarditis include infiltration of polymorphonuclear leukocytes, increased vascularity, and fibrin deposition. Inflammation may spread from the pericardium to the epicardium or pleura. The visceral pericardium may develop exudates or adhesions. Large effusions can lead to cardiac tamponade. The excess fluid compresses the heart within the pericardial sac, which impairs filling of the chambers and ventricular ejection.
Assessment
Vital sign assessment
Heart rate, heart rhythm, and BP are used to evaluate CO and perfusion.
• BP elevated on right arm: When BP is taken on both arms, if cardiac tamponade is ensuing, blood cannot flow into and through the constricted heart.
• Narrowing pulse pressure: May indicate effusion is exerting pressure around the heart
• Pulsus paradoxus: BP should be checked for a paradoxical pressure greater than 10 mm Hg. Normally the systolic pressure is slightly higher during the expiration and lower during inspiration. When effusions are present, this difference in systolic pressure across the respiratory cycle will be greater than 10 mm Hg.
• Tachycardia: Heart rate is usually rapid and regular.
• Beck’s triad: Hypotension, elevated venous pressure with jugular venous distention, and muffled heart sounds may occur in patients with cardiac tamponade, especially if sudden intrapericardial hemorrhage occurs.
Hemodynamic measurements
If used, the PA catheter reveals elevated CVP, PAP, and PAWP.
• As effusions increase: CO will decrease. If adhesions are present, the filling of the chambers may be restricted, resulting in reduced end-diastolic volumes and pressures.
• If cardiac tamponade is developing: Pressures in all heart chambers eventually equalize and the patient has a cardiac arrest.
12-lead electrocardiogram
• Heart rate: Diagnose type of tachycardia, bradycardia, or irregular rhythm. During acute episodes, atrial dysrhythmias such as PAT, PACs, atrial flutter, or atrial fibrillation may occur.
• PR, QRS, QT intervals: May lengthen or shorten
• ST-segment and T-wave changes: Depression or elevation
• Pacing and conduction: Regular, normal rate and velocity
• Late dysrhythmias: Include ventricular ectopy or bundle branch blocks if the inflammatory process involves the ventricles. Pericardial effusion may decrease the voltage of the QRS complex on the ECG. Diffuse ST-segment elevation can be documented as described in Table 5-9.
| Stage | Time of Change | Pattern |
| 1 | Onset of pain | ST segments have a concave elevation in all leads except AVL and V1; T waves are upright |
| 2 | 1–7 days | Return of ST segments to baseline with T wave flattening and invert |
| 3 | 1–2 wk | Inversion of T waves without R or Q changes |
| 4 | Weeks to months | ECG returns to prepericarditis state |
Diagnostic Tests for Acute Pericarditis
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Noninvasive Cardiology | ||
| Electrocardiogram (ECG) 12-, 15-, and 18-lead ECG |
Differentiate between ischemia versus inflammation | Will show ST-segment or T-wave changes, which often are confused with ischemic changes. In pericarditis, they are more diffuse and follow a four-stage pattern (see Table 5-9). |
| Blood Studies | ||
| Serial cardiac enzymes Myoglobin CK-MB isoform CK-MB Troponin I Troponin T |
Assess for enzyme changes indicative of myocardial tissue damage; diagnostic for MI; rule out MI. | May reveal elevation of the CK and MB bands if the epicardium is inflamed |
| Complete blood count (CBC) Hemoglobin (Hgb) Hematocrit (Hct) RBC count (RBCs) WBC count (WBCs) Anti–streptolysin O (ASO) titer C-reactive protein Sedimentation rate |
Assess for anemia, inflammation, and infection; assists with differential diagnosis of chest pain. | Decreased RBCs, Hgb, or Hct reflects anemia from blood loss. Anti–streptolysin O (ASO) titer is elevated when the cause of the pericarditis is an immunologic disorder. If the pericarditis is the result of an infection, blood cultures will identify the infecting organism. Other markers of inflammation can be seen (elevated WBCs, C-reactive protein [CRP], lactate dehydrogenase [LDH], or erythrocyte sedimentation rate [ESR]) unless the pericarditis is secondary to uremia. |
| Electrolytes Potassium (K+) Magnesium (Mg2+) Calcium (Ca2+) Sodium (Na+) |
Assess for electrolyte status. | Use to rule out other causes. |
| Coagulation profile Prothrombin time (PT) with international normalized ratio (INR) Partial thromboplastin time (PTT) Fibrinogen D-dimer |
Assess for causes of bleeding, clotting, and disseminated intravascular coagulation (DIC) indicative of abnormal clotting present in shock or ensuing shock. | Decreased PT with low INR promotes clotting; elevation promotes bleeding; elevated fibrinogen and D-dimer reflect abnormal clotting is present. In the presence of effusions, anticoagulants are contraindicated because of the high risk of cardiac tamponade, which can result from bleeding into the pericardium. |
| Radiology | ||
| Chest radiography (CXR) | Assess size of heart, mediastinum, thoracic cage (for fractures), thoracic aorta (for aneurysm), and lungs (pneumonia, pneumothorax); assists with differential diagnosis of chest pain. | Cardiac enlargement may reflect widening mediastinum and tamponade. Cardiac enlargement not always present with tamponade. |
| Computed tomography (CT) Cardiac CT scan |
Check for effusions, both pericardium and epicardium. Assess ventricular size, morphology, function, status of cardiac valves, and circulation. | Will differentiate restrictive pericarditis from constrictive cardiomyopathy by means of the appearance of thickened pericardium on the cross-sectional views of the thorax, which occurs with pericarditis |
| Ultrasound Echocardiography (echo) Transesophageal echo |
Assess for mechanical abnormalities related to effective pumping of blood from both sides of the heart. Assess for fluid in both the pericardial sac and pleural space. | Will show absence of echoes in the areas of effusion. This test, which is essential for quantifying and evaluating the trend of effusions, will appear normal if the pericarditis is present without effusions. TEE may be helpful in identifying some areas of effusion and provide an enhanced view of the posterior wall of the heart. |
| Magnetic resonance imaging (MRI) | Check for effusions, both pericardium and epicardium. | |
Collaborative management
Care priorities
• Oxygen: Usually 2 to 4 L/min by nasal cannula, or mode and rate as directed by arterial blood gas (ABG) values or pulse oximetry. Used to promote both myocardial and generalized increases in oxygenation. As oxygen (O2) delivery to the heart is enhanced, pain can be relieved. If the patient deteriorates, other methods of O2 delivery may be implemented (e.g., nonrebreather mask with reservoir and mechanical ventilation for those who deteriorate markedly).
• Aspirin: 160 to 320 mg ideally chewed. Should be given immediately if the patient has not been given aspirin prior to the hospital.
• Nonsteroidal anti-inflammatory drugs (NSAIDs): Preferred for reducing inflammation, particularly if the patient has had an MI or cardiac surgery, since these drugs do not delay healing as do corticosteroids. NSAIDs have fewer side effects than do steroids. Examples include aspirin, indomethacin, and ibuprofen. NSAIDs can increase fluid retention and may cause renal insufficiency and worsen HF, as well as pose risk for gastrointestinal bleeding.
• Colchicine: Reduces inflammation in the body; may be prescribed as a first-line treatment for pericarditis or as a treatment for recurrent symptoms. Colchicine can reduce the length of pericarditis symptoms and decreases the risk that the condition will recur. However, the drug is not safe for people with certain pre-existing health problems, such as liver or kidney disease. Carefully check the patient’s health history before prescribing colchicine.
• Prednisone: Given at 20 to 80 mg daily for 5 to 7 days if there is no response to NSAIDs. Corticosteroids are contraindicated if pericarditis occurs secondary to an AMI because they can cause thinning of the scar formation and increase risk of rupture. Must be tapered gradually to avoid adrenal insufficiency.
• Medications to manage the cause: Antibiotics, immunoglobulin, antifungals, chemotherapy
2. Prevent cardiac damage and manage pericardial effusions to prevent cardiac tamponade.
• Subxiphoid pericardiocentesis: Performed if effusions persist and cardiac status decompensates. A needle (used in a tamponade emergency) or catheter is used to remove the fluid compressing the heart. Echocardiography is used to guide the catheter tip and assess the amount of effusion remaining. The pericardial catheter may be removed after the fluid has been withdrawn or may be left in place for several days to allow for gradual removal of fluid. Usually 100 ml or more is withdrawn every 4 to 6 hours. The catheter is flushed with saline every 4 to 6 hours after withdrawal of the effusion to prevent clotting. Strict aseptic technique is essential for preventing infection.
• Pericardiectomy: A surgical procedure to prevent cardiac compression or relieve the restriction. It may be necessary in chronic pericarditis for patients with recurrent effusions or adhesions. This procedure is often required in severe and recurrent pericarditis associated with uremia.
CARE PLANS FOR ACUTE PERICARDITIS
related to guarding as a result of chest pain
Respiratory Status: Ventilation
1. Assess rate and depth of respirations along with the character and intensity of the chest pain. Provide prescribed pain medication as needed.
2. Teach patient to avoid aggravating factors such as a supine position. Encourage patient to alter his or her position to minimize the chest pain. The following positions may be helpful: side-lying, high Fowler’s, or sitting and leaning forward.
3. Assess lung sounds every 4 hours. If breath sounds are decreased, encourage patient to perform incentive spirometry exercises every 2 to 4 hours along with coughing and deep-breathing exercises.
4. To facilitate coughing and deep breathing, teach the patient to support the chest by splinting with pillows or by holding the arms around the chest.
Energy Conservation; Endurance; Activity Tolerance
1. Assess the patient for evidence of muscle weakness; assist with activities as needed.
2. Modify the activity plan for the patient with post-MI pericarditis who is receiving steroids. A lower activity level may help prevent thinning of the ventricular wall and reduce the risk of an aneurysm or rupture of the ventricle.
3. Teach patient to resume activities as tolerated, resting between activities.
4. For other interventions, see this nursing diagnosis in Prolonged Immobility, p. 149.
![]() Cardiac Care: Rehabilitative; Teaching: Prescribed Activity/Exercise
Cardiac Care: Rehabilitative; Teaching: Prescribed Activity/Exercise