CHAPTER 2 Cardiac Anatomy
The heart has four chambers, two superior atria and two inferior ventricles. A fibrous interatrial septum divides the atria and a muscular interventricular septum divides the ventricles (Figs. 2-1 and 2-2). The heart is enveloped in a serous membrane, the pericardium, which has a visceral and parietal layer that contains a minimal amount of fluid.

(From Netter Illustration Collection at www.netterimages.com © Elsevier Inc. All Rights Reserved. The author has added new labels to this image that are not part of the original image.)
Multidetector computed tomography (CT) and magnetic resonance imaging (MRI) have revolutionized cardiac imaging. The multiplanar capability of cross-sectional imaging enables the accurate diagnosis of cardiac pathology and function, and a thorough knowledge of cardiac anatomy is essential to interpret these examinations correctly. This chapter reviews cardiac anatomy, with an emphasis on the perspective of CT and MRI. Coronary artery anatomy will be covered in Chapter 3.
NORMAL ANATOMY
General Anatomic Descriptions
Pericardium
The pericardium is a two-layered membrane that envelops all four cardiac chambers and the origins of the great vessels. It consists of parietal and visceral layers separated by a small amount of serous fluid—normally, about 15 to 50 mL—that is mainly an ultrafiltrate of plasma. The pericardium limits the spread of infection and inflammation from adjacent mediastinal structures, prevents excessive dilation of the heart, and reduces friction between the heart and surrounding structures.1
CT and MRI are excellent modalities for imaging the pericardium. The normal pericardium is less than 2 mm thick and requires the presence of epicardial fat or pericardial fluid to distinguish it from the myocardium.2 The pericardium is visible over the right atrium and right ventricle but the lateral and posterior walls of the left ventricle are often bare (Fig. 2-3). Left atrial enlargement is attributed to its partial coverage by pericardium. Several pericardial recesses may be visible on CT and MRI scans. Small amounts of fluid may be present in these structures in healthy individuals. The superior pericardial recess, a curvilinear structure wrapped around the right wall of the ascending aorta, may be mistaken for an aortic dissection, mediastinal mass, lymph node, or thymus. The transverse pericardial sinus, which is dorsal to the ascending aorta, sometimes may be mistaken for aortic dissection or lymphadenopathy (Fig. 2-4). The oblique pericardial sinus, which is situated behind the left atrium, may be misinterpreted as esophageal lesions or bronchogenic cysts. Knowledge of the locations of these recesses can help the imaging specialist differentiate them from abnormal lesions.3
Atria
Right Atrium
The atria are divided into a venous component, vestibule of the atrioventricular valve, septal component, and appendage. The right atrium is embryologically derived from the sinus venosus and primitive auricle, with the former evolving into the venous component and the latter into the appendage. These corresponding parts are separated internally by a muscular band called the crista terminalis (Fig. 2-5), which could be misinterpreted as a right atrial mass.4 The posterior part of the atrium is derived from the sinus venosus and has a smooth interior, whereas the appendage, derived from the primitive auricle, has trabeculations and pectinate muscles that branch from the crista terminalis at right angles (Fig. 2-6). The venous component of the right atrium receives the superior and inferior vena cavae on its posterior surface and the coronary sinus at its junction with the septal component, just above the posterior interventricular groove (Fig. 2-7). The right atrial appendage has a triangular appearance and is best appreciated ventral to the junction of the superior vena cava (SVC) and right atrium. The smooth-walled vestibule incorporates the attachments for the tricuspid valve. The right atrium forms the right lateral border of the heart on a chest radiograph and enlargement of the appendage may be seen in the retrosternal space.
Left Atrium
The left atrium forms the upper posterior heart border on chest radiographs and lies just beneath the carina anterior to the esophagus. This latter characteristic makes it an ideal window in transesophageal echocardiography. The left atrium is also derived from the sinus venosus and the primitive auricle, with similar components. The posteriorly located smooth-walled venous component receives the four pulmonary veins (Fig. 2-8). The vestibule is also smooth-walled and supports the leaflets of the mitral valve.
The left atrial appendage is long and tubular in shape, with pectinate muscles that measure more than 1 mm (see Fig. 2-6).5 These can be mistaken for clots and are less prominent than those in the right atrium. The appendage contributes to the left cardiac contour on chest radiographs and is ventral to the left atrioventricular (AV) groove and left circumflex artery. Knowledge of the pulmonary venous anatomy and description of any accessory veins is important because of their propensity to form ectopic arrhythmogenic foci, and the multiplanar capability of CT and MRI is particularly useful for mapping the left atrial anatomy.6 Enlargement causes posterior displacement of the esophagus on the lateral radiograph and widening of the carinal angle on the frontal radiograph.
Atrial Septum
The atria are separated by the interatrial septum. Atrial septation is a complex process involving many components.7 The true septum is composed of the fossa ovalis, which is a remnant of the septum primum. The septum secundum, the superior rim of the fossa ovalis, is an enfolding of the atrial wall between the SVC and pulmonary vein. The atrial septum is visualized on MRI as a hypointense line, but the foramen ovale can sometimes be almost imperceptible and thus mistaken for an atrial septal defect (see Fig. 2-1). The fusion of the foramen ovale with the septum occurs in the first 2 years of life; incomplete fusion results in a patent foramen ovale in 25% of the population. Multidetector CT is a powerful tool for the demonstration of the patent foramen ovale.8 A patent foramen ovale can be associated with atrial septal aneurysms and Chiari networks, which are implicated in cryptogenic strokes.9
Ventricles
Right Ventricle
The right ventricle is the most anterior chamber in a normal heart, contributing to the inferior border. It has a characteristic external pyramidal shape and is supplied by the right coronary artery (Fig. 2-9). The left anterior descending artery in the anterior interventricular groove demarcates it from the left ventricle. The right ventricle (RV) has an inlet with the valvular apparatus, an apical trabecular component, and a subpulmonic outflow tract. The unique distinguishing features of the right ventricle are septal chordae tendinae for the septal leaflet of the tricuspid valve, a feature that is absent in the morphologic left ventricle. Coarse apical trabeculations and the moderator band, a continuation of the right bundle branch that extends from the interventricular septum to the free anterior ventricular wall, are other unique features (see Fig. 2-7).10 The pulmonary and tricuspid valves lack fibrous continuity, unlike on the left, and are separated by an infolding of the roof called the supraventricular crest. The more numerous papillary muscles are less prominent and are found on septal and free walls. The infundibulum is incorporated into the RV and forms the outflow tract. These characteristic features are integral to the recognition the morphologic right ventricle in congenital heart disease.10 The right ventricle is a thin-walled chamber normally measuring approximately 3 mm in thickness that pumps deoxygenated blood into the low-pressure pulmonary arterial bed (25 mm Hg). The physiologic apical thinning of the RV free wall must not be confused with arrhythmogenic right ventricular dysplasia (ARVD).
The right ventricle enlarges anteriorly, reducing the retrosternal clear space laterally and causing leftward displacement of the apex lifting off the hemidiaphragm. RV enlargement on cross-sectional imaging measured at the plane of the tricuspid valve is a sign of right heart strain in acute pulmonary embolism when the RV/LV ratio is more than 1.11
Left Ventricle
The morphologic left ventricle pumps oxygenated blood to the systemic circulation through the aorta and its branches. It is the most imaged chamber of the heart as a result of the consequences of its failure. It is a thick-walled conical chamber (see Fig. 2-9) that pumps blood when the pressure exceeds 120 mm Hg (the systemic peripheral resistance).
The left ventricle is characterized by the same chambers as the right—the inlet, apical, trabecular, and outlet. The inlet extends from the atrioventricular junction to the attachments of the papillary muscle and contains the mitral valve. The mitral valve, which has fibrous continuity with the aortic valve, has two cusps, anterior and posterior, with no septal attachments. The corresponding large papillary muscles are well seen on cross-sectional imaging (Fig. 2-10). The apex has fine trabeculations and the septum is smooth, thus distinguishing it from the morphologic right ventricle. The ventricular outlet is short and is deficient in conal muscles, in contrast to the right.
The left ventricular wall is not uniform in thickness. This is most accentuated in the longitudinal direction, toward the apex, where gradual wall thinning is noticed. The lateral wall segment thickness measures 7 to 8 mm in women and 9 mm in men. The apex can measure 3 mm. The circumferential wall thickness nonuniformity is less pronounced.12 Left ventricular enlargement tends to be in a leftward and inferior direction.
Interventricular Septum
The ventricles are separated by a thick-walled muscular layer, the septum. In the subaortic region, where the septum thins out, it is referred to as the membranous portion. Muscle fibers from the free ventricular walls contribute to the septum. The septum is normally slightly convex toward the right ventricle because of the higher pressure on the left , which is maintained throughout the cardiac cycle. This feature is best appreciated on short-axis and horizontal long-axis images (Figs. 2-11 and 2-12). Physiologic flattening of the septum in early diastole is called ventricular coupling. Pathologic flattening, or leftward bowing, is seen in constrictive pericarditis, cor pulmonale, and atrial septal defects.13
Valves
The AV valves form the conduit from the atria to the ventricle. The tricuspid on the right and mitral on the left are so named because the right sided AV valve has three cusps and the left-sided bicuspid valve resembles a bishop’s miter (Fig. 2-13). The mitral valve (MV) is composed of an anterior and posterior leaflet. The MV and aortic valve share fibrous continuity (Fig. 2-14). The MV annulus embedded in the myocardium is part of the cardiac skeleton.14 Calcification of the MV annulus is a common abnormality that makes identification of the difficult to visualize annulus possible with cardiac CT angiography. The papillary muscles (see earlier), with their chordae tendinae, are also a component of the MV apparatus (Fig. 2-15). The mitral valve has no septal attachments, thus distinguishing it from the tricuspid valve.
The tricuspid valve separates the right atrium (RA) from the RV and is composed of the same structures as the MV—leaflets, annulus, commissures (sites where two leaflets come together to attach to the aortic wall), papillary muscles, and chordae tendinae. It is normally connected to the morphologic RV (Fig. 2-16). As its name implies, the tricuspid valve is a trileaflet valve (anterior, posterior, and septal leaflets) and is separated from the pulmonary valve by the crista supraventricularis, a muscular ridge, unlike the MV, which is contiguous with the aortic valve. The tricuspid valve is more apically located.
The aortic valve separates the LV outflow tract from the ascending aorta. It is composed of an annulus, cusps, and commissures. No papillary muscles or chordae tendinae are associated with the aortic valve. The three cusps (right, left, and posterior or noncoronary) of the aortic valve form pocket-like outpouchings that are designed to direct blood into the sinuses of Valsalva during diastole and flatten against the aorta during systole (Fig. 2-17).14
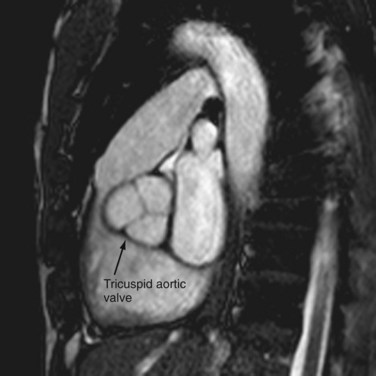
 FIGURE 2-17 This bSSFP image reveals a tricuspid aortic valve from a right ventricular long-axis stack.
FIGURE 2-17 This bSSFP image reveals a tricuspid aortic valve from a right ventricular long-axis stack.
The pulmonic valve separates the RV outflow tract from the main pulmonary artery but lacks fibrous continuity with the tricuspid valve (Fig. 2-18). It is otherwise essentially identical to the aortic valve, with right, left, and posterior leaflets.
Cardiac Veins
The anatomy of the cardiac venous system is variable. The coronary sinus is the most constant structure running in the left AV groove and emptying into the right atrium (see Fig. 2-7).14 The coronary sinus continues as the great cardiac vein, which courses in the left atrioventricular groove with the left circumflex artery and then becomes the anterior interventricular vein in the anterior ventricular groove adjacent to the left anterior descending artery. The first branch is the middle cardiac vein or posterior interventricular vein, running in the eponymously named groove from base to apex along with the posterior descending artery.13 The other two branches that are often variants are the posterior vein of the left ventricle and the left marginal vein, with the former being absent in up to 55% of individuals. Knowledge of their variant anatomy is important because the LV pacer lead of the implantable cardioverter defibrillator for the treatment of heart failure is inserted into one of these two veins.16
Malouf JF, Edwards WD, Tajik AJ, Seward JB. Functional anatomy of the heart. In Fuster V, Alexander RW, O’Rourke RA, et al, editors: Hurst’s the Heart, 11th ed, New York: McGraw-Hill, 2005.
O’Brien JP, Srichai MB, Hecht EM, et al. Anatomy of the heart at multidetector CT: what the radiologist needs to know. Radiographics. 2007;27:1569-1582.
1 Spodick DH. The normal and diseased pericardium: current concepts of pericardial physiology, diagnosis and treatment. J Am Coll Cardiol. 1983;1:240-251.
2 Sechtem U, Tscholakoff D, Higgins CB. MRI of the abnormal pericardium. AJR Am J Roentgenol. 1986;147:245-252.
3 M Levy-Ravetch YA, Rubenstein WA, Whalen JP, Kazam E. CT of the pericardial recesses. AJR Am J Roentgenol. 1985;144:707-714.
4 Menegus M, Greenberg M, Spindola-Franco H, Fayemi A. Magnetic resonance imaging of suspected atrial tumors. Am Heart J. 1992;123:1260-1268.
5 Veinot J, Harrity P, Gentile F, et al. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112-3115.
6 Jongbloed M, Dirksen M, Bax J, et al. Atrial fibrillation: multi-detector row CT of pulmonary vein anatomy prior to radiofrequency catheter ablation—initial experience. Radiology. 2005;234:702-709.
7 Anderson R, Brown N, Webb S. Development and structure of the atrial septum. Heart. 2002;88:104-110.
8 Saremi F, Attai S, Narula J. 64 multidetector CT in patent foramen ovale. Heart. 2007;93:505.
9 Mügge A, Daniel W, Angermann C, et al. Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91:2785-2792.
10 Choe Y, Kim Y, Han B, et al. MR imaging in the morphologic diagnosis of congenital heart disease. Radiographics. 1997;17:403-422.
11 Ghaye B, Ghuysen A, Bruyere P, et al. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006;26:23-39.
12 Bogaert J, Rademakers F. Regional nonuniformity of normal adult human left ventricle. Am J Physiol Heart Circ Physiol. 2001;280:H610-H620.
13 Higgins CB. Acquired heart disease. In: Higgins CB, Hricak H, Helms CA, editors. Magnetic Resonance Imaging of the Body. Philadelphia: Lippincott-Raven; 1997:409-460.
14 Malouf JF, Edwards WD, Tajik AJ, Seward JB. Functional anatomy of the heart. In Fuster V, Alexander RW, O’Rourke RA, et al, editors: Hurst’s the Heart, 11th ed, New York: McGraw-Hill, 2005.
15 Van de Veire N, Schuijf J, De Sutter J, et al. Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1832-1838.
16 Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy. II. Issues during and after device implantation and unresolved questions. J Am Coll Cardiol. 2005;46:2168-2182.

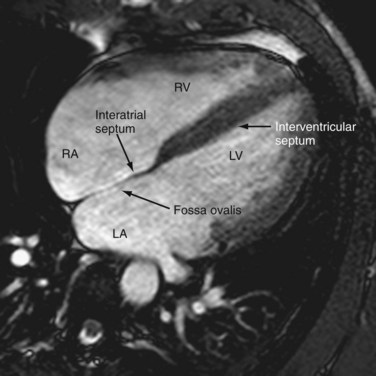
 FIGURE 2-1
FIGURE 2-1 FIGURE 2-2
FIGURE 2-2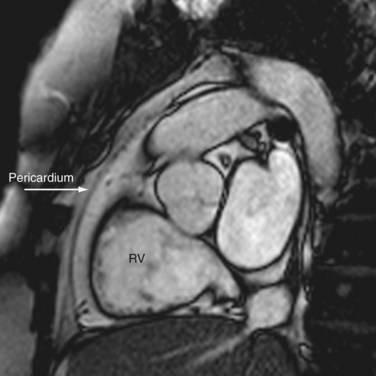
 FIGURE 2-3
FIGURE 2-3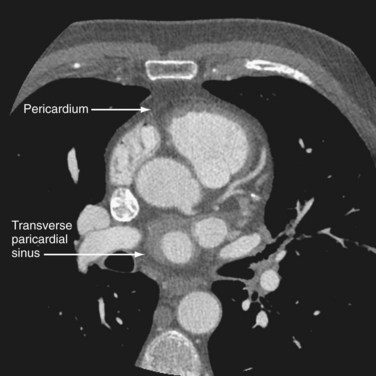
 FIGURE 2-4
FIGURE 2-4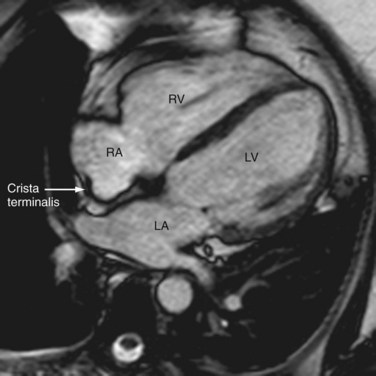
 FIGURE 2-5
FIGURE 2-5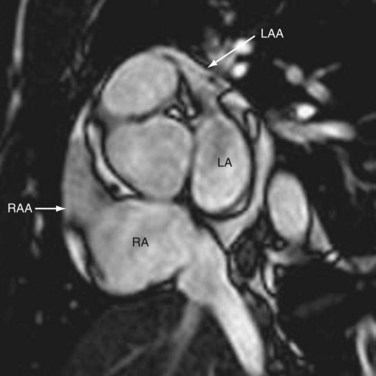
 FIGURE 2-6
FIGURE 2-6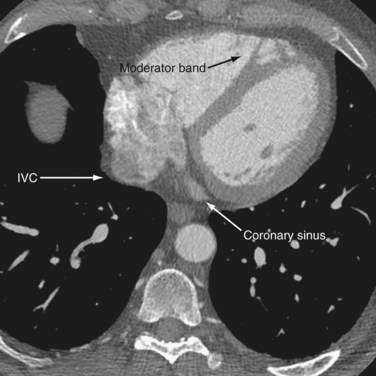
 FIGURE 2-7
FIGURE 2-7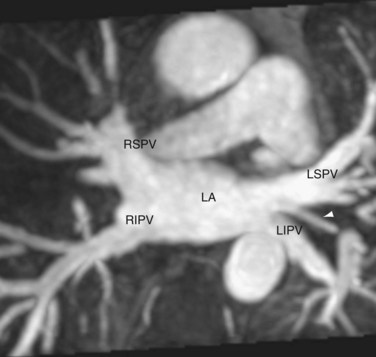
 FIGURE 2-8
FIGURE 2-8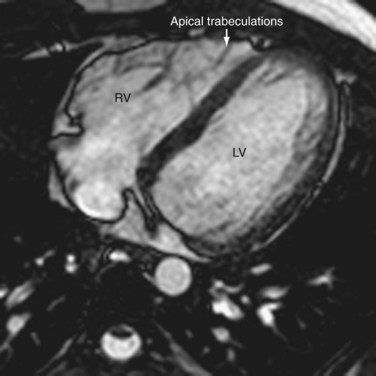
 FIGURE 2-9
FIGURE 2-9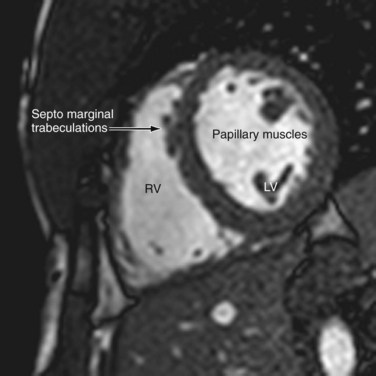
 FIGURE 2-10
FIGURE 2-10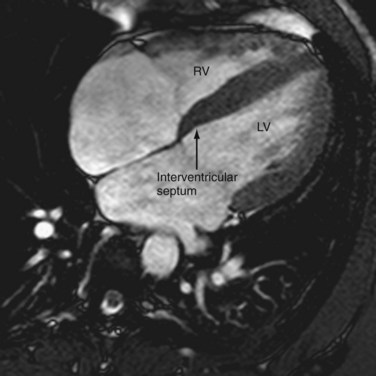
 FIGURE 2-11
FIGURE 2-11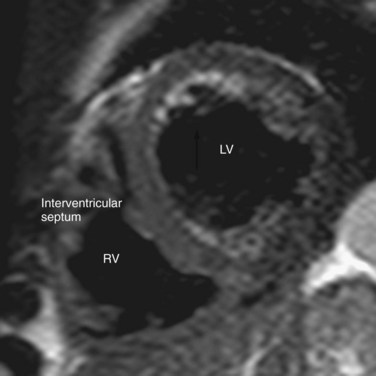
 FIGURE 2-12
FIGURE 2-12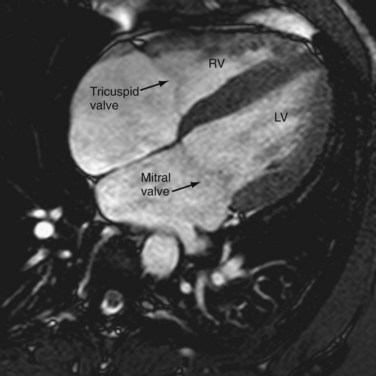
 FIGURE 2-13
FIGURE 2-13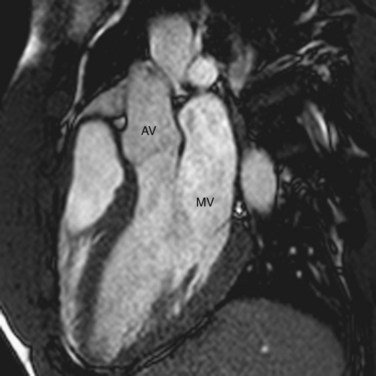
 FIGURE 2-14
FIGURE 2-14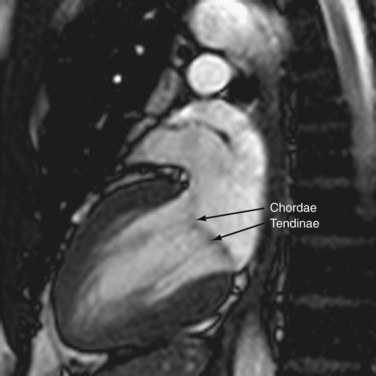
 FIGURE 2-15
FIGURE 2-15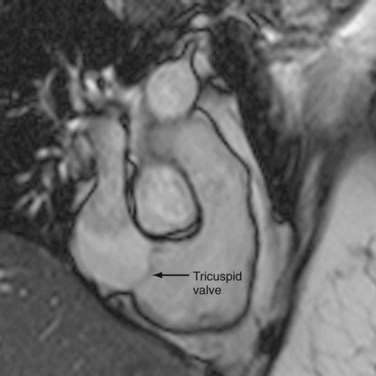
 FIGURE 2-16
FIGURE 2-16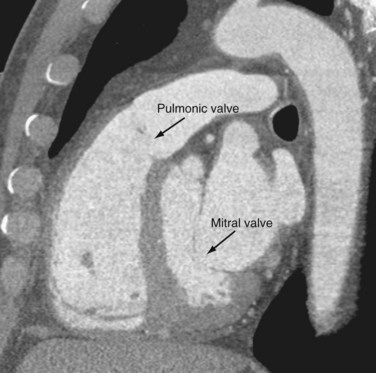
 FIGURE 2-18
FIGURE 2-18

