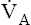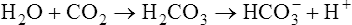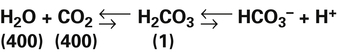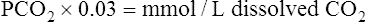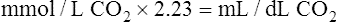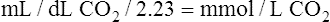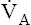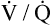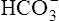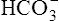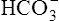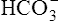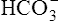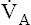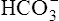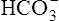Carbon Dioxide Equilibrium and Transport
After reading this chapter, you will be able to:
• Explain how blood levels of carbon dioxide partial pressure (PCO2), dissolved carbon dioxide (CO2), carbonic acid, and alveolar ventilation are interrelated
• Describe the way in which blood levels of CO2 play a role in the body’s acid-base balance
• Use the CO2 hydration reaction to explain how changes in alveolar ventilation affect blood levels of CO2 and hydrogen ions
• Explain why equal CO2 production and elimination rates can coexist with normal ventilation, hypoventilation, or hyperventilation
• Describe how CO2 is transported in different ways in the blood plasma and erythrocytes
• Explain how hemoglobin in the erythrocyte helps generate plasma bicarbonate ions (< ?xml:namespace prefix = "mml" />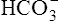 )
)
• Explain how the Bohr and Haldane effects are mutually enhancing with regard to oxygen and CO2 transport
Carbon Dioxide, Carbonic Acid, and Hydrogen Ion Equilibrium
Carbon Dioxide Hydration Reaction
The presence of CO2 in the blood creates a special problem because CO2 reacts with water (i.e., CO2 is hydrated) to form carbonic acid (H2CO3). The blood must possess effective buffering mechanisms to prevent harmful increases in acidity as it transports CO2. As the following reaction shows, when CO2 diffuses from tissues into capillary blood, the CO2 hydration reaction occurs:1
Hydration Reaction: Chemical Equilibrium (Le Chatelier’s Principle)
The short arrows pointing to the right and the long arrows pointing to the left indicate that at equilibrium the reaction is left shifted. The numbers in parentheses indicate relative concentrations of the constituents.2 At equilibrium, concentrations to the left of the arrows are greater than concentrations to the right of the arrows. Despite these concentration differences, the velocities of right-directed and left-directed reactions are identical at equilibrium.
According to Le Chatelier’s principle, if a system at equilibrium is placed under stress, it will react in such a way that counteracts the stress and creates a new equilibrium at a different point.3 For example, in reaction 2, if we add a certain amount of CO2 molecules to the left side of the reaction, we disrupt the equilibrium and drive the reaction to the right until a new equilibrium is established. Similarly, removing CO2 molecules from the left side “pulls” the reaction to the left. In other words, adding or subtracting CO2 molecules drives the reaction either toward the H+ and 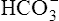 side or toward the CO2 and H2O side of the reaction.
side or toward the CO2 and H2O side of the reaction.
Relationship between Dissolved Carbon Dioxide and Carbonic Acid Concentrations
At 37° C, 0.03 mmol of CO2 dissolves in 1 L of plasma for each mm Hg PCO2; that is, mmol/L (CO2) = 0.03 mmol/L/mm Hg × PCO2. (The derivation of the conversion factor [0.03] to change PCO2 to millimoles per liter of dissolved CO2 is shown in Box 9-1.) Thus, if plasma PCO2 is 40 mm Hg, the concentration of dissolved CO2 in plasma is as follows: 40 mm Hg × 0.03 mmol/L/mm Hg = 1.2 mmol/L, which is the normal amount of dissolved CO2 in arterial blood. Figure 9-1 summarizes the relationship between gaseous CO2, dissolved CO2, and H2CO3. The slow uncatalyzed reaction between CO2 and H2O theoretically requires about 400 molecules of dissolved CO2 to produce 1 molecule of H2CO3.2 In other words, an extremely small amount of H2CO3 in plasma is in equilibrium with a relatively large pool of dissolved CO2. The reaction in Figure 9-1 shows that an increased alveolar PCO2 increases plasma PCO2, which increases the dissolved CO2 and H2CO3 concentration (law of mass action). Conversely, a decrease in alveolar PCO2 forces the hydration reaction to the left, decreasing the plasma H2CO3 concentration. Because it is in equilibrium with CO2 gas, H2CO3 is called a volatile acid. Plasma PCO2 is an important marker of the blood’s volatile acid content and the adequacy of alveolar ventilation.
Although the concentration of dissolved CO2 in plasma is not equal to the concentration of plasma H2CO3, its concentration is in direct proportion to the concentration of H2CO3; this makes it possible to treat CO2 as if it were the acid instead of H2CO3. Because dissolved CO2 and H2CO3 are indistinguishable from each other by chemical analysis,4 dissolved CO2, which can be easily measured, is commonly substituted for the concentration of H2CO3 in clinical acid-base equations (see Chapter 10).
Role of Ventilation in Regulating Arterial Carbon Dioxide Pressure and Volatile Acid
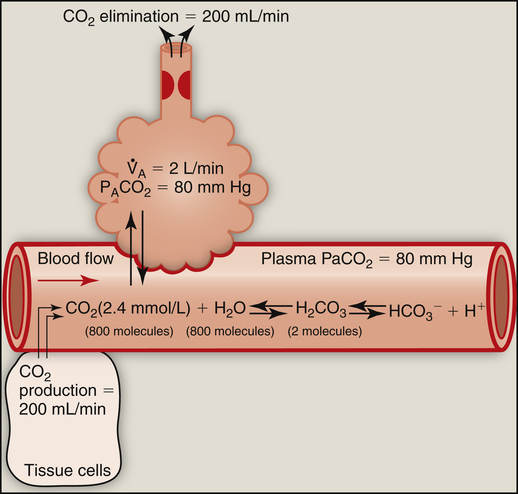
Changes in alveolar PCO2 ultimately change the plasma H2CO3 concentration (see Figure 9-1). (Chapter 4 discusses the reciprocal relationship between 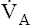 and PaCO2.) Figure 9-2 shows the chemical events that occur during hypoventilation. In Figure 9-2,
and PaCO2.) Figure 9-2 shows the chemical events that occur during hypoventilation. In Figure 9-2, 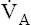 is halved, causing PACO2 to double. Subsequently, PaCO2 and dissolved CO2 double, pushing the hydration reaction to the right. The doubling of CO2 molecules on the left side of the reaction drives the reaction to the right to a new equilibrium point (Le Chatelier’s principle). Figure 9-2 shows that at equilibrium, the amount of H2CO3 molecules doubles, but the ratio of CO2 to H2CO3 molecules stays the same.
is halved, causing PACO2 to double. Subsequently, PaCO2 and dissolved CO2 double, pushing the hydration reaction to the right. The doubling of CO2 molecules on the left side of the reaction drives the reaction to the right to a new equilibrium point (Le Chatelier’s principle). Figure 9-2 shows that at equilibrium, the amount of H2CO3 molecules doubles, but the ratio of CO2 to H2CO3 molecules stays the same.
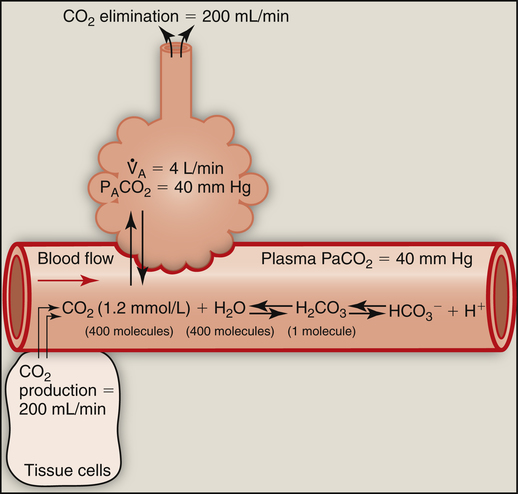
At the outset of hypoventilation, the rate of CO2 production momentarily exceeds the rate of alveolar CO2 elimination, causing alveolar and blood CO2 levels to increase. However, CO2 levels cannot continually increase; instead, a new equilibrium point (i.e., a new steady state) is reached at which CO2 production and elimination rates are again equal, but at higher PACO2 and PaCO2 levels. In Figure 9-1, a normal 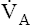 of 4 L per minute maintains a PACO2 of 40 mm Hg and eliminates 200 mL of CO2 per minute (an amount equal to its production rate). If the
of 4 L per minute maintains a PACO2 of 40 mm Hg and eliminates 200 mL of CO2 per minute (an amount equal to its production rate). If the 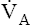 suddenly decreases to 2 L per minute with no change in CO2 production (see Figure 9-2), the PaCO2 and dissolved CO2 in the blood increase temporarily until a new steady state is reached, at which time CO2 production and elimination rates are again equal. However, for this steady state to occur at a
suddenly decreases to 2 L per minute with no change in CO2 production (see Figure 9-2), the PaCO2 and dissolved CO2 in the blood increase temporarily until a new steady state is reached, at which time CO2 production and elimination rates are again equal. However, for this steady state to occur at a 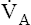 of 2 L per minute, PACO2 and PaCO2 must increase to 80 mm Hg, and dissolved plasma CO2 must increase to 2.4 mmol/L (80 × 0.03) (see Figure 9-2). A proportionate increase in H2CO3 levels is produced, increasing the blood’s acidity. This new steady state requires less ventilatory work to eliminate the same amount of CO2; for this reason, people with limited ventilatory capacity adopt this ventilatory strategy, but the “tradeoff” is increased blood acid levels.
of 2 L per minute, PACO2 and PaCO2 must increase to 80 mm Hg, and dissolved plasma CO2 must increase to 2.4 mmol/L (80 × 0.03) (see Figure 9-2). A proportionate increase in H2CO3 levels is produced, increasing the blood’s acidity. This new steady state requires less ventilatory work to eliminate the same amount of CO2; for this reason, people with limited ventilatory capacity adopt this ventilatory strategy, but the “tradeoff” is increased blood acid levels.
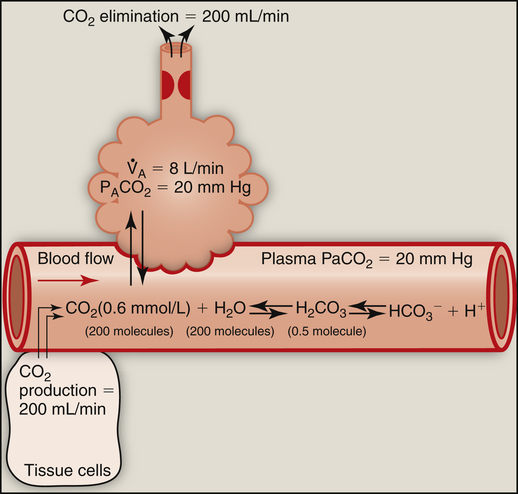
Hyperventilation also disrupts the chemical equilibrium of the hydration reaction (Figure 9-3). Hyperventilation pulls the reaction leftward to a new equilibrium point. At the new equilibrium, the ratio between dissolved CO2 and H2CO3 is preserved, while plasma H2CO3 concentration is decreased. In Figure 9-3, 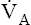 suddenly doubles from 4 L per minute to 8 L per minute, whereas CO2 production stays constant at 200 mL per minute. The CO2 elimination rate temporarily exceeds the CO2 production rate until a new equilibrium is established in which production and elimination rates are equal again. To eliminate only 200 mL of CO2 per minute at a
suddenly doubles from 4 L per minute to 8 L per minute, whereas CO2 production stays constant at 200 mL per minute. The CO2 elimination rate temporarily exceeds the CO2 production rate until a new equilibrium is established in which production and elimination rates are equal again. To eliminate only 200 mL of CO2 per minute at a 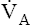 of 8 L per minute, the PACO2 must be at 20 mm Hg, which is associated
of 8 L per minute, the PACO2 must be at 20 mm Hg, which is associated
with a dissolved plasma CO2 of 0.6 mmol/L. A proportionate reduction in plasma H2CO3 concentration is produced, making the plasma less acidic or more alkaline. In this situation, more ventilatory work is required to eliminate the same amount of CO2 that is eliminated under normal conditions.
It is important that normal lungs eliminate CO2 at a rate equal to its production by the body, but it is equally important for 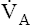 to keep the PACO2 near the 40-mm Hg range; otherwise, the blood becomes acidic or alkaline. For example, during heavy exercise,
to keep the PACO2 near the 40-mm Hg range; otherwise, the blood becomes acidic or alkaline. For example, during heavy exercise, 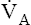 normally increases such that it eliminates CO2 at a rate proportional to its production; this maintains the PACO2 near 40 mm Hg and maintains normal blood acid (H2CO3) levels. An elaborate neurochemical control system (see Chapter 11) is responsible for regulating
normally increases such that it eliminates CO2 at a rate proportional to its production; this maintains the PACO2 near 40 mm Hg and maintains normal blood acid (H2CO3) levels. An elaborate neurochemical control system (see Chapter 11) is responsible for regulating 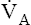 in this manner.
in this manner.
Ventilation also increases in response to abnormally high levels of nonvolatile (noncarbonic) acids in the blood (acidemia). Acids increase the blood’s H+ concentration, which chemically stimulates ventilation, eliminating more CO2 and decreasing PaCO2. These events pull the hydration reaction to the left, which decreases blood H2CO3 levels and offsets the blood’s high levels of nonvolatile acids. These events constitute an important compensatory mechanism. The opposite compensatory response occurs for abnormally low blood levels of nonvolatile acids; the normal acidic stimulus to ventilation is reduced, decreasing the 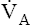 . These events drive the hydration reaction to the right, increasing blood H2CO3 concentration; this counteracts the effects of an abnormally low nonvolatile acid level. (These important compensatory ventilatory responses to nonrespiratory acid-base disturbances are discussed in detail in Chapter 10.)
. These events drive the hydration reaction to the right, increasing blood H2CO3 concentration; this counteracts the effects of an abnormally low nonvolatile acid level. (These important compensatory ventilatory responses to nonrespiratory acid-base disturbances are discussed in detail in Chapter 10.)
How Blood Carries Carbon Dioxide
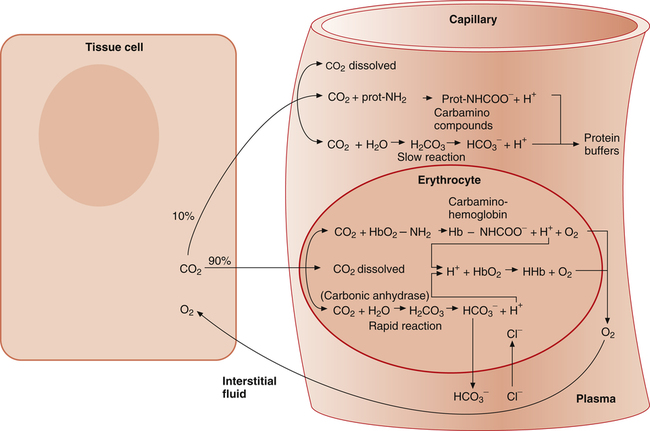
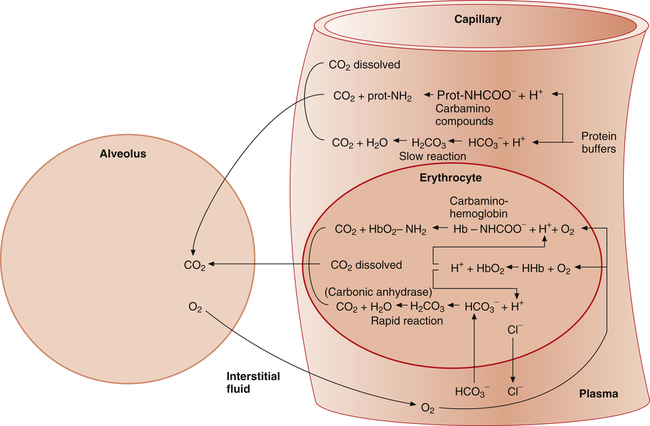
As with oxygen, CO2 is transported in blood plasma and erythrocytes. However, CO2 transport is more complex than oxygen transport. CO2 is carried in the following three major forms in the plasma and erythrocyte: (1) dissolved CO2 in the plasma, (2) plasma 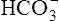 , and (3) protein compounds (carbamino compounds). Figure 9-4 illustrates all modes of transport. CO2 transport is intimately related to the blood’s acid-base status because CO2 reacts with water to form H2CO3.
, and (3) protein compounds (carbamino compounds). Figure 9-4 illustrates all modes of transport. CO2 transport is intimately related to the blood’s acid-base status because CO2 reacts with water to form H2CO3.
Dissolved Carbon Dioxide
As illustrated in Figure 9-4, when CO2 diffuses out of the tissues into the blood, a small portion physically dissolves in the plasma, but most diffuses into the erythrocyte. The small amount of dissolved CO2 in the plasma is in equilibrium with the plasma PCO2, which determines the direction and rate of CO2 diffusion at both tissue and alveolar levels. CO2 dissolves according to its solubility coefficient (PCO2 × 0.03 = mmol/L dissolved CO2). Thus, venous blood, with a PCO2 of about 46 mm Hg, contains 1.38 mmol/L of dissolved CO2 (46 × 0.03 = 1.38). This converts to about 3 mL/dL of dissolved CO2 in venous blood.
As the following equation shows, water hydrates a minuscule amount of plasma CO2 to form H2CO3:
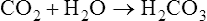
The rate of this reaction is quite slow in the plasma; no enzyme is present, as it is in the erythrocyte, to catalyze and speed up the reaction. The amount of CO2 carried as H2CO3 in the plasma is infinitesimally small because at the pH of normal body fluids, H2CO3 instantly dissociates into 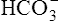 and H+.4 Because dissolved CO2 reacts with H2O to form H2CO3, dissolved CO2 plays a key role in determining the blood pH (see Chapter 10).
and H+.4 Because dissolved CO2 reacts with H2O to form H2CO3, dissolved CO2 plays a key role in determining the blood pH (see Chapter 10).
Bicarbonate
Most CO2 diffusing into the blood from tissue cells enters the erythrocyte rather than remaining in the plasma. Several reactions occur in the erythrocyte, ultimately generating large amounts of 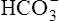 , which diffuse into the plasma (see Figure 9-4).
, which diffuse into the plasma (see Figure 9-4).
The reaction between CO2 and H2O occurs about 13,000 times faster in the erythrocyte than in the plasma because of the intracellular enzyme carbonic anhydrase.4 When CO2 enters the red blood cell, it is instantly converted into H2CO3 and its products, 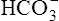 and H+. An extremely small amount of CO2 remains dissolved in the erythrocyte fluid; this explains why most of the CO2 molecules diffusing into the blood enter the erythrocyte rather than linger in the plasma. The rapid conversion of dissolved CO2 to
and H+. An extremely small amount of CO2 remains dissolved in the erythrocyte fluid; this explains why most of the CO2 molecules diffusing into the blood enter the erythrocyte rather than linger in the plasma. The rapid conversion of dissolved CO2 to 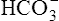 and H+ creates a concentration gradient that continually draws more plasma CO2 into the erythrocyte.
and H+ creates a concentration gradient that continually draws more plasma CO2 into the erythrocyte.
Chloride Shift
When the catalyzed hydration reaction forms H+ and 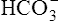 ions in the erythrocyte, hemoglobin immediately buffers the H+ ions, removing them from solution (see Figure 9-4). H+ buffering occurs at the same time hemoglobin releases oxygen; deoxygenated hemoglobin is a more effective buffer than oxygenated hemoglobin. Removal of H+ ions from solution by hemoglobin prevents their accumulation in the erythrocyte, producing the following three consequences: (1) the hydration reaction accelerates even further, (2) the reaction continually moves to the right, and (3)
ions in the erythrocyte, hemoglobin immediately buffers the H+ ions, removing them from solution (see Figure 9-4). H+ buffering occurs at the same time hemoglobin releases oxygen; deoxygenated hemoglobin is a more effective buffer than oxygenated hemoglobin. Removal of H+ ions from solution by hemoglobin prevents their accumulation in the erythrocyte, producing the following three consequences: (1) the hydration reaction accelerates even further, (2) the reaction continually moves to the right, and (3) 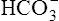 ions build up in the erythrocyte. Eventually, the erythrocyte’s
ions build up in the erythrocyte. Eventually, the erythrocyte’s 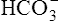 concentration builds up to a level higher than that of the plasma. Consequently,
concentration builds up to a level higher than that of the plasma. Consequently, 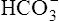 (a negatively charged ion) diffuses down its concentration gradient, out of the erythrocytes, and into plasma (see Figure 9-4). The outward movement of
(a negatively charged ion) diffuses down its concentration gradient, out of the erythrocytes, and into plasma (see Figure 9-4). The outward movement of 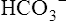 creates an electropositive environment inside the erythrocyte; to maintain electroneutrality, negatively charged plasma chloride (Cl−) ions diffuse into the erythrocyte. This event is known as the chloride shift.
creates an electropositive environment inside the erythrocyte; to maintain electroneutrality, negatively charged plasma chloride (Cl−) ions diffuse into the erythrocyte. This event is known as the chloride shift.
The chloride shift occurs for several reasons. Positively charged ions in the erythrocyte, such as Na+ or K+, do not accompany exit of 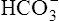 because the erythrocyte membrane is impermeable to positive ions. Before it buffers H+, the negative charges of the hemoglobin molecule are exactly balanced by the positive ions in the erythrocyte. Diffusion of CO2 into the erythrocyte generates H+ ions, which immediately combine with hemoglobin, reducing hemoglobin’s net negative charge. However, this reduction in negative charge is matched by newly generated
because the erythrocyte membrane is impermeable to positive ions. Before it buffers H+, the negative charges of the hemoglobin molecule are exactly balanced by the positive ions in the erythrocyte. Diffusion of CO2 into the erythrocyte generates H+ ions, which immediately combine with hemoglobin, reducing hemoglobin’s net negative charge. However, this reduction in negative charge is matched by newly generated 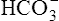 ions, maintaining electrical neutrality inside the erythrocyte. Because the
ions, maintaining electrical neutrality inside the erythrocyte. Because the 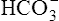 concentration increases above that of the surrounding plasma,
concentration increases above that of the surrounding plasma, 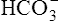 diffuses out of the erythrocyte, leaving the erythrocyte’s interior positive with respect to the plasma. In response to this electrostatic gradient, the most abundant negatively charged ion in the plasma, Cl−, moves into the erythrocytein exchange for
diffuses out of the erythrocyte, leaving the erythrocyte’s interior positive with respect to the plasma. In response to this electrostatic gradient, the most abundant negatively charged ion in the plasma, Cl−, moves into the erythrocytein exchange for 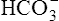 (see Figure 9-4). Hamburger, a Dutch physiologist, first described the chloride shift, which explains why it is also known as the Hamburger phenomenon.
(see Figure 9-4). Hamburger, a Dutch physiologist, first described the chloride shift, which explains why it is also known as the Hamburger phenomenon.
All the reactions involving CO2 are reversed in the lungs (Figure 9-5). CO2 diffuses out of the blood into alveoli because of its partial pressure gradient. According to the law of mass action, as CO2 leaves the blood, the hydration reaction in the erythrocyte shifts to the left. This shift causes 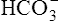 and H+ to rapidly form CO2 and H2O, quickly depleting the erythrocyte
and H+ to rapidly form CO2 and H2O, quickly depleting the erythrocyte 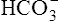 and H+ concentrations. Consequently, plasma
and H+ concentrations. Consequently, plasma 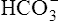 diffuses into the cell and hemoglobin releases H+. The CO2 produced from these reactions diffuses into ventilated alveoli and is exhaled to the atmosphere.
diffuses into the cell and hemoglobin releases H+. The CO2 produced from these reactions diffuses into ventilated alveoli and is exhaled to the atmosphere.
Carbamino Compounds
Plasma Carbamino Compounds
As illustrated in Figure 9-4, a small amount of CO2 reacts with the free amino groups (NH2) of plasma proteins to form carbamino compounds. This rapid reaction requires no catalyst (CO2 + prot-NH2 → prot-NHCOO− + H+). Prot-NH2 represents a protein molecule with a free amino group. When CO2 combines with the amino group, it displaces a H+ ion; a millimole of H+ is released for each millimole of carbamino compound (prot-NHCOO−) formed. These H+ ions and the H+ ions produced by the plasma hydration reaction are buffered by other plasma proteins (see Figure 9-4). (By convention, the carbamino compound amino group–CO2 combination is written as NHCOO− rather than 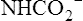 .)
.)
Erythrocyte Carbamino Compounds: Carbaminohemoglobin
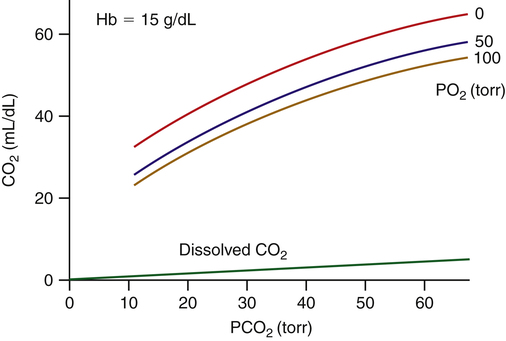
As with plasma proteins, CO2 combines with the hemoglobin protein molecule at free amino sites. This reaction is rapid, requiring no catalyst (CO2 + HbO2-NH2 → Hb-NHCOO− + H+). The resulting compound of hemoglobin and CO2 is called carbaminohemoglobin (Hb-NHCOO−). A millimole of H+ is released for each millimole of Hb-NHCOO− formed. These H+ ions are buffered by the hemoglobin molecule, along with H+ ions formed by the rapid hydration reaction in the erythrocyte (see Figure 9-4). The hemoglobin molecule carries oxygen and CO2 but not at the same binding site. Oxygen combines with the heme groups (see Chapter 8), whereas CO2 combines with the amino groups of α and β globin (protein) chains. However, the affinity of hemoglobin for CO2 is greater when it is not combined with oxygen, a phenomenon known as the Haldane effect. The presence of oxygen on the heme portion hinders carbaminohemoglobin formation. Conversely, carbaminohemoglobin has a decreased affinity for oxygen (Bohr effect). Oxygenated blood carries less CO2 for a given PCO2 than deoxygenated blood. Figure 9-6 shows the CO2 dissociation curve at different PO2 values. For a given PCO2, more CO2 is carried at lower PO2 values (lower saturations).
The CO2 dissociation curve is essentially linear over the physiological range (see Figure 9-6), in contrast to the sigmoid-shaped oxygen-hemoglobin dissociation curve (see Chapter 8). Thus, a change in alveolar ventilation is much more effective in changing arterial CO2 content than O2 content; that is, a doubling of the alveolar ventilation in a healthy person cuts the CO2 content in half but changes arterial O2 content very little because the hemoglobin is already nearly 100% saturated with normal ventilation.
Relative Contribution of Carbon Dioxide Transport Mechanisms
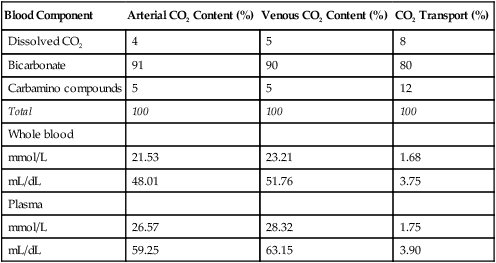
In discussing how much each blood mechanism contributes to CO2 transport, a distinction should be made between the amount of tissue CO2 taken up by venous blood and the total venous CO2 content. The amount of CO2 taken up at the tissues is equal to the mixed venous CO2 content minus the arterial CO2 content; this is the amount of CO2 actually transported to and eliminated by the lungs. Dissolved CO2, 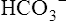 , and carbamino compounds are responsible for 8%, 80%, and 12% of CO2 transport.4 Table 9-1 shows the percentage contribution of each major blood mechanism to CO2 content and CO2 transport.4 (The percentage H2CO3 contributes is so small that it is not included in Table 9-1.) The milliliters per deciliter concentrations of CO2 are calculated by multiplying millimoles per liter by the conversion factor, 2.23. The CO2 concentration in plasma alone (see Table 9-1) is greater than that of whole blood (plasma plus erythrocytes) because the concentration of CO2 in erythrocytes is considerably less than that in the plasma. The erythrocyte volume (about 40% of the whole blood volume) represents a dilute solution of CO2, which when mixed with plasma, produces a lower CO2 concentration in whole blood (millimoles per liter) than exists in plasma alone.
, and carbamino compounds are responsible for 8%, 80%, and 12% of CO2 transport.4 Table 9-1 shows the percentage contribution of each major blood mechanism to CO2 content and CO2 transport.4 (The percentage H2CO3 contributes is so small that it is not included in Table 9-1.) The milliliters per deciliter concentrations of CO2 are calculated by multiplying millimoles per liter by the conversion factor, 2.23. The CO2 concentration in plasma alone (see Table 9-1) is greater than that of whole blood (plasma plus erythrocytes) because the concentration of CO2 in erythrocytes is considerably less than that in the plasma. The erythrocyte volume (about 40% of the whole blood volume) represents a dilute solution of CO2, which when mixed with plasma, produces a lower CO2 concentration in whole blood (millimoles per liter) than exists in plasma alone.
TABLE 9-1
Percentage Contribution of Blood Components to Carbon Dioxide Content and Transport
| Blood Component | Arterial CO2 Content (%) | Venous CO2 Content (%) | CO2 Transport (%) |
| Dissolved CO2 | 4 | 5 | 8 |
| Bicarbonate | 91 | 90 | 80 |
| Carbamino compounds | 5 | 5 | 12 |
| Total | 100 | 100 | 100 |
| Whole blood | |||
| mmol/L | 21.53 | 23.21 | 1.68 |
| mL/dL | 48.01 | 51.76 | 3.75 |
| Plasma | |||
| mmol/L | 26.57 | 28.32 | 1.75 |
| mL/dL | 59.25 | 63.15 | 3.90 |
Complementary Interaction between Oxygen and Carbon Dioxide Transport: Bohr and Haldane Effects
Haldane and Bohr effects are mutually enhancing. While CO2 is taken up at the tissues by various blood components, oxygen is diffusing down its partial pressure gradient from the blood plasma to the tissue cells (see Figure 9-4). Oxygen molecules dissociate from hemoglobin in response to decreasing plasma PO2. The dissociation of oxygen from hemoglobin is augmented by the following two simultaneous processes: (1) CO2 enters the erythrocyte and binds with the globin chains’ amino acid groups, and (2) H+ ions are formed by the rapid hydration of CO2 (see Figure 9-4). Both processes shift the oxyhemoglobin equilibrium curve to the right, decreasing hemoglobin’s affinity for oxygen (Bohr effect). Hemoglobin releases more oxygen than it otherwise would for a given PO2. The reaction illustrating the Bohr effect is shown in Figure 9-4 (H+ + HbO2 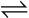 HHb + oxygen).
HHb + oxygen).