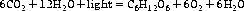Chapter 9 Carbohydrates
Plant Metabolism
Two systems are at work in a plant:
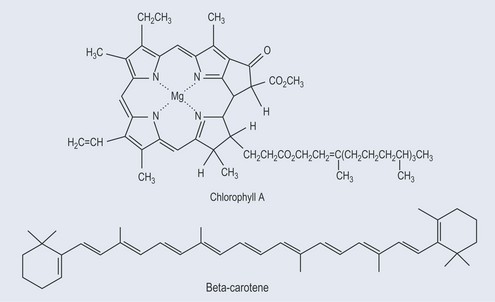
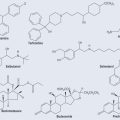
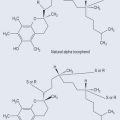
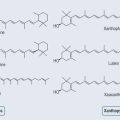
Photosynthesis is the means by which plants use sunlight to produce sugar. Through various chemical reactions in the plant, this ultimately produces energy that the plant can use. Structures called chloroplasts, which are present in the leaves, enable plants to absorb light. The chloroplasts contain photosynthetic pigments called chlorophylls; chlorophyll A is the main photosynthetic pigment in all organisms except bacteria. Combinations of pigments in the plant increase the spectrum of light that plants can absorb for photosynthesis. Carotenoids (Figure 9.1; see Chapter 22 ‘Terpenes’, p. 173) help to transfer this energy. Carbon dioxide and water combine to create chemical energy that can be stored.
Function of Carbohydrates
Monosaccharides
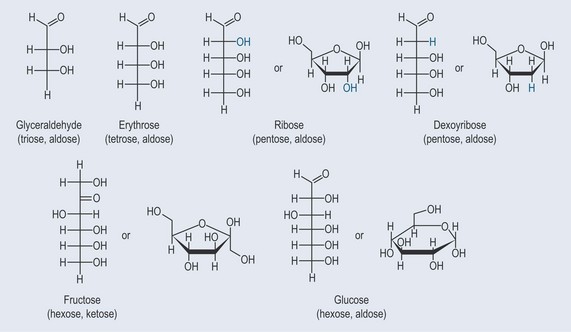
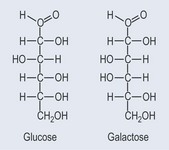
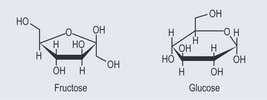
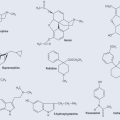
Monosaccharides are the simplest form of sugar. They have more than one hydroxyl group and are based on a backbone of 3, 4, 5; or 6 carbons (triose, tetrose, pentose, or hexose, respectively). Figure 9.2 demonstrates the varieties of sugars of different numbers of carbons:
General Characteristics of Simple Sugars
Nomenclature of Sugars
When reading the literature on sugars, particularly those as part of the structure of glycosides (see Chapter 24 ‘Glycosides’, p. 181) you might come across complex shorthand denoting the type of sugar or sugars to which the chemically active compound is attached. It is only really necessary to understand this if you want to pursue pharmacology to a very high level. Usually, it is enough to know you are looking at an active chemical attached to a glycoside.
Numbering Sugars
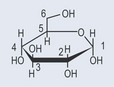
The carbon atoms in a sugar ring are numbered. This enables a chemist to see where functional groups are attached (Figure 9.5).
Abbreviations are used for some of the more common sugars, for example:
Isomers
d and l (+) and (−) might be used to indicate which isomer the sugar is (see Chapter 6 ‘Isomers’, p. 36).
Type of Ring Structure
p and f indicate pyranose and furanose rings.
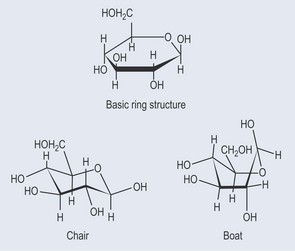
• ‘Boats and Chairs’
Sugars that form a ring structure, such as glucose, have several options as far as shape is concerned. Figure 9.6 demonstrates these variations.
The chair structure is most commonly seen attached to various plant chemicals to form a glycoside. Its shape is more conducive to forming a compact shape than a boat structure, and it is considered the most stable form. The lower bold edge of the ring represents this part of the molecule projecting out of the page towards the reader.
Polysaccharides
The Formation of Carbohydrate Polymers
Monosaccharides can join together by condensation (see Figure 4.7B, p. 26). When sugars are linked together the links can be one of two sorts: either at the fourth carbon (1–4 bond) or the sixth carbon (1–6 bond).
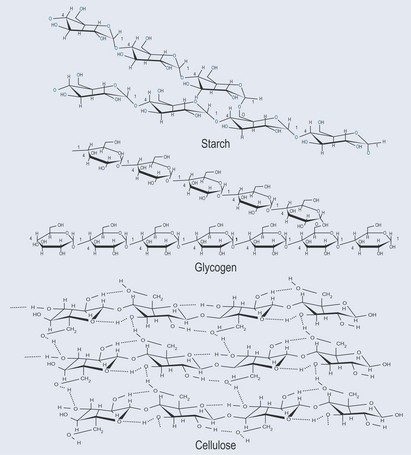
The bonds created between individual sugars can vary between the 1, 4 or 6 carbon atoms on each monosaccharide. This will lead to combinations that result in different chemical and physical properties (as shown in Figure 9.7).
Why is Cellulose Difficult to Break Down?
When beta-glucose (the —OH is on the same side as the C6 carbon) forms the polymer cellulose, the linkage occurs through beta 1–4 glycosidic bonds. This arrangement allows the hydroxyl groups sticking off the sugar backbone to form hydrogen bonds (see Figure 3.13, p. 19, Figure 4.7B, p. 26 and Figure 9.7) with other strings of cellulose. This effectively ‘glues’ the strings together, so that as a whole the structure has a high tensile strength. This is important in plant cells walls because it makes them rigid. It also explains why cellulose provides fibre in the diet; cellulose is difficult to break down.
Polysaccharides with Special Uses
Pectins
Pectins are a complex group of polysaccarides that are commonly found in plants. They are required in jam-making as they are what solidifies jam (Figure 9.8).
Mucilages
Chitin
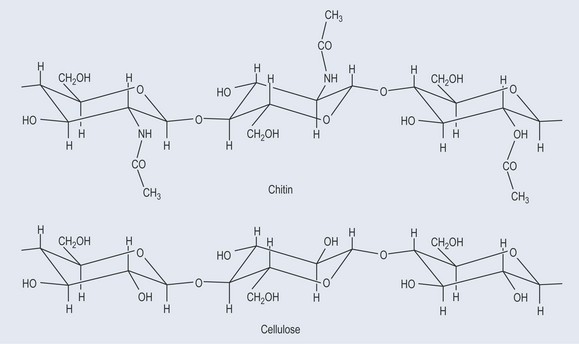
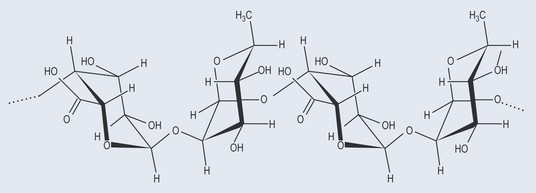
This is very similar to cellulose. It is the main component of fungal cell walls and arthropod exoskeletons (Figure 9.9).
Antibiotics
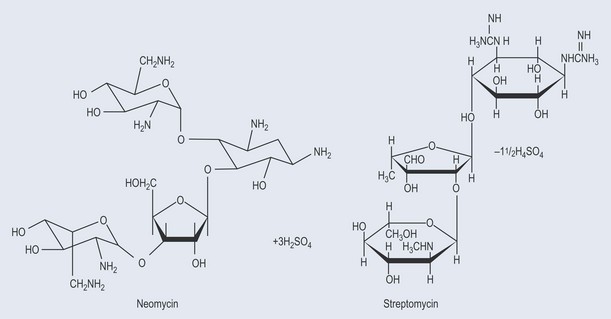
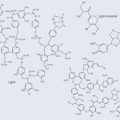
Many important antibiotics are oligosaccharides or contain oligosaccharide groups (Figure 9.10).
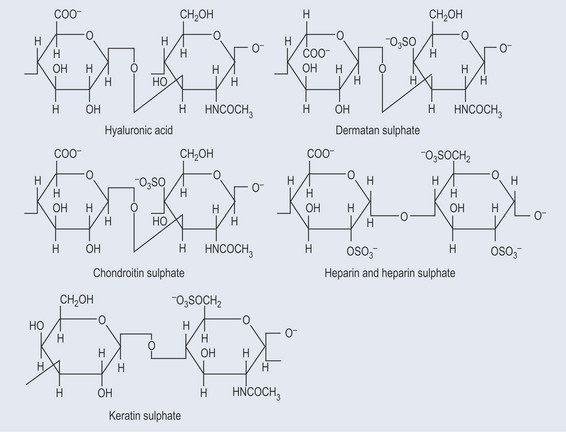
Glycosaminoglycans
Glycosaminoglycans (GAGs) are:
Examples of GAGs (Figure 9.11):