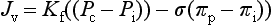CAPILLARY FUNCTION AND THE LYMPHATIC SYSTEM
Structure of capillaries
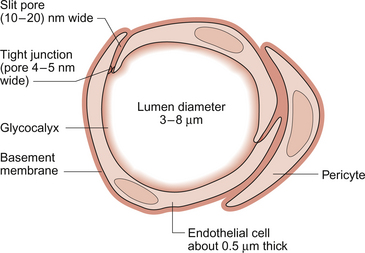
Aspects of the structure of capillaries are discussed, along with the other types of blood vessel, in Chapter 1. A diagram of a microcirculatory unit is shown in Figure 1.9. A capillary is a tube made up of a single layer of endothelial cells surrounded by a basement membrane. Capillaries have a lumen diameter in the range of 3–8 μm. As red cells have a diameter of about 8 μm they must deform to pass through capillaries. During passage along the 0.5–1.0 mm length of a capillary there is a volume of trapped plasma in between successive red cells. This flow pattern is known as bolus flow. Between adjacent endothelial cells there are gaps (pores or clefts) of varying size which contribute substantially to the permeability characteristics of the capillary wall. However, capillary structures are not all identical and three main types have been identified. These are designated continuous, fenestrated and discontinuous capillaries. It must be remembered that there are discrete variations in structure and function even within these three broad types of capillary.
Continuous capillaries (Fig. 11.1) are the least permeable group and they are found in skin, muscle, lungs, adipose tissue and the nervous system. They are the most widely distributed type of capillary. They have tight junctions in between adjacent endothelial cells which only allow the passage of relatively small molecules (Mr<10 000). Larger molecules are thought to cross endothelial cells via the extensive invaginations (caveolae) which cover both surfaces of endothelial cells. Caveolae are linked to the formation of vesicles which can cross the cells transporting larger molecules. An intact basement membrane, which consists mainly of collagen, surrounds the endothelial tube. Pericytes partially surround the capillary and provide support. In some circumstances they are contractile and may be relaxed by nitric oxide generated in the endothelial cells (Chapter 9). Even within the classification of ‘continuous’ capillary endothelia there are considerable variations in structure and function. The least leaky capillaries are found in the brain (see p. 109).
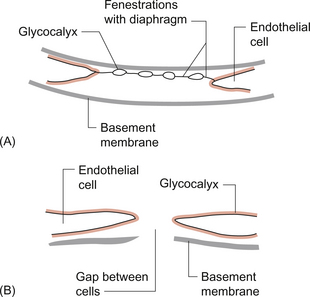
Fenestrated capillaries (Fig. 11.2A) are much more permeable to water than continuous endothelia and they are found in tissues that have a high transcapillary water flux. These include the glomerulus of the kidney, intestinal villi and the choroid plexus of the brain (the site of cerebrospinal fluid formation). There are fenestrae (round holes) in the endothelial cells which have a diameter of 50–60 nm. The fenestrae are not generally completely open holes but are covered by a very thin diaphragm which is derived from the glycocalyx. Viewed from the blood side of the capillary, these diaphragms are said to resemble a cartwheel-like structure with 14 wedge-shaped gaps. As with continuous capillaries, fenestrated capillaries have an intact basement membrane.
Discontinuous capillaries (Fig. 11.2B) are found in tissues such as bone marrow, liver and spleen where there is a need for red blood cells to enter or leave the circulation. There are gaps, which may be over 100 nm in diameter, in between adjacent endothelial cells and an incomplete basement membrane which also has gaps in it. Red cells have a diameter of about 8000 nm (8 μm) but they can deform to pass through quite small holes.
Movement of substances across capillary walls
1. Small lipid-soluble molecules, a group which includes oxygen, carbon dioxide and some drugs such as anaesthetic agents, can diffuse through the lipid bilayer which forms the plasma membrane of the endothelial cells. They can therefore cross almost the entire surface area of a capillary.
2. Small lipid-insoluble ions and molecules cannot easily cross cell membranes and so passage across capillary walls is confined to water-filled channels in between or through cells. There is a layer of negatively charged macromolecules (the glycocalyx) which covers the endothelial cells and lines the water filled channels. This also contributes to the permeability characteristics of the capillary wall. Small charged species (Na+, K+, Cl−, etc.) may cross capillary walls in this way but, in addition, larger hydrophilic compounds such as glucose, amino acids and many drugs also use this route.
3. Large lipid-insoluble molecules will be particularly affected by the structure of capillaries outlined above. In continuous endothelia for instance passage of these molecules will be very limited under normal conditions. In other tissues some plasma protein can leak out of the circulation and the concentration of protein in the interstitial space may be 20–70% of the plasma concentration. Many physiologically important compounds circulate bound to proteins. These include lipids, many hormones, some vitamins and micronutrients such as iron and copper. Delivery of these compounds to cells is dependent on transcapillary wall passage of their unbound form and this also applies to many drugs. A further mechanism by which proteins can cross capillary walls is via endocytotic vesicle formation.
Water movement across capillary walls
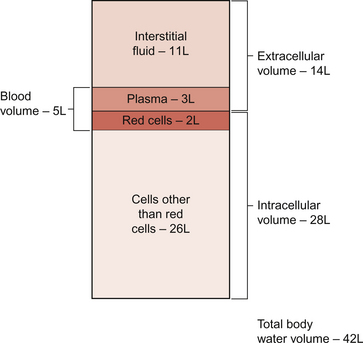
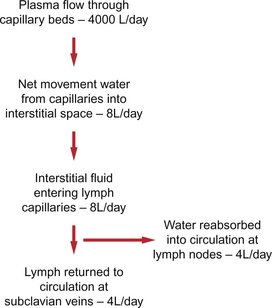
The textbook person (male, 70 kg body weight, aged 20–25 years) contains about 42 L of body water, i.e. 60% of body weight. Distribution of this water is typically about one third (14 L) in the extracellular compartment and two thirds (28 L) in the intracellular compartment (Fig. 11.3). The 14 L of extracellular fluid comprise 3 L of blood plasma and 11 L of interstitial fluid. The 2 L of red blood cells are included in the intracellular compartment. These compartments should not be regarded as ‘water-tight’ and there is a continuous flux of water between them. This process aids the distribution of nutrients around the body. Calculations based on typical values for cardiac output, and the fact that about 60% of blood volume is plasma, show that approximately 4000 L of plasma pass through the microcirculation of an adult in the course of a day (Fig. 11.4). In this period there is a net outflow of approximately 8 L of water passing across capillary walls and into the interstitial fluid. Correspondingly, 8 L of interstitial fluid drain into the lymphatic system and eventually return to the circulation by this route. Of the 8 L of fluid entering the lymph capillaries, about half is reabsorbed during passage through the lymph nodes and the other half will re-enter the circulation at the subclavian veins (see p. 132). Failure to maintain the correct distribution of body water leads to the excessive accumulation of interstitial fluid—oedema.
The mechanisms behind these fluid movements are now discussed and a case history of a patient with oedema is introduced in Case 11:11.
Osmotic pressure of body fluids
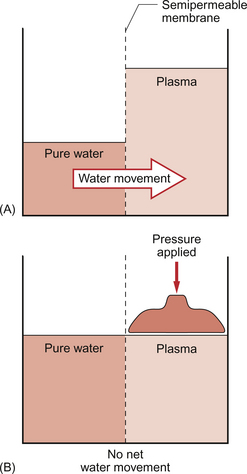
Water moves by osmosis from an area of low osmotic pressure (dilute solution) to an area of high osmotic pressure (strong solution). Osmotic pressure depends on the number of solute particles rather than their size or chemical identity. The total osmotic pressure exerted by the components of blood plasma is about 7.6 atmospheres (5800 mm Hg). In the simple model shown in Figure 11.5 this would be the pressure that would need to be exerted on top of the plasma compartment in order to just stop water moving by osmosis. Most of the osmotic pressure of plasma is generated by the small particles which have the highest concentrations in plasma, especially sodium and chloride. Capillary walls are quite freely permeable to the small particles and so the concentrations of sodium and chloride in plasma and in interstitial fluid are similar and there is no significant osmotic pressure gradient generated by the small particles. The concentrations of the small cations (Na+, K+) and the small anions (Cl−, HCO3−) each summate to approximately 150 mmol/L which means the osmolarity of plasma is approaching 300 mosmol/L. The normal range of plasma osmolarity is actually 275–290 mosmol/L. The fact that this is slightly less than 300 mosmol is partly due to electrostatic attraction between some anions and cations such as Na+ and Cl− so that a proportion are behaving as a single particle rather than two separate ones.
Colloids are large molecules which do not readily diffuse. Diffusion is discussed as a topic in Chapter 1. In plasma the natural colloids are the plasma proteins which, in most normal situations are too large to cross the capillary wall easily. Although their molar concentration in plasma is low, about 1 mmol/L, the concentration difference between plasma and interstitial fluid means there is a colloid osmotic pressure gradient of 21–29 mm Hg which will tend to move fluid from the interstitial compartment to the plasma compartment. The terms colloid osmotic pressure (COP) and oncotic pressure are used synonymously. The colloid osmotic pressure of plasma is attributable particularly to the albumin component. Albumin typically forms just over half of the total plasma protein concentration by weight (35–59 g/L out of a total of 70–78 g/L) but, in addition, albumin (Mr = 69 000) is much smaller than the globulin family of plasma proteins. The number of osmotically active particles per gram of albumin protein is therefore much higher than for the other proteins.
It must be stressed that the proportion of the total osmotic pressure of plasma (5800 mm Hg) contributed by the plasma proteins (21–29 mm Hg) is very small. Sometimes there is a clinical need to increase the colloid osmotic pressure of blood plasma. This can be achieved with administration of either human serum albumin or synthetic colloids such as the polyfructose polysaccharides called dextrans. These substances may be referred to as ‘plasma expanders’ in clinical practice because they osmotically draw fluid from the interstitial space. These strategies are used in the clinical management of circulatory shock (see Chapter 14).
The Starling hypothesis
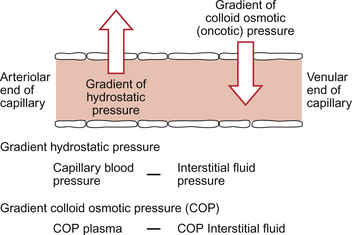
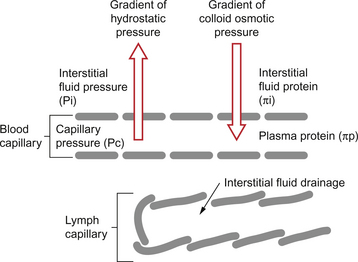
Starling published his ideas on capillary function in 1896 and they still form the basis of our understanding of capillary function today. Starling recognized that capillary walls are leaky to water but would retain the plasma protein. There is a gradient of hydrostatic pressure (capillary blood pressure—interstitial fluid pressure) which moves water out of capillaries and a gradient of colloid osmotic pressure (COP plasma—COP interstitial fluid) which would tend to move water back into capillaries (Fig. 11.6).
Until recently it was thought that water moved out of capillaries at the arterial end and moved back into capillaries at the venous end of a capillary. However the osmotic pressure gradient in the Starling equation (see below) is now thought to be rather smaller than previously estimated. There is a relatively small net movement of water out of capillaries (which enters the lymphatic system) but large scale movement of water is not now thought to occur. The gradient of colloid osmotic pressure should be viewed as a force opposing the capillary blood pressure hydrostatic force and therefore reducing its impact. Nevertheless the balance of Starling forces is an important concept and is central to an understanding of the mechanisms of oedema (see p. 132).
The Starling forces can be expressed in a mathematical format using standard notation as follows:
Typical values for the component ‘Starling forces’
Capillary hydrostatic pressure is not constant along the length of the capillary. The existence of blood flow from the arterial to the venous end means that there must be a drop in pressure along a capillary. Absolute values for capillary blood pressure will depend partly on the state of constriction of the vessels at either end of the capillary. Thus, arteriolar constriction will impose a high resistance to flow and therefore there will be a big pressure drop across this segment and the pressure at the start of a capillary will be relatively low. Correspondingly arteriolar vasodilatation, as occurs in inflammatory reactions, leads to an increase in capillary pressure. Constriction of the venule would also tend to increase capillary pressure. Typical numerical values in a well perfused capillary might be:
The lymphatic system
Although the two sets of ‘Starling forces’ almost balance, there is a net outflow of water from the circulation each day of about 8 L (Fig. 11.4). This volume is returned to the circulatory system via the lymphatic system. About half of the water component, but not the protein, is reabsorbed in lymph nodes. The remaining half of the volume and all the protein re-enters the circulation via ducts entering the venous drainage of the arms (see p. 131).
The functions of the lymphatic system can therefore be summarized as follows:
• tissue drainage system which helps to maintain appropriate body water distribution
• return of plasma proteins which have leaked out into the interstitial space back to the circulation
• absorption of digested fat in the form of chylomicrons into the lacteals (lymph capillaries) of the gut
• defence function at lymph nodes mediated by phagocytic cells and by lymphocytes.
Structure and function of lymph capillaries
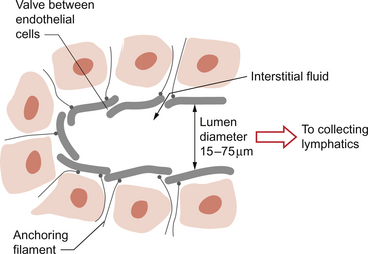
Lymph capillaries, like blood capillaries, consist of a single layer of endothelial cells but they are blind-ending tubes. The cells sit on an incomplete basement membrane. Adjacent endothelial cells overlap and so fluid entering a lymph capillary has to pass through a valve-like structure. This is aided by the presence of anchoring filaments which radiate out into the surrounding tissue and oppose the collapse of the lymph capillary especially when interstitial fluid pressure rises (Fig. 11.7).
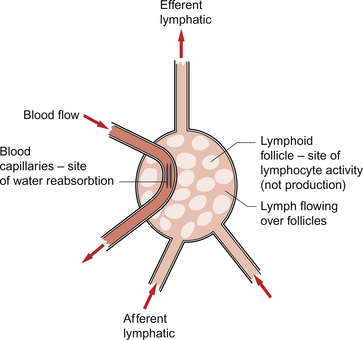
Large lymphatic vessels
At intervals along the lymph trunks there are lymph nodes (Fig. 11.8). These cavernous structures contain phagocytic cells and lymphocytes. The lymph nodes are also an important site for reabsorption of the water component of lymph back into the circulation. About half of the fluid which enters lymph capillaries will return to the circulation by this route. Several incoming (afferent) lymphatics drain into a series of sinuses within each lymph node. Bacteria and other foreign particulate matter are phagocytosed and antigens in the lymph trigger lymphoid tissues to increase the number of lymphocytes released into the lymph. Some of these will become antibody-producing cells.
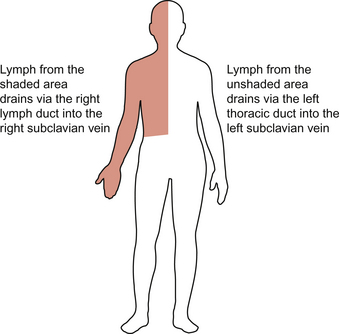
After the filtering process the lymph emerges in an efferent lymphatic. The lymphatic drainage from the lower limbs and the abdominal viscera enters a large lymphatic trunk on the posterior abdominal wall. This continues to collect tributaries including the lymphatic drainage from the left arm and the left side of the head until the largest lymphatic in the body, the left thoracic duct, enters the left subclavian vein. This vessel carries the lymph formed in most of the structures of the body except the ‘top right-hand corner’. The lymphatic drainage for the right arm, right side of the chest and the right side of the head enters the right subclavian vein via a smaller right lymphatic duct (Fig. 11.9). About half (3–4 L) of the lymph formed in the periphery returns to the circulation via the subclavian veins.
• Primary tumours of the lymph node (lymphomas) which include the groups of diseases known as Hodgkin’s lymphomas, non-Hodgkin’s lymphomas and chronic lymphocytic leukaemia.
• Secondary neoplasm in the lymph nodes arising as a result of metastasis from a tumour elsewhere.
• Specific infections of lymph nodes such as tuberculosis, infectious mononucleosis (glandular fever) or local infections.
• Inflammatory reactions which cause lymph node hyperplasia.
Oedema
Typical concentrations of Na+ and K+ in the intracellular and extracellular fluid compartments are shown in Table 11.1.There is therefore a concentration gradient for Na+ to leak into cells and Na+ is also attracted by the net negative charge, mainly held on proteins, on the inside of cells. Correspondingly there is a gradient for K+ to leak out of cells. Intracellular:extracellular ion gradients are maintained by the Na+/K+-ATPase (sodium pump) in the plasma membrane of cells. This expels 3Na+ to the extracellular compartment and returns 2 K+ to the intracellular compartment. Each cycle of the pump requires the hydrolysis of an ATP molecule to provide energy. Some cell membranes contain as many as 1 million sodium pumps which may work at a rate of about 30 times per second and consume an ATP molecule each time they pump 3Na+ out of the cell. As the textbook person has about 1014 cells this is obviously a major energy cost. Keeping the sodium pump running accounts for about 30–40% of our total energy consumption. The consequence of failing to expel the excess Na+ is osmotic swelling of cells, i.e. expansion of the intracellular fluid volume. This will occur in ischaemic tissues with inadequate ATP production which can swell to two to three times normal volume. This usually leads on to tissue death (see Chapter 1). Intracellular oedema can also occur as part of inflammatory reactions in which there is an increase in cellular sodium permeability.
Table 11.1
Typical concentrations of Na+ and K+ in the intracellular and extracellular fluid compartments
| Intracellular fluid | Extracellular fluid | |
| [Na+] | 10–20 mmol/L | 140 mmol/L |
| [K+] | 150 mmol/L | 4 mmol/L |
Extracellular oedema
• interstitial fluid pressure is normally slightly negative, i.e. less than atmospheric pressure
• interstitial fluid is effectively immobilized in a gel form by protein and carbohydrate macromolecules.
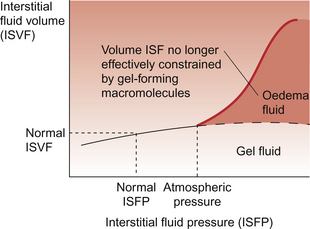
Any change in the Starling forces across capillary walls which tends to expand the interstitial fluid volume (ISFV) will lead to an increase in interstitial fluid pressure (ISFP). Initially, this will oppose further movement of fluid out of the capillary by reducing the gradient of hydrostatic pressure. If the amount of water entering the interstitial compartment is sufficient to force the macromolecules apart the ISFV will be able to expand more easily without a substantial change in ISFP. This is illustrated in Figure 11.10 which shows the relationship between ISFV and ISFP. Normal ISFP is about −2 mm Hg. When ISFP becomes the same as atmospheric pressure the interstitial fluid is no longer adequately constrained by the interstitial macromolecules and so expansion of ISFV occurs. Eventually, further expansion of the ISFV is limited by the restraining influence of the skin. This is why the top of the graph in Figure 11.10 flattens. Once the water is no longer adequately constrained by the macromolecules it is able to move under the influence of gravity and will accumulate in the lower limbs if the subject is mobile or over the sacrum if the subject is in bed.
The fundamental mechanisms of extracellular oedema can be illustrated by reference to Figure 11.11 which summarizes the Starling forces. The common disturbances of these forces which can lead to oedema are:
• an increase in capillary blood pressure (Pc)
• a decrease in plasma colloid osmotic pressure (πp)
• blockage of the lymphatic drainage
• an increase in capillary wall permeability (a change in Kf or σ).
Each of these mechanisms is now briefly discussed in relation to likely clinical scenarios.
Increase in capillary blood pressure
A common cause of oedema by this mechanism is in right sided heart failure. The compensatory response to ventricular failure is an increase in preload in order to raise stroke volume. This means an increase in the filling pressure of the right ventricle (right atrial pressure) mainly achieved by an increase in blood volume (see Chapter 6). The increase in right atrial pressure means that the pressure in the vessels preceding the atrium must be increased, i.e. in the vena cava, venules and capillaries if blood is to continue to flow back towards the heart. The rise in capillary pressure leads to extracellular oedema.
Hypertension per se does not lead to oedema. Blood pressure is usually high because of raised resistance to flow (Chapter 10). The main resistance vessels are the arterioles, which are pre-capillary vessels. Pressure in capillaries may therefore be normal as the high pressure in the arteries is dissipated moving the blood through the constricted arteriolar segment. It is only when hypertension leads to heart failure, due to the increased afterload effect, that people with high arterial blood pressure become oedematous.
Decrease in colloid osmotic pressure
Recognized causes of decreased plasma protein concentration include malnutrition (reduced amino acid availability to synthesize protein), liver disease (damage at the site of plasma protein synthesis), kidney disease (loss of protein in urine as a result of glomerular damage—the nephrotic syndrome) or in patients with extensive burns (a plasma protein-rich serous exudate covers the burned area and the water then evaporates leaving a dry protein coat). The case history in this chapter describes a patient with oedema secondary to renal disease.
Increase in capillary wall permeability
In many cases the increase in capillary permeability occurs locally and generates local oedema (swelling), as for example at the site of a skin lesion. Evidence has more recently emerged that oedema formation can occur rapidly during acute inflammatory responses as a result of primary changes in interstitial fluid pressure (Pi). This follows interactions between dermal cells and the macromolecules in the interstitial space which are mediated by the cell adhesion molecules, the β1-integrins (see Berg et al. 2001).
Aukland, K., Reed, R. K. Interstitial–lymphatic mechanisms in the control of extracellular fluid volume. Physiol. Rev.. 1993; 73:1–78.
Berg, A., Rubin, K., Reed, R. K. Cytochalasin D induces oedema formation and lowering of interstitial fluid pressure in rat dermis. Am. J. Physiol.. 2001; 281:H7–H13.
Browse, N., Burnand, K. G., Mortimer, P. S. Diseases of the Lymphatics. London: Hodder Arnold, 2003.
Levick, J. R. Cardiovascular Physiology, fifth ed. London: Arnold, 2009.
Michel, C. C. Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp. Physiol.. 1977; 82:1–30.
Michel, C. C., Curry, F. E. Microvascular permeability. Physiol. Rev.. 1999; 79:703–761.
Mortimer, P. S., Levick, J. R. Chronic peripheral oedema: the critical role of the lymphatic system. Clin. Med.. 2004; 4:448–453.