Chapter 57 Cancer Rehabilitation
Epidemiology
Cancer is a prevalent condition that becomes increasingly common with advancing age. Just under 1.5 million new cancers were diagnosed within the United States in 2009, and more than 560,000 people died of cancer.119 Cancer causes one in four deaths, and is second only to heart disease as the leading cause of mortality in the United States.119 Roughly 76% of all cancers occur in patients 55 years of age and older.7 Men are more commonly affected by cancer (excluding basal and squamous cell cancers of the skin), with a lifetime risk in the United States of one in two. The lifetime risk in women is one in three.7 Many cancers could be prevented through behavioral modification. One third of all cancer deaths are related to obesity, physical inactivity, and other lifestyle factors.7 Only 5% to 10% of cancers are hereditary and directly related to aberrantly expressed or regulated genes.
Survivorship
Cancer 5-year survival rates are increasing as a result of a variety of factors, including successful early detection efforts, improved multimodality treatments, and expansion of the chemotherapeutics and biopharmaceuticals available to treat patients with metastatic disease. Sixty-six percent of adult cancer patients live 5 years beyond diagnosis.107 These numbers do not accurately reflect current trends because the statistics pertain to patients who were treated 5 years ago, and the standards of care for many cancers have changed. The prevalence of cancer survivors will increase, given the anticipated persistence of factors responsible for current survivorship trends.107 First, the aging of the population will produce an increase in the incidence of age-related cancers such as colon, breast, and prostate cancer. Second, early detection efforts are being aggressively funded and implemented. We can expect that more and more cancers will be identified at early, curable stages. Last, clinical research continues to refine strategies for delivering established and novel anticancer therapies. During the past several years we have witnessed an unprecedented influx of targeted biopharmaceuticals into the cancer treatment arsenal. Collectively these efforts have consistently produced incremental outcome improvements. It is reasonable to assume that this trend will continue to benefit both patients who are cured and those who are living with cancer.
Disease Considerations
Prognosis and Metastatic Spread
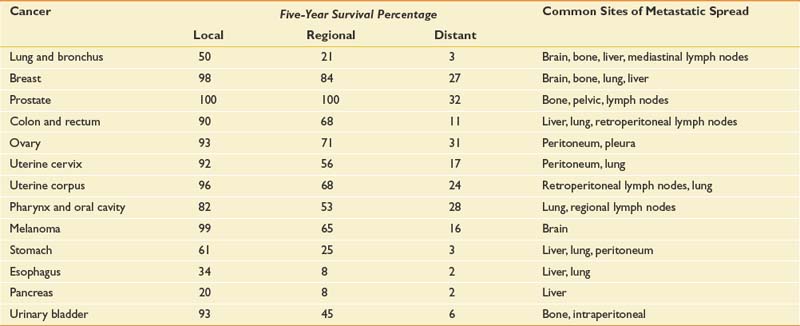
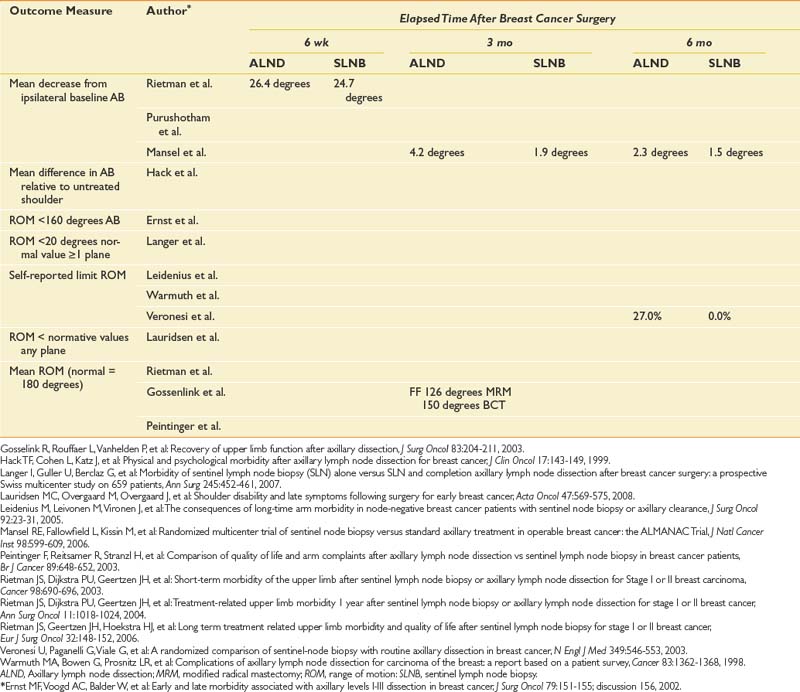
Table 57-1 presents 5-year survival statistics collected between 1996 and 2004 for different cancers.107,119 The implications of regional and distant spread at the time of diagnosis vary considerably by cancer type. For example, prostate cancer patients enjoy an excellent prognosis when their cancer is detected at the local or regional level, with virtually 100% 5-year survival. However, only 21% of lung cancer patients with regional spread are alive at 5 years. It is critical to bear in mind that survival statistics are mean values, with potentially wide confidence intervals that provide crude estimates—but are potentially imprecise when applied to individual patients.
This information informs rehabilitation goal setting, determines the level of emphasis placed on symptom-oriented versus disease-modifying treatments, and allows for the assessment of patients’ unduly optimistic or grim expectations. Table 57-1 also lists common sites of metastases for prevalent malignancies. Understanding patterns of metastatic spread can help clinicians focus the search for metastases. Lung, breast, colon, and melanoma commonly spread to the brain. Regular neurologic screening examinations should therefore be incorporated into posttreatment, surveillance care. Prostate, breast, and lung cancer commonly produce bone metastases. Musculoskeletal pain in these cancer populations can be due to the primary or secondary consequences of bony disease and should trigger an appropriate evaluation.
Phases of Cancer
For rehabilitation purposes, cancer can be divided into several distinct stages. This approach calls clinical attention to certain nodal points along the disease trajectory that should trigger a revaluation of functional deficits, reinvolvement of rehabilitation services, and redefinition of functional goals. Five distinct stages of malignant disease—initial diagnosis and treatment, surveillance, recurrence, temporization, and palliation—were initially outlined in a model proposed by Gerber et al.80 Phases of cancer should determine rehabilitation goals with interphase transitions mandating reassessment of goals. Attention to cancer phases ensures that significant shifts in prognosis and treatment requirements inform rehabilitative efforts.
Constitutional Symptoms
Fatigue
Fatigue is the most common symptom experienced by cancer patients.180 The prevalence of fatigue ranges from 70% to 100%, contingent on the type and stage of cancer. It is also related to whether patients are receiving anticancer treatments.180,219 A majority of patients in active treatment rate their fatigue as ‘‘severe’’ or 7 or more on an 11-point numerical rating scale.102 Because fatigue is an inherently subjective, definitions of fatigue understandably differ. The National Comprehensive Cancer Network defines CRF as: “an unusual, persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning.”181 Experts concur that fatigue reduces the energy, mental capacity, functional status, and psychologic resilience of cancer patients.181 The novel International Classification of Diseases, 10th edition criteria for CRF, listed in Box 57-1, reflect this consensus.
BOX 57-1 International Classification of Diseases, 10th edition, Criteria for Cancer-Related Fatigue
A discrete source of fatigue can be identified in some patients, leading to effective treatment and symptom reversal. More often the responsible mechanisms are multifactorial. Box 57-2 lists possible contributing factors. Anemia has typically received the greatest amount of attention as a source of fatigue, but this focus has shifted in recent years. Previous interest was due, in part, to the high prevalence of fatigue among cytopenic cancer patients receiving chemotherapy (38% to 86%),10,254 and to reports that the onset and severity of fatigue paralleled reductions in serum hemoglobin.34 More recent, comprehensive data demonstrate that the time course of fatigue differs from fluctuations in blood counts and that normalization of hemoglobin levels often fails to reduce fatigue. No specific decrement or increment in hemoglobin levels has been definitely associated with meaningful changes in patients’ quality of life (QOL). Of greater concern are the findings that patients receiving erythropoiesis-stimulating agents have an elevated risk of thromboembolism, that several randomized trials have demonstrated decreased survival times in cancer patients receiving erythropoiesis-stimulating agents, and that two randomized trials have demonstrated poorer “locoregional” control or progression-free survival in cancer patients receiving these agents.18,212
Despite this, erythropoiesis-stimulating agents continue to be used in the treatment of anemia related to cancer treatment. A case has been made for initiating therapeutic doses in appropriate patients receiving anticancer treatment. The American Society of Clinical Oncology/American Society of Hematology guidelines endorse the use of 10 g/dL as the threshold hemoglobin value to recommend initiating an erythropoiesis-stimulating agent.212 Starting doses should be determined by the package insert of the specific agent. Continuing erythropoiesis-stimulating agents beyond 6 to 8 weeks in nonresponding patients does not appear to be effective. Iron stores should be monitored and supplemented as required for patients treated with erythropoiesis-stimulating agents. Patients who have poor responses to epoetin therapy, intensely symptomatic anemia, hemoglobin levels less than 9 g/dL, or economic constraints to erythropoiesis-stimulating agents might require red blood cell transfusion.
Deconditioning secondary to inactivity is common among cancer patients.49 If deconditioning does not initiate fatigue, it frequently aggravates fatigue arising from other sources. Mood-related factors such as anxiety and depression are also prevalent among cancer patients. Thirty percent of patients develop clinical depression after a cancer diagnosis.69 Centrally acting medications should be carefully reviewed in patients complaining of fatigue. A reduction or withdrawal trial of nonessential drugs can identify those producing fatigue.181 Medications that commonly produce fatigue include opioids, benzodiazepines, antiemetics, antihistamines, tricyclic antidepressants, anticonvulsants (e.g., carbamazepine, gabapentin, and oxcarbazepine), thalidomide, and α2-adrenergic agonists (e.g., tizanidine).
In the absence of a discernible etiology, CRF might be associated with elevated cytokine levels. Cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6 have been implicated in CRF.87 The mechanism(s) by which elevations in circulating cytokine levels produce fatigue, however, and whether they are elaborated by host or tumor cells, remains unclear. Cytokine antagonists are not recommended at this time for the treatment of CRF.
When potentially reversible sources of fatigue (see Box 57-2) have been ruled out or definitively addressed, symptom-oriented fatigue management is indicated. The National Comprehensive Cancer Network endorses a multimodal approach that includes medications, exercise, psychologic interventions, and improved sleep hygiene as offering the greatest likelihood of success.181 The use of aerobic exercise to reduce CRF is discussed at length in the “Aerobic Conditioning” section of this chapter.
Methylphenidate has been used most extensively to treat fatigue in cancer patients. Four open-label studies in mixed cancer cohorts have demonstrated reduced fatigue with methylphenidate.23,91,105,224 A fifth open-label pilot study combining exercise and methylphenidate also reported benefit.232 However, results from five randomized, controlled, double-blinded studies conflict. Two studies published by Lower et al.150,151 detected reduced fatigue in patients who had completed cytotoxic chemotherapy. In three additional trials in mixed brain and breast cancer populations, however, methylphenidate did not differ from placebo in reducing CRF.25,27,155 These inconsistencies could be due to different maximal doses, trial duration, and inclusion criteria. Currently it is reasonable to trial methylphenidate at a starting dose of 5 to 10 mg/day. Dose-limiting toxicities associated with methylphenidate include anorexia, insomnia, anxiety, confusion, tremor, and tachycardia. Dose titration continues gradually until a therapeutic response is achieved or adverse side effects preclude further dose escalation. Doses greater than 60 mg/day are rarely required.
Modafinil has been less extensively studied in two open-label trials with disparate study populations. Both breast cancer survivors and patients with brain tumors reported less fatigue while taking modafinil.122,177 Modafinil is generally tolerated with few side effects (e.g., headache, anxiety, nausea). When present, these symptoms are rated as mild and resolve on discontinuation. Modafinil therapy can be initiated at 100 to 200 mg/day and titrated to a maximal dose of 400 mg/day.
Pain
The prevalence of cancer-related pain is 28% among patients with newly diagnosed cancer,269 50% to 70% among patients receiving antineoplastic therapy,198,199 and 64% to 80% among patients with advanced disease.29,63,257 Adequate pain control is an absolute requisite for successful rehabilitation. Cancer patients generally experience multiple concurrent pain syndromes. Thorough evaluation requires assessment of all relevant pain etiologies and pathophysiologic processes. Pain control might require the integrated use of anticancer treatments, agents from multiple analgesic classes, interventional techniques, topical agents, manual approaches, and modalities.
The unique disease context in which cancer pain develops distinguishes it from many other pain-associated diagnoses managed by physiatrists. Considerations in cancer pain management are listed in Box 57-3 and explained below. One of the most salient features of cancer pain management is the reliance on high-dose opioid therapy. The doses required by many cancer patients can extend far beyond the conventional levels used by physiatrists. Fifteen percent of a cohort of stage IV pancreatic cancer patients required more than the daily equivalent of 5 g of parenteral morphine.72 However, extensive international literature and multiple guidelines resoundingly endorse this approach.12,64,168,187
The majority of cancer pain is due to tumor effects. For this reason, disease-modifying, anticancer therapy plays a critical role in pain management. For example, a single radiation fraction of 8 Gy offers a definitive and effective means of controlling pain associated with symptomatic and uncomplicated bone metastases.277 Cancer progression frequently causes pain to worsen, and escalating analgesic requirements should be anticipated.72 Cancer-related depression, anxiety, and existential distress can exacerbate patients’ pain experience.246 For this reason, contributing psychiatric factors should be addressed.
Acute Pain
Acute pain after surgery or radiation therapy can be successfully treated using conventional algorithms for acute postoperative pain.3 Nerves are frequently severed, compressed, or stretched during tumor resection, making it possible for neuropathic pain to be a major factor during the postoperative period. Neural compromise contributes significantly to postmastectomy and postthoracotomy pain syndromes. Adjuvant analgesics (e.g., gabapentin) should be initiated when a neurogenic contribution to the pain is suspected. As with all postoperative pain that impedes function, aggressive opioid-based and antiinflammatory analgesia should be considered. Acute pain control allows movement and limits immobility. This is particularly important in cancer patients who face the debilitating effects of chemotherapy or radiation therapy shortly after surgery.
To allow patients whose cancers eventually recur or progress to benefit from opioid rotation, opioid use should be confined to the “immediate-” and “sustained-” or “continuous-release” formulations of a single drug. The dose threshold for switching opioids because of lack of efficacy in patients with poor prognoses should be high. In this way, patients’ exposure can be restricted to a limited number of opioids, allowing them to benefit from opioid rotation in the late stages of disease.112,166
Acute pain can also complicate the administration of chemotherapy, hormonal therapy, and irradiation. Most of the associated pain syndromes are transient but can produce intense discomfort that warrants aggressive analgesia. Acute pain syndromes associated with cancer therapy include paclitaxel-related arthralgias and myalgias,217 bisphosphonate-related bone pain,104 radiation mucositis,211 steroid pseudorheumatism (after withdrawal of corticosteroids),216 intravesicular Bacillus Calmette Guerin (BCG–induced cystitis, hepatic artery infusion pain,124 bone pain associated with colony-stimulating factor (CSF) and granulocyte macrophage CSF administration,266 and radiopharmaceutical-induced pain.
Chronic Pain
Chronic cancer-related pain can arise from visceral or neural structures but is most commonly associated with bone metastases.145 Bone metastases occur in 60% to 84% of patients with solid tumors. Pain intensity does not correlate with the number, size, or location of bone metastases. Pain intensity also does not correlate with tumor type because 25% of patients with bone metastases report no pain.210 Bone pain is particularly relevant to physiatrists because recruiting muscles that act on or loading affected structures can precipitate severe pain. Too often the excellent pain control achieved while patients remain in bed proves inadequate when they begin to transfer and ambulate. As mentioned above, bone pain responds well to local irradiation.277
Nonsteroidal Antiinflammatory Drugs for Bone Pain
Pharmacologic interventions reduce the intensity of bone pain. Prostaglandins have been implicated in pain associated with lytic bone metastases.167 Blockade of prostaglandin synthesis is likely the principal mechanism by which nonsteroidal antiinflammatory drugs (NSAIDs) alleviate bone pain.221 NSAIDs are considered first-line therapy for bone pain, and a trial is warranted unless contraindicated. Patients’ limited prognoses and the intensity of their suffering might eclipse cyclooxygenase (COX)-2 inhibitors’ worrisome cardiovascular risk profile. Although caution should be exercised, the significant potential benefits of COX-2 inhibitors outweigh their risks in many cancer patients with thrombocytopenia and/or gastropathy. Currently celecoxib is the only COX-2 inhibitor for oral use that remains available on the U.S. market.
COX nonselective inhibitors offer comparable or greater pain relief but a less desirable toxicity profile.223 Choline magnesium trisalicylate causes less inhibition of platelet aggregation than other COX nonselective inhibitors, but did not statistically outperform placebo when trialed in cancer-related bone pain.121 COX nonselective inhibitors with less desirable toxicity profiles have proven more effective. Several placebo-controlled, randomized trials found that ketoprofen reduced cancer pain to a greater extent than either codeine or morphine.249 Dosing NSAIDs for bone pain is no different from using them at antiinflammatory doses for pain of other etiologies.
Adjuvant for Bone Pain
Adjuvant and opioid analgesics can augment NSAID-related control of bone pain. A study found corticosteroids to be beneficial in relieving cancer pain,24 and extensive anecdotal experience supports their use. The toxicity profile of corticosteroids includes edema, bone demineralization, immunosuppression, and myopathies. This mandates that they be used transiently and rapidly tapered, except for patients in whom sustained analgesic benefit justifies the associated toxicity risk.
Use of calcitonin for bone pain is discouraged because of the weak supportive evidence and rapid tachyphylaxis.158,167 Evidence supporting the use of parenteral bisphosphonates in the management of bone pain is more robust.54,167,267 Aminobisphosphonates appear to have greater effectiveness in reducing bone pain than nonaminobisphosphonates (such as clodronate), and are preferred for patients with high pain scores. Effective doses include 30 to 90 mg of intravenous pamidronate every 4 weeks, 4 mg of intravenous zoledronic acid every 3 weeks, and 1600 mg of oral clodronate daily. Opioids enhance analgesia afforded by NSAIDs and can reduce the doses required for adequate pain relief.242
Opioids
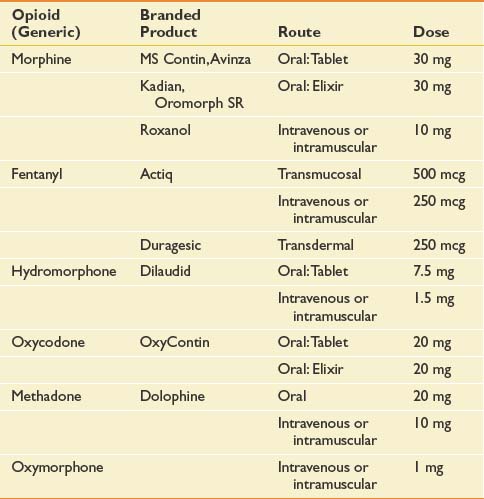
As previously mentioned, opioid-based pharmacotherapy is the current standard of care for the management of moderate to severe cancer pain, irrespective of its etiology.12,64,168,185 Opioid use should be restricted to pure μ-receptor agonists. Many μ-receptor agonists are commercially available in the United States. Those most commonly used in cancer pain management include morphine, hydromorphone, oxycodone, oxymorphone, fentanyl, and methadone. Opioid analgesic requirements change over time depending on whether a patient’s cancer progresses or responds to treatment. Ongoing dose adjustment maximizes pain control while reducing the incidence of side effects. The dominant paradigm for opioid administration has a well-established track record and has been reiterated by many experts in the field with few changes over the past.72,115,145
Opioid Conversion
Significant intraindividual variations in response to different opioids have long been recognized and are now presumed to result from genetically determined differences in pharmacokinetics and pharmacodynamics.75,214 An alternative opioid should be considered when an “adequate” trial of a particular agent has failed to achieve an acceptable decrement in pain intensity or has engendered refractory and untenable side effects. An adequate opioid trial in the cancer patient can entail use of high doses (e.g., >1 g/day intravenous morphine sulfate). Opioid dose conversion requires calculation of the equianalgesic dose of the novel agent (Table 57-2) and reduction by 50% for incomplete cross-tolerance. Incomplete cross-tolerance describes the property of opioids to induce analgesic tolerance with sustained high-dose opioid exposure. Tolerance is usually considerably lower to a novel agent. For this reason, patients often experience greater sedation and needless side effects when exposed to 100% of the equianalgesic dose. Reductions of 50% provide better estimates of the minimal effective opioid dose. If patients are being converted from methadone, reductions of 80% to 90% of the equianalgesic dose have been recommended because of methadone’s long half-life.274 Opioid conversions are based on imperfect dose equivalencies. Providing patients with liberal access to rescue doses is critical during the conversion period to avoid precipitation of pain crises.
Invasive and Intraspinal Analgesic Approaches
As mentioned previously, permanent ablation of central afferent tracts becomes tenable in the context of advanced cancer, and has been used with considerable success.36,88,255,272 More discrete neural blockade can effectively reduce pain transmitted by one or several adjacent peripheral nerves. Intercostal, paravertebral, genitofemoral, ilioinguinal, and trigeminal nerve blocks can afford dramatic relief and reduce analgesic requirements. Nociceptive impulses of visceral origin can be blocked by ablation of sympathetic ganglia. Celiac plexus blockade affords excellent relief of visceral cancer pain.67 Intraspinal opioid administration can reduce dose requirements and associated side effects.243 The potential benefits, however, must be weighed against the added cost, required maintenance, and risk of infection. Despite efforts to demonstrate cost savings through the use of implantable intrathecal opioid delivery systems,95 these devices are not widely used.
Impairments in Cancer
Impairments Caused by Tumor Effects
Bone Metastases
Bone metastases are an important source of cancer-related impairment and a critical consideration in rehabilitation.43 Surgical stabilization of acute or impending fractures produces impairments that warrant physiatric attention. Greater challenges arise when bone metastases produce severe, function-limiting pain or pose an uncertain fracture risk during therapeutic exercise. Bone metastases are highly prevalent because bone is the most common site of metastatic spread, and osseous lesions complicate the most frequently occurring cancers: lung, breast, and prostate. Thyroid cancer, lymphoma, renal cell carcinoma, myeloma, and melanoma also commonly spread to bone. Between 60% and 84% of patients with solid tumors will develop bone metastases.146,210 Management of bony metastatic pain is discussed in the preceding section on chronic pain. Of greatest physiatric concern are lesions involving the spine and long bones. These structures are critical for weight-bearing and mobility, and are the most prone to fracture. Bone metastases are managed with medications, radiopharmaceuticals, orthoses, radiation therapy, and/or surgical stabilization. The choice of intervention(s) will depend on lesion location, degree of associated pain, presence or risk for fracture, radiation responsiveness, and related neurologic compromise. The overall clinical context (e.g., prognosis, severity of medical comorbidities, and operative risk) must also be taken into consideration. Most patients with unfractured bony lesions can be treated nonoperatively with systemic therapy and radiation.
Bisphosphonates are the primary medications used to manage bone metastases. Use of these agents relieves pain and mitigates the spread and progression of bone metastases. Bisphosphonates are generally delivered parenterally. Bisphosphonates can reduce the risk of vertebral fracture (odds ratio, 0.69), nonvertebral fracture (odds ratio, 0.65), and hypercalcemia (odds ratio, 0.54).215 Bisphosphonates also significantly increase the time to first skeletal event after the initial detection of osseous metastases. Current evidence supports the empiric initiation of bisphosphonates in patients with bone metastases. Radiopharmaceuticals such as strontium-99 are predominantly used to manage severe, refractory pain associated with widely disseminated bone metastases. Drawbacks to radiopharmaceuticals include prolonged bone marrow suppression and potentially severe pain flares after administration.
Radiation delivered to bone metastasis offers an effective means of rapidly achieving local control of pain and tumor growth. Palliative radiation was formerly delivered in 10 fractions of 300 cGy. However, single fractions of 8 Gy also effectively alleviate pain.277 At present the protocols in use range between these extremes, with the choice of dose and schedule being heavily influenced by individual patient factors and institutional culture. Radiation can be delayed after surgical stabilization. It is an important adjunctive treatment, however, because it suppresses tumor growth in areas where surgical management could have distributed microscopic emboli.
Painful osteolytic lesions are predominantly responsible for pathologic fractures. The incidence of pathologic fracture among all cancer types is 8%.220 Breast carcinoma is responsible for roughly 53% of these. Other solid tumors associated with pathologic fractures are kidney, lung, thyroid cancer, and lymphoma. Sixty percent of all long bone fractures involve the femur, with 80% of these located in the proximal portion.210
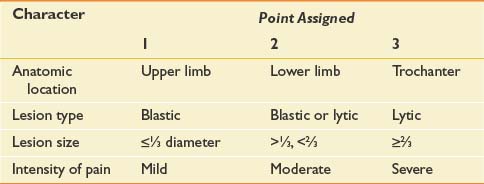
Management of osseous metastases that present a risk of fracture remains a source of clinical uncertainty. Precise quantification of fracture risk has been a persistent challenge in orthopedic oncology. Table 57-3 outlines Mirels’s proposed rating system for calculating fracture risk, whereby specific attributes are ascribed points.170 Neither this nor any other approach based on retrospective review has been adequately validated in clinical practice.
Pathologic fractures are generally managed through well-established surgical algorithms. Four main goals direct surgical management of pathologic fractures: pain relief, preservation or restoration of function, skeletal stabilization, and local tumor control.98 The general indications for surgery are life expectancy of more than 1 month with fracture of a weight-bearing bone, and more than 3 months for fracture of a non–weight-bearing bone. Internal fixation and prosthetic replacements with polymethylmethacrylate are the most effective ways of relieving pain and restoring function in patients with pathologic fractures.98 These procedures allow immediate weight-bearing. Intraoperative resection removes residual tumor that would impede bony healing, but healing rates are low after pathologic fractures. One review of 123 patients reported a 35% incidence of fracture healing.74
Fractures of the pelvis are generally treated conservatively, unless pain persists after radiation or they involve the acetabulum. In the latter case, patients are generally surgically reconstructed with screws or pins, and with an acetabular component. Vertebral fractures that are not associated with neurologic compromise are generally treated conservatively with radiation and bracing. Operative decompression and stabilization might be indicated for persistent pain refractory to aggressive analgesic therapy. Vertebroplasty can be considered for patients who are not at risk of tumor displacement into the spinal canal and associated myelopathy. Two large, recent randomized trials, however, have failed to demonstrate benefit in compression fractures related to osteoporosis.26,123 These results have raised skepticism regarding vertebroplasty’s benefit in cancer.
Brain Tumors: Primary and Metastases
Brain metastases occur in 15% to 40% of cancer patients, accounting for 200,000 new cases per year in the United States.78 They are the most common intracranial tumors.193 The incidence has increased in recent years, presumably as a result of prolonged patient survival and better early detection of small tumors through superior imaging modalities.66 Lung cancer is the most common primary source of brain metastases. As many as 64% of patients with stage IV lung cancer develop metastases.227 Breast cancer is the second most common source, followed by melanoma, with 2% to 25% and 4% to 20% of patients developing brain metastases, respectively.227 Brain metastases from colorectal cancers, genitourinary cancers, and sarcomas occur with considerably less frequency (1%).263 The distribution of metastases reflects cerebral blood flow, with 90% situated in the supratentorial region and 10% in the posterior fossa.263 Brain metastases are multiple in approximately 50% to 75% of cases.
Presentation
Lung cancer and melanoma often produce multiple metastases, whereas breast, colon, and renal cancer more commonly generate single lesions.263 Presenting symptoms at the time of diagnosis with brain metastasis, in order of decreasing frequency, are as follows (patients can have more than one): headache, 49%; mental disturbance, 32%; focal weakness, 30%; gait ataxia, 21%; seizures, 18%; speech difficulty, 12%; visual disturbance, 6%; sensory disturbance, 6%; and limb ataxia, 6%.201 Neurologic examination shows the following clinical signs at presentation: hemiparesis, 59%; impaired cognitive function, 58%; hemisensory loss, 21%; papilledema, 20%; gait ataxia, 19%; aphasia, 18%; visual field cut, 7%; and limb ataxia, 4%.201
Treatment
Corticosteroids are the first-line treatment, with dexamethasone being the drug of choice. By virtue of their ability to reduce peritumoral edema, corticosteroids reverse local brain compression and associated deficits. Treatment generally involves whole brain radiation therapy with stereotactic radiosurgery or surgical resection via craniotomy.125 Adjunctive chemotherapy can be used, contingent on patient performance status, type of cancer, and previous exposure to antineoplastics. Although seizures occur in 25% of patients with brain metastasis, studies and a metaanalysis have failed to show that antiepileptic drugs reduce their incidence.83,84
Prognosis
Untreated patients with brain metastases have a median survival of 1 to 2 months.154 Analyses performed by the Radiation Therapy Oncology Group that span multiple trials have produced a three-tiered classification scheme that predicts survival.77 Patients with the best prognoses (class 1), mean 7.1 months, had Karnofsky performance status (KPS) more than 70, age less than 65 years, controlled primary tumor, and no extracranial metastases. Patients with intermediate prognoses (class 2), mean 4.2 months, had KPS more than 70, with at least one of the following factors: more than 65 years of age, uncontrolled primary tumor, or systemic disease. Patients with poor prognoses (class 3), mean 2.3 months, had KPS less than 70. Although mean survival is less than 1 year for patients whose brain metastases are treated, the distribution is skewed, with some patients surviving more than 2 years with good functional preservation and QOL.
The rehabilitation needs of patients with brain metastases are determined by baseline functional status, prognoses, location and number of metastases, and antineoplastic treatment plan. The tremendous heterogeneity in the severity and type of associated impairments defies the formulation of a uniform algorithm. Cancer patients should be assessed on an individual basis using an approach analogous to that applied to patients with ischemic or traumatic intracranial lesions.
Epidural Spinal Cord Compression
Malignant spinal cord compression (SCC) occurs in up to 5% of patients.42 In contrast to brain metastases, which involve the brain parenchyma, most symptomatic tumors compress the spinal cord or cauda equina from the epidural space.200 Epidural lesions generally arise from vertebral metastases and rarely breach the dura.94 Invasion of the dural space accounts for only 5% of neoplastic SCC, and is due to either growth of tumor along the spinal roots or hematogenous spread to the cord.44,209 The cancers that most commonly cause SCC are those that produce vertebral metastases (e.g., breast, lung, myeloma, and prostate).17,276
Presentation
Pain is by far the most common initial (94%) and presenting (97% to 99%) symptom of malignant SCC.14,200 Radicular pain is present in 58% of patients at the time of diagnosis.14 Pain associated with SCC is generally exacerbated when supine or by coughing, sneezing, or the Valsalva maneuver. If malignant SCC is detected when pain is the only symptom, efforts to preserve function through surgical decompression or radiation therapy have high success rates.200 Unfortunately, this is rarely the case. Reports of symptom prevalence when the diagnosis of malignant SCC is eventually made are remarkably consistent. Weakness is present in 74% to 76% of patients, autonomic dysfunction in 52% to 57%, and sensory loss in 51% to 53%.81,200 The thoracic spine is the most common site of epidural SCC, followed by the lumbosacral and cervical spine in a ratio of 4:2:1.200
Diagnosis and Treatment
Magnetic resonance imaging (MRI) is the procedure of choice to evaluate the epidural space and spinal cord.251 MRI allows rapid evaluation of the entire spine with sagittal views. Computed tomography (CT) scans are helpful if there is an absolute contraindication to MRI, or if SCC is related to tumor encroachment through the foramina.
Prognosis
Tumors that cause rapid progression of neurologic deficits are associated with poorer functional outcomes after decompression.81 In general, patients remain ambulatory if able to walk at the time of definitive treatment. Motor and coordination deficits rarely resolve when present at the time of diagnosis. The recurrence rate for metastatic epidural SCC after successful treatment of the initial compression is 7% to 14%.42
Cranial Nerves
Cranial nerve palsies are caused by tumors that either originate near the base of the skull or metastasize there. Cancer can directly invade cranial nerves or exogenously compress them. Tumors often invade the neural foramina, which is seen in 15% to 35% of patients with nasopharyngeal carcinoma (a highly neurotrophic cancer).264 Bone metastases from lung, breast, and prostate cancers involving the base of the skull are also common sources of cranial nerve compromise.209 The incidence with which different cranial nerves are affected by cancer remains poorly quantified. One series of breast cancer patients reported a 13% incidence of cranial nerve dysfunction.90 The trigeminal and facial nerves were most frequently involved.
Clinical presentations vary depending on the cranial nerve being compressed. Evaluation should include MRI, which is the diagnostic test of choice.206 If patients have a bone-avid tumor (e.g., lung, breast, or prostate), a CT scan should be considered because bone destruction is more easily observed on CT scan.189 Positron emission tomography (PET) scanning, particularly in conjunction with CT scanning, can help to discretely localize tumor if extensive postradiation change or surgical alteration of the bony architecture has occurred. Acute management should include oral steroids, unless contraindicated, to preserve neurologic function until definitive treatment is delivered. Treatment generally involves chemotherapy and radiation.202
Spinal Roots
Malignant radiculopathies arise through direct hematogenous spread to the nerve roots or dorsal root ganglia, or more commonly by invasion from the paravertebral space. When the latter occurs, tumor can grow longitudinally in the paravertebral space and concurrently invade multiple foramina to produce a polyradiculopathy.202 Most cancer-related radiculopathies initially produce dysesthetic, aching, or burning pain in the affected dermatome, which can be associated with lancinations. Sympathetic hyperactivity or hypoactivity can be present.50 Involvement of the lower cervical or upper thoracic roots can produce Horner syndrome. In patients with a history of cancer, a new case of Horner syndrome should be attributed to malignancy until proven otherwise. Patients can complain of muscle cramps in affected myotomes.244
Nerve Plexuses
The brachial and lumbosacral plexi are commonly compressed or invaded by tumor. The frequency of neoplastic brachial plexopathy is 0.43%, and lumbosacral plexopathy 0.71%, based on retrospective case series.117,132 The most common sources of brachial plexopathy are tumors at the lung apex and regional spread of breast cancer.132 Because cancer generally grows superiorly to invade the lower brachial plexus, the inferior trunk and medial cord are most commonly involved. Occasionally head and neck neoplasms grow inferiorly to invade the upper trunk.116
Pain in the shoulder region and proximal arm occurs in 89% of patients with malignant brachial plexopathy and is the most common presenting symptom.133 The presence of pain helps to distinguish malignant from radiation-induced plexopathy. Only 18% of patients with radiation-induced plexopathy develop pain.133 Radiation plexopathies also differ in their propensity to cause progressive weakness in the C5–C6 myotomes as opposed to the lower cervical levels.133 Horner syndrome occurs in 23% of cancer patients with malignant brachial plexopathies.116 The presence of Horner syndrome suggests potential neuroforaminal encroachment and SCC. Numbness and paresthesias associated with malignant plexopathies typically are perceived in the C8 dermatome, especially digits 4 and 5.202 Loss of hand dexterity and power can be the initial motor complaint. Weakness subsequently extends proximally to involve the finger flexors, wrist extensors and flexors, and elbow extensors.202
Malignancies responsible for lumbosacral plexopathies include colorectal carcinomas; retroperitoneal sarcomas; or metastatic tumors from breast, lymphoma, uterus, cervix, bladder, melanoma, or prostate.117 When primary intrapelvic neoplasms are not responsible, the lumbosacral plexus is generally invaded from lymphatic and osseous metastases.116 Sacral plexopathies are more common than those in the lumbar region. Lumbar and sacral plexopathies can also occur concurrently.202 Lumbosacral plexopathies are bilateral in 25% of patients, particularly when the sacral plexus is more extensively involved.116,202 Incontinence and impotence strongly suggest bilateral involvement.117 Back, buttock, and/or leg pain is present in 98% of patients with malignant lumbosacral plexopathies. Among the 60% of patients who eventually develop neurologic deficits, 86% have leg weakness and 73% sensory loss.116 Positive straight leg raise is present in more than 50% of patients.117 As many as 33% of patients complain of a “hot dry foot” resulting from involvement of sympathetic components of the plexus.50
Diagnosis and Treatment
The evaluations of a suspected brachial plexopathy should include chest radiography to assess the lung apex. MRI with gadolinium is the diagnostic test of choice for evaluating the brachial and lumbosacral plexi.251 Cancerous invasion of plexi can extend along adjacent connective tissue or the epineurium of nerve trunks, without producing a discrete mass.65 For this reason, MRI findings can be erroneously interpreted as postradiation change. Electromyography can distinguish plexopathies from radiculopathies by defining the distribution of denervation. The presence of myokymia on needle examination is believed to be pathognomonic for radiation plexopathy.93
Acute treatment should include steroids for preservation of neurologic function. Radiation can effectively relieve pain but is less helpful in restoring lost function.116 Chemotherapy is commonly initiated or altered when plexus involvement heralds cancer progression; however, the success of this approach remains poorly characterized. Refractory pain requires aggressive coadministration of opioid and adjuvant analgesics, and potentially high cervical cordotomy or rhizotomy.113 Stellate ganglion blockade can relieve pain that is caused by sympathetically involvement.
Peripheral Nerves
Peripheral nerves are affected most often by cancer when extension of a bone metastasis produces a mononeuropathy.213 Rare polyneuropathy or mononeuritis multiplex resulting from myeloma, lymphoma, or leukemia has been reported.118,162 More commonly, nerves are compressed where they pass directly over an involved bone or through a bony canal.213 Common sites of nerve compression include the radial nerve at the humerus, obturator nerve at the obturator canal, ulnar nerve at the elbow and axilla, sciatic nerve in the pelvis, intercostal nerves, and peroneal nerve at the fibular head. Pain generally precedes motor and sensory loss.202
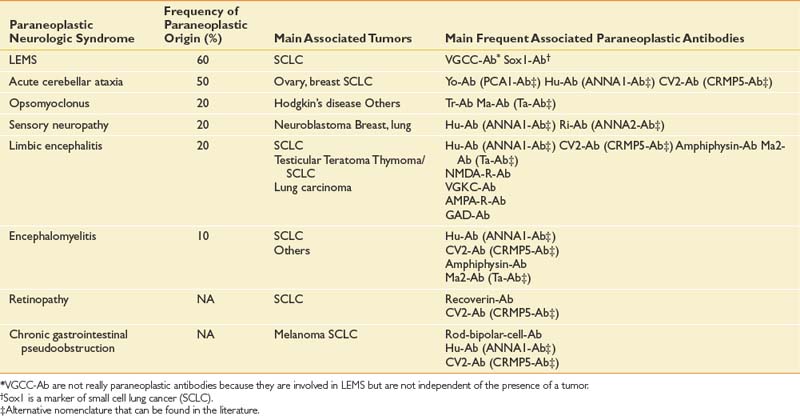
Paraneoplastic Syndromes
Paraneoplastic syndromes are pertinent to rehabilitation because they produce refractory neurologic deficits and severe disability.58 The incidence of paraneoplastic neurologic disorders (PNDs) is low, occurring in less than 1% of all cancer patients.106 PNDs can affect any level of the nervous system. Classic PNDs are listed in Table 57-4. These syndromes are produced when antibodies are made against tumors that express nervous system proteins. Discrete or multifocal neural degeneration produces diverse symptoms and deficits. Most PNDs are triggered during the early stages of cancer, when primary tumors and metastases might be undetectable by conventional imaging techniques. The emergence of a PND in a patient with known cancer should trigger workup for recurrent or progressive disease. PNDs are characterized by symptoms that develop and progress rapidly in days to weeks, and then stabilize. Spontaneous improvement is rare. Diagnostic workup can include serum and cerebrospinal fluid studies, brain MRI, and PET.5,47 Screening patients’ serum or cerebrospinal fluid for antineuronal antibodies known to be associated with particular cancers can direct the search for an occult malignancy. Timely diagnosis and treatment of the tumor offer the greatest chance of success in managing PNDs.11,35 PNDs do not generally respond solely to immunotherapies, including intravenous immunoglobulin, corticosteroids, and immunosuppressants. However, these can be useful adjuvant treatments.
Rehabilitation of patients with PNDs is determined by the type, distribution, and severity of the associated neurologic deficits. Potential improvement with planned antineoplastic therapy should be taken into consideration. Supportive and preventive measures to protect the integrity of the skin, affected joints, and genitourinary symptoms are critical while awaiting stabilization of neurologic deficits. Communication, respiratory, and nutritional issues should be addressed in patients with bulbar involvement.
Skin Metastases
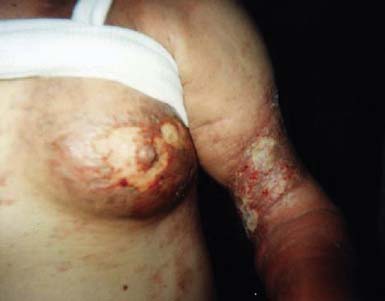
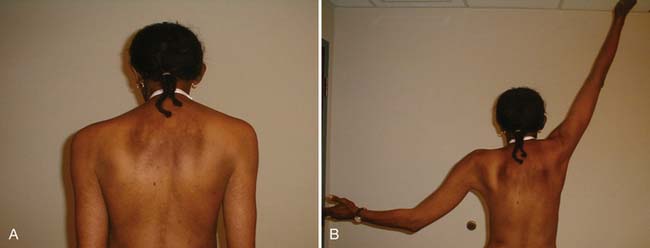
Dermal metastases occur in 5.3% of patients and are most common in breast cancer.134 Skin metastases can be a source of pain and an entry point for infectious pathogens. Because the associated wounds seldom heal, chronic wound care is necessary and becomes an integral part of patients’ rehabilitation needs. Figure 57-1 shows a breast cancer patient with dermal metastases involving the breast and proximal arm. Dermal metastases often engender or aggravate lymphedema. Use of compression is limited only by patient tolerance. Malignant wounds should be managed with nonadherent, bacteriostatic, hyperabsorbent dressings (e.g., SilvaSorb or Aquacel Ag). Associated pain must be managed aggressively to minimize adverse functional consequences. Proactive range of motion (ROM) activities can prevent the formation of contractures in joints adjacent to malignant wounds, facilitating hygiene and autonomous self-care.
Cardiopulmonary Metastases
Lung, pleural, and pericardial metastases involving the heart and lungs can produce dramatic and abrupt reductions in patients’ stamina and functional status. Virtually all cancers have the potential to spread to the lungs and pleura. At autopsy, 25% to 30% of all cancer patients have lung metastases.49 Pleural metastases occur in 12% of breast and 7% to 15% of lung cancers.135,138 Metastases to the heart and pericardium are less common, although their functional impact can be similarly devastating. A series of 4769 autopsies revealed the presence of cardiac metastases in 8.4% of cancer patients.237 Melanoma, mesothelioma, lung tumors, and renal neoplasms had the highest prevalence of cardiac spread. The clinical diagnosis of heart or lung metastases can be generally made by CT scans. PET scans and plain radiographs can also be helpful, depending on the clinical context.
Treatment of lung, pleural, pericardial, or cardiac metastases varies considerably. The type and efficacy of anticancer treatment will depend on the primary tumor, number and location of metastases, previous antineoplastic therapies, overall medical condition of the patient, and degree of associated symptomatic distress. Surgical metastectomy has the potential to definitively eliminate disease in certain patients.260 Discrete metastases that are not resectable might be amenable to radiation therapy. Patients with extrathoracic metastases are commonly treated with systemic chemotherapy.
Malignant pleural effusions should be evacuated when patients become symptomatic. The associated dyspnea often arises from other causes as well, and reducing the effusion might fail to alleviate patients’ shortness of breath if the lung is trapped because of parenchymal or pleural disease. Reaccumulation of malignant effusions can be managed through intermittent thoracentesis or pleurodesis, or placement of an indwelling pleural catheter.39 Chemical pleurodesis has an overall complete response rate of 64% when all sclerotic agents are considered.138 Talc appears the most effective, with a complete response rate of 91%.
Impairments Caused by Cancer Treatment
Donor Site Morbidity
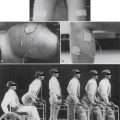
By virtue of the high incidence of breast cancer, significant donor site morbidity is most prevalent with autogenous tissue transposition for breast reconstruction. Transverse rectus abdominis muscle (TRAM), gluteus maximus, and latissimus myocutaneous flaps are used, with the former being more common. With a relatively low complication rate (25.3%) and potentially excellent cosmesis (Figure 57-2), the TRAM flap procedure is an increasingly common choice, given the potential to create a natural-looking breast with normal ptosis and an inframammary fold. More patients are electing to undergo immediate breast reconstruction to reduce the risk associated with repeat operations and the psychologic distress engendered by mastectomy.
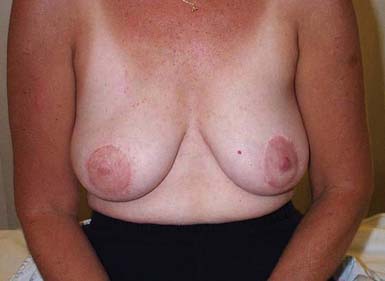
FIGURE 57-2 Excellent cosmesis achieved with bilateral transverse rectus abdominis flap breast reconstructions.
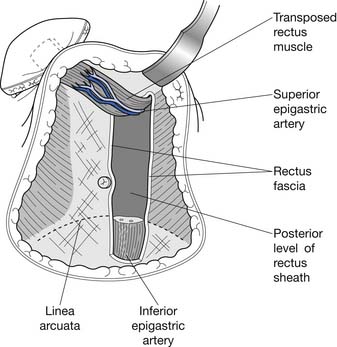
The TRAM procedure involves the transposition of muscle and adipose tissue to match preoperative breast appearance (Figure 57-3). Other advantages of the TRAM procedure include relatively hidden scars and a satisfactory donor site resulting in a flat abdomen.188 The TRAM flap can be divided into the pedicled or free flap procedures. These procedures differ in that the pedicled, or conventional, procedure uses the epigastric vessels supplying the rectus muscle to perfuse the subumbilical fat. Subumbilical adipose tissue is tunneled under the abdominal skin to repair the defect created by mastectomy. The inferior end of the contralateral rectus abdominis muscle is tunneled with the fat (see Figure 57-3). In contrast, the free flap procedure involves the creation of anastomoses with vessels in the chest, such as the thoracodorsal or internal mammary arteries. Although the free flap procedure requires increased operative time, it is associated with decreased incidence of partial flap loss resulting from fat necrosis.9
Despite declining perioperative complication rates, the adverse musculoskeletal sequelae of TRAM flap breast reconstruction can be significant.175 Fat necrosis within the reconstructed breast can significantly undermine cosmesis.38 Donor site complications include abdominal wall bulge (2.9% to 3.8%), abdominal hernia (2.6% to 2.9%), and dehiscence (3.8%).40,136 Patients experience abdominal weakness and reduced exertional tolerance, particularly those undergoing bilateral procedures.171 Because the TRAM procedure produces a defect in the abdominal wall, patients have difficulty stabilizing the trunk while transferring from supine and seated positions. Partial denervation of the abdominal wall also leads to deficits in proprioception and truncal balance. Weakness of the abdominal wall can lead to exaggerated lumbar lordosis and an increased incidence of back pain. An algorithm for treatment of patients post–TRAM flap reconstruction is presented in the “Rehabilitation of Specific Cancer Populations” section of this chapter.
Radiation Therapy–Related Impairments
Radiation therapy has become an integral part of combined modality and organ preservation therapy for many cancers. Approximately 50% of cancer patients undergo radiation therapy during the course of their disease. Although highly effective in eliminating radiosensitive tumors, controlling regional disease, and palliating symptomatic metastases, radiation therapy also injures normal tissue. The tolerance of normal tissues surrounding tumors is the most important radiation dose-limiting consideration.96 Radiation injury is multiphasic, characterized by discrete acute and late phases mediated by distinct pathophysiologic processes. Acute injury is predominantly caused by inflammation and the death of rapidly proliferating cell types. Cell death occurs through the induction of apoptosis and free radical-mediated DNA damage. Patients can develop desquamation of the dermis and mucous membranes, visceral inflammation (e.g., colitis, cystitis, and enteritis), and muscle hypertonicity, among other symptoms. Biologic response modifiers released from injured tumor cells are thought to mediate systemic radiation effects such as fatigue and malaise.143 The time course of acute radiation effects on normal tissue varies significantly by tissue type and radiation dose. Most patients return to their preradiation baseline by the second month after treatment. The distribution is highly skewed, however, and some patients remain symptomatic as many as 12 months after treatment.
The deleterious effects of late radiation injury are being subjected to increasing scrutiny. Adverse late effects can be attributed to tissue necrosis and fibrosis. The mechanisms underlying these end processes are being actively investigated. Microvascular injury predisposes to thrombus formation and produces a hypoxic interstitial environment.68 Hypoxia is believed to favor the generation of free radical species that produce further damage, and ultimately a self-perpetuating cycle of tissue injury and fibrosis.268 In addition to compression from fibrosis, neural and microvascular injury can occur from occlusion of the vasonervorum, vasorum, and lymphorum with resultant infarction. Dysregulated transforming growth factor β (TGF-β)–mediated fibroblast activation has also been implicated, as has aberrant signaling of TGF-β–related pathways.197
The adverse late effects of radiation therapy depend on the extent and location of the radiation field. Identifying the tattoos placed during radiation therapy simulation can help delineate the extent of the irradiated tissue. This is particularly helpful when clinical records are unavailable. Table 57-5 lists conditions caused by delayed radiation toxicity by system. Late radiation effects most relevant to rehabilitation medicine include those involving connective tissue, muscles, and nerves. Fibrosis occurs to some degree in all muscles and connective tissue within a radiation portal. In the absence of ongoing ROM, patients can develop contractures. Because free radical-mediated and TGF-β–mediated late radiation injury is an ongoing and potentially self-perpetuating process, ranging of affected muscles and fascia should continue indefinitely.
| System | Adverse Late Effects |
|---|---|
| Endocrine | Hypothyroidism, hypogonadism, adrenal insufficiency, glucose intolerance caused by pancreatic insufficiency |
| Exocrine | Xerostomia, pancreatic enzyme deficiency |
| Neural | Myelopathy, plexopathy, cerebrovascular ischemia, dementia, leukoencephalopathy, cranial neuropathy |
| Lymphatic | Lymph node necrosis, lymphedema |
| Gastrointestinal | Dysmotility, malabsorption, neuroconstipation, obstruction, perforation, dysgeusia |
| Dermis | Atrophy, ulceration, delayed healing, hyperpigmentation |
| Auditory | Progressive loss of acuity, tinnitus |
| Vascular | Premature atherosclerosis, venous sclerosis |
| Pulmonary or | Parenchymal fibrosis tracheal stenosis, |
| upper respiratory | dysphonia secondary to laryngeal fibrosis |
| Musculoskeletal | Fibrosis, osteonecrosis, osteoporosis, soft tissue necrosis joint contracture, epimysial fibrosis |
| Ocular | Corneal ulceration, retinopathy, scleral necrosis |
| Genitourinary | Neurogenic bladder, renal failure, obstruction, perforation |
The most devastating neural effects of radiation therapy include myelopathy, plexopathy, and encephalopathy. Delayed radiation myelopathy produces symptoms 12 to 50 months after radiation therapy, and progresses over weeks or months to paraparesis or quadriparesis.62,228 Symptoms can worsen or stabilize, producing deficits ranging from mild to complete motor weakness. Although radiation hyperfractionation has reduced the incidence of myelopathy, it has been reported to affect 5% of patients who survive 18 months after receiving 5000 cGy (1 Gy = 100 rads, 1 cGy = 1 rad) to the mediastinum for lung cancer.271 Risk factors include radiation therapy fraction size greater than 180 cGy and older age.218 The presenting symptom is usually a Brown-Séquard syndrome, which begins distally and ascends to reach the irradiated level of the cord.203 MRI is useful in distinguishing radiation from malignant myelopathy. Hyperbaric oxygen might offer benefit if initiated soon after the onset of weakness; however, this remains controversial.
Radiation plexopathies occur in 1.8% to 4.9% of treated patients.196,207 Risk is dose-related and seems to increase with radiation therapy exposure greater than 5000 cGy.126 Concurrent administration of chemotherapy increases the risk.185 Radiation therapy-induced brachial plexopathies develop between 3 months and 14 years (median, 1.5 years) after therapy.132 Lumbosacral plexopathies develop between 1 month and 31 years (generally 1 to 5 years) after radiation therapy.258 Characteristics of radiation therapy plexopathies that distinguish them from malignant plexopathies include lower incidence of pain (18%), pain that develops after weakness, and the presence of myokymia on electromyography.
Delayed radiation therapy encephalopathy resulting from necrosis of brain parenchyma occurs in 3% to 5% of patients receiving more than 5000 cGy, and in 5% to 15% of patients receiving 6000 cGy.157 Symptoms generally develop 2 years after completion of radiation therapy. The clinical presentation often resembles that of the primary malignancy, raising the question of local recurrence. PET scanning is of greater usefulness in distinguishing tumor from radiation necrosis than either MRI or CT because radiation necrosis is hypometabolic.21,57
Cerebral atrophy occurs more commonly than radiation therapy necrosis, being present invariably after whole brain radiation therapy of 3000 cGy in 10 fractions.203 Virtually all patients complain of memory loss, which can be sufficiently severe to compromise vocational viability.37 Memory loss progresses in 10% to 20% of patients to involve other cognitive domains, potentially leading to dementia.52 Patients can also develop gait abnormalities and urinary urgency.52
Medical treatment of radiation therapy-associated neural compromise can include short-term steroids, anticoagulation, and/or hyperbaric oxygen therapy.82,208 Focal radiation necrosis of brain parenchyma can require surgical resection. Increasing use of bevacizumab to reverse radiation fibrosis is based on anecdotal successes and a tenuous but growing evidence base.147,261 Pentoxifylline (800 mg/day) coadministered with tocopherol attenuates radiation fibrosis.55,56 The benefits of pentoxifylline have yet to be assessed in radiation therapy-related neural compromise.
Chemotherapy
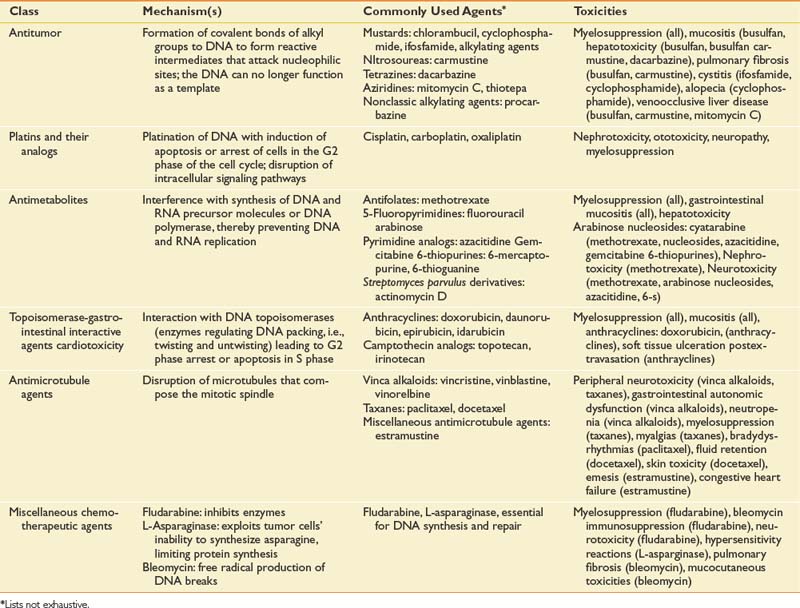
A staggering array of chemotherapeutic agents, or antineoplastics, is currently used in oncologic practice. Antineoplastic drugs can be mechanistically grouped into a manageable number of subclasses for the nononcologist, which include alkylating agents, platins and their analogs, antimetabolites, topoisomerase interactive agents, antimicrotubule agents, differentiation agents, and miscellaneous agents. Table 57-6 lists antineoplastics by class.
Antineoplastics are distinguished by their capacity to preferentially injure rapidly dividing cancer cells while sparing normal cells, but all are associated with significant potential for normal tissue toxicity. The chemotherapeutic toxicities that most commonly produce functional impairments are peripheral neuropathy, cognitive dysfunction, cardiomyopathy, and pulmonary fibrosis. Fortunately, with proactive screening, the incidence of significant cardiopulmonary toxicity has been substantially reduced. Bleomycin produces pulmonary fibrosis in 10% of patients.192 The risk of doxorubicin-associated cardiac toxicity directly parallels increases in cumulative dose. With cumulative doses of 550, 600, and 700 mg/m2, the incidence is 7%, 15%, and 30%, respectively.101 Cardiomyopathy becomes a real concern in stage IV breast cancer patients who resume doxorubicin treatment after having received it in the context of primary adjuvant therapy. Trastuzumab produces cardiac toxicity in 3% to 5% of patients receiving monotherapy and in 28% of patients who concurrently receive anthracyclines.128
Chemotherapeutic neuropathy is a prevalent and functionally morbid complication of cancer treatment. The vinca alkaloids, cisplatin, ixabepilone, the taxanes, and thalidomide are among the most important drugs inducing peripheral neurotoxicity.238 These drugs are widely used for various malignancies, such as ovarian and breast cancer, and hematologic cancers. Chemotherapeutic neuropathy is related to cumulative dose or dose intensity.30 Patients who already have neuropathic symptoms resulting from diabetes mellitus, hereditary neuropathies, or earlier treatment with neurotoxic chemotherapy are believed to be at higher risk.
All platin compounds (e.g., cisplatin, carboplatin, and oxaliplatin) have the potential to produce sensory neuropathy. Cisplatin is a more frequent source of neurotoxicity than the latter two compounds. Symptoms often occur after completion of treatment.148,236 Large sensory fibers are preferentially affected, leading to proprioceptive deficits. Pinprick and temperature sensation, as well as motor function, are relatively spared.30 Lower-limb muscle stretch reflexes often disappear. Autonomic nerves remain unaffected. Nerve conduction studies show decreased sensory nerve action potentials and prolonged sensory distal latencies, whereas nerve conduction velocities are minimally impaired.148,236
Peripheral neuropathy related to vinca alkaloid treatment is observed most commonly with vincristine. Paresthesias in the distal extremities are the initial symptoms, and loss of lower-limb muscle stretch reflexes is the initial sign. Weakness of the wrist and digital extensors can occur. Autonomic neuropathy is common and might lead to paralytic ileus, orthostatic hypotension, and impotence.89 Vibration sense generally remains intact.30 Nerve conduction studies show decreased distal motor and sensory nerve action potentials, with only slight reduction in nerve conduction velocities, indicating an axonal rather than a demyelinating mechanism of injury.20
Taxanes have become first-line therapy in the treatment of primary breast, ovarian, and lung cancers. Docetaxel is a more frequent and severe source of neuropathy. Signs and symptoms that characterize taxane neuropathy include paresthesias, loss of muscle stretch reflexes, and diminished vibration sense.205 Patients can develop mild proximal muscle weakness that resolves spontaneously.73 Autonomic neuropathy occurs uncommonly.120 Nerve conduction studies show reduction of sensory nerve action potentials in patients treated with taxanes.222 Reduced motor nerve action potentials and diminished sensory and motor nerve conduction velocity have been reported.222
Novel targeted biopharmaceuticals are increasingly displacing established treatment standards. These include monoclonal antibodies to epidermal growth factor receptors (pertuzumab), small molecule tyrosine kinase inhibitors that targeted the various epidermal growth factor receptors (gefitinib, erlotinib), monoclonal antibodies directed at the vascular endothelial growth factor (bevacizumab), and the small tyrosine kinase inhibitors that target the vascular endothelial growth factor receptor.28 The risk profiles of many of these agents remain inadequately characterized, particularly when administered to elderly and infirm patients who differ considerably from the cohorts studied in trials. Thromboembolic events are of concern in patients receiving therapies targeting the vascular endothelial growth factor receptor.85
Rehabilitation Approaches
General Strategies
Rehabilitation of Bone Metastases
Although theoretically appealing, evidence is lacking for the usefulness of therapeutic exercise in the prevention of pathologic fractures. Regardless, patients at risk for vertebral fractures routinely tolerate exercise programs designed to strengthen the abdominal and spinal extensor muscles and to enhance their awareness of body positioning. A comprehensive exercise program should include postural and balance training, as well as truncal strengthening. Simple environmental modifications can significantly reduce patients’ fracture risk. Throw rugs and other hazards that increase fall risk should be removed. Railings can be added to stairwells and bathrooms as appropriate. Patients’ prognoses should obviously be considered in the zeal and expense with which such modifications are implemented.
Exercise
Aerobic Conditioning and Resistive Exercise
Trials of aerobic conditioning in cancer populations have been predominantly conducted to determine whether exercise attenuates treatment-associated fatigue and enhances QOL. Breast cancer patients receiving adjuvant chemotherapy have comprised the majority of study cohorts, although Dimeo et al.59–61 contributed significantly to the literature with studies of aerobic conditioning immediately after bone marrow transplantation.
Studies in breast and other cancer populations currently under or after cancer treatments have consistently noted improved symptom burden: fatigue,172,173,229–231 insomnia,172 nausea,275 and emotional distress.172,173 Trials have varied considerably in the intensity, frequency, and duration of aerobic training, the targeted interval in cancer treatment (e.g., active, posttreatment), as well as in the level of investigator supervision.226,239 Self-paced exercise regimens have reliably achieved modest improvements in 12-minute walk time.172,173,229–231 Use of more rigorous, structured programs (more than three exercise sessions per week at 60% to 90% of maximal heart rate) increases relative lean body mass275 and VO2max.97,111,153,184 Protocols involving less intense exercise, for example, five times per week at 50% to 60% of VO2max, have not consistently achieved statistically significant improvements in oxidative capacity (VO2max).233 This suggests a potential exertional threshold below which physiologic benefits are limited, but this remains speculative. The literature suggests that at virtually all points along the cancer trajectory, patients benefit from incremental aerobic exercise, and that exercise intensities as high as 90% of maximal heart rate three times weekly can be safely tolerated. The common sense caveat regarding the need for program oversight and individualization by clinicians knowledgeable in both rehabilitation approaches and patients’ disease and treatment specifics applies.
Aerobic conditioning reduces symptom burden and mitigates the physiologic impact of high-dose chemotherapy delivered in the context of bone marrow transplantation as well. Performance of cardiovascular cycling at 50% of heart rate reserve reduced participants’ decline in physical performance (e.g., walking distance and speed), physiologic parameters, neutropenia and thrombocytopenia, and psychologic distress relative to those of control subjects.59,61 Training on a treadmill after high-dose chemotherapy administration at an intensity set to increase blood lactate concentrations to 3 mmol/L produced similar improvements in mean blood lactate concentrations,60 and training distances improved more than 100%.60
Investigations of the impact of aerobic exercise on immunologic parameters in cancer patients have produced mixed results. Short-term (2-week) aerobic training in stomach cancer patients using arm and cycle ergometers at 60% of maximal heart rate caused a mean 27.9% increase in natural killer (NK) cell activity.180 Cardiovascular training at 60% of maximal heart rate during a 7-month intervention in breast cancer survivors similarly improved NK cell activity without increasing NK cell numbers.194,195 A mixed aerobic (75% of heart rate maximum) and resistance training program failed to alter NK cell activity among breast cancer survivors.182 This study was inadequately powered, however, with a sample size of only six per group. The limited literature available suggests that exercise can modulate immunologic parameters. Defining the magnitude, duration, and reproducibility of the exercise effect requires further investigation, as does the clinical relevance of alterations in immunologic parameters.
Initially programs combining resistance training with aerobic conditioning yielded inconsistent improvement in overall QOL, with some studies failing to note change1,45,233 and others showing improvement.131,234 Recent, randomized, and adequately powered trials, however, have consistently demonstrated marked improvements in fatigue, physical functioning, and mental health.2,76,99,101,169 No study has reported compromised QOL associated with participation in exercise programs, irrespective of their intensity. Of note, integrated physical training approaches appear to be superior to psychocognitive approaches in enhancing physical well-being and QOL.159
Limited trials have evaluated the impact of resistance training in cancer populations.48 Definitive improvement was reported with resistance training among prostate cancer patients receiving androgen deprivation therapy,234 as well as in breast4,225 and head and neck cancer survivors.163 A single trial that compared resistance and aerobic training found both to be effective, but the former afforded longer-term improvements.235 Several studies suggest that resistance training might be an effective means to reduce bone loss in postmenopausal breast cancer survivors.265,270 The exercise interventions were well tolerated without adverse effects in both resistance trials.
The number of trials evaluating exercise interventions in cancer populations has burgeoned in recent years. Trials have consistently demonstrated that exercise is safe, but not always effective, contingent on study endpoints. A comprehensive summary is well beyond the scope of this chapter. Interested readers are referred to multiple excellent and recently published systematic reviews.∗
Rehabilitation of Cardiopulmonary Dysfunction.
Exertional intolerance resulting from cardiopulmonary factors occurs commonly among cancer patients. Surgical pneumonectomy or lobectomy, the current standard of care for management of local and regional lung cancer, abruptly reduces aerobic capacity. Radiation of the thorax produces fibrosis of lung parenchyma, visceral pleura, and pericardium. Review of patients’ radiation treatment records can be invaluable in gauging their risk of cardiopulmonary fibrosis. Many patients requiring treatment for intrathoracic tumor have smoking histories and some degree of premorbid subclinical chronic obstructive pulmonary or reactive airway disease.253 As a consequence, resection or irradiation of lung tissue can result in far greater dyspnea and functional compromise than anticipated. Chemotherapy and intrathoracic metastases can also produce cardiopulmonary dysfunction.
Rehabilitation of cardiopulmonary dysfunction in cancer patients uses protocols well established in cardiac and pulmonary rehabilitation (see Chapters 33 and 34). Incremental aerobic conditioning with supplemental oxygen as needed usually produces a reduction in exertional intolerance. Similar to both cardiac and pulmonary rehabilitation, aerobic conditioning has limited beneficial impact on heart and lung physiology. Improvements in stamina and perceived exertion are due to muscle-training effects.
Flexibility Exercises
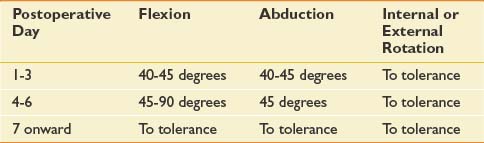
Protocols for the prevention or treatment of radiation contractures have not been published or empirically assessed. Patients are generally provided with a series of active-assisted ROM activities that target all affected muscle groups, with emphasis placed on restricted planes of motion and instructions to hold each stretch for three to five deep breaths. Stretching should be performed at least twice per day during the first year after treatment. If soft tissue restrictions progress despite adequate compliance, the duration, frequency, and degree of active assistance should be increased. As with any restriction in soft tissue excursion, patients should be examined for secondary myofascial dysfunction, tightness in muscles outside the radiation field, and biomechanical imbalance. A single report describes the successful treatment of refractory radiation-induced contractures with botulinum toxin injections.248
Comprehensive Inpatient Rehabilitation
The appropriateness and potential benefits of comprehensive inpatient rehabilitation must be assessed on a case-by-case basis. Cancer patients’ candidacy is generally deemed appropriate when their deficits conform to a neurologic or musculoskeletal syndrome familiar in the inpatient rehabilitation setting, that is, hemiparesis, paraplegia, or amputation. Several studies have reported equal functional independence measure (FIM) efficacies when patients with malignant SCC are compared with patients with similar but traumatically and ischemically induced impairments. Patients with malignant SCC achieve less functional improvement but, because of shorter lengths of stay, have comparable FIM efficiencies relative to patients with traumatic spinal cord injury.161 Home discharge rates are equal, 84% in a retrospective case series,160 or higher among patients with malignant SCC.
Retrospective case series of patients transferred to rehabilitation after treatment for primary brain tumors and intracranial metastases describe substantial gains in cognitive ADL and mobility domains.110,156 The functional gains achieved by brain tumor patients are similar to those of patients with acute stroke108 and traumatic brain injury.109,183 Patients with brain tumors are consistently discharged to the community more than 80% of the time109 and have significantly shorter lengths of stay.108,182 Studies have differed on the impact of concurrent radiation therapy. Some describe greater FIM mobility efficiencies with radiation, whereas others report the opposite.183
A recent comparison of patients admitted for inpatient rehabilitation with wide-ranging cancer-related impairments noted no significant differences in FIM efficiencies or length of stay relative to noncancer patients. This suggests that inpatient admissions should be considered for cancer patients whose debilities arise from impairments other that intracranial or epidural metastases.256 That said, roughly 31% of cancer patients admitted for acute inpatient rehabilitation undergo unplanned transfer back to acute care units. The predictors for transfer are low albumin, elevated creatinine, and a requirement for tube feeding or a Foley catheter.86
Lymphedema Management
Lymphedema is a chronic and currently incurable condition that frequently complicates cancer therapy. After resection or irradiation of lymph nodes and vessels, lymphatic congestion can develop in any region of the body drained by the affected structures. If congestion becomes sufficiently severe, swelling can result from accumulation of protein-rich fluid.273 Far from being a treatment-refractory and inexorably progressive condition, lymphedema is now amenable to highly effective and widely available therapy. Complete (or complex) decongestive therapy (CDT) represents the current international standard of care for lymphedema management.13 This was formalized in a white paper published by the International Society of Lymphology in 2001.13 CDT is an intensive integration of manual approaches and is able to achieve and maintain substantial volume reduction for the majority of lymphedema patients. Surgical, dietary, and pharmacologic approaches offer equivocal benefit at best but can be considered when appropriate manual and compression therapy fail to adequately reduce lymphedema.252
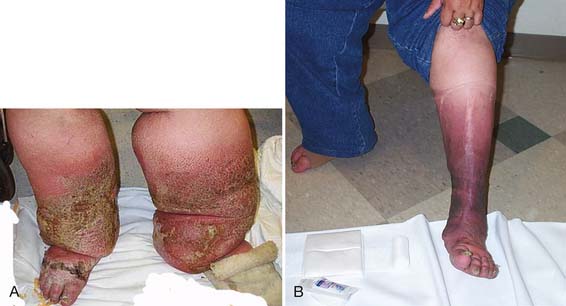
CDT is a two-phase, multimodal system that incorporates manual lymphatic drainage (MLD), short-stretch compressive bandaging, skin care, therapeutic exercise, and elastic compression garments. The initial phase, sometimes designated with a Roman numeral I or described by the term reductive, has as its primary goal decreasing lymphedema volume.70 During daily phase I CDT sessions, patients receive approximately 45 minutes of MLD, followed by the application of compression bandages and performance of remedial exercises. Compressive bandages are left in place 21 to 24 hr/day. The efficacy of treatment delivered at this intensity has been demonstrated in numerous case series.71,130,176 Figure 57-4 shows pre- and post-CDT images in a patient with bilateral stage 3 lymphedema. After maximal volume reduction, patients are gradually transitioned to a long-term maintenance program (phase II). In this phase, compressive garments are used during the day, with application of compressive bandages overnight. Patients perform remedial exercises daily while bandaged and receive MLD as needed.
Compression forms the basis of virtually all successful lymphedema therapy. During both CDT phases I (day and night) and II (night-time only), compression is achieved through the use of short-stretch bandages. Short-stretch bandages have a high working pressure by virtue of contractions in the underlying muscles.190,191,245 The bandages exert low pressure while the muscles are resting. A distal to proximal compression gradient is achieved by applying more layers of bandages distally, rather than varying the amount of tension used to apply the bandages. Compression garments are added to patients’ phase II regimens for daytime compression. Compression garments achieve the following:
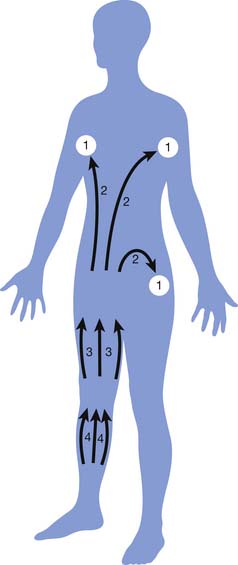
MLD or “lymphatic massage” is a highly specialized technique designed to enhance the sequestration and transport of lymph. Specific stroke duration, orientation, pressure, and sequence characterize MLD. MLD stimulates the intrinsic contractility of the lymph vessels, leading to increased sequestration and transformation of macromolecules in the interstitium.33 Through gentle and rhythmic skin distention, congested lymph is directed through residual lymph vessels into intact lymph node beds. MLD permits shifting of congested lymph to lymphotomes (anatomic regions drained by a specific lymph node bed with preserved drainage, as illustrated in Figure 57-5). The massage is light and superficial, limited to finger or hand pressures of around 30 to 45 mm Hg. MLD treatments are initiated proximally in lymphostatic regions adjacent to functioning lymphotomes. Lymph is constantly directed toward functional lymphotomes and lymph node beds with strategic hand movement. Treatments gradually progress distally to terminate in the regions farthest removed from intact lymphatics.
Remedial lymphedema exercises refer to repetitive movements designed to encourage rhythmic, serial muscle contractions in lymphedematous territories. Remedial exercises are always performed with external compression, most commonly compressive garments or bandages. Remedial exercises repeatedly compress the lymph vessels through sequential muscle contraction and relaxation, thereby triggering smooth muscle contraction in lymph vessel walls.186 An internal pumping mechanism is established that encourages congested lymph to flow along the compression gradient created with bandages or garments.141,142 Progressive strength training, when supervised and gradually progressed, was shown to reduce lymphedema flares in a large, randomized controlled trial. Based on this finding, strength training should be integrated into the routine management of breast cancer–related lymphedema.225
Skin care is stressed in manual approaches to lymphedema. The goals of skin care include controlling skin colonization with bacteria and fungi, eliminating overgrowth in skin crevices, and hydrating the skin to eliminate microfissuring. Daily cleansing with mineral oil-based soap will remove debris and bacteria while moisturizing the skin.32
The current shortage of adequately trained and experienced lymphedema therapists in the United States is a consistent impediment to successful treatment. The Lymphology Association of North America (LANA) has developed a certification examination to identify therapists with the requisite knowledge and manual training. A list of certified therapists is available through the LANA website (http://www.cltlana.org). The National Lymphedema Network (http://www.lymphnet.org) offers an extensive list of lymphedema-related resources. Patients with lymphedema complicated by aggressive recurrent cancer, dermal metastases, chemotherapeutic neuropathies, or pain will require specialized care generally available only at tertiary medical and comprehensive cancer centers.
Rehabilitation of Specific Cancer Populations
Breast Cancer
Persistent deficits in shoulder ROM occur in as many as 35% of patients after ALND140 (Table 57-7). Even after SNLB, 16% of patients have self-reported limitations.144 Lotze et al.149 reported that vigorous shoulder mobilization in the immediate postoperative period led to an increase in seroma formation. The timeline for shoulder mobilization presented in Table 57-8 adequately restores shoulder mobility without increasing the incidence of postsurgical complications but has not been empirically evaluated. MRM and ALND are performed as same-day procedures at some institutions. In such cases, a gradual, supervised, and progressive ROM program is not possible. In such cases patients are often provided with illustrated exercise sheets covering “wall walking,” forward flexion assisted by the unaffected arm, shoulder rolls, etc. A growing literature suggests that physical therapy after surgery for breast cancer offers a number of compelling benefits, including reduced pain, shoulder limitations, and lymphedema, as well as enhanced psychologic well-being.15,19,139,262 This evidence is arguably strong enough to mandate the inclusion of physical therapy as standard care in postsurgical breast cancer management.
Axillary web syndrome (Figure 57-6) refers to the presence of taut, palpable cords originating in the axilla and extending distally along the anterior surface of the arm, often below the elbow.178 What precise tissues comprise the cords remains a source of speculation. In a limited case series resected cords were pathologically evaluated and contained either lymphatic vessels or veins and surrounding connective tissue.178 The clinical relevance of axillary web syndrome arises from its potential for painful restrictions in shoulder ROM. In severe cases, the cords tether the humerus, preventing full shoulder flexion or abduction. Pain generally responds to NSAIDs, but opioid analgesics might be necessary during passive and active assisted ROM if the pain is severe. Therapy involves incremental ROM activities, topical heat, manipulation to soften and potentially “pop” the cords, as well as provision with a home exercise program. Heat should be used briefly, if at all, given the risk of lymphedema and the almost universal presence of intercostal brachial neuropathy in the axilla and upper arm.
The surgical community has increasingly recognized the need for rehabilitation after TRAM flap breast reconstruction. The procedure denervates and disrupts the integrity of the abdominal wall, producing significant deficits in truncal stability, particularly during functional transfers. The goals of post-TRAM rehabilitation are to prevent subdermal fibrosis and adhesions, restore truncal alignment, minimize stress on the lumbar spine, optimize proprioceptive acuity in residual abdominal muscles, and encourage normal muscle recruitment patterns. The algorithm for post-TRAM flap rehabilitation (Table 57-9) was well tolerated and eliminated lasting impairment in a cohort of 52 patients.247 No patients developed donor site herniation or wound dehiscence.
| Weeks | Activities |
|---|---|
| 0-3 | Patient education |
| Lymphedema precautions | |
| Body mechanics | |
| Back safety | |
| (Driving permitted 3-4 weeks) | |
| 3-5 | Active upper extremity range of motion (to tolerance) |
| Supine forward flexion with wand | |
| Supine external rotation with wand | |
| Standing abduction, wall walking | |
| Postural body mechanics | |
| Shoulder retraction—active with mirror cues | |
| Upright standing | |
| Head up or chin tucks | |
| Manual techniques (as needed) | |
| Manual lymphatic drainage | |
| Scar mobilization (if healed) | |
| Gentle myofascial release if restrictions are notable | |
| Walking program if needed | |
| 6-7 | Postural body mechanics |
| Shoulder retraction—active with mirror cues | |
| Pectoral stretch (corner stretch) | |
| If good alignment with retraction, may initiate | |
| Resistive Thera-Band at yellow level for shoulder | |
| retraction | |
| Upright standing posture—posterior pelvic tilt in supine | |
| 8-12 | Stabilization or strengthening exercises |
| Prone lying | |
| Isometric pelvic or lumbar stabilization (in supine) | |
| Lumbar extensor strengthening or stabilization | |
| Abdominal or oblique stabilization or strengthening | |
| Physioball activities | |
| Aerobic exercise | |
| Biking | |
| Treadmill | |
| Manual techniques | |
| Manual scar mobilization | |
| Myofascial release |
Spinal Accessory Nerve Palsy
The severity and distribution of trapezius weakness secondary to spinal accessory nerve palsy (Figure 57-7) are subject to great individual variability. The upper, middle, and lower trapezius muscles can be innervated solely by the spinal accessory nerve or receive partial or total innervation from the cervical plexus.22 When the spinal accessory nerve was routinely sacrificed during radical neck dissections, some patients developed little to no shoulder compromise, suggesting that innervation was predominantly derived from the cervical plexus. Baseline anatomic variability is compounded by inconsistency in the type and degree of intraoperative nerve injury. The spinal accessory nerve can be entirely spared or subject to neurapraxic, axonotmetic, or neurotmetic insult, all with different rates and degrees of recovery. Electrocautery of blood vessels can undermine blood supply to the vasonervorum, producing ischemic nerve injury.
Rehabilitative efforts should by constantly informed by the rate of reinnervation. Patients with complete, persistent spinal accessory nerve palsy can be fitted with an orthosis. To date, none of the braces designed to substitute for absent or weak trapezius muscles have enjoyed widespread success. The relevant literature does not extend beyond limited case reports. For patients plagued by levator scapulae fatigue and spasms, a “shelf” orthosis (Figure 57-8) designed to encircle the waist, and to provide a ledge on which patients can rest their affected arms when not in use, reduces symptoms.
Cervical Contracture
Progressive fibrosis of the anterior and lateral cervical soft tissue can be highly problematic for head and neck cancer patients, particularly those who receive external beam radiation. A general approach to radiation-induced fibrosis was outlined previously in this chapter. Because of the high radiation doses delivered to some head and neck cancer patients, proactive ROM in all planes of neck motion should be initiated as soon as safely possible. Cervical ROM can continue throughout radiation therapy in the absence of significant skin breakdown. Ranging activities should ideally begin immediately after surgery and before radiation. The delicate balance between flexibility and postsurgical wound healing must be respected. Surgeons should be consulted regarding the length of the postoperative interval before ROM activity can begin. For an uncomplicated radical or functional neck dissection, 3 days is generally considered safe. Reconstruction with tissue transposition requires a longer recovery period. ROM activities should be delayed until all drains are removed to avoid seroma formation.
Irradiated patients should perform ROM activities twice daily during the first 2 years after cancer treatment and daily thereafter. As previously mentioned, radiation-induced fibrosis can be indefinitely progressive. Figure 57-9 demonstrates the head-forward posture and thoracic kyphosis characteristic of head and neck cancer patients with severe anterior cervical soft tissue fibrosis. For optimal results, patients should be taught to provide additional stretch during end-range lateral bending or rotation by exerting additional pressure with the contralateral hand. Stretches should be held for five deep breaths and repeated between 5 and 10 times per session. Isometric strengthening of the cervical extensors and postural modification with visual cuing are beneficial.
Manual fibrous release techniques are indicated when ROM is restricted by robust soft tissue fibrosis or tethering of the skin to subdermal tissues. Patients can be taught self-massage to augment the efficacy of ROM activities. Compression of severely fibrotic areas breaks down established scar tissue and inhibits re-formation. Compression garments, either off the shelf or customized, are a convenient means of applying compression. Custom-cut foam pieces that are strategically inserted can achieve greater focal pressure on stubborn areas. Constant vigilance must be maintained to ensure that friable, irradiated skin is not compromised. Botulinum toxin injection can be trialed in refractory cases.248
Aphonia and Dysphonia
Various approaches to restore communication can be used depending on the anticipated duration, severity, and nature of the deficit. The most common compensatory strategies used by acutely voiceless adults include mouthing words, gestures, writing, and head nods.8,92 Patients rendered chronically aphonic by total laryngectomy can communicate through esophageal speech, tracheoesophageal speech, or use of an electrolarynx. The frequency with which these options are offered to and accepted by patients varies considerably across physician practices, institutions, and geographic regions.165 The following frequencies for different types of alaryngeal speech have been reported: esophageal speech, 1% to 32%; tracheoesophageal speech, 20% to 45%; electrolarynx, 0% to 50%; and nonvocal, 17% to 26% (see Chapter 3).79,103,165,241
Both esophageal and tracheoesophageal speech use the oropharynx, lips, and tongue to produce intelligible sound. Esophageal speech is time consuming and difficult to learn. Among a cohort of laryngectomized esophageal speakers, a subjectively “good enough” result was achieved by 41% in less than 6 months, by an additional 20% in 6 to 12 months, and by a further 10% in more than 12 months.278 Despite the challenges of mastery, tracheoesophageal puncture, or the TEP procedure, represents an increasingly common approach to voice restoration. The TEP procedure creates a stoma between the trachea and esophagus. A one-way valved prosthesis is inserted in the stoma, allowing pulmonary air to enter the esophageal reservoir when patients manually occlude their tracheostomies. Although guttural, the resultant speech can be exceedingly intelligible, with natural-sounding inflection. Compared with other types of alaryngeal speech, TEP speakers most closely approximate the frequency and intensity of the normal voice.104 In two surveys, TEP speech received higher satisfaction ratings than alternative methods, particularly with telephone use.41,278
For patients who elect not to undergo TEP, speech is most often accomplished through use of an electrolarynx. Many head and neck cancer patients who fail to achieve intelligible esophageal speech eventually opt for an artificial larynx. The currently marketed devices vary with respect to placement of the transducer. Some sense vibrations within the oral cavity, whereas others are placed on the submental or buccal skin. Training is essential to the achievement of acceptable voice quality. Various transducers should be trialed because patients’ preferences vary. A significant downside of electrolaryngeal speech is its mechanical and monotonic quality.
Precautions in Cancer Rehabilitation
Modalities
A climate of exaggerated caution too often limits cancer rehabilitation. Specific therapeutic precautions reflect a fear of injuring patients, or worse, spreading their cancer. Although it is important to appreciate that cancer patients are predisposed to a host of adverse complications (e.g., hemorrhage and disease recurrence), it is equally important to recognize that a causal relationship has not been established between such complications and physiatric interventions. Inactivity causes far greater long-term difficulty for the majority of cancer patients. Most precautions are not supported by empiric data, and they frequently reinforce ambivalence toward structured, incremental physical activity.
Questioning the current rigid precautions against the use of heating modalities in cancer patients might be moot. Deep heat is rarely of clinical usefulness, or therapeutic goals can be realized by alternative means. If the clinical context warrants a trial of ultrasound or related modalities, however, the option should not be reflexively abandoned because of unsubstantiated warnings. In the author’s experience, patients with widespread tumor have benefited from the discrete use of ultrasound in areas of dense radiation-induced fibrosis and postsurgical scarring. Massage has the potential to greatly benefit cancer patients through its antispasmodic, fibrolytic, and counterstimulatory effects. Additionally, MLD is an integral part of lymphedema management. Aside from vigorous massage in the immediate vicinity of established tumor, massage is likely to be of far greater benefit than harm.
1. Abramsen L., Midtgaard J., Rorth M., et al. Feasibility, physical capacity, and health benefits of a multidimensional exercise program for cancer patients undergoing chemotherapy. Support Care Cancer. 2003;11:707-716.
2. Adamsen L., Quist M., Andersen C., et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410.
3. Agency for Health Care Policy and Research. Acute pain management: operative or medical procedures and trauma. Washington, DC: U.S. Departmente of Health and Human Services; 1992.
4. Ahmed R.L., Thomas W., Yee D., et al. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24:2765-2772.
5. Alamowitch S., Graus F., Uchuya M., et al. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120(Pt 6):923-928.
6. Altekruse SF, Kosary CL, Krapcho M, et al., editors: SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda, MD, http://seer.canser.gov/csr/1975_2007/. Based on November 2009 SEER data submission, posted to the SEER web site, 2010. Accessed October 1, 2010
7. American Cancer Society: Cancer facts & figures. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf. Accessed October 1, 2010
8. Ashworth P.M. Staff-patient communication in coronary care units. J Adv Nurs. 1984;9:35-42.
9. Baldwin B.J., Schusterman M.A., Miller M.J., et al. Bilateral breast reconstruction: conventional versus free TRAM. Plast Reconstr Surg. 1994;93:1410-1416. discussion 1417
10. Barrett-Lee P.J., Bailey N.P., O’Brien M.E., et al. Large-scale UK audit of blood transfusion requirements and anaemia in patients receiving cytotoxic chemotherapy. Br J Cancer. 2000;82:93-97.
11. Bataller L., Graus F., Saiz A., et al. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain. 2001;124:437-443.
12. Benedetti C., Brock C., Cleeland C., et al. NCCN Practice Guidelines for Cancer Pain. Oncology (Williston Park). 2000;14:135-150.
13. Bernas M.J., Witte C.L., Witte M.H. The diagnosis and treatment of peripheral lymphedema: draft revision of the 1995 Consensus Document of the International Society of Lymphology Executive Committee for discussion at the September 3-7, 2001, XVIII International Congress of Lymphology in Genoa, Italy. Lymphology. 2001;34:84-91.
14. Bernat J.L., Greenberg E.R., Barrett J. Suspected epidural compression of the spinal cord and cauda equina by metastatic carcinoma. Clinical diagnosis and survival. Cancer. 1983;51:1953-1957.
15. Beurskens C.H., van Uden C.J., Strobbe L.J., et al. The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer. 2007;7:166.
16. Bicego D., Brown K., Ruddick M., et al. Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J. 2009;15:45-51.
17. Boccardo M., Ruelle A., Mariotti E., et al. Spinal carcinomatous metastases: retrospective study of 67 surgically treated cases. J Neurooncol. 1985;3:251-257.
18. Bohlius J., Schmidlin K., Brillant C., et al. Erythropoietin or Darbepoetin for patients with cancer–meta-analysis based on individual patient data. Cochrane Database Syst Rev. 2009. CD007303
19. Box R.C., Reul-Hirche H.M., Bullock-Saxton J.E., et al. Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res Treat. 2002;75:35-50.
20. Bradley W.G., Lassman L.P., Pearce G.W., et al. The neuromyopathy of vincristine in man: clinical, electrophysiological and pathological studies. J Neurol Sci. 1970;10:107-131.
21. Brennan K.M., Roos M.S., Budinger T.F., et al. A study of radiation necrosis and edema in the canine brain using positron emission tomography and magnetic resonance imaging. Radiat Res. 1993;134:43-53.
22. Brown H. Anatomy of the spinal accessory nerve plexus: relevance to head and neck cancer and atherosclerosis. Exp Biol Med (Maywood). 2002;227:570-578.
23. Bruera E., Driver L., Barnes E.A., et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol. 2003;21:4439-4443.
24. Bruera E., Roca E., Cedaro L., et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep. 1985;69:751-754.
25. Bruera E., Valero V., Driver L., et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24:2073-2078.
26. Buchbinder R., Osborne R.H., Ebeling P.R., et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568.
27. Butler J.M.Jr., Case L.D., Atkins J., et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69:1496-1501.
28. Campos S.M., Ghosh S. A current review of targeted therapeutics for ovarian cancer. J Oncol. 2010;149362:2010.
29. Caraceni A., Portenoy R.K. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain. 1999;82:263-274.
30. Carla C., Verstappen J., Heimans K., et al. Neurotoxin complications of chemotherapy in patients with cancer. Drugs. 2003;63:1549-1563.
31. Casley-Smith JR: Modern treatment of lymphedema. 1. Complex physical therapy: the first 200 Australian limbs, Australas J Dermatol 33:61-68, 1992.
32. Casley-Smith: J.R., Boris M., Weindorf S., Lasinski B. Treatment for lymphedema of the arm–the Casley-Smith method: a noninvasive method produces continued reduction. Cancer. 1998;83:2843-2860.
33. Casley-Smith J.R., Casley-Smith J.R. The pathophysiology of lymphedema and the action of benzo-pyrones in reducing it. Lymphology. 1988;21:190-194.
34. Cella D. Factors influencing quality of life in cancer patients: anemia and fatigue. Semin Oncol. 1998;25:43-46.
35. Chalk C.H., Murray N.M., Newsom-Davis J., O’Neill J.H., Spiro S.G. Response of the Lambert-Eaton myasthenic syndrome to treatment of associated small-cell lung carcinoma. Neurology. 1990;40:1552-1556.
36. Chambers W.A. Nerve blocks in palliative care. Br J Anaesth. 2008;101:95-100.
37. Chang E.L., Wefel J.S., Hess K.R., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037-1044.
38. Cheema B., Gaul C.A., Lane K., et al. Progressive resistance training in breast cancer: a systematic review of clinical trials. Breast Cancer Res Treat. 2008;109:9-26.
39. Chen H., Brahmer J. Management of malignant pleural effusion. Curr Oncol Rep. 2008;10:287-293.
40. Chun YS, Sinha I, Turko A, et al. Outcomes and patient satisfaction following breast reconstruction with bilateral pedicled TRAM flaps in 105 consecutive patients, Plast Reconstr Surg 125:1-9
41. Clements K., Rassekh C., Seikaly H., et al. Communications after laryngectomy: an assessment of patient satisfaction. Otolaryngol Head Neck Surg. 1997;123:493-496.
42. Cole J.S., Patchell R.A. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7:459-466.
43. Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
44. Costigan D.A., Winkelman M.D. Intramedullary spinal cord metastasis: a clinicopathological study of 13 cases. J Neurosurg. 1985;62:227-233.
45. Courneya K.S., Friedenreich C.M., Quinney H.A., et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl). 2003;12:347-357.
46. Cramp F., Daniel J.: Exercise for the management of cancer-related fatigue in adults, Cochrane Database Syst Rev CD006145, 2008
47. Crotty E., Patz E.F.Jr. FDG-PET imaging in patients with paraneoplastic syndromes and suspected small cell lung cancer. J Thorac Imaging. 2001;16:89-93.
48. Cunningham A., Morris G., Cheney C. Effects of resistance exercise on skeletal muscle in marrow transplant recipients receiving total parenteral nutrition. J Parenter Enteral Nutr. 1986;10:558-563.
49. Dahele M., Skipworth R.J., Wall L., et al. Objective physical activity and self-reported quality of life in patients receiving palliative chemotherapy. J Pain Symptom Manage. 2007;33:676-685.
50. Dalmau J., Graus F., Marco M. ‘Hot and dry foot’ as initial manifestation of neoplastic lumbosacral plexopathy. Neurology. 1989;39:871-872.
51. Davidson R.S., Nwogu C.E., Brentjens M.J., et al. The surgical management of pulmonary metastasis: current concepts. Surg Oncol. 2001;10:35-42.
52. De Backer I.C., Schep G., Backx F.J., et al. Resistance training in cancer survivors: a systematic review. Int J Sports Med. 2009;30:703-712.
53. De Marinis F., Eberhardt W., Harper P.G., et al. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a european expert panel. J Thorac Oncol. 2009.
54. DeAngelis L.M., Delattre J.Y., Posner J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
55. Delanian S., Lefaix J.L. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17:99-107.
56. Delanian S., Porcher R., Balla-Mekias S., et al. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21:2545-2550.
57. DiChiro G., Oldfield E., Wright D., et al. Cerebral necrosis after radiotherapy and/or intracranial chemotherapy for brain tumors: PET and neuropathic studies. Am J Roentgenol. 1988;150:189-197.
58. Didelot A., Honnorat J. Update on paraneoplastic neurological syndromes. Curr Opin Oncol. 2009;21:566-572.
59. Dimeo F., Fetscher S., Lange W., et al. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90:3390-3394.
60. Dimeo F., Rumberger B., Keul J. Aerobic exercise as therapy for cancer fatigue. Med Sci Sports. 1998;30:475-478.
61. Dimeo F.C., Stieglitz R.D., Novelli-Fischer U., et al. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273-2277.
62. Dische S., Warburton M.F., Saunders M.I. Radiation myelitis and survival in the radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys. 1988;15:75-81.
63. Donnelly S., Walsh D. The symptoms of advanced cancer. Semin Oncol. 1995;22:67-72.
64. Dy S.M., Asch S.M., Naeim A., et al. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26:3879-3885.
65. Ebner I., Anderl H., Mikuz G., et al. [Plexus neuropathy: tumor infiltration or radiation damage]. Rofo. 1990;152:662-666.
66. Eichler A.F., Loeffler J.S. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884-898.
67. Erdek M.A., Halpert D.E., Fernandez M.G., et al. assessment of celiac plexus block and neurolysis outcomes and technique in the management of refractory visceral cancer pain. Pain Med. 2009.
68. Fajardo L.F. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13-22.
69. Fisch M. Treatment of depression in cancer. J Natl Cancer Inst Monogr. 2004;(32:):105-111.
70. Foldi E., Foldi M., Weissleder H. Conservative treatment of lymphoedema of the limbs. Angiology. 1985;36:171-180.
71. Foldi M., Foldi E. Komplexe physikalische enstauungstherapie des chronischen gliedmaben lymphnodems. Folia Angiol. 1981;29:161-168.
72. Foley K.M. The treatment of cancer pain. N Engl J Med. 1985;313:84-95.
73. Freilich R.J., Balmaceda C., Seidman A.D., et al. Motor neuropathy due to docetaxel and paclitaxel. Neurology. 1996;47:115-118.
74. Gainor G., Buchert P. Fracture healing in metastatic bone disease. Clin Orthop. 1983;178:297-302.
75. Galer B.S., Coyle N., Pasternak G.W., et al. Individual variability in the response to different opioids: report of five cases. Pain. 1992;49:87-91.
76. Galvao DA, Taaffe DR, Spry N, et al: Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial, J Clin Oncol 28:340-347.
77. Gaspar L.C., Scott M., Rotman S., et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
78. Gavrilovic I.T., Posner J.B. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5-14.
79. Geraghty J.A., Wenig B.L., Smith B.E., et al. Long-term follow-up of tracheoesophageal puncture results. Ann Otol Rhinol Laryngol. 1996;105:501-503.
80. Gerber L., Hicks J., Klaiman M., et al. Rehabilitation in the cancer patient. In: Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams and Wilkins; 1997.
81. Gilbert R.W., Kim J.H., Posner J.B. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3:40-51.
82. Glantz M.J., Burger P.C., Friedman A.H., et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020-2027.
83. Glantz M.J., Cole B.F., Forsyth P.A., et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886-1893.
84. Glantz M.J., Cole B.F., Friedberg M.H., et al. A randomized, blinded, placebo-controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology. 1996;46:985-991.
85. Grivas A.A., Trafalis D.T., Athanassiou A.E. Implication of bevacizumab in fatal arterial thromboembolic incidents. J BUON. 2009;14:115-117.
86. Guo Y., Persyn L., Palmer J.L., et al. Incidence of and risk factors for transferring cancer patients from rehabilitation to acute care units. Am J Phys Med Rehabil. 2008;87:647-653.
87. Gutstein H. The biological basis for fatigue. Cancer. 2001;92:1678-1683.
88. Gybels J., Swet W. Neurosurgical treatment of persistent pain: physiological and pathological mechanisms of human pain. Basel: Karger; 1989.
89. Haim N., Epelbaum R., Ben-Shahar M., et al. Full dose vincristine (without 2-mg dose limit) in the treatment of lymphomas. Cancer. 1994;73:2515-2519.
90. Hall S.M., Buzdar A.U., Blumenschein G.R. Cranial nerve palsies in metastatic breast cancer due to osseous metastasis without intracranial involvement. Cancer. 1983;52:180-184.
91. Hanna A., Sledge G., Mayer M.L., et al. A phase II study of methylphenidate for the treatment of fatigue. Support Care Cancer. 2006;14:210-215.
92. Happ M.B. Interpretation of nonvocal behavior and the meaning of voicelessness in critical care. Soc Sci Med. 2000;50:1247-1255.
93. Harper C., Thomas J., Cascino T., et al. Distinction between neoplastic and radiation-induced brachial plexopathy with emphasis on role of EMG. Neurology. 1989;39:502-506.
94. Harrington K: Metastatic disease of the spine, J Bone Joint Surg Am 68: 1110-1115, 1988.
95. Hassenbusch S.J. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology (Williston Park). 1999;13:63-67.
96. Hauer-Jensen M., Fink L.M., Wang J. Radiation injury and the protein C pathway. Crit Care Med. 2004;32:S325-S330.
97. Hayes S., Davies P.S., Parker T., et al. Total energy expenditure and body composition changes following peripheral blood stem cell transplantation and participation in an exercise programme. Bone Marrow Transplant. 2003;31:331-338.
98. Healey J.H., Brown H.K. Complications of bone metastases: surgical management. Cancer. 2000;88:2940-2951.
99. Heim M.E., v d Malsburg M.L., Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor-related chronic fatigue. Onkologie. 2007;30:429-434.
100. Herrero F., San Juan A.F., Fleck S.J., et al. Combined aerobic and resistance training in breast cancer survivors: a randomized, controlled pilot trial. Int J Sports Med. 2006;27:573-580.
101. Hershman D.L., Shao T. Anthracycline cardiotoxicity after breast cancer treatment. Oncology (Williston Park). 2009;23:227-234.
102. Hickok J.T., Roscoe J.A., Morrow G.R., et al. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772-1778.
103. Hillman R.E., Walsh M.J., Wolf G.T., et al. Functional outcomes following treatment for advanced laryngeal cancer. Part I. Voice preservation in advanced laryngeal cancer. Part II. Laryngectomy rehabilitation: the state of the art in the VA System. Research Speech-Language Pathologists. Department of Veterans Affairs Laryngeal Cancer Study Group. Ann Otol Rhinol Laryngol Suppl. 1998;172:1-27.
104. Hillner B., Ingle N., Berenson J., et al. American Society of Clinical Oncology guideline in the role of bisphosphonates in breast cancer: American Society of Clinical Oncology Bisphosphonates Expert Panel. J Clin Oncol. 2000;18:1378-1391.
105. Homsi J., Nelson K.A., Sarhill N., et al. A phase II study of methylphenidate for depression in advanced cancer. Am J Hosp Palliat Care. 2001;18:403-407.
106. Honnorat J., Cartalat-Carel S. Advances in paraneoplastic neurological syndromes. Curr Opin Oncol. 2004;16:614-620.
107. Horner MJ, Ries LAG, Krapcho M, et al. (eds). SEER cancer statistics review, 1975-2006. Available at: http://seer.cancer.gov/csr/1975_2006/. Accessed 2009.
108. Huang M.E., Cifu D.X., Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79:1386-1390.
109. Huang M.E., Cifu D.X., Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79:327-335.
110. Huang M.E., Wartella J.E., Kreutzer J.S. Functional outcomes and quality of life in patients with brain tumors: a preliminary report. Arch Phys Med Rehabil. 2001;82:1540-1546.
111. Hutnick N.A., Williams N.I., Kraemer W.J., et al. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005;37:1827-1835.
112. Indelicato R.A., Portenoy R.K. Opioid rotation in the management of refractory cancer pain. J Clin Oncol. 2002;20:348-352.
113. Ischia S., Ischia A., Luzzani A., et al. Results up to death in the treatment of persistent cervico-thoracic (Pancoast) and thoracic malignant pain by unilateral percutaneous cervical cordotomy. Pain. 1985;21:339-355.
114. Jacobsen P.B., Donovan K.A., Vadaparampil S.T., et al. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26:660-667.
115. Jacox A., Carr D.B., Payne R. New clinical-practice guidelines for the management of pain in patients with cancer. N Engl J Med. 1994;330:651-655.
116. Jaeckle K.A. Neurological manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol. 2004;24:385-393.
117. Jaeckle K.A., Young D.F., Foley K.M. The natural history of lumbosacral plexopathy in cancer. Neurology. 1985;35:8-15.
118. Jellinger K., Radiaszkiewicz T. Involvement of the central nervous system in malignant lymphomas. Virchows Arch A Pathol Anat Histol. 1976;370:345-362.
119. Jemal A., Siegel R., Ward E., et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
120. Jerian S.M., Sarosy G.A., Link C.J.Jr., et al. Incapacitating autonomic neuropathy precipitated by taxol. Gynecol Oncol. 1993;51:277-280.
121. Johnson J.R., Miller A.J. The efficacy of choline magnesium trisalicylate (CMT) in the management of metastatic bone pain: a pilot study. Palliat Med. 1994;8:129-135.
122. Kaleita T.A., WD, Graham C.A., et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors. J Clin Oncol ASCO Meeting Abstract. 1503, 2006.
123. Kallmes D.F., Comstock B.A., Heagerty P.J., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569-579.
124. Kemeny M.M. Continuous hepatic artery infusion (CHAI) as treatment of liver metastases: are the complications worth it? Drug Saf. 1991;6:159-165.
125. Khuntia D., Brown P., Li J., et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295-1304.
126. Killer H.E., Hess K. Natural history of radiation-induced brachial plexopathy compared with surgically treated patients. J Neurol. 1990;237:247-250.
127. Kirshbaum M.N. A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2007;16:104-121.
128. Kirsten J., Schimmel K., Richel D., et al. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30:181-191.
129. Knols R., Aaronson N.K., Uebelhart D., et al. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23:3830-3842.
130. Ko D.S., Lerner R., Klose G., et al. Effective treatment of lymphedema of the extremities. Arch Surg. 1998;133:452-458.
131. Kolden G.G., Strauman T.J., Ward A., et al. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psychooncology. 2002;11:447-456.
132. Kori S.H., Foley K.M., Posner J.B. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31:45-50.
133. Kori SH, Foley KM, Posner JB: Brachial plexus lesions in patients with cancer: 100 cases. Neurology 1981; 31(1):45-50
134. Krathen R.A., Orengo I.F., Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
135. Kreisman H., Wolkove N., Finkelstein H.S., et al. Breast cancer and thoracic metastases: review of 119 patients. Thorax. 1983;38:175-179.
136. Kroll S.S., Shusterman M.A., Reece G.P., et al. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96:616-619.
137. Kuchinski A.M., Reading M., Lash A.A. Treatment-related fatigue and exercise in patients with cancer: a systematic review. Medsurg Nurs. 2009;18:174-180.
138. Kvale P.A., Simoff M., Prakash U.B. Lung cancer. Palliative care. Chest. 2003;123:284S-311S.
139. Lauridsen M.C., Christiansen P., Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol. 2005;44:449-457.
140. Lauridsen M.C., Overgaard M., Overgaard J., et al. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47:569-575.
141. Leduc O., Klein P., Demaret P., et al: Dynamic pressure variation under bandages with different stiffness. In Boccalon H, editor: Vascular medicine, Amsterdam, 1993, Elsevier
142. Leduc O., Peeters A., Bourgeois P.: Bandages: scintigraphic demonstration of its efficacy on colloidal protein reabsorption during muscular activity. In Nishi M, Uctino S, Yabuki S, editors: Progress in lymphology, Tokyo, 1993, Congress Book
143. Lee B.N., Dantzer R., Langley K.E., et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279-292.
144. Leidenius M., Leivonen M., Vironen J., et al. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92:23-31.
145. Levy M.H. Pharmacologic treatment of cancer pain. N Engl J Med. 1996;335:1124-1132.
146. Lipton A. Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol. 2004;2:205-213. discussion 213-204, 216-207, 219–220
147. Liu A.K., Macy M.E., Foreman N.K. Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys. 2009;75:1148-1154.
148. LoMonaco M., Milone M., Batocchi A.P., et al. Cisplatin neuropathy: clinical course and neurophysiological findings. J Neurol. 1992;239:199-204.
149. Lotze M.T., Duncan M.A., Gerber L.H., et al. Early versus delayed shoulder motion following axillary dissection: a randomized prospective study. Ann Surg. 1981;193:288-295.
150. Lower E.FS, Cooper A., et al: A phase III, randomized placebo-controlled trial of the safety and efficacy of d-MPH as a new treatment of fatigue and “chemobrain” in adult cancer patients, J Clin Oncol Proceedings of the 2005 ASCO Annual Meeting, 23(16 suppl):8000, 2005
151. Lower E.E., Fleishman S., Cooper A., et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38:650-662.
152. Luctkar-Flude M.F., Groll D.L., Tranmer J.E., et al. Fatigue and physical activity in older adults with cancer: a systematic review of the literature. Cancer Nurs. 2007;30:E35-E45.
153. MacVicar M.G., Winningham M.L., Nickel J.L. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989;38:348-351.
154. Manon R., O’Neill A., Knisely J., et al. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol. 2005;23:8870-8876.
155. Mar Fan H.G., Clemons M., Xu W., et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577-583.
156. Marciniak C.M., Sliwa J.A., Heinemann A.W., et al. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82:457-463.
157. Marks J.E., Wong J. The risk of cerebral radionecrosis in relation to dose, time and fractionation: a follow-up study. Prog Exp Tumor Res. 1985;29:210-218.
158. Martinez-Zapata M.J., Roque M., Alonso-Coello P., et al. Calcitonin for metastatic bone pain. Cochrane Database Syst Rev.. 2006;3:CD003223.
159. May A.M., Van Weert E., Korstjens I., et al. Improved physical fitness of cancer survivors: a randomised controlled trial comparing physical training with physical and cognitive-behavioural training. Acta Oncol. 2008;47:825-834.
160. McKinley W.O., Conti-Wyneken A.R., Vokac C.W., et al. Rehabilitation functional outcome of patients with neoplastic spinal cord compressions. Arch Phys Med Rehabil. 1996;77:892-895.
161. McKinley W.O., Huang M.E., Tewksbury M.A. Neoplastic vs. traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79:138-144.
162. McLeod, JG: Peripheral neuropathy associated with lymphomas, leukemias, and polycythemia vera. In Dyck PJ, Thomas PK, editors: Peripheral neuropathy, philadelphia, 1993, WB Saunders.
163. McNeely M.L., Campbell K.L., Rowe B.H., et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34-41.
164. McNeely M.L., Parliament M.B., Seikaly H., et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer. 2008;113:214-222.
165. Mendenhall W.M., Morris C.G., Stringer S.P., et al. Voice rehabilitation after total laryngectomy and postoperative radiation therapy. J Clin Oncol. 2002;20:2500-2505.
166. Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer. 1999;86:1856-1866.
167. Mercadante S., Fulfaro F. Management of painful bone metastases. Curr Opin Oncol. 2007;19:308-314.
168. Miaskowski C., Cleary J., Burney R., et al. Guideline for the management of Cancer pain in adults and children. Glenview, IL: American Pain Society; 2005.
169. Milne H.M., Wallman K.E., Gordon S., et al. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108:279-288.
170. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989:256-264.
171. Mizgala C.L., Hartrampf C.R.Jr., Bennett G.K. Abdominal function after pedicled TRAM flap surgery. Clin Plast Surg. 1994;21:255-272.
172. Mock V., Dow K.H., Meares C.J., et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24:991-1000.
173. Mock V., Pickett M., Ropka M.E., et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9:119-127.
174. Monninkhof E.M., Elias S.G., Vlems F.A., et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137-157.
175. Monteiro M. Physical therapy implications following the TRAM procedure. Phys Ther. 1997;77:765-770.
176. Morgan R.G., Casley-Smith J.R., Mason M.R., et al. Complex physical therapy for the lymphoedematous arm. J Hand Surg Br. 1992;17:437-441.
177. Morrow G.R., Gilles L.J., Hickok J.T., et al. The positive effect of the psycho-stimulant modafinil on fatigue from cancer that persists after treatment is completed. J Clin Oncol Proceedings of the 2005 ASCO Annual meetings,. 2005;23(16 suppl):8012.
178. Moskovitz A.H., Anderson B.O., Yeung R.S., et al. Axillary web syndrome after axillary dissection. Am J Surg. 2001;181:434-439.
179. Mustian K.M., Morrow G.R., Carroll J.K., et al: Integrative nonpharmacologic behavioral interventions for the management of cancer-related fatigue, Oncologist 12(suppl 1):52-67, 2007
180. Na Y.M., Kim M.Y., Kim Y.K., et al. Exercise therapy effect on natural killer cell cytotoxic activity in stomach cancer patients after curative surgery. Arch Phys Med Rehabil. 2000;81:777-779.
181. National Comprehensive Cancer Network Clinical practice guidelines in oncology: Cancer-related fatigue, v1.2006. Available at: http://www.nccn.org/professionals/physician_glf/PDF/fatigue.pdf. Accessed 2006.
182. Nieman D.C., Cook V.D., Henson D.A., et al. Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. Int J Sports Med. 1995;16:334-337.
183. O’Dell M.W., Barr K., Spanier D., et al. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79:1530-1534.
184. Oldervoll L.M., Kaasa S., Knobel H., et al. Exercise reduces fatigue in chronic fatigued Hodgkins disease survivors: results from a pilot study. Eur J Cancer. 2003;39:57-63.
185. Olsen N.K., Pfeiffer P., Mondrup K., et al. Radiation-induced brachial plexus neuropathy in breast cancer patients. Acta Oncol. 1990;29:885-890.
186. Olszewski W., Engeset A.: Vasomotoric function of lymphatics and lymph transport in limbs during massage and with elastic support. In Partsch H, editor: Progress in lymphology XI, Amsterdam, 1988, Elsevier
187. Organization W.H. Cancer pain relief and palliative care: report of a WHO expert committee. Geneva: World Health Organization; 1990.
188. Paige K.T. J., Bostwick, Bried J.T.3rd, et al. A comparison of morbidity from bilateral, unipedicled and unilateral, unipedicled TRAM flap breast reconstructions. Plast Reconstr Surg. 1998;101:1819-1827.
189. Paling M.R., Black W.C., Levine P.A., et al. Tumor invasion of the anterior skull base: a comparison of MR and CT studies. J Comput Assist Tomogr. 1987;11:824-830.
190. Partsch H. Do we need firm compression stocking exerting high pressure? Vasa. 1984;13:52-57.
191. Partsch H. Verbesserte forderleistung der wadenmuskelpumpe unter kompressionstrumpfen bei varizen und venoser insuffizienz. Phlebol Proktol. 1978;7:58.
192. Pavinen L., Kikku P., Maekinen E., et al. Factors affecting the pulmonary toxicity of bleomycin. Acta Radiol. 1983;22:417.
193. Peacock K.H., Lesser G.J. Current therapeutic approaches in patients with brain metastases. Curr Treat Options Oncol. 2006;7:479-489.
194. Peters C., Lotzerich H., Niemeier B., et al. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer Res. 1994;14:1033-1036.
195. Peters C., Lotzerich H., Niemeir B., et al. Exercise, cancer and the immune response of monocytes. Anticancer Res. 1995;15:175-179.
196. Pierce S.M., Recht A., Lingos T.I., et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915-923.
197. Pohlers D., Brenmoehl J., Loffler I., et al. TGF-beta and fibrosis in different organs – molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746-756.
198. Portenoy R., Kornblith A., Wong G. Pain in ovarian cancer patients: prevalence, characteristics, and associated symptoms. Cancer. 1994;74:907-915.
199. Portenoy R., Miransky J., Thaler H. Pain in ambulatory patients with lung or colon cancer: prevalence, characteristics, and effect. Cancer. 1992;70:1616-1624.
200. Posner J. Spinal metastases: clinical findings. Philadelphia: FA Davis, 1995.
201. Posner J. Management of brain metastases. In: Neurological complications of cancer. Philadelphia: Davis; 1992.
202. Posner J.B.: Cancer involving cranial and peripheral nerves. In Neurological complications of cancer, Philadelphia, 1995, FA Davis
203. Posner J.B.: Side effects of radiation therapy. In Neurological complications of cancer, Philadelphia, 1995, FA Davis
204. Post J., Quencer R., Green B., et al. Intramedullary spinal cord metastases, mainly of nonneurogenic origin. Am J Roentgenol. 1987;148:1015-1022.
205. Postma T.J., Vermorken J.B., Liefting A.J., et al. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6:489-494.
206. Potts D., Zimmerman R. Nuclear magnetic resonance imaging of skull base lesions. J Neurol Sci. 1975;12:327-331.
207. Powell S., Cooke J., Parsons C. Radiation-induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol. 1990;18:213-220.
208. Pritchard J., Anand P., Broome J., et al. Double-blind randomized phase II study of hyperbaric oxygen in patients with radiation-induced brachial plexopathy. Radiother Oncol. 2001;58:279-286.
209. Ransom D.T., Dinapoli R.P., Richardson R.L. Cranial nerve lesions due to base of the skull metastases in prostate carcinoma. Cancer. 1990;65:586-589.
210. Riccio A.I., Wodajo F.M., Malawer M. Metastatic carcinoma of the long bones. Am Fam Physician. 2007;76:1489-1494.
211. Rider C.A. Oral mucositis. A complication of radiotherapy. N Y State Dent J. 1990;56:37-39.
212. Rizzo J.D., Somerfield M.R., Hagerty K.L., et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26:132-149.
213. Rodgers L., Borkowski G., Albers J., et al. Obturator mononeuropathy caused by pelvic cancer: six cases. Neurology. 1993;43:1489-1492.
214. Rollason V., Samer C., Piguet V., et al. Pharmacogenetics of analgesics: toward the individualization of prescription. Pharmacogenomics. 2008;9:905-933.
215. Ross J.R., Saunders Y., Edmonds P.M., et al. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ. 2003;327:469.
216. Rotstein J., Good R. Steroid pseudorheumatism. Arch Intern Med. 1957;99:545-555.
217. Rowinsky E.K., Chaudhry V., Cornblath D.R., et al. Neurotoxicity of Taxol. J Natl Cancer Inst Monogr 107-115. 1993.
218. Ruifrok A.C., Kleiboer B.J., van der Kogel A.J. Radiation tolerance and fractionation sensitivity of the developing rat cervical spinal cord. Int J Radiat Oncol Biol Phys. 1992;24:505-510.
219. Ryan J.L., Carroll J.K., Ryan E.P., et al. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22-34.
220. Saarto T., Janes R., Tenhunen M., et al. Palliative radiotherapy in the treatment of skeletal metastases. Eur J Pain. 2002;6:323-330.
221. Sabino M.A., Ghilardi J.R., Jongen J.L., et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002;62:7343-7349.
222. Sahenk Z., Barohn R., New P., et al. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch Neurol. 1994;51:726-729.
223. Saito O., Aoe T., Yamamoto T. Analgesic effects of nonsteroidal antiinflammatory drugs, acetaminophen, and morphine in a mouse model of bone cancer pain. J Anesth. 2005;19:218-224.
224. Sarhill N., Walsh D., Nelson K.A., et al. Methylphenidate for fatigue in advanced cancer: a prospective open-label pilot study. Am J Hosp Palliat Care. 2001;18:187-192.
225. Schmitz K.H., Ahmed R.L., Troxel A., et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664-673.
226. Schmitz K.H., Holtzman J., Courneya K.S., et al. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588-1595.
227. Schouten L.J., Rutten J., Huveneers H.A., et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698-2705.
228. Schultheiss T.E., Stephens L.C., Peters L.J. Survival in radiation myelopathy. Int J Radiat Oncol Biol Phys. 1986;12:1765-1769.
229. Schwartz A.L. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract. 2000;8:16-24.
230. Schwartz A.L. Fatigue mediates the effects of exercise on quality of life. Qual Life Res. 1999;8:529-538.
231. Schwartz A.L., Mori M., Gao R., et al. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med Sci Sports Exerc. 2001;33:718-723.
232. Schwartz A.L., Thompson J.A., Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum. 2002;29:E85-E90.
233. Segal R., Evans W., Johnson D., et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657-665.
234. Segal R.J., Reid R.D., Courneya K.S., et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653-1659.
235. Segal R.J., Reid R.D., Courneya K.S., et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344-351.
236. Siegal T., Haim N. Cisplatin-induced peripheral neuropathy: frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer. 1990;66:1117-1123.
237. Silvestri F., Bussani R., Pavletic N., et al. Metastases of the heart and pericardium. G Ital Cardiol. 1997;27:1252-1255.
238. Sioka C., Kyritsis A.P. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63:761-767.
239. Speck RM, Courneya KS, Masse LC, et al: An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis, J Cancer Surv 4(2):87-100, 2010.
240. Spence R.R., Heesch K.C., Brown W.J.: Exercise and cancer rehabilitation: a systematic review, Cancer Treat Rev, 36(2):185-194 2009
241. St Guily J.L., Angelard B., el-Bez M., et al. Postlaryngectomy voice restoration: a prospective study in 83 patients. Arch Otolaryngol Head Neck Surg. 1992;118:252-255.
242. Stambaugh J.E.Jr., Drew J. The combination of ibuprofen and oxycodone/acetaminophen in the management of chronic cancer pain. Clin Pharmacol Ther. 1988;44:665-669.
243. Stearns L., Boortz-Marx R., Du Pen S., et al. Intrathecal drug delivery for the management of cancer pain: a multidisciplinary consensus of best clinical practices. J Support Oncol. 2005;3:399-408.
244. Steiner I., Siegal T. Muscle cramps in cancer patients. Cancer. 1989;63:574-577.
245. Stemmer R., Marescaux J., Furderer C. Compression treatment of the lower extremities particularly with compression stockings. Dermatologist. 1980;31:355-365.
246. Strang P. Emotional and social aspects of cancer pain. Acta Oncol. 1992;31:323-326.
247. Stout Gergich N, Smith H: Core stabilization and rehabilitation after breast cancer treatment involving breast reconstruction, Combined sections American physical Therapy Association, 2005 San Diego, CA
248. Stubblefield M.D., Levine A., Custodio C.M., et al. The role of botulinum toxin type A in the radiation fibrosis syndrome: a preliminary report. Arch Phys Med Rehabil. 2008;89:417-421.
249. Sunshine A., Olson N. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. J Clin Pharmacol. 1998;28:S47-S54.
250. Sureka J., Cherian R.A., Alexander M., et al. MRI of brachial plexopathies. Clin Radiol. 2009;64:208-218.
251. Sze G. Magnetic resonance imaging in the evaluation of spinal tumors. Cancer. 1991;67:1229-1241.
252. Szuba A., Rockson S.G. Lymphedema: classification, diagnosis and therapy. Vasc Med. 1998;3:145-156.
253. Tanoue L.T. Preoperative evaluation of the high-risk surgical patient for lung cancer resection. Semin Respir Crit Care Med. 2000;21:421-432.
254. Tas F., Eralp Y., Basaran M., et al. Anemia in oncology practice: relation to diseases and their therapies. Am J Clin Oncol. 2002;25:371-379.
255. Tasker R. Percutaneous cordotomy for persistent pain. In: Textbook of stereotactic and functional neurosurgery. New York: McGraw-Hill; 1998.
256. Tay S.S., Ng Y.S., Lim P.A. Functional outcomes of cancer patients in an inpatient rehabilitation setting. Ann Acad Med Singapore. 2009;38:197-201.
257. Tay W.K., Shaw R.J., Goh C.R. A survey of symptoms in hospice patients in Singapore. Ann Acad Med Singapore. 1994;23:191-196.
258. Thomas J., Cascino T., Earle J. Differential diagnosis between radiation and tumor plexopathy of the pelvis. Neurology. 1985;35:1-7.
259. Thompson E., Sola I., Subirana M. Non-invasive interventions for improving well-being and quality of life in patients with lung cancer: a systematic review of the evidence. Lung Cancer. 2005;50:163-176.
260. Todd T.R. The surgical treatment of pulmonary metastases. Chest. 1997;112:287S-290S.
261. Torcuator R., Zuniga R., Mohan Y.S., et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94:63-68.
262. Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, et al: Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ 340:b5396.
263. Tosoni A., Ermani M., Brandes A.A. The pathogenesis and treatment of brain metastases: a comprehensive review. Crit Rev Oncol Hematol. 2004;52:199-215.
264. Turgman J., Braham J., Modan B., et al. Neurological complications in patients with malignant tumors of the nasopharynx. Eur Neurol. 1978;17:149-154.
265. Twiss J.J., Waltman N.L., Berg K., et al. An exercise intervention for breast cancer survivors with bone loss. J Nurs Scholarsh. 2009;41:20-27.
266. Vial T., Descotes J. Clinical toxicity of cytokines used as haemopoietic growth factors. Drug Saf. 1995;13:371-406.
267. von Moos R., Strasser F., Gillessen S., et al. Metastatic bone pain: treatment options with an emphasis on bisphosphonates. Support Care Cancer. 2008;16:1105-1115.
268. Vujaskovic Z., Anscher M.S., Feng Q.F., et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50:851-855.
269. Vuorinen E. Pain as an early symptom in cancer. Clin J Pain. 1993;9:272-278.
270. Waltman N.L., Twiss J.J., Ott C.D., et al: The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial, Osteoporos Int, 2009 21(8): 1361-1369, 2009
271. Wara W.M., Phillips T.L., Sheline G.E., et al. Radiation tolerance of the spinal cord. Cancer. 1975;35:1558-1562.
272. Watling CJ, Payne R, Allen RR, et al: Commissural myelotomy for intractable cancer pain: report of two cases, Clin J Pain 12(2): 151-156, 1996
273. Weissleder H, Schuchhardt, editors: Lymphedema: diagnosis and therapy, ed 2, Bonn, 1997, Kagerer Kommunikation
274. Weschules D.J., Bain K.T. A systematic review of opioid conversion ratios used with methadone for the treatment of pain. Pain Med. 2008;9:595-612.
275. Winningham M.L., MacVicar M.G. The effect of aerobic exercise on patient reports of nausea. Oncol Nurs Forum. 1988;15:447-450.
276. Woo E., Yu Y.L., Ng M., et al. Spinal cord compression in multiple myeloma: who gets it? Aust N Z J Med. 1986;16:671-675.
277. Wu J.S., Wong R.K., Lloyd N.S., et al. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases: an evidence-based practice guideline. BMC Cancer. 2004;4:71.
278. Zeine L., Larson M. Pre- and postoperative counseling for laryngectomees and their spouses: an update. J Commun Disorders. 1999;32:51-71.