Chapter 34 Cancer of Unknown Primary
Introduction*
Unproved theories regarding the localization of the primary CUP site include (1) the primary tumor has involuted and only the metastatic disease is evident and (2) metastatic disease is favored over the primary growth based on the phenotype and genotype of the tumor.1
Epidemiology and Risk Factors
Worldwide, CUP is one of the 10 most frequent cancers constituting about 3% to 5% of all cancer cases.2 In 2011, according to the American Cancer Society, there were an estimated 30,500 cases of “Other & unspecified primary sites” in the United States or approximately 2% of all cancer cases and an estimated 44,260 deaths.2 At presentation, the median age is approximately 60 years and slightly more frequent in males.3
Anatomy and Pathology
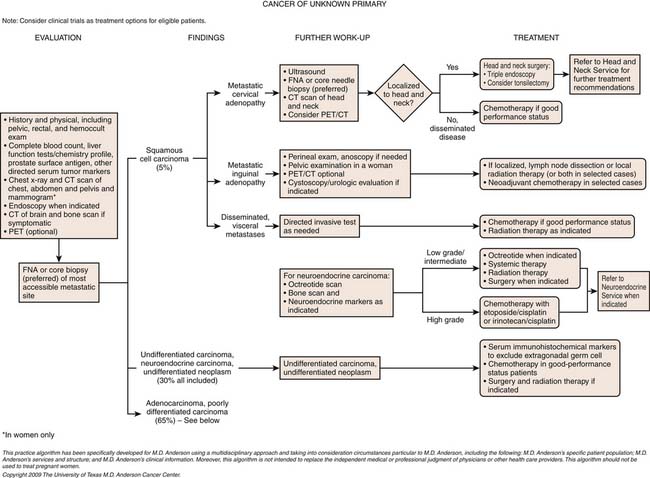
Because only carcinomas are included in the diagnosis of CUP, four main histologic types of CUP have been described: well to moderately differentiated adenocarcinomas (50%), undifferentiated or poorly differentiated carcinomas (30%), squamous cell carcinomas (15%), and undifferentiated neoplasms (5%). The latter group includes lymphomas, sarcomas, germ cell tumors, poorly differentiated carcinomas, neuroendocrine tumors, and embryonal malignancies that can be characterized by immunohistologichemistry.4 In children, CUPs represent less than 1% of solid tumors and the majority of these tumors are embryonal malignancies.2
The chromosomal and molecular abnormalities identified in CUPs are beyond the scope of this chapter.
Clinical Presentation
These patients face a grim prognosis with reported survival rates of 6 to 10 months for those enrolled in clinical studies.2
However, those CUP patients not enrolled in clinical trials have reported life expectancies of 2 to 3 months.5 Given the diverse clinical presentation of these patients, it is best to classify them into favorable and unfavorable subsets, with the former having a better prognosis.6
1. Squamous cell carcinoma involving cervical lymph nodes (Figure 34-1).
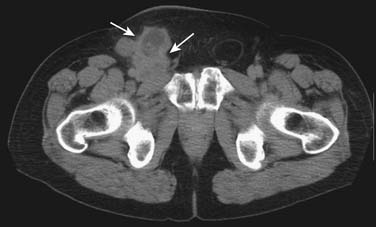
2. Women with adenocarcinoma involving only axillary lymph nodes.
3. Women with peritoneal cavity papillary serous adenocarcinoma.
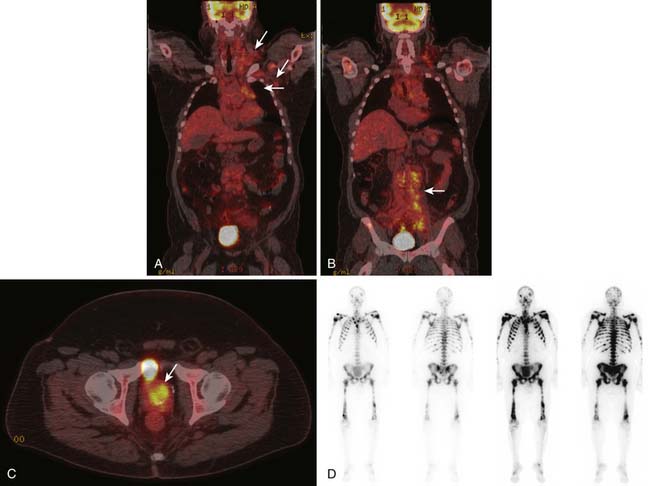
4. Men with elevated PSA and blastic osseous metastases (Figure 34-2).
5. Poorly differentiated carcinoma with midline distribution (extragonadal germ cell tumor syndrome).
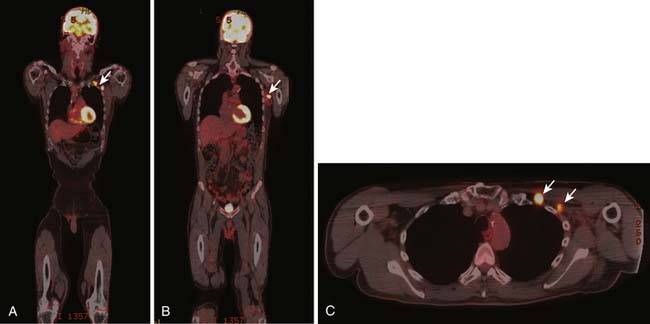
6. Isolated inguinal adenopathy with squamous cell carcinoma (Figure 34-3).
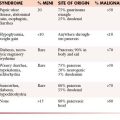
7. Poorly differentiated neuroendocrine carcinomas.
8. Patient with a single, small, potentially resectable metastasis.
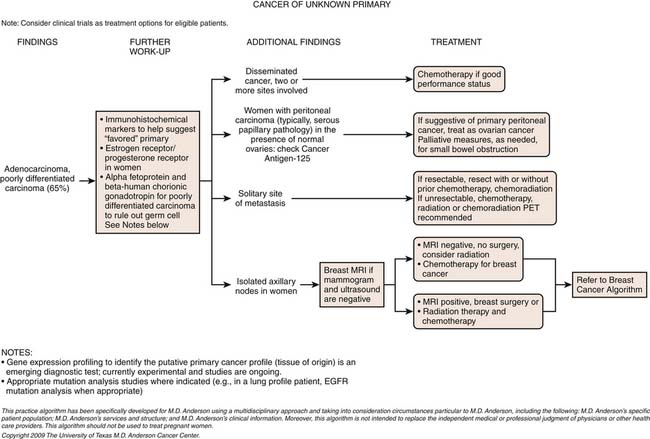
1. Adenocarcinoma primarily metastatic to the liver.
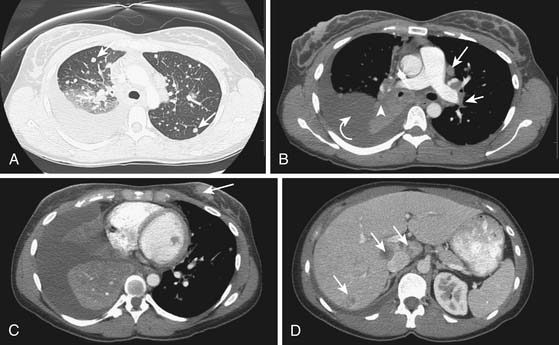
2. Adenocarcinoma with multiple metastases to the lungs (Figure 34-4).
3. Adenocarcinoma with mainly diffuse osseous metastatic disease.
4. Multiple cerebral metastases.
5. Malignant ascites with nonpapillary adenocarcinoma histology.
To complicate the clinical presentation, more than 50% of CUP patients present with multiple sites of metastatic disease and approximately 30% have three or more organs involved with metastatic disease. In comparison, in patients with known primary tumors, fewer than 15% have metastatic disease in three or more sites.5,7,8
Patterns of Tumor Spread
The most common sites for CUP metastatic disease are the liver, lungs, bones, and lymph nodes. However, when compared with tumors of known origin, CUPs typically present with unpredictable patterns of spread. For example, when pancreatic and hepatic carcinomas present as CUPs, there is a higher incidence of lung and osseous metastatic disease compared with a known primary originating in the pancreas and liver. Brain and osseous metastatic disease from a CUP originating in the gastrointestinal tract is more commonly seen than that from a known gastrointestinal primary carcinoma. Approximately 50% of pulmonary carcinomas have osseous metastatic disease, whereas CUPs of pulmonary origin have a lower incidence of osseous metastatic disease. Hepatic and pulmonary metastatic disease is seen more frequently in cases that present as a CUP from a prostatic carcinoma than from a known prostatic carcinoma.9–12
Staging Evaluation
To stage a malignancy, the tumor needs to be identified for the tumor-node-metastasis (TNM) system. However, in only approximately 11% to 20% are the CUPs identified after a thorough clinical workup.13,14 The postmortem tumor identification yield has been reported to be higher at approximately 51% to 79%.10,12,15 CUPs do not allow for the typical staging of tumors, given their diverse clinical presentation, different subtypes, different histologies, and metastatic patterns. The best approach for optimal patient management is to identify the subset to which that patient best fits.
Imaging
An example of tailored imaging in a favorable subset would be that of a female with axillary adenopathy but with a nondiagnostic mammographic study. This patient would benefit from an MRI evaluation of the breasts, in which there is a reported higher detection rate of 70% to 86%.16–18
A testicular sonogram may help to identify a neoplasm in a male with midline adenopathy.
Although an optional imaging study, PET and PET/CT are being more frequently used to identify not only the tumor but also any unrecognized metastatic disease. These imaging modalities utilize 2-deoxy-2-[F-18]fluoro-D-glucose (FDG)–PET and provide functional results, with PET/CT providing better anatomic information, a disadvantage for FDG-PET only. In a study reported by Kolesnikov-Gauthier and coworkers,19 CUP patients underwent FDG per imaging. The primary tumor site was identified in 6 of the 24 patients and all known metastatic sites were visualized.
An excellent paper by Seve and colleagues20 reviewed 10 published studies (1998-2006) involving a combined total of 221 CUP patients. This review included a heterogeneous patient population, study design, and diagnostic workup; however, 94% of these patients had a single metastatic site. Each study evaluated the role of FDG-PET in identifying the unknown primary cancer site. FDG-PET detected the primary tumor sites in 41% of these patients. Previously unrecognized metastases were detected in 37% of patients. Of the detected tumors, 59% were in the lungs. A false-positive rate of 58% was noted in those tumors of the lower digestive tract and likely due to the inherent bowel activity. The clinical management was changed in approximately 35% of these patients. Specific chemotherapy was given to those patients (53%) with lung and pancreatic cancers. Specific therapy (12%) was given for breast, ovarian, and prostate cancers, and 14% of these patients underwent potential curative surgery.
Based on FDG-PET, the clinical management of metastatic cervical lymph adenopathy was altered in 25% of patients as reported by Johansen and associates.21
In a systematic review and meta-analysis of 11 published studies with a cohort of 433 patients with CUP, Kwee and Kwee22 reported a primary tumor detection rate of 37% and a pooled sensitivity and specificity of 84% and 84%, respectively. Across the studies, the sensitivity was heterogeneous and the specificity was homogeneous. Another review article on the clinical utility of FDG-PET/CT is presented and compared with other imaging modalities.23 It also addresses the potential role for radiation therapy planning and for monitoring response or lack of response to therapy.
Key Points Imaging and imaging evaluation
• Clinical problem is to identify the primary tumor, mainly using CT of the chest, abdomen, and pelvis. A mammogram, breast MRI, or bone scan is ordered as indicated. CT or brain MRI is used if the patient is symptomatic.
• PET/CT may become a more global evaluation of a CUP patient and forego all or some other forms of imaging.
• The vast majority of these patients will be managed by the medical oncologist with radiation therapy as needed. To define the burden and location of disease, a surgeon may be needed for a biopsy.
• There is no set staging or TNM system available because the primary tumor is occult.
Key Points The radiology report
• The locations of metastatic disease should be identified.
• More isolated lesions should be identified and used as index lesions.
• Describe all soft tissue, osseous sites of disease to include any lymphadenopathy.
• Suggest possible primary tumor etiology, if possible, based on radiographic pattern of metastatic disease.
• Suggest alternative imaging modalities that may help in evaluating the possible primary tumor or to manage potential complications such as spinal cord involvement.
Role of Molecular Profiling to Determine Tissue of Origin
Molecular profiling of CUP cancers offers a promising technique to determine the tissue of origin (ToO) with a possible impact on therapy. Several studies demonstrated the feasibility of using gene expression profiling with DNA microarray to classify uncertain tumors based on their ToO, identifying gene subsets whose expression typify each cancer class. These studies achieved accuracy in identifying the tissue of origin in 78% to 89% of tumors.24–29
General Considerations
The median survival duration of patients with disseminated CUP is approximately 6 to 10 months. Patients with favorable prognosis do better because they have a cancer biology that is either more indolent or more responsive to therapies. Prognostic factors include performance status, locations of and number of metastases, response to chemotherapy, and serum lactate dehydrogenase level (LDH). Culine and coworkers30 developed and retrospectively validated a prognostic model that uses performance status and serum LDH, allowing patients to be assigned to one of two subgroups with divergent outcomes. Further prospective trials using this prognostic model are warranted. Management of some of the favorable subsets and of disseminated CUP outside of favorable subset is discussed later.
Prognostically Favorable Subsets of Cancers of Unknown Primary: Treatment Considerations
These subgroups are important to note because therapies can significantly affect survival.
1. Isolated axillary adenopathy with adenocarcinoma or carcinoma in women. Women with isolated axillary adenopathy and adenocarcinoma or carcinoma are often treated for stage II or III breast cancer and are candidates for breast MRI if their mammography and sonography results are negative (as discussed previously). Beside breast markers on immunohistochemisty including mammoglobin and GCDFP-15, estrogen and progesterone receptors and HER2 neu are warranted to determine the appropriate treatment. If the clinical and imaging presentation suggests breast cancer, neoadjuvant or adjuvant chemotherapy and axillary lymph node dissection and radiation therapy (and hormonal therapy if appropriate) is the standard therapy.
2. Peritoneal carcinomatosis suggestive of primary peritoneal carcinoma in women. The term primary peritoneal papillary serous carcinoma (PPSC) refers to CUP with carcinomatosis and the pathologic and laboratory (elevated CA125 antigen) characteristics of a müllerian cancer without an obvious ovarian primary identified on transvaginal sonography or laparotomy. Patients with PPSC are candidates for cytoreductive surgery followed by adjuvant taxane- and platinum-based chemotherapy and suggest median progression-free and overall survival durations of 7 and 15 months or longer, respectively (median follow-up 60 mo).
3. Cervical adenopathy with squamous cell carcinoma. Patients with cervical adenopathy with squamous cell carcinoma should undergo triple endoscopy with biopsies of inconspicuous sites, bilateral tonsillectomy, and CT or PET/CT of the neck and chest to search for the primary and determine the stage of the tumor. Patients with early-stage disease are candidates for node dissection and radiation therapy, which can result in long-term survival. The utility of chemotherapy in these patients is unknown, although chemoradiation therapy or induction chemotherapy is often used and is beneficial in bulky N2 and N3 disease.
4. Low-grade neuroendocrine carcinoma. It is important to differentiate between low-grade and high-grade neuroendocrine CUP. High-grade neuroendocrine cancers have a high mitotic index (Ki-67), necrosis, and hemorrhage on pathologic evaluation. Patients with low-grade neuroendocrine carcinoma may present with an indolent disease course; thus, treatment decisions are based on symptoms and tumor bulk. Current therapies include treatment with somatostatin analogues alone for hormone-related symptoms and locoregional therapies including bland or chemoembolization or SIR Spheres microspheres. Systemic therapy including anti–vascular endothelial growth factor (VEGF) therapies and mammalian target of rapamycin (mTOR) inhibitors are showing promise in these tumors and are indicated in patients with symptomatic or progressive disease.
5. Solitary metastases. Patients with solitary metastatic disease most often present with nodal, liver, bone, or brain metastasis. Some of these are candidates for aggressive management, which can result in prolonged disease-free survival and even a cure. Immunohistochemical studies, pattern of spread, age, gender, and risk factors help determine the best therapy for these patients. In selected patients, neoadjuvant chemotherapy is used to treat micrometastatic disease and downstage the cancer to maximize the potential for a margin-negative resection as well as study its biology. We do not advocate that all patients with solitary metastatic CUP follow this approach, and this is not intended to define the standard of care in this setting.
Detecting Recurrences and Complications
Traditionally, cisplatin-based combination chemotherapy regimens have been used to treat CUP patients. In a phase II study by Greco and colleagues,31 55 CUP patients (51 of whom were chemotherapy-naïve) were treated with paclitaxel, carboplatin, and oral etoposide every 3 weeks. The overall response rate was 47%, and the median overall survival was 13.4 months. Briasoulis and associates32 reported similar results in 77 CUP patients treated with paclitaxel and carboplatin (without etoposide). Patients with nodal or pleural disease and peritoneal carcinomatosis had a higher response rate (than did patients with visceral disseminated disease) and overall survival durations of 13 and 15 months, respectively. More recent studies have incorporated newer agents, such as gemcitabine, irinotecan, and targeted agents. In a phase II randomized trial by Culine and coworkers,33 80 patients were randomly assigned to receive gemcitabine plus cisplatin or irinotecan plus cisplatin. Seventy-eight patients were assessable for effectiveness and toxicity. Objective responses were observed in 21 patients (55%) in the gemcitabine and cisplatin arm and 15 (38%) in the irinotecan and cisplatin arm. The median survival durations were 8 and 6 months in the gemcitabine and cisplatin and irinotecan and cisplatin arms, respectively (median follow-up 22 mo).
Key Points Detecting recurrences and complications
• The response to therapy is for control, prolonged disease-free survival, and occasional cure. Recurrence is guided by the clinical examination and appropriate imaging studies.
• Given the use of multiple chemotherapeutic agents, the complications are varied based on the agent(s) used and their specific toxicities to organs and systems in the body.
Preliminary data on combination targeted therapy are available. Hainsworth and colleagues34 determined the effectiveness of bevacizumab and erlotinib in 51 patients, 25% of who were chemotherapy naïve with advanced bone or liver metastases and 75% of who had been treated with one or two chemotherapy regimens. Responses were noted in 4 patients (8%), and 30 patients (59%) experienced stable disease or a minor response. The median overall survival duration was 8.9 months, with 42% of patients alive at 1 year.
1. Lenzi R., Raber M.N., Frost P., et al. Phase II study of cisplatin, 5-fluorouracil and folinic acid in patients with carcinoma of unknown primary origin. Eur J Cancer. 1993;29A:1634.
2. Pavlidis N., Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69:271-278.
3. Siegel R., Ward E., Brawley O., et al. Cancer Statistics, 2011. The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236.
4. Greco F.A., Hainsworth J.D.. Cancer of unknown primary site. DeVita V.T., Hellman S., Rosenberg S.A., editors. 8th ed. Cancer: Principles and Practice of Oncology. Philadelphia: JB Lippincott; 2008:2363-2386.
5. van de Wouw A.J., Janssen-Heijnen M.L., Coebergh J.W., et al. Epidemiology of unknown primary tumors: incidence and population-based survival of 1285 patients in Southeast Netherlands, 1984-1992. Eur Cancer. 2002;38:409-413.
6. Abbruzzese J.L., Abbruzzese M.C., Hess K.R., et al. Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol. 1994;12:1272-1280.
7. Hess K.R., Abbruzzese M.C., Lenzi R., et al. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res.. 1999;5:3403-3410.
8. Culine S., Kramar A., Saghatchian M., et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol. 2002;20:4679-4683.
9. Blaszyk H., Hartmann A., Bjornsson J. Cancer of unknown primary: clinicopathologic correlations. APMIS. 2003;111:1089-1094.
10. Mayordomo J.I., Guerra J.M., Guijaro C., et al. Neoplasms of unknown primary site: a clinicopathologic study of autopsied patients. Tumori. 1993;79:321-324.
11. Jordan W.E., Shidi R.A. Adenocarcinoma of unknown primary site. The Brooke Army Medical Center experience. Cancer. 1985;55:857-860.
12. Le Chevalier T., Cvitkovic E., Caille P., et al. Early metastatic cancer of unknown primary origin at presentation. A clinical study of 302 consecutive studied patients. Arch Intern Med. 1988;148:2035-2039.
13. Kirsten F., Chi C.H., Leary J.A., et al. Metastatic adeno or undifferentiated carcinoma from an unknown primary site—natural history or guidelines for identification of treatable subsets. Q J Med. 1987;62:143-161.
14. Nystrom J.S., Weiner J.M., Heffelfinger-Juttner J., et al. Metastatic and histologic presentations in unknown primary cancer. Semin Oncol. 1977;4:53-58.
15. AI-Brahim N., Ross C., Carter B., et al. The value of postmortem examination in cases of metastasis of unknown origin—20 year retrospective data from a tertiary center. Ann Diagn Pathol. 2005;9:77-90.
16. Orel S.G., Wienstein S.P., Schnall M.D., et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999;212:543-549.
17. Olson J.A., Morris E.A., Van Zee K.J., et al. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000;7:411-425.
18. Obdeijn I.M., Brouwers-Kuyper E.M., Tilanus-Linthorst M.M., et al. MR imaging-guided sonography followed by fine-needle aspiration cytology in occult carcinoma of the breast. AJR Am J Roentgenol. 2000;174:1079-1084.
19. Kolesnikov-Gauthier H., Levy E., Merlet P., et al. FDG PET in patients with cancer of an unknown primary. Nucl Med Commun. 2005;26:1059-1066.
20. Seve P., Billotey C., Broussolle C., et al. The role of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography in disseminated carcinoma of unknown primary site. Cancer. 2006;109:292-299.
21. Johansen J., Buus S., Loft A., et al. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck. 2008;30:471-478.
22. Kwee T.C., Kwee R.M. Combined FDG-PET/CT for the detection of unknown primary tumors: systemic review and meta-analysis. Eur Radiol. 2009;19:731-744.
23. Podoloff D.A. PET/CT and occult primary tumors. J Natl Compr Canc Netw. 2009;7:239-244.
24. Tothill R.W., Kowalczyk A., Rischin D., et al. An expression-based site of origin diagnostic method designed for clinical application to cancer of unknown origin. Cancer Res.. 2005;65:4031-4040.
25. Ma X.J., Patel R., Wang X., et al. Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch Pathol Lab Med. 2006;130:465-473.
26. Monzon F.A., Koen T.J. Diagnosis of metastatic neoplasms: molecular approaches for identification of tissue of origin. Arch Pathol Lab Med. 2010;134:216-224.
27. Varadhachary G.R., Talantov D., Raber M.N., et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol. 2008;26:4442-4448.
28. Horlings H.M., van Laar R.K., Kerst J.M., et al. Gene expression profiling to identify the histogenetic origin of metastatic adenocarcinomas of unknown primary. J Clin Oncol. 2008;26:4435-4441.
29. Pentheroudakis G., Greco F.A., Pavlidis N. Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: a systematic literature review. Cancer Treat Rev.. 2009;35:221-227.
30. Culine S., Kramar A., Saghatchian M., et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol. 2002;20:4679-4683.
31. Greco F.A., Hainsworth J.D. One-hour paclitaxel, carboplatin, and extended-schedule etoposide in the treatment of carcinoma of unknown primary site. Semin Oncol. 24(6 Suppl. 19), 1997. S19-101-S19-105
32. Briasoulis E., Kalofonos H., Bafaloukos D., et al. Carboplatin plus paclitaxel in unknown primary carcinoma: a phase II Hellenic Cooperative Oncology Group Study. J Clin Oncol. 2000;18:3101-3107.
33. Culine S., Lortholary A., Voigt J.-J., et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study—trial for the French Study Group on Carcinomas of Unknown Primary (GEFCAPI 01). J Clin Oncol. 2003;21:3479-3482.
34. J.D. Hainsworth, D.R. Spigel, D.S. Thompson. et al. Bevacizumab plus erlotinib in patients (pts) with carcinoma of unknown primary site: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2006;24:3033