FIGURE 103e-1 Gompertzian tumor growth. The growth fraction of a tumor declines exponentially over time (top). The growth rate of a tumor peaks before it is clinically detectable (middle). Tumor size increases slowly, goes through an exponential phase, and slows again as the tumor reaches the size at which limitation of nutrients or autoregulatory or host regulatory influences can occur. The maximum growth rate occurs at 1/e, the point at which the tumor is about 37% of its maximum size (marked with an X). Tumor becomes detectable at a burden of about 109 (1 cm3) cells and kills the patient at a tumor cell burden of about 1012 (1 kg). Efforts to treat the tumor and reduce its size can result in an increase in the growth fraction and an increase in growth rate.
LOCALIZED CANCER TREATMENTS
SURGERY
Surgery is unquestionably the most effective means of treating cancer. Today at least 40% of cancer patients are cured by surgery. Unfortunately, a large fraction of patients with solid tumors (perhaps 60%) have metastatic disease that is not accessible for removal. However, even when the disease is not curable by surgery alone, the removal of tumor can obtain important benefits, including local control of tumor, preservation of organ function, debulking that permits subsequent therapy to work better, and staging information on extent of involvement. Cancer surgery aiming for cure is usually planned to excise the tumor completely with an adequate margin of normal tissue (the margin varies with the tumor and the anatomy), touching the tumor as little as possible to prevent vascular and lymphatic spread, and minimizing operative risk. Such a resection is defined as an R0 resection. R1 and R2 resections, in contrast, are imprecisely defined pathologically as having microscopic or macroscopic tumor at resection margins. Such outcomes may be necessitated by proximity of the tumor to vital structures or recognition only in the resected specimen of the extent of tumor involvement, and may be the basis for reoperation to obtain optimal margins if feasible. Extending the procedure to resect draining lymph nodes obtains prognostic information and may, in some anatomic locations, improve survival.
Increasingly, laparoscopic approaches are being used to address primary abdominal and pelvic tumors. Lymph node spread may be assessed using the sentinel node approach, in which the first draining lymph node a spreading tumor would encounter is defined by injecting a dye or radioisotope into the tumor site at operation and then resecting the first node to turn blue or collect label. The sentinel node assessment is continuing to undergo clinical evaluation but appears to provide reliable information without the risks (lymphedema, lymphangiosarcoma) associated with resection of all the regional nodes. Advances in adjuvant chemotherapy (chemotherapy given systemically after removal of all disease by operation and without evidence of active metastatic disease) and radiation therapy following surgery have permitted a substantial decrease in the extent of primary surgery necessary to obtain the best outcomes. Thus, lumpectomy with radiation therapy is as effective as modified radical mastectomy for breast cancer, and limb-sparing surgery followed by adjuvant radiation therapy and chemotherapy has replaced radical primary surgical procedures involving amputation and disarticulation for childhood rhabdomyosarcomas and osteosarcomas. More limited surgery is also being used to spare organ function, as in larynx and bladder cancer. The magnitude of operations necessary to optimally control and cure cancer has also been diminished by technical advances; for example, the circular anastomotic stapler has allowed narrower (<2 cm) margins in colon cancer without compromise of local control rates, and many patients who would have had colostomies are able to maintain normal anatomy.
In some settings (e.g., bulky testicular cancer or stage III breast cancer), surgery is not the first treatment modality used. After an initial diagnostic biopsy, chemotherapy and/or radiation therapy is delivered to reduce the size of the tumor and clinically control undetected metastatic disease. Such therapy is followed by a surgical procedure to remove residual masses; this is called neoadjuvant therapy. Because the sequence of treatment is critical to success and is different from the standard surgery-first approach, coordination among the surgical oncologist, radiation oncologist, and medical oncologist is crucial.
Surgery may be curative in a subset of patients with metastatic disease. Patients with lung metastases from osteosarcoma may be cured by resection of the lung lesions. In patients with colon cancer who have fewer than five liver metastases restricted to one lobe and no extrahepatic metastases, hepatic lobectomy may produce long-term disease-free survival in 25% of selected patients. Surgery can also be associated with systemic antitumor effects. In the setting of hormonally responsive tumors, oophorectomy and/or adrenalectomy may eliminate estrogen production, and orchiectomy may reduce androgen production, hormones that drive certain breast and all prostate cancers, respectively; both procedures can have useful effects on metastatic tumor growth. If resection of the primary lesion takes place in the presence of metastases, acceleration of metastatic growth has also been described in certain cases, perhaps based on the removal of a source of angiogenesis inhibitors and mass-related growth regulators in the tumor.
In selecting a surgeon or center for primary cancer treatment, consideration must be given to the volume of cancer surgeries undertaken by the site. Studies in a variety of cancers have shown that increased annual procedure volume appears to correlate with outcome. In addition, facilities with extensive support systems—e.g., for joint thoracic and abdominal surgical teams with cardiopulmonary bypass, if needed—may allow resection of certain tumors that would otherwise not be possible.
Surgery is used in a number of ways for palliative or supportive care of the cancer patient, not related to the goal of curing the cancer. These include insertion and care of central venous catheters, control of pleural and pericardial effusions and ascites, caval interruption for recurrent pulmonary emboli, stabilization of cancer-weakened weight-bearing bones, and control of hemorrhage, among others. Surgical bypass of gastrointestinal, urinary tract, or biliary tree obstruction can alleviate symptoms and prolong survival. Surgical procedures may provide relief of otherwise intractable pain or reverse neurologic dysfunction (cord decompression). Splenectomy may relieve symptoms and reverse hypersplenism. Intrathecal or intrahepatic therapy relies on surgical placement of appropriate infusion portals. Surgery may correct other treatment-related toxicities such as adhesions or strictures. Surgical procedures are also valuable in rehabilitative efforts to restore health or function. Orthopedic procedures may be necessary to ensure proper ambulation. Breast reconstruction can make an enormous impact on the patient’s perception of successful therapy. Plastic and reconstructive surgery can correct the effects of disfiguring primary treatment.
Surgery is also a tool valuable in the prevention of cancers in high-risk populations. Prophylactic mastectomy, colectomy, oophorectomy, and thyroidectomy are mainstays of prevention of genetic cancer syndromes. Resection of premalignant skin and uterine cervix lesions and colonic polyps prevents progression to frank malignancy.
RADIATION
Radiation Biology and Medicine Therapeutic radiation is ionizing; it damages any tissue in its path. The selectivity of radiation for causing cancer cell death may be due to defects in a cancer cell’s ability to repair sublethal DNA and other damage. Ionizing radiation causes breaks in DNA and generates free radicals from cell water that may damage cell membranes, proteins, and organelles. Radiation damage is augmented by oxygen; hypoxic cells are more resistant. Augmentation of oxygen presence is one basis for radiation sensitization. Sulfhydryl compounds interfere with free radical generation and may act as radiation protectors. X-rays and gamma rays are the forms of ionizing radiation most commonly used to treat cancer. They are both electromagnetic, nonparticulate waves that cause the ejection of an orbital electron when absorbed. This orbital electron ejection is called ionization. X-rays are generated by linear accelerators; gamma rays are generated from decay of atomic nuclei in radioisotopes such as cobalt and radium. These waves behave biologically as packets of energy, called photons. Particulate ionizing radiation using protons has also become available. Most radiation-induced cell damage is due to the formation of hydroxyl radicals from tissue water:
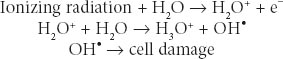
Radiation is quantitated based on the amount of radiation absorbed by the tumor in the patient; it is not based on the amount of radiation generated by the machine. The International System (SI) unit for radiation absorbed is the Gray (Gy): 1 Gy refers to 1 J/kg of tissue; 1 Gy equals 100 centigrays (cGy) of absorbed dose. A historically used unit appearing in the oncology literature, the rad (radiation absorbed dose), is defined as 100 ergs of energy absorbed per gram of tissue and is equivalent to 1 cGy. Radiation dosage is defined by the energy absorbed per mass of tissue. Radiation dose is measured by placing detectors at the body surface or based on radiating phantoms that resemble human form and substance, containing internal detectors. The features that make a particular cell more sensitive or more resistant to the biologic effects of radiation are not completely defined and critically involve DNA repair proteins that, in their physiologic role, protect against environmentally related DNA damage.
Localized Radiation Therapy Radiation effect is influenced by three determinants: total absorbed dose, number of fractions, and time of treatment. A frequent error is to omit the number of fractions and the duration of treatment. This is analogous to saying that a runner completed a race in 20 s; without knowing how far he or she ran, the result is difficult to interpret. The time could be very good for a 200-m race or very poor for a 100-m race. Thus, a typical course of radiation therapy should be described as 4500 cGy delivered to a particular target (e.g., mediastinum) over 5 weeks in 180-cGy fractions. Most curative radiation treatment programs are delivered once a day, 5 days a week, in 150- to 200-cGy fractions.
A number of parameters influence the damage done to tissue (normal and tumor) by radiation. Hypoxic cells are relatively resistant. Nondividing cells are more resistant than dividing cells, and this is one rationale for delivering radiation in repeated fractions, to ultimately expose a larger number of tumor cells that have entered the division cycle. In addition to these biologic parameters, physical parameters of the radiation are also crucial. The energy of the radiation determines its ability to penetrate tissue. Low-energy orthovoltage beams (150–400 kV) scatter when they strike the body, much like light diffuses when it strikes particles in the air. Such beams result in more damage to adjacent normal tissues and less radiation delivered to the tumor. Megavoltage radiation (>1 MeV) has very low lateral scatter; this produces a skin-sparing effect, more homogeneous distribution of the radiation energy, and greater deposit of the energy in the tumor, or target volume. The tissues that the beam passes through to get to the tumor are called the transit volume. The maximum dose in the target volume is often the cause of complications to tissues in the transit volume, and the minimum dose in the target volume influences the likelihood of tumor recurrence. Dose homogeneity in the target volume is the goal. Computational approaches and delivery of many beams to converge on a target lesion are the basis for “gamma knife” and related approaches to deliver high doses to small volumes of tumor, sparing normal tissue.
Therapeutic radiation is delivered in three ways: (1) teletherapy, with focused beams of radiation generated at a distance and aimed at the tumor within the patient; (2) brachytherapy, with encapsulated sources of radiation implanted directly into or adjacent to tumor tissues; and (3) systemic therapy, with radionuclides administered, for example, intravenously but targeted by some means to a tumor site. Teletherapy with x-ray or gamma-ray photons is the most commonly used form of radiation therapy. Particulate forms of radiation are also used in certain circumstances, such as the use of proton beams. The difference between photons and protons relates to the volume in which the greatest delivery of energy occurs. Typically protons have a much narrower range of energy deposition, theoretically resulting in more precise delivery of radiation with improvement in the degree to which adjacent structures may be affected, in comparison to photons. Electron beams are a particulate form of radiation that, in contrast to photons and protons, have a very low tissue penetrance and are used to treat cutaneous tumors. Apart from sparing adjacent structures, particulate forms of radiation are in most applications not superior to x-rays or gamma rays in clinical studies reported thus far, but this is an active area of investigation.
Certain drugs used in cancer treatment may also act as radiation sensitizers. For example, compounds that incorporate into DNA and alter its stereochemistry (e.g., halogenated pyrimidines, cisplatin) augment radiation effects at local sites, as does hydroxyurea, another DNA synthesis inhibitor. These are important adjuncts to the local treatment of certain tumors, such as squamous head and neck, uterine cervix, and rectal cancers.
Toxicity of Radiation Therapy Although radiation therapy is most often administered to a local region, systemic effects, including fatigue, anorexia, nausea, and vomiting, may develop that are related in part to the volume of tissue irradiated, dose fractionation, radiation fields, and individual susceptibility. Injured tissues release cytokines that act systemically to produce these effects. Bone is among the most radioresistant organs, with radiation effects being manifested mainly in children through premature fusion of the epiphyseal growth plate. By contrast, the male testis, female ovary, and bone marrow are the most sensitive organs. Any bone marrow in a radiation field will be eradicated by therapeutic irradiation. Organs with less need for cell renewal, such as heart, skeletal muscle, and nerves, are more resistant to radiation effects. In radiation-resistant organs, the vascular endothelium is the most sensitive component. Organs with more self-renewal as a part of normal homeostasis, such as the hematopoietic system and mucosal lining of the intestinal tract, are more sensitive. Acute toxicities include mucositis, skin erythema (ulceration in severe cases), and bone marrow toxicity. Often these can be alleviated by interruption of treatment.
Chronic toxicities are more serious. Radiation of the head and neck region often produces thyroid failure. Cataracts and retinal damage can lead to blindness. Salivary glands stop making saliva, which leads to dental caries and poor dentition. Taste and smell can be affected. Mediastinal irradiation leads to a threefold increased risk of fatal myocardial infarction. Other late vascular effects include chronic constrictive pericarditis, lung fibrosis, viscus stricture, spinal cord transection, and radiation enteritis. A serious late toxicity is the development of second solid tumors in or adjacent to the radiation fields. Such tumors can develop in any organ or tissue and occur at a rate of about 1% per year beginning in the second decade after treatment. Some organs vary in susceptibility to radiation carcinogenesis. A woman who receives mantle field radiation therapy for Hodgkin’s disease at age 25 years has a 30% risk of developing breast cancer by age 55 years. This is comparable in magnitude to genetic breast cancer syndromes. Women treated after age 30 years have little or no increased risk of breast cancer. No data suggest that a threshold dose of therapeutic radiation exists below which the incidence of second cancers is decreased. High rates of second tumors occur in people who receive as little as 1000 cGy.
OTHER LOCALIZED CANCER TREATMENTS
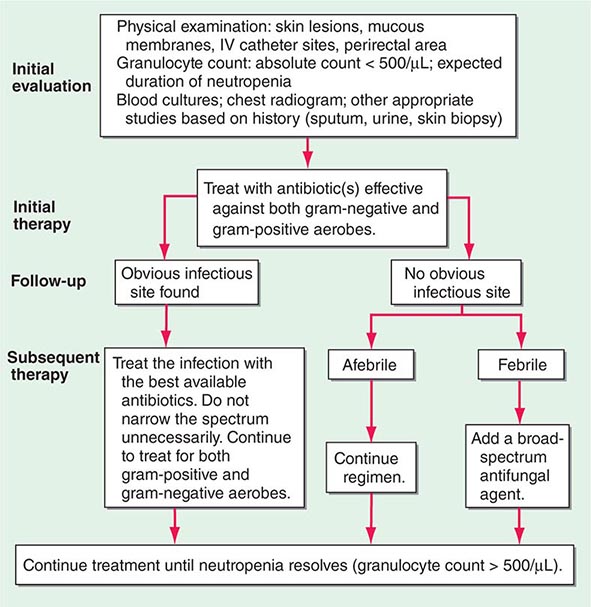
Endoscopy techniques may allow the placement of stents to unblock viscera by mechanical means, palliating, for example, gastrointestinal or biliary obstructions. Radiofrequency ablation (RFA) refers to the use of focused microwave radiation to induce thermal injury within a volume of tissue. RFA can be useful in the control of metastatic lesions, particularly in liver, that may threaten biliary drainage (as one example) and threaten quality and duration of useful life in patients with otherwise unresectable disease. Cryosurgery uses extreme cold to sterilize lesions in certain sites, such as prostate and kidney, when at a very early stage, eliminating the need for modalities with more side effects such as surgery or radiation.
Some chemicals (porphyrins, phthalocyanines) are preferentially taken up by cancer cells by mechanisms not fully defined. When light, usually delivered by a laser, is shone on cells containing these compounds, free radicals are generated and the cells die. Hematoporphyrins and light (phototherapy) are being used with increasing frequency to treat skin cancer; ovarian cancer; and cancers of the lung, colon, rectum, and esophagus. Palliation of recurrent locally advanced disease can sometimes be dramatic and last many months.
Infusion of chemotherapeutic or biologic agents or radiation-bearing delivery devices such as isotope-coated glass spheres into local sites through catheters inserted into specific vascular sites such as liver or an extremity have been used in an effort to control disease limited to that site; in selected cases, prolonged control of truly localized disease has been possible.
SYSTEMIC CANCER TREATMENTS
The concept that systemically administered agents may have a useful effect on cancers was historically derived from three sets of observations. Paul Ehrlich in the nineteenth century observed that different dyes reacted with different cell and tissue components. He hypothesized the existence of compounds that would be “magic bullets” that might bind to tumors, owing to the affinity of the agent for the tumor. A second observation was the toxic effects of certain mustard gas derivatives on the bone marrow during World War I, leading to the idea that smaller doses of these agents might be used to treat tumors of marrow-derived cells. Finally, the observation that certain tumors from hormone-responsive tissues, e.g., breast tumors, could shrink after oophorectomy led to the idea that endogenous substances promoting the growth of a tumor might be antagonized. Chemicals achieving each of the goals are actually or intellectually the forbearers of the currently used cancer chemotherapy agents.
Systemic cancer treatments are of four broad types. Conventional “cytotoxic” chemotherapy agents were historically derived by the empirical observation that these “small molecules” (generally with molecular mass <1500 Da) could cause major regression of experimental tumors growing in animals. These agents mainly target DNA structure or segregation of DNA as chromosomes in mitosis. Targeted agents refer to small molecules or “biologics” (generally macromolecules such as antibodies or cytokines) designed and developed to interact with a defined molecular target important in maintaining the malignant state or expressed by the tumor cells. As described in Chap. 102e, successful tumors have activated biochemical pathways that lead to uncontrolled proliferation through the action of, e.g., oncogene products, loss of cell cycle inhibitors, or loss of cell death regulation, and have acquired the capacity to replicate chromosomes indefinitely, invade, metastasize, and evade the immune system. Targeted therapies seek to capitalize on the biology behind the aberrant cellular behavior as a basis for therapeutic effects. Hormonal therapies (the first form of targeted therapy) capitalize on the biochemical pathways underlying estrogen and androgen function and action as a therapeutic basis for approaching patients with tumors of breast, prostate, uterus, and ovarian origin. Biologic therapies are often macromolecules that have a particular target (e.g., antigrowth factor or cytokine antibodies) or may have the capacity to regulate growth of tumor cells or induce a host immune response to kill tumor cells. Thus, biologic therapies include not only antibodies but also cytokines and gene therapies.
CANCER CHEMOTHERAPY
Principles The usefulness of any drug is governed by the extent to which a given dose causes a useful result (therapeutic effect; in the case of anticancer agents, toxicity to tumor cells) as opposed to a toxic effect to the host. The therapeutic index is the degree of separation between toxic and therapeutic doses. Really useful drugs have large therapeutic indices, and this usually occurs when the drug target is expressed in the disease-causing compartment as opposed to the normal compartment. Classically, selective toxicity of an agent for a tissue or cell type is governed by the differential expression of a drug’s target in the “sensitive” cell type or by differential drug accumulation into or elimination from compartments where greater or lesser toxicity is experienced, respectively. Currently used chemotherapeutic agents have the unfortunate property that their targets are present in both normal and tumor tissues. Therefore, they have relatively narrow therapeutic indices.
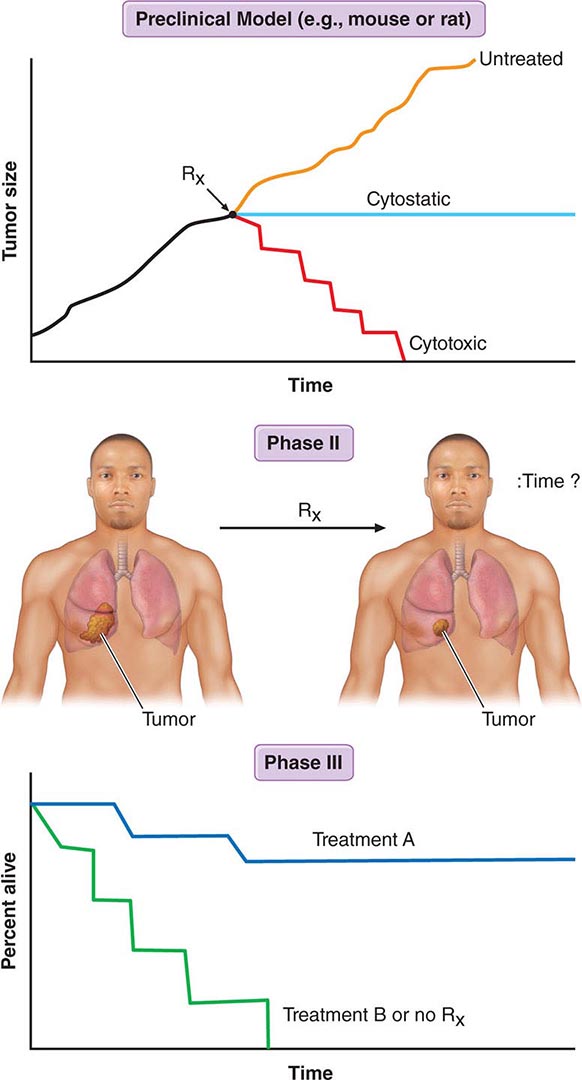
Figure 103e-2 illustrates steps in cancer drug development. Following demonstration of antitumor activity in animal models, potentially useful anticancer agents are further evaluated to define an optimal schedule of administration and arrive at a drug formulation designed for a given route of administration and schedule. Safety testing in two species on an analogous schedule of administration defines the starting dose for a phase 1 trial in humans, usually but not always in patients with cancer who have exhausted “standard” (already approved) treatments. The initial dose is usually one-sixth to one-tenth of the dose just causing easily reversible toxicity in the more sensitive animal species. Escalating doses of the drug are then given during the human phase 1 trial until reversible toxicity is observed. Dose-limiting toxicity (DLT) defines a dose that conveys greater toxicity than would be acceptable in routine practice, allowing definition of a lower maximum-tolerated dose (MTD). The occurrence of toxicity is, if possible, correlated with plasma drug concentrations. The MTD or a dose just lower than the MTD is usually the dose suitable for phase 2 trials, where a fixed dose is administered to a relatively homogeneous set of patients with a particular tumor type in an effort to define whether the drug causes regression of tumors. In a phase 3 trial, evidence of improved overall survival or improvement in the time to progression of disease on the part of the new drug is sought in comparison to an appropriate control population, which is usually receiving an acceptable “standard of care” approach. A favorable outcome of a phase 3 trial is the basis for application to a regulatory agency for approval of the new agent for commercial marketing as safe and possessing a measure of clinical effectiveness.
FIGURE 103e-2 Steps in cancer drug discovery and development. Preclinical activity (top) in animal models of cancers may be used as evidence to support the entry of the drug candidate into phase 1 trials in humans to define a correct dose and observe any clinical antitumor effect that may occur. The drug may then be advanced to phase 2 trials directed against specific cancer types, with rigorous quantitation of antitumor effects (middle). Phase 3 trials then may reveal activity superior to standard or no treatment (bottom).
Response, defined as tumor shrinkage, is the most immediate indicator of drug effect. To be clinically valuable, responses must translate into clinical benefit. This is conventionally established by a beneficial effect on overall survival, or at least an increased time to further progression of disease. Karnofsky was among the first to champion the evaluation of a chemotherapeutic agent’s benefit by carefully quantitating its effect on tumor size and using these measurements to objectively decide the basis for further treatment of a particular patient or further clinical evaluation of a drug’s potential. A partial response (PR) is defined conventionally as a decrease by at least 50% in a tumor’s bidimensional area; a complete response (CR) connotes disappearance of all tumor; progression of disease signifies an increase in size of existing lesions by >25% from baseline or best response or development of new lesions; and stable disease fits into none of the above categories. Newer evaluation systems, such as Response Evaluation Criteria in Solid Tumors (RECIST), use unidimensional measurement, but the intent is similar in rigorously defining evidence for the activity of the agent in assessing its value to the patient. An active chemotherapy agent conventionally has PR rates of at least 20–25% with reversible non-life-threatening side effects, and it may then be suitable for study in phase 3 trials to assess efficacy in comparison to standard or no therapy. Active efforts are being made to quantitate effects of anticancer agents on quality of life. Cancer drug clinical trials conventionally use a toxicity grading scale where grade 1 toxicities do not require treatment, grade 2 toxicities may require symptomatic treatment but are not life-threatening, grade 3 toxicities are potentially life-threatening if untreated, grade 4 toxicities are actually life-threatening, and grade 5 toxicities are those that result in the patient’s death.
Development of targeted agents may proceed quite differently. While phase 1–3 trials are still conducted, molecular analysis of human tumors may allow the precise definition of target expression in a patient’s tumor that is necessary for or relevant to the drug’s action. This information might then allow selection of patients expressing the drug target for participation in all trial phases. These patients may then have a greater chance of developing a useful response to the drug by virtue of expressing the target in the tumor. Clinical trials may be designed to incorporate an assessment of the behavior of the target in relation to the drug (pharmacodynamic studies). Ideally, the plasma concentration that affects the drug target is known, so escalation to MTD may not be necessary. Rather, the correlation of host toxicity while achieving an “optimal biologic dose” becomes a more relevant endpoint for phase 1 and early phase 2 trials with targeted agents.
Useful cancer drug treatment strategies using conventional chemotherapy agents, targeted agents, hormonal treatments, or biologics have one of two valuable outcomes. They can induce cancer cell death, resulting in tumor shrinkage with corresponding improvement in patient survival, or increase the time until the disease progresses. Another potential outcome is to induce cancer cell differentiation or dormancy with loss of tumor cell replicative potential and reacquisition of phenotypic properties resembling normal cells. A blocking in normal cellular differentiation may be a key feature in the pathogenesis of certain leukemias.
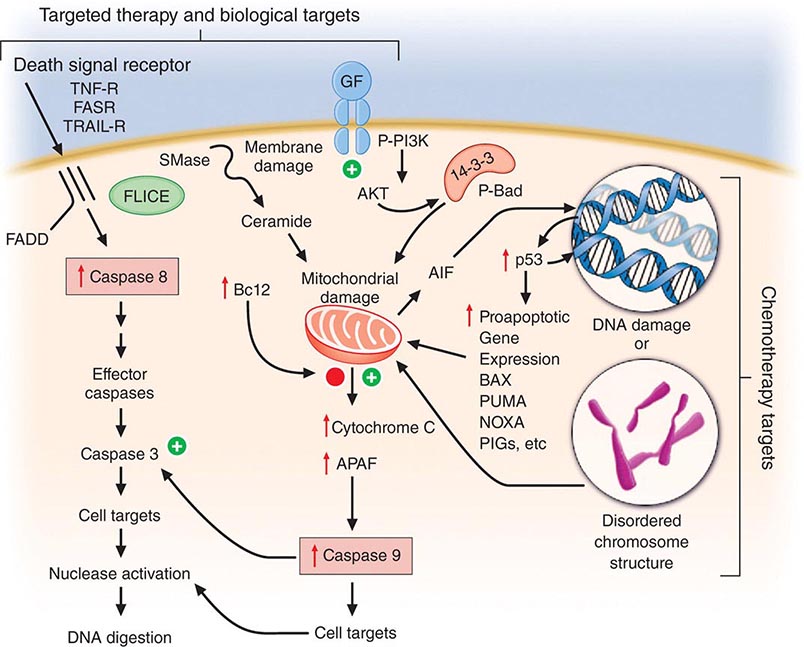
Cell death is a closely regulated process. Necrosis refers to cell death induced, for example, by physical damage with the hallmarks of cell swelling and membrane disruption. Apoptosis, or programmed cell death, refers to a highly ordered process whereby cells respond to defined stimuli by dying, and it recapitulates the necessary cell death observed during the ontogeny of the organism. Cancer chemotherapeutic agents can cause both necrosis and apoptosis. Apoptosis is characterized by chromatin condensation (giving rise to “apoptotic bodies”), cell shrinkage, and, in living animals, phagocytosis by surrounding stromal cells without evidence of inflammation. This process is regulated either by signal transduction systems that promote a cell’s demise after a certain level of insult is achieved or in response to specific cell-surface receptors that mediate physiologic cell death responses, such as occurs in the developing organism or in the normal function of immune cells. Influencing apoptosis by manipulation of signal transduction pathways has emerged as a basis for understanding the actions of drugs and designing new strategies to improve their use. Autophagy is a cellular response to injury where the cell does not initially die but catabolizes itself in a way that can lead to loss of replicative potential. A general view of how cancer treatments work is that the interaction of a chemotherapeutic drug with its target induces a “cascade” of further signaling steps. These signals ultimately lead to cell death by triggering an “execution phase” where proteases, nucleases, and endogenous regulators of the cell death pathway are activated (Fig. 103e-3).
FIGURE 103e-3 Integration of cell death responses. Cell death through an apoptotic mechanism requires active participation of the cell. In response to interruption of growth factor (GF) or propagation of certain cytokine death signals (e.g., tumor necrosis factor receptor [TNF-R]), there is activation of “upstream” cysteine aspartyl proteases (caspases), which then directly digest cytoplasmic and nuclear proteins, resulting in activation of “downstream” caspases; these cause activation of nucleases, resulting in the characteristic DNA fragmentation that is a hallmark of apoptosis. Chemotherapy agents that create lesions in DNA or alter mitotic spindle function seem to activate aspects of this process by damage ultimately conveyed to the mitochondria, perhaps by activating the transcription of genes whose products can produce or modulate the toxicity of free radicals. In addition, membrane damage with activation of sphingomyelinases results in the production of ceramides that can have a direct action at mitochondria. The antiapoptotic protein bcl2 attenuates mitochondrial toxicity, while proapoptotic gene products such as bax antagonize the action of bcl2. Damaged mitochondria release cytochrome C and apoptosis-activating factor (APAF), which can directly activate caspase 9, resulting in propagation of a direct signal to other downstream caspases through protease activation. Apoptosis-inducing factor (AIF) is also released from the mitochondrion and then can translocate to the nucleus, bind to DNA, and generate free radicals to further damage DNA. An additional proapoptotic stimulus is the bad protein, which can heterodimerize with bcl2 gene family members to antagonize apoptosis. Importantly, though, bad protein function can be retarded by its sequestration as phospho-bad through the 14-3-3 adapter proteins. The phosphorylation of bad is mediated by the action of the AKT kinase in a way that defines how growth factors that activate this kinase can retard apoptosis and promote cell survival.
Targeted agents differ from chemotherapy agents in that they do not indiscriminately cause macromolecular lesions but regulate the action of particular pathways. For example, the p210bcr-abl fusion protein tyrosine kinase drives chronic myeloid leukemia (CML), and HER2/neu stimulates the proliferation of certain breast cancers. The tumor has been described as “addicted” to the function of these molecules in the sense that without the pathway’s continued action, the tumor cell cannot survive. In this way, targeted agents directed at p210bcr-abl or HER2/neu may alter the “threshold” tumors driven by these molecules may have for undergoing apoptosis without actually creating any molecular lesions such as direct DNA strand breakage or altered membrane function.
While apoptotic mechanisms are important in regulating cellular proliferation and the behavior of tumor cells in vitro, in vivo it is unclear whether all of the actions of chemotherapeutic agents to cause cell death can be attributed to apoptotic mechanisms. However, changes in molecules that regulate apoptosis are correlated with clinical outcomes (e.g., bcl2 overexpression in certain lymphomas conveys poor prognosis; proapoptotic bax expression is associated with a better outcome after chemotherapy for ovarian carcinoma). A better understanding of the relationship of cell death and cell survival mechanisms is needed.
Chemotherapy agents may be used for the treatment of active, clinically apparent cancer. The goal of such treatment in some cases is cure of the cancer, that is, elimination of all clinical and pathologic evidence of cancer and return of the patient to an expected survival no different than the general population. Table 103e-3, A lists those tumors considered curable by conventionally available chemotherapeutic agents when used to address disseminated or metastatic cancers. If a tumor is localized to a single site, serious consideration of surgery or primary radiation therapy should be given, because these treatment modalities may be curative as local treatments. Chemotherapy may then be used after the failure of these modalities to eradicate a local tumor or as part of multimodality approaches to offer primary treatment to a clinically localized tumor. In this event, it can allow organ preservation when given with radiation, as in the larynx or other upper airway sites, or sensitize tumors to radiation when given, e.g., to patients concurrently receiving radiation for lung or cervix cancer (Table 103e-3, B). Chemotherapy can be administered as an adjuvant, i.e., in addition to surgery or radiation (Table 103e-3, C), even after all clinically apparent disease has been removed. This use of chemotherapy has curative potential in breast and colorectal neoplasms, as it attempts to eliminate clinically unapparent tumor that may have already disseminated. As noted above, small tumors frequently have high growth fractions and therefore may be intrinsically more susceptible to the action of antiproliferative agents. Neoadjuvant chemotherapy refers to administration of chemotherapy prior to any surgery or radiation to a local tumor in an effort to enhance the effect of the local treatment.
|
CURABILITY OF CANCERS WITH CHEMOTHERAPY |
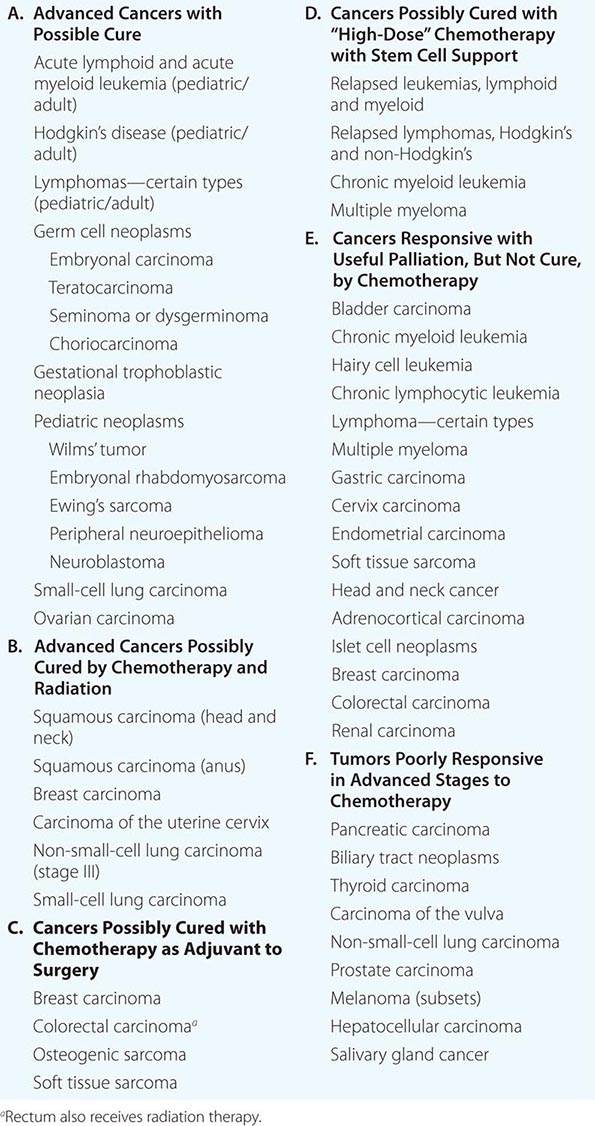
Chemotherapy is routinely used in “conventional” dose regimens. In general, these doses produce reversible acute side effects, primarily consisting of transient myelosuppression with or without gastrointestinal toxicity (usually nausea), which are readily managed. “High-dose” chemotherapy regimens are predicated on the observation that the dose-response curve for many anticancer agents is rather steep, and increased dose can produce markedly increased therapeutic effect, although at the cost of potentially life-threatening complications that require intensive support, usually in the form of hematopoietic stem cell support from the patient (autologous) or from donors matched for histocompatibility loci (allogeneic), or pharmacologic “rescue” strategies to repair the effect of the high-dose chemotherapy on normal tissues. High-dose regimens have definite curative potential in defined clinical settings (Table 103e-3, D).
If cure is not possible, chemotherapy may be undertaken with the goal of palliating some aspect of the tumor’s effect on the host. In this usage, value is perceived by the demonstration of improved symptom relief, progression-free survival, or overall survival at a certain time from the inception of treatment in the treated population, compared to a relevant control population established as the result of clinical research protocol or other organized comparative study. Such clinical research protocols are the basis for U.S. Food and Drug Administration (FDA) approval of a particular cancer treatment as safe and effective and are the benchmark for an evidence-based approach to the use of chemotherapeutic agents. Common tumors that may be meaningfully addressed by chemotherapy with palliative intent are listed in Table 103e-3, E.
Usually, tumor-related symptoms manifest as pain, weight loss, or some local symptom related to the tumor’s effect on normal structures. Patients treated with palliative intent should be aware of their diagnosis and the limitations of the proposed treatments, have access to supportive care, and have suitable “performance status,” according to assessment algorithms such as the one developed by Karnofsky (see Table 99-4) or by the Eastern Cooperative Oncology Group (ECOG) (see Table 99-5). ECOG performance status 0 (PS0) patients are without symptoms; PS1 patients are ambulatory but restricted in strenuous physical activity; PS2 patients are ambulatory but unable to work and are up and about 50% or more of the time; PS3 patients are capable of limited self-care and are up <50% of the time; and PS4 patients are totally confined to bed or chair and incapable of self-care. Only PS0, PS1, and PS2 patients are generally considered suitable for palliative (noncurative) treatment. If there is curative potential, even poor–performance status patients may be treated, but their prognosis is usually inferior to that of good–performance status patients treated with similar regimens.
An important perspective the primary care provider may bring to patients and their families facing incurable cancer is that, given the limited value of chemotherapeutic approaches at some point in the natural history of most metastatic cancers, palliative care or hospice-based approaches, with meticulous and ongoing attention to symptom relief and with family, psychological, and spiritual support, should receive prominent attention as a valuable therapeutic plan (Chaps. 10 and 99). Optimizing the quality of life rather than attempting to extend it becomes a valued intervention. Patients facing the impending progression of disease in a life-threatening way frequently choose to undertake toxic treatments of little to no potential value, and support provided by the primary caregiver in accessing palliative and hospice-based options in contrast to receiving toxic and ineffective regimen can be critical in providing a basis for patients to make sensible choices.
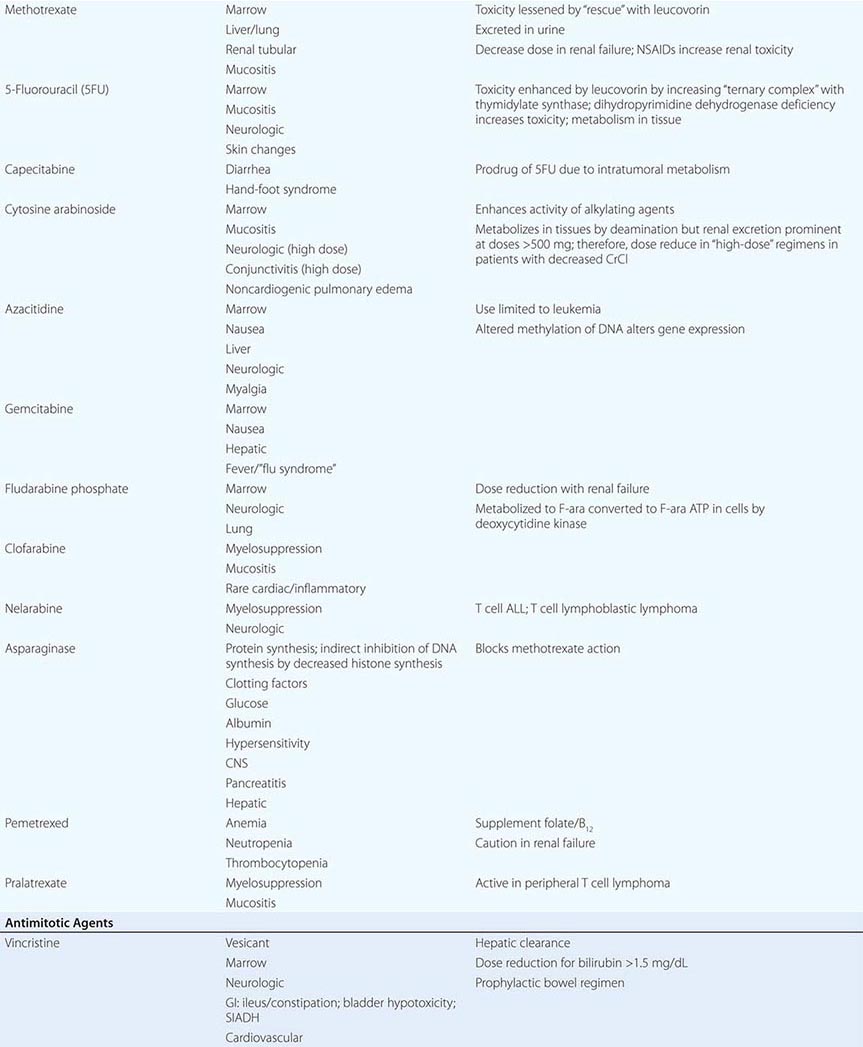
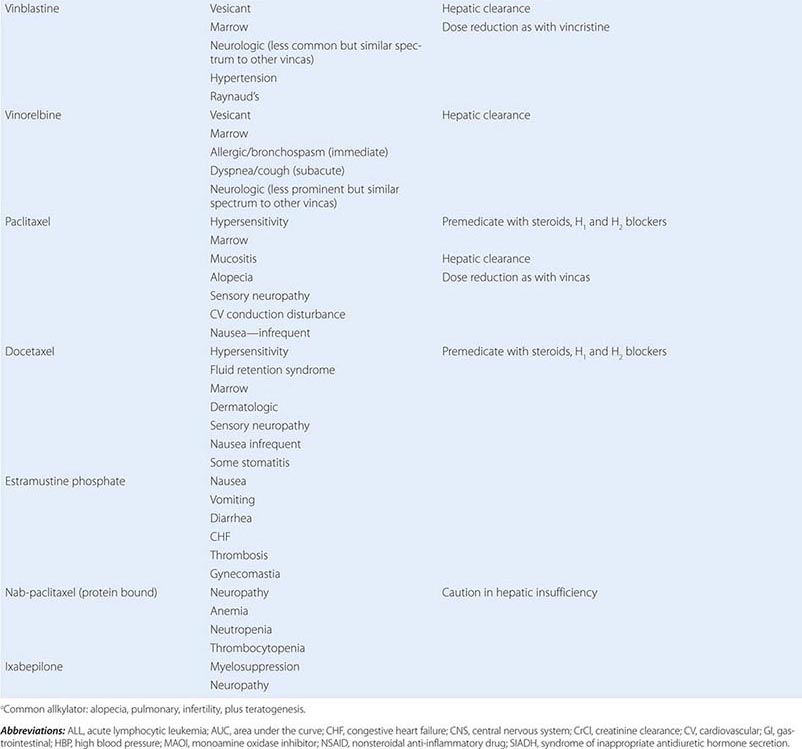
Cytotoxic Chemotherapy Agents Table 103e-4 lists commonly used cytotoxic cancer chemotherapy agents and pertinent clinical aspects of their use, with particular reference to adverse effects that might be encountered by the generalist in the care of patients. The drugs listed may be usefully grouped into two general categories: those affecting DNA and those affecting microtubules.
|
CYTOTOXIC CHEMOTHERAPY AGENTS |
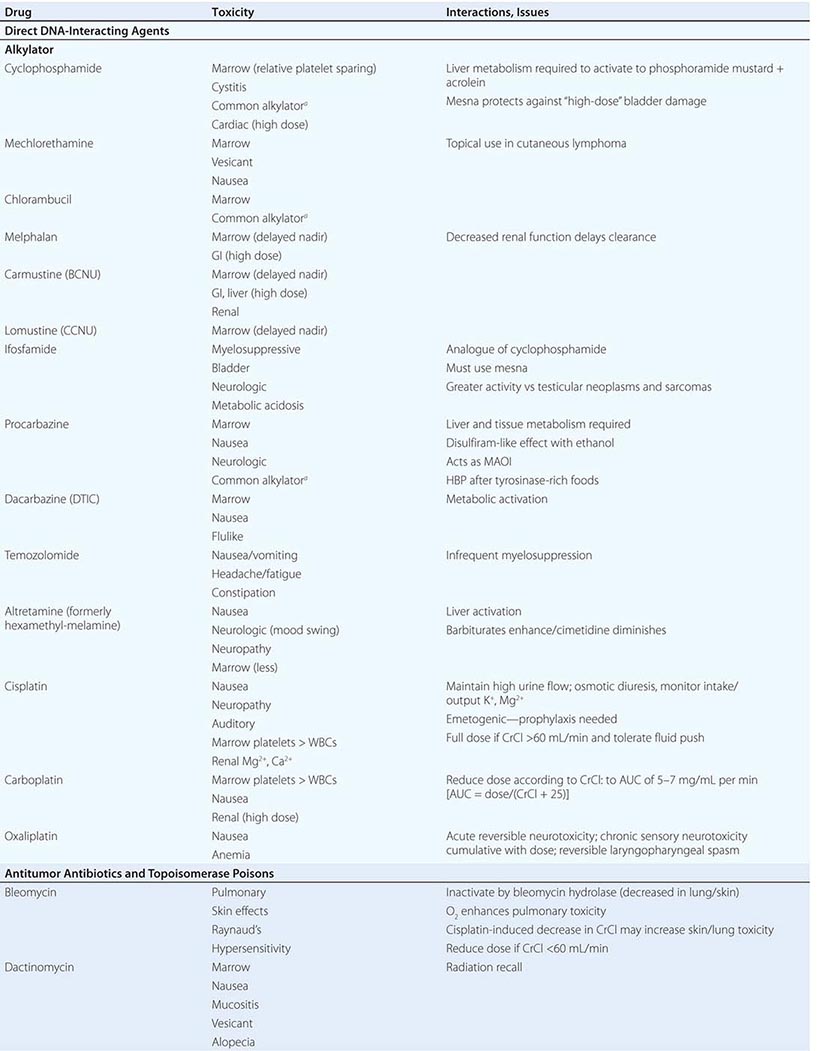
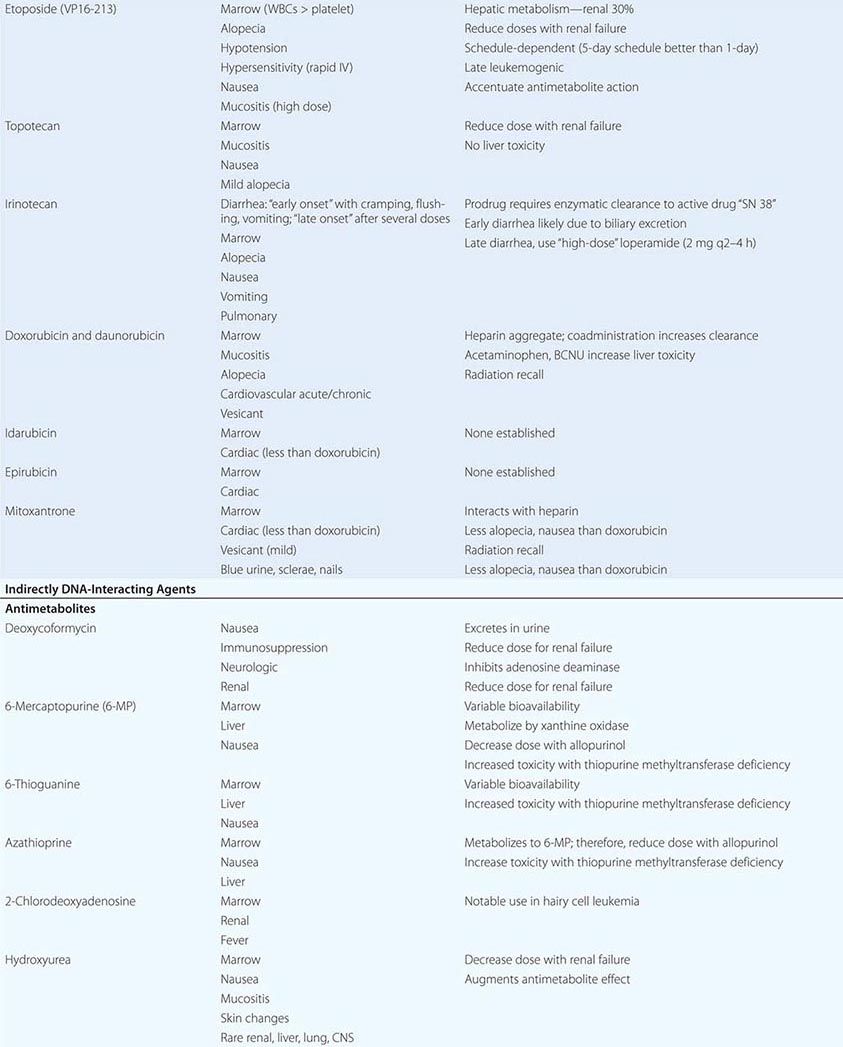
DIRECT DNA-INTERACTIVE AGENTS DNA replication occurs during the synthesis or S-phase of the cell cycle, with chromosome segregation of the replicated DNA occurring in the M, or mitosis, phase. The G1 and G2 “gap phases” precede S and M, respectively. Historically, chemotherapeutic agents have been divided into “phase-nonspecific” agents, which can act in any phase of the cell cycle, and “phase-specific” agents, which require the cell to be at a particular cell cycle phase to cause greatest effect. Once the agent has acted, cells may progress to “checkpoints” in the cell cycle where the drug-related damage may be assessed and either repaired or allowed to initiate apoptosis. An important function of certain tumor-suppressor genes such as p53 may be to modulate checkpoint function.
Alkylating agents as a class are cell cycle phase–nonspecific agents. They break down, either spontaneously or after normal organ or tumor cell metabolism, to reactive intermediates that covalently modify bases in DNA. This leads to cross-linkage of DNA strands or the appearance of breaks in DNA as a result of repair efforts. “Broken” or cross-linked DNA is intrinsically unable to complete normal replication or cell division; in addition, it is a potent activator of cell cycle checkpoints and further activates cell-signaling pathways that can precipitate apoptosis. As a class, alkylating agents share similar toxicities: myelosuppression, alopecia, gonadal dysfunction, mucositis, and pulmonary fibrosis. They differ greatly in a spectrum of normal organ toxicities. As a class, they share the capacity to cause “second” neoplasms, particularly leukemia, many years after use, particularly when used in low doses for protracted periods.
Cyclophosphamide is inactive unless metabolized by the liver to 4-hydroxy-cyclophosphamide, which decomposes into an alkylating species, as well as to chloroacetaldehyde and acrolein. The latter causes chemical cystitis; therefore, excellent hydration must be maintained while using cyclophosphamide. If severe, the cystitis may be prevented from progressing or prevented altogether (if expected from the dose of cyclophosphamide to be used) by mesna (2-mercaptoethanesulfonate). Liver disease impairs cyclophosphamide activation. Sporadic interstitial pneumonitis leading to pulmonary fibrosis can accompany the use of cyclophosphamide, and high doses used in conditioning regimens for bone marrow transplant can cause cardiac dysfunction. Ifosfamide is a cyclophosphamide analogue also activated in the liver, but more slowly, and it requires coadministration of mesna to prevent bladder injury. Central nervous system (CNS) effects, including somnolence, confusion, and psychosis, can follow ifosfamide use; the incidence appears related to low body surface area or decreased creatinine clearance.
Several alkylating agents are less commonly used. Nitrogen mustard (mechlorethamine) is the prototypic agent of this class, decomposing rapidly in aqueous solution to potentially yield a bifunctional carbonium ion. It must be administered shortly after preparation into a rapidly flowing intravenous line. It is a powerful vesicant, and infiltration may be symptomatically ameliorated by infiltration of the affected site with 1/6 M thiosulfate. Even without infiltration, aseptic thrombophlebitis is frequent. It can be used topically as a dilute solution or ointment in cutaneous lymphomas, with a notable incidence of hypersensitivity reactions. It causes moderate nausea after intravenous administration. Bendamustine is a nitrogen mustard derivative with evidence of activity in chronic lymphocytic leukemia and certain lymphomas.
Chlorambucil causes predictable myelosuppression, azoospermia, nausea, and pulmonary side effects. Busulfan can cause profound myelosuppression, alopecia, and pulmonary toxicity but is relatively “lymphocyte sparing.” Its routine use in treatment of CML has been curtailed in favor of imatinib (Gleevec) or dasatinib, but it is still used in transplant preparation regimens. Melphalan shows variable oral bioavailability and undergoes extensive binding to albumin and α1-acidic glycoprotein. Mucositis appears more prominently; however, it has prominent activity in multiple myeloma.
Nitrosoureas break down to carbamylating species that not only cause a distinct pattern of DNA base pair–directed toxicity but also can covalently modify proteins. They share the feature of causing relatively delayed bone marrow toxicity, which can be cumulative and long-lasting. Procarbazine is metabolized in the liver and possibly in tumor cells to yield a variety of free radical and alkylating species. In addition to myelosuppression, it causes hypnotic and other CNS effects, including vivid nightmares. It can cause a disulfiram-like syndrome on ingestion of ethanol. Altretamine (formerly hexa-methylmelamine) and thiotepa can chemically give rise to alkylating species, although the nature of the DNA damage has not been well characterized in either case. Dacarbazine (DTIC) is activated in the liver to yield the highly reactive methyl diazonium cation. It causes only modest myelosuppression 21–25 days after a dose but causes prominent nausea on day 1. Temozolomide is structurally related to dacarbazine but was designed to be activated by nonenzymatic hydrolysis in tumors and is bioavailable orally.
Cisplatin was discovered fortuitously by observing that bacteria present in electrolysis solutions with platinum electrodes could not divide. Only the cis diamine configuration is active as an antitumor agent. It is hypothesized that in the intracellular environment, a chloride is lost from each position, being replaced by a water molecule. The resulting positively charged species is an efficient bifunctional interactor with DNA, forming Pt-based cross-links. Cisplatin requires administration with adequate hydration, including forced diuresis with mannitol to prevent kidney damage; even with the use of hydration, gradual decrease in kidney function is common, along with noteworthy anemia. Hypomagnesemia frequently attends cisplatin use and can lead to hypocalcemia and tetany. Other common toxicities include neurotoxocity with stocking-and-glove sensorimotor neuropathy. Hearing loss occurs in 50% of patients treated with conventional doses. Cisplatin is intensely emetogenic, requiring prophylactic antiemetics. Myelosuppression is less evident than with other alkylating agents. Chronic vascular toxicity (Raynaud’s phenomenon, coronary artery disease) is a more unusual toxicity. Carboplatin displays less nephro-, oto-, and neurotoxicity. However, myelosuppression is more frequent, and because the drug is exclusively cleared through the kidney, adjustment of dose for creatinine clearance must be accomplished through use of various dosing nomograms. Oxaliplatin is a platinum analogue with noteworthy activity in colon cancers refractory to other treatments. It is prominently neurotoxic.
ANTITUMOR ANTIBIOTICS AND TOPOISOMERASE POISONS Antitumor antibiotics are substances produced by bacteria that in nature appear to provide a chemical defense against other hostile microorganisms. As a class, they bind to DNA directly and can frequently undergo electron transfer reactions to generate free radicals in close proximity to DNA, leading to DNA damage in the form of single-strand breaks or cross-links. Topoisomerase poisons include natural products or semisynthetic species derived ultimately from plants, and they modify enzymes that regulate the capacity of DNA to unwind to allow normal replication or transcription. These include topoisomerase I, which creates single-strand breaks that then rejoin following the passage of the other DNA strand through the break. Topoisomerase II creates double-strand breaks through which another segment of DNA duplex passes before rejoining. DNA damage from these agents can occur in any cell cycle phase, but cells tend to arrest in S-phase or G2 of the cell cycle in cells with p53 and Rb pathway lesions as the result of defective checkpoint mechanisms in cancer cells. Owing to the role of topoisomerase I in the procession of the replication fork, topoisomerase I poisons cause lethality if the topoisomerase I–induced lesions are made in S-phase.
Doxorubicin can intercalate into DNA, thereby altering DNA structure, replication, and topoisomerase II function. It can also undergo reduction reactions by accepting electrons into its quinone ring system, with the capacity to undergo reoxidation to form reactive oxygen radicals after reoxidation. It causes predictable myelosuppression, alopecia, nausea, and mucositis. In addition, it causes acute cardiotoxicity in the form of atrial and ventricular dysrhythmias, but these are rarely of clinical significance. In contrast, cumulative doses >550 mg/m2 are associated with a 10% incidence of chronic cardiomyopathy. The incidence of cardiomyopathy appears to be related to schedule (peak serum concentration), with low-dose, frequent treatment or continuous infusions better tolerated than intermittent higher-dose exposures. Cardiotoxicity has been related to iron-catalyzed oxidation and reduction of doxorubicin, and not to topoisomerase action. Cardiotoxicity is related to peak plasma dose; thus, lower doses and continuous infusions are less likely to cause heart damage. Doxorubicin’s cardiotoxicity is increased when given together with trastuzumab (Herceptin), the anti-HER2/neu antibody. Radiation recall or interaction with concomitantly administered radiation to cause local site complications is frequent. The drug is a powerful vesicant, with necrosis of tissue apparent 4–7 days after an extravasation; therefore, it should be administered into a rapidly flowing intravenous line. Dexrazoxane is an antidote to doxorubicin-induced extravasation. Doxorubicin is metabolized by the liver, so doses must be reduced by 50–75% in the presence of liver dysfunction. Daunorubicin is closely related to doxorubicin and was actually introduced first into leukemia treatment, where it remains part of curative regimens and has been shown preferable to doxorubicin owing to less mucositis and colonic damage. Idarubicin is also used in acute myeloid leukemia treatment and may be preferable to daunorubicin in activity. Encapsulation of daunorubicin into a liposomal formulation has attenuated cardiac toxicity and antitumor activity in Kaposi’s sarcoma, other sarcomas, multiple myeloma, and ovarian cancer.
Bleomycin refers to a mixture of glycopeptides that have the unique feature of forming complexes with Fe2+ while also bound to DNA. It remains an important component of curative regimens for Hodgkin’s disease and germ cell neoplasms. Oxidation of Fe2+ gives rise to superoxide and hydroxyl radicals. The drug causes little, if any, myelosuppression. The drug is cleared rapidly, but augmented skin and pulmonary toxicity in the presence of renal failure has led to the recommendation that doses be reduced by 50–75% in the face of a creatinine clearance <25 mL/min. Bleomycin is not a vesicant and can be administered intravenously, intramuscularly, or subcutaneously. Common side effects include fever and chills, facial flush, and Raynaud’s phenomenon. Hypertension can follow rapid intravenous administration, and the incidence of anaphylaxis with early preparations of the drug has led to the practice of administering a test dose of 0.5–1 unit before the rest of the dose. The most feared complication of bleomycin treatment is pulmonary fibrosis, which increases in incidence at >300 cumulative units administered and is minimally responsive to treatment (e.g., glucocorticoids). The earliest indicator of an adverse effect is usually a decline in the carbon monoxide diffusing capacity (DLco) or coughing, although cessation of drug immediately upon documentation of a decrease in DLco may not prevent further decline in pulmonary function. Bleomycin is inactivated by a bleomycin hydrolase, whose concentration is diminished in skin and lung. Because bleomycin-dependent electron transport is dependent on O2, bleomycin toxicity may become apparent after exposure to transient very high fraction of inspired oxygen (FIO2). Thus, during surgical procedures, patients with prior exposure to bleomycin should be maintained on the lowest FIo2 consistent with maintaining adequate tissue oxygenation.
Mitoxantrone is a synthetic compound that was designed to recapitulate features of doxorubicin but with less cardiotoxicity. It is quantitatively less cardiotoxic (comparing the ratio of cardiotoxic to therapeutically effective doses) but is still associated with a 10% incidence of cardiotoxicity at cumulative doses of >150 mg/m2. It also causes alopecia. Cases of acute promyelocytic leukemia (APL) have arisen shortly after exposure of patients to mitoxantrone, particularly in the adjuvant treatment of breast cancer. Although chemotherapy-associated leukemia is generally of the acute myeloid type, APL arising in the setting of prior mitoxantrone treatment had the typical t(15;17) chromosome translocation associated with APL, but the breakpoints of the translocation appeared to be at topoisomerase II sites that would be preferred sites of mitoxantrone action, clearly linking the action of the drug to the generation of the leukemia.
Etoposide was synthetically derived from the plant product podophyllotoxin; it binds directly to topoisomerase II and DNA in a reversible ternary complex. It stabilizes the covalent intermediate in the enzyme’s action where the enzyme is covalently linked to DNA. This “alkali-labile” DNA bond was historically a first hint that an enzyme such as a topoisomerase might exist. The drug therefore causes a prominent G2 arrest, reflecting the action of a DNA damage checkpoint. Prominent clinical effects include myelosuppression, nausea, and transient hypotension related to the speed of administration of the agent. Etoposide is a mild vesicant but is relatively free from other large-organ toxicities. When given at high doses or very frequently, topoisomerase II inhibitors may cause acute leukemia associated with chromosome 11q23 abnormalities in up to 1% of exposed patients.
Camptothecin was isolated from extracts of a Chinese tree and had notable antileukemia activity in preclinical mouse models. Early human clinical studies with the sodium salt of the hydrolyzed camptothecin lactone showed evidence of toxicity with little antitumor activity. Identification of topoisomerase I as the target of camptothecins and the need to preserve lactone structure allowed additional efforts to identify active members of this series. Topoisomerase I is responsible for unwinding the DNA strand by introducing single-strand breaks and allowing rotation of one strand about the other. In S-phase, topoisomerase I–induced breaks that are not promptly resealed lead to progress of the replication fork off the end of a DNA strand. The DNA damage is a potent signal for induction of apoptosis. Camptothecins promote the stabilization of the DNA linked to the enzyme in a so-called cleavable complex, analogous to the action of etoposide with topoisomerase II. Topotecan is a camptothecin derivative approved for use in gynecologic tumors and small-cell lung cancer. Toxicity is limited to myelosuppression and mucositis. CPT-11, or irinotecan, is a camptothecin with evidence of activity in colon carcinoma. In addition to myelosuppression, it causes a secretory diarrhea related to the toxicity of a metabolite called SN-38. Levels of SN-38 are particularly high in the setting of Gilbert’s disease, characterized by defective glucuronyl transferase and indirect hyperbilirubinemia, a condition that affects about 10% of the white population in the United States. The diarrhea can be treated effectively with loperamide or octreotide.
INDIRECT MODULATORS OF NUCLEIC ACID FUNCTION: ANTIMETABOLITES A broad definition of antimetabolites would include compounds with structural similarity to precursors of purines or pyrimidines, or compounds that interfere with purine or pyrimidine synthesis. Some antimetabolites can cause DNA damage indirectly, through misincorporation into DNA, abnormal timing or progression through DNA synthesis, or altered function of pyrimidine and purine biosynthetic enzymes. They tend to convey greatest toxicity to cells in S-phase, and the degree of toxicity increases with duration of exposure. Common toxic manifestations include stomatitis, diarrhea, and myelosuppression. Second malignancies are not associated with their use.
Methotrexate inhibits dihydrofolate reductase, which regenerates reduced folates from the oxidized folates produced when thymidine monophosphate is formed from deoxyuridine monophosphate. Without reduced folates, cells die a “thymine-less” death. N5-Tetrahydrofolate or N5-formyltetrahydrofolate (leucovorin) can bypass this block and rescue cells from methotrexate, which is maintained in cells by polyglutamylation. The drug and other reduced folates are transported into cells by the folate carrier, and high concentrations of drug can bypass this carrier and allow diffusion of drug directly into cells. These properties have suggested the design of “high-dose” methotrexate regimens with leucovorin rescue of normal marrow and mucosa as part of curative approaches to osteosarcoma in the adjuvant setting and hematopoietic neoplasms of children and adults. Methotrexate is cleared by the kidney via both glomerular filtration and tubular secretion, and toxicity is augmented by renal dysfunction and drugs such as salicylates, probenecid, and nonsteroidal anti-inflammatory agents that undergo tubular secretion. With normal renal function, 15 mg/m2 leucovorin will rescue 10–8 to 10–6 M methotrexate in three to four doses. However, with decreased creatinine clearance, doses of 50–100 mg/m2 are continued until methotrexate levels are <5 × 10–8 M. In addition to bone marrow suppression and mucosal irritation, methotrexate can cause renal failure itself at high doses owing to crystallization in renal tubules; therefore, high-dose regimens require alkalinization of urine with increased flow by hydration. Methotrexate can be sequestered in third-space collections and diffuse back into the general circulation, causing prolonged myelosuppression. Less frequent adverse effects include reversible increases in transaminases and hypersensitivity-like pulmonary syndrome. Chronic low-dose methotrexate can cause hepatic fibrosis. When administered to the intrathecal space, methotrexate can cause chemical arachnoiditis and CNS dysfunction.
Pemetrexed is a novel folate-directed antimetabolite. It is “multitargeted” in that it inhibits the activity of several enzymes, including thymidylate synthetase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase, thereby affecting the synthesis of both purine and pyrimidine nucleic acid precursors. To avoid significant toxicity to the normal tissues, patients receiving pemetrexed should also receive low-dose folate and vitamin B12 supplementation. Pemetrexed has notable activity against certain lung cancers and, in combination with cisplatin, also against mesotheliomas. Pralatrexate is an antifolate approved for use in T cell lymphoma that is very efficiently transported into cancer cells.
5-Fluorouracil (5FU) represents an early example of “rational” drug design in that it originated from the observation that tumor cells incorporate radiolabeled uracil more efficiently into DNA than normal cells, especially gut. 5FU is metabolized in cells to 5´FdUMP, which inhibits thymidylate synthetase (TS). In addition, misincorporation can lead to single-strand breaks, and RNA can aberrantly incorporate FUMP. 5FU is metabolized by dihydropyrimidine dehydrogenase, and deficiency of this enzyme can lead to excessive toxicity from 5FU. Oral bioavailability varies unreliably, but orally administered analogues of 5FU such as capecitabine have been developed that allow at least equivalent activity to many parenteral 5FU-based approaches. Intravenous administration of 5FU leads to bone marrow suppression after short infusions but to stomatitis after prolonged infusions. Leucovorin augments the activity of 5FU by promoting formation of the ternary covalent complex of 5FU, the reduced folate, and TS. Less frequent toxicities include CNS dysfunction, with prominent cerebellar signs, and endothelial toxicity manifested by thrombosis, including pulmonary embolus and myocardial infarction.
Cytosine arabinoside (ara-C) is incorporated into DNA after formation of ara-CTP, resulting in S-phase–related toxicity. Continuous infusion schedules allow maximal efficiency, with uptake maximal at 5–7 μM. Ara-C can be administered intrathecally. Adverse effects include nausea, diarrhea, stomatitis, chemical conjunctivitis, and cerebellar ataxia. Gemcitabine is a cytosine derivative that is similar to ara-C in that it is incorporated into DNA after anabolism to the triphosphate, rendering DNA susceptible to breakage and repair synthesis, which differs from that in ara-C in that gemcitabine-induced lesions are very inefficiently removed. In contrast to ara-C, gemcitabine appears to have useful activity in a variety of solid tumors, with limited nonmyelosuppressive toxicities.
6-Thioguanine and 6-mercaptopurine (6MP) are used in the treatment of acute lymphoid leukemia. Although administered orally, they display variable bioavailability. 6MP is metabolized by xanthine oxidase and therefore requires dose reduction when used with allopurinol. 6MP is also metabolized by thiopurine methyltransferase; genetic deficiency of thiopurine methyltransferase results in excessive toxicity.
Fludarabine phosphate is a prodrug of F-adenine arabinoside (F-ara-A), which in turn was designed to diminish the susceptibility of ara-A to adenosine deaminase. F-ara-A is incorporated into DNA and can cause delayed cytotoxicity even in cells with low growth fraction, including chronic lymphocytic leukemia and follicular B cell lymphoma. CNS and peripheral nerve dysfunction and T cell depletion leading to opportunistic infections can occur in addition to myelosuppression. 2-Chlorodeoxyadenosine is a similar compound with activity in hairy cell leukemia. 2-Deoxycoformycin inhibits adenosine deaminase, with resulting increase in dATP levels. This causes inhibition of ribonucleotide reductase as well as augmented susceptibility to apoptosis, particularly in T cells. Renal failure and CNS dysfunction are notable toxicities in addition to immunosuppression. Hydroxyurea inhibits ribonucleotide reductase, resulting in S-phase block. It is orally bioavailable and useful for the acute management of myeloproliferative states.
Asparaginase is a bacterial enzyme that causes breakdown of extracellular asparagine required for protein synthesis in certain leukemic cells. This effectively stops tumor cell DNA synthesis, as DNA synthesis requires concurrent protein synthesis. The outcome of asparaginase action is therefore very similar to the result of the small-molecule antimetabolites. Because asparaginase is a foreign protein, hypersensitivity reactions are common, as are effects on organs such as pancreas and liver that normally require continuing protein synthesis. This may result in decreased insulin secretion with hyperglycemia, with or without hyperamylasemia and clotting function abnormalities. Close monitoring of clotting functions should accompany use of asparaginase. Paradoxically, owing to depletion of rapidly turning over anticoagulant factors, thromboses particularly affecting the CNS may also be seen with asparaginase.
MITOTIC SPINDLE INHIBITORS Microtubules are cellular structures that form the mitotic spindle, and in interphase cells, they are responsible for the cellular “scaffolding” along which various motile and secretory processes occur. Microtubules are composed of repeating noncovalent multimers of a heterodimer of α and β isoform of the protein tubulin. Vincristine binds to the tubulin dimer with the result that microtubules are disaggregated. This results in the block of growing cells in M-phase; however, toxic effects in G1 and S-phase are also evident, reflecting effects on normal cellular activities of microtubules. Vincristine is metabolized by the liver, and dose adjustment in the presence of hepatic dysfunction is required. It is a powerful vesicant, and infiltration can be treated by local heat and infiltration of hyaluronidase. At clinically used intravenous doses, neurotoxicity in the form of glove-and-stocking neuropathy is frequent. Acute neuropathic effects include jaw pain, paralytic ileus, urinary retention, and the syndrome of inappropriate antidiuretic hormone secretion. Myelosuppression is not seen. Vinblastine is similar to vincristine, except that it tends to be more myelotoxic, with more frequent thrombocytopenia and also mucositis and stomatitis. Vinorelbine is a vinca alkaloid that appears to have differences in resistance patterns in comparison to vincristine and vinblastine; it may be administered orally.
The taxanes include paclitaxel and docetaxel. These agents differ from the vinca alkaloids in that the taxanes stabilize microtubules against depolymerization. The “stabilized” microtubules function abnormally and are not able to undergo the normal dynamic changes of microtubule structure and function necessary for cell cycle completion. Taxanes are among the most broadly active antineoplastic agents for use in solid tumors, with evidence of activity in ovarian cancer, breast cancer, Kaposi’s sarcoma, and lung tumors. They are administered intravenously, and paclitaxel requires use of a Cremophor-containing vehicle that can cause hypersensitivity reactions. Premedication with dexamethasone (8–16 mg orally or intravenously 12 and 6 h before treatment) and diphenhydramine (50 mg) and cimetidine (300 mg), both 30 min before treatment, decreases but does not eliminate the risk of hypersensitivity reactions to the paclitaxel vehicle. Docetaxel uses a polysorbate 80 formulation, which can cause fluid retention in addition to hypersensitivity reactions, and dexamethasone premedication with or without antihistamines is frequently used. A protein-bound formulation of paclitaxel (called nab-paclitaxel) has at least equivalent antineoplastic activity and decreased risk of hypersensitivity reactions. Paclitaxel may also cause hypersensitivity reactions, myelosuppression, neurotoxicity in the form of glove-and-stocking numbness, and paresthesia. Cardiac rhythm disturbances were observed in phase 1 and 2 trials, most commonly asymptomatic bradycardia but also, much more rarely, varying degrees of heart block. These have not emerged as clinically significant in the majority of patients. Docetaxel causes comparable degrees of myelosuppression and neuropathy. Hypersensitivity reactions, including bronchospasm, dyspnea, and hypotension, are less frequent but occur to some degree in up to 25% of patients. Fluid retention appears to result from a vascular leak syndrome that can aggravate preexisting effusions. Rash can complicate docetaxel administration, appearing prominently as a pruritic maculopapular rash affecting the forearms, but it has also been associated with fingernail ridging, breakdown, and skin discoloration. Stomatitis appears to be somewhat more frequent than with paclitaxel. Cabazitaxel is a taxane with somewhat better activity in prostate cancers than earlier generations of taxanes, perhaps due to superior delivery to sites of disease.
Resistance to taxanes has been related to the emergence of efficient efflux of taxanes from tumor cells through the p170 P-glycoprotein (mdr gene product) or the presence of variant or mutant forms of tubulin. Epothilones represent a class of novel microtubule-stabilizing agents that have been conscientiously optimized for activity in taxane-resistant tumors. Ixabepilone has clear evidence of activity in breast cancers resistant to taxanes and anthracyclines such as doxorubicin. It retains acceptable expected side effects, including myelosuppression, and can also cause peripheral sensory neuropathy. Eribulin is a microtubule-directed agent with activity in patients who have had progression of disease on taxanes and is more similar to vinca alkaloids in its action but has similar side effects as vinca alkaloids and taxanes.
Estramustine was originally synthesized as a mustard derivative that might be useful in neoplasms that possessed estrogen receptors. However, no evidence of interaction with DNA was observed. Surprisingly, the drug caused metaphase arrest, and subsequent study revealed that it binds to microtubule-associated proteins, resulting in abnormal microtubule function. Estramustine binds to estramustine-binding proteins (EMBPs), which are notably present in prostate tumor tissue, where the drug is used. Gastrointestinal and cardiovascular adverse effects related to the estrogen moiety occur in up to 10% of patients, including worsened heart failure and thromboembolic phenomena. Gynecomastia and nipple tenderness can also occur.
Targeted Chemotherapy • HORMONE RECEPTOR–DIRECTED THERAPY Steroid hormone receptor–related molecules have emerged as prominent targets for small molecules useful in cancer treatment. When bound to their cognate ligands, these receptors can alter gene transcription and, in certain tissues, induce apoptosis. The pharmacologic effect is a mirror or parody of the normal effects of the agents acting on nontransformed normal tissues, although the effects on tumors are mediated by indirect effects in some cases. While in some cases, such as breast cancer, demonstration of the target hormone receptor is necessary, in other cases such prostate cancer (androgen receptor) and lymphoid neoplasms (glucocorticoid receptor), the relevant receptor is always present in the tumor.
Glucocorticoids are generally given in “pulsed” high doses in leukemias and lymphomas, where they induce apoptosis in tumor cells. Cushing’s syndrome and inadvertent adrenal suppression on withdrawal from high-dose glucocorticoids can be significant complications, along with infections common in immunosuppressed patients, in particular Pneumocystis pneumonia, which classically appears a few days after completing a course of high-dose glucocorticoids.
Tamoxifen is a partial estrogen receptor antagonist; it has a 10-fold greater antitumor activity in breast cancer patients whose tumors express estrogen receptors than in those who have low or no levels of expression. It might be considered the prototypic “molecularly targeted” agent. Owing to its agonistic activities in vascular and uterine tissue, side effects include a somewhat increased risk of cardiovascular complications, such as thromboembolic phenomena, and a small increased incidence of endometrial carcinoma, which appears after chronic use (usually >5 years). Progestational agents—including medroxyprogesterone acetate, androgens including fluoxymesterone (Halotestin), and, paradoxically, estrogens—have approximately the same degree of activity in primary hormonal treatment of breast cancers that have elevated expression of estrogen receptor protein. Estrogen itself is not used often owing to prominent cardiovascular and uterotropic activity.
Aromatase refers to a family of enzymes that catalyze the formation of estrogen in various tissues, including the ovary and peripheral adipose tissue and some tumor cells. Aromatase inhibitors are of two types, the irreversible steroid analogues such as exemestane and the reversible inhibitors such as anastrozole or letrozole. Anastrozole is superior to tamoxifen in the adjuvant treatment of breast cancer in postmenopausal patients with estrogen receptor–positive tumors. Letrozole treatment affords benefit following tamoxifen treatment. Adverse effects of aromatase inhibitors may include an increased risk of osteoporosis.
Prostate cancer is classically treated by androgen deprivation. Diethylstilbestrol (DES) acting as an estrogen at the level of the hypothalamus to downregulate hypothalamic luteinizing hormone (LH) production results in decreased elaboration of testosterone by the testicle. For this reason, orchiectomy is equally as effective as moderate-dose DES, inducing responses in 80% of previously untreated patients with prostate cancer but without the prominent cardiovascular side effects of DES, including thrombosis and exacerbation of coronary artery disease. In the event that orchiectomy is not accepted by the patient, testicular androgen suppression can also be effected by luteinizing hormone–releasing hormone (LHRH) agonists such as leuprolide and goserelin. These agents cause tonic stimulation of the LHRH receptor, with the loss of its normal pulsatile activation resulting in decreased output of LH by the anterior pituitary. Therefore, as primary hormonal manipulation in prostate cancer, one can choose orchiectomy or leuprolide, but not both. The addition of androgen receptor blockers, including flutamide or bicalutamide, is of uncertain additional benefit in extending overall response duration; the combined use of orchiectomy or leuprolide plus flutamide is referred to as total androgen blockade. Enzalutamide also binds to the androgen receptor and antagonizes androgen action in a mechanistically distinct way. Somewhat analogous to inhibitors of aromatase, agents have been derived that inhibit testosterone and other androgen synthesis in the testis, adrenal gland, and prostate tissue. Abiraterone inhibits 17 α-hydroxylase/C17,20 lyase (CYP 17A1) and has been shown to be active in prostate cancer patients experiencing progression despite androgen blockade.
Tumors that respond to a primary hormonal manipulation may frequently respond to second and third hormonal manipulations. Thus, breast tumors that had previously responded to tamoxifen have, on relapse, notable response rates to withdrawal of tamoxifen itself or to subsequent addition of an aromatase inhibitor or progestin. Likewise, initial treatment of prostate cancers with leuprolide plus flutamide may be followed after disease progression by response to withdrawal of flutamide. These responses may result from the removal of antagonists from mutant steroid hormone receptors that have come to depend on the presence of the antagonist as a growth-promoting influence.
Additional strategies to treat refractory breast and prostate cancers that possess steroid hormone receptors may also address adrenal capacity to produce androgens and estrogens, even after orchiectomy or oophorectomy, respectively. Thus, aminoglutethimide or ketoconazole can be used to block adrenal synthesis by interfering with the enzymes of steroid hormone metabolism. Administration of these agents requires concomitant hydrocortisone replacement and additional glucocorticoid doses administered in the event of physiologic stress.
Humoral mechanisms can also result in complications from an underlying malignancy producing the hormone. Adrenocortical carcinomas can cause Cushing’s syndrome as well as syndromes of androgen or estrogen excess. Mitotane can counteract these by decreasing synthesis of steroid hormones. Islet cell neoplasms can cause debilitating diarrhea, treated with the somatostatin analogue octreotide. Prolactin-secreting tumors can be effectively managed by the dopaminergic agonist bromocriptine.
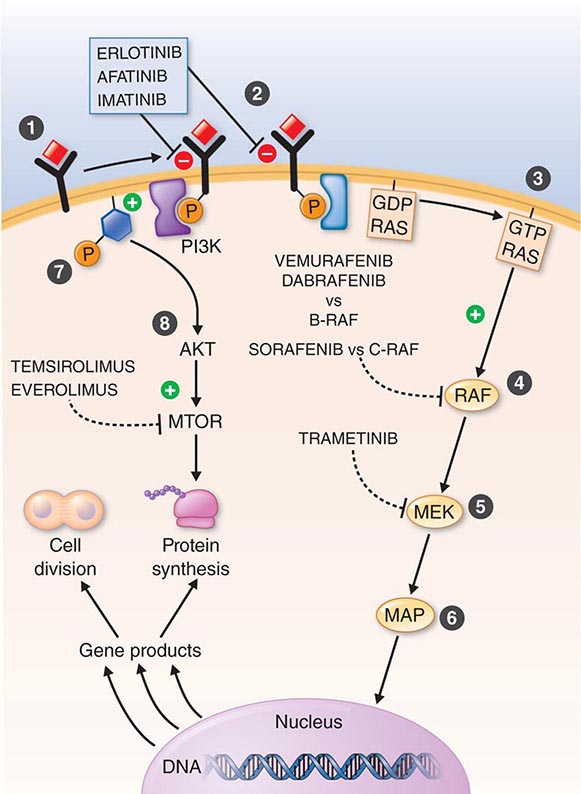
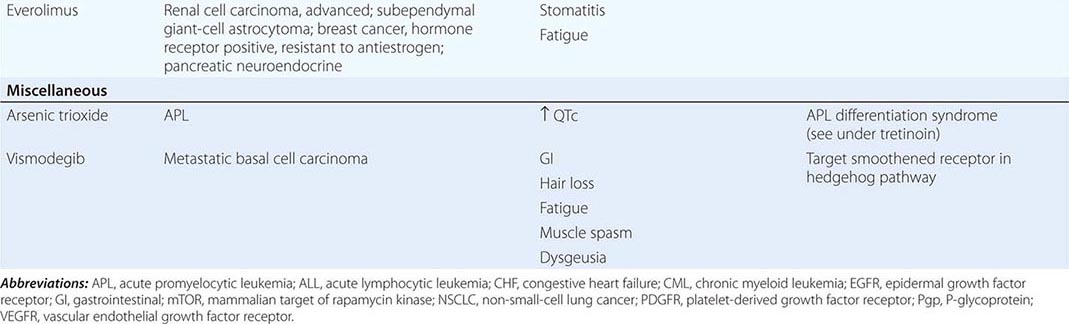
DIAGNOSTICALLY GUIDED THERAPY The basis for discovery of drugs of this type was the prior knowledge of the importance of the drugs’ molecular target to drive tumors in different contexts. Figure 103e-4 summarizes how FDA-approved targeted agents act. In the case of diagnostically guided targeted chemotherapy, prior demonstration of a specific target is necessary to guide the rational use of the agent, while in the case of targeted agents directed at oncogenic pathways, specific diagnosis of pathway activation is not yet necessary or in some cases feasible, although this is an area of ongoing clinical research. Table 103e-5 lists currently approved targeted chemotherapy agents, with features of their use.
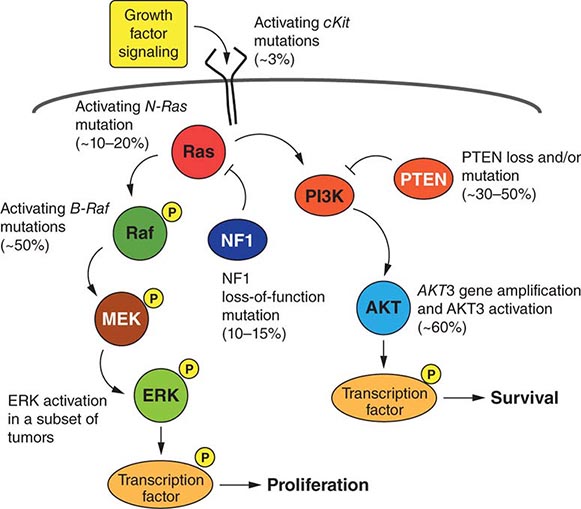
FIGURE 103e-4 Targeted chemotherapeutic agents act in most instances by interrupting cell growth factor-mediated signaling pathways. After a growth factor binds to is cognate receptor (1), in many cases there is activation of tyrsosine kinase activity particularly after dimerization of the receptors (2). This leads to autophosphorylation of the receptor and docking of “adaptor” proteins. One important pathway activated occurs after exchange of GDP for GTP in the RAS family of proto-oncogene products (3). GTP-RAS activates the RAF proto-oncogene kinase (4), leading to a phosphorylation cascade of kinases (5, 6) that ultimately impart signals to regulators of gene function to produce transcripts which activate cell cycle progression and increase protein synthesis. In parallel, tyrosine phosphorylated receptors can activate the phosphatidylinositol-3-kinase to produce the phosphorylated lipid phosphatidyl-inositol-3- phosphate (7). This leads to the activation of the AKT kinase(8) which in turn stimulates the mammalian “Target of Rapamycin” kinase (mTOR), which directly increases the translation of key mRNAs for gene products regulating cell growth. Erlotinib and afatinib, are examples of Epidermal Growth Factor receptor tyrosine kinase inhibitors; imatinib can act on the nonreceptor tyrosine kinase bcr-abl or c-KIT membrane bound tyrosine kinase. Vemurafenib and Dabrafenib act on the B isoform of RAF uniquely in melanoma, and c-RAF is inhibited by sorafenib. Trametinib acts on MEK. Temsirolimus and everolimus inhibit mTOR kinase to downregulate translation of oncogenic mRNAs.
|
MOLECULARLY TARGETED AGENTS |
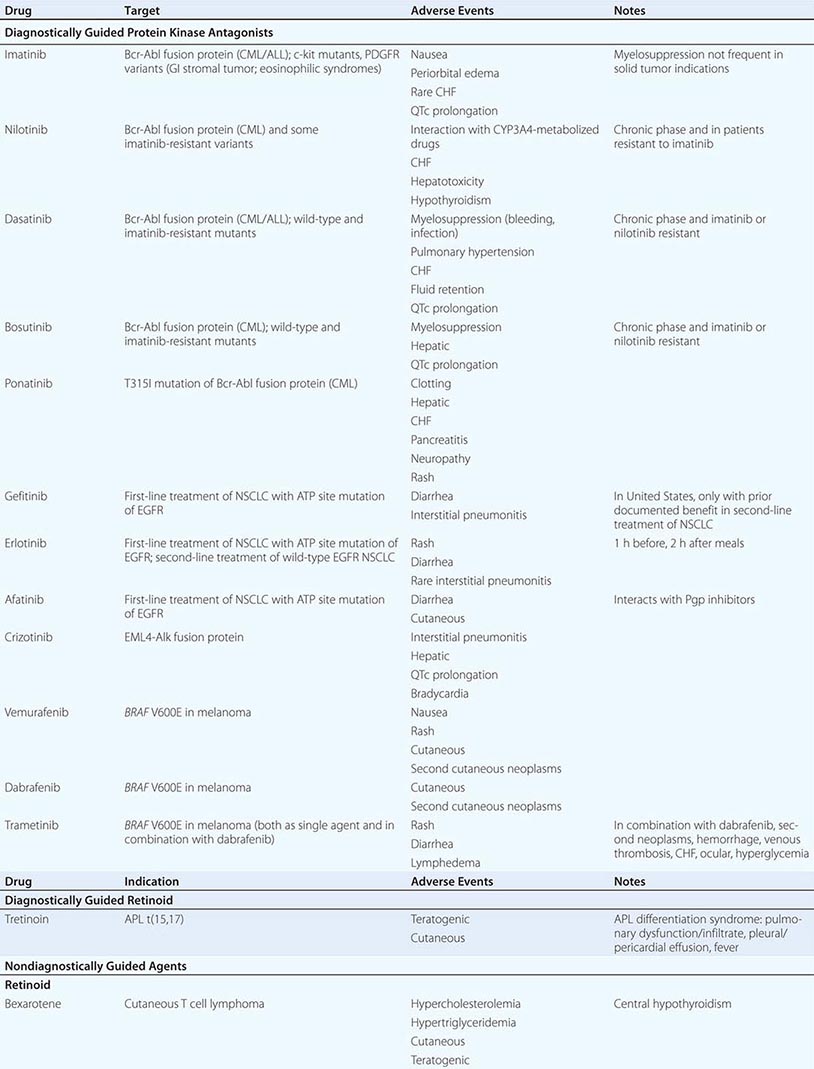
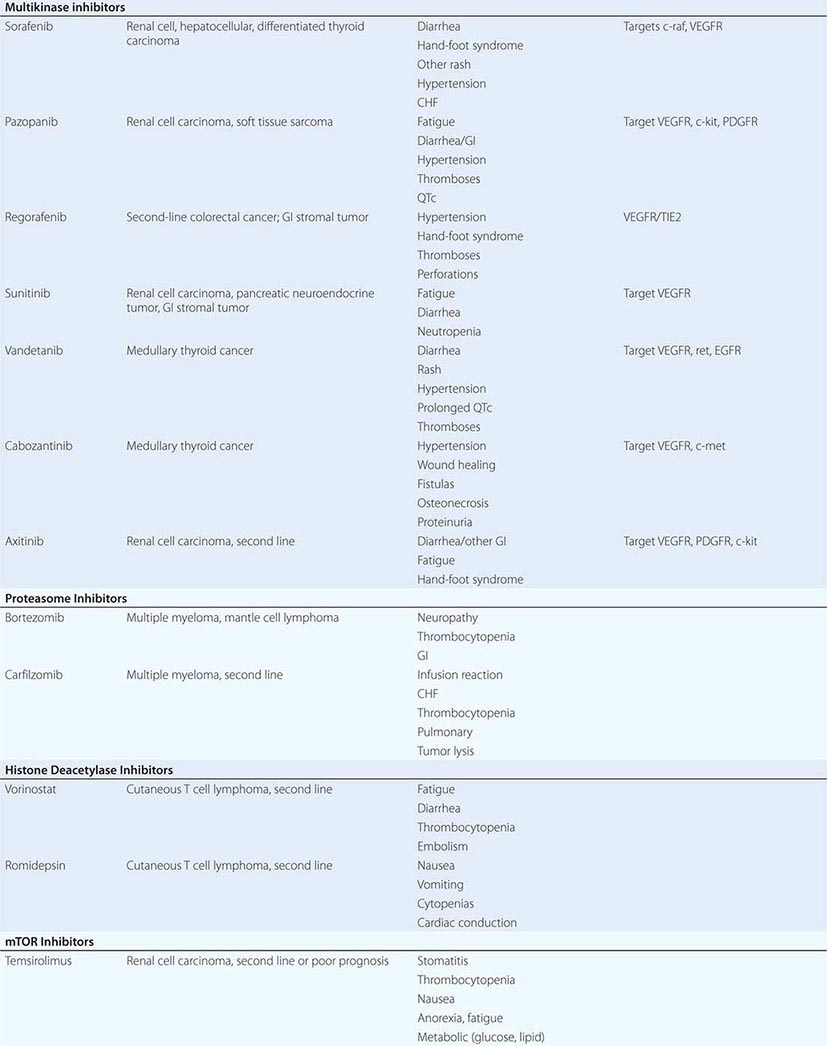
In hematologic tumors, the prototypic agent of this type is imatinib, which targets the ATP binding site of the p210bcr-abl protein tyrosine kinase that is formed as the result of the chromosome 9;22 translocation producing the Philadelphia chromosome in CML. Imatinib is superior to interferon plus chemotherapy in the initial treatment of the chronic phase of this disorder. It has lesser activity in the blast phase of CML, where the cells may have acquired additional mutations in p210bcr-abl itself or other genetic lesions. Its side effects are relatively tolerable in most patients and include hepatic dysfunction, diarrhea, and fluid retention. Rarely, patients receiving imatinib have decreased cardiac function, which may persist after discontinuation of the drug. The quality of response to imatinib enters into the decision about when to refer patients with CML for consideration of transplant approaches. Nilotinib is a tyrosine protein kinase inhibitor with a similar spectrum of activity to imatinib, but with increased potency and perhaps better tolerance by certain patients. Dasatinib, another inhibitor of the p210bcr-abl oncoproteins, is active in certain mutant variants of p210bcr-abl that are refractory to imatinib and arise during therapy with imatinib or are present de novo. Dasatinib also has inhibitory action against kinases belonging to the src tyrosine protein kinase family; this activity may contribute to its effects in hematopoietic tumors and suggest a role in solid tumors where src kinases are active. The T315I mutant of p210bcr-abl is resistant to imatinib, nilotinib, bosutinib, and dasatinib; ponatinib has activity in patients with this p210bcr-abl variant, but ponatinib has noteworthy associated thromboembolic toxicity. Use of this class of targeted agents is thus critically guided not only by the presence of the p210bcr-abl tyrosine kinase, but also by the presence of different mutations in the ATP binding site.
All-trans-retinoic acid (ATRA) targets the PML-retinoic acid receptor (RAR) α fusion protein, which is the result of the chromosome 15;17 translocation pathogenic for most forms of APL. Administered orally, it causes differentiation of the neoplastic promyelocytes to mature granulocytes and attenuates the rate of hemorrhagic complications. Adverse effects include headache with or without pseudotumor cerebri and gastrointestinal and cutaneous toxicities.
In epithelial solid tumors, the small-molecule epidermal growth factor (EGF) antagonists act at the ATP binding site of the EGF receptor tyrosine kinase. In early clinical trials, gefitinib showed evidence of responses in a small fraction of patients with non-small-cell lung cancer (NSCLC). Side effects were generally acceptable, consisting mostly of rash and diarrhea. Subsequent analysis of responding patients revealed a high frequency of activating mutations in the EGF receptor. Patients with such activating mutations who initially responded to gefitinib but who then had progression of the disease then acquired additional mutations in the enzyme, analogous functionally to mutational variants responsible for imatinib resistance in CML. Erlotinib is another EGF receptor tyrosine kinase antagonist with a superior outcome in clinical trials in NSCLC; an overall survival advantage was demonstrated in subsets of patients who were treated after demonstrating progression of disease and who also had not been preselected for the presence of activating mutations. Thus, although even patients with wild-type EGF receptors may benefit from erlotinib treatment, the presence of EGF receptor tyrosine kinase mutations has recently been shown to be a basis for recommending erlotinib and afatinib for first-line treatment of advanced NSCLC. Likewise, crizotinib targeting the alk protooncogene fusion protein has value in the initial treatment of alk-positive NSCLC. Lapatinib is a tyrosine kinase inhibitor with both EGF receptor and HER2/neu antagonist activity, which is important in the treatment of breast cancers expressing the HER2/neu oncoprotein.
In addition to the p210bcr-abl kinase, imatinib also has activity against the c-kit tyrosine kinase (the receptor for the steel growth factor, also called stem cell factor) and the platelet-derived growth factor receptor (PDGFR), both of which can be expressed in gastrointestinal stromal sarcoma (GIST). Imatinib has found clinical utility in GIST, a tumor previously notable for its refractoriness to chemotherapeutic approaches. Imatinib’s degree of activity varies with the specific mutational variant of kit or PDGFR present in a particular patient’s tumor.
The BRAF V600E mutation has been detected in a notable fraction of melanomas, thyroid tumors, and hairy cell leukemia, and preclinical models supported the concept that BRAF V600E drives oncogenic signaling in these tumors. Vemurafenib and dabrafenib, with selective capacity to inhibit the BRAF V600E serine kinase activity, were each shown to cause noteworthy responses in patients with BRAF V600E–mutated melanomas, although early relapse occurred in many patients treated with the drugs as single agents. Trametinib, acting downstream of BRAF V600E by directly inhibiting the MEK serine kinase by a non-ATP binding site mechanism, also displayed noteworthy responses in BRAF V600E–mutated melanomas, and the combination of trametinib and dabrafenib is even more active, by targeting the BRAF V600E–driven pathway at two points in the pathway leading to gene activation.
ONCOGENICALLY ACTIVATED PATHWAYS This group of agents also targets specific regulatory molecules in promoting the viability of tumor cells, but they do not require the diagnostically verified presence of a particular target or target variant at this time.
“Multitargeted” kinase antagonists are small-molecule ATP site-directed antagonists that inhibit more than one protein kinase and have value in the treatment of several solid tumors. Drugs of this type with prominent activity against the vascular endothelial growth factor receptor (VEGFR) tyrosine kinase have activity in renal cell carcinoma. Sorafenib is a VEGFR antagonist with activity against the raf serine-threonine protein kinase, and regorafenib is a closely related drug with value in relapsed advanced colon cancer. Pazopanib also prominently targets VEGFR and has activity in renal carcinoma and soft tissue sarcomas. Sunitinib has anti-VEGFR, anti-PDGFR, and anti-c-kit activity. It causes prominent responses and stabilization of disease in renal cell cancers and GISTs. Side effects for agents with anti-VEGFR activity prominently include hypertension, proteinuria, and, more rarely, bleeding and clotting disorders and perforation of scarred gastrointestinal lesions. Also encountered are fatigue, diarrhea, and the hand-foot syndrome, with erythema and desquamation of the distal extremities, in some cases requiring dose modification, particularly with sorafenib.
Temsirolimus and everolimus are mammalian target of rapamycin (mTOR) inhibitors with activity in renal cancers. They produce stomatitis, fatigue, and some hyperlipidemia (10%), myelosuppression (10%), and rare lung toxicity. Everolimus is also useful in patients with hormone receptor–positive breast cancers displaying resistance to hormonal inhibition and in certain neuroendocrine and brain tumors, the latter arising in patients with sporadic or inherited mutations in the pathway activating mTOR.
In hematologic neoplasms, bortezomib is an inhibitor of the proteasome, the multisubunit assembly of protease activities responsible for the selective degradation of proteins important in regulating activation of transcription factors, including nuclear factor-κB (NF-κB) and proteins regulating cell cycle progression. It has activity in multiple myeloma and certain lymphomas. Adverse effects include neuropathy, orthostatic hypotension with or without hyponatremia, and reversible thrombocytopenia. Carfilzomib is a proteasome inhibitor chemically unrelated to bortezomib without prominent neuropathy, but with evidence of a cytokine release syndrome, which can be a cardiopulmonary stress. Other agents active in multiple myeloma and certain other hematologic neoplasms include the immunomodulatory agents related to thalidomide, including lenalidomide and pomalidomide. All these agents collectively inhibit aberrant angiogenesis in the bone marrow microenvironment, as well as influence stromal cell immune functions to alter the cytokine milieu supporting the growth of myeloma cells. Thalidomide, although clinically active, has prominent cytopenic, neuropathic, procoagulant, and CNS toxicities that have been somewhat attenuated in the other drugs of the class, although use of these agents frequently entails concomitant anticoagulant prophylaxis.
Ibrutinib is representative a novel class of inhibitors directed at Bruton’s tyrosine kinase, which is important in the function of B cells. Initially approved for use in mantle cell lymphoma, it is potentially applicable to a number of B cell neoplasms that depend on signals through the B cell antigen receptor. Janus kinases likewise function downstream of a variety of cytokine receptors to amplify cytokine signals, and Janus kinase inhibitors including ruxolitinib have approved activity in myelofibrosis to ameliorate splenomegaly and systemic symptoms.
Vorinostat is an inhibitor of histone deacetylases, which are responsible for maintaining the proper orientation of histones on DNA, with resulting capacity for transcriptional readiness. Acetylated histones allow access of transcription factors to target genes and therefore increase expression of genes that are selectively repressed in tumors. The result can be differentiation with the emergence of a more normal cellular phenotype, or cell cycle arrest with expression of endogenous regulators of cell cycle progression. Vorinostat is approved for clinical use in cutaneous T cell lymphoma, with dramatic skin clearing and very few side effects. Romidepsin is a distinct molecular class of histone deacetylase inhibitor also active in cutaneous T cell lymphoma. An active retinoid in cutaneous T cell lymphoma is the synthetic retinoid × receptor ligand bexarotene.
DNA methyltransferase inhibitors, including 5-aza-cytidine and 2´-deoxy-5-azacytidine (decitabine), can also increase transcription of genes “silenced” during the pathogenesis of a tumor by causing demethylation of the methylated cytosines that are acquired as an “epigenetic” (i.e., after the DNA is replicated) modification of DNA. These drugs were originally considered antimetabolites but have clinical value in myelodysplastic syndromes and certain leukemias when administered at low doses.
CANCER BIOLOGIC THERAPY
Principles The goal of biologic therapy is to manipulate the host–tumor interaction in favor of the host, potentially at an optimum biologic dose that might be different than the MTD. As a class, biologic therapies may be distinguished from molecularly targeted agents in that many biologic therapies require an active response (e.g., reexpression of silenced genes or antigen expression) on the part of the tumor cell or on the part of the host (e.g., immunologic effects) to allow therapeutic effect. This may be contrasted with the more narrowly defined antiproliferative or apoptotic response that is the ultimate goal of molecularly targeted agents discussed above. However, there is much commonality in the strategies to evaluate and use molecularly targeted and biologic therapies.
Immune Cell–Mediated Therapies Tumors have a variety of means of avoiding the immune system: (1) they are often only subtly different from their normal counterparts; (2) they are capable of downregulating their major histocompatibility complex antigens, effectively masking them from recognition by T cells; (3) they are inefficient at presenting antigens to the immune system; (4) they can cloak themselves in a protective shell of fibrin to minimize contact with surveillance mechanisms; and (5) they can produce a range of soluble molecules, including potential immune targets, that can distract the immune system from recognizing the tumor cell or can kill or inactivate the immune effector cells. Some of the cell products initially polarize the immune response away from cellular immunity (shifting from TH1 to TH2 responses; Chap. 372e) and ultimately lead to defects in T cells that prevent their activation and cytotoxic activity. Cancer treatment further suppresses host immunity. A variety of strategies are being tested to overcome these barriers.
Cell-Mediated Immunity The strongest evidence that the immune system can exert clinically meaningful antitumor effects comes from allogeneic bone marrow transplantation. Adoptively transferred T cells from the donor expand in the tumor-bearing host, recognize the tumor as being foreign, and can mediate impressive antitumor effects (graft-versus-tumor effects). Three types of experimental interventions are being developed to take advantage of the ability of T cells to kill tumor cells.
1. Transfer of allogeneic T cells. This occurs in three major settings: in allogeneic bone marrow transplantation; as purified lymphocyte transfusions following bone marrow recovery after allogeneic bone marrow transplantation; and as pure lymphocyte transfusions following immunosuppressive (nonmyeloablative) therapy (also called minitransplants). In each of these settings, the effector cells are donor T cells that recognize the tumor as being foreign, probably through minor histocompatibility differences. The main risk of such therapy is the development of graft-versus-host disease because of the minimal difference between the cancer and the normal host cells. This approach has been highly effective in certain hematologic cancers.
2. Transfer of autologous T cells. In this approach, the patient’s own T cells are removed from the tumor-bearing host, manipulated in several ways in vitro, and given back to the patient. There are three major classes of autologous T-cell manipulation. First, tumor antigen–specific T cells can be developed and expanded to large numbers over many weeks ex vivo before administration. Second, the patient’s T cells can be activated by exposure to polyclonal stimulators such as anti-CD3 and anti-CD28 after a short period ex vivo, and then amplified in the host after transfer by stimulation with IL-2, for example. Short periods removed from the patient permit the cells to overcome the tumor-induced T cell defects, and such cells traffic and home to sites of disease better than cells that have been in culture for many weeks. In a third approach, genes that encode for a T cell receptor specific for an antigen expressed by the tumor along with genes that facilitate T cell activation can be introduced into subsets of a patient’s T cells, which, after transfer back into the patient, allow homing of cytotoxic T cells to tumor cells expressing the antigen.
3. Tumor vaccines aimed at boosting T cell immunity. The finding that mutant oncogenes that are expressed only intracellularly can be recognized as targets of T cell killing greatly expanded the possibilities for tumor vaccine development. No longer is it difficult to find something different about tumor cells. However, major difficulties remain in getting the tumor-specific peptides presented in a fashion to prime the T cells. Tumors themselves are very poor at presenting their own antigens to T cells at the first antigen exposure (priming). Priming is best accomplished by professional antigen-presenting cells (dendritic cells). Thus, a number of experimental strategies are aimed at priming host T cells against tumor-associated peptides. Vaccine adjuvants such as granulocyte-macrophage colony-stimulating factor (GM-CSF) appear capable of attracting antigen-presenting cells to a skin site containing a tumor antigen. Such an approach has been documented to eradicate microscopic residual disease in follicular lymphoma and give rise to tumor-specific T cells. Purified antigen-presenting cells can be pulsed with tumor, its membranes, or particular tumor antigens and delivered as a vaccine. One such vaccine, Sipuleucel-T, is approved for use in patients with hormone-independent prostate cancer. In this approach, the patient undergoes leukapheresis, wherein mononuclear cells (that include antigen-presenting cells) are removed from the patient’s blood. The cells are pulsed in a laboratory with an antigenic fusion protein comprising a protein frequently expressed by prostate cancer cells, prostate acid phosphatase, fused to GM-CSF, and matured to increase their capacity to present the antigen to immune effector cells. The cells are then returned to the patient in a well-tolerated treatment. Although no objective tumor response was documented in clinical trials, median survival was increased by about 4 months. Tumor cells can also be transfected with genes that attract antigen-presenting cells.
Another important vaccine strategy is directed at infectious agents whose action ultimately is tied to the development of human cancer. Hepatitis B vaccine in an epidemiologic sense prevents hepatocellular carcinoma, and a tetravalent human papillomavirus vaccine prevents infection by virus types currently accounting for 70% of cervical cancer. Unfortunately, these vaccines are ineffective at treating patients who have developed a virus-induced cancer.
Antibody-Mediated Therapeutic Approaches In general, antibodies are not very effective at killing cancer cells. Because the tumor seems to influence the host toward making antibodies rather than generating cellular immunity, it is inferred that antibodies are easier for the tumor to fend off. Many patients can be shown to have serum antibodies directed at their tumors, but these do not appear to influence disease progression. However, the ability to grow very large quantities of high-affinity antibody directed at a tumor by the hybridoma technique has led to the application of antibodies in the treatment of cancer. In this approach, antibodies are derived where the antigen-combining regions are grafted onto human immunoglobulin gene products (chimerized or humanized) or derived de novo from mice bearing human immunoglobulin gene loci. Three general strategies have emerged using antibodies. Tumor-regulatory antibodies target tumor cells directly or indirectly to modulate intracellular functions or attract immune or stromal cells. Immunoregulatory antibodies target antigens expressed on the tumor cells or host immune cells to modulate primarily the host’s immune responsiveness to the tumor. Finally, antibody conjugates can be made with the antibody linked to drugs, toxins, or radioisotopes to target these “warheads” for delivery to the tumor. Table 103e-6 lists features of currently used or promising antibodies for cancer treatment.
|
ANTIBODIES USED IN CANCER TREATMENT |
TUMOR-REGULATORY ANTIBODIES Humanized antibodies against the CD20 molecule expressed on B cell lymphomas (rituximab and ofatumumab) are exemplary of antibodies that affect both signaling events driving lymphomagenesis as well as activating immune responses against B cell neoplasms. They are used as single agents and in combination with chemotherapy and radiation in the treatment of B cell neoplasms. Obinutuzumab is an antibody with an altered glycosylation that enhances its ability to fix complement; it is also directed against CD20 and is of value in chronic lymphocytic leukemia. It seems to be more effective in this setting than rituximab.
The HER2/neu receptor overexpressed on epithelial cancers, especially breast cancer, was initially targeted by trastuzumab, with noteworthy activity in potentiating the action of chemotherapy in breast cancer as well as some evidence of single-agent activity. Trastuzumab also appears to interrupt intracellular signals derived from HER2/neu and to stimulate immune mechanisms. The anti-HER2 antibody pertuzumab, specifically targeting the domain of HER2/neu responsible for dimerization with other HER2 family members, is more specifically directed against HER2 signaling function and augments the action of trastuzumab.
EGF receptor (EGFR)-directed antibodies (such as cetuximab and panitumumab) have activity in colorectal cancer refractory to chemotherapy, particularly when used to augment the activity of an additional chemotherapy program, and in the primary treatment of head and neck cancers treated with radiation therapy. The mechanism of action is unclear. Direct effects on the tumor may mediate an antiproliferative effect as well as stimulate the participation of host mechanisms involving immune cell or complement-mediated response to tumor cell–bound antibody. Alternatively, the antibody may alter the release of paracrine factors promoting tumor cell survival.
The anti-VEGF antibody bevacizumab shows little evidence of antitumor effect when used alone, but when combined with chemotherapeutic agents, it improves the magnitude of tumor shrinkage and time to disease progression in colorectal and nonsquamous lung cancers. The mechanism for the effect is unclear and may relate to the capacity of the antibody to alter delivery and tumor uptake of the active chemotherapeutic agent. Ziv-aflibercept is not an antibody, but a solubilized VEGF receptor VEGF binding domain, and therefore may have a distinct mechanism of action with comparable side effects.
Unintended side effects of any antibody use include infusion-related hypersensitivity reactions, usually limited to the first infusion, which can be managed with glucocorticoid and/or antihistamine prophylaxis. In addition, distinct syndromes have emerged with different antibodies. Anti-EGFR antibodies produce an acneiform rash that poorly responds to glucocorticoid cream treatment. Trastuzumab (anti-HER2) can inhibit cardiac function, particularly in patients with prior exposure to anthracyclines. Bevacizumab has a number of side effects of medical significance, including hypertension, thrombosis, proteinuria, hemorrhage, and gastrointestinal perforations with or without prior surgeries; these adverse events also occur with small-molecule drugs modulating VEGFR function.
IMMUNOREGULATORY ANTIBODIES Purely immunoregulatory antibodies stimulate immune responses to mediate tumor-directed cytotoxicity. First-generation approaches sought to activate complement and are exemplified by antibodies to CD52; these are active in chronic lymphoid leukemia and T cell malignancies. A more refined understanding of the tumor–host interface has defined that cytotoxic tumor-directed T cells are frequently inhibited by ligands upregulated in the tumor cells. The programmed death ligand 1 (PD-L1; also known as B7-homolog 1) was initially recognized as an entity that induced T cell death through a receptor present on T cells, termed the PD receptor (Fig 103e-5), which physiologically exists to regulate the intensity of the immune response. The PD family of ligands and receptors also regulates macrophage function, present in tumor stroma. These actions raised the hypothesis that antibodies directed against the PD signaling axis (both anti-PD-L1 and anti-PD) might be useful in cancer treatment by allowing reactivation of the immune response against tumors. Indeed, nivolumab and lambrolizumab, both anti-PD antibodies, have shown evidence of important immune-mediated actions against certain solid tumors, including melanoma and lung cancers.
FIGURE 103e-5 Tumors possess a microenvironment (tumor stroma) with immune cells including both helper T cells, suppressor T cells (both “regulatory” of other immune cell function), macrophages, and cytotoxic T cells. Cytokines found in the stroma and deriving from macrophawsges and regulatory T cells modulate the activities of cytotoxic T cells, which have the potential to kill tumor cells. Antigens released by tumor cells are taken up by Antigen Presenting Cells (APCs), also in the stroma. Antigens are processed by the APCs to peptides presented by the Major Histocompatibility Complex to T-cell antigen receptors, thus providing an (+) activation signal for the cytotoxic tumor cells to kill tumor cells bearing that antigen. Negative (–) signals inhibiting cytoxic T cell action include the CTLA4 receptor (on T cells), interacting with the B7 family of negative regulatory signals from APCs, and the PD receptor (on T cells), interacting with the PD-L1 (–) signal coming from tumor cells expressing the PD-1 ligand (PD-l1). As both CTLA4 and PD1 signals attenuate the anti-tumor T cell response, strategies which inhibit CTLA4 and PD1 function are a means of stimulating cytotoxic T cell activity to kill tumor cells. Cytokines from other immune cells and macrophages can provide both (+) and (–) signals for T cell action, and are under investigation as novel immunoregulatory therapeutics.
Already approved for clinical use in melanoma is ipilimumab, an antibody directed against the anti-CTLA4 (cytotoxic T lymphocyte antigen 4), which is expressed on T cells (not tumor cells), responds to signals from antigen-presenting cells (Fig. 103e-5), and also downregulates the intensity of the T cell proliferative response to antigens derived from tumor cells. Indeed, manipulation of the CTLA4 axis was the first demonstration that purely immunoregulatory antibody strategies directed at T cell physiology could be safe and effective in the treatment of cancer, although it acts at a very early stage in T cell activation and can be considered somewhat nonspecific in its basis for T cell stimulation. Pembrolizumab, an anti-PD ligand blocking agent was also approved for melanoma, with a similar spectrum of potential adverse events, but acting in the tumor microenvironment. Indeed, prominent activation of autoimmune hepatic, endocrine, cutaneous, neurologic, and gastrointestinal responses is a basis for adverse events with the use of ipilimumab; the emergent use of glucocorticoids may be required to attenuate severe toxicities, which unfortunately can cause potential attenuation of antitumor effect. Importantly for the general internist, these events may occur late after exposure to ipilimumab while the patient may otherwise be enjoying sustained control of tumor growth owing to the beneficial actions of ipilimumab.
Another class of immunoregulatory antibody is the “bispecific” antibody blinatumomab, which was constructed to have an anti-CD19 antigen combining site as one valency of an antibody with anti-CD3 binding site as the other valency. This antibody thus can bring T cells (with its anti-CD3 activity) close to B cells bearing the CD19 determinant. Blinatumomab is active in B cell neoplasms such as acute lymphocytic leukemia, which may not have prominent expression of the CD20 targeted by rituximab.
ANTIBODY CONJUGATES Conjugates of antibodies with drugs and isotopes have also been shown to be effective in the treatment of cancer and have the intent of increasing the therapeutic index of the drug or isotope by delivering the toxic “warhead” directly to the tumor cell or tumor microenvironment. Ado-trastuzumab is a conjugate of the HER2/neu-directed trastuzumab and a highly toxic microtubule targeted drug (emtansine), which by itself is too toxic for human use; the antibody-drug conjugate shows valuable activity in patients with breast cancer who have developed resistance to the “naked” antibody. Brentuximab vedotin is an anti-CD30 antibody drug conjugate with a distinct microtubule poison with activity in neoplasms such as Hodgkin’s lymphoma where the tumor cells frequently express CD30. Radioconjugates targeting CD20 on lymphomas have been approved for use (ibritumomab tiuxetan [Zevalin], using yttrium-90 or 131I-tositumomab). Toxicity concerns have limited their use.
Cytokines There are >70 separate proteins and glycoproteins with biologic effects in humans: interferon (IFN) α, β, and γ; interleukin (IL) 1 through 29 (so far); the tumor necrosis factor (TNF) family (including lymphotoxin, TNF-related apoptosis-inducing ligand [TRAIL], CD40 ligand, and others); and the chemokine family. Only a fraction of these has been tested against cancer; only IFN-α and IL-2 are in routine clinical use.
About 20 different genes encode IFN-α, and their biologic effects are indistinguishable. IFN induces the expression of many genes, inhibits protein synthesis, and exerts a number of different effects on diverse cellular processes. The two recombinant forms that are commercially available are IFN-α2a and -α2b. Interferon is not curative for any tumor but can induce partial responses in follicular lymphoma, hairy cell leukemia, CML, melanoma, and Kaposi’s sarcoma. It has been used in the adjuvant setting in stage II melanoma, multiple myeloma, and follicular lymphoma, with uncertain effects on survival. It produces fever, fatigue, a flulike syndrome, malaise, myelosuppression, and depression and can induce clinically significant autoimmune disease. IFN-α is not generally the treatment of choice for any cancer.
IL-2 exerts its antitumor effects indirectly through augmentation of immune function. Its biologic activity is to promote the growth and activity of T cells and natural killer (NK) cells. High doses of IL-2 can produce tumor regression in certain patients with metastatic melanoma and renal cell cancer. About 2–5% of patients may experience complete remissions that are durable, unlike any other treatment for these tumors. IL-2 is associated with myriad clinical side effects, including intravascular volume depletion, capillary leak syndrome, adult respiratory distress syndrome, hypotension, fever, chills, skin rash, and impaired renal and liver function. Patients may require blood pressure support and intensive care to manage the toxicity. However, once the agent is stopped, most of the toxicities reverse completely within 3–6 days.
Ligand Receptor–Directed Constructs High-affinity receptors for cytokines have led to the design of cytokine-toxin recombinant fusion proteins, such as IL-2 expressed in frame with a fragment of diphtheria toxin. A commercially available construct has activity against certain T cell lymphomas. Likewise, the high-affinity folate receptor is the target for folate conjugated to chemotherapeutic agents. In both cases, the drug’s utility derives from the internalization of the targeted receptor and cleavage of the active drug or toxin moiety.
SYSTEMIC RADIATION THERAPY
Although total-body irradiation has a role in preparing a patient to received allogeneic stem cells, and antibodies as described above can specifically target radioisotopes, systemically administered isotopes of iodide salts have an important role in the treatment of thyroid neoplasms, owing to the selective upregulation of the iodide transporter in the tumor cell compartment. Likewise, isotopes of samarium and radium have been found useful in the palliation of symptoms from advanced bony metastases of prostate cancer owing to their selective deposition at the tumor–bone matrix interface, thereby potentially affecting the function of both tumor and stromal cells in the progressive growth of the metastatic deposit.
RESISTANCE TO CANCER TREATMENTS
Resistance mechanisms to the conventional cytotoxic agents were initially characterized in the late twentieth century as defects in drug uptake, metabolism, or export by tumor cells. The multidrug resistance (mdr) gene defined in vitro in cell lines exposed to increasing concentrations of drugs led to the definition of a family of transport proteins that, when overexpressed, result in the facile transport of a variety of hydrophobic drugs out of the cancer cell. Although efforts to manipulate this transporter to promote drug residence in tumor cells have been pursued, none are clinically useful at this time. Drug-metabolizing enzymes such as cytidine deaminase are upregulated in resistant tumor cells, and this is the basis for so-called “high-dose cytarabine” regimens in the treatment of leukemia. Another resistance mechanism defined during this era involved increased expression of a drug’s target, exemplified by amplification of the dihydrofolate reductase gene, in patients who had lost responsiveness to methotrexate, or mutation of topoisomerase II in tumors that relapsed after topoisomerase II modulator treatment.
A second class of resistance mechanisms involves loss of the cellular apoptotic mechanism activated after the engagement of a drug’s target by the drug. This occurs in a way that is heavily influenced by the biology of the particular tumor type. For example, decreased alkylguanine alkyltransferase defines a subset of glioblastoma patients with the prospect of greatest benefit from treatment with temozolomide, but has no predictive value for benefit from temozolomide in epithelial neoplasms. Likewise, ovarian cancers resistant to platinating agents have decreased expression of the proapoptotic gene bax. These types of findings have prompted the idea that responsive tumors to chemotherapeutic agents are populated by cells that express drug-related cell death controlling genes, creating in effect a state of “synthetic lethality” of the drug (Chap. 102e) with the genes expressed in responsive tumors, analogous to the existence in yeast of mutations that are well tolerated in the absence of a physiologic stressor but become lethal in the presence of that stressor. In the case of tumors, the chemotherapy inducing the cell death response is the analogous physiologic stressor.
A third class of resistance mechanisms emerged from sequencing of the targets of agents directed at oncogenic kinases. Thus, patients with CML resistant to imatinib have acquired mutations in the ATP binding domain of p210bcr-abl in some cases, leading to the screening and design of agents with activity against the mutant proteins. Entirely analogous resistance mechanisms have emerged in patients with lung cancer treated with the EGFR antagonists gefitinib and erlotinib.
A final category of tumor resistance mechanisms to targeted agents includes the upregulation of alternate means of activating the pathway targeted by the agent. Thus melanomas initially responsive to BRAF V600E antagonists such as vemurafenib may reactivate raf signaling by upregulating isoforms that can bypass the variant blocked by the drug. Likewise, inhibition of HER2/neu signaling in breast cancer cells can lead to the emergence of variants with distinct oncogenic signaling pathways such as PI3 kinase. Analogously in NSCLC, EGFR inhibitor treatment leads to the emergence of cells with a predominance of c-met protooncogene–dependent signaling in the resistant tumors.
The susceptibility of a tumor to different treatments as a function of its expression of potential drug targets or their mutational profile has led to efforts to define the dominant pathways driving a patient’s tumor by genomic techniques including whole exome sequencing. The difficulty with applying such data to patient treatment is recognizing that these pathways may change during the natural history of a tumor and that different sites in a single patient may have tumors with different patterns of gene mutation.
SUPPORTIVE CARE DURING CANCER TREATMENT
MYELOSUPPRESSION
The common cytotoxic chemotherapeutic agents almost invariably affect bone marrow function. Titration of this effect determines the MTD of the agent on a given schedule. The normal kinetics of blood cell turnover influences the sequence and sensitivity of each of the formed elements. Polymorphonuclear leukocytes (PMNs; t1/2 = 6–8 h), platelets (t1/2 = 5–7 days), and red blood cells (RBCs; t1/2 = 120 days) have most, less, and least susceptibility, respectively, to usually administered cytotoxic agents. The nadir count of each cell type in response to classes of agents is characteristic. Maximal neutropenia occurs 6–14 days after conventional doses of anthracyclines, antifolates, and antimetabolites. Alkylating agents differ from each other in the timing of cytopenias. Nitrosoureas, DTIC, and procarbazine can display delayed marrow toxicity, first appearing 6 weeks after dosing.
Complications of myelosuppression result from the predictable sequelae of the missing cells’ function. Febrile neutropenia refers to the clinical presentation of fever (one temperature ≥38.5°C or three readings ≥38°C but ≤38.5°C per 24 h) in a neutropenic patient with an uncontrolled neoplasm involving the bone marrow or, more usually, in a patient undergoing treatment with cytotoxic agents. Mortality from uncontrolled infection varies inversely with the neutrophil count. If the nadir neutrophil count is >1000/μL, there is little risk; if <500/μL, risk of death is markedly increased. Management of febrile neutropenia has conventionally included empirical coverage with antibiotics for the duration of neutropenia (Chap. 104). Selection of antibiotics is governed by the expected association of infections with certain underlying neoplasms; careful physical examination (with scrutiny of catheter sites, dentition, mucosal surfaces, and perirectal and genital orifices by gentle palpation); chest x-ray; and Gram stain and culture of blood, urine, and sputum (if any) to define a putative site of infection. In the absence of any originating site, a broadly acting β-lactam with anti-Pseudomonas activity, such as ceftazidime, is begun empirically. The addition of vancomycin to cover potential cutaneous sites of origin (until these are ruled out or shown to originate from methicillin-sensitive organisms) or metronidazole or imipenem for abdominal or other sites favoring anaerobes reflects modifications tailored to individual patient presentations. The coexistence of pulmonary compromise raises a distinct set of potential pathogens, including Legionella, Pneumocystis, and fungal agents that may require further diagnostic evaluations, such as bronchoscopy with bronchoalveolar lavage. Febrile neutropenic patients can be stratified broadly into two prognostic groups. The first, with expected short duration of neutropenia and no evidence of hypotension or abdominal or other localizing symptoms, may be expected to do well even with oral regimens, e.g., ciprofloxacin or moxifloxacin, or amoxicillin plus clavulanic acid. A less favorable prognostic group is patients with expected prolonged neutropenia, evidence of sepsis, and end organ compromise, particularly pneumonia. These patients require tailoring of their antibiotic regimen to their underlying presentation, with frequent empirical addition of antifungal agents if fever and neutropenia persists for 7 days without identification of an adequately treated organism or site.
Transfusion of granulocytes has no role in the management of febrile neutropenia, owing to their exceedingly short half-life, mechanical fragility, and clinical syndromes of pulmonary compromise with leukostasis after their use. Instead, colony-stimulating factors (CSFs) are used to augment bone marrow production of PMNs. Early-acting factors such as IL-1, IL-3, and stem cell factor have not been as useful clinically as late-acting, lineage-specific factors such as granulocyte colony-stimulating factor (G-CSF) or GM-CSF, erythropoietin (EPO), thrombopoietin, IL-6, and IL-11. CSFs may easily become overused in oncology practice. The settings in which their use has been proved effective are limited. G-CSF, GM-CSF, EPO, and IL-11 are currently approved for use. The American Society of Clinical Oncology has developed practice guidelines for the use of G-CSF and GM-CSF (Table 103e-7).
|
INDICATIONS FOR THE CLINICAL USE OF G-CSF OR GM-CSF |
Abbreviations: CSF, cerebrospinal fluid; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Source: From the American Society of Clinical Oncology: J Clin Oncol 24:3187, 2006.
Primary prophylaxis (i.e., shortly after completing chemotherapy to reduce the nadir) administers G-CSF to patients receiving cytotoxic regimens associated with a 20% incidence of febrile neutropenia. “Dose-dense” regimens, where cycling of chemotherapy is intended to be completed without delay of administered doses, may also benefit, but such patients should be on a clinical trial. Administration of G-CSF in these circumstances has reduced the incidence of febrile neutropenia in several studies by about 50%. Most patients, however, receive regimens that do not have such a high risk of expected febrile neutropenia, and therefore most patients initially should not receive G-CSF or GM-CSF. Special circumstances—such as a documented history of febrile neutropenia with the regimen in a particular patient or categories of patients at increased risk, such as patients older than age 65 years with aggressive lymphoma treated with curative chemotherapy regimens; extensive compromise of marrow by prior radiation or chemotherapy; or active, open wounds or deep-seated infection—may support primary treatment with G-CSF or GM-CSF. Administration of G-CSF or GM-CSF to afebrile neutropenic patients or to patients with low-risk febrile neutropenia is not recommended, and patients receiving concomitant chemoradiation treatment, particularly those with thoracic neoplasms, likewise are not generally recommended for treatment. In contrast, administration of G-CSF to high-risk patients with febrile neutropenia and evidence of organ compromise including sepsis syndrome, invasive fungal infection, concurrent hospitalization at the time fever develops, pneumonia, profound neutropenia (<0.1 × 109/L), or age >65 years is reasonable.
Secondary prophylaxis refers to the administration of CSFs in patients who have experienced a neutropenic complication from a prior cycle of chemotherapy; dose reduction or delay may be a reasonably considered alternative. G-CSF or GM-CSF is conventionally started 24–72 h after completion of chemotherapy and continued until a PMN count of 10,000/μL is achieved, unless a “depot” preparation of G-CSF such as pegfilgrastim is used, where one dose is administered at least 14 days before the next scheduled administration of chemotherapy. Also, patients with myeloid leukemias undergoing induction therapy may have a slight reduction in the duration of neutropenia if G-CSF is commenced after completion of therapy and may be of particular value in elderly patients, but the influence on long-term outcome has not been defined. GM-CSF probably has a more restricted utility than G-CSF, with its use currently limited to patients after autologous bone marrow transplants, although proper head-to-head comparisons with G-CSF have not been conducted in most instances. GM-CSF may be associated with more systemic side effects.
Dangerous degrees of thrombocytopenia do not frequently complicate the management of patients with solid tumors receiving cytotoxic chemotherapy (with the possible exception of certain carboplatin-containing regimens), but they are frequent in patients with certain hematologic neoplasms where marrow is infiltrated with tumor. Severe bleeding related to thrombocytopenia occurs with increased frequency at platelet counts <20,000/μL and is very prevalent at counts <5000/μL.
The precise “trigger” point at which to transfuse patients has been defined as a platelet count of 10,000/μL or less in patients without medical comorbidities that may increase the risk of bleeding. This issue is important not only because of the costs of frequent transfusion, but unnecessary platelet transfusions expose the patient to the risks of allosensitization and loss of value from subsequent transfusion owing to rapid platelet clearance, as well as the infectious and hypersensitivity risks inherent in any transfusion. Prophylactic transfusions to keep platelets >20,000/μL are reasonable in patients with leukemia who are stressed by fever or concomitant medical conditions (the threshold for transfusion is 10,000/μL in patients with solid tumors and no other bleeding diathesis or physiologic stressors such as fever or hypotension, a level that might also be reasonably considered for leukemia patients who are thrombocytopenic but not stressed or bleeding). In contrast, patients with myeloproliferative states may have functionally altered platelets despite normal platelet counts, and transfusion with normal donor platelets should be considered for evidence of bleeding in these patients. Careful review of medication lists to prevent exposure to nonsteroidal anti-inflammatory agents and maintenance of clotting factor levels adequate to support near-normal prothrombin and partial thromboplastin time tests are important in minimizing the risk of bleeding in the thrombocytopenic patient.
Certain cytokines in clinical investigation have shown an ability to increase platelets (e.g., IL-6, IL-1, thrombopoietin), but clinical benefit and safety are not yet proven. IL-11 (oprelvekin) is approved for use in the setting of expected thrombocytopenia, but its effects on platelet counts are small, and it is associated with side effects such as headache, fever, malaise, syncope, cardiac arrhythmias, and fluid retention. Eltrombopag and romiplostim are thrombopoietin agonists with demonstrated efficacy in certain thrombocytopenic states, but they have not been systematically studied in chemotherapy-induced thrombocytopenia.
Anemia associated with chemotherapy can be managed by transfusion of packed RBCs. Transfusion is not undertaken until the hemoglobin falls to <80 g/L (8 g/dL), compromise of end organ function occurs, or an underlying condition (e.g., coronary artery disease) calls for maintenance of hemoglobin >90 g/L (9 g/dL). Patients who are to receive therapy for >2 months on a “stable” regimen and who are likely to require continuing transfusions are also candidates for erythropoietin (EPO). Randomized trials in certain tumors have raised the possibility that EPO use may promote tumor-related adverse events. This information should be considered in the care of individual patients. In the event EPO treatment is undertaken, maintenance of hemoglobin of 90–100 g/L (9–10 g/dL) should be the target. In the setting of adequate iron stores and serum EPO levels <100 ng/mL, EPO, 150 U three times a week, can produce a slow increase in hemoglobin over about 2 months of administration. Depot formulations can be administered less frequently. It is unclear whether higher hemoglobin levels, up to 110–120 g/L (11–12 g/dL), are associated with improved quality of life to a degree that justifies the more intensive EPO use. Efforts to achieve levels at or above 120 g/L (12 g/dL) have been associated with increased thromboses and mortality rates. EPO may rescue hypoxemic cells from death and contribute to tumor radioresistance.
NAUSEA AND VOMITING
The most common side effect of chemotherapy administration is nausea, with or without vomiting. Nausea may be acute (within 24 h of chemotherapy), delayed (>24 h), or anticipatory of the receipt of chemotherapy. Patients may be likewise stratified for their risk of susceptibility to nausea and vomiting, with increased risk in young, female, heavily pretreated patients without a history of alcohol or drug use but with a history of motion or morning sickness. Antineoplastic agents vary in their capacity to cause nausea and vomiting. Highly emetogenic drugs (>90%) include mechlorethamine, streptozotocin, DTIC, cyclophosphamide at >1500 mg/m2, and cisplatin; moderately emetogenic drugs (30–90% risk) include carboplatin, cytosine arabinoside (>1 mg/m2), ifosfamide, conventional-dose cyclophosphamide, and anthracyclines; low-risk (10–30%) agents include 5FU, taxanes, etoposide, and bortezomib, with minimal risk (<10%) afforded by treatment with antibodies, bleomycin, busulfan, fludarabine, and vinca alkaloids. Emesis is a reflex caused by stimulation of the vomiting center in the medulla. Input to the vomiting center comes from the chemoreceptor trigger zone (CTZ) and afferents from the peripheral gastrointestinal tract, cerebral cortex, and heart. The different emesis “syndromes” require distinct management approaches. In addition, a conditioned reflex may contribute to anticipatory nausea arising after repeated cycles of chemotherapy. Accordingly, antiemetic agents differ in their locus and timing of action. Combining agents from different classes or the sequential use of different classes of agent is the cornerstone of successful management of chemotherapy-induced nausea and vomiting. Of great importance are the prophylactic administration of agents and such psychological techniques as the maintenance of a supportive milieu, counseling, and relaxation to augment the action of antiemetic agents.
Serotonin antagonists (5-HT3) and neurokinin 1 (NK1) receptor antagonists are useful in “high-risk” chemotherapy regimens. The combination acts at both peripheral gastrointestinal and CNS sites that control nausea and vomiting. For example, the 5-HT3 blocker dolasetron, 100 mg intravenously or orally; dexamethasone, 12 mg; and the NK1 antagonist aprepitant, 125 mg orally, are combined on the day of administration of severely emetogenic regimens, with repetition of dexamethasone (8 mg) and aprepitant (80 mg) on days 2 and 3 for delayed nausea. Alternate 5-HT3 antagonists include ondansetron, given as 0.15 mg/kg intravenously for three doses just before and at 4 and 8 h after chemotherapy; palonosetron at 0.25 mg over 30 s, 30 min before chemotherapy; and granisetron, given as a single dose of 0.01 mg/kg just before chemotherapy. Emesis from moderately emetic chemotherapy regimens may be prevented with a 5-HT3 antagonist and dexamethasone alone for patients not receiving doxorubicin and cyclophosphamide combinations; the latter combination requires the 5-HT3/dexamethasone/aprepitant on day 1 but aprepitant alone on days 2 and 3. Emesis from low-emetic-risk regimens may be prevented with 8 mg of dexamethasone alone or with non-5-HT3, non-NK1 antagonist approaches including the following.
Antidopaminergic phenothiazines act directly at the CTZ and include prochlorperazine (Compazine), 10 mg intramuscularly or intravenously, 10–25 mg orally, or 25 mg per rectum every 4–6 h for up to four doses; and thiethylperazine, 10 mg by potentially all of the above routes every 6 h. Haloperidol is a butyrophenone dopamine antagonist given at 1 mg intramuscularly or orally every 8 h. Antihistamines such as diphenhydramine have little intrinsic antiemetic capacity but are frequently given to prevent or treat dystonic reactions that can complicate use of the antidopaminergic agents. Lorazepam is a short-acting benzodiazepine that provides an anxiolytic effect to augment the effectiveness of a variety of agents when used at 1–2 mg intramuscularly, intravenously, or orally every 4–6 h. Metoclopramide acts on peripheral dopamine receptors to augment gastric emptying and is used in high doses for highly emetogenic regimens (1–2 mg/kg intravenously 30 min before chemotherapy and every 2 h for up to three additional doses as needed); intravenous doses of 10–20 mg every 4–6 h as needed or 50 mg orally 4 h before and 8 and 12 h after chemotherapy are used for moderately emetogenic regimens. 5-9-Tetrahydrocannabinol (Marinol) is a rather weak antiemetic compared to other available agents, but it may be useful for persisting nausea and is used orally at 10 mg every 3–4 h as needed.
DIARRHEA
Regimens that include 5FU infusions and/or irinotecan may produce severe diarrhea. Similar to the vomiting syndromes, chemotherapy-induced diarrhea may be immediate or can occur in a delayed fashion up to 48–72 h after the drugs. Careful attention to maintained hydration and electrolyte repletion, intravenously if necessary, along with antimotility treatments such as “high-dose” loperamide, commenced with 4 mg at the first occurrence of diarrhea, with 2 mg repeated every 2 h until 12 h without loose stools, not to exceed a total daily dose of 16 mg. Octreotide (100–150 μg), a somatostatin analogue, or opiate-based preparations may be considered for patients not responding to loperamide.
MUCOSITIS
Irritation and inflammation of the mucous membranes particularly afflicting the oral and anal mucosa, but potentially involving the gastrointestinal tract, may accompany cytotoxic chemotherapy. Mucositis is due to damage to the proliferating cells at the base of the mucosal squamous epithelia or in the intestinal crypts. Topical therapies, including anesthetics and barrier-creating preparations, may provide symptomatic relief in mild cases. Palifermin or keratinocyte growth factor, a member of the fibroblast growth factor family, is effective in preventing severe mucositis in the setting of high-dose chemotherapy with stem cell transplantation for hematologic malignancies. It may also prevent or ameliorate mucositis from radiation.
ALOPECIA
Chemotherapeutic agents vary widely in causing alopecia, with anthracyclines, alkylating agents, and topoisomerase inhibitors reliably causing near-total alopecia when given at therapeutic doses. Antimetabolites are more variably associated with alopecia. Psychological support and the use of cosmetic resources are to be encouraged, and “chemo caps” that reduce scalp temperature to decrease the degree of alopecia should be discouraged, particularly during treatment with curative intent of neoplasms, such as leukemia or lymphoma, or in adjuvant breast cancer therapy. The richly vascularized scalp can certainly harbor micrometastatic or disseminated disease.
GONADAL DYSFUNCTION AND PREGNANCY
Cessation of ovulation and azoospermia reliably result from alkylating agent– and topoisomerase poison–containing regimens. The duration of these effects varies with age and sex. Males treated for Hodgkin’s disease with mechlorethamine- and procarbazine-containing regimens are effectively sterile, whereas fertility usually returns after regimens that include cisplatin, vinblastine, or etoposide and after bleomycin for testicular cancer. Sperm banking before treatment may be considered to support patients likely to be sterilized by treatment. Females experience amenorrhea with anovulation after alkylating agent therapy; they are likely to recover normal menses if treatment is completed before age 30 but unlikely to recover menses after age 35. Even those who regain menses usually experience premature menopause. Because the magnitude and extent of decreased fertility can be difficult to predict, patients should be counseled to maintain effective contraception, preferably by barrier means, during and after therapy. Resumption of efforts to conceive should be considered in the context of the patient’s likely prognosis. Hormone replacement therapy should be undertaken in women who do not have a hormonally responsive tumor. For patients who have had a hormone-sensitive tumor primarily treated by a local modality, conventional practice would counsel against hormone replacement, but this issue is under investigation.
Chemotherapy agents have variable effects on the success of pregnancy. All agents tend to have increased risk of adverse outcomes when administered during the first trimester, and strategies to delay chemotherapy, if possible, until after this milestone should be considered if the pregnancy is to continue to term. Patients in their second or third trimester can be treated with most regimens for the common neoplasms afflicting women in their childbearing years, with the exception of antimetabolites, particularly antifolates, which have notable teratogenic or fetotoxic effects throughout pregnancy. The need for anticancer chemotherapy per se is infrequently a clear basis to recommend termination of a concurrent pregnancy, although each treatment strategy in this circumstance must be tailored to the individual needs of the patient.
SPECIAL ISSUES WITH TARGETED TREATMENTS
Treatment with EGFR-directed small molecules (e.g., erlotinib, afatinib, lapatinib), antibodies (e.g., cetuximab, panitumumab), and mTOR antagonists (e.g., everolimus, temsirolimus) reliably produces an acneiform rash that can be a source of distress to patients and can be ameliorated with topically applied clindamycin gels and low-potency corticosteroid creams. Diarrhea frequently accompanies tyrosine kinase inhibitor administration and may respond to antimotility agents such as loperamide or stool-bulking agents.
Anti-VEGFR-directed treatments, including the specific antibody bevacizumab, and the “multikinase” inhibitors with anti VEGFR activity, such as sorafenib, sunitinib, and pazopanib, reliably produce hypertension in a significant fraction of patients that typically can be treated with lisinopril, amlodipine, or clonidine alone or in combination. More difficult to treat is proteinuria with resultant azotemia; this can be a basis for discontinuing treatment depending on the clinical context. Thyroid function is prominently affected by chronic exposure to this group of multikinase inhibitors including sorafenib and pazopanib, and periodic surveillance of thyroid-stimulating hormone and thyroxine (T4) levels during treatment is reasonable. Gastrointestinal perforations, arterial thromboses, and hemorrhage likewise have no specific treatments and may be a basis to avoid this class of agents. Palmar-plantar dysesthesia (“hand-foot syndrome”) can be seen after administration of these agents (as well as some cytotoxic agents including gemcitabine and liposomal preparations of doxorubicin) and is a basis for considering dose reduction if not responsive to topical emollients and analgesics.
Protein kinase antagonists as a class have been associated with poorly predicted hepatic and cardiac toxicities (imatinib, dasatinib, sorafenib, pazopanib) or cardiac conduction deficits including prolonged QT interval (pazopanib). The occurrence of new cardiac or liver abnormalities in a patient receiving treatment with a protein kinase antagonist should lead to a consideration of the risk versus benefit and the possible relation of the agent to the new adverse event. The existence of prior cardiac dysfunction is a relative contraindication to the use of certain targeted therapies (e.g., trastuzumab), although each patient’s needs should be individualized. Chronic effects of cancer treatment are reviewed in Chap. 125.
104 |
Infections in Patients with Cancer |
Infections are a common cause of death and an even more common cause of morbidity in patients with a wide variety of neoplasms. Autopsy studies show that most deaths from acute leukemia and half of deaths from lymphoma are caused directly by infection. With more intensive chemotherapy, patients with solid tumors have also become more likely to die of infection. Fortunately, an evolving approach to prevention and treatment of infectious complications of cancer has decreased infection-associated mortality rates and will probably continue to do so. This accomplishment has resulted from three major steps:
1. The practice of using “early empirical” antibiotics reduced mortality rates among patients with leukemia and bacteremia from 84% in 1965 to 44% in 1972. Recent studies suggest that the mortality rate due to infection in febrile neutropenic patients dropped to <10% by 2013. This dramatic improvement is attributed to early intervention with appropriate antimicrobial therapy.
2. “Empirical” antifungal therapy has also lowered the incidence of disseminated fungal infection, with dramatic decreases in mortality rates. An antifungal agent is administered—on the basis of likely fungal infection—to neutropenic patients who, after 4–7 days of antibiotic therapy, remain febrile but have no positive cultures.
3. Use of antibiotics for afebrile neutropenic patients as broad-spectrum prophylaxis against infections has decreased both mortality and morbidity even further. The current approach to treatment of severely neutropenic patients (e.g., those receiving high-dose chemotherapy for leukemia or high-grade lymphoma) is based on initial prophylactic therapy at the onset of neutropenia, subsequent “empirical” antibacterial therapy targeting the organisms whose involvement is likely in light of physical findings (most often fever alone), and finally “empirical” antifungal therapy based on the known likelihood that fungal infection will become a serious issue after 4–7 days of broad-spectrum antibacterial therapy.
A physical predisposition to infection in patients with cancer (Table 104-1) can be a result of the neoplasm’s production of a break in the skin. For example, a squamous cell carcinoma may cause local invasion of the epidermis, which allows bacteria to gain access to subcutaneous tissue and permits the development of cellulitis. The artificial closing of a normally patent orifice can also predispose to infection; for example, obstruction of a ureter by a tumor can cause urinary tract infection, and obstruction of the bile duct can cause cholangitis. Part of the host’s normal defense against infection depends on the continuous emptying of a viscus; without emptying, a few bacteria that are present as a result of bacteremia or local transit can multiply and cause disease.
|
DISRUPTION OF NORMAL BARRIERS THAT MAY PREDISPOSE TO INFECTIONS IN PATIENTS WITH CANCER |
A similar problem can affect patients whose lymph node integrity has been disrupted by radical surgery, particularly patients who have had radical node dissections. A common clinical problem following radical mastectomy is the development of cellulitis (usually caused by streptococci or staphylococci) because of lymphedema and/or inadequate lymph drainage. In most cases, this problem can be addressed by local measures designed to prevent fluid accumulation and breaks in the skin, but antibiotic prophylaxis has been necessary in refractory cases.
A life-threatening problem common to many cancer patients is the loss of the reticuloendothelial capacity to clear microorganisms after splenectomy, which may be performed as part of the management of hairy cell leukemia, chronic lymphocytic leukemia (CLL), and chronic myelogenous leukemia (CML) and in Hodgkin’s disease. Even after curative therapy for the underlying disease, the lack of a spleen predisposes such patients to rapidly fatal infections. The loss of the spleen through trauma similarly predisposes the normal host to overwhelming infection throughout life. The splenectomized patient should be counseled about the risks of infection with certain organisms, such as the protozoan Babesia (Chap. 249) and Capnocytophaga canimorsus, a bacterium carried in the mouths of animals (Chaps. 167e and 183e). Because encapsulated bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis) are the organisms most commonly associated with postsplenectomy sepsis, splenectomized persons should be vaccinated (and revaccinated; Table 104-2 and Chap. 148) against the capsular polysaccharides of these organisms. Many clinicians recommend giving splenectomized patients a small supply of antibiotics effective against S. pneumoniae, N. meningitidis, and H. influenzae to avert rapid, overwhelming sepsis in the event that they cannot present for medical attention immediately after the onset of fever or other signs or symptoms of bacterial infection. A few tablets of amoxicillin/clavulanic acid (or levofloxacin if resistant strains of S. pneumoniae are prevalent locally) are a reasonable choice for this purpose.
|
VACC INATION OF CANCER PATIENTS RECEIVING CHEMOTHERAPYa |
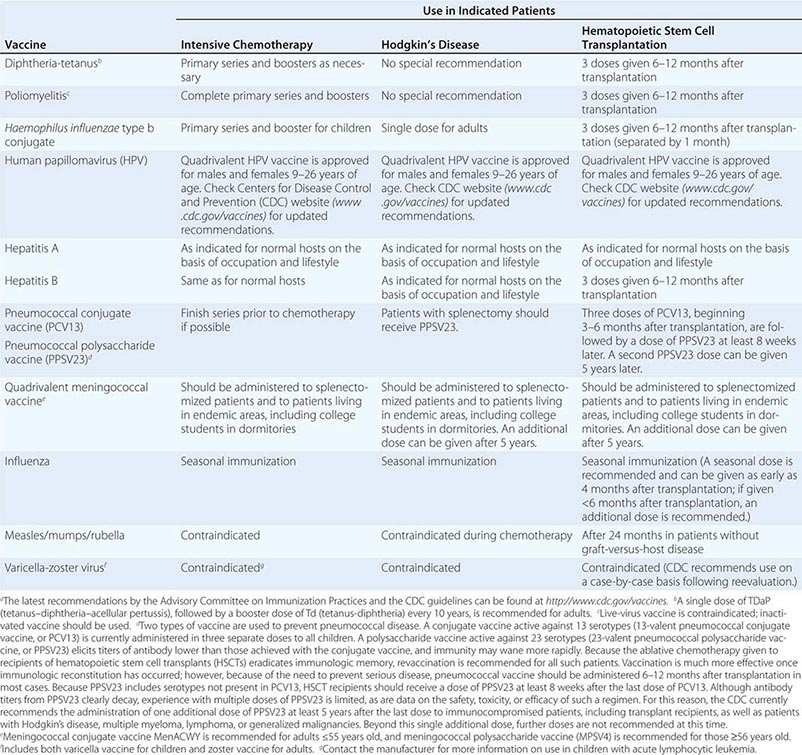
The level of suspicion of infections with certain organisms should depend on the type of cancer diagnosed (Table 104-3). Diagnosis of multiple myeloma or CLL should alert the clinician to the possibility of hypogammaglobulinemia. While immunoglobulin replacement therapy can be effective, in most cases prophylactic antibiotics are a cheaper, more convenient method of eliminating bacterial infections in CLL patients with hypogammaglobulinemia. Patients with acute lymphocytic leukemia (ALL), patients with non-Hodgkin’s lymphoma, and all cancer patients treated with high-dose glucocorticoids (or glucocorticoid-containing chemotherapy regimens) should receive antibiotic prophylaxis for Pneumocystis infection (Table 104-3) for the duration of their chemotherapy. In addition to exhibiting susceptibility to certain infectious organisms, patients with cancer are likely to manifest their infections in characteristic ways. For example, fever—generally a sign of infection in normal hosts—continues to be a reliable indicator in neutropenic patients. In contrast, patients receiving glucocorticoids and agents that impair T cell function and cytokine secretion may have serious infections in the absence of fever. Similarly, neutropenic patients commonly present with cellulitis without purulence and with pneumonia without sputum or even x-ray findings (see below).
|
INFECTIONS ASSOCIATED WITH SPECIFIC TYPES OF CANCER |
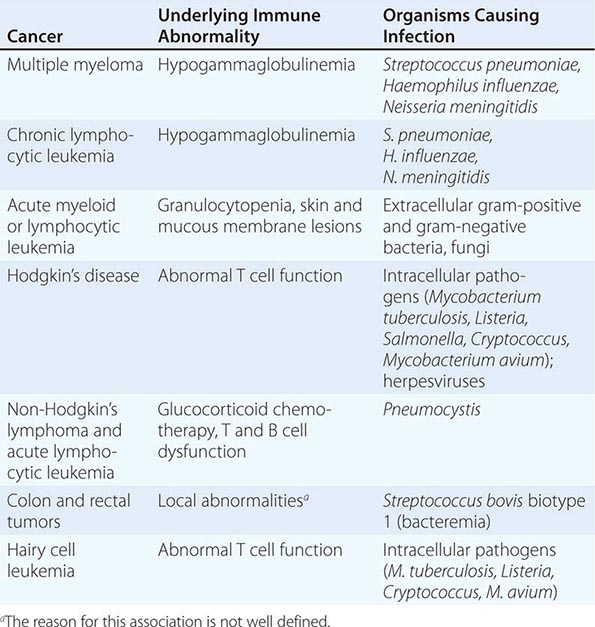
The use of monoclonal antibodies that target B and T cells as well as drugs that interfere with lymphocyte signal transduction events is associated with reactivation of latent infections. The use of rituximab, the antibody to CD20 (a B cell surface protein), is associated with the development of reactivation tuberculosis as well as other latent viral infections, including hepatitis B and cytomegalovirus (CMV) infection. Like organ transplant recipients (Chap. 169), patients with latent bacterial disease (like tuberculosis) and latent viral disease (like herpes simplex or zoster) should be carefully monitored for reactivation disease.
SYSTEM-SPECIFIC SYNDROMES
SKIN-SPECIFIC SYNDROMES
Skin lesions are common in cancer patients, and the appearance of these lesions may permit the diagnosis of systemic bacterial or fungal infection. While cellulitis caused by skin organisms such as Streptococcus or Staphylococcus is common, neutropenic patients—i.e., those with <500 functional polymorphonuclear leukocytes (PMNs)/μL—and patients with impaired blood or lymphatic drainage may develop infections with unusual organisms. Innocent-looking macules or papules may be the first sign of bacterial or fungal sepsis in immunocompromised patients (Fig. 104-1). In the neutropenic host, a macule progresses rapidly to ecthyma gangrenosum (see Fig. 25e-35), a usually painless, round, necrotic lesion consisting of a central black or gray-black eschar with surrounding erythema. Ecthyma gangrenosum, which is located in nonpressure areas (as distinguished from necrotic lesions associated with lack of circulation), is often associated with Pseudomonas aeruginosa bacteremia (Chap. 189) but may be caused by other bacteria.
FIGURE 104-1 A. Papules related to Escherichia coli bacteremia in a patient with acute lymphocytic leukemia. B. The same lesions on the following day.
Candidemia (Chap. 240) is also associated with a variety of skin conditions (see Fig. 25e-38) and commonly presents as a maculopapular rash. Punch biopsy of the skin may be the best method for diagnosis.
Cellulitis, an acute spreading inflammation of the skin, is most often caused by infection with group A Streptococcus or Staphylococcus aureus, virulent organisms normally found on the skin (Chap. 156). Although cellulitis tends to be circumscribed in normal hosts, it may spread rapidly in neutropenic patients. A tiny break in the skin may lead to spreading cellulitis, which is characterized by pain and erythema; in the affected patients, signs of infection (e.g., purulence) are often lacking. What might be a furuncle in a normal host may require amputation because of uncontrolled infection in a patient presenting with leukemia. A dramatic response to an infection that might be trivial in a normal host can mark the first sign of leukemia. Fortunately, granulocytopenic patients are likely to be infected with certain types of organisms (Table 104-4); thus the selection of an antibiotic regimen is somewhat easier than it might otherwise be (see “Antibacterial Therapy,” below). It is essential to recognize cellulitis early and to treat it aggressively. Patients who are neutropenic or who have previously received antibiotics for other reasons may develop cellulitis with unusual organisms (e.g., Escherichia coli, Pseudomonas, or fungi). Early treatment, even of innocent-looking lesions, is essential to prevent necrosis and loss of tissue. Debridement to prevent spread may sometimes be necessary early in the course of disease, but it can often be performed after chemotherapy, when the PMN count increases.
|
ORGANISMS LIKELY TO CAUSE INFECTIONS IN GRANULOCYTOPENIC PATIENTS |
Sweet syndrome, or febrile neutrophilic dermatosis, was originally described in women with elevated white blood cell (WBC) counts. The disease is characterized by the presence of leukocytes in the lower dermis, with edema of the papillary body. Ironically, this disease now is usually seen in neutropenic patients with cancer, most often in association with acute myeloid leukemia (AML) but also in association with a variety of other malignancies. Sweet syndrome usually presents as red or bluish-red papules or nodules that may coalesce and form sharply bordered plaques (see Fig. 25e-41). The edema may suggest vesicles, but on palpation the lesions are solid, and vesicles probably never arise in this disease. The lesions are most common on the face, neck, and arms. On the legs, they may be confused with erythema nodosum (see Fig. 25e-40). The development of lesions is often accompanied by high fevers and an elevated erythrocyte sedimentation rate. Both the lesions and the temperature elevation respond dramatically to glucocorticoid administration. Treatment begins with high doses of glucocorticoids (prednisone, 60 mg/d) followed by tapered doses over the next 2–3 weeks.
Data indicate that erythema multiforme (see Fig. 25e-25) with mucous membrane involvement is often associated with herpes simplex virus (HSV) infection and is distinct from Stevens-Johnson syndrome, which is associated with drugs and tends to have a more widespread distribution. Because cancer patients are both immunosuppressed (and therefore susceptible to herpes infections) and heavily treated with drugs (and therefore subject to Stevens-Johnson syndrome [see Fig. 46e-4]), both of these conditions are common in this population.
Cytokines, which are used as adjuvants or primary treatments for cancer, can themselves cause characteristic rashes, further complicating the differential diagnosis. This phenomenon is a particular problem in bone marrow transplant recipients (Chap. 169), who, in addition to having the usual chemotherapy-, antibiotic-, and cytokine-induced rashes, are plagued by graft-versus-host disease.
CATHETER-RELATED INFECTIONS
Because IV catheters are commonly used in cancer chemotherapy and are prone to cause infection (Chap. 168), they pose a major problem in the care of patients with cancer. Some catheter-associated infections can be treated with antibiotics, whereas in others the catheter must be removed (Table 104-5). If the patient has a “tunneled” catheter (which consists of an entrance site, a subcutaneous tunnel, and an exit site), a red streak over the subcutaneous part of the line (the tunnel) is grounds for immediate device removal. Failure to remove catheters under these circumstances may result in extensive cellulitis and tissue necrosis.
|
APPROACH TO CATHETER INFECTIONS IN IMMUNOCOMPROMISED PATIENTS |
More common than tunnel infections are exit-site infections, often with erythema around the area where the line penetrates the skin. Most authorities (Chap. 172) recommend treatment (usually with vancomycin) for an exit-site infection caused by coagulase-negative Staphylococcus. Treatment of coagulase-positive staphylococcal infection is associated with a poorer outcome, and it is advisable to remove the catheter if possible. Similarly, most clinicians remove catheters associated with infections due to P. aeruginosa and Candida species, because such infections are difficult to treat and bloodstream infections with these organisms are likely to be deadly. Catheter infections caused by Burkholderia cepacia, Stenotrophomonas species, Agrobacterium species, Acinetobacter baumannii, Pseudomonas species other than aeruginosa, and carbapenem-resistant Enterobacteriaceae are likely to be very difficult to eradicate with antibiotics alone. Similarly, isolation of Bacillus, Corynebacterium, and Mycobacterium species should prompt removal of the catheter.
GASTROINTESTINAL TRACT–SPECIFIC SYNDROMES
Upper Gastrointestinal Tract Disease
INFECTIONS OF THE MOUTH The oral cavity is rich in aerobic and anaerobic bacteria (Chap. 201) that normally live in a commensal relationship with the host. The antimetabolic effects of chemotherapy cause a breakdown of mucosal host defenses, leading to ulceration of the mouth and the potential for invasion by resident bacteria. Mouth ulcerations afflict most patients receiving cytotoxic chemotherapy and have been associated with viridans streptococcal bacteremia. Candida infections of the mouth are very common. Fluconazole is clearly effective in the treatment of both local infections (thrush) and systemic infections (esophagitis) due to Candida albicans. Other azoles (e.g., voriconazole) as well as echinocandins offer similar efficacy as well as activity against the fluconazole-resistant organisms that are associated with chronic fluconazole treatment (Chap. 240).
Noma (cancrum oris), commonly seen in malnourished children, is a penetrating disease of the soft and hard tissues of the mouth and adjacent sites, with resulting necrosis and gangrene. It has a counterpart in immunocompromised patients and is thought to be due to invasion of the tissues by Bacteroides, Fusobacterium, and other normal inhabitants of the mouth. Noma is associated with debility, poor oral hygiene, and immunosuppression.
Viruses, particularly HSV, are a prominent cause of morbidity in immunocompromised patients, in whom they are associated with severe mucositis. The use of acyclovir, either prophylactically or therapeutically, is of value.
ESOPHAGEAL INFECTIONS The differential diagnosis of esophagitis (usually presenting as substernal chest pain upon swallowing) includes herpes simplex and candidiasis, both of which are readily treatable.
Lower Gastrointestinal Tract Disease Hepatic candidiasis (Chap. 240) results from seeding of the liver (usually from a gastrointestinal source) in neutropenic patients. It is most common among patients being treated for AML and usually presents symptomatically around the time the neutropenia resolves. The characteristic picture is that of persistent fever unresponsive to antibiotics, abdominal pain and tenderness or nausea, and elevated serum levels of alkaline phosphatase in a patient with hematologic malignancy who has recently recovered from neutropenia. The diagnosis of this disease (which may present in an indolent manner and persist for several months) is based on the finding of yeasts or pseudohyphae in granulomatous lesions. Hepatic ultrasound or CT may reveal bull’s-eye lesions. MRI scans reveal small lesions not visible by other imaging modalities. The pathology (a granulomatous response) and the timing (with resolution of neutropenia and an elevation in granulocyte count) suggest that the host response to Candida is an important component of the manifestations of disease. In many cases, although organisms are visible, cultures of biopsied material may be negative. The designation hepatosplenic candidiasis or hepatic candidiasis is a misnomer because the disease often involves the kidneys and other tissues; the term chronic disseminated candidiasis may be more appropriate. Because of the risk of bleeding with liver biopsy, diagnosis is often based on imaging studies (MRI, CT). Treatment should be directed to the causative agent (usually C. albicans but sometimes Candida tropicalis or other less common Candida species).
Typhlitis Typhlitis (also referred to as necrotizing colitis, neutropenic colitis, necrotizing enteropathy, ileocecal syndrome, and cecitis) is a clinical syndrome of fever and right-lower-quadrant (or generalized abdominal) tenderness in an immunosuppressed host. This syndrome is classically seen in neutropenic patients after chemotherapy with cytotoxic drugs. It may be more common among children than among adults and appears to be much more common among patients with AML or ALL than among those with other types of cancer. Physical examination reveals right-lower-quadrant tenderness, with or without rebound tenderness. Associated diarrhea (often bloody) is common, and the diagnosis can be confirmed by the finding of a thickened cecal wall on CT, MRI, or ultrasonography. Plain films may reveal a right-lower-quadrant mass, but CT with contrast or MRI is a much more sensitive means of diagnosis. Although surgery is sometimes attempted to avoid perforation from ischemia, most cases resolve with medical therapy alone. The disease is sometimes associated with positive blood cultures (which usually yield aerobic gram-negative bacilli), and therapy is recommended for a broad spectrum of bacteria (particularly gram-negative bacilli, which are likely to be found in the bowel flora). Surgery is indicated in the case of perforation.
Clostridium difficile–Induced Diarrhea Patients with cancer are predisposed to the development of C. difficile diarrhea (Chap. 161) as a consequence of chemotherapy alone. Thus, they may test positive for C. difficile even without receiving antibiotics. Obviously, such patients are also subject to C. difficile–induced diarrhea as a result of antibiotic pressure. C. difficile should always be considered as a possible cause of diarrhea in cancer patients who have received either chemotherapy or antibiotics.
CENTRAL NERVOUS SYSTEM–SPECIFIC SYNDROMES
Meningitis The presentation of meningitis in patients with lymphoma or CLL and in patients receiving chemotherapy (particularly with glucocorticoids) for solid tumors suggests a diagnosis of cryptococcal or listerial infection. As noted previously, splenectomized patients are susceptible to rapid, overwhelming infection with encapsulated bacteria (including S. pneumoniae, H. influenzae, and N. meningitidis). Similarly, patients who are antibody-deficient (e.g., those with CLL, those who have received intensive chemotherapy, or those who have undergone bone marrow transplantation) are likely to have infections caused by these bacteria. Other cancer patients, however, because of their defective cellular immunity, are likely to be infected with other pathogens (Table 104-3). Central nervous system (CNS) tuberculosis should be considered, especially in patients from countries where tuberculosis is highly prevalent in the population.
Encephalitis The spectrum of disease resulting from viral encephalitis is expanded in immunocompromised patients. A predisposition to infections with intracellular organisms similar to those encountered in patients with AIDS (Chap. 226) is seen in cancer patients receiving (1) high-dose cytotoxic chemotherapy, (2) chemotherapy affecting T cell function (e.g., fludarabine), or (3) antibodies that eliminate T cells (e.g., anti-CD3, alemtuzumab, anti-CD52) or cytokine activity (anti–tumor necrosis factor agents or interleukin 1 receptor antagonists). Infection with varicella-zoster virus (VZV) has been associated with encephalitis that may be caused by VZV-related vasculitis. Chronic viral infections may also be associated with dementia and encephalitic presentations. A diagnosis of progressive multifocal leukoencephalopathy (Chap. 164) should be considered when a patient who has received chemotherapy (rituximab in particular) presents with dementia (Table 104-6). Other abnormalities of the CNS that may be confused with infection include normal-pressure hydrocephalus and vasculitis resulting from CNS irradiation. It may be possible to differentiate these conditions by MRI.
|
DIFFERENTIAL DIAGNOSIS OF CENTRAL NERVOUS SYSTEM INFECTIONS IN PATIENTS WITH CANCER |
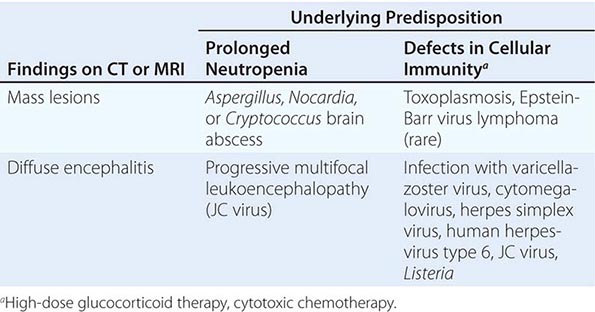
Brain Masses Mass lesions of the brain most often present as headache with or without fever or neurologic abnormalities. Infections associated with mass lesions may be caused by bacteria (particularly Nocardia), fungi (particularly Cryptococcus or Aspergillus), or parasites (Toxoplasma). Epstein-Barr virus (EBV)–associated lymphoma may also present as single—or sometimes multiple—mass lesions of the brain. A biopsy may be required for a definitive diagnosis.
PULMONARY INFECTIONS
Pneumonia (Chap. 153) in immunocompromised patients may be difficult to diagnose because conventional methods of diagnosis depend on the presence of neutrophils. Bacterial pneumonia in neutropenic patients may present without purulent sputum—or, in fact, without any sputum at all—and may not produce physical findings suggestive of chest consolidation (rales or egophony).
In granulocytopenic patients with persistent or recurrent fever, the chest x-ray pattern may help to localize an infection and thus to determine which investigative tests and procedures should be undertaken and which therapeutic options should be considered (Table 104-7). In this setting, a simple chest x-ray is a screening tool; because the impaired host response results in less evidence of consolidation or infiltration, high-resolution CT is recommended for the diagnosis of pulmonary infections. The difficulties encountered in the management of pulmonary infiltrates relate in part to the difficulties of performing diagnostic procedures on the patients involved. When platelet counts can be increased to adequate levels by transfusion, microscopic and microbiologic evaluation of the fluid obtained by endoscopic bronchial lavage is often diagnostic. Lavage fluid should be cultured for Mycoplasma, Chlamydia, Legionella, Nocardia, more common bacterial pathogens, fungi, and viruses. In addition, the possibility of Pneumocystis pneumonia should be considered, especially in patients with ALL or lymphoma who have not received prophylactic trimethoprim-sulfamethoxazole (TMP-SMX). The characteristics of the infiltrate may be helpful in decisions about further diagnostic and therapeutic maneuvers. Nodular infiltrates suggest fungal pneumonia (e.g., that caused by Aspergillus or Mucor). Such lesions may best be approached by visualized biopsy procedures. It is worth noting that while bacterial pneumonias classically present as lobar infiltrates in normal hosts, bacterial pneumonias in granulocytopenic hosts present with a paucity of signs, symptoms, or radiographic abnormalities; thus, the diagnosis is difficult.
|
DIFFERENTIAL DIAGNOSIS OF CHEST INFILTRATES IN IMMUNOCOMPROMISED PATIENTS |
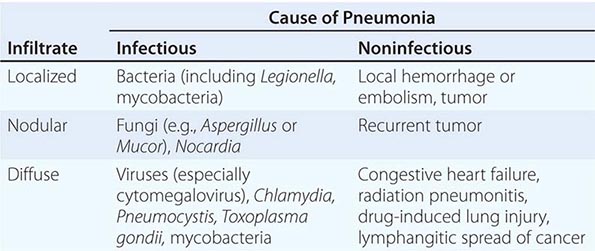
Aspergillus species (Chap. 241) can colonize the skin and respiratory tract or cause fatal systemic illness. Although this fungus may cause aspergillomas in a previously existing cavity or may produce allergic bronchopulmonary disease in some patients, the major problem posed by this genus in neutropenic patients is invasive disease, primarily due to Aspergillus fumigatus or Aspergillus flavus. The organisms enter the host following colonization of the respiratory tract, with subsequent invasion of blood vessels. The disease is likely to present as a thrombotic or embolic event because of this ability of the fungi to invade blood vessels. The risk of infection with Aspergillus correlates directly with the duration of neutropenia. In prolonged neutropenia, positive surveillance cultures for nasopharyngeal colonization with Aspergillus may predict the development of disease.
Patients with Aspergillus infection often present with pleuritic chest pain and fever, which are sometimes accompanied by cough. Hemoptysis may be an ominous sign. Chest x-rays may reveal new focal infiltrates or nodules. Chest CT may reveal a characteristic halo consisting of a mass-like infiltrate surrounded by an area of low attenuation. The presence of a “crescent sign” on chest x-ray or chest CT, in which the mass progresses to central cavitation, is characteristic of invasive Aspergillus infection but may develop as the lesions are resolving.
In addition to causing pulmonary disease, Aspergillus may invade through the nose or palate, with deep sinus penetration. The appearance of a discolored area in the nasal passages or on the hard palate should prompt a search for invasive Aspergillus. This situation is likely to require surgical debridement. Catheter infections with Aspergillus usually require both removal of the catheter and antifungal therapy.
Diffuse interstitial infiltrates suggest viral, parasitic, or Pneumocystis pneumonia. If the patient has a diffuse interstitial pattern on chest x-ray, it may be reasonable, while considering invasive diagnostic procedures, to institute empirical treatment for Pneumocystis with TMP-SMX and for Chlamydia, Mycoplasma, and Legionella with a quinolone or azithromycin. Noninvasive procedures, such as staining of induced sputum smears for Pneumocystis, serum cryptococcal antigen tests, and urine testing for Legionella antigen, may be helpful. Serum galactomannan and β-D-glucan tests may be of value in diagnosing Aspergillus infection, but their utility is limited by their lack of sensitivity and specificity. The presence of an elevated level of β-D-glucan in the serum of a patient being treated for cancer who is not receiving prophylaxis against Pneumocystis suggests the diagnosis of Pneumocystis pneumonia. Infections with viruses that cause only upper respiratory symptoms in immunocompetent hosts, such as respiratory syncytial virus (RSV), influenza viruses, and parainfluenza viruses, may be associated with fatal pneumonitis in immunocompromised hosts. CMV reactivation occurs in cancer patients receiving chemotherapy, but CMV pneumonia is most common among HSCT recipients (Chap. 169). Polymerase chain reaction testing now allows rapid diagnosis of viral pneumonia, which can lead to treatment in some cases (e.g., influenza). Multiplex studies that can detect a wide array of viruses in the lung and upper respiratory tract are now available and will lead to specific diagnoses of viral pneumonias.
Bleomycin is the most common cause of chemotherapy-induced lung disease. Other causes include alkylating agents (such as cyclophosphamide, chlorambucil, and melphalan), nitrosoureas (carmustine [BCNU], lomustine [CCNU], and methyl-CCNU), busulfan, procarbazine, methotrexate, and hydroxyurea. Both infectious and noninfectious (drug- and/or radiation-induced) pneumonitis can cause fever and abnormalities on chest x-ray; thus, the differential diagnosis of an infiltrate in a patient receiving chemotherapy encompasses a broad range of conditions (Table 104-7). The treatment of radiation pneumonitis (which may respond dramatically to glucocorticoids) or drug-induced pneumonitis is different from that of infectious pneumonia, and a biopsy may be important in the diagnosis. Unfortunately, no definitive diagnosis can be made in ∼30% of cases, even after bronchoscopy.
Open-lung biopsy is the gold standard of diagnostic techniques. Biopsy via a visualized thoracostomy can replace an open procedure in many cases. When a biopsy cannot be performed, empirical treatment can be undertaken; a quinolone or an erythromycin derivative (azithromycin) and TMP-SMX are used in the case of diffuse infiltrates, and an antifungal agent is administered in the case of nodular infiltrates. The risks should be weighed carefully in these cases. If inappropriate drugs are administered, empirical treatment may prove toxic or ineffective; either of these outcomes may be riskier than biopsy.
CARDIOVASCULAR INFECTIONS
Patients with Hodgkin’s disease are prone to persistent infections by Salmonella, sometimes (and particularly often in elderly patients) affecting a vascular site. The use of IV catheters deliberately lodged in the right atrium is associated with a high incidence of bacterial endocarditis, presumably related to valve damage followed by bacteremia. Nonbacterial thrombotic endocarditis (marantic endocarditis) has been described in association with a variety of malignancies (most often solid tumors) and may follow bone marrow transplantation as well. The presentation of an embolic event with a new cardiac murmur suggests this diagnosis. Blood cultures are negative in this disease of unknown pathogenesis.
ENDOCRINE SYNDROMES
Infections of the endocrine system have been described in immunocompromised patients. Candida infection of the thyroid may be difficult to diagnose during the neutropenic period. It can be defined by indium-labeled WBC scans or gallium scans after neutrophil counts increase. CMV infection can cause adrenalitis with or without resulting adrenal insufficiency. The presentation of a sudden endocrine anomaly in an immunocompromised patient can be a sign of infection in the involved end organ.
MUSCULOSKELETAL INFECTIONS
Infection that is a consequence of vascular compromise, resulting in gangrene, can occur when a tumor restricts the blood supply to muscles, bones, or joints. The process of diagnosis and treatment of such infection is similar to that in normal hosts, with the following caveats:
1. In terms of diagnosis, a lack of physical findings resulting from a lack of granulocytes in the granulocytopenic patient should make the clinician more aggressive in obtaining tissue rather than more willing to rely on physical signs.
2. In terms of therapy, aggressive debridement of infected tissues may be required. However, it is usually difficult to operate on patients who have recently received chemotherapy, both because of a lack of platelets (which results in bleeding complications) and because of a lack of WBCs (which may lead to secondary infection). A blood culture positive for Clostridium perfringens—an organism commonly associated with gas gangrene—can have a number of meanings (Chap. 179). Clostridium septicum bacteremia is associated with the presence of an underlying malignancy. Bloodstream infections with intestinal organisms such as Streptococcus bovis biotype 1 and C. perfringens may arise spontaneously from lower gastrointestinal lesions (tumor or polyps); alternatively, these lesions may be harbingers of invasive disease. The clinical setting must be considered in order to define the appropriate treatment for each case.
RENAL AND URETERAL INFECTIONS
Infections of the urinary tract are common among patients whose ureteral excretion is compromised (Table 104-1). Candida, which has a predilection for the kidney, can invade either from the bloodstream or in a retrograde manner (via the ureters or bladder) in immunocompromised patients. The presence of “fungus balls” or persistent candiduria suggests invasive disease. Persistent funguria (with Aspergillus as well as Candida) should prompt a search for a nidus of infection in the kidney.
Certain viruses are typically seen only in immunosuppressed patients. BK virus (polyomavirus hominis 1) has been documented in the urine of bone marrow transplant recipients and, like adenovirus, may be associated with hemorrhagic cystitis.
ABNORMALITIES THAT PREDISPOSE TO INFECTION
THE LYMPHOID SYSTEM
It is beyond the scope of this chapter to detail how all the immunologic abnormalities that result from cancer or from chemotherapy for cancer lead to infections. Disorders of the immune system are discussed in other sections of this book. As has been noted, patients with antibody deficiency are predisposed to overwhelming infection with encapsulated bacteria (including S. pneumoniae, H. influenzae, and N. meningitidis). Infections that result from the lack of a functional cellular immune system are described in Chap. 226. It is worth mentioning, however, that patients undergoing intensive chemotherapy for any form of cancer will have not only defects due to granulocytopenia but also lymphocyte dysfunction, which may be profound. Thus, these patients—especially those receiving glucocorticoid-containing regimens or drugs that inhibit either T cell activation (calcineurin inhibitors or drugs like fludarabine, which affect lymphocyte function) or cytokine induction—should be given prophylaxis for Pneumocystis pneumonia.
Patients receiving treatment that eliminates B cells (e.g., with anti-CD20 antibodies or rituximab) are especially vulnerable to intercurrent viral infections. The incidence of progressive multifocal leukoencephalopathy (caused by JC virus) is elevated in these patients.
THE HEMATOPOIETIC SYSTEM
![]() Initial studies in the 1960s revealed a dramatic increase in the incidence of infections (fatal and nonfatal) among cancer patients with a granulocyte count of <500/μL. The use of prophylactic antibacterial agents has reduced the number of bacterial infections, but 35–78% of febrile neutropenic patients being treated for hematologic malignancies develop infections at some time during chemotherapy. Aerobic pathogens (both gram-positive and gram-negative) predominate in all series, but the exact organisms isolated vary from center to center. Infections with anaerobic organisms are uncommon. Geographic patterns affect the types of fungi isolated. Tuberculosis and malaria are common causes of fever in the developing world and may present in this setting as well.
Initial studies in the 1960s revealed a dramatic increase in the incidence of infections (fatal and nonfatal) among cancer patients with a granulocyte count of <500/μL. The use of prophylactic antibacterial agents has reduced the number of bacterial infections, but 35–78% of febrile neutropenic patients being treated for hematologic malignancies develop infections at some time during chemotherapy. Aerobic pathogens (both gram-positive and gram-negative) predominate in all series, but the exact organisms isolated vary from center to center. Infections with anaerobic organisms are uncommon. Geographic patterns affect the types of fungi isolated. Tuberculosis and malaria are common causes of fever in the developing world and may present in this setting as well.
Neutropenic patients are unusually susceptible to infection with a wide variety of bacteria; thus, antibiotic therapy should be initiated promptly to cover likely pathogens if infection is suspected. Indeed, early initiation of antibacterial agents is mandatory to prevent deaths. Like most immunocompromised patients, neutropenic patients are threatened by their own microbial flora, including gram-positive and gram-negative organisms found commonly on the skin and mucous membranes and in the bowel (Table 104-4). Because treatment with narrow-spectrum agents leads to infection with organisms not covered by the antibiotics used, the initial regimen should target all pathogens likely to be the initial causes of bacterial infection in neutropenic hosts. As noted in the algorithm shown in Fig. 104-2, administration of antimicrobial agents is routinely continued until neutropenia resolves—i.e., the granulocyte count is sustained above 500 μL for at least 2 days. In some cases, patients remain febrile after resolution of neutropenia. In these instances, the risk of sudden death from overwhelming bacteremia is greatly reduced, and the following diagnoses should be seriously considered: (1) fungal infection, (2) bacterial abscesses or undrained foci of infection, and (3) drug fever (including reactions to antimicrobial agents as well as to chemotherapy or cytokines). In the proper setting, viral infection or graft-versus-host disease should be considered. In clinical practice, antibacterial therapy is usually discontinued when the patient is no longer neutropenic and all evidence of bacterial disease has been eliminated. Antifungal agents are then discontinued if there is no evidence of fungal disease. If the patient remains febrile, a search for viral diseases or unusual pathogens is conducted while unnecessary cytokines and other drugs are systematically eliminated from the regimen.
FIGURE 104-2 Algorithm for the diagnosis and treatment of fever and neutropenia.
PREVENTION OF INFECTION IN CANCER PATIENTS
EFFECT OF THE ENVIRONMENT
Outbreaks of fatal Aspergillus infection have been associated with construction projects and materials in several hospitals. The association between spore counts and risk of infection suggests the need for a high-efficiency air-handling system in hospitals that care for large numbers of neutropenic patients. The use of laminar-flow rooms and prophylactic antibiotics has decreased the number of infectious episodes in severely neutropenic patients. However, because of the expense of such a program and the failure to show that it dramatically affects mortality rates, most centers do not routinely use laminar flow to care for neutropenic patients. Some centers use “reverse isolation,” in which health care providers and visitors to a patient who is neutropenic wear gowns and gloves. Since most of the infections these patients develop are due to organisms that colonize the patients’ own skin and bowel, the validity of such schemes is dubious, and limited clinical data do not support their use. Hand washing by all staff caring for neutropenic patients should be required to prevent the spread of resistant organisms.
The presence of large numbers of bacteria (particularly P. aeruginosa) in certain foods, especially fresh vegetables, has led some authorities to recommend a special “low-bacteria” diet. A diet consisting of cooked and canned food is satisfactory to most neutropenic patients and does not involve elaborate disinfection or sterilization protocols. However, there are no studies to support even this type of dietary restriction. Counseling of patients to avoid leftovers, deli foods, undercooked meat, and unpasteurized dairy products is recommended.
PHYSICAL MEASURES
Although few studies address this issue, patients with cancer are predisposed to infections resulting from anatomic compromise (e.g., lymphedema resulting from node dissections after radical mastectomy). Surgeons who specialize in cancer surgery can provide specific guidelines for the care of such patients, and patients benefit from common-sense advice about how to prevent infections in vulnerable areas.
IMMUNOGLOBULIN REPLACEMENT
Many patients with multiple myeloma or CLL have immunoglobulin deficiencies as a result of their disease, and all allogeneic bone marrow transplant recipients are hypogammaglobulinemic for a period after transplantation. However, current recommendations reserve intravenous immunoglobulin replacement therapy for those patients with severe (<400 mg of total IgG/dL), prolonged hypogammaglobulinemia and a history of repeated infections. Antibiotic prophylaxis has been shown to be cheaper and is efficacious in preventing infections in most CLL patients with hypogammaglobulinemia. Routine use of immunoglobulin replacement is not recommended.
SEXUAL PRACTICES
The use of condoms is recommended for severely immunocompromised patients. Any sexual practice that results in oral exposure to feces is not recommended. Neutropenic patients should be advised to avoid any practice that results in trauma, as even microscopic cuts may result in bacterial invasion and fatal sepsis.
ANTIBIOTIC PROPHYLAXIS
Several studies indicate that the use of oral fluoroquinolones prevents infection and decreases mortality rates among severely neutropenic patients. Prophylaxis for Pneumocystis is mandatory for patients with ALL and for all cancer patients receiving glucocorticoid-containing chemotherapy regimens.
VACCINATION OF CANCER PATIENTS
In general, patients undergoing chemotherapy respond less well to vaccines than do normal hosts. Their greater need for vaccines thus leads to a dilemma in their management. Purified proteins and inactivated vaccines are almost never contraindicated and should be given to patients even during chemotherapy. For example, all adults should receive diphtheria–tetanus toxoid boosters at the indicated times as well as seasonal influenza vaccine. However, if possible, vaccination should not be undertaken concurrent with cytotoxic chemotherapy. If patients are expected to be receiving chemotherapy for several months and vaccination is indicated (e.g., influenza vaccination in the fall), the vaccine should be given midcycle—as far apart in time as possible from the antimetabolic agents that will prevent an immune response. The meningococcal and pneumococcal polysaccharide vaccines should be given to patients before splenectomy, if possible. The H. influenzae type b conjugate vaccine should be administered to all splenectomized patients.
In general, live virus (or live bacterial) vaccines should not be given to patients during intensive chemotherapy because of the risk of disseminated infection. Recommendations on vaccination are summarized in Table 104-2 (see www.cdc.gov/vaccine for updated recommendations).
105 |
Cancer of the Skin |
MELANOMA
Pigmented lesions are among the most common findings on skin examination. The challenge is to distinguish cutaneous melanomas, which account for the overwhelming majority of deaths resulting from skin cancer, from the remainder, which are usually benign. Cutaneous melanoma can occur in adults of all ages, even young individuals, and people of all colors; its location on the skin and its distinct clinical features make it detectable at a time when complete surgical excision is possible. Examples of malignant and benign pigmented lesions are shown in Fig. 105-1.
FIGURE 105-1 Atypical and malignant pigmented lesions. The most common melanoma is superficial spreading melanoma (not pictured). A. Acral lentiginous melanoma is the most common melanoma in blacks, Asians, and Hispanics and occurs as an enlarging hyperpigmented macule or plaque on the palms and soles. Lateral pigment diffusion is present. B. Nodular melanoma most commonly manifests as a rapidly growing, often ulcerated or crusted black nodule. C. Lentigo maligna melanoma occurs on sun-exposed skin as a large, hyperpigmented macule or plaque with irregular borders and variable pigmentation. D. Dysplastic nevi are irregularly pigmented and shaped nevomelanocytic lesions that may be associated with familial melanoma.
EPIDEMIOLOGY
Melanoma is an aggressive malignancy of melanocytes, pigment-producing cells that originate from the neural crest and migrate to the skin, meninges, mucous membranes, upper esophagus, and eyes. Melanocytes in each of these locations have the potential for malignant transformation. Cutaneous melanoma is predominantly a malignancy of white-skinned people (98% of cases), and the incidence correlates with latitude of residence, providing strong evidence for the role of sun exposure. Men are affected slightly more than women (1.3:1), and the median age at diagnosis is the late fifties. Dark-skinned populations (such as those of India and Puerto Rico), blacks, and East Asians also develop melanoma, albeit at rates 10–20 times lower than those in whites. Cutaneous melanomas in these populations are diagnosed more often at a higher stage, and patients tend to have worse outcomes. Furthermore, in nonwhite populations, there is a much higher frequency of acral (subungual, plantar, palmar) and mucosal melanomas. In 2014, more than 76,000 individuals in the United States were expected to develop melanoma, and approximately 9700 were expected to die. There will be nearly 50,000 annual deaths worldwide as a result of melanoma. Data from the Connecticut Tumor Registry support an unremitting increase in the incidence and mortality of melanoma. In the past 60 years, there have been 17-fold and 9-fold increases in incidence for men and women, respectively. In the same six decades, there has been a tripling of mortality rates for men and doubling for women. Mortality rates begin to rise at age 55, with the greatest increase in men age >65 years. Of particular concern is the increase in rates among women <40 years of age. Much of this increase is believed to be associated with a greater emphasis on tanned skin as a marker of beauty, the increased availability and use of indoor tanning beds, and exposure to intense ultraviolet (UV) light in childhood. These statistics highlight the need to promote prevention and early detection.
RISK FACTORS
Presence of Nevi The risk of developing melanoma is related to genetic, environmental, and host factors (Table 105-1). The strongest risk factors for melanoma are the presence of multiple benign or atypical nevi and a family or personal history of melanoma. The presence of melanocytic nevi, common or dysplastic, is a marker for increased risk of melanoma. Nevi have been referred to as precursor lesions because they can transform into melanomas; however, the actual risk for any specific nevus is exceedingly low. About one-quarter of melanomas are histologically associated with nevi, but the majority arise de novo. The number of clinically atypical moles may vary from one to several hundred, and they usually differ from one another in appearance. The borders are often hazy and indistinct, and the pigment pattern is more highly varied than that in benign acquired nevi. Individuals with clinically atypical moles and a strong family history of melanoma have been reported to have a >50% lifetime risk for developing melanoma and warrant close follow-up with a dermatologist. Of the 90% of patients whose disease is sporadic (i.e., who lack a family history of melanoma), ∼40% have clinically atypical moles, compared with an estimated 5–10% of the population at large.
|
FACTORS ASSOCIATED WITH INCREASED RISK OF MELANOMA |
Congenital melanocytic nevi, which are classified as small (≤1.5 cm), medium (1.5–20 cm), and giant (>20 cm), can be precursors for melanoma. The risk is highest for the giant melanocytic nevus, also called the bathing trunk nevus, a rare malformation that affects 1 in 30,000–100,000 individuals. Since the lifetime risk of melanoma development is estimated to be as high as 6%, prophylactic excision early in life is prudent. This usually requires staged removal with coverage by split-thickness skin grafts. Surgery cannot remove all at-risk nevus cells, as some may penetrate into the muscles or central nervous system (CNS) below the nevus. Small- to medium-size congenital melanocytic nevi affect approximately 1% of persons; the risk of melanoma developing in these lesions is not known but appears to be relatively low. The management of small- to medium-size congenital melanocytic nevi remains controversial.
Personal and Family History Once diagnosed, patients with melanoma require a lifetime of surveillance because their risk of developing another melanoma is 10 times that of the general population. First-degree relatives have a higher risk of developing melanoma than do individuals without a family history, but only 5–10% of all melanomas are truly familial. In familial melanoma, patients tend to be younger at first diagnosis, lesions are thinner, survival is improved, and multiple primary melanomas are common.
![]() Genetic Susceptibility Approximately 20–40% of cases of hereditary melanoma (0.2–2% of all melanomas) are due to germline mutations in the cell cycle regulatory gene cyclin-dependent kinase inhibitor 2A (CDKN2A). In fact, 70% of all cutaneous melanomas have mutations or deletions affecting the CDKN2A locus on chromosome 9p21. This locus encodes two distinct tumor-suppressor proteins from alternate reading frames: p16 and ARF (p14ARF). The p16 protein inhibits CDK4/6-mediated phosphorylation and inactivation of the retinoblastoma (RB) protein, whereas ARF inhibits MDM2 ubiquitin-mediated degradation of p53. The end result of the loss of CDKN2A is inactivation of two critical tumor-suppressor pathways, RB and p53, which control entry of cells into the cell cycle. Several studies have shown an increased risk of pancreatic cancer among melanoma-prone families with CDKN2A mutations. A second high-risk locus for melanoma susceptibility, CDK4, is located on chromosome 12q13 and encodes the kinase inhibited by p16. CDK4 mutations, which also inactivate the RB pathway, are much rarer than CDKN2A mutations. Germline mutations in the melanoma lineage-specific oncogene microphthalmia-associated transcription factor (MITF) predispose to both familial and sporadic melanomas.
Genetic Susceptibility Approximately 20–40% of cases of hereditary melanoma (0.2–2% of all melanomas) are due to germline mutations in the cell cycle regulatory gene cyclin-dependent kinase inhibitor 2A (CDKN2A). In fact, 70% of all cutaneous melanomas have mutations or deletions affecting the CDKN2A locus on chromosome 9p21. This locus encodes two distinct tumor-suppressor proteins from alternate reading frames: p16 and ARF (p14ARF). The p16 protein inhibits CDK4/6-mediated phosphorylation and inactivation of the retinoblastoma (RB) protein, whereas ARF inhibits MDM2 ubiquitin-mediated degradation of p53. The end result of the loss of CDKN2A is inactivation of two critical tumor-suppressor pathways, RB and p53, which control entry of cells into the cell cycle. Several studies have shown an increased risk of pancreatic cancer among melanoma-prone families with CDKN2A mutations. A second high-risk locus for melanoma susceptibility, CDK4, is located on chromosome 12q13 and encodes the kinase inhibited by p16. CDK4 mutations, which also inactivate the RB pathway, are much rarer than CDKN2A mutations. Germline mutations in the melanoma lineage-specific oncogene microphthalmia-associated transcription factor (MITF) predispose to both familial and sporadic melanomas.
The melanocortin-1 receptor (MC1R) gene is a moderate-risk inherited melanoma susceptibility factor. Solar radiation stimulates the production of melanocortin (α-melanocyte-stimulating hormone [α-MSH]), the ligand for MC1R, which is a G-protein-coupled receptor that signals via cyclic AMP and regulates the amount and type of pigment produced. MC1R is highly polymorphic, and among its 80 variants are those that result in partial loss of signaling and lead to the production of red/yellow pheomelanins, which are not sun-protective and produce red hair, rather than brown/black eumelanins that are photoprotective. This red hair color (RHC) phenotype is associated with fair skin, red hair, freckles, increased sun sensitivity, and increased risk of melanoma. In addition to its weak UV shielding capacity relative to eumelanin, increased pheomelanin production in patients with inactivating polymorphisms of MC1R also provides a UV-independent carcinogenic contribution to melanomagenesis via oxidative damage.
A number of other more common, low-penetrance polymorphisms that have small effects on melanoma susceptibility include other genes related to pigmentation, nevus count, immune responses, DNA repair, metabolism, and the vitamin D receptor.
PREVENTION AND EARLY DETECTION
Primary prevention of melanoma and nonmelanoma skin cancer (NMSC) is based on protection from the sun. Public health initiatives, such as the SunSmart program that started in Australia and now is operative in Europe and the United States, have demonstrated that behavioral change can decrease the incidence of NMSC and melanoma. Preventive measures should start early in life because damage from UV light begins early despite the fact that cancers develop years later. Biological factors are increasingly being understood, such as tanning addiction, which is postulated to involve stimulation of reward centers in the brain involving dopamine pathways, and cutaneous secretion of β-endorphins after UV exposure, and may represent another area for preventive intervention. Regular use of broad-spectrum sunscreens that block UVA and UVB with a sun protection factor (SPF) of at least 30 and protective clothing should be encouraged. Avoidance of tanning beds and midday (10:00 A.M. to 2:00 P.M.) sun exposure is recommended.
Secondary prevention comprises education, screening, and early detection. Patients should be educated in the clinical features of melanoma (ABCDEs; see following “Diagnosis” section) and advised to report any growth or other change in a pigmented lesion. Brochures are available from the American Cancer Society, the American Academy of Dermatology, the National Cancer Institute, and the Skin Cancer Foundation. Self-examination at 6- to 8-week intervals may enhance the likelihood of detecting change. Although the U.S. Preventive Services Task Force states that evidence is insufficient to recommend for or against skin cancer screening, a full-body skin exam seems to be a simple, practical way to approach reducing the mortality rate for skin cancer. Depending on the presence or absence of risk factors, strategies for early detection can be individualized. This is particularly true for patients with clinically atypical moles (dysplastic nevi) and those with a personal history of melanoma. For these individuals, surveillance should be performed by the dermatologist and include total-body photography and dermoscopy where appropriate. Individuals with three or more primary melanomas and families with at least one invasive melanoma and two or more cases of melanoma and/or pancreatic cancer among first- or second-degree relatives on the same side of the family may benefit from genetic testing. Precancerous and in situ lesions should be treated early. Early detection of small tumors allows the use of simpler treatment modalities with higher cure rates and lower morbidity.
DIAGNOSIS
The main goal is to identify a melanoma before tumor invasion and life-threatening metastases have occurred. Early detection may be facilitated by applying the ABCDEs: asymmetry (benign lesions are usually symmetric); border irregularity (most nevi have clear-cut borders); color variegation (benign lesions usually have uniform light or dark pigment); diameter >6 mm (the size of a pencil eraser); and evolving (any change in size, shape, color, or elevation or new symptoms such as bleeding, itching, and crusting). Benign nevi usually appear on sun-exposed skin above the waist, rarely involving the scalp, breasts, or buttocks; atypical moles usually appear on sun-exposed skin, most often on the back, but can involve the scalp, breasts, or buttocks. Benign nevi are present in 85% of adults, with 10–40 moles scattered over the body; atypical nevi can be present in the hundreds.
The entire skin surface, including the scalp and mucous membranes, as well as the nails should be examined in each patient. Bright room illumination is important, and a hand lens is helpful for evaluating variation in pigment pattern. Any suspicious lesions should be biopsied, evaluated by a specialist, or recorded by chart and/or photography for follow-up. A focused method for examining individual lesions, dermoscopy, employs low-level magnification of the epidermis and may allow a more precise visualization of patterns of pigmentation than is possible with the naked eye. Complete physical examination with attention to the regional lymph nodes is part of the initial evaluation in a patient with suspected melanoma. The patient should be advised to have other family members screened if either melanoma or clinically atypical moles (dysplastic nevi) are present. Patients who fit into high-risk groups should be instructed to perform monthly self-examinations.
Biopsy Any pigmented cutaneous lesion that has changed in size or shape or has other features suggestive of malignant melanoma is a candidate for biopsy. An excisional biopsy with 1- to 3-mm margins is suggested. This facilitates pathologic assessment of the lesion, permits accurate measurement of thickness if the lesion is melanoma, and constitutes definitive treatment if the lesion is benign. For lesions that are large or on anatomic sites where excisional biopsy may not be feasible (such as the face, hands, and feet), an incisional biopsy through the most nodular or darkest area of the lesion is acceptable; this should include the vertical growth phase of the primary tumor, if present. Incisional biopsy does not appear to facilitate the spread of melanoma. For suspicious lesions, every attempt should be made to preserve the ability to assess the deep and peripheral margins and to perform immunohistochemistry. Shave biopsies are an acceptable alternative, particularly if the suspicion of malignancy is low, but they should be deep and include underlying fat; cauterization should be avoided. The biopsy should be read by a pathologist experienced in pigmented lesions, and the report should include Breslow thickness, mitoses per square millimeter for lesions ≤1 mm, presence or absence of ulceration, and peripheral and deep margin status. Breslow thickness is the greatest thickness of a primary cutaneous melanoma measured on the slide from the top of the epidermal granular layer, or from the ulcer base, to the bottom of the tumor. To distinguish melanomas from benign nevi in cases with challenging histology, fluorescence in situ hybridization (FISH) with multiple probes and comparative genome hybridization (CGH) can be helpful.
CLINICAL CLASSIFICATION
Four major types of cutaneous melanoma have been recognized (Table 105-2). In three of these types—superficial spreading melanoma, lentigo maligna melanoma, and acral lentiginous melanoma—the lesion has a period of superficial (so-called radial) growth during which it increases in size but does not penetrate deeply. It is during this period that the melanoma is most capable of being cured by surgical excision. The fourth type—nodular melanoma—does not have a recognizable radial growth phase and usually presents as a deeply invasive lesion that is capable of early metastasis. When tumors begin to penetrate deeply into the skin, they are in the so-called vertical growth phase. Melanomas with a radial growth phase are characterized by irregular and sometimes notched borders, variation in pigment pattern, and variation in color. An increase in size or change in color is noted by the patient in 70% of early lesions. Bleeding, ulceration, and pain are late signs and are of little help in early recognition. Superficial spreading melanoma is the most common variant observed in the white population. The back is the most common site for melanoma in men. In women, the back and the lower leg (from knee to ankle) are common sites. Nodular melanomas are dark brown-black to blue-black nodules. Lentigo maligna melanoma usually is confined to chronically sun-damaged sites in older individuals. Acral lentiginous melanoma occurs on the palms, soles, nail beds, and mucous membranes. Although this type occurs in whites, it occurs most frequently (along with nodular melanoma) in blacks and East Asians. A fifth type of melanoma, desmoplastic melanoma, is associated with a fibrotic response, neural invasion, and a greater tendency for local recurrence. Occasionally, melanomas appear clinically to be amelanotic, in which case the diagnosis is established microscopically after biopsy of a new or a changing skin nodule. Melanomas can also arise in the mucosa of the head and neck (nasal cavity, paranasal sinuses and oral cavity), the gastrointestinal tract, the CNS, the female genital tract (vulva, vagina), and the uveal tract of the eye.
|
HISTOLOGIC SUBTYPES OF MALIGNANT MELANOMA |
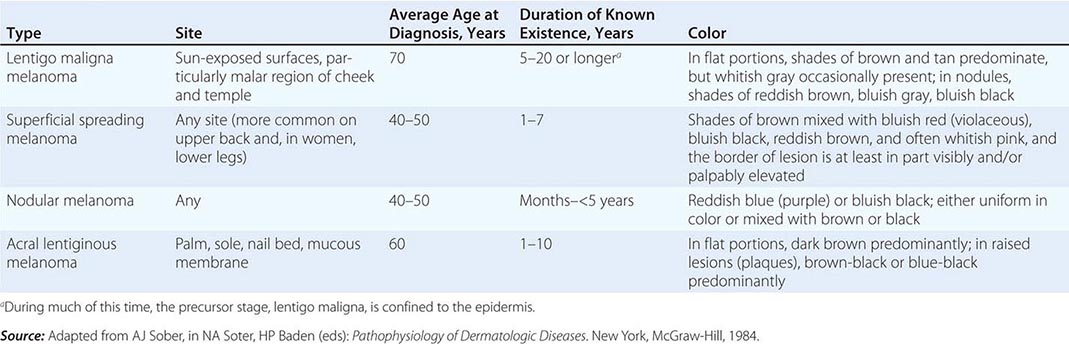
Although cutaneous melanoma subtypes are clinically and histopathologically distinct, this classification does not have independent prognostic value. Histologic subtype is not part of American Joint Committee on Cancer (AJCC) staging, although the College of American Pathologists (CAP) recommends inclusion in the pathology report. Newer classifications will increasingly emphasize molecular features of each melanoma (see below). The molecular analysis of individual melanomas will provide a basis for distinguishing benign nevi from melanomas, and determination of the mutational status of the tumor will help elucidate the molecular mechanisms of tumorigenesis and be used to identify targets that will guide therapy.
PATHOGENESIS AND MOLECULAR CLASSIFICATION
Considerable evidence from epidemiologic and molecular studies suggests that cutaneous melanomas arise via multiple causal pathways. There are both environmental and genetic components. UV solar radiation causes genetic changes in the skin, impairs cutaneous immune function, increases the production of growth factors, and induces the formation of DNA-damaging reactive oxygen species that affect keratinocytes and melanocytes. A comprehensive catalog of somatic mutations from a human melanoma revealed more than 33,000 base mutations with damage to almost 300 protein-coding segments compared with normal cells from the same patient. The dominant mutational signature reflected DNA damage due to UV light exposure. The melanoma also contained previously described driver mutations (i.e., mutations that confer selective clonal growth advantage and are implicated in oncogenesis). These driver mutations affect pathways that promote cell proliferation and inhibit normal pathways of apoptosis in response to DNA repair (see below). The altered melanocytes accumulate DNA damage, and selection occurs for all the attributes that constitute the malignant phenotype: invasion, metastasis, and angiogenesis.
An understanding of the molecular changes that occur during the transformation of normal melanocytes into malignant melanoma would not only help classify patients but also would contribute to the understanding of etiology and aid the development of new therapeutic options. A genome-wide assessment of melanomas classified into four groups based on their location and degree of exposure to the sun has confirmed that there are distinct genetic pathways in the development of melanoma. The four groups were cutaneous melanomas on skin without chronic sun-induced damage, cutaneous melanomas with chronic sun-induced damage, mucosal melanomas, and acral melanomas. Distinct patterns of DNA alterations were noted that varied with the site of origin and were independent of the histologic subtype of the tumor. Thus, although the genetic changes are diverse, the overall pattern of mutation, amplification, and loss of cancer genes indicates they have convergent effects on key biochemical pathways involved in proliferation, senescence, and apoptosis. The p16 mutation that affects cell cycle arrest and the ARF mutation that results in defective apoptotic responses to genotoxic damage were described earlier. The proliferative pathways affected were the mitogen-activated protein (MAP) kinase and phosphatidylinositol 3’ kinase/AKT pathways (Fig. 105-2).
FIGURE 105-2 Major pathways involved in melanoma. The MAP kinase and PI3K/AKT pathways, which promote proliferation and inhibit apoptosis, respectively, are subject to mutations in melanoma. ERK, extracellular signal-regulated kinase; MEK, mitogen-activated protein kinase kinase; NF-1; neurofibromatosis type 1 gene; PTEN, phosphatase and tensin homolog.
RAS and BRAF, members of the MAP kinase pathway, which classically mediates the transcription of genes involved in cell proliferation and survival, undergo somatic mutation in melanoma and thereby generate potential therapeutic targets. N-RAS is mutated in approximately 20% of melanomas, and somatic activating BRAF mutations are found in most benign nevi and 40–60% of melanomas. Neither mutation by itself appears to be sufficient to cause melanoma; thus, they often are accompanied by other mutations. The BRAF mutation is most commonly a point mutation (T→A nucleotide change) that results in a valine-to-glutamate amino acid substitution (V600E). V600E BRAF mutations do not have the standard UV signature mutation (pyrimidine dimer); they are more common in younger patients and are present in most melanomas that arise on sites with intermittent sun exposure and are less common in melanomas from chronically sun-damaged skin.
Melanomas also harbor mutations in AKT (primarily in AKT3) and PTEN (phosphatase and tensin homolog). AKT can be amplified, and PTEN may be deleted or undergo epigenetic silencing that leads to constitutive activation of the PI3K/AKT pathway and enhanced cell survival by antagonizing the intrinsic pathway of apoptosis. Loss of PTEN, which dysregulates AKT activity, and mutation of AKT3 both prolong cell survival through inactivation of BAD, Bc12-antagonist of cell death, and activation of the forkhead transcription factor FOXO1, which leads to synthesis of prosurvival genes. A loss-of-function mutation in NF1, which can affect both MAP kinase and PI3K/AKT pathways, has been described in 10–15% of melanomas. In melanoma, these two signaling pathways (MAP kinase and PI3K/AKT) enhance tumorigenesis, chemoresistance, migration, and cell cycle dysregulation. Targeted agents that inhibit these pathways have been developed, and some are available for clinical use (see below). Optimal treatment of patients with melanoma may require simultaneous inhibition of both MAPK and PI3K pathways as well as promotion of immune eradication of malignancy.
PROGNOSTIC FACTORS
The prognostic factors of greatest importance to a newly diagnosed patient are included in the staging classification (Table 105-3). The best predictor of metastatic risk is the lesion’s Breslow thickness. The Clark level, which defines melanomas on the basis of the layer of skin to which a melanoma has invaded, does not add significant prognostic information and has minimal influence on treatment decisions. The anatomic site of the primary is also prognostic; favorable sites are the forearm and leg (excluding the feet), and unfavorable sites include the scalp, hands, feet, and mucous membranes. In general, women with stage I or II disease have better survival than men, perhaps in part because of earlier diagnosis; women frequently have melanomas on the lower leg, where self-recognition is more likely and the prognosis is better. The effect of age is not straightforward. Older individuals, especially men over 60, have worse prognoses, a finding that has been explained in part by a tendency toward later diagnosis (and thus thicker tumors) and in part by a higher proportion of acral melanomas in men. However, there is a greater risk of lymph node metastasis in young patients. Other important adverse factors recognized via the staging classification include high mitotic rate, presence of ulceration, microsatellite lesions and/or in-transit metastases, evidence of nodal involvement, elevated serum lactate dehydrogenase (LDH), and presence and site of distant metastases.
|
STAGING CRITERIA FOR MELANOMA |
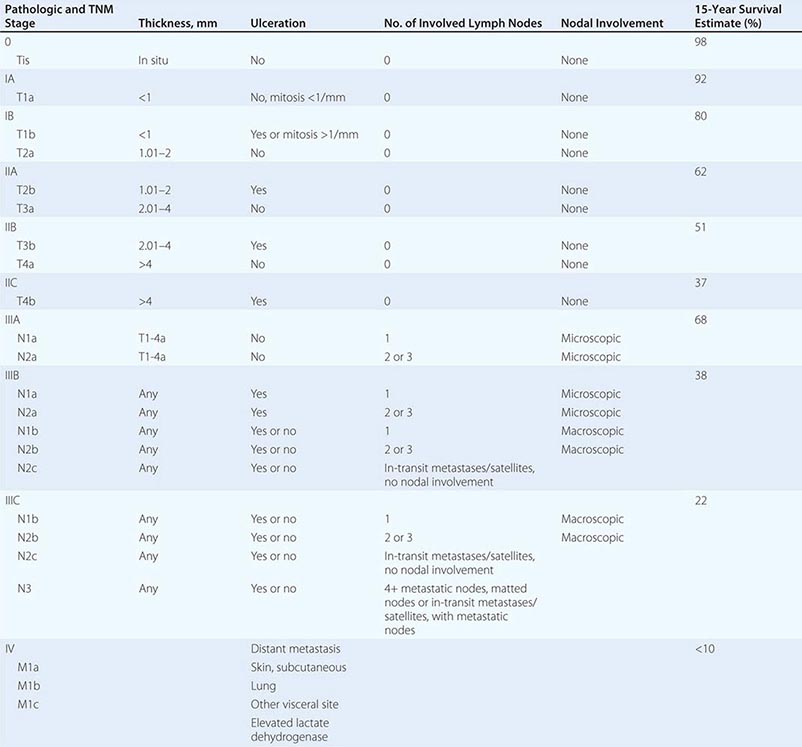
STAGING
Once the diagnosis of melanoma has been made, the tumor must be staged to determine the prognosis and treatment. Staging helps determine prognosis and aids in treatment selection. The current melanoma staging criteria and estimated 15-year survival by stage are depicted in Table 105-3. The clinical stage of the patient is determined after the pathologic evaluation of the melanoma skin lesion and clinical/radiologic assessment for metastatic disease. Pathologic staging also includes the microscopic evaluation of the regional lymph nodes obtained at sentinel lymph node biopsy or completion lymphadenectomy as indicated. All patients should have a complete history, with attention to symptoms that may represent metastatic disease such as malaise, weight loss, headaches, visual changes, and pain, and physical examination directed to the site of the primary melanoma, looking for persistent disease or for dermal or subcutaneous nodules that could represent satellite or in-transit metastases, and to the regional draining lymph nodes, CNS, liver, and lungs. A complete blood count (CBC), complete metabolic panel, and LDH should be performed. Although these are low-yield tests for uncovering occult metastatic disease, a microcytic anemia would raise the possibility of bowel metastases, particularly in the small bowel, and an unexplained elevated LDH should prompt a more extensive evaluation, including computed tomography (CT) scan or possibly a positron emission tomography (PET) (or CT/PET combined) scan. If signs or symptoms of metastatic disease are present, appropriate diagnostic imaging should be performed. At initial presentation, more than 80% of patients will have disease confined to the skin and a negative history and physical exam, in which case imaging is not indicated.