Chapter 29 Cancer
AETIOLOGY
Cancer is a multifactorial disease that is still not completely understood. Many theories exist as to what may cause cancer and only a few cancers have definitive risk factors (for example, cigarette smoking and lung cancer,1 or human papilloma (HPV) and cervical cancer).2 However, even with these connections, not everyone who smokes gets lung cancer and not everyone with HPV gets cervical cancer. Table 29.1 shows some possible general theories incorporating medical and naturopathic causes of cancer.
| POSSIBLE CAUSE | EXPLANATION |
|---|---|
| Genetics | Certain cancers, such as breast, prostate and colon cancer, have been found to have hereditary links. There are certain genetic markers that the medical fraternity can test for to check if someone has the genetic markers for that cancer, e.g. the BRCA gene for breast cancer.3 |
| Viruses and infection | Viruses such as the HPV have been linked with the development of certain cancers. Examples of these include human papillomavirus (cervical cancer);2 Epstein-Barr virus (non-Hodgkin’s lymphoma);4 HIV (Kaposi’s sarcoma, primary central nervous system lymphoma, invasive cervical cancer and non-Hodgkin’s lymphoma);5 hepatitis B virus (hepatocellular carcinomas);6 retroviruses (adult T-cell leukaemia);7 Helicobacter pylori (gastric cancer in people with certain polymorphisms);8 simian virus 40 (non-Hodgkin’s lymphoma)9 |
| Mitochondrial dysfunction | Cancer progression has been linked with mitochondrial dysfunction. This can be a result of mitochondrial DNA (mtDNA) mutations and/or depletion. These mutations or mitochondrial depletions have also been linked with an increase of drug resistance within cells. This mitochondrial dysfunction has been found in certain cancers such as prostate cancer.10 |
| Environmental influences | Over the years, an increase in cancer in the Western world has been found.11 Epidemiological studies have found an association with this increase and the exposure to environmental toxins such as organochlorides and synthetic pesticides.12 Further research is being conducted but it seems at this stage that fetuses, infants, children and young adults are most at risk.12 There are specific cancers, such as mesothelioma, which have been directly linked with exposure to asbestos.13 |
| Immune system | It has been postulated that a lowered immune system may increase the chance of cancer developing. The natural killer cells are the main surveillance system of the body, protecting against cancer formation and infection. These natural killer cells are a main part of the innate immune system and, if they are not functioning correctly, can allow tumours to develop through abnormal cell development.14 |
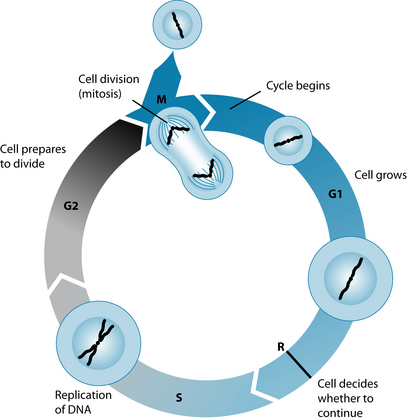
| Cell cycle mitosis malfunction | In cell replication certain checkpoints and cellular activity maintain the health of the cell. If these checkpoints, such as p53, have mutations15 or there are abnormal centrosomes in a cell, cancer cells may develop.16 The elderly in particular are prone to malignant tumours that are related to the accumulation of damaged DNA from malfunctioning cell mitosis.17 |
| Oxidative damage | There are several schools of thoughts that oxidative damage, especially to DNA and cellular components, can be attributed to the development of cancer. Reactive species such as ROS (reactive oxygen species) and RNS (reactive nitrogen species) are a natural by-product of normal biochemical and physiological reactions in the body. These reactive species can cause damage to the DNA, cellular membrane and cellular organelles, and can interfere with cellular regulators if there is not enough antioxidants to counteract reactive species damaging effect.18 |
| Mind–body connection | A different way of looking at cancer is the psychological aspect. There are theories on a connection between the mind and the development of certain cancers, but this has not been confirmed. However, there is good evidence that mind–body medicine should be taken into consideration when addressing cancer.19 |
| Polymorphisms | There are numerous studies on polymorphisms and genetic mutations increasing the risk of nearly all cancers. As this is a relatively new area of research, more studies will emerge on new polymorphisms that can be linked with certain cancers, and testing procedures will become more accessible and less expensive.20–22 |
| Epigenetics | The increase of scientific knowledge on how chromatin organisation modulates gene transcriptions has highlighted how epigenetic mechanisms are involved in the initiation and progression of human cancer. These epigenetic changes have been found to affect nearly every step in tumour progression, especially the aberrant promoter hypermethylation that is associated with gene silencing.23 |
Certain cancers have been linked with certain dietary and lifestyle choices (see Table 29.2).
Table 29.2 Links between cancer and lifestyle
| FOOD OR BEVERAGE | IMPLICATED CANCER |
|---|---|
| Smoking | |
| Alcohol | |
| Low fruit and vegetable, fibre intake, high intake of processed meats, nitrosamines, highly salted foods | |
| Obesity |
CANCER PREVENTION
A basic list of areas to consider in the prevention of cancer includes:
CAM TREATMENT OF COMMON SIDE EFFECTS OF CHEMOTHERAPY AND RADIATION
Table 29.3 provides specific evidence pertaining to integrative cancer treatment only; see the relevant chapters for generalised information.
Table 29.3 CAM treatment of common side effects of chemotherapy and radiation
| SIDE EFFECT | CAM THERAPY |
|---|---|
| Mouth ulcers | Calcium phosphate, honey, zinc sulfate39 |
| Diarrhoea | Glutamine40 |
| Constipation | Senna41 |
| Intestinal permeability | Glutamine42 |
| Radiation enteritis or enteropathy | Hyperbaric oxygen chambers,43 probiotics during radiation,44 glutamine during radiation45 |
| Neutrophilia | Vitamin E46 |
| Anaemia | Iron47 |
| Fibrosis | Lipoic acid48 |
| Fatigue | L-carnitine,49 coenzyme Q1050 |
| Memory loss | Egg phosphocholine and low-dose vitamin B1251 |
| Weight loss | Omega-3 fatty acids52 |
| Weight gain | Dietary suggestions to keep BMI in range53 |
| Stress/anxiety | l-theanine54 |
| Peripheral neuropathy | Vitamin B1, B6, B1255 |
| Cardiomyopathy | Coenzyme Q1056 |
POTENTIAL INTERACTIONS
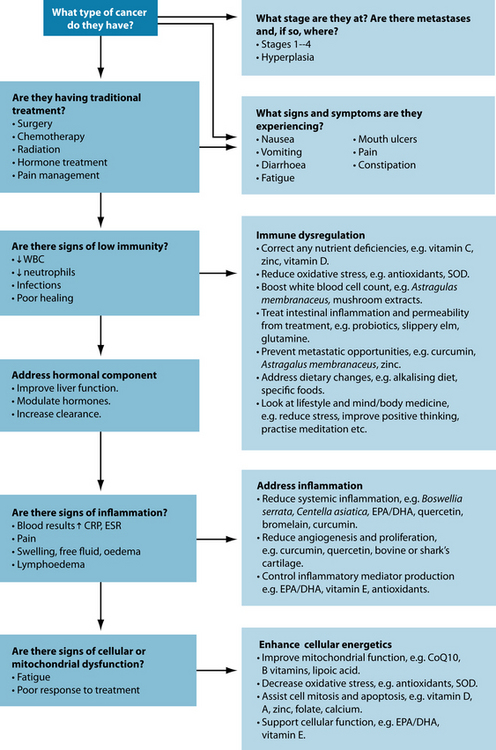
Potential interactions between orthodox and complementary therapies need to be taken into consideration when treating a patient with cancer. As there are many different drugs used for cancer, it is important for the naturopath to address each drug and check for any possible interactions both beneficial and contradictory (see Figure 29.1). Unfortunately, most potential interactions of cancer drugs and complementary medicine still require clinical research as there is little human data (most studies are animal or in vitro based).57 A chart of potential interactions between naturopathic medicines and chemotherapeutics is in the appendices at the back of the book.
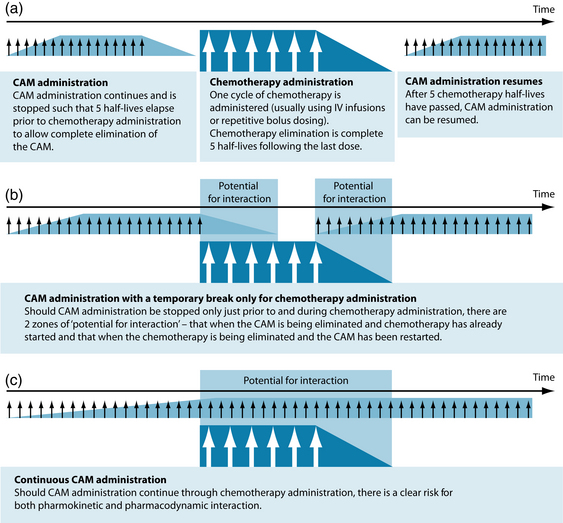
Figure 29.1 Risk of interactions associated with different CAM treatment protocols
Source: Seely D, et al58.
There are, however, ways in which the potential risk for interaction with chemotherapy can be moderated. As many naturopathic medicines can protect and support not only the body from deleterious effects of chemotherapy but the cancerous tissue as well, it may be prudent to apply a cautionary approach to CAM prescription.58 In principle it takes five half-lives to reach a steady state and five half-lives to eliminate virtually all of a drug in the body. Knowing both the half-lives of naturopathic medicine and chemotherapy can allow for a dosing regimen that can reduce risk of possible interactions (see Table 29.4).
Table 29.4 Selected antioxidant naturopathic medicines and their half-life58
| NATUROPATHIC INTERVENTION | HALF-LIFE |
|---|---|
| Green tea | 3.4 h ± 0.3 h |
| Curcumin | < 1 h |
| Selenium | 102-252 days |
| Vitamin C | 30 min |
RISK FACTORS
The risk factors vary depending on the type of cancer. These may include diet and lifestyle factors, viruses, genetics, environmental exposure and lowered immune system. Other risk factors include lack of physical exercise,59 high BMI/obesity,59 exposure or lack of exposure to sun (due to low vitamin D),60,61 nutrient deficiencies,62 medication (in some cases medication can increase the risk of other cancers, such as Tamoxifen
COMMON TUMOUR MARKERS
causing endometrial carcinoma),63 country/nationality,64 stress,65 gender (for example, prostate cancer in males and ovarian cancer in females), age17 and occupation.66–68
NATUROPATHIC APPROACHES TO CANCER PATHOPHYSIOLOGY
Cell mitosis
Oncogenes and tumour-suppressor genes
Oncogenic processes have long been associated with cancer development and progression. These genes stimulate the process of cell mitosis and DNA replication and studies have shown that these genes are altered in cancer. The two main genes that are focused on are the proto-oncogenes and tumour-suppressor genes.69
Recent studies have identified a specific small class of RNA molecules called microRNAs (miRNAs). These miRNAs negatively regulate gene expression post-transcriptionally and aid the development of cell identity. These are important as they can affect cellular transformation, carcinogenesis and metastasis because they can act as an oncogene or tumour-suppressor gene.70
According to naturopathic treatment, keeping cellular oncogenes working efficiently without the development of cancer requires both dietary regulation and the maintenance of levels of particular nutrients in the body. Epidemiological and experimental data have found that Western diets that are high in fat and low in calcium and vitamin D can accelerate tumour growth by causing genetic manipulation.71 Maintaining a diet low in detrimental fats and increasing calcium and vitamin D levels may therefore prevent gene alteration.
p53, p21
Abnormalities in the regulatory marker p53 have been found in most cancer cells. Cancer cells may have either mutations of the p53 gene or an accumulation of p53 proteins in the cells.72,73 p21-activated kinases (Paks) aid controlling G(2)/M transition and mitosis and are tumour-suppressor genes regulated by p53. Therefore they are necessary for cell proliferation, mitotic progression and other regulators.74,75
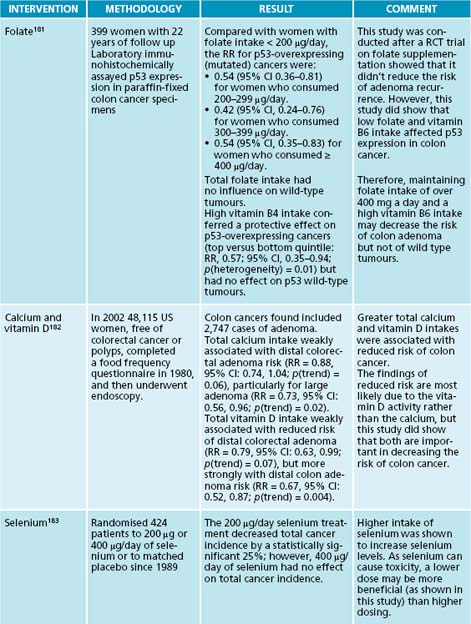
A number of different nutrients can affect the p53 and p21 genes and be of benefit for cancer. Low levels of folate and vitamin B6 can affect p53, increasing the risk of colorectal cancer.76 The mechanism of this deficiency involves the mutation of the p53 gene, therefore changing its expression. It is recommended that people should consume over 400 μg of folate a day to prevent this occurring.
Resveratrol has also been found to modulate p53 to induce apoptosis (programmed cell death that is an integral part of normal biological processes to kill abnormal cell development) as well as knocking out excessive p53, having a beneficial effect in preventing cancer. Resveratrol has also been found to induce apoptosis via modulating other cellular molecules such as cyclin D1, p21, BCL2, BAX, Bcl XL, caspase 9 and p27.77,78
Green tea is another nutrient that has been found to affect p53 and p21. One study has found that green tea enhanced Zizyphus jujuba’s selective cytotoxic activity by causing cell death via up-regulation of p53 and p21 while decreasing cyclin E levels in HepG2 cells.79 Green tea has also been found to inhibit tumour growth and angiogenesis as well as induce apoptosis.80 Genistein has been found to influence p21 transcription by markedly inhibiting proliferative activity and inducing the expression of p21 plus ERbeta.81
Vitamin D also influences p53 and p21. Both p21 and p53 have vitamin D binding sites (VDR).75 If a patient is deficient in vitamin D, both p53 and p21 may be affected, possibly influencing cancer development or progression. Flavonoids—such as quercetin—have also been found to influence p53 in relation to cancer. One study found that quercetin helped to stabilise p53 at both the mRNA and the protein level to reactive p53-dependent cell cycle arrest and apoptosis.82 This was achieved by quercetin inducing p53 phosphorylation and total p53 protein, but not up-regulating p53 mRNA at transcription. It also stimulates p21 expression and suppresses cyclin D1 to activate cell cycle arrest. Quercetin was also found to inhibit p53 mRNA degradation post transcriptionally.
Centrosomes
These microtubules aid cell mitosis by creating the polar rejection force that pushes the chromosomes away from the spindle poles by wobbling at high frequencies. A rise in intracellular calcium aid the regulation of the polar force at the onset of chromosomal splitting. If this is defective, chromosomal instability (characteristic of most cancers) can occur.83 It has also been found that cells that have dysfunctional p53s have more centrosomes.84
Vitamin A plays a key role in centrosomes and chromosome replication through the retinoblastoma protein, which is a regulating protein of cyclins D and E, cdk 4 and 6, cdk inhibitors p16, p15 and p53.85 A vitamin B12 deficiency has been associated with enlarged, disrupted centrioles in monocytes and neutrophils.86
Proteasomes play an important role in a variety of cellular processes such as cell cycle progression, signal transduction and the immune response, and are important in maintaining rapid turnover of short-lived proteins. They also prevent accumulation of misfolded or damaged proteins. Zinc increases proteasome substrates such as p5 and p21, as well as decreasing the enzyme that degrades these substrates in centrosomes.87
Folate also plays a critical role in the prevention of chromosome breakage and hypomethylation of DNA. A folate deficiency can cause centromere defects that can induce abnormal distribution of replicated chromosomes during mitosis. It has also been found to be a risk factor for chromosomes 17 and 21 aneuploidy, which has been observed in breast cancer and leukaemia.88
Glycolysis and p53
A common theory sometimes postulated in the naturopathic treatment of cancer is that tumours feed on sugar. In part this is true, as cancer cells work differently to other cells biochemically. Research has found that most cancer cells have higher rates of glycolysis than normal cells.73 Research has found that, after a loss of functional p53, there were mitochondrial changes, up-regulation of rate-limiting enzymes and proteins in glycolysis and intracellular pH regulation, hypoxia-induced switching to anaerobic metabolism leading to higher lactic acid levels, and metabolic reprogramming.
Extrapolation of these results has led to various diets being developed to ‘starve’ the cancer. This theory has not yet been proven to work; however, it is still recommended that people with cancer decrease their sugar intake as it has been found that insulin-like growth factor 1 is involved in a number of cancers.89,90 One animal study has shown delayed growth of gastric cancer cells with administration of a ketogenic diet high in omega-3 fats.91
Another theory (the Warburg effect) suggests ‘oxygenating’ the body, as cancer cells use more anaerobic glycolysis than normal cells do and normal cells use the aerobic system of the mitochondria.92 Increasing oxygen availability (for example, through breathing exercises and circulatory stimulants) to cells more generally may aid the normal cells rather than the cancer cells and also aid the removal of toxins and carcinogens.
Inflammation
Inflammation has been linked with a variety of different cancers as inflammatory markers aid tumour progression, promotion and growth. It has been found that a cellular inflammatory microenvironment and an increased level of nitric oxide can stimulate or accelerate tumour development.93 Cyclooxygenase enzymes such as prostaglandins and thromboxanes play an important role in cancer, particularly in gastrointestinal, oesophageal, gastric, liver, pancreatic and colorectal cancers.94
A key approach to cancer is to modulate the inflammatory cascade; this can have a positive effect on cancer initiation, promotion, progression and metastasis. Anti-inflammatory nutrients such as curcumin,95 bromelain,96 quercetin96 and EPA/DHA96 can decrease the progressive effect of the inflammation in cancer. Herbal medicines such as Zingiber officinale, Curcuma longa,95 Withania somnifera97 and Boswellia serrata may also be useful.96
Immunological factors
A functioning immune system appropriately monitors and attacks any foreign cells or pathogenic invaders. A dysregulated immune system can allow cancer cells to develop and spread.98 States that can cause a dysregulated immune system affecting cancer include stress,99,100 diabetes,101 HIV (and other viruses)102 and nutritional deficiencies.103,104
Nutrients and herbs that support immune function can be very important in assisting people with cancer. Those clinical nutritional interventions with particular benefit in cancer include vitamin D,105 antioxidants,106 vitamin C,106 zinc106 and medicinal mushrooms such as shiitake or reishi.107,108 Herbal medicines which may be of benefit include Astragalus membranaceaus,109 Uncaria tomentosa,110 Eleutherococcus senticosus,111 Rhodiola rosacea,111 Schisandra chinensis,111 Panax ginseng112 and Echinacea spp.113
Hormonal factors
Some specific cancers, including breast,114 ovarian, endometrial,115 cervical, prostate and testicular cancers, are well known to have hormonal influences.116 Other cancers, including both gastric and lung cancer, may also have hormonal influences.117,118
Breast cancer can be stimulated by oestrogen and/or progesterone,114 whereas ovarian, endometrial and cervical cancers may be oestrogen-stimulated via receptor sites on the cancer cells.115 Testosterone is the key hormone related to prostate- or male genitalia-based cancers, whereas testicular cancer may be related to either oestrogen or androgens, and approximately 7–11% of males who get testicular cancer have also been found to have gynaecomastia.116
Oestrogen may have protective effects on gastric cancer, as the prevalence of gastric cancer is higher in men and lower in premenopausal women, women on hormone replacement and men undergoing oestrogen therapy.117 The actual mechanism of protection is unclear, but it is thought that oestrogen may increase the expression of trefoil factor proteins, which have been found to protect mucous epithelia or inhibit the expression of c-erb-2 oncogene.
Lung cancer has also been found to have an oestrogen connection. Adenocarcinoma is the most common type of lung cancer in non-smokers, and it has been suggested that there is a distinct pattern concerning oestrogen beta receptors.118
A number of nutrients and herbs can have a positive effect in the integrative treatment of hormonal tumours. For oestrogen- and progesterone-stimulated tumours, cruciferous indoles—particularly indole-3-carbonol—aids in a number of different ways: it aids the induction of apoptosis in breast and cervical cancer cells by inducing DNA breakage in the nucleus of the cells,119 and it alters the metabolism of active oestrogen hormones indicated in urinary excretion (16-oestrodiol to 2-oestrodiol).120
Other nutrients that have been found to be of assistance include zinc for testosterone via the enzyme 5 alpha-reductase;121 resveratrol via inhibiting the cytochrome p450 19 enzymes—also called aromatase catalases—which are the rate limiting step of oestrogen synthesis;122 and genistein, which, although controversial and having mixed results, has shown positive results by inhibiting cell proliferation and inducing apoptosis in breast cancer cell lines.123 However, it has also been shown to stimulate growth in oestrogen-dependent human tumour cells, MCF-7, and may negate the inhibitory effect of Tamoxifen.124
Herbs that have shown to be of potential benefit include Serenoa repens for prostate cancer. Serenoa has been found to induce growth arrest of prostate cancer cells as well as increasing p21 and p53 in the prostate cells.125 Cimicifuga racemosa has been found to improve symptoms associated with breast cancer by alleviating vasomotor symptoms associated with the decrease of oestrogen.126
Trifolium pratense has also been found to be of benefit for oestrogen-based cancers. The isoflavone constituent biochanin A has been found to inhibit cytochrome p19 (aromatase) activity and gene expression, therefore decreasing the effect of active oestrogen.127
Mitochondrial function
Mitochondrial dysfunction has been linked with the increased risk of cancer.128 Most of the mitochondrial influence is via its link to apoptosis (p53) and anaerobic glycolysis; however, there may be another area in which mitochondrial function plays an important role. Cancer cells may have impaired mitochondria and mitochondrial activity, which may support Warburg’s theory as to why cancer cells use the anaerobic glycolysis pathway rather than the aerobic system.129 Concentrating on mitochondrial reprogramming and support may therefore assist in addressing cancer. Nutrients that have been found to assist mitochondrial function include coenzyme Q10,130 lipoic acid,131 carnitine132 and B vitamins such as niacin (NAD).133
Angiogenesis
Angiogenesis (developing their own blood supply to aid their proliferation and growth) in tumours is well recognised.128 Many chemotherapeutics currently target this angiogenic process. However, some naturopathic medicines have also been found to possess an anti-angiogenic action. These include intravenous vitamin C,134 Curcuma longa,135,136 Artemisia annua, Viscum album, Scutellaria baicalensis, resveratrol and proanthocyanidins, Magnolia officinalis, Camellia sinensis, Ginkgo biloba, quercetin, Poria cocos, Zingiber officinales, Panax ginseng and Rabdosia rubescens hora.137 Using any number of these naturopathic medicines may aid the reduction of angiogenesis, by virtue of possibly having a positive effect on decreasing tumour initiation, promotion, progression and metastasis.
DIET AND CANCER
There have been a number of studies on diet and its effect on cancer. Certain dietary deficiencies have been associated with cancer development. Dietary factors account for approximately 30% of cancer cases in developed countries and 20% in developing nations, and are second only to tobacco as a preventable cause of cancer.138 Other studies have been conducted on specific foods or beverages linked with cancer. There are also a multitude of diets that are promoted as ‘anti-cancer diets’, but most of these have not been studied specifically and are mostly based on broader empirical evidence.
The most common dietary link to cancer is the crude lack of fruit and vegetables.139 An increased intake of fruit and vegetables has been found to be protective against lung,140 oesophageal141 and colon cancer.142 Fruit and vegetable intake has also been suggested to be protective from breast and ovarian cancer although results at this stage state that the lack of fruit and vegetables is not a risk factor for either.143,144
In addition to an increased consumption of fruit and vegetables, a low intake of meat and potatoes has been found to be protective against colorectal and oesophageal cancer.141,142 High meat consumption—particularly through adolescence—has also been found to be a risk factor for premenopausal breast cancer.145 Both high meat consumption and high sugar intake have also been linked as risk factors for pancreatic cancer.146 High poultry intake and high-fat dairy intake are associated with an increased risk of gastric cancer.141 Adherence to a Western-style diet and high alcohol intake are also associated with an increased risk of gastric cancer.147 A high intake of alcohol has been associated with not only gastric cancer but also breast148 and liver cancer.26
Fat intake is another area of concern in relation to cancer. High-fat diets have been linked with numerous cancers, including breast, prostate and colon cancers.149,150 Studies have also found that high fat intake increases the oxidation of DNA and encourages damaged cell proliferation.149 However, specific fats, such as omega-3 fatty acids as found in fish oil, have demonstrated protective effects for breast, prostate and colon cancer.149,150 The current Western diet has a disproportionate ratio of omega-6 fatty acids to omega-3 fatty acids compared to more traditional diets; this may result in increased risk of cancer development.150
Iodine has been studied for a number of cancers, including thyroid cancer and breast cancer. Both a deficiency and an excessive amount of iodine have been associated with an increased risk of thyroid cancer.151,152 For breast cancer, recent studies have suggested that iodine may have a protective effect on breast tissue by inhibiting cancer development via the modulation of the oestrogen pathway.152
Green tea153 has also been extensively studied for cancer with positive results, and black tea154 and coffee155 have also shown convincing beneficial effects. Green tea and black tea have demonstrated benefit across a broad range of cancers; however, coffee seems to be mainly beneficial in protection against liver cancer. The protective effect of coffee seems to be related to moderate intake with studies looking at only one coffee drink a day, or less than 250 mg of caffeine intake a day.155
Studies looking at vegetarian or plant-based diets have also achieved reasonable results, though nothing conclusive has been found at this stage. It seems that a vegetarian or plant-based diet or a macrobiotic diet may be of benefit, particularly for colorectal, prostate and breast cancer.156,157 This may be related not merely to the vegetarian nature of the diet, but also to the increased proportions of fibre, fruit and vegetable intakes often associated with these diets.
INTEGRATIVE MEDICAL CONSIDERATIONS
Acupuncture
There are many studies proving the beneficial effects of acupuncture for cancer, for a variety of different reasons. Studies using acupuncture to treat cancer pain,163 vasomotor and fatigue symptoms of both breast and prostate cancer164,165 show that it boosts the immune system and decrease tumour growth166 and is beneficial for the treatment of nausea and other side effects.167 Acupuncture has had documented success in improving nausea symptoms associated with chemotherapy.168–170 Electroacupuncture seems more effective than manual acupuncture for this purpose. Other studies on acupuncture and specific cancer conditions show benefit, suggesting it may be a beneficial adjuvant treatment.128
Psychosocial and mind–body interventions
Meditation can be of assistance to people with cancer by reducing stress, improving mood and quality of life and aiding sleep problems.171 Counselling has been found to assist with the anxiety, depression and life decisions that these people experience.172
Yoga is another therapy that has growing support to assist with quality of life, sleep and mood. Other therapies that may be of assistance include creative therapies such as
ALTERATIVES/DEPURATIVES AND CANCER—ESSIAC
One of the best known CAM treatments used a depurative for cancer is the Essiac® tea. It is a mixture of four herbs: Arctium lappa, Rumex acetosella, Rheum officinale and Ulmus fulva. It was originally a herbal formulation from the Ojibway Indians in Canada and was discovered in 1922 by Rene Caisse, who reports to have ‘cured’ hundreds of people with cancer with this formula.158
Rumex acetosella is said to destroy cancer cells while the other three herbs aid purifying the blood. A chiropractor, Gary Glum, stated that the burdock root also contains inulin, which is considered to be a powerful immune modulator.158 Most of the information is anecdotal; however, there have been a few recent studies on the mechanism of action of Essiac, although there are also studies that found that there was no real benefit to Essiac.
The first real investigation of Essiac was in 2007 by the Department of Research and Clinical Epidemiology in the Canadian College of Naturopathic Medicine in Toronto.159 This study comes about as a response to the negative recommendations from the Task Force on Alternative Therapies of the Canadian Breast Cancer Research Initiative, as very little research has been conducted on this formula.159
The activity of Essiac was measured using assays to establish the antioxidant, fibrinolytic, antimicrobial, anti-inflammatory, immune modulation, cell-specific cytotoxicity and effect on cytochrome P450 enzymes. It was found that Essiac exhibited significant antioxidant activity and had immunomodulating effects through stimulation of granulocyte phagocytosis, increasing CD8+ cell activation and moderately inhibiting inflammatory pathways. It also exhibited significant cell-specific cytotoxicity towards ovarian epithelial carcinoma cells. In regards to cytochrome P450 enzymes, it inhibited CYP1A2 and CYP2C19. Clot fibrinolytic activity was dose dependent.159
Two other in vitro studies found that Essiac had positive results, with one indicating inhibition of tumour growth in prostate cells.160 However, one of the studies also showed that Essiac tea stimulated some pro-inflammatory molecules as well as nitric oxide.161 This same study found that that it had no anti-proliferative affect on leukaemic cell lines but that it inhibited 50% of MCF7 breast cancer cell lines, as did Flor-Essence tea—a similar product containing Arctium lappa, Rumex acetosella and Ulmus fulva, but also containing Nasturtium officinale, Rheum palmatum, Cnicus benedictus and Trifolium pratense.161
Only one in vivo study has been conducted on animals (mice); the tea was administered orally, mimicking human intake. The result found that it did demonstrate a modest gastric protective effect by reducing ethanol-induced gastric ulceration. However, there was no demonstration of hepatoprotective, hypoglycaemic or immunomodulatory effects, not supporting the suggested actions of Essiac.162
visual arts, painting, dance and music, which may help patients with cancer express their feelings and help them cope with what they are experiencing.171 A review of 50 studies found that Qi gong was mostly associated with positive outcomes in cancer patients.173
However, it should be noted that while such interventions, like support groups, can help cancer patients in numerous ways, evidence does not at this stage seem to suggest that this translates into prolonging their lives.174 Religious or spiritual beliefs have been found to be of benefit for cancer patients. The supportive environment and thought of an afterlife can be very important for cancer patients;175 however, this should not translate to the encouragement of false hope.
Homoeopathy
A Cochrane review on homoeopathy and cancer treatment involving eight controlled trials and a total of 664 participants found preliminary data to support the efficacy of topical homoeopathic Calendula treatment for prophylaxis of acute dermatitis during radiotherapy and the proprietary homoeopathic Traumeel S mouthwash in the treatment of chemotherapy-induced stomatitis.176 They found no convincing evidence for the efficacy of homoeopathic medicines for other adverse effects of cancer treatment. Pilot studies from the UK hospitals have demonstrated positive patient outcomes when homoeopathy has been used in conjunction with conventional treatment.177
Massage
Massage has been found to give immediate beneficial effects for pain and improving mood for patients with cancer.178 While positive outcomes have been found for stress and anxiety as well, adverse effects from massage therapy—including pathological fractures of metastatic bones and bruising in patients with coagulopathy—may be more likely in cancer patients.179
KEY POINTS
 Cancer is a complex condition with a myriad of different causes and interactions. It is important to address this multifactorial condition from many different aspects by addressing each stage rather than everything at once.
Cancer is a complex condition with a myriad of different causes and interactions. It is important to address this multifactorial condition from many different aspects by addressing each stage rather than everything at once. Keep supplementation and diet simple, and don’t overwhelm the patient with too many things to take, especially if they are also undergoing traditional treatment.
Keep supplementation and diet simple, and don’t overwhelm the patient with too many things to take, especially if they are also undergoing traditional treatment.Overview
The aetiology of cancer has been previously discussed; however, there are certain aspects that are more relevant to colon cancer. Diet and lifestyle are associated with the development of colon cancer, but the most clearly defined aetiological factors appear to be hereditary factors, inflammatory bowel disease, papillomavirus and acquired immunodeficiency syndrome (AIDS).180
Example treatment
Prescription
This patient has started with a very good diet and has adequate fibre intake, so minimal dietary changes are required. The dietary changes include adding cabbage to the juice she is consuming daily. Due to weight loss, it would be beneficial to drink a whey-based protein drink twice a day as well as increasing her intake of sardines and salmon for the omega-3 fatty acids. Using fats such as flax seed, chai seeds, nuts and seeds, olive oil and ghee in her diet will also be of benefit for both good fats and maintaining weight. She should drink at least 2–3 cups of sencha green tea, making sure to let it stand for 10 minutes before drinking to ensure catechin release.
Herbal formula
| Echinacea angustifolia 1:2 | 30 mL |
| Astragalus membranaceus 1:2 | 40 mL |
| Eleutherococcus senticosus 1:2 | 30 mL |
| 5 mL t.d.s. | 100 mL |
| Silybum marianum thistle 10 g 1 tablet b.d. | |
Nutritional prescription
| Folic acid tablet | 500 mg/day |
| Selenium capsule | 200 μg/day |
| Vitamin D3 capsule | 1000 μg/day |
| Activated B6 | 50 mg/day |
Note: No supplements should be taken the day before and 1–2 days after chemotherapy.
For exercise she is advised to continue yoga on a daily basis. Her spiritual beliefs and supportive environment will be good for her wellbeing.
Expected outcomes and follow-up protocols
This patient has a very good state of mind and will to live, as well as a supportive environment that will benefit her greatly. However, as the primary site has not been found, her long-term results are quite difficult to predict. In all, the diet and supplements should decrease her side effects, increase her immune system, aid cellular function, decrease inflammation and support body functions. She has specific foods for bowel cancer and support for her liver.
KEY POINTS
OVERVIEW
The aetiology of breast cancer is multifaceted. There are many suggested causes of this disease and there are a variety of different types of breast cancer from benign to hormone sensitive to inflammatory. Breast cancer can be oestrogen positive, progesterone positive or both. Research has found that longer exposure to oestrogen and higher levels of oestrogen exposure do increase the risk of developing breast cancer. Pregnancy, late menarche and early menopause are all considered to be protective factors.187 High concentrations of oestrogen even as a fetus have been found to have an effect on breast cancer development.188
Another risk factor for breast cancer is an energy-rich diet during puberty and adolescence as it develops pre-cancerous lesions in the breast. Full-term pregnancy decreases these developments; however, continuation of an energy-rich diet late in life also contributes
to an increase in obesity and growth of existing subclinical breast cancer lesions. The mechanism behind this dietary factor is through endocrine factors such as oestrogen.189
Other suggested risk factors include iodine deficiencies,190,152 vitamin D deficiencies,191,192 high intake of red meat,145 environmental exposure to xenoestrogens193 and organochlorides,194,195 factors such as make-up, deodorants and creams,196,197 low melatonin or sleep deprivation (including that from shiftworking)198 and other diseases such as diabetes.199
Example treatment
Prescriptions
Diet: As this patient had a good diet; all she needed was more variety. Diet suggestions include increasing cold water oily fish, salmon or sardines four to five times a week, and including fruits such as berries, strawberries, paw paw, red apples, cherries, red grapes and pears. For grains, include quinoa, buckwheat or black rice porridge using coconut milk. She can also include legumes such as chickpeas, akuzi beans and broad beans. She can use quinoa or brown rice for lunch and/or dinner for added grains. Organic red meat can be consumed 1–2 times a week if needed, low-mercury fish as much as she wants, and organic chicken 2–3 times a week. Almond or quinoa milk can be used where required. One drink of a red wine such as pinot noir a night for 3–4 nights or fewer a week if she wants (this is for resveratrol).
Nutritional prescription
CoQ10 200–300 mg/day with food
Lipoic acid 400 mg–600 mg/day with food
Vitamin B complex 1 capsule a day after breakfast
Quercetin 600 mg and vitamin C 3 g split throughout the day in between food
5-hydroxytryptophan 100 mg at night away from food, for anxiety
Calcium 800 mg and vitamin D 5–100 μg at night before bed
Indole-3-carbinole or diindolylmethane (DIM) 2–4 capsules a day
Lifestyle: Take up yoga or pilates at least once a week. Meditate every day for around 5–20 minutes, or more if possible. Look at getting massage when needed to aid relaxation. Look at stress management techniques.
Supplements: All supplements are explained above in the overview. Indole-3-carbinole (not discussed above) has been found to help the conversion of oestrogen to 16-hydroxyestrone (16-OHE1) instead of 2-hydroxyestrone (2-OHE1). Women with breast cancer have been found to have higher levels of 2-OHE1, which is considered to be detrimental as it is associated with the development of breast cancer.203
Note: No supplements are to be taken at least 3 hours before or after the chemotherapy tablet.
Other prescriptive options
Many other supplements, including liver nutrients, taurine, glutathione, mixed antioxidants and berry punches, can be used. It is important to ascertain which is most appropriate at each particular stage for the patient without overwhelming them.
Expected outcomes and follow-up protocols
KEY POINTS
Overview
Like the other cancers, the aetiology is not completely proven and is multifactorial. The one clear factor that has been linked with prostate cancer is testosterone and its active metabolite, dihydrotestosterone.204 Genetic factors have also been found to be involved with prostate cancer. In this case, there is a complex interaction between genetic factors and the environment, which seem to be the predisposing factors for prostate cancer.204
Occupational factors that increase the risk of prostate cancer include jobs that have exposure to cadmium and other heavy metals in men with lower zinc levels. These include occupations such as farmers, miners, painters and printers.204 The dietary factors associated with increased risk of prostate cancer include fats such as animal fats and alpha-linoleic vegetable fats. The fat on meats in particular has been found to be a high indicator.204
Sexual habits have also been linked with increased risk of prostate cancer. This includes the age of first intercourse, the number of sexual partners and a history of sexually transmitted diseases. All of these have been found to increase the risk; however, studies are still inconclusive.204
Example treatment
Prescription
Herbal formula
| Uncaria tomentosa | 1:2 50 mL |
| Andrographis paniculata | 1:2 25 mL |
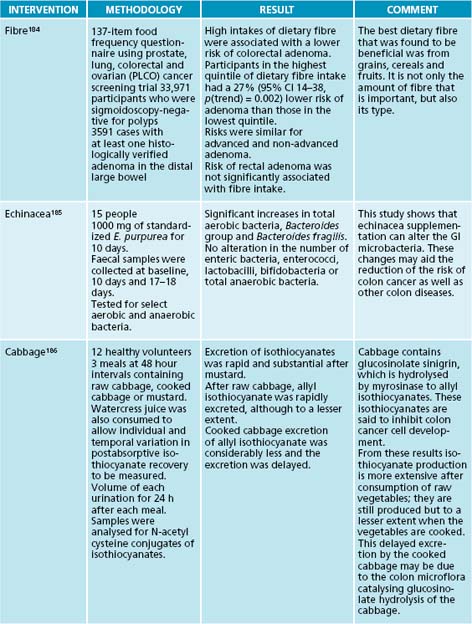
| Matricaria recutita | 1:2 25 mL |
| 5 mL t.d.s. | 100 mL |
| Serenoa repens 3 g tablet b.d. | |
Diet: Alkalise the diet as much as possible, aiming for a low-fat, vegan-style diet with the exception of eating fish. It is important to include a lot of fruit and vegetables, especially cruciferous vegetables such as broccoli, cabbage, kale and brussels sprouts for the sulforazanes, as well as berries, especially raspberries. Cooked tomatoes is also important for lycopene, and carrots and high betacarotene foods are also beneficial. Garlic, onions, shallots as well as spices such as turmeric and cumin are also very good to include in the diet. Reduce as many animal products as possible, including fatty meats, cream and dairy. Increase fish as a protein, plus nuts, seeds and legumes. Recommend that he drink ginger punch, which has extra antioxidants in it, including turmeric, green tea and resveratrol. Suggest that he stops drinking alcohol for the 3 months,
decreases his coffee consumption to one ‘real’ coffee a day and changes to drinking a green tea such as sencha (remembering to let it sit for 10 minutes before drinking).
Supplements: The nutritional supplementation has been explained above. Although no RCT studies have been conducted on Serenoa repens, it has shown to be of benefit for prostate cancer patients.125 Andrographis paniculata has been found to induce apoptosis of prostate cancer cells via the activation of caspase 3, up-regulation of bax, down-regulation of bcl-2 and inhibition of vascular endothelial growth factor.208 Matricaria recutita has also been found to be antiproliferative and can induce apoptosis in cancer cells but not normal cells.209 Uncaria tomentosa has also been found to have antiproliferative effects against cancer.110 Monitoring the selenium and zinc as well as copper levels by blood tests on a regular basis, ideally every 2 months, is also advised.
Other prescriptive options
As in the breast cancer case, supplements that could be very beneficial and can be added later include calcium D-glucarate, which aids the excretion of environmental or pharmacological xenobiotics by increasing glucuronidation in phase II liver detoxification. Citrus pectin has been suggested to be of assistance, particularly in people with metastasis. Melatonin has many chemoprotective and antioxidant properties that have been found to be beneficial for people with cancer. Mushroom extracts such as shitake or reishi may also be used to boost the immune system.
Expected outcomes and follow-up protocols
It is important to remember that every patient responds differently. This client has allowed 3 months to see what can happen and he could expect to see his PSA decrease but not to levels low enough for him not to consider other treatment. The decision remains the patient’s; he may decide that as there has been enough of a decrease that he will continue with the CAM interventions rather than the orthodox for as long as possible. The older the patient, the more likely it seems that the PSA will decrease and that patients can continue with complementary therapies. However, younger men seem to not decrease their PSA as much and may be less inclined to continue complementary treatment alone.128
KEY POINTS
Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009. In press
Bartsch H., et al. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20(12):2209-2218.
Dewell A., et al. A very-low-fat vegan diet increases intake of protective dietary factors and decreases intake of pathogenic dietary factors. J Am Diet Assoc. 2008;108(2):347-356.
Fleet J.C. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29(6):388-396.
Gissel T., et al. Intake of vitamin D and risk of breast cancer—a meta-analysis. J Steroid Biochem Mol Biol. 2008;111(3–5):195-199.
Littrell J. The mind-body connection: not just a theory anymore. Soc Work Health Care. 2008;46(4):17-37.
Mumber M., editor. Integrative oncology: principles and practice. London: Taylor & Francis, 2005.
Oh K., et al. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. 2007;165(10):1178-1186.
Reid M.E., et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60(2):155-163.
Schernhammer E.S., et al. Folate and vitamin B6 intake and risk of colon cancer in relation to p53 expression. Gastroenterology. 2008;135(3):770-780.
Mason M., Moffet L. Prostate cancer: the facts. New York: Oxford University press, 2003.
Northrup, Northrup C. Breast health CD set. ISBN: 1564553116.
Stoddard F.R., et al. Iodine alters gene expression in the MCF7 breast cancer cell line: evidence for an anti-estrogen effect of iodine. Int J Med Sci. 2008;5(4):189-196.
Urbaniak E., et al. Healing your prostate: natural cures that work. Gig Harbor: Harbor press, 1998.
Wallace J.M. Nutritional and botanical modulation of the inflammatory cascade—eicosanoids, cyclooxygenases, and lipoxygenases—as an adjunct in cancer therapy. Integr Cancer Ther. 2002;1(1):7-37.
1. Cancer Council NSW. Lung cancer: causes and symptoms. myDR Australian Government, 2002.
2. Krambeck W.M., et al. HPV detection and genotyping as an earlier approach in cervical cancer screening of the female genital tract. Clin Exp Obstet Gynecol. 2008;35(3):175-178.
3. Bourret P. BRCA patients and clinical collectives: new configurations of action in cancer genetics practices. Soc Stud Sci. 2005;35(1):41-68.
4. Queiroga E.M., et al. Viral studies in Burkitt lymphoma: association with Epstein-Barr virus but not HHV-8. Am J Clin Pathol. 2008;130(2):186-192.
5. Nutankalva L., et al. Malignancies in HIV: pre- and post-highly active antiretroviral therapy. J Natl Med Assoc. 2008;100(7):817-820.
6. Tao X., et al. The role of hepatitis B virus x gene in development of primary hepatocellular carcinoma. Sci China C Life Sci. 2000;43(3):293-301.
7. Yoshida M. Identification of adult T-cell leukemia virus and its gene structure. Gan To Kagaku Ryoho. 1983;10(2):680-689.
8. Zhou S.Z., et al. [Association of single nucleotide polymorphism at interleukin-10 gene 1082 nt with the risk of gastric cancer in Chinese population]. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(8):1335-1338.
9. Amara K., et al. Presence of simian virus 40 DNA sequences in diffuse large B-cell lymphomas in Tunisia correlates with aberrant promoter hypermethylation of multiple tumor suppressor genes. Int J Cancer. 2007;121(12):2693-2702.
10. Moro L., et al. Mitochondrial DNA depletion reduces PARP-1 levels and promotes progression of the neoplastic phenotype in prostate carcinoma. Cell Oncol. 2008;30(4):307-322.
11. Kamangar F., et al. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137-2150.
12. Newby J.A., Howard C.V. Environmental influences in cancer aetiology. J Nutr Environ Med. 2006:1-59.
13. Frost G., et al. Occupational exposure to asbestos and mortality among asbestos removal workers: a Poisson regression analysis. Br J Cancer. 2008;99(5):822-829.
14. Schmitt C., et al. NK cells and surveillance in humans. Reprod Biomed Online. 2008;16(2):192-201.
15. Bell H.S., Ryan K.M. Targeting the p53 family for cancer therapy: ‘big brother’ joins the fight. Cell Cycle. 2007;6(16):1995-2000.
16. Pelletier L. Centrosomes: keeping tumors in check. Curr Biol. 2008;18(16):R702-R704.
17. Arai T., et al. Role of DNA repair systems in malignant tumor development in the elderly. Geriatr Gerontol Int. 2008;8(2):65-72.
18. Hoye A.T., et al. Targeting mitochondria. Acc Chem Res. 2008;41(1):87-97.
19. Gordon J.S. Mind-body medicine and cancer. Hematol Oncol Clin North Am. 2008;22(4):683-708.
20. Ma Z., et al. Polymorphisms of fibroblast growth factor receptor 4 have association with the development of prostate cancer and benign prostatic hyperplasia and the progression of prostate cancer in a Japanese population. Int J Cancer. 2008;123:2574-2579.
21. Mosor M., et al. Polymorphisms and haplotypes of the NBS1 gene in childhood acute leukaemia. Eur J Cancer. 2008;44:627-630.
22. Zhang Z., et al. Genetic variants in RUNX3 and risk of bladder cancer: a haplotype-based analysis. Carcinogenesis. 2008;29(10):1973-1978.
23. Jones P.A., Baylin S.B. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415-428.
24. Hussain S.K., et al. Cervical and vulvar cancer risk in relation to the joint effects of cigarette smoking and genetic variation in interleukin 2. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1790-1799.
25. Inoue M., et al. Alcohol drinking and total cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2007;37(9):692-700.
26. Gomaa A.I., et al. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14(27):4300-4308.
27. Deandrea S., et al. Alcohol and breast cancer risk defined by estrogen and progesterone receptor status: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2025-2028.
28. Mizoue T., et al. Alcohol drinking and colorectal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2006;36(9):582-597.
29. Liu C., Russell R.M. Nutrition and gastric cancer risk: an update. Nutr Rev. 2008;66(5):237-249.
30. Gravaghi C., et al. Obesity enhances gastrointestinal tumorigenesis in Apc-mutant mice. Int J Obes (Lond). 2008;32:1716-1719.
31. Perera C.N., et al. Leptin regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin regulated mammary tumor growth and progression. J Endocrinol. 2008;199(2):221-233.
32. Amin A.R., et al. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27(16):12-25.
33. Thomas C.C., et al. Endometrial cancer risk among younger, overweight women. Obstet Gynecol. 2009;114(1):22-27.
34. Andrykowski M.A., et al. Psychological health in cancer survivors. Semin Oncol Nurs. 2008;24(3):193-201.
35. Blask D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257-264.
36. Mystakidou K., et al. Exploring the relationships between depression, hopelessness, cognitive status, pain, and spirituality in patients with advanced cancer. Arch Psychiatr Nurs. 2007;21(3):150-161.
37. Frieden T.R., et al. A public health approach to winning the war against cancer. Oncologist. 2008;13(12):1306-1313.
38. Stanley M. Prevention strategies against the human papillomavirus: the effectiveness of vaccination. Gynecol Oncol. 2007;107(2 Suppl 1):S19-S23.
39. Worthington H.V., et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 17(4), 2007. CD000978
40. Daniele B., et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomized trial. Gut. 2001;48(1):28-33.
41. Thorpe D.M. Management of opioid-induced constipation. Curr Pain Headache Rep. 2001;5(3):237-240.
42. Li Y., et al. Oral glutamine ameliorates chemotherapy-induced changes of intestinal permeability and does not interfere with the antitumor effect of chemotherapy in patients with breast cancer: a prospective randomized trial. Tumori. 2006;92(5):396-401.
43. Marshall G.T., et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34(1):35-42.
44. Demirer S., et al. Effects of probiotics on radiation-induced intestinal injury in rats. Nutrition. 2006;22(2):179-186.
45. Erbil Y., et al. The effect of glutamine on radiation-induced organ damage. Life Sci. 2005;78(4):376-382.
46. Branda R.F., et al. Vitamin E but not St John’s wort mitigates leukopenia caused by cancer chemotherapy in rats. Transl Res. 2006;148(6):315-324.
47. Wojtukiewicz M.Z., et al. The Polish Cancer Anemia Survey (POLCAS): a retrospective multicenter study of 999 cases. Int J Hematol. 2009;89(3):276-284.
48. Liu R., et al. Therapeutic effects of alpha-lipoic acid on bleomycin-induced pulmonary fibrosis in rats. Int J Mol Med. 2007;10(6):865-873.
49. Cruciani R.A., et al. Safety, tolerability and symptom outcomes associated with L-carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: a phase I/II study. J Pain Symptom Manage. 2006;32(6):551-559.
50. Nicolson G.L., Conklin K.A. Reversing mitochondrial dysfunction, fatigue and the adverse effects of chemotherapy of metastatic disease by molecular replacement therapy. Clin Exp Metastasis. 2008;25(2):161-169.
51. Masuda Y., et al. EGG phosphatidylcholine combined with vitamin B12 improved memory impairment following lesioning of nucleus basalis in rats. Life Sci. 1998;62(9):813-822.
52. MacDonald N. Cancer cachexia and targeting chronic inflammation: a unified approach to cancer treatment and palliative/supportive care. J Support Oncol. 2007;5(4):157-162.
53. Campbell K.L., et al. Resting energy expenditure and body mass changes in women during adjuvant chemotherapy for breast cancer. Cancer Nurs. 2007;30(2):95-100.
54. Yamada T., et al. Effects of theanine, r-glutamylethylamide, on neurotransmitter release and its relationship with glutamic acid neurotransmission. Nutr Neurosci. 2005;8(4):219-226.
55. Caram-Salas N.L., et al. Thiamine and cyanocobalamin relieve neuropathic pain in rats: synergy with dexamethasone. Pharmacology. 2006;77(2):53-62.
56. Conklin K.A. Coenzyme q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther. 2005;4(2):110-130.
57. Engdal S., et al. Identification and exploration of herb-drug combinations used by cancer patients. Integr Cancer Ther. 2009;8(1):29-36.
58. Seely D., et al. A strategy for controlling potential interactions between natural health products and chemotherapy: a review in pediatric oncology. J Pediatr Hematol Oncol. 2007;29(1):32-47.
59. Moayyedi P. The epidemiology of obesity and gastrointestinal and other diseases: an overview. Dig Dis Sci. 2008;53(9):2293-2299.
60. Dobbinson S., et al. Prevalence and determinants of Australian adolescents’ and adults’ weekend sun protection and sunburn, summer 2003–2004. J Am Acad Dermatol. 2008;59:602-614.
61. Bouillon R., et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;16:200-257.
62. Ames B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103(47):17589-17594.
63. Bläuer M., et al. Effects of tamoxifen and raloxifene on normal human endometrial cells in an organotypic in vitro model. Eur J Pharmacol. 2008;592(1–3):13-18.
64. Moore M., et al. Cancer registration literature update (2006–2008). Asian Pac J Cancer Prev. 2008;9(2):165-185.
65. Chan C., et al. Stress-associated hormone, norepinephrine, increases proliferation and IL-6 levels of human pancreatic duct epithelial cells and can be inhibited by the dietary agent, sulforaphane. Int J Oncol. 2008;33(2):415-419.
66. Lahti T.A., et al. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123(9):2148-2151.
67. Karunanayake C.P., et al. Occupational exposures and non-Hodgkin’s lymphoma: Canadian case-control study. Environ Health. 2008;7(1):44.
68. Swiatkowska B., et al. [Occupational risk factors for lung cancer—a case-control study, Lódź industrial center]. Med Pr. 2008;59(1):25-34.
69. Furney S.J., et al. Prioritization of candidate cancer genes—an aid to oncogenomic studies. Nucleic Acids Res. 36(18), 2008. e115
70. Medina P.P., Slack F.J. MicroRNAs and cancer: an overview. Cell Cycle. 2008;7(16):2485-2492.
71. Yang K., et al. Dietary components modify gene expression: implications for carcinogenesis. J Nutr. 2005;135(11):2710-2714.
72. Azarhoush R., et al. Relationship between p53 expression and gastric cancers in cardia and antrum. Arch Iran Med. 2008;11(5):502-506.
73. Yeung S.J., et al. Roles of p53, MYC and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981-3999.
74. Maroto B., et al. P21-activated kinase is required for mitotic progression and regulates P1k1. Oncogene. 2008;27(36):4900-4908.
75. Saramäki A., et al. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34(2):543-554.
76. Schernhammer E.S., et al. Folate and vitamin B6 intake and risk of colon cancer in relation to p53 expression. Gastroenterology. 2008;135:770-780.
77. Cecconi D., et al. Induction of apoptosis in Jeko-1 mantle cell lymphoma cell line by resveratrol: a proteomic analysis. J Proteome Res. 2008;7(7):2670-2680.
78. Chan J.Y., et al. Resveratrol displays converse dose-related effects on 5-fluorouracil-evoked colon cancer cell apoptosis: the roles of caspase-6 and p53. Cancer Biol Ther. 7(8), 2008. [Epub ahead of print]
79. Huang X., et al. Green tea extract enhances the selective cytotoxic activity of Zizyphus jujuba extracts in HepG2 Cells. Am J Chin Med. 2008;36(4):729-744.
80. Lee S.C., et al. Effect of a prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr Cancer. 2008;60(4):483-491.
81. Matsumura K., et al. Involvement of the estrogen receptor beta in genistein-induced expression of p21(waf1/cip1) in PC-3 prostate cancer cells. Anticancer Res. 2008;28(2A):709-714.
82. Tanigawa S., et al. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem. 2008;72(3):797-804.
83. Wells J. Do centrioles generate a polar ejection force? Riv Biol. 2005;98(1):71-95.
84. Dutertre S., et al. The absence of p53 aggravates polyploidy and centrosome number abnormality induced by Aurora-C overexpression. Cell Cycle. 2005;4(12):1783-1787.
85. Krämer A., et al. Centrosome replication, genomic instability and cancer. Leukemia. 2002;16(5):767-775.
86. Crist W.M., et al. Dysgranulopoietic neutropenia and abnormal monocytes in childhood vitamin B12 deficiency. Am J Hematol. 1980;9(1):89-107.
87. Kim I., et al. Pyrrolidine dithiocarbamate and zinc inhibit proteasome-dependent proteolysis. Exp Cell Res. 2004;298(1):229-238.
88. Wang X., et al. Folate deficiency induces aneuploidy in human lymphocytes in vitro-evidence using cytokinesis-blocked cells and probes specific for chromosomes 17 and 21. Mutat Res. 2004;551(1–2):167-180.
89. Kuramoto H., et al. Immunohistochemical evaluation of insulin-like growth factor I receptor status in cervical cancer specimens. Acta Med Okayama. 2008;62(4):251-259.
90. Haluska P., et al. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther. 2008;7(9):2589-2598.
91. Otto C., et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122.
92. Samudio I., et al. The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res. 2008;68(13):5198-5205.
93. Hussain S.P., et al. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 2008;68(17):7130-7136.
94. Wang D., DuBois R.N. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett. 2008;267(2):197-203.
95. Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2008;41:40-59.
96. Wallace J.M. Nutritional and botanical modulation of the inflammatory cascade—eicosanoids, cyclooxygenases, and lipoxygenases—as an adjunct in cancer therapy. Integr Cancer Ther. 2002;1(1):7-37.
97. Kaileh M., et al. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J Ethnopharmacol. 2007;113(3):510-516.
98. de Visser K.E., et al. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37.
99. Caserta M.T., et al. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain Behav Immun. 2008;22(6):933-940.
100. Littrell J. The mind-body connection: not just a theory anymore. Soc Work Health Care. 2008;46(4):17-37.
101. Bartella V., et al. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 2008;68(12):4919-4927.
102. Silberstein J., et al. HIV and prostate cancer: a systematic review of the literature. Prostate Cancer Prostatic Dis. 2008;12(1):6-12.
103. Pérez-López F.R. Sunlight, the vitamin D endocrine system, and their relationships with gynaecologic cancer. Maturitas. 2008;59(2):101-113.
104. Friedlander A.H., et al. The relationship between measures of nutritional status and masticatory function in untreated patients with head and neck cancer. J Oral Maxillofac Surg. 2008;66(1):85-92.
105. Fleet J.C. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29(6):388-396.
106. Maggini S., et al. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(Suppl 1):S29-S35.
107. Shen J., et al. Potentiation of intestinal immunity by micellary mushroom extracts. Biomed Res. 2007;28(2):71-77.
108. Nozaki H., et al. Mushroom acidic glycosphingolipid induction of cytokine secretion from murine T cells and proliferation of NK1.1 alpha/beta TCR-double positive cells in vitro. Biochem Biophys Res Commun. 2008;373(3):435-439.
109. Dong J.C., Dong X.H. [Comparative study on effect of astragulus injection and interleukin-2 in enhancing anti-tumor metastasis action of dendrite cells]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25(3):236-239.
110. Pilarski R., et al. Antiproliferative activity of various Uncaria tomentosa preparations on HL-60 promyelocytic leukemia cells. Pharmacol Rep. 2007;59(5):565-572.
111. Kormosh N., et al. Effect of a combination of extract from several plants on cell-mediated and humoral immunity of patients with advanced ovarian cancer. Phytother Res. 2006;20(5):424-425.
112. Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29(9):1109-1118.
113. Zhai Z., et al. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J Med Food. 2007;10(3):423-434.
114. Ogba N., et al. HEXIM1 regulates 17beta-estradiol/estrogen receptor-alpha-mediated expression of cyclin D1 in mammary cells via modulation of P-TEFb. Cancer Res. 2008;68(17):7015-7024.
115. Tan D.S., et al. ESR1 amplification in endometrial carcinomas: hope or hyperbole? J Pathol. 2008;216:271-274.
116. Hassan H.C., et al. Gynaecomastia: an endocrine manifestation of testicular cancer. Andrologia. 2008;40(3):152-157.
117. Chandanos E., Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44(16):2397-2403.
118. Alì G., et al. Different estrogen receptor beta expression in distinct histologic subtypes of lung adenocarcinoma. Hum Pathol. 2008;39(10):1465-1473.
119. Chen D.Z., et al. Indole-3-carbinol and diindolylmethane induce apoptosis of human cervical cancer cells and in murine HPV16-transgenic preneoplastic cervical epithelium. J Nutr. 2001;131(12):3294-3302.
120. Higdon J.V., et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224-236.
121. Om A.S., Chung K.W. Dietary zinc deficiency alters 5 alpha-reduction and aromatization of testosterone and androgen and estrogen receptors in rat liver. J Nutr. 1996;126(4):842-848.
122. Wang Y., et al. A positive feedback pathway of estrogen biosynthesis in breast cancer cells is contained by resveratrol. Toxicology. 2008;248(2–3):130-135.
123. Li Z., et al. Genistein induces cell apoptosis in MDA-MB-231 breast cancer cells via the mitogen-activated protein kinase pathway. Toxicol In Vitro. 2008;22:1749-1753.
124. Ju Y.H., et al. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29(11):2162-2168.
125. Yang Y., et al. Saw palmetto induces growth arrest and apoptosis of androgen-dependent prostate cancer LNCaP cells via inactivation of STAT 3 and androgen receptor signaling. Int J Oncol. 2007;31(3):593-600.
126. Kanadys W.M., et al. [Efficacy and safety of black cohosh (Actaea/ Cimicifuga racemosa) in the treatment of vasomotor symptoms–review of clinical trials]. Ginekol Pol. 2008;79(4):287-296.
127. Wang Y., et al. The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br J Nutr. 2008;99(2):303-310.
128. Mumber M., editor. Integrative oncology: principles and practice. London: Taylor & Francis, 2005.
129. Ortega A.D., et al. Glucose avidity of carcinomas. Cancer Lett. 2008;276:125-135.
130. Littarru G.P., Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37(1):31-37.
131. McCarty M.F., et al. The ‘rejuvenatory’ impact of lipoic acid on mitochondrial function in aging rats may reflect induction and activation of PPAR-gamma coactivator-1alpha. Med Hypotheses. 2009;72(1):29-33.
132. Inazu M., Matsumiya T. [Physiological functions of carnitine and carnitine transporters in the central nervous system]. Nihon Shinkei Seishin Yakurigaku Zasshi. 2008;28(3):113-120.
133. Ahn B.H., et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;05(38):14447-14452.
134. Mikirova N.A., et al. Anti—angiogenic effect of high doses of ascorbic acid. J Transl Med. 2008;6(1):50.
135. Anand P., et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590-1611.
136. Binion D.G., et al. Curcumin inhibits VEGF mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut. 2008;57:1509-1517.
137. Sagar S.M., et al. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-part 2. Curr Oncol. 2006;13(3):99-107.
138. Lock K., et al. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83(2):100-108.
139. Riboli E., Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3 Supp):559S-569S.
140. Wright M.E., et al. Intakes of fruit, vegetables, and specific botanical groups in relation to lung cancer risk in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2008;168:1024-1034.
141. Navarro Silvera S.A., et al. Food group intake and risk of subtypes of esophageal and gastric cancer. Int J Cancer. 2008;123(4):852-860.
142. Flood A., et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr. 2008;88(1):176-184.
143. van Gils C.H., et al. Consumption of vegetables and fruits and risk of breast cancer. JAMA. 2005;293(2):183-193.
144. Koushik A., et al. Fruits and vegetables and ovarian cancer risk in a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2160-2167.
145. Linos E., et al. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2146-2151.
146. Hart A.R., et al. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6(3):275-282.
147. Bahmanyar S., Ye W. Dietary patterns and risk of squamous-cell carcinoma and adenocarcinoma of the esophagus and adenocarcinoma of the gastric cardia: a population-based case-control study in Sweden. Nutr Cancer. 2008;54(2):171-178.
148. Duffy C.M., et al. Alcohol and folate intake and breast cancer risk in the WHI Observational Study. Breast Cancer Res Treat. 2008;168(9):1024-1034.
149. Bartsch H., et al. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20(12):2209-2218.
150. Berquin I.M., et al. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269(2):363-377.
151. Dal Maso L., et al. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2008;42(2–3):53-61.
152. Stoddard F.R.2nd, et al. Iodine alters gene expression in the MCF7 breast cancer cell line: evidence for an anti-estrogen effect of iodine. Int J Med Sci. 2008;5(4):189-196.
153. Ishii T., et al. Covalent modification of proteins by green tea polyphenol (–)–epigallocatechin-3-gallate through autoxidation. Free Radic Biol Med. 2008;45:384-1394.
154. Arts I.C. A review of the epidemiological evidence on tea, flavonoids, and lung cancer. J Nutr. 2008;138(8):1561S-1566S.
155. Tao K.S., et al. The multifaceted mechanisms for coffee’s anti-tumorigenic effect on liver. Med Hypotheses. 2008;71:730-736.
156. Young G.P., Le Leu R.K. Preventing cancer: dietary lifestyle or clinical intervention? Asia Pac J Clin Nutr. 2002;11(Suppl 3):S618-S631.
157. Kushi L.H., et al. The macrobiotic diet in cancer. J Nutr. 2001;131(11 Suppl):3056S-3064S.
158. Majchrowicz M.A. Essiac. Notes Undergr. 1995;29:6-7.
159. Seely D., et al. In vitro analysis of the herbal compound Essiac. Anticancer Res. 2007;27(6B):3875-3882.
160. Ottenweller J., et al. Inhibition of prostate cancer-cell proliferation by Essiac. J Altern Complement Med. 2004;10(4):687-691.
161. Cheung S., et al. Antioxidant and anti-inflammatory properties of ESSIAC and Flor-Essence. Oncol Rep. 2005;14(5):1345-1350.
162. Leonard B.J., et al. An in vivo analysis of the herbal compound essiac. Anticancer Res. 2006;26(4B):3057-3063.
163. Robb K., et al. A Cochrane Systematic Review of transcutaneous electrical nerve stimulation for cancer pain. J Pain Symptom Manage. 2009;37(4):746-753.
164. Harding C., et al. Auricular acupuncture: a novel treatment for vasomotor symptoms associated with luteinizing-hormone releasing hormone agonist treatment for prostate cancer. BJU Int. 2008;103(2):186-190.
165. Johnston M.F., et al. Acupuncture and fatigue: current basis for shared communication between breast cancer survivors and providers. J Cancer Surviv. 2007;1(4):306-312.
166. Lai M., et al. [Effects of electroacupuncture on tumor growth and immune function in the Walker-256 model rat]. Zhongguo Zhen Jiu. 2008;28(8):607-609.
167. Naeim A., et al. Evidence-based recommendations for cancer nausea and vomiting. J Clin Oncol. 2008;26(23):3903-3910.
168. Streitberger K., et al. Acupuncture for nausea and vomiting: an update of clinical and experimental studies. Auton Neurosci. 2006;129:107-117.
169. Choo S.P., et al. Electroacupuncture for refractory acute emesis caused by chemotherapy. J Altern Complement Med. 2006;12:963-969.
170. Ezzo J.M., et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database Syst Rev. (2):2006. CD002285
171. Carlson L., Bultz B. Mind-body interventions in oncology. Curr Treat Options Oncol. 2008;9(2–3):127-134.
172. Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27(1):32.
173. Chen K., Yeung R. Exploratory studies of Qigong therapy for cancer in China. Integr Cancer Ther. 2002;1(4):345-370.
174. Smedslund G., Ringdal G. Meta-analysis of the effects of psychosocial interventions on survival time in cancer patients. J Psychosom Res. 2004;57(2):123-131.
175. Breitbart W. Spirituality and meaning in supportive care: spirituality- and meaning-centered group psychotherapy interventions in advanced cancer. Support Care Cancer. 2002;10(4):272-280.
176. Kassab S., et al. Homoeopathic medicines for adverse effects of cancer treatments. Cochrane Database Syst Rev. 15(2), 2009. CD004845
177. Thompson E.A., et al. Towards standard setting for patient-reported outcomes in the NHS homoeopathic hospitals. Homoeopathy. 2008;97(3):114-121.
178. Kutner J.S., et al. Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: a randomized trial. Ann Intern Med. 2008;149(6):369-379.
179. Corbin L. Safety and efficacy of massage therapy for patients with cancer. Cancer Control. 2005;12(3):158-164.
180. Ponz de Leon M., et al. Aetiology of colorectal cancer and relevance of monogenic inheritance. Gut. 2004;53:115-122.
181. Schernhammer E.S., et al. Folate and vitamin B6 intake and risk of colon cancer in relation to p53 expression. Gastroenterology. 2008;135(3):770-780.
182. Oh K., et al. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. 2007;165(10):1178-1186.
183. Reid M.E., et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60(2):155-163.
184. Peters U., et al. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361(9368):1491-1495.
185. Hill L.L., et al. Echinacea purpurea supplementation stimulates select groups of human gastrointestinal tract microbiota. J Clin Pharm Ther. 2006;31(6):599-604.
186. Rouzaud G., et al. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol Biomarkers Prev. 2004;13(1):125-131.
187. Key T.J., Verkasalo P.K. Endogenous hormones and the aetiology of breast cancer. Breast Cancer Res. 1999;1(1):18-21.
188. Swerdlow A.J., et al. Risks of breast and testicular cancers in young adult twins in England and Wales: evidence on prenatal and genetic aetiology. Lancet. 1997;350(9093):1723-1728.
189. de Waard F., Trichopoulos D. A unifying concept of the aetiology of breast cancer. Int J Cancer. 1988;41(5):666-669.
190. Patrick L. Iodine: deficiency and therapeutic considerations. Altern Med Rev. 2008;13(2):116-127.
191. Blackmore K.M., et al. Vitamin D from dietary intake and sunlight exposure and the risk of hormone-receptor-defined breast cancer. Am J Epidemiol. 2008;168:915.
192. Gissel T., et al. Intake of vitamin D and risk of breast cancer—a meta-analysis. J Steroid Biochem Mol Biol. 2008;111(3–5):195-199.
193. Fénichel P. B-DF. Breast risk cancer and environmental endocrine disruptors. Gynecol Obstet Fertil. 2008.
194. Krieger N., et al. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst. 1994;86(8):589-599.
195. Wolff M.S., et al. Environmental exposures and puberty in inner-city girls. Environ Res. 2008;107(3):393-400.
196. Darbre P.D. Aluminium, antiperspirants and breast cancer. J Inorg Biochem. 2005;99(9):1912-1919.
197. Darbre P.D., Harvey P.W. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008;28(5):561-578.
198. Korkmaz A., et al. Role of melatonin in the epigenetic regulation of breast cancer. Breast Cancer Res Treat. 2009;115(1):13.
199. Hjartåker A., et al. Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med Biol. 2008;630:72-93.
200. Loprinzi C.L., et al. Mayo Clinic and North Central Cancer Treatment Group hot flash studies: a 20-year experience. Menopause. 2008;15(4 Pt 1):655-660.
201. Yuvaraj S., et al. Augmented antioxidant status in Tamoxifen treated postmenopausal women with breast cancer on co-administration with Coenzyme Q10, Niacin and Riboflavin. Cancer Chemother Pharmacol. 2008;61(6):933-941.
202. van Dalen E.C., et al. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. (2):2008. CD003917
203. Dalessandri K.M., et al. Pilot study: effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50(2):161-167.
204. O’Rielly P.H. Aetiology and pathology of prostate cancer. Pharm J. 1999;6:65-67.
205. Mikhak B., et al. Manganese superoxide dismutase (MnSOD) gene polymorphism, interactions with carotenoid levels, and prostate cancer risk. Carcinogenesis. 2008;29(1):2335-2340.
206. Grainger E.M., et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60(2):145-154.
207. Dewell A., et al. A very-low-fat vegan diet increases intake of protective dietary factors and decreases intake of pathogenic dietary factors. J Am Diet Assoc. 2008;108(2):347-356.
208. Zhao F., et al. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J Asian Nat Prod Res. 2008;10(5–6):467-473.
209. Srivastava J.K., Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem. 2007;55(23):9470-9478.