103 Calculous and Acalculous Cholecystitis
Acute cholecystitis has long been recognized as a complication of surgery or acute critical illness. The first reported case of acute postoperative cholecystitis, described in 1844, was a lethal complication that occurred in a patient who had been treated for a strangulated femoral hernia.1 In 1902, Kocher and Matti described successful operation for gangrenous cholecystitis complicating a ventral herniorrhaphy.2 In 1962, Thompson et al. reported a series of 98 patients who developed acute cholecystitis in the postoperative period.3 Seventy-six percent were men, and 47% did not have gallstones. Twelve percent of the patients developed perforation of the gallbladder. It is noteworthy that Glenn and Becker showed that the incidence of acalculous and postoperative cholecystitis increased between 1955 and 1979.4
The pathophysiology of cholecystitis in critically ill patients is different from that in the general population. At least half of the cases are acalculous.5 Understanding this disease process can help increase the index of suspicion and lead to early diagnosis and treatment, which is necessary for good outcomes in the already critically ill patient.
 Risk Factors and Pathophysiology
Risk Factors and Pathophysiology
Acalculous cholecystitis also can spontaneously occur under certain circumstances. In outpatients, risk factors for acalculous cholecystitis include diabetes mellitus, vasculitis, older age, and male sex.6 Acalculous cholecystitis also has been reported in cancer patients and patients with systemic infections and the acquired immunodeficiency syndrome (AIDS). Indeed, acute cholecystitis is the most common indication for exploratory laparotomy or laparoscopy in AIDS patients.7 Most have acalculous disease. Not surprisingly, the mortality rate is high. In children, the majority of cases of acute cholecystitis are acalculous.8 The etiology appears to be dehydration or lymphadenopathy secondary to viral infections. Congenital biliary tract anomalies also need to be considered.
Acute cholecystitis has been described in multiple reports as a complication of a variety of surgical procedures,9–14 trauma,15–20 burns,21 sepsis,22 cardiovascular diseases, and malignancy.23,24 There also has been an association with total parenteral nutrition and biliary stasis.25–28 The pathophysiology, however, remains unclear.
Theories regarding the pathogenesis of acalculous cholecystitis in critically ill and postoperative patients have evolved over the years. Sparkman was the first to suggest that gastrointestinal hypomotility and biliary stasis were causative factors.29 Glenn and Wantz added that the lack of enteral feeding in the postoperative period increased the concentration of bile salts and cholesterol in bile.30 They further noted acute onset of cholecystitis with refeeding, suggesting impaction of stones or viscous bile in the cystic duct, with gallbladder contractions. Thompson et al., having noted gallbladder mucosal necrosis, arterial thrombosis, gangrene, and perforation, suggested that hypoperfusion may be the critical mechanism for acalculous cholecystitis.3 A recent histopathologic study found that two-thirds of surgical and trauma patients who developed acute cholecystitis had ischemic cholecystitis histologically.31 Hakala et al. performed ex vivo microangiography of gallbladders immediately after cholecystectomy.32 Patients with stones had normal vasculature, whereas those with acalculous disease had poor and irregular capillary filling, suggesting that microvascular disturbances may play a role in the pathogenesis of this disease. Hypoperfusion, particularly of the splanchnic circulation, is common in critically ill patients. Etiologic factors include hemorrhage, dehydration, heart failure, and/or sepsis. The use of vasopressors can exacerbate the situation. Mechanical ventilation with positive end-expiratory pressure (PEEP) can increase hepatic venous pressure and thereby decrease portal perfusion.34
Orlando et al. suggested that in addition to hypoperfusion, increased intraluminal pressure may be a critical factor.33 Biliary stasis secondary to fasting and narcotics may play a critical role in increasing intraluminal pressure in the gallbladder. The combination of hypoperfusion and increased luminal pressure leads to a decrease in gallbladder perfusion pressure. Bacterial invasion can subsequently occur in the ischemic tissue.
The use of parenteral nutrition has been implicated in the pathogenesis of acalculous cholecystitis. In addition to the effects of fasting, parenteral nutrition can decrease bile production, worsening biliary stasis. Biliary sludge can be found in almost all patients on long-term parenteral nutrition.25–28 Many go on to form gallstones. Trauma patients also develop sludge over time, and this factor may play a role in the development of cholecystitis, as well as pancreatitis.35
Eosinophilic infiltration of the inflamed gallbladder has been seen in patients developing acute acalculous cholecystitis after administration of antibiotics for other reasons, suggesting the possibility that a hypersensitivity reaction to the antibiotic played an etiologic role.36 This theory has not been substantiated.
It has been suggested that the pigment load from massive transfusions can lead to changes in the relative concentrations of bile pigments compared to cholesterol and lecithin in bile, increasing risk of acalculous cholecystitis. Long et al., however, found no relationship between transfusion requirements and risk of cholecystitis.34
 Incidence
Incidence
The incidence of acute cholecystitis in the ICU is difficult to determine given the great diversity of ICU patient populations and illness severity. Among cardiac surgical patients, acute cholecystitis is second only to upper gastrointestinal hemorrhage as an indication for abdominal operation.37 About half the cases of acute cholecystitis in this population are due to acalculous disease. Visceral hypoperfusion related to left ventricular dysfunction has been implicated as an etiologic factor. Rady et al. found that early predictors of acute cholecystitis included arterial occlusive disease, low preoperative oxygen delivery, longer cardiopulmonary bypass times, need for surgical re-exploration, cardiac arrhythmias, mechanical ventilation for ≥ 3 days, bacteremia, and nosocomial infections.13 The common threads of these factors are decreased tissue perfusion and oxygenation, significant surgical trauma (which would be expected to lead to production of inflammatory mediators), and perhaps bacterial translocation from the gut lumen. These authors went so far as to suggest that patients who have had a complicated postoperative course should be followed by serial ultrasonography of the gallbladder. Hagino et al. found that 6 of 7 patients who developed cholecystitis after aortic reconstruction had prolonged hypotension and developed multiple organ dysfunction; 5 died.14
In the general population of postoperative patients, acute cholecystitis appears to occur with or without gallstones. Mortality is about 30%. Among trauma patients, about 90% of cases of acute cholecystitis are acalculous.15–20 The percentage of cases of acute cholecystitis that are acalculous has increased significantly over time.4 Because the incidence of the disease is low, but the many risk factors for the disease are common, it is difficult to identify specific groups of ICU patients who might benefit from selective screening for acute cholecystitis.
 Clinical Presentation
Clinical Presentation
The most consistent laboratory finding is a leukocytosis. Elevated circulating levels of liver enzymes and bilirubin are common, but not necessarily present. Clinical findings and laboratory studies are not very sensitive or specific for cholecystitis even in the general population38,39 and are less so in critically ill patients. Consequently, radiologic studies are necessary.
 Imaging Studies
Imaging Studies
Ultrasonography has proven to be an accurate radiologic test for acute cholecystitis in the general population. In the ICU, the presence or absence of gallstones does not help with the diagnosis. The most useful ultrasonographic findings indicative of acute cholecystitis are thickening of the gallbladder wall and pericholecystic fluid (Figure 103-1). Ultrasonographic findings correlate well with operative findings. False-positive findings may occur with sludge, non-shadowing stones, cholesterolosis, ascites, hypoalbuminemia, and portal hypertension. Other ultrasonographic findings indicative of acute cholecystitis include the “double wall sign,” representing edema of the gallbladder wall; the “halo sign,” representing sloughed gallbladder mucosa; intramural gas; distention of the gallbladder; and the “sonographic Murphy’s sign,” demonstrating point tenderness over the gallbladder. The sensitivity of ultrasound for detecting acalculous cholecystitis is 81% to 92%. The specificity is 60% to 96%.38–42,47,48 One problem is that the typical ultrasonographic findings of cholecystitis can be seen in ICU patients without other evidence of cholecystitis. For example, Boland et al. performed ultrasound examinations of the gallbladder twice a week in a variety of ICU patients.40 Half of the patients without calculi developed at least one ultrasonographic finding of acute cholecystitis. Helbich et al.41 attempted to apply a scoring system to the ultrasonographic findings characteristic of acute cholecystitis, suggesting that patients with several findings should undergo more aggressive diagnostic evaluation and perhaps therapeutic interventions.40 In equivocal cases, serial examinations may demonstrate increasing wall thickness which should increase the suspicion for cholecystitis.42
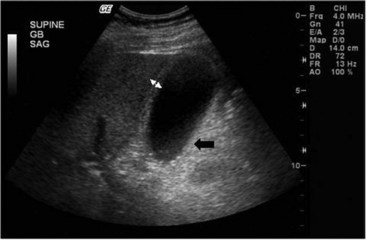
Figure 103-1 Ultrasound of gallbladder, demonstrating wall thickening (double arrows) and sludge (black arrow).
Scintigraphy of the gallbladder frequently has been used when acute cholecystitis is suspected, but the findings from other tests such as ultrasound are inconclusive or contradictory. Gallbladder scintigraphy is performed by administering technetium-labeled iminodiacetic acid (IDA). Cholecystitis is diagnosed if the radioactive tracer is visualized in the small bowel without visualization of the gallbladder within 4 hours, suggesting occlusion of the cystic duct (Figure 103-2). Delayed visualization of the gallbladder may represent chronic cholecystitis. The rate of false-positive tests is significant in fasting patients, particularly those receiving parenteral nutrition. The use of intravenous morphine to increase tone in the sphincter of Oddi and thereby increase pressure within the biliary system can decrease the risk of a falsely positive test.43 The sensitivity of scintigraphy is 91% to 97%. The specificity is 38% to 99%.43,44,48 Scintigraphy is a useful complement to ultrasonography when ultrasonography alone does not provide enough information to permit a sufficiently early decision regarding intervention.44
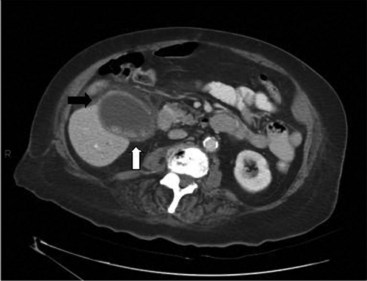
CT of the abdomen can be used to make the diagnosis of acute cholecystitis.45,46 The criteria for a positive study include wall thickness greater than 4 mm, pericholecystic fluid, intramural gas, sloughed mucosa, or subserosal edema without ascites (Figure 103-3). If intravenous contrast is administered, enhancement of the gallbladder wall may be seen. Although CT may not be as sensitive as the other studies for determining the presence of gallstones or acute cholecystitis, it has the advantage of being able to detect or rule out other causes of an acute abdomen. A great disadvantage for critically ill patients, however, is the need to transport the patient to the scanner.
In critically ill patients, ultrasound is usually the first test requested because it can be performed at the bedside in the ICU and carries no risk. It also can be repeated readily. Because the study is operator-dependent, the reliability of the test, particularly its sensitivity, can be variable.47 Specificity is good. Frequently, however, additional studies are necessary. Ultrasound and scintigraphy, in particular, complement each other well.48 The results of any imaging studies need to be considered in the context of the patient’s underlying disease(s), physical findings, and laboratory studies.
 Management
Management
The standard initial medical treatment for acute cholecystitis includes antibiotics, analgesia, and, at least during the early phase, bowel rest. Antibiotics for uncomplicated cholecystitis should cover enterococcal species and gram-negative rods, particularly Escherichia coli and Klebsiella spp.49 Among patients who have previously received antibiotics, more resistant and unusual organisms are often cultured from gallbladder bile in patients with acute cholecystitis. These organisms can include Staphylococcus spp., resistant gram-negative bacilli, anaerobic bacteria, and fungi. Older patients are also more apt to have infected bile. In patients with empyema of the gallbladder, Tseng et al. found that bile cultures were positive in 83% of the cases.50 Gram-negative bacteria (e.g., E. coli, K. pneumoniae, Morganella morganii, Pseudomonas aeruginosa, and Salmonella spp.) were found in 75%, gram-positive bacteria (e.g., Enterococcus spp.) in 30%, and obligate anaerobes in 7%. Broader coverage may be required for empirical coverage until cultures are obtained and coverage can be more tailored.
Image-Directed Drainage
Image-directed cholecystostomy can readily be performed using either ultrasound or CT. This procedure was first used for palliation of obstructive jaundice in 1979.51 In 1980, successful drainage of empyema of the gallbladder was reported.52 The first large series of percutaneous cholecystostomy for acute cholecystitis was reported in 198553; 113 of 114 patients were treated successfully.
Percutaneous cholecystostomy and bile culture have been performed occasionally in patients with unexplained sepsis in the ICU. In patients who have cholecystitis, cultures are often positive if performed 72 hours after the onset of symptoms. Culture of bile is sterile in approximately 50% of patients with acute cholecystitis.49 Boland et al. tested the efficacy of percutaneous cholecystostomy as a diagnostic and therapeutic maneuver in 82 patients in the ICU with persistent unexplained sepsis54; 48 of 82 patients improved. Sonographic findings were not helpful in predicting response to percutaneous cholecystostomy. In a separate study of 24 such patients, 14 patients improved after cholecystostomy.55 Of the remaining patients, three had pneumonia and the others did not have a source of sepsis identified. Of the patients who improved, only four had positive bile cultures. Thus, in critically ill patients without a definitive diagnosis of acute cholecystitis, the role of percutaneous cholecystostomy and bile culture remains unclear. Since the risk of this procedure is low, percutaneous cholecystostomy should be considered when the index of suspicion for acute cholecystitis is high enough.
Van Sonnenberg et al. reported a series of percutaneous cholecystostomies in 127 patients.56 Indications included acute cholecystitis, obstructive jaundice, gallbladder perforation, need for percutaneous removal or dissolution of gallstones, need for diagnostic cholecystocholangiography, and gallbladder biopsy. The procedure was successful in 125 cases. Eleven patients (8.7%) had major complications, including bile peritonitis, bleeding, vagal reactions, hypotension, catheter dislodgement, and acute respiratory distress. Five (3.9%) had minor complications. No deaths were related to the procedure itself.
Overall mortality for percutaneous cholecystostomy is about 10%, similar to open cholecystostomy.56–59 The limiting factor for success of percutaneous drainage is the viability of the gallbladder. Focal ischemia or necrosis is unlikely to improve without cholecystectomy and predisposes the patient to perforation. Cholecystectomy should be considered in patients who do not improve with cholecystostomy. Lo et al. found in their series that all six patients who failed to respond to cholecystostomy had transmural inflammation; five had a gangrenous gallbladder wall.57
Appropriate management following cholecystostomy is not completely clear. Once the patient has recovered, one can readily obtain a cholangiogram through the catheter. If gallstones are present, elective cholecystectomy at a later date is recommended. On the other hand, if no stones are present, cholecystostomy may obviate the need for cholecystectomy, as patients do well without cholecystectomy.58,59
A novel technique for drainage of the gallbladder involves a transpapillary endoscopic approach.60 This approach may be helpful if other indications for endoscopic evaluation or intervention are present. It seems that the intervention is more successful if the ultrasound demonstrates that the gallbladder is not severely distended or thick.61
Surgical Management
Cholecystectomy may be advantageous compared to cholecystostomy, since it allows one to examine the entire right upper quadrant for other pathology and to completely drain any fluid collections around the gallbladder. It also alleviates the risk of gallbladder perforation. When cholecystectomy is performed, a laparoscopic approach can usually be attempted, recognizing that one may need to abandon the attempt and proceed with an open procedure because of difficulty with the dissection. The timing of cholecystectomy for acute cholecystitis remains controversial62 but definitely should be considered if the patient is not responding to nonoperative management. If a patient undergoes cholecystostomy, it may be beneficial to delay the cholecystectomy for at least 2 weeks.
Bedside laparoscopy can be performed for evaluation of the acute abdomen in critically ill patients. If acute cholecystitis is identified, a cholecystostomy can be performed readily, or the patient can be taken to the operating room for a cholecystectomy.63,64 If the diagnosis of cholecystitis is excluded, the patient may be spared an unnecessary trip to the operating room.
 Complications and Outcome
Complications and Outcome
Complications of acute cholecystitis are much more common in critically ill patients than in the general population. Elderly patients are particularly at risk. Among patients with acalculous cholecystitis, Kalliafas et al. found that 17 of 27 had gangrene, four had perforation, and one had an abscess.38 Mortality was 41%.
Gangrene may be present in as many as 59% of cases.9–22 Shapiro et al. found gangrene or frank necrosis in 13 of 22 patients undergoing cholecystectomy for acute cholecystitis that developed in the ICU.22 Cornwell et al. found necrosis or gangrene in 6 of 14 trauma patients who developed acute acalculous cholecystitis.65
Compared to patients without gangrene, those with gangrene are at greater risk of perforation or failure of percutaneous drainage. Some of these patients have emphysematous cholecystitis (gas in the wall of the gallbladder), a diagnosis that carries an even greater risk of perforation. Emphysema can be identified by plain abdominal radiographs, CT, or ultrasound. Antibiotics should cover gas-forming anaerobic organisms. Although percutaneous drainage may be effective,66 early cholecystectomy is indicated if the patient does not improve promptly.
Perforation of the gallbladder occurs in approximately 10% of cases.9–22 Usually the resulting fluid collection is localized and amenable to percutaneous drainage. Free perforation also can occur, and when it does, the risk of mortality is markedly increased.67 The clinical problem, however, is that preoperative imaging may not demonstrate evidence of perforation.68 The risk of perforation increases with delay in drainage or operation. Cholecystectomy is indicated for free perforation or for patients failing to rapidly respond to percutaneous drainage.
Empyema of the gallbladder also greatly increases mortality.69 This complication may be amenable to percutaneous drainage,50,70 but the risks of failure or perforation are substantial.
The risk of mortality from cholecystitis in the ICU mainly reflects the underlying disease processes and comorbidities. Overall mortality is around 30%.9–22 Hadas-Halpern et al. found that 10 of 80 patients undergoing percutaneous cholecystostomy for acute cholecystitis died of comorbid disease, whereas only two died of biliary peritonitis.71
 Prevention
Prevention
No intervention has been shown conclusively to prevent development of cholecystitis in ICU patients. If the theories regarding the pathophysiologic mechanisms are correct, the incidence of the disease should be reduced by aggressively resuscitating patients with shock, avoiding biliary stasis by implementing early enteral feeding, and minimizing the use of narcotics. Intermittent doses of cholecystokinin or deoxycholic acid have been shown to increase bile flow and, therefore, may decrease the risk of acalculous cholecystitis in patients receiving parenteral nutrition,72–74 though studies in ICU patients are needed.
Key Points
Boland G, Lee MJ, Mueller PR. Acute cholecystitis in the intensive care unit. New Horiz. 1993;1:246-260.
Thompson JWIII, Ferris DO, Beggenstoss AH. Acute cholecystitis complicating operation for other diseases. Ann Surg. 1962;155:489.
Helbich TH, Mallek R, Madl C, Wunderbaldinger P, Breitenseher M, Tscholakoff D, et al. Sonomorphology of the gallbladder in critically ill patients. Value of a scoring system and follow-up examinations. Acta Radiol. 1997;38:129-134.
Flancbaum L, Alden SM, Trooskin SZ. Use of cholescintigraphy with morphine in critically ill patients with suspected cholecystitis. Surgery. 1989;106:668-673.
vanSonnenberg E, D’Agostino HB, Goodacre BW, Sanchez RB, Casola G. Percutaneous gallbladder puncture and cholecystostomy: results, complications, and caveats for safety. Radiology. 1992;183:167-170.
1 Duncan J. Femoral hernia: Gangrene of the gallbladder; extravasation of bile; peritonitis; death. North Med J. 1844;2:151-153.
2 Kocher T, Matti H. Uber 100 operationen an den gallenwegen mit berucksichtigung der dauererfolge. Arch F Min Chir. 1906;81:655.
3 Thompson JWIII, Ferris DO, Beggenstoss AH. Acute cholecystitis complicating operation for other diseases. Ann Surg. 1962;155:489.
4 Glenn F, Becker CG. Acute acalculous cholecystitis: An increasing entity. Ann Surg. 1982;195:131-136.
5 Boland G, Lee MJ, Mueller PR. Acute cholecystitis in the intensive care unit. New Horiz. 1993;1:246-260.
6 Ryu JK, Ryu KH, Kim KH. Clinical features of acute acalculous cholecystitis. J Clin Gastroenterol. 2003;36:166-169.
7 LaRaja RD, Rothenberg RE, Odom JW, Mueller SC. The incidence of intraabdominal surgery in acquired immunodeficiency syndrome: A statistical review of 904 patients. Surgery. 1989;105:175-179.
8 Lobe TE. Cholelithiasis and cholecystitis in children. Semin Pediatr Surg. 2000;9:170-176.
9 Laws HL, Elliott RL. Postoperative acalculous gangrenous cholecystitis. Am Surg. 1971;37:371-374.
10 Jonsson PE, Andersson A. Postoperative acute acalculous cholecystitis. Arch Surg. 1976;111:1097-1101.
11 Ottinger LW. Acute cholecystitis as a postoperative complication. Ann Surg. 1976;184:162-165.
12 Devine RM, Farrell MB, Mucha PJr. Acute cholecystitis as a complication in surgical patients. Arch Surg. 1984;119:1389-1393.
13 Rady MY, Kodavatiganti R, Ryan T. Perioperative predictors of acute cholecystitis after cardiovascular surgery. Chest. 1998;114:76-84.
14 Hagino RT, Valentine RJ, Clagett GP. Acalculous cholecystitis after aortic reconstruction. J Am Coll Surg. 1997;184:245-248.
15 DuPriest RW, Khancja SC, Cowley RA. Acute cholecystitis complicating trauma. Ann Surg. 1979;189:84-89.
16 Rice J, Williams HC, Flint LM, Richardson JD. Posttraumatic acalculous cholecystitis. South Med J. 1980;73:14-17.
17 Herlin P, Ericcson M, Holmin T, Jonsson PE. Acute acalculous cholecystitis following trauma. Br J Surg. 1982;69:475-476.
18 Flancbaum L, Majerus TC, Cox EF. Acute posttraumatic acalculous cholecystitis. Am J Surg. 1985;150:252-256.
19 Buckley PM, Hunter JM. Acute acalculous cholecystitis following multiple skeletal trauma. Anesthesia. 1985;40:23-26.
20 Fabian TC, Hickerson WL, Mangiante EC. Post-traumatic and postoperative acute cholecystitis. Am Surg. 1986;52:188-192.
21 McDermott MW, Scudamore CH, Boileau LO, et al. Acalculous cholecystitis: Its role as a complication of major burn injury. Can J Surg. 1985;28:529-533.
22 Shapiro MJ, Luchtefeld WB, Kurzweil S, et al. Acute acalculous cholecystitis in the critically ill. Am Surg. 1994;60:335-339.
23 Lafon PC, Reed K, Rosenthal D. Acute cholecystitis associated with hepatic arterial infusion of floxuridine. Am J Surg. 1985;150:687-689.
24 Chung-Park M, Kim B, Marmolya G, et al. Acalculous lymphoeosinophilic cholecystitis associated with interleukin-2 and lymphokine-activated killer cell therapy. Arch Pathol Lab Med. 1990;114:1073-1075.
25 Petersen SR, Sheldon GF. Acute acalculous cholecystitis: A complication of hyperalimentation. Am. J. Surg. 1979;138:814-817.
26 Roslyn JJ, Pitt HA, Mann LL, et al. Gallbladder disease in patients on long-term parenteral nutrition. Gastroenterology. 1983;84:148-154.
27 Messing B, Bories C, Kunstlinger F, Bernier JJ. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology. 1983;84:1012-1019.
28 Allen B, Bernhoft R, Blanckaert N, et al. Sludge is calcium bilirubinate associated with bile stasis. Am J Surg. 1981;141:51-56.
29 Sparkman RD. Abdominal emergencies following unrelated surgical procedures. Ann Surg. 1952;135:863.
30 Glenn F, Wantz GE. Acute cholecystitis following the surgical treatment of unrelated disease. Surg Gynecol Obstet. 1956;102:145.
31 Warren BL, Carstens CA, Falck VG. Acute acalculous cholecystitis–a clinical-pathological disease spectrum. S Afr J Surg. 1999;37:99-104.
32 Hakala T, Nuutinen PJ, Ruokonen ET, Alhava E. Microangiopathy in acute acalculous cholecystitis. Br J Surg. 1997;84:1249-1252.
33 Orlando R, Gleason E, Drezner AD. Acute acalculous cholecystitis in the critically ill patient. Am J Surg. 1983;145:472-476.
34 Long TN, Heimbach DM, Carrico CJ. Acalculous cholecystitis in critically ill patients. Am. J. Surg. 1978;136:31-36.
35 Toursarkissian B, Kearney PA, Holley DT, et al. Biliary sludging in critically ill trauma patients. South Med J. 1995;88:420-424.
36 Parry SW, Pelias ME, Browder W. Acalculous hypersensitivity cholecystitis: Hypothesis of a new clinicopathologic entity. Surgery. 1988;104:911-916.
37 Leitman IM, Paull DE, Barie PS, et al. Intra-abdominal complications of cardiopulmonary bypass operations. Surg Gynecol Obstet. 1987;165:251-254.
38 Kalliafas S, Ziegler DW, Flancbaum L, Choban PS. Acute acalculous cholecystitis: Incidence, risk factors, diagnosis, and outcome. Amer Surg. 1998;64:471-475.
39 Trowbridge RL, Rutkowski NK, Shojania KG. Does this patient have acute cholecystitis? JAMA. 2003;289:80-86.
40 Boland GW, Slater G, Lu DS, et al. Prevalence and significance of gallbladder abnormalities seen on sonography in intensive care unit patients. Am J Roentgenol. 2000;174:973-977.
41 Helbich TH, Mallek R, Madl C, et al. Sonomorphology of the gallbladder in critically ill patients. Value of a scoring system and follow-up examinations. Acta Radiol. 1997;38:129-134.
42 Jeffrey RBJr, Sommer FG. Follow-up sonography in suspected acalculous cholecystitis: preliminary clinical experience. J Ultrasound Med. 1993;12:183-187.
43 Flancbaum L, Alden SM, Trooskin SZ. Use of cholescintigraphy with morphine in critically ill patients with suspected cholecystitis. Surgery. 1989;106:668-673.
44 Prevot N, Mariat G, Mahul P, et al. Contribution of cholescintigraphy to the early diagnosis of acute acalculous cholecystitis in intensive-care-unit patients. Eur J Nucl Med. 1999;26:1317-1325.
45 Varma DG, Faust JM. Computed tomography of gangrenous acute postoperative acalculous cholecystitis. J Comput Tomogr. 1988;12:29-31.
46 Mirvis SE, Whitley NO, Miller JW. CT diagnosis of acalculous cholecystitis. J Comput Assist Tomogr. 1987;11:83-87.
47 Puc MM, Tran HS, Wry PW, Ross SE. Ultrasound is not a useful screening tool for acute acalculous cholecystitis in critically ill trauma patients. Am Surg. 2002;68:65-69.
48 Mariat G, Mahul P, Prevot N, et al. Contribution of ultrasonography and cholescintigraphy to the diagnosis of acute acalculous cholecystitis in intensive care unit patients. Intensive Care Med. 2000;26:1658-1663.
49 Barie PS, Jacobson IM. Gallbladder Disease. In: Zakim D., Boyer T.D., editors. Hepatology. A Textbook of Liver Disease. 2nd ed. Philadelphia: Saunders; 1990:1516-1532.
50 Tseng LJ, Tsai CC, Mo LR, et al. Palliative percutaneous transhepatic gallbladder drainage of gallbladder empyema before laparoscopic cholecystectomy. Hepatogastroenterology. 2000;47:932-936.
51 Elyaderani M, Gabriele OF. Percutaneous cholecystostomy and cholangiography in patients with obstructive jaundice. Radiology. 1979;130:601-602.
52 Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging Clin Med. 1980;49:330-333.
53 Eggermont AM, Lameris JS, Jeekel J. Ultrasound guided percutaneous transhepatic cholecystostomy for acute acalculous cholecystitis. Arch Surg. 1985;120:1354-1356.
54 Boland GW, Lee MJ, Leung J, Mueller PR. Percutaneous cholecystostomy in critically ill patients: early response and final outcome in 82 patients. Am J Roentgenol. 1994;163:339-342.
55 Lee MJ, Saini S, Brink JA, et al. Treatment of critically ill patients with sepsis of unknown cause: Value of percutaneous cholecystostomy. Am J Roentgenol. 1991;156:1163-1166.
56 van Sonnenberg E, D’Agostino HB, Goodacre BW, et al. Percutaneous gallbladder puncture and cholecystostomy: Results, complications, and caveats for safety. Radiology. 1992;183:167-170.
57 Lo LD, Vogelzang RL, Braun MA, Nemcek AAJr. Percutaneous cholecystostomy for the diagnosis and treatment of acute calculous and acalculous cholecystitis. J Vasc Interv Radiol. 1995;6:629-634.
58 Patel M, Miedema BW, James MA, Marshall JB. Percutaneous cholecystostomy is an effective treatment for high-risk patients with acute cholecystitis. Am Surg. 2000;66:33-37.
59 Pearse DM, Hawkins IF, Shaver R, Vogel S. Percutaneous cholecystostomy in acute cholecystitis and common duct obstruction. Radiology. 1984;152:365-367.
60 Mutignani M, Iacopini F, Perri V, et al. Endoscopic gallbladder drainage for acute cholecystitis: technical and clinical results. Endoscopy. 2009;41:539-546.
61 Ogawa O, Yoshikumi H, Maruoka N, et al. Predicting the success of endoscopic transpapillary gallbladder drainage for patients with acute cholecystitis during pretreatment evaluation. Can J Gastroenterol. 2008;22:681-685.
62 Kim Ho, Ho Son B, Yoo Ch, Ho Shin J. Impact of delayed laparoscopic cholecystectomy after percutaneous transhepatic gallbladder drainage for patients with complicated acute cholecystitis. Surg Laparosc Endosc Percutan Tech. 2009;19:20-24.
63 Yang HK, Hodgson WJ. Laparoscopic cholecystostomy for acute acalculous cholecystitis. Surg Endosc. 1996;10:673-675.
64 Almeida J, Sleeman D, Sosa JL, et al. Acalculous cholecystitis: the use of diagnostic laparoscopy. J Laparoendosc Surg. 1995;5:227-231.
65 Cornwell EE3rd, Rodriguez A, Mirvis SE, Shorr RM. Acute acalculous cholecystitis in critically injured patients. Preoperative diagnostic imaging. Ann Surg. 1989;210:52-55.
66 Vingan HL, Wohlgemuth SD, Bell JSIII. Percutaneous cholecystostomy drainage for the treatment of acute emphysematous cholecystitis. Am J Roentgenol. 1990;155:1013-1014.
67 Felice PR, Trowbridge PE, Ferrara JJ. Evolving changes in the pathogenesis and treatment of the perforated gallbladder. A combined hospital study. Am J Surg. 1985;149:466-473.
68 Tsai M-J, Chen J-D, Tiu C-M, et al. Can acute cholecystitis with gallbladder perforation be detected preoperatively by computed tomography in ED? Correlation with clinical data and computed tomography features. Am J Emerg Med. 2009;27:574-581.
69 Fry DE, Cox RA, Harbrecht PJ. Empyema of the gallbladder: A complication in the natural history of acute cholecystitis. Am J Surg. 1981;141:366-369.
70 Van Steenbergen W, Ponette E, Marchal G, et al. Percutaneous transhepatic cholecystostomy for acute complicated cholecystitis in elderly patients. Am J Gastroenterol. 1990;85:1363-1369.
71 Hadas-Halpern I, Patlas M, Knizhnik M, Zaghal I, Fisher D. Percutaneous cholecystostomy in the management of acute cholecystitis. Isr Med Assoc J. 2003;5:170-171.
72 Sitzmann JV, Pitt HA, Steinborn PA, et al. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg Gynecol Obstet. 1990;170:25-31.
73 Teitelbaum DH, Han-Markey T, Drongowski RA, et al. Use of cholecystokinin to prevent the development of parenteral nutrition-associated cholestasis. J Parenter Enteral Nutr. 1997;21:100-103.
74 Gunsar C, Melek M, Karaca I, et al. The biochemical and histopathological effects of ursodeoxycholic acid and metronidazole on total parenteral nutrition-associated hepatic dysfunction: an experimental study. Hepatogastroenterology. 2002;49:497-500.