Burns
Over the past several decades, burn-related hospital admissions and deaths have decreased by 50% in large part due to advancements in burn care, introduction of topical antimicrobial agents, and a better understanding of fluid resuscitation and aggressive surgical management.1–3 Despite these advances, nearly 100,000 children age 14 years and under were treated for burns in hospital emergency rooms in 2007. Of these, 20% occurred in children less than 4 years old.4 Thus, burns remain a leading cause of morbidity and mortality in children. Optimal management requires a team of health care providers, therapists, and social workers.
Pathophysiology
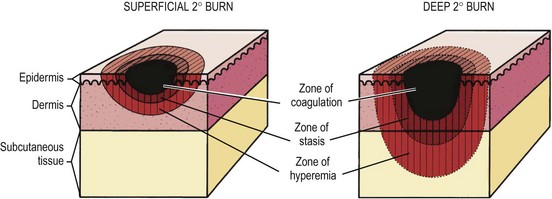
In 1953, Jackson described the zones of burn injury that remain important to the understanding and management of burns today (Fig. 13-1).5 The zone of coagulation occurs at the site of maximal damage and is defined by irreversible tissue loss due to protein coagulation. The zone of stasis surrounds this area and has decreased tissue perfusion, but remains salvageable with aggressive resuscitation. Outside the zone of stasis is the zone of hyperemia where tissue perfusion is increased and often survives unless faced with infection or hypotension.
The systemic response to burn injury is mediated by the release of inflammatory mediators such as thromboxane A2, bradyknin, oxidants, and cytokines which can impair flow to the zone of stasis through thrombosis, vasoconstriction, and capillary blockage.6 The administration of antioxidants, bradykinin antagonists, and thromboxane A2 inhibitors can improve blood flow and mitigate injury.7–9 In addition, the administration of β-glucan, through its immunomodulatory effects, antioxidant properties, and ability to reduce the inflammatory response, has been shown to improve re-epithelialization in a rat model of burn injury.10
The systemic effects of burn injury extend beyond these three zones and can potentially lead to multiorgan dysfunction. The cardiovascular system can experience myocardial depression and hypovolemia. Pulmonary vasoconstriction and edema lead to respiratory failure.11 Splanchnic vasoconstriction can result in gut dysmotility and malabsorption by causing epithelial apoptosis and decreased epithelial proliferation.12–14 This results in atrophy of small bowel mucosa, increased intestinal permeability, bacterial translocation, and sepsis.15 Splanchnic vasoconstriction and activation of stress-induced hormones and mediators, such as angiotensin, aldosterone, and vasopressin, leads to a decrease in renal perfusion that can lead to oliguria.16 When unrecognized, this can progress to acute tubular necrosis, renal failure, and, ultimately, death.17,18 Finally, there is a systemic decrease in immune function due to impaired production and function of neutrophils, macrophages and T-lymphocytes, placing the patient at risk for infectious complications.19,20
Initial Management
The majority of pediatric burns are minor, often resulting from scald accidents and affecting less than 10% total body surface area (TBSA), or from thermal injuries isolated to the hands. Such burns are usually limited to partial-thickness injury of the skin and can be managed on an outpatient basis, which is beyond the scope of this chapter. Unfortunately, larger burns require inpatient treatment and special attention. The initial step is completion of the primary and secondary surveys.21 Issues with airway, breathing, and circulation should be addressed immediately. Signs and symptoms including increased respiratory effort, wheezing, stridor, and tachypnea should raise concern for impending loss of the airway. The decision to intubate a patient with a tenuous airway should be made early. Inhalation injury can result in edema that may worsen over the first few hours, so repeated evaluations of the airway are important. Burn injuries can have a negative effect on breathing mechanics through smoke inhalation, a blast injury causing blunt chest trauma, and the restrictive effects of a burn eschar that limits full chest expansion. Escharotomy should be performed for the latter. Patients need support with 100% supplemental oxygen. If inhalation injury is suspected, arterial blood gas analysis and carboxyhemoglobin level are needed. Two large bore IVs should be placed and aggressive fluid resuscitation is important. When extremity burns limit peripheral IV access or if there is difficulty obtaining central venous access, intraosseus access should be utilized as a temporary (<24 hours) alternate route for fluid administration. A urinary catheter should be inserted, and heart rate, blood pressure and urine output should be monitored as tachycardia and low urine output signal a low intravascular volume state.
A decision should be made after initial evaluation, resuscitation, and wound dressing as to whether or not the patient needs to be transferred to a burn center. The American Burn Association and American College of Surgeons have clear recommendation regarding which patients should be referred to these centers (Box 13-1).22 However, a recent study raises concern that resources are often wasted by transferring less severe injuries to burn centers.23
There are several techniques to estimate the TBSA of the burn. The Wallace rule of nines estimates burn area fairly well for adolescents. Each upper extremity and the head represent 9% of the TBSA. The lower extremities and the anterior and posterior trunks are 18% each. The perineum, genitalia, and neck each measures 1%. Due to differences in body proportions for infants and children, the rule of nines has been modified to more accurately determine burn area in these patients. In this modification, the head represents 18% of the TBSA and each leg is 13.5%. Other modifications have been proposed to better estimate TBSA burn in obese patients.24 The Lund and Browder chart may provide more accurate determination of burn area in children as it compensates for variations in body shape and proportions (Fig. 13-2). For a rapid estimation of burn size, the palmar method can be used. The palmar surface is approximately 1% of the TBSA and is best used for estimating small surface area burns. First-degree burns should not be included in burn size calculations using any of these techniques. A recent study found that estimates of burn area are inaccurate in approximately 80% of children with the majority being overestimated.25 The authors concluded that this may be due to the variety of techniques used, or inclusion of first-degree burns in the calculation. This can also lead to unnecessary transfer of patients to burn care centers as discussed previously.
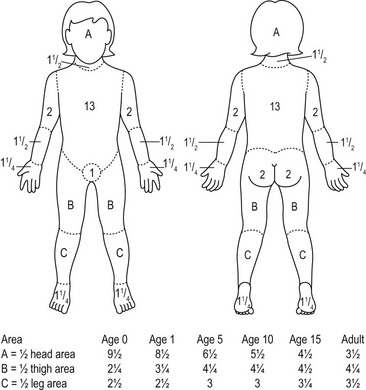
FIGURE 13-2 The Lund–Browder burn diagram is depicted for estimating the body surface area for burns in children.
A key component to the initial management of the severely burned patient includes escharotomy when indicated (Fig. 13-3). Full-thickness circumferential burns on the extremities can produce a constricting eschar which, together with the associated edema, can impede venous outflow and impair arterial flow. If pulses are absent, a bedside escharotomy should be performed with a scalpel or electrocautery along the lateral and medial aspects of the affected extremity. Incisions can be carried onto the hypothenar and thenar eminences and dorsolateral aspects of the digits if the hands or fingers are involved (Fig. 13-4). The resulting ischemia reperfusion injury and edema can cause significant increases in fascial compartment pressures. Although rare, fasciotomy may be necessary to relieve pressure and prevent necrosis of the underlying muscles and neurovascular bundles.
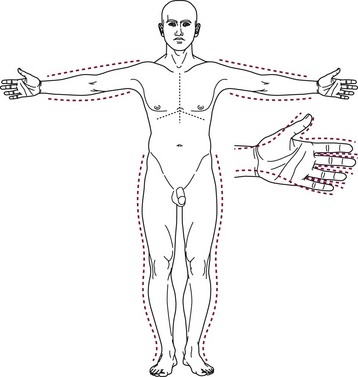
FIGURE 13-3 Escharatomies. The incisions are made on the medial and lateral aspects of the extremity. Hand escharotomies are performed on the medial and lateral digits and on the dorsum of the hand. (From Eichelberger MR [ed]; Pediatric Trauma: Prevention, Acute Care, Rehabilitation. St Louis, Mosby, 1993.)
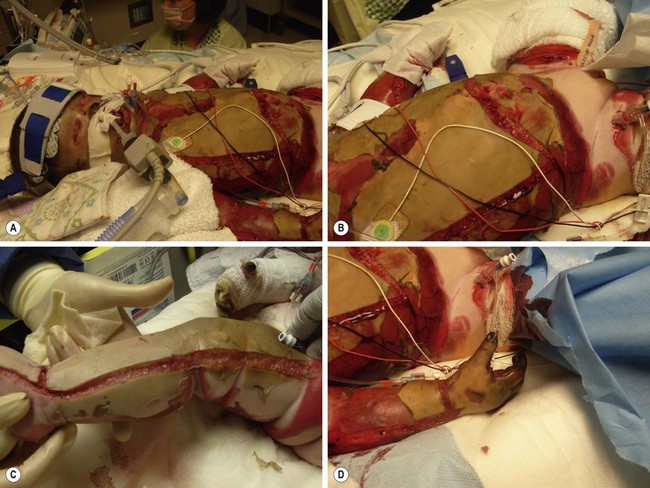
FIGURE 13-4 A 3-year-old male transferred after being rescued from a house fire. He required extensive escharotomies of the (A,B) chest and (C) distal extremities during the initial resuscitation. (D) Incisions should be carried onto the hypothenar and thenar eminences and dorsolateral aspects of the digits if the hands or fingers are involved.
Fluid Resuscitation
Once adequate intravenous or intraosseous access is obtained, fluid resuscitation is initiated. There are several formulas available to guide this resuscitation (Table 13-1). The Parkland formula is the most widely used, but is not as applicable in young children because children have greater TBSA relative to their body weight than adults. As a result, weight-based formulas can under-resuscitate children with minor burns and can grossly over-resuscitate children with extensive burns.26 TBSA-based formulas, such as the Shriners–Galveston formula, are therefore better at estimating fluid requirements in children less than 20 kg.27 TBSA is assessed from height and weight using standard nomograms or calculated using formulas (Table 13-2). Dextrose-containing solutions, such as 5% dextrose with 0.25 to 0.5 normal saline, are used as the primary solution. Children younger than 2 years of age are susceptible to hypoglycemia due to limited glycogen stores. Therefore, lactated Ringer’s solution with 5% dextrose is given during the first 24 hours in these patients.
TABLE 13-1
| Formula | First 24 Hours | Fluid Solution |
| Parkland | 4 mL/kg per %TBSA burn | Lactated Ringer’s (LR) |
| Brooke | 1.5 mL/kg per %TBSA burn | LR + colloid 0.5 mL/kg per %TBSA burn |
| Shriners–Galveston | 5000 mL/m2 burned + 2000 mL/m2 total | LR + 12.5 g albumin |
TABLE 13-2
Formulas for Body Surface Area (BSA)
| Dubois Formula | BSA (m2) = height (cm)0.725 × weight (kg)0.425 × 0.007184 |
| Jacobson Formula | BSA (m2) = [height (cm) + weight (kg) − 60]/100 |
Regardless of which formula is utilized, there must be close monitoring of the patient’s physiologic response to the fluid administration. Urine output should be maintained at 1 mL/kg/h. Inadequate resuscitation can result in hypoperfusion to the zone of stasis, with subsequent deepening of the burn, as well as hypoperfusion of major organs. During the first six to 12 hours, capillary permeability is increased and fluid moves from the intravascular space to the interstitial tissues with worsening edema. Overly aggressive fluid administration can result in significant tissue edema, tissue hypoxia, and elevated compartment pressures.28
Several authors have shown that the addition of colloid solutions such as albumin can reduce crystalloid requirements and more rapidly establish a balanced fluid intake to output ratio.29,30 However, a 2011 Cochrane database review found that albumin does not lower mortality in adults with major burns.31 Thus, the benefit of the more expensive albumin instead of standard crystalloid solutions is questionable. Other solutions, such as hypertonic saline, have been used in an effort to provide a high osmotic pressure which is thought to keep more volume in the intravascular space. Hypertonic saline also may have anti-inflammatory effects. However, it should be used with caution as it causes hypernatremia, and has not been shown to improve outcomes for hypotensive trauma patients.32
An increased incidence of pneumonia, bloodstream infection, acute respiratory distress syndrome (ARDS), multiple-organ failure, and death may be associated with over-resuscitation during the first 24 hours postburn.33 Interestingly, ‘permissive hypovolemia’ helps avoid these complications and has been shown to decrease multiple-organ dysfunction.34
Inhalation Injury
While inhalation injury is less common in children than adults, the mortality increases to nearly 40% when it occurs.35 The heat of inhaled gas causes upper airway injury. Inhaled toxins can result in upper airway injury in addition to causing tracheobronchial irritation and bronchoconstriction. Damaged epithelium releases vasoactive substances (thromboxanes A2, C3a, and C5a) that lead to hypoxia, increased airway resistance, decreased pulmonary compliance, increased alveolar epithelial permeability, and increased pulmonary vascular resistance.36 Carbon monoxide (CO) is produced during combustion and can contribute to the inhalational injury by displacing oxygen from hemoglobin-binding sites. The oxyhemoglobin dissociation curve is shifted to the left as the ability of hemoglobin to unload oxygen in the tissues is decreased. Secondary injury is due to impaired ciliary clearance of airway debris. Neutrophil infiltration occurs, macrophages are destroyed, and bacteria accumulate leading to pneumonia.
An inhalation injury should be recognized early through a detailed history of the burn and the initial evaluation. On examination, patients may be found to have facial burns, singed hairs in the nose, eyebrows or head, and/or carbonaceous sputum. Patients with inhalation injury who are obtunded are likely to have a carboxyhemoglobin level greater than 10%. Additional signs of significant injury are an altered voice along with hoarseness and stridor. Airway security in these patients is imperative. Fiberoptic bronchoscopy continues to be the preferred technique for documenting inhalation injury. Inflammatory changes in the tracheal mucosa such as edema, hyperemia, mucosal ulceration, and sloughing can be visualized. A ventilation scan with xenon-133 can also identify regions of inhalation injury by assessing respiratory exchange and excretion of xenon-133 from the lungs.37 When combined, bronchoscopy and xenon-133 scanning are over 90% accurate in the diagnosis of inhalation injury. However, the clinical applicability of xenon-133 scans remains unclear due to false positives in children with pulmonary disease.38,39 They may also be potentially dangerous due to the need to transport a critically ill burn patient to a nuclear medicine suite. Chest radiographs are not useful as they are often normal immediately following injury.38,40 Bedside bronchoscopy and a heightened clinical suspicion remain paramount in the diagnosis and treatment of these injuries.
After the airway is secured, an inhalation treatment protocol is utilized in the intensive care unit that focuses on the clearance of secretions and control of bronchospasm. One hundred per cent high flow, humidified oxygen should be administered to displace CO from hemoglobin. Early and aggressive pulmonary therapy consisting of chest physiotherapy, frequent suctioning, and early mobilization of the patient should be started. Bronchodilators and racemic epinephrine are used to treat bronchospasm. Clearance of secretions can be assisted with inhalation treatments composed of heparin and acetylcysteine. Human autopsy and animal models have shown nebulized heparin (5000–10,000 units/3 mL of NS q5 hours) to reduce tracheobronchial cast formation improves minute ventilation and decreases peak inspiratory pressures after smoke inhalation.41–43 The addition of 20% acetylcysteine (3 mL q4 hours) also improves the clearance of tracheobronchial secretions and minimizes bronchospasm. Pediatric and adult studies have shown this combination of medications to decrease reintubation rates and reduce mortality.44–46
Assessment of Burn Depth
Burn injury may involve one or both layers of the skin and may even extend to the subcutaneous fat, muscle, and bone.47 First-degree burns involve the epidermis. They are erythematous and very painful. Most sunburns fit this category of superficial, epidermal injury. For first-degree burns, topical ointments may be used for symptom relief and the involved areas should be kept out of the sunlight.
Superficial second-degree burns (superficial dermal burns) extend into the papillary dermis. They characteristically form blisters, but these blisters may not immediately follow the injury, making determination of depth difficult (Fig. 13-5). These wounds are painful when uncovered. The wound blanches with pressure due to increased vascularity secondary to vasodilatation. Superficial second-degree burns are managed with daily dressing changes with topical antimicrobials. They may also be treated with application of petroleum gauze or a synthetic dressing to allow for rapid spontaneous re-epithelialization. With appropriate care, these wounds will heal spontaneously within two to three weeks without the need for excision and grafting.
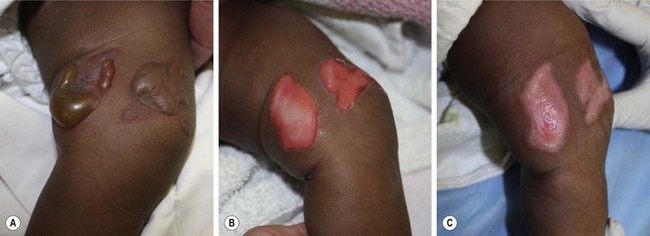
FIGURE 13-5 A superficial second-degree burn of the lower extremity is shown. (A) The characteristic blisters seen with this degree of burn injury. (B) After removal of the blister, the wounds are painful and blanch when pressure is applied. (C) The progress of healing after several days of daily dressing changes with topical antimicrobials.
Deep second-degree burns (deep dermal burns) extend into the reticular dermis and healing takes several weeks. These burns also blister, but the surface of the wound remains mottled and white (Fig. 13-6). Blanching occurs very slowly. Patients complain of pressure and discomfort rather than pain. These burns will require operative treatment if healing does not occur by 3 weeks.
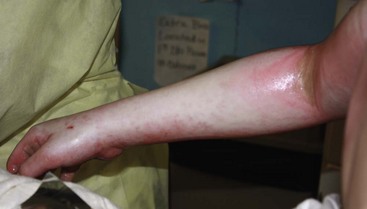
FIGURE 13-6 A deep second-degree burn of the right upper extremity is seen. Note the mottled and white appearance of the extremity.
Third-degree or full-thickness burns involve the entire dermis and extend into the subcutaneous tissue. These appear charred, leathery, and firm (Fig. 13-7). Patients typically are insensate in the burned regions and may not feel pressure. Blanching does not occur when pressure is applied. Full-thickness injuries should be excised and grafted early.48
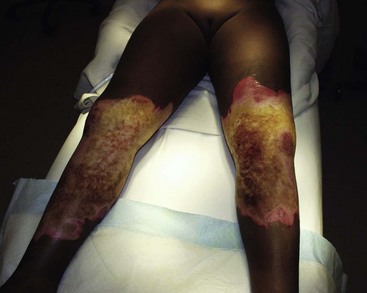
FIGURE 13-7 Bilateral lower extremity burns are shown. The outside areas of the burns are second degree while the central areas are third degree with eschar. Note the ‘leathery’ appearance of the areas that are third degree in depth.
Determination of burn wound depth can sometimes be difficult. Initial evaluation by an experienced surgeon as to whether an indeterminate dermal burn will heal in three weeks is only about 50–70% accurate.49–51 Scald injuries are particularly difficult to assess for depth and extent of injury. A number of techniques or tools have been described to improve accuracy. These techniques utilize the physiology of the skin and the alterations that occur with burn injury. Detection of dead cells or denatured collagen using ultrasound, biopsy, or vital dyes has been trialed.52–55 Other technologies such as analyzing altered blood flow using fluorescein, laser doppler imaging, and thermography have shown some promise.56–58 Unfortunately, many of these techniques have not been adopted due to variable reproducibility. Laser Doppler imaging has been shown to increase the accuracy of burn depth assessment when compared with experienced burn surgeons.59 Videomicroscopy has also been found to be accurate, especially when used after the first 24 hours.60 This technique is relatively inexpensive and easy to learn.
Wound Care
Most partial-thickness burns can be managed nonoperatively for ten to 14 days with topical therapies and dressings. Using this strategy, the goal of burn care is to provide an optimal environment for re-epithelialization by providing a warm and moist environment, removal of exudate and potentially contaminated or necrotic material (eschar), and control of bacterial proliferation. Burns should be excised and grafted if there are no signs of healing by three weeks. Desai et al confirmed the validity of this strategy with scald injuries in a randomized trial.61
Antimicrobial agents
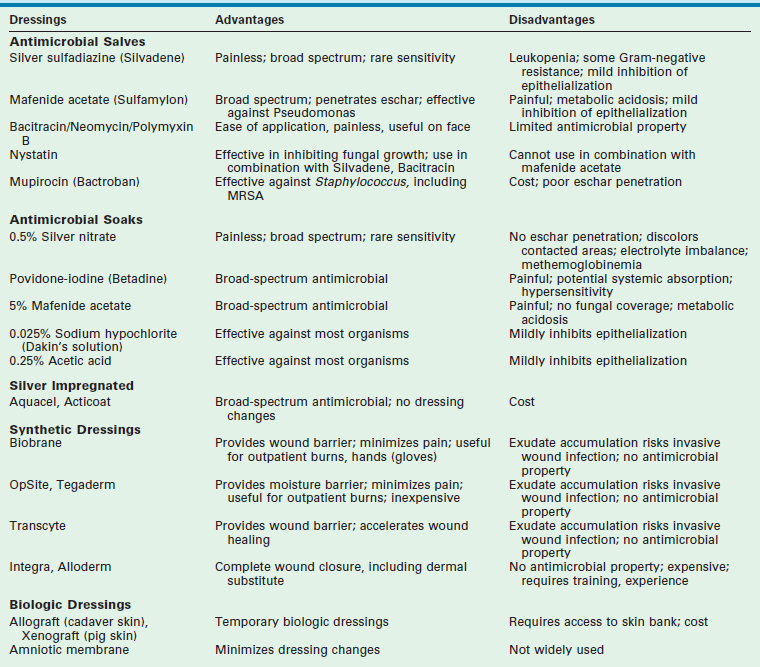
The initial treatment of partial-thickness burns is debridement and coverage with a topical agent that has antibacterial properties and allows for separation of the burn eschar.62–64 Various topical antimicrobial agents have been used (Table 13-3). These agents decrease bacterial content, but they do not eradicate or prevent colonization. In general, quantitative wound biopsy cultures that exceed 100,000 organisms per gram are consistent with, although not diagnostic of, invasive infection.
Silver sulfadiazine (Silvadene, Monarch Pharmaceuticals Inc., Bristol, TN) is currently the most commonly used topical antimicrobial agent for burn care. It is a white, highly insoluble compound synthesized from silver nitrate and sodium sulfadiazine.65 It has a broad spectrum of efficacy including Staphylococcus aureus, Escherichia coli, Klebsiella, Pseudomonas, and Proteus species. It also possesses an analgesic effect but does not penetrate eschar well. The combination of silver sulfadiazine with nystatin has significantly reduced the incidence of Candida infection in burned patients.66 The most common side effect from silver sulfadiazine is leukopenia which is caused by margination of leukocytes and is usually transient.67 It occurs somewhere between 5–15% of patients treated.68 Changing to another topical antimicrobial agent usually resolves this side effect.
Mafenide acetate (Sulfamylon, UDC Laboratories, Inc, Rockford IL) was introduced as a topical burn agent in the mid-1960s. It is more effective in penetrating eschar and is frequently used in third-degree burns. It has broad activity against most Gram-negative and Gram-positive pathogens, but unfortunately has minimal antifungal activity.69 The application of Sulfamylon can be painful which limits its practical use in the outpatient setting. Sulfamylon is a potent carbonic anhydrase inhibitor and can therefore cause metabolic acidosis.70 This side effect can usually be avoided by limiting its use to only 20% TBSA at any given time, and rotating application sites every several hours with another topical antimicrobial agent.
Silver nitrate (0.5%) was also introduced in the mid-1960s. It is typically used to soak gauze dressings thereby avoiding frequent dressing changes with the potential loss of grafts or healing cells. Silver nitrate is painless on application and has broad coverage. Unfortunately, the compound can cause hyponatremia and hypochloremia while also creating dark gray or black stains. Another important but infrequent complication is methemoglobinemia, which occurs as a result of nitrate reduction by wound bacteria followed by the systemic absorption of the toxic nitrite. Dressings containing biologically active silver ions (Aquacel, ConvaTec Ltd., UK; Acticoat, Smith & Nephew, London, UK) hold promise for retaining the effectiveness of silver nitrate but without its side effects. Several favorable clinical trials utilizing these products have been conducted and have found these products to be as effective as traditional dressings utilizing silver nitrate. The products were also noted to be less painful than traditional dressings when applied and removed, and also were associated with decreased burn wound cellulitis.71–73
Facial burns, small areas of partial-thickness burns, and healing donor sites require special mention. On superficial facial wounds, silver sulfadiazine may retard epithelialization and is not usually placed on the face.74 An alternative is petroleum-based antimicrobial ointments. These include polymyxin B, bacitracin, and polysporin. Their application is painless and transparent which allows for easier monitoring. These agents are mostly effective against Gram-positive organisms.
Proteolytic enzymatic agents have been utilized to debride wounds, including proteases (sultilains) elaborated from Bacillus subtilis, collagenase, and papaine-urea. Collagen is a protein that is found normally in skin (~75% of dry weight of skin) and is the dominant protein that must be lysed to allow for eschar separation. Collagenase is an exogenous enzyme that breaks down denatured collagen but does not lyse healthy, normal collagen. Collagenase Santyl ointment (Healthpoint Biotherapeutics, Fort Worth, TX) is used in many burn units for the treatment of partial-thickness burns. A multicenter trial of 79 patients ranging in age from 5 to 60 years suggested a slight acceleration of wound closure compared to silver sulfadiazine.75 A recent prospective randomized study in children comparing collagenase to silver sulfadiazine showed equivalent outcomes with regards to skin graft rates, hospitalization, and hospital charges.76
Burn Wound Dressings
The concept of an ‘optimal environment’ is derived from the work carried out by Winter in 1962.77 In young pigs, he found that partial-thickness wounds that were kept moistened with polyethylene film epithelialized twice as fast as those left exposed to air. Hinman and Maiback confirmed this observation with a series of human volunteers.78 Therefore, for 50 years, it has been felt that a burn dressing should provide an ‘optimal environment’ while also possessing bacterial inhibition. Typically, burn dressings consist of mesh that either contains or are placed over antimicrobial compounds. Nonadherent dressings such as Telfa (Tyco Healthcare Group LP, Mansfield, MA), Xeroform (Tyco Healthcare Group LP, Mansfield, MA), Adaptic (Johnson & Johnson, New Brunswick, NJ), or Mepitel (Molnlycke Health Care AB Gothenburg, Sweden) can be placed directly on the wound to help reduce both the pain associated with dressing changes and the friction associated damage to the wound or skin graft. The nonadherent dressing and antimicrobial compound serve to provide the ‘optimal environment’ for re-epithelialization.
An example of a synthetic mesh product is Biobrane (UCL Laboratories, Rockford IL). It is a bilaminate thin membrane composed of thin semipermeable silicone bonded to a layer of nylon mesh, which is coated with a monomolecular layer of type I collagen of porcine origin. This dressing provides a hydrophilic coating for fibrin ingrowth which promotes wound adherence. The dressing is placed on a clean fresh superficial second-degree burn wound and can be secured using steri-strips and/or bandages. This dressing is easily removed from the wound bed as the wound epithelializes underneath it. Fluid can accumulate under the dressing and can be aspirated if needed. However, if a foul-smelling exudate is detected, the Biobrane should be removed and an antimicrobial dressing applied. Biobrane is now widely used in the management of superficial second-degree burns as it reduces pain, fluid, and electrolyte loss. These advantages make it an ideal dressing for use in the outpatient setting.79
Dressings that are commonly utilized for coverage of postoperative incisions may also be used as small superficial second-degree burn dressings. These alternatives include Duoderm (ER Squibb & Sons, Inc. Princeton NJ), Opsite (Smith & Nephew, London, UK), and Tegaderm (3M Pharmaceuticals, St Paul MN). Despite lacking special biological factors (collagen and growth factors), these dressings provide a cheap and transparent alternative to more expensive dressings. Also, Duoderm has been found to be less expensive than Biobrane and therefore could be a first-line treatment option for intermediate thickness burn wounds in children.80
The disfigurement resulting from full-thickness burns has been decreased with the advent of combined synthetic and biologic materials. Integra (Integra LifeSciences Corp, Plainsboro NJ) has an inner layer composed of a porous matrix of bovine collagen and the glycosaminoglycan chrondroitin-6-sulfate which facilitates fibrovascular ingrowth.81 The outer layer is a polysiloxane polymer with vapor transmission characteristics similar to normal epithelium. Integra acts as a dermal replacement. It provides a matrix for the infiltration of fibroblasts, macrophages, lymphocytes, and capillaries from the wound bed, and promotes rapid neo-dermis formation. Approximately two weeks after engraftment, the outer silicone layer is removed and is replaced with an epidermal split thickness autograft (Fig. 13-8). Integra covered wounds have less scarring, but are susceptible to infection and must be monitored carefully. Its advantages were validated in a randomized study in children with large TBSA burns.82 Children treated with Integra demonstrated significantly decreased resting energy expenditure as well as increased bone mineral content and density. Also, improved scarring was found at 24 months after burn injury.
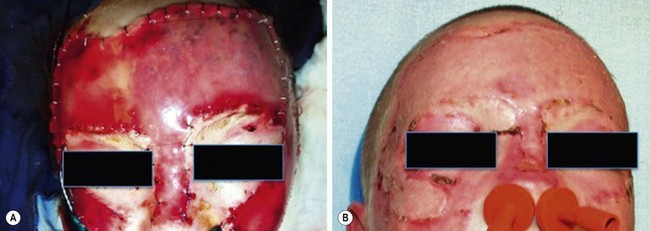
FIGURE 13-8 The use of Integra for a superior cosmetic result in a child with facial burns is depicted. (A) The application of the Integra with the silicone layer in place. Approximately two weeks after placement, the silicone layer is removed and split thickness skin grafts are placed. (B) The same child after skin grafting over the Integra.
Excision and Grafting
Prompt burn excision and grafting has been shown to improve survival, decrease length of hospitalization, and reduce costs in burn patients of all ages. Children particularly have benefited from more timely and extensive operative management.81–86 Once a burn is considered ‘deep’ or fails to heal with topical care, tangential excision is needed. This technique was originally described by Janzekovic in 1970.87 The eschar is sequentially shaved using a dermatome, knife blade or, more recently, a Versajet (Smith & Nephew, Inc., London, UK) water dissector until a viable tissue bed is obtained.88 In a prospective randomized trial, the Versajet technique was shown to produce a more precise and faster excision than hand-held dermatome escharectomy.89
After excision, coverage is ideally completed with an autograph. Split-thickness autografts (0.008–0.010 inch thickness) are harvested and utilized as a sheet (unmeshed) or meshed graft. Sheet grafts provide better long-term aesthetic outcomes, but are complicated by the development of a seroma or hematoma and also limited coverage. Narrow meshed autografts (1 : 1 or 1 : 2) have the advantages of limiting the total surface area of donor harvest and allowing better drainage of fluid under the grafted sites. In larger burns (>20–30%), coverage may require a combination of meshed autograft and allografts. The meshed autografts (4 : 1 to 6 : 1) can be covered with meshed allograft (2 : 1) overlays.90 Alternatively, grafting with sequential harvesting of split-thickness autograft from limited donor sites until the entire burn wound is covered may be needed. The use of a widely meshed graft is avoided on the face and functionally important parts of the hand. Full-thickness grafts that include both dermal and epidermal components are commonly obtained from the lower abdomen, groin, or upper arm. These grafts provide the best outcome for wound coverage with diminished contracture and a better pigment match, but their use is generally limited due to the lack of donor sites.
Nonthermal Injuries
Electrical Burns
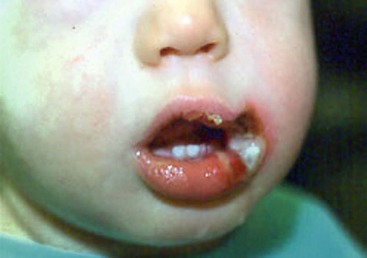
Three to 9% of all admitted burn patients are injured from electrical contact.91 Electrical burns are categorized into low- and high-voltage injuries. Low voltage (<1000 V) injuries typically occur at home where electrical cords are bitten especially by younger children (Fig. 13-9). High-voltage (>1000 V) injuries may result from power lines or lightning strikes, and are characterized by a varying degree of local burn with destruction of deep tissues.92 The electrical current enters the body and travels preferentially through the low resistance tissues (nerves, blood vessels, and muscles). As skin has high resistance, it is mostly spared leaving little visible evidence of injury. Primary and secondary surveys, including electrocardiography, are very important. If the initial electrocardiogram is normal, further cardiac monitoring is not needed. However, any abnormal findings require continued monitoring for 48 hours and appropriate management of dysrhythmias if detected.93 Injuries to deep tissues and organs must be identified and treated. As tissue edema worsens, patients may develop compartment syndromes requiring fasciotomy to avoid limb loss. Myoglobinuria can lead to renal failure and should be treated with vigorous hydration with sodium bicarbonate and mannitol. Low-voltage injury is typically limited to superficial thermal burn and can be treated with topical wound care.
References
1. Lund, CC, Browder, NC. The estimation of areas of burns. Surg Gynecol Obstet. 1944; 79:352–358.
2. Brigham, PA, McLoughlin, E. Burn incidence and medical care use in the United States: Estimates, trends, and data sources. J Burn Care Rehabil. 1996; 17:95–107.
3. Herndon, DN, Gore, D, Cole, M, et al. Determinants of mortality in pediatric patients with greater than 70% full-thickness total body surface area thermal injury treated by early total excision and grafting. J Trauma. 1987; 27:208–212.
4. Hettiaratchy, S, Dziewulski, P. ABC of burns. Introduction. BMJ. 2004; 328:1366–1368.
5. Jackson, DM. The diagnosis of the depth of burning. Br J Surgery. 1953; 40:588–596.
6. Vo, LT, Papworth, GD, Delaney, PM, et al. A study of vascular response to thermal injury on hairless mice by fiberoptic confocal imaging, laser Doppler flowmetry and conventional histology. Burns. 1998; 24:319–324.
7. Demling, RH, LaLonde, C. Early postburn lipid peroxidation: Effect of ibuprofen and allopurinol. Surgery. 1990; 107:85–93.
8. Nwariaku, FE, Sikes, PJ, Lightfoot, E, et al. Effect of a bradykinin antagonist on the local inflammatory response following thermal injury. Burns. 1996; 22:324–327.
9. DelBeccaro, EJ, Robson, MC, Heggers, JP, et al. The use of specific thromboxane inhibitors to preserve the dermal microcirculation after burning. Surgery. 1980; 87:137–141.
10. Firat, C, Samdancı, E, Erbatur, S, et al. β-Glucan treatment prevents progressive burn ischaemia in the zone of stasis and improves burn healing: An experimental study in rats. Burns. 2013; 39:105–112.
11. Evers, LH, Bhavsar, D, Mailänder, P. The biology of burn injury. Exp Dermatol. 2010; 19:777–783.
12. Chung, DH, Evers, BM, Townsend, CM, Jr., et al. Role of polyamine biosynthesis during gut mucosal adaptation after burn injury. Am J Surg. 1993; 165:144–149.
13. Chung, DH, Evers, BM, Townsend, CM, Jr., et al. Burn-induced transcriptional regulation of small intestinal ornithine decarboxylase. Am J Surg. 1992; 163:157–163.
14. Wolf, SE, Ikeda, H, Matin, S, et al. Cutaneous burn increases apoptosis in the gut epithelium of mice. J Am Coll Surg. 1999; 188:10–16.
15. Carter, EA, Gonnella, A, Tompkins, RG. Increased transcellular permeability of rat small intestine after thermal injury. Burns. 1992; 18:117–120.
16. Myers, SI, Minei, JP, Casteneda, A, et al. Differential effects of acute thermal injury on rat splanchnic and renal blood flow and prostanoid release. Prostaglandins Leukot Essent Fatty Acids. 1995; 53:439–444.
17. Jeschke, MG, Barrow, RE, Wolf, SE, et al. Mortality in burned children with acute renal failure. Arch Surg. 1998; 133:752–756.
18. Brusselaers, N, Monstrey, S, Colpaert, K, et al. Outcome of acute kidney injury in severe burns: A systematic review and meta-analysis. Intensive Care Med. 2010; 36:915–925.
19. Gamelli, RL, He, LK, Liu, H. Macrophage suppression of granulocyte and macrophage growth following burn wound infection. J Trauma. 1994; 37:888–892.
20. Hunt, JP, Hunter, CT, Brownstein, MR, et al. The effector component of the cytotoxic T-lymphocyte response has a biphasic pattern after burn injury. J Surg Res. 1998; 80:243–251.
21. Hettiaratchy, S, Papini, R. Initial management of a major burn: I–overview. BMJ. 2004; 328(7455):1555–1557.
22. American Burn Association/American College of Surgeons. Guidelines for the operation of burn centers. J Burn Care Res. 2007; 28:134–141.
23. Vercruysse, GA, Ingram, WL, Feliciano, DV. Overutilization of regional burn centers for pediatric patients–a healthcare system problem that should be corrected. Am J Surg. 2011; 202:802–808.
24. Neaman, KC, Andres, LA, McClure, AM, et al. A new method for estimation of involved BSAs for obese and normal-weight patients with burn injury. J Burn Care Res. 2011; 32:421–428.
25. Chan, QE, Barzi, F, Cheney, L, et al. Burn size estimation in children: Still a problem. Emerg Med Australas. 2012; 24:181–186.
26. Carvajal, HG. Fluid resuscitation of pediatric burn victims: A critical appraisal. Pediatr Nephrol. 1994; 8:357–366.
27. Ansermino, JM, Vandebeek, CA, Myers, D. An allometric model to estimate fluid requirements in children following burn injury. Paediatr Anaesth. 2010; 20:305–312.
28. Du, GB, Slater, H, Goldfarb, IW. Influences of different resuscitation regimens on acute early weight gain in extensively burned patients. Burns. 1991; 17:147–150.
29. Lawrence, A, Faraklas, I, Watkins, H, et al. Colloid administration normalizes resuscitation ratio and ameliorates ‘fluid creep’. J Burn Care Res. 2010; 31:40–47.
30. Faraklas, I, Cochran, A, Saffle, J. Review of a fluid resuscitation protocol: ‘Fluid creep’ is not due to nursing error. J Burn Care Res. 2012; 33:74–83.
31. Roberts, I, Blackhall, K, Alderson, P, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. (11):2011.
32. Wigginton, JG, Roppolo, L, Pepe, PE. Advances in resuscitative trauma care. Minerva Anestesiol. 2011; 77:993–1002.
33. Klein, MB, Hayden, D, Elson, C, et al. The association between fluid administration and outcome following major burn: A multicenter study. Ann Surg. 2007; 245:622–628.
34. Arlati, S, Storti, E, Pradella, V, et al. Decreased fluid volume to reduce organ damage: A new approach to burn shock resuscitaion? A preliminary study. Resuscitation. 2007; 72:371–378.
35. Herndon, DN, Thompson, PB, Traber, DL. Pulmonary injury in burned patients. Crit Care Clin. 1985; 1:79–96.
36. Traber, DL, Herndon, DN, Stein, MD, et al. The pulmonary lesions of smoke inhalation in an ovine model. Circ Shock. 1986; 18:311–323.
37. Moylan, JA, Jr., Wilmore, DW, Mouton, DE, et al. Early diagnosis of inhalation injury using 133 xenon lung scans. Ann Surg. 1972; 176:477–484.
38. Clark, WR, Jr. Smoke inhalation: Diagnosis and treatment. World J Surg. 1992; 16:24–29.
39. Chou, SH, Lin, SD, Chuang, HY, et al. Fiberoptic bronchoscopic classification of inhalation injury: Prediction of acute lung injury. Surg Endosc. 2004; 18:1377–1379.
40. Peitzman, AB, Shires, GT, III., Teixidor, HS, et al. Smoke inhalation injury: Evaluation of radiographic manifestations and pulmonary dysfunction. J Trauma. 1989; 29:1232–1238.
41. Micak, RP, Suman, OE, Herndon, DN. Respiratory management of inhalation injury. Burns. 2007; 33:2–13.
42. Miller, AC, Rivero, A, Ziad, S, et al. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009; 30:249–256.
43. Cancio, LC. Current concepts in the pathophysiology and treatment of inhalation injury. Trauma. 2005; 7:19–35.
44. Saliba, MJ, Jr. Heparin in the treatment of burns: A review. Burns. 2001; 27:349–358.
45. Desai, MH, Micak, R, Richardson, J, et al. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcysteine] therapy. J Burn Care Rehabil. 1998; 19:210–212.
46. Brown, M, Desai, M, Traber, LD, et al. Dimethylsulfoxide with heparin in the treatment of smoke inhalation injury. J Burn Care Rehabil. 1988; 9:22–25.
47. Forage, AV. The history of the classification of burns (diagnosis of depth). Br J Plastic Surg. 1963; 16:239–242.
48. Engrav, LH, Heimbach, DM, Reus, JL, et al. Early excision and grafting vs. nonoperative treatment of burns of indeterminate depth: A randomized prospective study. J Trauma. 1983; 11:1001–1004.
49. Hlava, P, Moserová, J, Königová, R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir Plas. 1983; 25:202–208.
50. Niazi, ZB, Essex, TJ, Papini, R, et al. New laser Doppler scanner, a valuable adjunct in burn depth assessment. Burns. 1993; 19:485–489.
51. Yeong, EK, Mann, R, Goldberg, M, et al. Improved accuracy of burn wound assessment using laser Doppler. J Trauma. 1996; 40:956–961.
52. Ho-Asjoe, M, Chronnell, CM, Frame, JD, et al. Immunohistochemical analysis of burn depth. J Burn Care Rehabil. 1999; 20:207–211.
53. Moserová, J, Hlava, P, Malínský, J. Scope for ultrasound diagnosis of the depth of thermal damage. Preliminary report. Acta Chir Plast. 1982; 24:235–242.
54. Cantrell, JH, Jr. Can ultrasound assist an experienced surgeon in estimating burn depth? J Trauma. 1984; 24:S64–S70.
55. Kaufman, T, Hurwitz, DJ, Heggers, JP. The india ink injection technique to assess the depth of experimental burn wounds. Burns Incl Therm Inj. 1984; 10:405–408.
56. Black, KS, Hewitt, CW, Miller, DM, et al. Burn depth evaluation with fluorometry: Is it really definitive? J Burn Care Rehabil. 1986; 7:313–317.
57. Pape, SA, Skouras, CA, Byrne, PO. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns. 2001; 27:233–239.
58. Hackett, ME. The use of thermography in the assessment of depth of burn and blood supply of flaps, with preliminary reports on its use in Dupuytren’s contracture and treatment of varicose ulcers. Br J Plast Surg. 1974; 27:311–317.
59. Erba, P, Espinoza, D, Koch, N, et al. FluxEXPLORER: A new high-speed laser Doppler imaging system for the assessment of burn injuries. Skin Res Technol. 2012; 18:456–461.
60. Mihara, K, Shindo, H, Ohtani, M, et al. Early depth assessment of local burns by videomicroscopy: 24 h after injury is a critical time point. Burns. 2011; 37:986–993.
61. Desai, MH, Rutan, RL, Herndon, DN. Conservative treatment of scald burns is superior to early excision. J Burn Care Rehabil. 1991; 12:482–484.
62. Kumar, RJ, Kimble, RM, Boots, R, et al. Treatment of partial-thickness burns: A prospective, randomized trial using Transcyte. ANZ J Surg. 2004; 74:622–626.
63. Costagliola, M, Agrosì, M. Second-degree burns: A comparative, multicenter, randomized trial of hyaluronic acid plus silver sulfadiazine vs. silver sulfadiazine alone. Curr Med Res Opin. 2005; 21:1235–1240.
64. Soroff, HS, Sasvary, DH. Collagenase ointment and polymyxin B sulfate/bacitracin spray versus silver sulfadiazine cream in partial-thickness burns: A pilot study. J Burn Care Rehabil. 1994; 15:13–17.
65. Fox, CL, Jr. Silver sulfadiazine–a new topical therapy for Pseudomonas in burns. Therapy of Pseudomonas infection in burns. Arch Surg. 1968; 96:184–188.
66. Desai, MH, Rutan, RL, Heggers, JP, et al. Candida infection with and without nystatin prophylaxis. A 11-year experience with patients with burn injury. Arch Surg. 1992; 127:159–162.
67. Jarrett, F, Ellerbe, S, Demling, R. Acute leukopenia during topical burn therapy with silver sulfadiazine. Am J Surg. 1978; 135:818–819.
68. Choban, PS, Marshall, WJ. Leukopenia secondary to silver sulfadiazine: frequency, characteristics and clinical consequences. Am Surg. 1987; 53:515–517.
69. Lindberg, RB, Moncrief, JA, Mason, AD, Jr. Control of experimental and clinical burn wounds sepsis by topical application of sulfamylon compounds. Ann N Y Acad Sci. 1968; 150:950–960.
70. Asch, MJ, White, MG, Pruitt, BA, Jr. Acid base changes associated with topical Sulfamylon therapy: Retrospective study of 100 burn patients. Ann Surg. 1970; 172:946–950.
71. Fong, J, Wood, F, Fowler, B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: Comparative patient care audits. Burns. 2005; 31:562–567.
72. Tredget, EE, Shankowsky, HA, Groeneveld, A, et al. A matched-pair, randomized study evaluating the efficacy and safety of Acticoat silver-coated dressing for the treatment of burn wounds. J Burn Care Rehabil. 1998; 19:531–537.
73. Varas, RP, O’Keeffe, T, Namias, N, et al. A prospective, randomized trial of Acticoat versus silver sulfadiazine in the treatment of partial-thickness burns: Which method is less painful? J Burn Care Rehabil. 2005; 26:344–347.
74. Muller, MJ, Hollyoak, MA, Moaveni, Z, et al. Retardation of wound healing by silver sulfadiazine is reversed by Aloe vera and nystatin. Burns. 2003; 29:834–836.
75. Hansbrough, JF, Achauer, B, Dawson, J, et al. Wound healing in partial-thickness burn wounds treated with collagenase ointment versus silver sulfadiazine cream. J Burn Care Rehabil. 1995; 16:241–247.
76. Ostlie, DJ, Juang, D, Aguayo, P, et al. Topical silver sulfadiazine vs collagenase ointment for the treatment of partial thickness burns in children: A prospective randomized trial. J Pediatr Surg. 2012; 47:1204–1207.
77. Winter, GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962; 193:293–294.
78. Hinman, CD, Maibach, H. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963; 200:377–378.
79. Paddock, HN, Fabia, R, Giles, S, et al. A silver-impregnated antimicrobial dressing reduces hospital length of stay for pediatric patients with burns. J Burn Care Res. 2007; 28:409–411.
80. Cassidy, C, St Peter, SD, Lacey, S, et al. Biobrane versus duoderm for the treatment of intermediate thickness burns in children: A prospective, randomized trial. Burns. 2005; 31:890–893.
81. Tompkins, RG, Burke, JF. Progress in burn treatment and the use of artificial skin. World J Surg. 1990; 14:819–824.
82. Branski, LK, Herndon, DN, Pereira, C, et al. Longitudinal assessment of Integra in primary burn management: A randomized pediatric clinical trial. Crit Care Med. 2007; 35:2615–2623.
83. Herndon, DN, Parks, DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma. 1986; 26:149–152.
84. Herndon, DN, Gore, D, Cole, M, et al. Determinants of mortality in pediatric patients with greater than 70% full-thickness total body surface area thermal injury treated by early total excision and grafting. J Trauma. 1987; 27:208–212.
85. Muller, MJ, Herndon, DN. The challenge of burns. Lancet. 1994; 343:216–220.
86. Bull, JP, Fisher, AJ. A study of mortality in a burns unit: A revised estimate. Ann Surg. 1954; 139:269–274.
87. Janzekovic, Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970; 10:1103–1108.
88. Klein, MB, Hunter, S, Heimbach, DM, et al. The Versajet water dissector: A new tool for tangential excision. J Burn Care Rehabil. 2005; 26:483–487.
89. Gravante, G, Delogu, D, Esposito, G, et al. Versajet hydrosurgery versus classic escharectomy for burn debridment: A prospective randomized trial. J Burn Care Res. 2007; 28:720–724.
90. Alexander, JW, MacMillan, BG, Law, E, et al. Treatment of severe burns with widely meshed skin autograft and meshed skin allograft overlay. J Trauma. 1981; 21:433–438.
91. Celik, A, Ergun, O, Ozok, G. Pediatric electrical injuries: A review of 38 consecutive patients. J Pediatr Surg. 2004; 39:1233–1237.
92. Laberge, LC, Ballard, PA, Daniel, RK. Experimental electrical burns: Low voltage. Ann Plast Surg. 1984; 13:185–190.
93. Robson, MC, Smith, DJ. Care of the thermal injured victim. In: Jurkiewicz MJ, Krizek TJ, Mathes SJ, Ariyan S, eds. Plastic Surgery: Principles and Practice. St. Louis, CV: Mosby, 1990.