Chapter 58 Burn Rehabilitation
Modern burn care has resulted in a significant increase in survival after burn injury. Survivors of burn injury can have scarring, amputations, neuromuscular complications, and significant psychologic impairments. Most survivors of burn injury are young adults, making such issues as community reintegration and return to work important. It is estimated that 1 million annually will require treatment for burn injuries in the United States alone, but an even larger number of burns will occur in the developing world.1 Burn care in the future will face several challenges because of an aging population, the expense and complexity of burn treatment, and a current and predicted shortage of qualified burn surgeons.76
Epidemiology of Burn Injury
The American Burn Association (ABA) has led an effort to catalogue burn injuries through a burn registry project. Between 1995 and 2005, burn centers in the United States and Canada contributed information to the National Burn Repository (NBR). From approximately 126,000 records, several conclusions could be drawn about burn injury in North America. Specifically, most burn patients are male (70%) with a mean age of 33 years. Most burn injuries covered less than 10% of the total body surface area (TBSA). The most common etiologies of burn injuries were flames and scalds. Approximately 95% of patients survived their hospitalization.115 Burn centers, however, also reported admitting patients with unusual burn etiologies such as radiation. Skin diseases make up a small number of admissions to burn centers, and the complexity of diseases such as Stevens-Johnson syndrome and necrotizing fasciitis add to the complexity of care in the burn unit.43,115
Several negative predictors have been noted for survival after burn injury. Increasing age, burn injury over a higher TBSA, and the presence of inhalation injury are all significantly associated with increased mortality.115,170
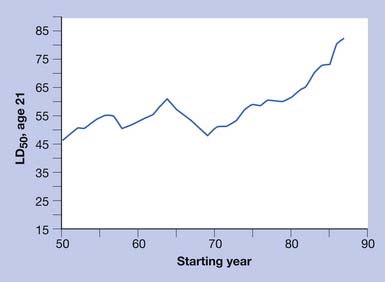
Increasing TBSA is associated with higher mortality rates, especially in patients older than 60 years of age and even more so in patients older than 70.115 In elderly patients the presence of medical comorbidities, inhalation injury, and burn size best predict mortality within 1 year of burn injury (Figures 58-1 and 58-2).108
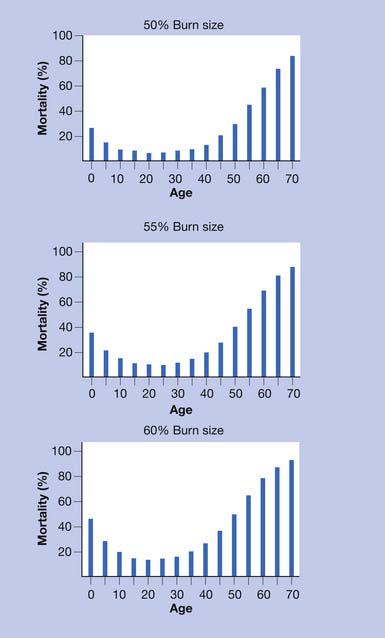
FIGURE 58-1 Effect of age on percent mortality at three discrete burn sizes.
(From Pruitt BA Jr, Goodwin CW, Mason AD: Epidemiological, demographic, and outcome characteristics of burn injury. In Herndon DN, editor: Total burn care, ed 2, New York, 2002, Saunders, with permission.)
With the large majority of burn patients surviving their hospital stay, however, a shift away from mortality as a primary outcome measure toward measurements of quality of life and function has been advocated by burn practitioners.134 The National Institute on Disability and Rehabilitation Research (NIDRR) has funded the Burn Model Systems project92 to further research the long-term outcomes of burn survivors.
Acute Physiatric Assessment of the Burn Patient
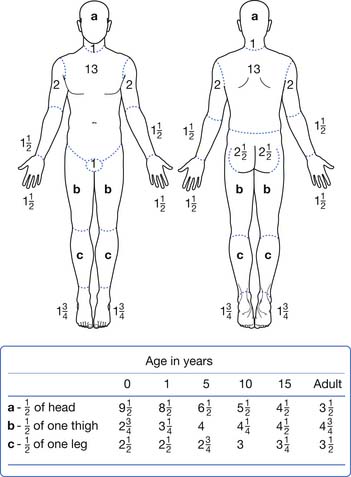
The size and location of the burn injury are important for several reasons. Larger burn injuries are associated with higher mortality and also are more likely to be associated with significant complications.13,78,106 Relative proportions of the TBSA made up by the head and limbs can be different by age. Estimations of the TBSA burned can be calculated using a tool such as the Lund and Browder chart (Figure 58-3). Other estimators of burn size can be made by following the “rule of nines” or estimating that the palm of the patient’s hand is approximately 1% of the TBSA.74
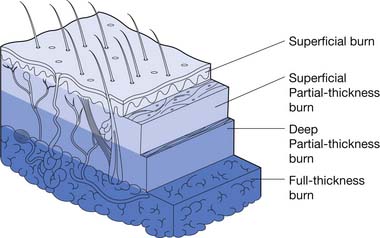
Partial-thickness burn injuries are divided into superficial partial thickness and deep partial thickness. Superficial partial-thickness burns have mild to moderate wound exudate. This depth of burn injures the superficial layers of the dermis. Loose or tense blisters can be filled with serous fluid. These wounds are painful but typically heal within 7 to 14 days. The risk of scarring from this injury is low, but there might be pigmentation changes in the skin in the long term.
A summary of burn depth and skin sections is shown in Figures 58-4 and 58-5.
Acute Surgical Procedures in Burn Injuries
In full-thickness burn injuries, the damaged tissues are adherent to underlying structures. With the development of wound edema, combined with the fluids required for resuscitation after burn injury, there is a risk for development of compartment syndromes in the limbs. Thick eschar in the trunk region can inhibit respiration. The surgical team can perform escharotomy, which is a surgical incision through the eschar, to relieve this pressure. The pattern of the incision can be important in avoiding future contractures from the resulting scar. Escharotomies in the limbs and trunk typically are left open in the acute phase.74 Limbs requiring escharotomies should be elevated and splinted in a neutral position for 24 hours before initiating passive range of motion.
Early excision and autologous skin grafting has greatly improved survival after burn injury. This reduces the inflammatory stimulation from the burn eschar and reduces the risk of infection. The entire devitalized tissue is removed by the surgical team with tangential excision, followed by split-thickness autologous skin grafting. Donor skin is removed from an unburned area of skin through the use of a powered dermatome (Figure 58-6). The donor skin is then prepared and placed on the surgically prepared wound bed. The donor skin is held in place by staples, sutures, or, in some cases, dermal glues.126 A compressive postoperative dressing is then applied to the skin graft area, and an appropriate dressing is applied to the skin graft donor site. Because the donor skin is typically partial thickness, the donor site heals spontaneously, usually over 2 weeks. Although hypertrophic scarring is not usually seen in the donor site, hyperpigmentation and hypertrophic scarring might be seen.

FIGURE 58-6 Donor skin is removed from an unburned area of skin through the use of a powered dermatome.
The donor skin can be prepared by meshing to broaden the area of wound covered. Meshing also allows for any blood or wound exudates to escape from underneath the skin graft. The donor skin can be applied as a sheet over areas of special function such as the hands and face. Sheet grafts generally do not scar as much as meshed grafts and can result in improved function.89 Because there is no route of escape from beneath the unmeshed sheet graft, however, seromas or hematomas can form and even cause loss of the skin graft.
To improve adherence of the skin graft to the donor site, the grafted site is typically immobilized in the immediate postoperative phase. The immobilization phase time is now shorter than in the past because early initiation of therapies and mobilization improve patient function and reduce length of hospital stay.24
Presence of Inhalation Injury
Inhalation injury associated with burns is a significant risk factor for morbidity, especially in children and older adults.68,108,129,170 Little has been documented regarding the incidence of hypoxic brain injury in burn patients with inhalation injury, but the reduction of available oxygen, combined with toxic smoke components such as carbon monoxide and cyanide, puts the burn patient with inhalation injury at risk for brain injury.4 Burn patients with inhalation injuries are at risk for developing pneumonia, adult respiratory distress syndrome, and multisystem organ failure during the acute periods of recovery.75 Early tracheostomy in patients likely to require prolonged intubation has not been shown to change pulmonary outcomes, but it does offer advantages for oral hygiene and preventing of microstomia.139 It is not known whether inhalation injury predisposes burn patients to pneumonia in the rehabilitation setting, and as a result, routine oxygen monitoring during therapies varies among practitioners.
Polytrauma and Burns
Approximately 5% of all trauma patients will also have some degree of burn injury.147 Fractures outside of the burned area can be treated with standard fracture care. An individualized approach is adopted, however, when the fractures occur in regions that also have burn injury.91 Patients with a history of major trauma can have delayed diagnoses, including fractures or other neurologic and musculoskeletal injuries, which the physiatrist should consider during the assessment of the burn patient.22,42
Electrical Injury
Electrical injuries are arbitrarily divided into high-voltage (>1000 V) and low-voltage (<1000 V) injuries. High-voltage injuries are typically associated with work-related accidents, whereas most electrical injuries that occur in the home are low voltage.11,69 Electrical injuries have special characteristics compared with other thermal injuries. For example, the degree of soft tissue injury can be greater than expected based on the cutaneous injury alone. Electric current follows the tissues of least electrical resistance, which can result in significant peripheral and central nervous system damage. A risk exists for myocardial necrosis and arrythmias.96 Those who do not have an arrhythmia within the first 24 hours of injury, however, are typically not at risk for later arrhythmias that can be associated with the electrical injury.11
Survivors of high-voltage electrical injuries account for a large number of amputations in the burn unit, as well as longer lengths of stay.11 These patients are also at risk for both peripheral and central nervous system complications, which might not present until late after injury. High-voltage electrical injuries are associated with impairments in function, but even low-voltage injuries can complicate return to work and other functional tasks.113,166
Lightning is a rare cause of electrical injury, but can cause a spectrum of neurologic complications, including myelopathy and encephalopathy.11,27 Lightning injuries can also cause both sensorineural and mechanical hearing loss.125
Ocular complications of electrical injury, such as the development of cataracts or macular holes, can present acutely or late after injury.20
Peripheral Neuropathies
Approximately 10% of burn patients will develop peripheral neuropathies from a variety of etiologies, such as direct thermal injury, electrical current, compression, and metabolic derangements.97 The patients most likely to develop neuropathies with electrical injuries include those with a history of alcohol abuse and long stays in the intensive care unit.97 Several patterns of neuropathy are seen, including mononeuropathies, peripheral polyneuropathies, and patterns that resemble mononeuritis multiplex.97,106 Deeper and larger TBSA burns are more associated with axonal rather than demyelinating neuropathies.106
The physiatrist performing electrodiagnostic testing on burn patients should be mindful that the changes after burn injury can alter the results of both nerve conduction testing and electromyography. The increased skin thickness seen with hypertrophic burn scars can have an inverse relationship with the amplitude of the responses seen in nerve conduction testing.70 After large burn injuries there can be up-regulation of nicotinic acetylcholine receptors in the muscle cell membrane that results in electrodiagnostic features suggestive of membrane instability or acute denervation.79,111 As a result, electromyographic studies must be interpreted with caution in the burn patient because these neuromuscular changes can be indistinguishable from true neuropathic changes.
Median sensory neuropathies are the most common peripheral nerve abnormality after burn injury. Conservative electrodiagnostic studies can help to define the extent of nerve injuries in burn patients. Over the course of approximately 1 year, repeated nerve conduction tests typically show improvement in burn-related neuropathy without requiring intervening surgical treatments.61
Catabolism and Metabolic Abnormalities
In all patients with burn injuries greater than 30% TBSA, the physiatrist should anticipate a significant metabolic abnormality that results in loss of bone mineral density and lean body mass and increased insulin resistance.73,82,84 These metabolic abnormalities mean that patients with large burn injuries have increased caloric and nutritional needs that should be addressed with early enteral feeding.26 Progressive loss of lean body mass is associated with increasing loss of function and an increased incidence of medical complications, such as pneumonia and poor wound healing.37
The management of the hypermetabolic state in large burn patients has been improved with the use of anabolic agents, β-blockers, and exercise. Oxandrolone is a synthetic testosterone analogue that has been shown to reduce mortality and length of hospital stay.135,184 Lean body mass and bone mineral density might be improved by maintaining patients on oxandrolone beyond their hospital discharge because the hypermetabolic state can persist beyond the time of wound closure. The typical dosage of oxandrolone for burn patients is 10 mg twice a day.114,117 Although human growth hormone (HGH) has shown some promise as an additional anabolic agent in burn injury, reports of increased mortality with the use of HGH in critically ill adults have limited research into the use of HGH.83,165
The stress response after burn injury is thought to be mediated by circulating catecholamines. An association of increased cortisol and catecholamine levels with infections and poor wound healing has been demonstrated in children.123 β-Blockade is helpful in managing the increased catecholamines and the stress response to burn injury. The administration of propranolol in doses high enough to reduce resting heart rate by 20% has been shown in children to reduce the metabolic rate and help preserve protein and lean body mass.72 β-Blockade might also have benefits for improving wound healing and preventing posttraumatic stress disorder (PTDS) in both adults and children.157,162
Dysfunction in insulin action is also observed as part of the metabolic response to large burn injury. The mechanism for this is not entirely clear, but it can be related to insulin antagonists emanating from inflammatory cells in the burn region.40 Patients with burn injuries greater than 40% TBSA should have close monitoring of blood glucose, with a goal of euglycemia in the acute phase. They might also need glucose monitoring for months to years after injury.62 Besides maintaining appropriate blood glucose levels, insulin has an added benefit to improving the metabolic state of the burn patient to help with minimizing lean body mass losses.81
Mobilization and exercise should be initiated early in the burn patient. An increase over time has occurred in the frequency of active and passive therapeutic interventions by the rehabilitation team in caring for acute care of burn patients.180 In the acute stages after a large burn, passive therapies, including splinting and positioning, should be initiated to minimize the risk of burn scar contracture, improve respiration, and reduce the risk of ventilator-associated pneumonias.5,66 As soon as the patient is able, however, active exercise should be started to help maintain function and to address the hypermetabolic state. Exercise is also an essential component for maximizing the benefits of anabolic hormones.163
Nutrition and Swallowing in Burns
Enteral feeding should be instituted early for patients unable to feed normally. This helps maintain gut immunity and motility, while providing the necessary calories and nutrients to counter the hypermetabolic response to burn injury.99,105 The total caloric requirements for adults with burn injuries can be estimated. Daily caloric requirements for an adult patient can be close to 25 kcal/kg plus 40 kcal/1% TBSA burn/day.33
In addition to the changes in lipid and carbohydrate metabolism seen in patients with a large burn injury, important changes in protein and amino acid metabolism are also of special importance. The administration of glutamine appears to have benefits in patients with large burn injuries and is the subject of ongoing research.133 Using body weight measurement to assess nutritional status can be difficult because of surgical procedures, dressings, and fluid changes. The serum prealbumin level can be very helpful as a marker of protein synthesis.26
Dysphagia can be a barrier to achieving adequate nutrition (see also Chapter 27). Dysphagia can develop from inhaled irritants, mechanical complications of tube placement, or neurologic injury. Larger TBSA burns, higher number of days with a tracheostomy, and higher number of days on a ventilator are all correlated with dysphagia after burn injury.39 An abnormal bedside swallowing assessment is predictive of abnormal barium swallowing studies, but there is still uncertainty regarding the best protocols for assessing swallowing in burn patients.41 Although some very complex cases might have prolonged dysphagia, studies have described between 42% and 90% of patients having normal swallowing function by the time of hospital discharge.148,177
Heterotopic Ossification
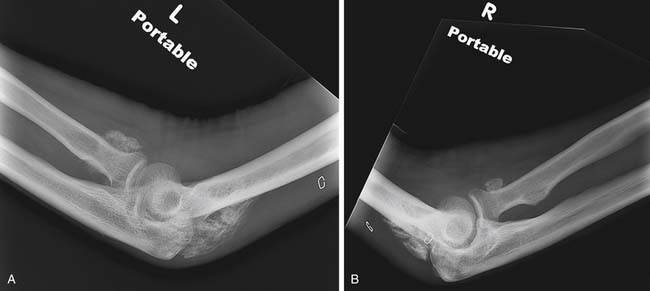
In patients with burn injuries greater than 30% TBSA, there is a risk of development of heterotopic ossification. The most common site of heterotopic ossification in burn patients is the posterior elbow (Figure 58-7). Heterotopic ossification can develop in an unburned limb but is more common in an affected limb and can be associated with delayed wound closure over the elbow.93
The best treatment and prevention for heterotopic ossification in burns is still controversial. This is particularly the case regarding the use of bisphosphonates, with some evidence suggesting etidronate is not helpful.78,154 It is possible that etidronate is ineffective because of the significant postinjury inflammatory state seen in burn injury, and higher potency bisphosphonates might be more effective.149 Some practitioners might be concerned regarding the potential development of osteonecrosis of the jaw with bisphosphonate administration, but this complication has been described primarily in patients receiving zoledronate or pamidronate in the setting of cancer treatment. No osteonecrosis was reported in osteoporosis trials using risedronate or alendronate.143 Therapies should be maintained in limbs affected by heterotopic ossification, and surgical resection should be performed when the bone is mature. Heterotopic ossification can restrict the performance of activities of daily living (see Chapter 26) or result in neurovascular compromise.78
Recurrence is common even after resection and the use of postoperative continuous passive motion. The timing of surgical resection is made based on the severity of functional deficit.78 Surgical resection, however, might have to be delayed in burns compared with other diagnoses to allow for adequate soft tissue coverage in the proposed surgical site.
Dressing Choices and Hydrotherapy
In superficial and partial-thickness wounds not treated with tangential excision and skin grafting, there are a large number of products available. Not many high quality studies have been performed on the various types of dressings that can be used for these wounds. There appears, however, to be a trend toward better healing with the use of hydrocolloid, biosynthetic, and antimicrobial dressings rather than with silver sulfadiazine dressings.178
The use of immersion hydrotherapy in burn wound care is less commonly practiced in today’s burn centers.67 Hydrotherapy tanks can be a source of gram-negative bacteria such as Pseudomonas.155 The minimization of hydrotherapy techniques has been associated with a reduction in serious skin infections in the burn unit.112 Further minimization of hydrotherapy is possible with the use of hydrocolloid and biosynthetic dressings instead of creams such as silver sulfadiazine. On the outpatient side, third-party reimbursement typically does not cover the costs for hydrotherapy. The pain associated with dressing changes can be minimized by adequate analgesia, distraction, and the use of longer-lasting dressings that reduce the frequency of changes.85,156,185
Hypertrophic Scarring
Hypertrophic scarring is the most common complication after burn injury (prevalence of 67%).19 Hypertrophic scars and keloids are both dermatoproliferative disorders of the skin. Hypertrophic scars are described as raised, red, painful, pruritic, and contractile. Hypertrophic scars stay within the margins of the original injury.44 Keloid scars have some of the same characteristics as hypertrophic scars, but they also extend beyond the original injury and invade into local soft tissues.153 Hypertrophic burn scars tend to develop in the first few months of injury, while increasing in volume and erythema. After several months they can regress, becoming less erythematous and flatter, but the skin never returns to its original state.44
Although there have been some advances in the prediction of wound healing, there is no accurate predictor of who will develop hypertrophic burn scars.145 It is thought that younger patients, particularly adolescents and those with darker skin pigmentation, tend to have a higher incidence of hypertrophic scarring.44 Wounds with a prolonged inflammatory wound healing phase and wounds that are open longer than 3 weeks are more likely to develop hypertrophic scars (Figure 58-8).10
One of the limitations of studying hypertrophic burn scarring is the lack of a widely accepted animal model for burn scarring.44 Although some progress has been made in creating thick scars in an excisional porcine wound model, there are no well-validated scar models in other species.44
Despite this, some progress has been made in the understanding of the development of hypertrophic burn scars. A key signal in scar development appears to be transforming growth factor–β(TGF-β).174 TGF-β is a ubiquitous protein and part of a larger family of proteins known as bone morphogenic proteins. It exists in a latent form that can be activated through a variety of inflammatory signals. TGF-β acts through a dimerized cell membrane receptor that activates the Smad signaling proteins to increase the expression of proteins related to increased extracellular matrix production.10,60 Beyond local inflammatory signals, there also are likely systemic effects on the development of hypertrophic burn scars from bone marrow–derived fibrocytes that become present in burn wounds and scars.176 Over time, fibroblasts can develop an autocrine feedback loop between TGF-β and the Smad proteins that might perpetuate the excessive production of extracellular matrix.186
The overexpression of extracellular matrix alone does not entirely account for the development of hypertrophic scar. A balance of both extracellular matrix formation and remodeling of the matrix and new skin is necessary for normal wound healing. In dermatoproliferative disorders, an imbalance in both production and breakdown is present. Important components in the balance of burn scarring are likely also derived from keratinocyte signals.64 As an example, keratinocyte-derived stratifin has been demonstrated to activate matrix metalloproteinases, which can be an important component of extracellular matrix breakdown and reorganization.63
Many treatment options are available for addressing the symptoms associated with hypertrophic burn scars, but none completely remove the scar. The best treatment is to prevent the scar through adequate wound care. When scars are present, early and aggressive treatment is indicated.
The first-line treatment for any burn scar is regular moisturizer cream, applied several (four to six) times per day, avoidance of mechanical insults, and the minimization of direct heat and sun exposure.15 Deficiencies in skin, such as sweat and sebaceous glands, can cause scars to be dry and pruritic, and a moisturizer cream is helpful in managing these problems.15,65 Patients with burn scars should also be instructed to minimize direct sunlight and heat exposure through the use of clothing and sunscreen.187
Pressure garments have been used in burn scar treatment for decades. They are thought to improve the appearance of burn scars by making the scar flatter and less erythematous and by offering some environmental protection.171 Some evidence exists that pressure aids in remodeling hypertrophic burn scar.31 The overall clinical effectiveness overall is controversial, however, and in metaanalysis of research into pressure garment use, the overall effect seems small, with minor benefits on scar height but not necessarily on secondary measures of scar.8 If they are used, pressure garments should be prescribed with monitoring by the rehabilitation physician or therapist for adequate fit and wear tolerance because friction in the garment can create or perpetuate superficial wounds in the burn scar.
Silicone gel sheeting can also be used alone or in combination with pressure garments. If worn for several (12 to 24) hours per day, silicone is thought to change hypertrophic burn scars through a combination of temperature and perfusion changes.118 Some evidence exists that silicone sheeting might be helpful in improving the volume in hypertrophic burn scars.116 Reviews indicate that the evidence for the use of silicone sheeting in the treatment or prevention of hypertrophic burn scars, however, is weak.124
Intralesional corticosteroid injection can also be of some limited benefit in the treatment of hypertrophic burn scar.94 The injections are done with an injection tangential to the skin. The injection is usually painful, and force is necessary to infiltrate the injectate into the fibrotic scar tissue. Some authors combine triamcinolone with other agents such as 5-fluorouracil or add a second modality such as pulsed dye laser treatment to potentiate the effects of the intralesional steroid.12,34
The use of pulsed dye laser in the treatment of hypertrophic scars is also controversial. In some studies the use of laser resulted in the formation of new wounds and little clinically relevant benefit in the scar.6,183 Other studies have shown benefit with this type of laser for the treatment of hyperpigmentation in burn scars.21 Pulsed dye laser appears to have some benefits compared with carbon dioxide or argon lasers, which have also been used to attempt to obliterate hypertrophic scars and keloids.188 Significant scar recurrence with carbon dioxide laser has been described.9 The use of pulsed dye laser in burn scar treatment is not yet routine in most burn centers in North America. Some early preclinical evidence supports the effect of interferon-α as a promising treatment for hypertrophic burn scars, but larger human studies have yet to be done.169,175
Massage is routinely applied to hypertrophic burn scars, although the evidence for its effectiveness is lacking.30,131 Massage of hypertrophic burn scars can be applied by the therapist or patient, but the skin must be monitored for potential development of pain and superficial wounds in the scar.30
Contractures
In addition to massage, it is routine for burn patients to stretch their scars. It is typical for a burn patient to report “tightness” in the scars, particularly in the morning after relative immobility. Contracture after burn injury is common, with the shoulder, elbow, and knee being the most common joints affected in patients discharged from the burn unit.151 The contractures seen in burn scars might be due to the effect of myofibroblasts and free actin in the scar.88,98 Basic research on the mechanical effects of stretch is difficult to translate into clinical practice because small forces observed in the in vitro setting would suggest that stretching can increase the risk of contracture.98 The large forces applied through splinting, positioning, and range of motion exercise, however, can likely overcome the changes seen at the cellular level to give a more acceptable clinical outcome.
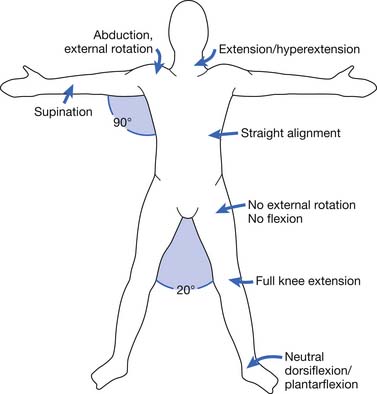
The appropriate positioning for burn patients has been described in an attempt to minimize burn scar contracture development (Figure 58-9). More burn centers are making use of appropriate positioning and splinting in the acute care setting.179 Because any anatomic site can be affected by burn injury, however, there is a need for a number of different devices. The specific features of any particular burn splint can vary significantly between centers and individual therapists.144
There might be concerns regarding the application of splints in areas of acute burn wound because of the unknown effect on the wound. In the acute setting, however, an affected area can be splinted while the patient is either sedated or asleep in an effort to minimize edema. A wrist and hand splint can be used to minimize the “intrinsic minus” position.144 When the patient’s mental status improves, the burned regions can be maintained in splints in positions of function during periods of sleep or sedation, and removed for wound checks and active motion. Splints for the “position of function” can be safely applied even over fresh skin grafts.144 A variety of splinting materials and techniques are available, none of which have been shown to be superior to others.86,144
Because of the frequency of axillary contractures, special consideration for the positioning and splinting of the axilla is warranted. Evidence supports early splinting and therapy for deep burns of the axilla to prevent contracture formation.173 Concern exists that positioning the axilla in greater than 90 degrees of abduction can result in brachial plexopathy; however, this complication has infrequently been described.
Another clinical feature of burn scars is the onset of pruritus in the subacute period. The exact mechanism of burn scar pruritus is not clear, but it might be related to increased mast cell and histamine presence in the burn scar.36 Increased nerve endings and substance P can also add to both the inflammatory state of the immature hypertrophic burn scar and the pruritic nature of scarring.152
A variety of local and systemic treatments for pruritic burn scars are available. A topical moisturizer should be applied to all burned areas several times per day. For small regions of pruritic burn scar, topical treatments such as colloidal oatmeal and topicals such as diphenhydramine, doxepin, and gabapentin can be helpful. Oral diphenhydramine, selective antihistamines, doxepin, hydroxyzine, and gabapentin have all shown some efficacy for large regions of burn scar.15 Newer studies show some evidence for the use of naltrexone as an antihistamine antipruritic agent.87,100 Nonpharmacologic treatments with some evidence for efficacy include laser, massage, and transcutaneous nerve stimulation. The evidence for nonpharmacologic treatments, however, is not as strong as for the pharmacologic approaches.15
Scar Measurement
A problem for clinicians and researchers is an adequate tool for the measurement of hypertrophic burn scars. A commonly applied tool for scar measurement is the modified Vancouver Scar Scale. The modified Vancouver Scar Scale is a subjective measurement made by a clinician that scores a scar in four domains: pliability, height, vascularity, and pigmentation.14 Although the modified Vancouver Scar Scale can be applied quickly to measure scars, the subjective nature of the scale results in questionable validity compared with measures such as volume.121
Scar pliability can be measured with a Cutometer, which is a device that measures deformity in the scar by applying a negative pressure to the scar through an aperture in a wand attachment. Scar pliability is then measured by an optical system that quantifies the amount of skin or scar brought up by the vacuum device.119
A Mexameter can be used to measure the pigmentation and vascularity of a scar. This device measures reflected light at specified wavelengths to quantify the amount of erythema as determined by the presence of hemoglobin and pigmentation as measured by the light absorbed by melanin.119
Transcutaneous ultrasound can provide measurements of scar thickness. A maximum thickness can be generated by measuring the distance from the echogenic components of skin and scar and the hypoechogenic subcutaneous fat.119
Each of these measurement tools has been shown to have superior interrater reliability than the modified Vancouver Scar Scale,119 but there are limitations to the use of these instruments. An upper limit exists on measurement with the Cutometer in very hard tissues that can be seen in hypertrophic scar.120 The erythema index used in the Mexameter might not discriminate well between normal skin and hypertrophic scar.120 More objective measures of scar will be important for future scar research.
Sleep After Burn Injury
Survivors of burn injury can have altered sleep both in the acute and rehabilitation settings. Several factors can lead to disturbed sleep in patients with large burn injuries admitted to the intensive care unit setting. The timing of procedures such as phlebotomy, surgeries, and drug administration can all lead to disruptions in sleep.57 Several environmental factors can also disrupt sleep patterns, such as inconsistent light schedules, noise from monitors, and ventilator settings.57,59 The overall clinical effects of disturbed sleep in the intensive care setting are not yet well known, but in animal model research, disturbed sleep has been correlated with immune system abnormalities in sepsis models.49,58
Sleep disturbance can continue as burn patients move from the intensive care unit to the acute care setting. Sleep can also affect burn pain level because patients with more disturbed sleep tend to require more analgesics the day after a night of abnormal sleep. It should be noted that the pain level experienced during the day might not predict a night of disturbed sleep.141,142
Although the organic implications of sleep disturbance are not entirely clear, insomnia in the hospital is associated with worse pain after hospital discharge after burn injuries.159 In one study a large proportion of burn survivors reported ongoing sleep disturbance even months after hospital discharge.101
Managing sleep disturbance for the burn patient can be divided into pharmacologic and nonpharmacologic treatments. Some combination might be necessary at different stages of recovery.80 For example, hypnotic medication might be necessary in combination with a sleep–wake ventilator setting for the patient in the intensive care setting.57 Although a rare complication of burn injury, obstructive sleep apneas should be considered in patients reporting excessive snoring, headaches, or daytime drowsiness.80 Secondary causes for sleep apnea could include the use of compressive garments or tracheal stenosis after prolonged intubation or tracheostomy.77,95
Inpatient Rehabilitation Admission for Burn Patients
Some patients require admission to an inpatient rehabilitation facility to maximize function. Patients who are admitted to inpatient rehabilitation facilities tend to be older patients and have larger burn injuries (>40% TBSA burned).158 A majority of burn centers have reported facilities available for both inpatient and outpatient burn rehabilitation services.32 Specialized rehabilitation units in the acute care setting have been shown to reduce length of hospital stay and improve function.38 Centers that have a medium to high volume of annual admissions (20+ or 100+) have a significantly higher proportion of uncomplicated discharges to home than small-volume centers.127 Although burn injury is a qualifying diagnosis for inpatient rehabilitation, it is unknown whether burn patients are sometimes discharged to nursing homes, even though inpatient rehabilitation might be more appropriate.
The Functional Independence Measure (FIM) has been used to decide who to admit to inpatient rehabilitation and to describe changes during burn rehabilitation. A FIM score in the acute care setting less than 110 has been associated with admission to an inpatient rehabilitation facility.28 In other studies using FIM as an outcome measure, however, admission FIM scores have ranged from means of 64.6 to 93, discharge FIM scores have ranged from means of 90.8 to 113, and mean days length of stay in rehabilitation has varied between 23 and 78.6.161 Although FIM is used in most rehabilitation facilities, it has not been shown to correlate with the size of burn injury, and the cognitive subdomain of the instrument might not be sensitive to change in burn injury.158 Other outcome measures have been developed and refined specific to burn injury such as the Burn-Specific Health Scale, but there is ongoing development in creating outcome measures for burn patient rehabilitation.56,90
Psychosocial Adjustment
Comprehensive interdisciplinary rehabilitation treatment of burn injuries should focus on both physical and psychologic complications.45 Burn injury survivors are at risk of developing psychopathologies such as depression and anxiety disorders.172 Depression and anxiety disorders are reported to be the most prevalent (25% to 65%) in burn survivors during the first year after injury.110,132 The largest prospective cohort study of patients with burn injury55 found that clinically significant psychologic distress is common and tends to persist for at least 2 years after burn injury in this population.
A wide discrepancy exists in the prevalence rates of depression reported across studies. Ptacek et al.138 found a 53% prevalence rate of moderate to severe symptoms of depression within 3 days of inpatient admission, which decreased to 35% by day 5 and to 15% by day 10. Although most symptoms were reported to subside after the first year postinjury,132,172 a follow-up study182 found a 53% prevalence rate of moderate to severe symptoms of depression at 1 month, 34% at 1 year, and 45% at 2 years postinjury. Another study, however, found less depression with prevalence of at least mild to moderate symptoms of depression among burn injury survivors to be between 23% and 26% during hospitalization, at 1 week postdischarge, and at 2 months postdischarge.167 No psychologic treatment studies for depression in burn survivors have been published.48 A list of risk factors for development of depression in burn survivors is presented in Box 58-1.48,52,53,182
BOX 58-1 Risk Factors for Development of Depression in Burn Survivors
In-hospital depression was found to predict change in physical health after burn injury.167 It is important to screen patients for depression, both in-hospital and during rehabilitation after discharge, because depression can have a negative impact on recovery.167
The diagnostic criteria for PTSD are presented in Box 58-2.7
BOX 58-2 Diagnostic Criteria for Posttraumatic Stress Disorder?
From American Psychiatric Association: Diagnostic and statistical manual of mental disorders: DSM-IV-TR, Washington, DC, 2000, American Psychiatric Association.
The prevalence rates for PTSD (excluding the duration criterion) were found to be 11% to 25% when validated assessment methods were used, and the rates ranged from 20% to 45% in studies using validated structured clinical interviews.48 A summary of identified risk factors for developing PTSD after a burn injury is presented in Box 58-3.48,52,54,172
No criteria exist for determining clinically significant levels of body image distress in burn patients.48 Predictors of poorer body image include burn characteristics, social and emotional variables,102 and coping style.51 Lawrence et al.103 found issues related to appearance and attractiveness are more important among female burn survivors, and body image esteem is lower among female burn survivors. Another study by Thombs et al.168 found female sex, TBSA burn, and importance of appearance predicted body image dissatisfaction. Body image dissatisfaction increased for women and individuals with larger burns from hospitalization up to 1 year, and body image dissatisfaction predicted psychosocial function at 1 year. Lawrence et al.102 found psychologic variables such as depression, self-acceptance, and social comfort to be more important than burn severity and scar location in predicting body esteem. Young burn survivors appear to cope well compared with their normal peers and in some areas can be coping better and reporting better body esteem.104,136 Routine psychologic screening for body image distress during hospitalization and after discharge is important.168
The inevitable alteration in body image and subsequent challenge to one’s self-esteem is one of the most devastating consequences of a burn injury.181 Sexuality is a complex human phenomenon that encompasses a broad range of feelings and behaviors and is more than just physical sexual behavior (see also Chapter 31).107 An individual’s expression of sexuality remains inseparable from body image and self-esteem.181 Assessment of alterations in sexual health is as important as assessments of all other areas of human function,107 but sexuality concerns of patients with burns rarely receive attention. Disfiguring scars affect sexuality because hypertrophic burn scars cause alterations in skin sensitivity.71 Other causes of sexual dysfunction in burn patients can be related to psychopathologic factors, psychodynamic factors, adverse effects of medication, and surgical and pain factors.35 After physiologic stabilization, completing a sexual health history serves to initiate a discussion of this delicate issue.181 Sexual health promotion and peer counseling are two types of interventions described in the literature.172 The treatment of patients with burns should provide the possibility of a therapeutic dialogue regarding sexuality. If that is not possible for any reason, however, the patient should be referred to a specialized counselor, sexologist, or psychiatrist as appropriate.18 It is important for the burn team to be aware that pediatric survivors of burns, even when appearing to be adjusting well, can harbor grave self-deprecating feelings.2 Other helpful avenues valuable for exchanging information and experience18 include self-help groups, face-to-face peer counseling, and professionally facilitated self-help by mail.25
Individuals suffering from altered body image report social functioning as a major area of difficulty causing considerable distress.122 Every person who has been touched by the experience of facial disfigurement (survivors, family members, and professionals caring for these individuals) is well aware of the challenges encountered in the course of daily life.137 A study investigating psychosocial adjustment of patients 5 years after their burn injury found social reintegration of burn survivors with poor physical function unsuccessful, with feelings of social isolation, increased depression, and loss in circle of friends.128 Individuals with clearly apparent burn scars appeared less frequently in public and also reported significant impairments in sexuality.128 Social introversion is significantly associated with the development of burn-related pathologic feelings of shame.164 Problems with social interaction include difficulties in meeting new people, making friends, and developing intimate relationships.130,146 Commonly reported reactions in public include stares, startle reactions, remarks, and personal questions.109,160
Community Integration
The long-term psychosocial adaptation of burn survivors depends largely on their successful integration into community life.17 Community integration is an important aspect of every burn rehabilitation program48 because the ultimate goal is to return the patient as close as possible to preinjury life (i.e., work, school, home, recreational, and community activities). Burn survivors have difficulty in the area of home integration, social integration, and productivity.47
Because a large number of burn injuries happen either at work or to individuals of working age, return to work is of particular concern for the patient and the burn rehabilitation team (see also Chapter 35). People with burn injuries who were employed at the time of injury are more likely to sustain hand burns and have hand surgery than the unemployed.50 The size of the burn is inversely related to the probability of returning to work, and most burn survivors are able to return to work at an average of 17 weeks after injury.23 A study identifying barriers to return to work after burn injuries found that although about 80% of patients had returned to work by 1 year, more than 30% of them had long-term disabilities.46 Risk factors identified23,150 for longer time off from work or no return to work, and barriers46,150 to return to work after a major burn injury are listed in Box 58-4.
Early identification of those at risk for prolonged unemployment, referral to comprehensive rehabilitative services that include work hardening and vocational training programs, assessment of the workplace, and identification of appropriate workplace accommodations are important steps to facilitate a quicker and successful return to work.46,150
In school-aged patients surviving burn injuries, returning to school is an important indicator of functional aptitude and emotional adjustment.29 Male gender, age, and length of hospitalization were found significantly associated with longer time to return to school. The average time to school reentry was 10.5 days.29
According to burn survivors, the single most important issue is the social anxiety and social strain accompanying community integration.16 In the area of social adjustment, social skills training should be considered as a promising intervention to prevent social anxiety among those who suffer from interpersonal difficulties.17,172
Two well-known programs have been developed in the area of social skills training. One of them is Changing Faces,130 a charitable organization in the United Kingdom founded by James Partridge, a burn survivor. The program focuses on scientific documentation of the clinical efficacy of its interventions137 and includes life skills for children, young people, adults, and families to develop self-confidence and self-esteem. A video entitled REACH OUT (acronym for Reassurance, Effort, Assertiveness, Courage, Humor, Over There (Distraction), Understanding, and Try Again) shows a variety of communication skills to help people cope with self-consciousness and other people’s reactions to their appearance. The “3-2-1 Go” strategy includes strategies such as 3 things to do if someone stares at them, 2 things to say if someone asks what happened, and 1 thing to think if someone turns away. The second program available is Behavior Skills and Image Enhancement Program, also known as the BEST program, and was developed in the United States by Barbara Kammerer-Quayle, a burn survivor. This program is offered through the Phoenix Society for Burn Survivors (Grand Rapids, MI). The program includes methods and strategies to enable burn survivors and their families to make an optimal transition back to their communities. STEPS (an acronym for Self-Talk, Tone of Voice, Eye Contact, Posture, Smile) to social comfort and confidence; behavioral skills to manage stares, questions, and teasing; corrective cosmetic application; and color analysis techniques are taught to children, teens, and adults.140
It is encouraging to note the formation of a national Aftercare Reintegration Committee (ARC), a joint committee of the ABA (the national organization for burn care providers) and the Phoenix Society for Burn Survivors (the national organization of burn survivors and their caregivers). ARC’s mission is to “… coordinate the efforts of the ABA and the Phoenix Society to establish standards of aftercare for those affected by burn trauma in the areas of rehabilitation and reintegration.” A survivor-focused consensus conference (March 27 to 29, 2008 at the John E. Fetzer Institute in Kalamazoo, MI.) held by the ARC established a psychosocial rehabilitation research agenda concerning the key areas of peer support, body image, and social skills training.3 Bringing together burn survivors, clinical providers, and researchers to set clear research priorities and active working groups to help establish an evidence base for raising the standard of psychosocial care for burn survivors3 is clearly a movement in the right direction.
1. Aarabi S., Longaker M.T., Gurtner G.C. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PloS Med. 2007;4:1464-1470.
2. Abdullah A., Blakeney P., Hunt R., et al. Visible scars and self-esteem in pediatric patients with burns. J Burn Care Rehabil. 1994;15(2):164-168.
3. Acton A., Badger K., Fauerbach J.A., et al. A survivor-focused consensus conference: establishing a psychosocial rehabilitation research agenda. J Burn Care Res. 2009;30(2):S148.
4. Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32(4):259-289.
5. Alexiou VG, Ierodiakonou V, Dimopoulos G, et al. Impact of patient position on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. J Crit Care 24(4):515-522, 2009.
6. Allison K.P., Kiernan M.N., Waters R.A., et al. Pulsed dye laser treatment of burn scars: alleviation or irritation? Burns. 2003;29(3):207-213.
7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
8. Anzarut A., Olson J., Singh P., et al. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis, J.Plast Reconstr Aesthet Surg 62(1):77-84. 2009.
9. Apfelberg D.B., Maser M.R., White D.N., et al. Failure of carbon-dioxide laser excision of keloids. Lasers Surg Med. 1989;9(4):382-388.
10. Armour A., Scott P.G., Tredget E.E. Cellular and molecular pathology of HTS: basis for treatment. Wound Repair Regen. 2007;15:S6-S17.
11. Arnoldo B.D., Purdue G.F., Kowalske K., et al. Electrical injuries: a 20-year review. J Burn Care Rehabil. 2004;25(6):479-484.
12. Asilian A., Darougheh A., Shariati F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32(7):907-915.
13. Atiyeh B.S., Gunn S.W.A., Dibo S.A. Metabolic implications of severe burn injuries and their management: a systematic review of the literature. World J Surg. 2008;32(8):1857-1869.
14. Baryza M.J., Baryza G.A. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16(5):535-538.
15. Bell P.L., Gabriel V. Evidence based review for the treatment of post-burn pruritus. J Burn Care Res. 2009;30(1):55-61.
16. Blakeney P., Partridge J., Rumsey N. Community integration. J Burn Care Res. 2007;28(4):598-601.
17. Blakeney P., Thomas C., Holzer C.3rd, et al. Efficacy of a short-term, intensive social skills training program for burned adolescents. J Burn Care Rehabil. 2005;26(6):546-555.
18. Bogaerts F., Boeckx W. Burns and sexuality. J Burn Care Rehabil. 1992;13(1):39-43.
19. Bombaro K.M., Engrav L.H., Carrougher G.J., et al. What is the prevalence of hypertrophic scarring following burns? Burns. 2003;29(4):299-302.
20. Boozalis G.T., Purdue G.F., Hunt J.L., et al. Ocular changes from electrical burn injuries: a literature review and report of cases. J Burn Care Rehabil. 1991;12(5):458-462.
21. Bowes L.E., Nouri K., Berman B., et al. Treatment of pigmented hypertrophic scars with the 585 nm pulsed dye laser and the 532 nm frequency-doubled ND: yag laser in the Q-switched and variable pulse modes: a comparative study. Dermatol Surg. 2002;28(8):714-719.
22. Brooks A., Holroyd B., Riley B. Missed injury in major trauma patients. Injury. 2004;35(4):407-410.
23. Brych S.B., Engrav L.H., Rivara F.P., et al. Time off work and return to work rates after burns: systematic review of the literature and a large two-center series. J Burn Care Rehabil. 2001;22(6):401-405.
24. Burnsworth B., Krob M.J., Langer-Schnepp M. Immediate ambulation of patients with lower-extremity grafts. J Burn Care Rehabil. 1992;13(1):89-96.
25. Cahners S.S. Young women with breast burns: A self-help “group by mail”. J Burn Care Rehabil. 1992;13(1):44-47.
26. Chan M.M., Chan G.M. Nutritional therapy for burns in children and adults. Nutrition. 2009;25(3):261-269.
27. Cherington M. Spectrum of neurologic complications of lightning injuries. Neurorehabilitation. 2005;20(1):3-8.
28. Choo B., Umraw N., Gomez M., et al. The utility of the functional independence measure (FIM) in discharge planning for burn patients. Burns. 2006;32(1):20-23.
29. Christiansen M., Carrougher G.J., Engrav L.H., et al. Time to school re-entry after burn injury is quite short. J Burn Care Res. 2007;28(3):478-481. discussion 482–473
30. Claude R. Massage applied to scars. Wound Repair Regen. 2002;10(2):126-128.
31. Costa AMA, Peyrol S, Porto LC, et al. Mechanical forces induce scar remodeling: study in non-pressure-treated versus pressure-treated hypertrophic scars, Am J Pathol 155(5):1671-1679, 1999.
32. Cromes G.F., Helm P.A. The status of burn rehabilitation services in the united states: results of a national survey. J Burn Care Rehabil. 1992;13(6):656-662.
33. Curreri P.W., Richmond D., Marvin J., et al. Dietary requirements of patients with major burns. J Am Diet Assoc. 1974;65(4):415-417.
34. Darougheh A., Asilian A., Shariati F. Intralesional triamcinolone alone or in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. Clin Exp Dermatol. 2009;34(2):219-223.
35. de Rios M.D., Novac A., Achauer B.H. Sexual dysfunction and the patient with burns. J Burn Care Rehabil. 1997;18(1 Pt 1):37-42.
36. Demling R.H., DeSanti L. Topical doxepin significantly decreases itching and erythema in the chronically pruritic burn scar. Wounds Compend Clin Res Pract. 2003;15(6):195-200.
37. Demling R.H., Seigne P. Metabolic management of patients with severe burns. World J Surg. 2000;24(6):673-680.
38. DeSanti L., Lincoln L., Egan F., et al. Development of a burn rehabilitation unit: impact on burn center length of stay and functional outcome. J Burn Care Rehabil. 1998;19(5):414-419.
39. DuBose C.M., Groher M.G., Mann G.C., et al. Pattern of dysphagia recovery after thermal burn injury. J Burn Care Rehabil. 2005;26(3):233-237.
40. Duffy S.L., Lagrone L., Herndon D.N., et al. Resistin and postburn insulin dysfunction. J Trauma. 2009;66(1):250-254.
41. Edelman D.A., Sheehy-Deardorff D.A., White M.T. Bedside assessment of swallowing is predictive of an abnormal barium swallow examination. J Burn Care Res. 2008;29(1):89-96.
42. Enderson B.L., Reath D.B., Meadors J., et al. The tertiary trauma survey – a prospective-study of missed injury. J Trauma. 1990;30(6):666-670.
43. Endorf F.W., Klein M.B., Mack C.D., et al. Necrotizing soft-tissue infections: differences in patients treated at burn centers and non-burn centers. J Burn Care Res. 2008;29(6):933-938.
44. Engrav L.H., Garner W.L., Tredget E.E. Hypertrophic scar, wound contraction and hyper-hypopigmentation. J Burn Care Res. 2007;28(4):593-597.
45. Esselman P.C. Burn rehabilitation: an overview. Arch Phys Med Rehabil. 2007;88(12 suppl 2):S3-S6.
46. Esselman P.C., Askay S.W., Carrougher G.J., et al. Barriers to return to work after burn injuries. Arch Phys Med Rehabil. 2007;88(12 suppl 2):S50-S56.
47. Esselman P.C., Ptacek J.T., Kowalske K., et al. Community integration after burn injuries. J Burn Care Rehabil. 2001;22(3):221-227.
48. Esselman P.C., Thombs B.D., Magyar-Russell G., et al. Burn rehabilitation: state of the science. Am J Phys Med Rehabil. 2006;85(4):383-413.
49. Everson CA, Thalacker CD, Hogg N. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery, Am J Physiol Regul Integt Comp Physiol 295(6): R2067-R2074, 2008.
50. Fauerbach J.A., Engrav L., Kowalske K., et al. Barriers to employment among working-aged patients with major burn injury. J Burn Care Rehabil. 2001;22(1):26-34.
51. Fauerbach J.A., Heinberg L.J., Lawrence J.W., et al. Coping with body image changes following a disfiguring burn injury. Health Psychol. 2002;21(2):115-121.
52. Fauerbach J.A., Lawrence J., Haythornthwaite J., et al. Preburn psychiatric history affects posttrauma morbidity. Psychosomatics. 1997;38(4):374-385.
53. Fauerbach J.A., Lawrence J.W., Bryant A.G., et al. The relationship of ambivalent coping to depression symptoms and adjustment. Rehabil Psychol. 2002;47(4):387-401.
54. Fauerbach J.A., Lawrence J.W., Schmidt C.W.Jr., et al. Personality predictors of injury-related posttraumatic stress disorder. J Nerv Ment Dis. 2000;188(8):510-517.
55. Fauerbach J.A., McKibben J., Bienvenu O.J., et al. Psychological distress after major burn injury. Psychosom Med. 2007;69(5):473-482.
56. Forbes-Duchart L., Cooper J., Nedelec B., et al. Burn therapists’ opinion on the application and essential characteristics of a burn scar outcome measure. J Burn Care Res. 2009;30(5):792-800.
57. Friese R.S. Sleep and recovery from critical illness and injury: a review of theory, current practice, and future directions. Crit Care Med. 2008;36(3):697-705.
58. Friese R.S., Bruns B., Sinton C.M. Sleep deprivation after septic insult increases mortality independent of age. J Trauma. 2009;66(1):50-54.
59. Gabor J.Y., Cooper A.B., Hanly P.J. Sleep disruption in the intensive care unit. Curr Opin Crit Care. 2001;7(1):21-27.
60. Gabriel V. Transforming growth factor-beta and angiotensin in fibrosis and burn injuries. J Burn Care Res. 2009;30(3):471-481.
61. Gabriel V., Kowalske K.J., Holavanahalli R.K. Assessment of recovery from burn-related neuropathy by electrodiagnostic testing. J Burn Care Res. 2009;30(4):668-693.
62. Gauglitz G.G., Herndon D.N., Kulp G.A., et al. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94(5):1656-1664.
63. Ghaffari A., Li Y.Y., Karami A., et al. Fibroblast extracellular matrix gene expression in response to keratinocyte-releasable stratifin. J Cell Biochem. 2006;98(2):383-393.
64. Ghahary A., Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15:S46-S53.
65. Ghahary A., Shen Y.J., Wang R.J., et al. Expression and localization of insulin-like growth factor-1 in normal and post-burn hypertrophic scar tissue in human. Mol Cell Biochem. 1998;183(1-2):1-9.
66. Giuliani C.A., Perry G.A. Factors to consider in the rehabilitation aspect of burn care. Phys Ther. 1985;65(5):619-623.
67. Gomez M., Cartotto R., Knighton J., et al. Improved survival following thermal injury in adult patients treated at a regional burn center. J Burn Care Res. 2008;29(1):130-137.
68. Gore D.C., Hawkins H.K., Chinkes D.L., et al. Assessment of adverse events in the demise of pediatric burn patients. J Trauma. 2007;63:814-818.
69. Handschin A.E., Vetter S., Jung F.J., et al. A case-matched controlled study on high-voltage electrical injuries vs thermal burns. J Burn Care Res. 2009;30(3):400-407.
70. Hasanzadeh P., Oveisgharan S., Sedighi N., et al. Effect of skin thickness on sensory nerve action potential amplitude. Clin Neurophysiol. 2008;119(8):1824-1828.
71. Helm P.A. Burn rehabilitation: Dimensions of the problem. Clin Plast Surg. 1992;19(3):551-559.
72. Herndon D.N., Hart D.W., Wolf S.E., et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345(17):1223-1229.
73. Herndon D.N., Tompkins R.G. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895-1902.
74. Hettiaratchy S., Papini R. Abc of burns: initial management of a major burn. II. A assessment and resuscitation. BMJ. 2004;329(7457):101-103.
75. Hollingsed TC, Saffle JR, Barton RG, et al: etiology and consequences of respiratory-failure in thermally injured patients, Am J Surg 166(6):592-596, 1993.
76. Holmes J.H. Critical issues in burn care. J Burn Care Res. 2008;29(6):S180-S187.
77. Hubbard M., Masters I.B., Williams G.R., et al. Severe obstructive sleep apnoea secondary to pressure garments used in the treatment of hypertrophic burn scars. Eur Resp J. 2000;16(6):1205-1207.
78. Hunt J.L., Arnoldo B.D., Kowalske K., et al. Heterotopic ossification revisited: a 21-year surgical experience. J Burn Care Res. 2006;27(4):535-540.
79. Ibebunjo C., Martyn J. Disparate dysfunction of skeletal muscles located near and distant from burn site in the rat. Muscle Nerve. 2001;24(10):1283-1294.
80. Jaffe S.E., Patterson D.R. Treating sleep problems in patients with burn injuries: practical considerations. J Burn Care Rehabil. 2004;25(3):294-305.
81. Jeschke M.G., Boehning D.F., Finnerty C.C., et al. Effect of insulin on the inflammatory and acute phase response after burn injury. Crit Care Med. 2007;35(uppl 9):S519-S523.
82. Jeschke M.G., Chinkes D.L., Finnerty C.C., et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387-400.
83. Jeschke M.G., Finnerty C.C., Kulp G.A., et al. Combination of recombinant human growth hormone and propranolol decreases hypermetabolism and inflammation in severely burned children. Ped Crit Care Med. 2008;9(2):209-216.
84. Jeschke M.G., Mlcak R.P., Finnerty C.C., et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007:11.
85. Jones M.L. Minimising pain at dressing changes. Nurs Stand. 2004;18(24):65-68. 70
86. Jordan R.B., Daher J., Wasil K. Splints and scar management for acute and reconstructive burn care. Clin Plast Surg. 2000;27(1):71-85.
87. Jung S.I., Seo C.H., Jang K., et al. Efficacy of naltrexone in the treatment of chronic refractory itching in burn patients: preliminary report of an open trial. J Burn Care Res. 2009;30(2):257-260.
88. Junker J.P.E., Kratz C., Tollback A., et al. Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns. 2008;34(7):942-946.
89. Kamolz L.-P., Kitzinger H.B., Karle B., et al. The treatment of hand burns. Burns. 2009;35(3):327-337.
90. Kildal M., Andersson G., Fugl-Meyer A.R., et al. Development of a brief version of the Burn Specific Health Scale (BSHS-B). J Trauma. 2001;51(4):740-746.
91. Klein L., Dousa P., Zajicek R., et al. Specific aspects of the treatment of patients with multiple mechanical and burn injuries. Acta Chir Plast. 2008;50(1):17-22.
92. Klein MB, Lezotte DL, Fauerbach JA, et al: The National Institute on Disability and Rehabilitation Research Burn Model system database: a tool for the multicenter study of the outcome of burn injury, J Burn Care Res 28(1):84-96, 2007.
93. Klein M.B., Logsetty S., Costa B., et al. Extended time to wound closure is associated with increased risk of heterotopic ossification of the elbow. J Burn Care Res. 2007;28(3):447-450.
94. Koc E., Arca E., Surucu B., et al. An open, randomized, controlled, comparative study of the combined effect of intralesional triamcinolone acetonide and onion extract gel and intralesional triamcinolone acetonide alone in the treatment of hypertrophic scars and keloids. Dermatol Surg. 2008;34(11):1507-1514.
95. Koshkareva Y., Gaughan J.P., Soliman A.M.S. Risk factors for adult laryngotracheal stenosis: a review of 74 cases. Ann Otol Rhinol Laryngol. 2007;116(3):206-210.
96. Koumbourlis A.C. Electrical injuries. Crit Care Med. 2002;30(11):S424-S430.
97. Kowalske K., Holavanahalli R., Helm P. Neuropathy after burn injury. J Burn Care Rehabil. 2001;22(5):353-357.
98. Kratz C., Tollback A., Kratz G. Effects of continuous stretching on cell proliferation and collagen synthesis in human burn scars. Scand J Plast Reconstr Surg Hand Surg. 2001;35(1):57-63.
99. Lam N.N., Tien N.G., Khoa C.M. Early enteral feeding for burned patients: an effective method which should be encouraged in developing countries. Burns. 2008;34(2):192-196.
100. LaSalle L., Rachelska G., Nedelec B. Naltrexone for the management of post-burn pruritus: a preliminary report. Burns. 2008;34(6):797-802.
101. Lawrence J.W., Fauerbach J., Eudell E., et al. The 1998 clinical research award: sleep disturbance after burn injury: A frequent yet understudied complication. J Burn Care Rehabil. 1998;19(6):480-486.
102. Lawrence J.W., Fauerbach J.A., Heinberg L., et al. Visible vs hidden scars and their relation to body esteem. J Burn Care Rehabil. 2004;25(1):25-32.
103. Lawrence J.W., Fauerbach J.A., Thombs B.D. A test of the moderating role of importance of appearance in the relationship between perceived scar severity and body-esteem among adult burn survivors. Body Image. 2006;3(2):101-111.
104. Lawrence J.W., Rosenberg L.E., Fauerbach J.A. Comparing the body esteem of pediatric survivors of burn injury with the body esteem of an age-matched comparison group without burns. Rehabil Psychol. 2007;52(4):370-379.
105. Lee J.O., Benjamin D., Herndon D.N. Nutrition support strategies for severely burned patients. Nutr Clin Pract. 2005;20(3):325-330.
106. Lee M.Y., Liu G., Kowlowitz V., et al. Causative factors affecting peripheral neuropathy in burn patients. Burns. 2009;35(3):412-416.
107. LeMone P., Jones D. Nursing assessment of altered sexuality: a review of salient factors and objective measures. Nurs Diagn. 1997;8(3):120-128.
108. Lundgren R.S., Kramer C.B., Rivara F.P., et al. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30(2):307-314.
109. Macgregor F.C. Facial disfigurement: Problems and management of social interaction and implications for mental health. Aesthetic Plast Surg. 1990;14(4):249-257.
110. Malt U. Long-term psychosocial follow-up studies of burned adults: review of the literature. Burns. 1980;6(3):190-197.
111. Martyn J.A.J., Fagerlund M.J., Eriksson L.I. Basic principles of neuromuscular transmission. Anaesthesia. 2009;64(Suppl 1):1-9.
112. Mayhall C.G. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37(4):543-550.
113. Mazzetto-Betti K.C., Amâncio A.C., Farina J.A.Jr., et al. High-voltage electrical burn injuries: functional upper extremity assessment. Burns. 2009;35:707-713.
114. Miller J.T., Btaiche I.F. Oxandrolone treatment in adults with severe thermal injury. Pharmacotherapy. 2009;29(2):213-226.
115. Miller S.F., Bessey P.Q., Schurr M.J., et al. National burn repository 2005: a ten-year review. J Burn Care Res. 2006;27(4):411-436.
116. Momeni M., Hafezi F., Rahbar H., et al. Effects of silicone gel on burn scars. Burns. 2009;35(1):70-74.
117. Murphy K.D., Thomas S., Mlcak R.P., et al. Effects of long-term oxandrolone administration in severely burned children. Surgery. 2004;136(2):219-224.
118. Musgrave M.A., Umraw N., Fish J.S., et al. The effect of silicone gel sheets on perfusion of hypertrophic burn scars. J Burn Care Rehabil. 2002;23(3):208-214.
119. Nedelec B., Correa J.A., Rachelska G., et al. Quantitative measurement of hypertrophic scar: interrater reliability and concurrent validity. J Burn Care Res. 2008;29(3):501-511.
120. Nedelec B., Correa J.A., Rachelska G., et al. Quantitative measurement of hypertrophic scar: intrarater reliability, sensitivity, and specificity. J Burn Care Res. 2008;29(3):489-500.
121. Nedelec B., Shankowsky H.A., Tredget E.E. Rating the resolving hypertrophic scar: comparison of the Vancouver Scar Scale and scar volume. J Burn Care Rehabil. 2000;21(3):205-212.
122. Newell R.J. Altered body image: a fear-avoidance model of psycho-social difficulties following disfigurement. J Adv Nurs. 1999;30(5):1230-1238.
123. Norbury W.B., Herndon D.N., Branski L.K., et al. Urinary cortisol and catecholamine excretion after burn injury in children. J Clin Endocrinol Metab. 2008;93(4):1270-1275.
124. O’Brien L., Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Sys Rev. 2006;25(1):CD003826.
125. Offiah C., Heran M., Graeb D. Lightning strike: a rare cause of bilateral ossicular disruption. Am J Neuroradiol. 2007;28(5):974-975.
126. Orgill D.P. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360(9):893-901.
127. Pacella S.J., Butz D.A., Comstock M.C., et al. Hospital volume outcome and discharge disposition of burn patients. Plast Reconstr Surg. 2006;117(4):1296-1305.
128. Pallua N., Kunsebeck H.W., Noah E.M. Psychosocial adjustments 5 years after burn injury. Burns. 2003;29(2):143-152.
129. Palmieri T.L., Warner P., Mlcak R.P., et al. Inhalation injury in children: A 10 year experience at Shriners Hospitals for Children. J Burn Care Res. 2009;30(1):206-208.
130. Partridge J. About changing faces: Promoting a good quality of life for people with visible disfigurements. Burns. 1997;23(2):186-187.
131. Patino O., Novick C., Merlo A., et al. Massage in hypertrophic scars. J Burn Care Rehabil. 1999;20(3):268-271.
132. Patterson D.R., Everett J.J., Bombardier C.H., et al. Psychological effects of severe burn injuries. Psychol Bull. 1993;113(2):362-378.
133. Peng X., Yan H., You Z.Y., et al. Clinical and protein metabolic efficacy of glutamine granules-supplemented enteral nutrition in severely burned patients. Burns. 2005;31(3):342-346.
134. Pereira C., Murphy K., Herndon D. Outcome measures in burn care: Is mortality dead? Burns. 2004;30(8):761-771.
135. Pham T.N., Klein M.B., Gibran N.S., et al. Impact of oxandrolone treatment on acute outcomes after severe burn injury. J Burn Care Res. 2008;29(6):902-906.
136. Pope S.J., Solomons W.R., Done D., et al. Body image, mood and quality of life in young burn survivors. Burns. 2007;33(6):747-755.
137. Pruzinsky T. Rehabilitation challenges for burn survivors with residual disfigurement: promising directions for intervention, research, and collaboration. J Burn Care Rehabil. 1998;19(2):169-173.
138. Ptacek J.T., Patterson D.R., Heimbach D.M. Inpatient depression in persons with burns. J Burn Care Rehabil. 2002;23(1):1-9.
139. Purdue G.F. To trach or not to trach. J Burn Care Res. 2009;30(1):192-193.
140. Quayle B. Behavioral skills and image enhancement training for burn survivors: essential interventions for improving quality of life and community integration. In: Sood R., Achauer B.M., editors. Achauer and Sood’s burn surgery: Reconstruction and rehabilitation. Philadelphia: Saunders Elsevier, 2006.
141. Raymond I., Ancoli-Israel S., Choiniere M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5(6):551-559.
142. Raymond I., Nielsen T.A., Lavigne G., et al. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;92(3):381-388.
143. Reid I.R. Osteonecrosis of the jaw: who gets it, and why? Bone. 2009;44(1):4-10.
144. Richard R., Ward R.S. Splinting strategies and controversies. J Burn Care Rehabil. 2005;26(5):392-396.
145. Riordan CL, McDonough M, Davidson JM, et al: Noncontact laser Doppler imaging in burn depth analysis of the extremities, J Burn Care Rehabil 24:177-186, 2003.
146. Robinson E., Rumsey N., Partridge J. An evaluation of the impact of social interaction skills training for facially disfigured people. Br J Plast Surg. 1996;49(5):281-289.
147. Rosenkranz K.M., Sheridan R. Management of the burned trauma patient: balancing conflicting priorities. Burns. 2002;28(7):665-669.
148. Rumbach A.F., Ward E.C., Cornwell P.L., et al. The challenges of dysphagia management and rehabilitation after extensive thermal burn injury: a complex case. J Burn Care Res. 2009;30(5):901-905.
149. Russell R.G.G., Watts N.B., Ebetino F.H., et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19(6):733-759.
150. Schneider J.C., Bassi S., Ryan C.M. Barriers impacting employment after burn injury. J Burn Care Res. 2009;30(2):294-300.
151. Schneider J.C., Holavanahalli R., Heim P., et al. Contractures in burn injury: defining the problem. J Burn Care Res. 2006;27(4):508-514.
152. Scott JR, Muangman PR, Tamura RN, et al. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar, Plastic Reconstr Surg 115(4):1095-1102, 2005.
153. Seifert O., Mrowietz U. Keloid scarring: bench and bedside. Arch Dermatol Res. 2009;301(4):259-272.
154. Shafer D.M., Bay C., Caruso D.M., et al. The use of eidronate disodium in the prevention of heterotopic ossification in burn patients. Burns. 2008;34(3):355-360.
155. Shankowsky H.A., Callioux L.S., Tredget E.E. North American survey of hydrotherapy in modern burn care. J Burn Care Rehabil. 1994;15(2):143-146.
156. Sharar S.R., Miller W., Teeley A., et al. Applications of virtual reality for pain management in burn-injured patients. Expert Rev Neurother. 2008;8(11):1667-1674.
157. Sivamani R.K., Pullar C.E., Manabat-Hidalgo C.G., et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PloS Med. 2009;6(1):105-115.
158. Sliwa J.A., Heinemann A., Semik P. Inpatient rehabilitation following burn injury: patient demographics and functional outcomes. Arch Phys Med Rehabil. 2005;86(10):1920-1923.
159. Smith M.T., Klick B., Kozachik S., et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138(3):497-506.
160. Solomon S.E. On an island by myself: Women of color with facial distinctions. J Burn Care Rehabil. 1998;19(3):268-278.
161. Spires M.C., Bowden M.L., Ahrns K.S., et al. Impact of an inpatient rehabilitation facility on functional outcome and length of stay of burn survivors. J Burn Care Rehabil. 2005;26(6):532-538.
162. Strawn J.R., Geracioti T.D. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
163. Suman O.E., Thomas S.J., Wilkins J.P., et al. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol. 2003;94(6):2273-2281.
164. Taal L., Faber A.W. Posttraumatic stress and maladjustment among adult burn survivors 1 to 2 years postburn. II. The interview data. Burns. 1998;24(5):399-405.
165. Takala J., Ruokonen E., Webster N.R., et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341(11):785-792.
166. Theman K., Singerman J., Gomez M., et al. Return to work after low voltage electrical injury. J Burn Care Res. 2008;29(6):959-964.
167. Thombs B.D., Bresnick M.G., Magyar-Russell G., et al. Symptoms of depression predict change in physical health after burn injury. Burns. 2007;33(3):292-298.
168. Thombs B.D., Notes L.D., Lawrence J.W., et al. From survival to socialization: a longitudinal study of body image in survivors of severe burn injury. J Psychosom Res. 2008;64(2):205-212.
169. Tredget E., Wang J., Jiao H., et al. Decreased fibrocytes in post-burn hypertrophic scar after treatment with interferon alpha-2b. Wound Repair Regen. 2008;16(2):126.
170. Tredget E.E., Shankowsky H.A., Taerum T.V., et al. The role of inhalation injury in burn trauma: a Canadian experience. Ann Surg. 1990;212(6):720-727.
171. Van den Kerckhove E., Stappaerts K., Fieuws S., et al. The assessment of erythema and thickness on burn related scars during pressure garment therapy as a preventive measure for hypertrophic scarring. Burns. 2005;31(6):696-702.
172. Van Loey N.E.E., Van Song M.J.M. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4(4):245-272.
173. Vehmeyer-Heeman M., Lommers B., Van den Kerckhove E., et al. Axillary burns: extended grafting and early splinting prevents contractures. J Burn Care Rehabil. 2005;26(6):539-542.
174. Wang J., Jiao H., Stewart T.L., et al. Increased TGF-beta-producing CD4+ T lymphocytes in postburn patients and their potential interaction with dermal fibroblasts in hypertrophic scarring. Wound Repair Regen. 15(6), 2007. 940–940
175. Wang J.F., Chen H., Shankowsky H.A., et al. Improved scar in postburn patients following interferon-alpha 2b treatment is associated with decreased angiogenesis mediated by vascular endothelial cell growth factor. J Interferon Cytokine Res. 2008;28(7):423-434.
176. Wang J.F., Jiao H.Y., Stewart T.L., et al. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15(1):113-121.
177. Ward E.C., Uriarte M., Conroy A.L. Duration of dysphagic symptoms and swallowing outcomes after thermal burn injury. J Burn Care Rehabil. 2001;22(6):441-453.
178. Wasiak J., Cleland H., Campbell F. Dressings for superficial and partial thickness burns. Cochrane Database Sys Rev. 2008;8(4):CD002106.
179. Whitehead C, Serghiou M. A 12-year comparison of common therapeutic interventions in the burn unit 30(2):281-287, 2009.
180. Whitehead C., Serghiou M.A. 12-year comparison of common therapeutic interventions in the burn unit. J Burn Care Res. 2009;30(2):281-287.
181. Whitehead T.L. Sexual health promotion of the patient with burns. J Burn Care Rehabil. 1993;14(2 Pat 1):221-226.
182. Wiechman S.A., Ptacek J.T., Patterson D.R., et al. Rates, trends, and severity of depression after burn injuries. J Burn Care Rehabil. 2001;22(6):417-424.
183. Wittenberg GP, Fabian BG, Bogomilsky JL, et al: Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment, Arch Dermatol 135:1049-1055, 1999.
184. Wolf S.E., Edelman L.S., Kemalyan N., et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res. 2006;27(2):131-139.
185. Wyatt D., McGowan D.N., Najarian M.P. Comparison of a hydrocolloid dressing and silver sulfadiazine cream in the outpatient management of 2nd-degree burns. J Trauma. 1990;30(7):857-865.
186. Xie J.L., Qi S.H., Pan S., et al. Expression of Smad protein by normal skin fibroblasts and hypertrophic scar fibroblasts in response to transforming growth factor beta 1. Dermatol Surg. 2008;34(9):1216-1225.
187. Zeitlin R.E.K., Jarnberg J., Somppi E.J., et al. Long-term functional sequelae after paediatric burns. Burns. 1998;24(1):3-6.
188. Ziegler U.E. International clinical recommendations on scar management. Zentralblatt Chirurgie. 2004;129(4):296-306.