Chapter 77 Budd-Chiari syndrome and venoocclusive disease
Overview
Budd-Chiari syndrome (BCS) is a group of disorders caused by occlusion of the major hepatic veins or the inferior vena cava (IVC) or both at or near the level of the hepatic vein ostia. Although a brief discussion of these disorders first appeared in a book by Budd in 1845, Lambron in 1842 is said to have reported the first case. In 1899, Chiari collected 10 cases and reported three personal cases and presented the first thorough clinical and pathologic description of the syndrome, including the hypothesis that the underlying mechanism is endophlebitis of the hepatic veins. The weight of evidence favors the current opinion, however, that the primary process is usually thrombotic rather than inflammatory. Since publication of the initial description, more than 8000 cases of BCS have been described in the medical literature. In recent years, the incidence has increased substantially, most likely as a result of increased awareness of BCS, improvements in diagnostic methods, and widespread use of thrombogenic agents, such as oral contraceptives (Maddrey, 1987; Valla et al, 1986). Nevertheless, BCS remains a relatively uncommon condition.
Predisposing Conditions for Budd-Chiari Syndrome
Box 77.1 lists the specific conditions that are known to predispose to the development of BCS and VOD. Over the past 50 years, a marked change has been observed in the frequency with which a known cause or predisposing condition has been identified in cases of BCS. In the classic collective review of 164 cases of BCS reported by Parker in 1959, a predisposing condition or etiology could not be identified in 70% of the patients. In recent years, the incidence of idiopathic cases of BCS has plummeted to less than 30% (Mahmoud et al, 1996; Menon et al, 2004; Mitchell et al, 1982; Murad et al, 2009; Valla, 2003; Plessier & Valla, 2008), an improvement attributed to two factors: 1) a greater awareness of BCS and 2) improved diagnostic tools for identifying the anatomic lesions and for diagnosing thrombogenic hematologic disorders.
Box 77.1 Conditions Predisposing to Budd-Chiari Syndrome and Venoocclusive Disease
Budd-Chiari Syndrome
Membranous obstruction of the inferior vena cava
Miscellaneous rare conditions (inflammatory bowel disease, hepatic torsion, lipoid nephrosis, protein-losing enteropathy)
The distinct difference has been recognized for some time between the East and West in the conditions that predispose to the development of BCS and in the anatomic pattern of BCS. Table 77.1 displays these differences. Membranous obstruction of the vena cava (MOVC) is rare in the West, but it is a frequent cause of BCS in Eastern countries such as Japan, China, and India and in South Africa. In the West, thrombosis of the major hepatic veins alone is substantially more common than thrombosis or occlusion of the IVC; whereas in India, China, and Japan, IVC occlusion is far more common than hepatic vein occlusion alone. In North America, the acute or subacute forms of BCS predominate, and chronic BCS is observed less frequently, whereas in the East, the reverse is observed. In the West, BCS is seldom found during pregnancy or the postpartum period, whereas in India, pregnancy is a major predisposing condition for BCS. The same difference is seen in the incidence of infections such as hepatic amebiasis (see Chapter 67), which are rare in the West but are reported frequently in series of BCS from India. Finally, oral contraceptives are frequently associated with BCS in the United States, where use of these agents is widespread, whereas use of birth control pills is seldom associated with BCS in Eastern countries, where women use oral contraceptives much less.
Table 77.1 Differences Between West and East in Predisposing Conditions and Anatomic Patterns of Budd-Chiari Syndrome
| Feature | West | East |
|---|---|---|
| Membranous obstruction of the IVC | Rare | Frequent |
| Hepatic vein occlusion predominates | + | − |
| IVC occlusion predominates | − | + |
| Acute or subacute BCS predominates | + | − |
| Chronic BCS predominates | − | + |
| Pregnancy/postpartum | Uncommon | Frequent |
| Infection | Rare | Common |
| Oral contraceptives | Frequent | Uncommon |
BCS, Budd-Chiari syndrome; IVC, inferior vena cava
Hematologic Disorders
Hematologic diseases that cause vascular thrombosis are the most common conditions that predispose to BCS in North America and Western Europe. Of disorders with thrombotic tendencies, polycythemia rubra vera is the most frequent, constituting 8.5% of the cases of BCS in the collected series of Parker (1959) and 10.4% of the cases in the collected series of Mitchell and colleagues (1982). In our series of 77 cases of BCS, 31% had polycythemia rubra vera, which is associated with BCS in some distinctly different ways compared with the classic disease. For one thing, it is found in young adults, rather than in middle-aged and elderly patients, and it is responsive to treatment with hydroxyurea or anagrelide, which should be started as soon as the disease is discovered and should be continued for life. In our experience, the disease runs a benign course if treated. Finally, polycythemia rubra vera associated with BCS is compatible with a long life, if it is treated with hydroxyurea.
Paroxysmal nocturnal hemoglobinuria is another hematologic disorder associated with BCS (Hartmann et al, 1980; Hoekstra et al, 2009; Liebowitz & Hartmann, 1981; Valla et al, 1987). It was responsible for 6.7% of the cases in the collected series of Mitchell and colleagues (1982) and 12% of the cases in the series of Valla and colleagues (1987). In all of the hematologic disorders associated with hepatic vein thrombosis, but particularly in paroxysmal nocturnal hemoglobinuria, thrombosis of other blood vessels outside of the liver has sometimes been observed. These cases of multiple sites of thrombosis have involved the portal, splenic, and superior mesenteric veins; pelvic veins; deep calf veins; splanchnic arteries; pulmonary artery; coronary arteries; and cerebral arteries (Peytremann et al, 1972).
As hematologic diagnosis has become progressively more sophisticated, many other thrombogenic conditions have been identified in BCS, including other myeloproliferative states—such as essential thrombocythemia, primary erythrocytosis, and myelofibrosis—and thrombophilic states, such as protein C deficiency, protein S deficiency, antithrombin III deficiency, and antiphospholipid syndrome with lupus anticoagulant or anticardiolipin antibodies or both (Bertina et al, 1994; Boughton, 1991; Dahlback, 1995; Dahlback et al, 1993; Espinosa et al, 2001; Koster et al, 1993; Mahmoud et al, 1995; Menon et al, 2004; Pelletier et al, 1994; Svensson & Dahlback, 1994; Valla, 2003; Vandenbroucke et al, 1994). Subjects with the factor V Leiden mutation, which leads to activated protein C resistance, have a 5-fold to 10-fold increase in the risk of thrombosis if they are heterozygotic and a 50-fold to 100-fold increase if they are homozygotic (Dahlback, 1995; Deltenre et al, 2001; Janssen et al, 2000). More recent evidence indicates that multiple prothrombotic factors acting concurrently are involved in a substantial percentage of patients with BCS (Denninger et al, 2000; Janssen et al, 2000). Rarely, hematologic malignancies, such as acute leukemia and lymphoma, have been associated with BCS.
In 2005, identification of the underlying cause of BCS was enhanced by the discovery of a very reliable and noninvasive marker for chronic myeloproliferative disorders. The marker is the gain-of-function mutation V617F of the JAK2 gene (Baxter et al, 2005; James et al, 2005; Jelinek et al, 2005; Jones et al, 2005; Kralovics et al, 2005; Levine et al, 2005; Steensma et al, 2005; Zhao et al, 2005). By combining identification of this marker with results of bone marrow histology and clonality assay, over 50% of the cases of BCS have been found to be due to an underlying chronic myeloproliferative disorder (Primignani et al, 2006).
It cannot be overemphasized that every patient found to have BCS should undergo a thorough hematologic evaluation. The workup that we perform is an expansion of the workup proposed by Mahmoud and Elias (1996) and others (Hirschberg et al, 2000; Valla, 2009) and is shown in Box 77.2. With these studies, it should be possible to diagnose all of the predisposing thrombogenic hematologic disorders known to be associated with BCS. If an evaluation such as this is done uniformly, it is highly likely that the incidence of idiopathic BCS will continue to decline.
Oral Contraceptives
An increased incidence of thromboembolic phenomena involving various blood vessels and organs in women taking oral contraceptives has been well established. The first case of BCS associated with use of oral contraceptives was reported by Ecker and McKittrick (1966), 5 years after these drugs became available commercially. Since then, more than 200 cases of BCS in patients taking oral contraceptive have been described (Janssen et al, 2000; Lewis et al, 1983; Maddrey, 1987; Valla et al, 1986; Zafrani et al, 1983), and the increasing overall incidence of BCS in recent years has been attributed partly to the widespread use of these agents. In the collective review reported by Mitchell and colleagues (1982), use of oral contraceptives was believed to be responsible for 9.4% of the cases of BCS during the period 1960 to 1980. Valla and associates (1986) reported that the relative risk of hepatic vein thrombosis among oral contraceptive users was close to that of stroke, myocardial infarction, and venous thromboembolism. In our series of 77 cases of BCS, 23% of patients gave a history of oral contraceptive use. The incidence is even higher if the denominator consists only of the number of women in each series. In our series, 49% of the 37 women with BCS used oral contraceptives.
Some authors have proposed that oral contraceptives are not a primary cause of BCS but contribute to thrombosis only if there is an underlying hematologic disorder (Valla et al, 1986). In our series, no hematologic disease was identified in users of oral contraceptives, but until relatively recently, our patients did not undergo the extensive hematologic workup shown in Box 77.2. The duration of usage of oral contraceptives before the diagnosis of BCS has ranged from 2 weeks to 10 years. In addition to causing BCS, oral contraceptives have been linked to other liver disorders, including VOD, portal vein thrombosis, cholestasis, hepatocellular adenoma, focal nodular hyperplasia, and possibly hepatocellular carcinoma and angiosarcoma (Zafrani et al, 1983).
Box 77.2
Screening for Hematologic Disorders in Budd-Chiari Syndrome
Complete blood count, prothrombin time, partial thromboplastin time, fibrinogen
Red blood cell mass, plasma volume
Bone marrow biopsy, cell culture, karyotype
JAK2 gene, V617F mutation in peripheral blood granulocytes
Activated protein C resistance or Factor V Leiden mutation or both
Endogenous erythroid colony assay
Flow cytometry for blood cells deficient in CD55 and CD59 (PNH)
Molecular test for G20210A prothrombin gene mutation
PNH, paroxysmal nocturnal hemoglobinurea.
Pregnancy and Postpartum
BCS has been observed in women during pregnancy and, more commonly, during the postpartum period. The first case of BCS reported by Chiari (1899) occurred in a woman who developed the disorder after childbirth. In the collective review by Mitchell and colleagues (1982), 9.9% of the cases of BCS occurred during pregnancy or postpartum, and in a series of 105 patients with BCS observed from 1963 through 1978, Khuroo and Datta (1980) reported 16 cases (15.2%) of BCS after pregnancy; 8 of the patients died, and 7 were lost to follow-up after discharge from the hospital. The hypercoagulable state that is known to occur during pregnancy is presumed to be responsible for the association of BCS with this condition, although only 1 of our 77 cases of BCS occurred during pregnancy or postpartum.
Malignant Neoplasms
Malignant neoplasms were responsible for 13.4% of the collected cases of BCS reported by Parker (1959), 8.8% of the collected cases reported by Mitchell and colleagues (1982), and 12% of the cases reported by Powell-Jackson and colleagues (1982). Because we do not operate on patients with BCS caused by cancer, none of our 77 patients had malignant neoplasms. Occlusion of the suprahepatic IVC by invasive tumors has been the cause of BCS in many cases. The most common cancers associated with BCS are hepatocellular carcinoma, renal cell carcinoma, adrenal carcinoma, and leiomyosarcoma of the IVC. Other malignancies that rarely have caused BCS include carcinomas of the lung, pancreas, and stomach; melanoma; reticulum cell sarcoma; adrenal sarcoma; and sarcoma of the right atrium.
Infections
Infections involving the liver were believed responsible for 3% of the collected cases of BCS reviewed by Parker (1959), 9.9% of the collected cases reported by Mitchell and colleagues (1982), and none of the cases in sizable series reported in more recent years, including our own series. The most common infections associated with BCS are those caused by parasites, particularly amebic liver abscess, hydatid disease, and schistosomiasis (see Chapters 67 and 68). Syphilitic gumma of the liver accounted for 1.8% of the cases in Parker’s review but has not been reported as a cause of BCS in recent years. Aspergillosis involving the hepatic veins and IVC has been a rare cause of BCS. In India, Victor and colleagues (1994) provided evidence that filariasis can cause BCS.
Trauma
Abdominal trauma has uncommonly predisposed to the development of BCS. Trauma was responsible for 1.2% of the collected cases of BCS reported by Parker (1959) and 2.4% of the collected cases reviewed by Mitchell and colleagues (1982). Blunt and penetrating trauma have been implicated in occasional cases of BCS.
Connective Tissue Disorders
Occasional cases of BCS have been reported in association with various connective tissue and autoimmune diseases, most of which are known to have thrombotic tendencies. Included among these are Behçet disease, Sjögren syndrome, mixed connective tissue disease, sarcoidosis, and rheumatoid arthritis. Numerous cases of BCS in patients with Behçet disease, including five of our own, have been described (Bazraktar et al, 1997; Orloff & Orloff, 1999).
Membranous Obstruction of the Vena Cava
More than 600 cases of BCS resulting from MOVC have been reported from Japan (Hirooka & Kimura, 1970; Kimura et al, 1972; Okuda, 2002; Ono et al, 1983; Taneja et al, 1979; Yamamoto et al, 1968), China (Wang, 1989; Wang et al, 1989; Wu et al, 1990) and other parts of Asia, India (Khuroo & Datta, 1980), and South Africa (Semson, 1982). In the United States and Europe, MOVC is rare. A congenital cause of this condition has been proposed, but evidence strongly suggests it represents the end result of acquired thrombosis (Kage et al, 1992; Okuda 2002; Okuda et al, 1995).
MOVC usually runs a chronic course over many years, and most patients will have developed extensive hepatic fibrosis and cirrhosis and portal hypertension by the time they come to medical attention. An increased incidence of hepatocellular carcinoma has been observed in association with MOVC (Okuda, 2002; Semson, 1982). The therapeutic implications of this condition and other forms of IVC occlusion are distinctly different from those of occlusion confined to the major hepatic veins.
Causes of Venoocclusive Disease
In 1954, Bras and colleagues proposed the term venoocclusive disease to describe a serious and common liver disease in Jamaican children in which there was occlusion of the central and sublobular hepatic veins and surrounding centrilobular necrosis of the liver parenchyma. More recently, the condition has been called the sinusoidal obstruction syndrome. Shortly after his initial description, Bras and others (Bras & McLean, 1963; Brooks et al, 1970; Gore et al, 1961; Stuart & Bras, 1957) showed that VOD was due to ingestion of “bush teas” made from plants of the Crotolaria and Senecio genera, which contain well-known hepatotoxic pyrrolizidine alkaloids. It has long been known that these plants and plants of the Heliotropium genus produce liver failure in herbivorous animals and are common causes of poisoning of grazing cows and horses. Since the initial descriptions by Bras and others, VOD caused by ingestion of toxic pyrrolizidines has been observed in Israel (Ghanem & Hershko, 1981), Egypt (Safouh & Shehata, 1965), Iraq (Al-Hasany & Mohamed, 1970), Afghanistan (Mohabbat et al, 1976), India (Aikat et al, 1978; Tandon et al, 1976), Venezuela (Grases & Beker, 1972), Ecuador (Lyford et al, 1976), South Africa (Steenkamp et al, 2000; Zuckerman et al, 2002), and the United States (Abbott, 1988; Bach et al, 1989; Ridker & McDermott, 1989; Ridker et al, 1985; Stillman et al, 1977). In addition to poisoning by drinking bush teas, humans have been poisoned by eating flour milled from grain contaminated by the seeds of these plants and by taking herbal remedies.
Antineoplastic drugs have been identified as another cause of VOD. Included among these are cytosine arabinoside, thioguanine, and gemtuzumab ozogamicin (Mylotarg) used in the treatment of acute myelocytic leukemia (Cruz et al, 1983; Giles et al, 2001; Gill et al, 1982; Griner et al, 1976); carmustine used in the treatment of diffuse histiocytic lymphoma (McIntyre et al, 1981); dacarbazine used in the treatment of melanoma (Asbury et al, 1980); oxaliplatin used in treatment of metastatic colorectal adenocarcinoma to the liver (Cleary et al, 2009; Pawlik et al, 2007; Mehta et al, 2008; Kandutsch et al, 2008; Rubbia-Brandt et al, 2004); and gemtuzumab ozogamicin and mitomycin C combined with bone marrow transplantation (Kumar et al, 2003; Lazarus et al, 1982; McDonald et al, 1993; Wadleigh et al, 2003). Fatal VOD in renal transplant recipients has been attributed to azathioprine (Liano et al, 1989; Marubbio & Danielson, 1975).
Hepatic injury caused by therapeutic irradiation of malignant neoplasms in and near the liver is an important cause of VOD (Fajardo & Colby, 1980; Reed & Cox, 1966). Although radiation-induced VOD usually does not produce clinical manifestations with doses less than 3000 rad, the condition has been observed after smaller amounts of radiotherapy (Fajardo & Colby, 1980).
Currently, the most common cause of VOD in the Western Hemisphere is bone marrow transplantation (Ayash et al, 1990; Berk et al, 1979; Jones et al, 1987; Kriegshauser et al, 1988; Kumar et al, 2003; McDonald et al, 1984, 1993; Wadleigh et al, 2003; Senzolo et al, 2007; Helmz, 2006; Cacchione et al, 2008). It has been estimated that approximately one fourth of recipients of bone marrow transplants develop the disorder. It is uncertain whether VOD in these patients is caused by the marrow transplant, or if it is a result of the radiation and chemotherapy used in the pretransplant conditioning regimen. Berk and colleagues (1979) proposed that graft-versus-host disease might be responsible for VOD after marrow transplantation, but the studies of McDonald and colleagues (1984) cast doubt on this hypothesis. Finally, there have been rare reports of VOD in association with systemic lupus erythematosus (Pappas et al, 1984), familial immunodeficiency (Mellis & Bale, 1976), and the use of oral contraceptives (Alpert, 1976).
Pathology
Budd-Chiari Syndrome
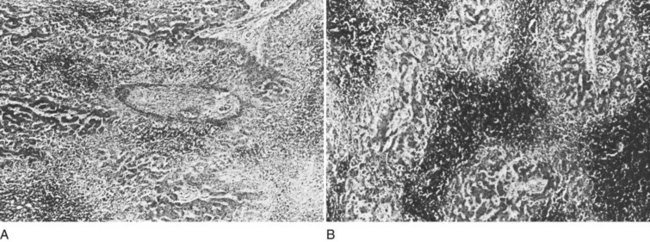
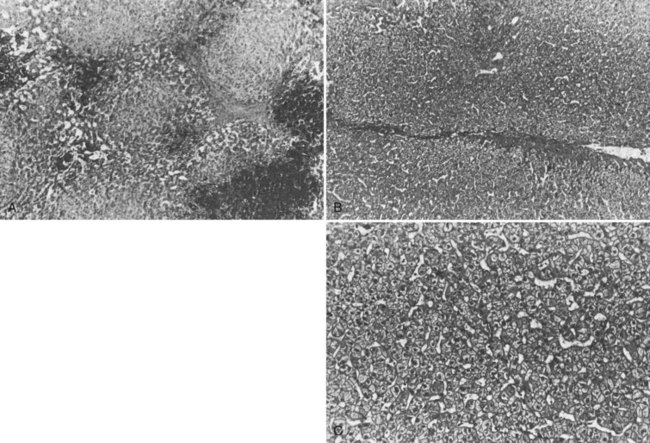
The liver receives about one fourth of the cardiac output via its dual afferent blood supply, the portal vein and hepatic artery. After perfusing the sinusoids, the blood is returned to the heart through the hepatic veins and IVC. Obstruction to the egress of blood from the liver at any point along the outflow route results in numerous serious hemodynamic and morphologic alterations. There is a marked increase in intrahepatic pressure, which is reflected by a similar increase in portal pressure. The increased intrahepatic pressure causes extravasation of plasma from the liver sinusoids and lymphatics with formation of ascites. Obstruction to the egress of blood from the liver also results in dilation of the sinusoids and intense centrilobular congestion of the hepatic parenchyma, which is greatest around the terminal hepatic venules (central veins; Fig. 77.1). Ischemia, pressure necrosis, and atrophy of the parenchymal cells in the center of the liver lobule are apparent. With persistence of the obstruction, the necrotic parenchyma is replaced by fibrous tissue and regenerating nodules of liver tissue. The end result is cirrhosis of the type associated with chronic congestive heart failure. The rapidity with which cirrhosis develops is related to the severity of outflow obstruction, but it is not unusual for cirrhosis to occur within a matter of months (Parker, 1959).
In most cases of BCS, occlusion of the hepatic veins is caused by thrombosis (Parker, 1959). The thrombus undergoes organization and ultimately is converted to fibrous tissue that permanently occludes the veins. Although recanalization of the occluded veins sometimes occurs, it rarely results in effective new outflow channels. Retrograde propagation of the thrombus into smaller hepatic veins is commonly found. Prograde propagation of the thrombus from the hepatic veins into the IVC, with partial or complete occlusion of the IVC, sometimes occurs and markedly changes the therapeutic approach and prognosis. It is important to determine by imaging studies that include angiography and pressure measurements whether the IVC has become involved in the occlusive process.
MOVC has been reported to be the most common cause of BCS in Japan (Hirooka & Kimura, 1970; Kimura et al, 1972; Okuda et al, 1995; Ono et al, 1983; Taneja et al, 1979; Yamamoto et al, 1968), India (Khuroo & Datta, 1980), China (Wang, 1989; Wang et al, 1989, 2005; Wu et al, 1990), and in the Bantu population of South Africa (Semson, 1982). The “membrane” varies from very thin to several centimeters thick and usually contains fibrous tissue, smooth muscle, and elastic tissue. The location and extent of the membrane vary considerably, and in some cases a long segment of IVC has been replaced by fibrous tissue. Occlusion of one or more of the major hepatic veins often has been associated with membranous obstruction of the IVC. Although some experienced authors have proposed a congenital cause (Hirooka & Kimura, 1970; Kimura et al, 1972; Ono et al, 1983; Semson, 1982; Taneja et al, 1979), a strong argument has been made that suggests MOVC is the end result of thrombosis of the IVC, often occurring early in life (Okuda et al, 2002). Most of the cases have run a chronic course before discovery, and when first seen by a physician, patients have had extensive hepatic fibrosis or cirrhosis with portal hypertension and all of its manifestations. The therapeutic considerations in patients with MOVC differ from those in patients with BCS caused by obstruction of the hepatic veins.
Venoocclusive Disease
VOD of the liver, more recently called sinusoidal obstruction syndrome, may mimic BCS clinically, because both conditions involve hepatic venous outflow obstruction. VOD involves the sinusoids and the central and sublobular hepatic veins within the liver, however, rather than the hepatic veins (Kumar et al, 2003; Shulman et al, 1987, 1994). The underlying process in VOD is subendothelial sclerosis of the hepatic veins and sinusoids secondary to endothelial injury caused by a toxic agent, such as a pyrrolizidine alkaloid, antineoplastic drug, radiation, or a stem cell transplant. Thrombosis of the small hepatic veins may occur after damage to the venous intima. Electron microscopic studies of liver biopsy specimens obtained from children with VOD caused by pyrrolizidine poisoning showed marked endothelial damage in the sinusoids and subterminal and terminal hepatic veins in all zones of the liver, with extravasation of erythrocytes into the space of Disse and narrowing of the lumen where the sinusoid entered the central vein (Brooks et al, 1970).
Clinical Manifestations of Budd-Chiari Syndrome
In our series of 77 patients with BCS, 12 were referred with advanced cirrhosis as a result of prolonged hepatic outflow obstruction and were not candidates for portal decompression surgery. The other 65 patients were referred at a mean 14 weeks after onset of BCS (range 4 to 78 weeks). Fifty-nine of the 65 patients (91%) were referred less than 18 weeks after onset of symptoms, relatively early in the course of BCS. The remaining 6 patients were operated on 19, 21, 23, 25, 26, and 78 weeks after onset of symptoms. In the collected series of Mitchell and colleagues (1982), which excluded cases of MOVC, two thirds had had symptoms for less than 3 months, and 83% had had symptoms for 6 months or less at the time of diagnosis. In Parker’s (1959) collected series of 133 cases, he observed that 57% had had symptoms for 3 months or less, and 71% had been symptomatic for 6 months or less.
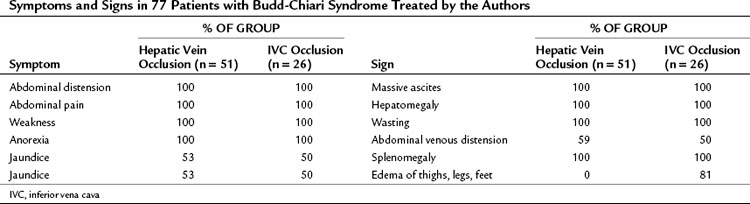
Table 77.2 presents the frequency of symptoms and signs in our series of 77 patients with BCS, 51 of whom had hepatic vein occlusion alone, and 26 of whom had IVC occlusion and hepatic vein occlusion. The only difference between the two groups—and it is an important one—was the absence of lower extremity edema in the group without IVC thrombosis and the high incidence of edema from feet to thighs (81%) that resulted from IVC occlusion.
Symptoms
Abdominal Distension
The initial symptom in all of our patients was abdominal distension secondary to ascites, which increased progressively over a few weeks. Abdominal distension caused by ascites occurs at some time in almost every patient with BCS. In the large collected series (Mitchell et al, 1982; Parker, 1959), abdominal distension was among the first symptoms experienced by most patients.
Anorexia
Anorexia was reported by all patients in our series and has been experienced by many patients in the reports of others (Mitchell et al, 1982).
Jaundice
Clinical jaundice was observed in 52% of the patients in our series but in only 28% of the patients in Parker’s collective review (1959) and in only 17% of the patients collected by Mitchell and colleagues (1982). Usually, jaundice has been mild.
Symptoms of Chronic Liver Disease
Patients with the chronic forms of BCS, such as MOVC, often have the usual symptoms of cirrhosis and portal hypertension, including upper gastrointestinal (GI) bleeding secondary to ruptured esophagogastric varices (see Chapter 75A, Chapter 75B, Chapter 75C ), hepatic encephalopathy, hepatorenal syndrome, and edema of the lower extremities (see Chapters 72 and 73). Peripheral edema is particularly prominent in patients with MOVC, and some develop varicose veins of the legs (Parker, 1959; Semson, 1982). In our series of patients who underwent surgical portal decompression for acute BCS secondary to occlusion of the hepatic veins, one patient had these symptoms.
Findings on Physical Examination (Signs)
Ascites
All 77 patients in our series had the abdominal signs of massive ascites on physical examination at the time of diagnosis. The incidence of ascites was 93% in the collective review of Parker (1959) and 83% in the series of Mitchell and colleagues (1982).
Hepatomegaly
All patients in our series and in the series of Mitchell and colleagues (1982) had marked hepatomegaly resulting from severe congestion of the liver. In chronic forms of BCS, hepatomegaly may not be as striking, but it is usually present.
Distension of Abdominal Veins
Forty-three of the 77 patients in our series (56%) and 55% of the patients in the collected series of Parker (1959) had distension of the superficial veins of the anterior abdominal wall. Abdominal venous distension is a manifestation of portal hypertension and the formation of portosystemic collaterals early in the course of BCS.
Splenomegaly
Splenomegaly, another manifestation of portal hypertension, was observed in all of the patients in our series, in 50% of those in the collected series of Mitchell and colleagues (1982), and in 30% in the collected series of Parker (1959). Enlargement and congestion of the spleen was sometimes accompanied by the hematologic manifestations of secondary hypersplenism.
Jaundice
Clinical jaundice was observed in 52% of the patients in our series but in only 28% of the patients in the collected series of Parker (1959) and in only 17% of the patients in the collected series of Mitchell and colleagues (1982). The jaundice in our patients who underwent surgical portal decompression was invariably mild; the highest serum bilirubin level was only 6.8 mg/dL.
Symptoms and Signs of Venoocclusive Disease
VOD has been observed in individuals of all ages, including infants and adults in their sixth decade; however, VOD caused by pyrrolizidine alkaloids has been seen most commonly in infants and children. The clinical manifestations depend on the disease stage at which the patient seeks medical treatment (Brooks et al, 1970; Ghanem & Hershko, 1981; Gore et al, 1961; Safouh & Shehata, 1965; Stuart & Bras, 1957). The acute stage is often preceded for 1 or 2 weeks by a febrile illness with upper respiratory symptoms, vomiting and diarrhea, or both. The patient then experiences abrupt onset of abdominal pain, weakness, anorexia, fever, and abdominal distension secondary to ascites (Kumar et al, 2003; Wadleigh et al, 2003; Senzolo et al, 2007). Jaundice is the rule, and splenomegaly with thrombocytopenia is common. Some patients develop edema of the feet and occasionally of the hands and face, and physical examination in the acute phase invariably shows hepatomegaly and ascites. Many patients have splenomegaly, some have distension of the superficial veins of the abdominal wall, and some have peripheral edema and a pleural effusion. Bone marrow transplant recipients usually develop the clinical features of VOD within 3 weeks after transplantation. Many patients have died during the acute stage from liver failure, bleeding esophageal varices, or intercurrent infection.
Patients other than bone marrow transplant recipients may be initially seen in the chronic stage of VOD with the usual clinical manifestations of cirrhosis of the liver: ascites, hepatomegaly, splenomegaly, wasting, abdominal venous distension, spider angiomata, palmar erythema, asterixis, and peripheral edema. Bleeding from esophageal varices is a major cause of death in the chronic stage, and cirrhosis of the liver has been observed 3 months after acute onset of VOD (Gore et al, 1961).
Diagnostic Studies in Budd-Chiari Syndrome
Hepatic Angiography and Pressures
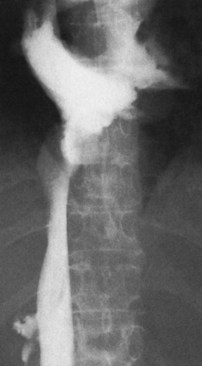
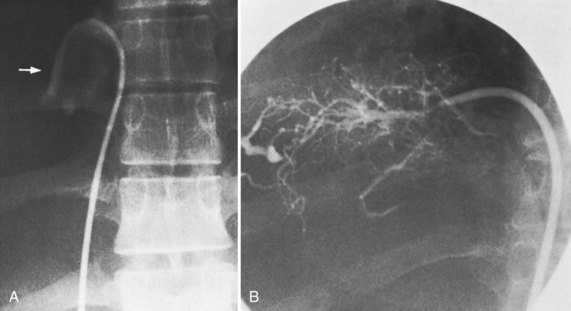
The diagnostic study of greatest value in BCS, particularly if surgical therapy is contemplated and venous pressure measurements are required, is angiographic examination of the IVC and hepatic veins with pressure measurements (Clain et al, 1967; Kreel et al, 1967; Redman, 1975; Tavill et al, 1975). This study usually is combined with hepatic and superior mesenteric arteriography and indirect portography. In BCS confined to the hepatic veins, the IVC is patent, and IVC pressure is relatively normal for subjects with ascites (Fig. 77.2). Patency of the IVC is a prerequisite for portacaval shunt (PCS) and is a crucial finding (see Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E ). In some patients, the IVC is moderately compressed in its retrohepatic course by the enlarged liver and, in particular, by a hypertrophied caudate lobe (Fig. 77.3). This finding usually is not clinically significant (Clain et al, 1967; Kreel et al, 1967; Redman, 1975; Tavill et al, 1975).
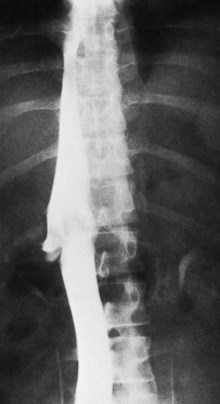
The most important angiographic finding is the demonstration by hepatic venography of occlusion or marked narrowing of the major hepatic veins. Sometimes it is not possible to find patent orifices of any of the hepatic veins, which is indirect evidence that all of the major hepatic veins are occluded. Usually it is possible, however, to enter at least one major hepatic vein and to show the presence of a thrombus or of narrowing and distortion of the vein (Fig. 77.4A). Injection of dye in the wedged position often shows a characteristic spiderweb pattern of small hepatic venous collaterals connecting to portal or systemic veins (see Fig. 77.4B). Wedged hepatic vein pressure (WHVP) usually is markedly elevated, which reflects the obstruction to hepatic venous outflow. In patients with hepatic vein occlusion alone, IVC pressure is substantially lower than WHVP.
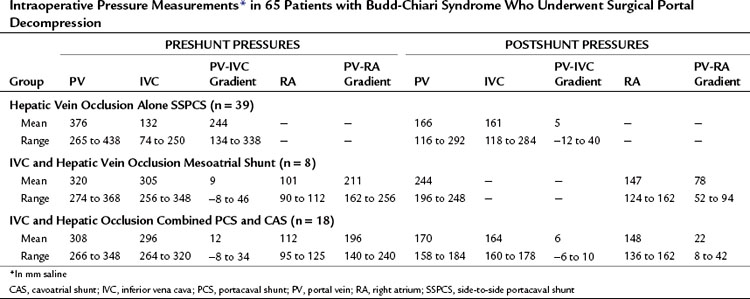
A spiderweb pattern was observed in 48 patients (62%). WHVP was markedly elevated in all patients in whom it was measured. In the 51 patients with thrombosis confined to the hepatic veins, the IVC was patent, with relatively normal pressures for patients with ascites, ranging from 62 to 160 mm saline (Table 77.3). Many patients had moderate compression of the retrohepatic IVC by the enlarged liver, but invariably WHVP was substantially higher than IVC pressure—a crucial finding. In contrast, in the 26 patients with IVC occlusion, IVC pressure and WHVP were similar. All patients had stretched and attenuated hepatic artery branches within the liver and a patent portal vein (PV).
Table 77.3 Results of Diagnostic Studies in 77 Patients with Budd-Chiari Syndrome Treated by the Authors
| % OF GROUP | ||
|---|---|---|
| Hepatic Vein Occlusion (n = 51) | IVC Occlusion (n = 26) | |
| Angiography and Pressures | ||
| Occluded hepatic veins | 100 | 100 |
| Occluded IVC near hepatic veins | 0 | 100 |
| Spiderweb pattern of hepatic veins | 69 | 50 |
| Patent portal and splenic veins | 100 | 100 |
| Stretched hepatic arteries | 100 | 100 |
| IVC pressure much lower than WHVP | 100 | 0 |
| High WHVP | 100 | 100 |
| Percutaneous Needle Liver Biopsy (73 Patients) | ||
| Centrilobular congestion | 100 | 100 |
| Centrilobular hepatocyte loss and necrosis | 100 | 100 |
| Fibrosis | 76 | 58 |
| Cirrhosis (included also under fibrosis) | 34 | 8 |
| Hepatic Scintiscan | ||
| Decreased hepatic uptake | 100 | 100 |
| Nonhomogeneous hepatic uptake | 100 | 100 |
| Excessive extrahepatic uptake | 74 | 77 |
| Central hot spot in liver | 31 | 12 |
| Computed Tomography (64 Patients) | ||
| Absence of hepatic veins | 100 | 100 |
| Abnormal hepatic uptake of contrast | 100 | 100 |
| Occlusion of IVC | 0 | 100 |
| Ascites | 100 | 100 |
| Real-Time and Doppler Duplex Ultrasound (61/76 Patients) | ||
| Absence of hepatic veins with flat or reversed flow | 100 | 100 |
| Abnormal venous structures in liver | 100 | 100 |
| IVC thrombosis with flat or reversed flow | 0 | 100 |
| Ascites | 100 | 100 |
| Abnormal Liver Function Tests | ||
| Bromosulfophthalein or indocyanine green retention (23% to 90%; 24 patients) | 100 | 100 |
| Serum alkaline phosphatase (138 to 458 IU) | 100 | 100 |
| Prothrombin time (12.4 to 16.5 seconds) | 98 | 92 |
| Partial thromboplastin time (31 to 59 seconds) | 98 | 92 |
| Serum albumin (2.1 to 2.8 g/dL) | 96 | 95 |
| Aspartate aminotransferase (76 to 540 IU) | 90 | 88 |
| Serum bilirubin (3.8 to 6.8 mg/dL) | 54 | 42 |
| Upper Gastrointestinal Radiographs (53 Patients) | ||
| Normal | 60 | 0 |
| Esophageal varices | 40 | 100 |
| Esophagogastroscopy (28 Patients) | ||
| Esophageal varices | 44 | 100 |
IVC, inferior vena cava; WHVP, wedged hepatic venous pressure
An additional advantage of hepatic venography is that it facilitates performing a transjugular liver biopsy. Some investigators have stated that “at present, there is almost no place for direct or retrograde venography for the sole purpose of making a diagnosis of BCS” (Valla, 2009; Janssen et al, 2003). We hasten to point out that our use of hepatic venography was an essential guide to surgical therapy, not simply a means of making the diagnosis of BCS, as others have agreed (Kamath, 2006; Erdon, 2007).
Liver Biopsy
Percutaneous or transjugular needle liver biopsy yields histologic findings characteristic of BCS early in the course of the disease; along with hepatic angiography, it provides conclusive diagnostic information (Tang et al, 2001). The diagnostic features are quite spectacular and include intense centrilobular congestion combined with centrilobular loss of parenchyma and necrosis (see Fig. 77.1). Mild to moderate fibrosis of the liver parenchyma is found in many patients. As the disease evolves and becomes chronic, cirrhosis of the cardiac type develops. Cirrhosis has been observed within months of the onset of symptoms. Only two other conditions, constrictive pericarditis and congestive heart failure, produce a histologic picture similar to that seen in the acute stage of BCS. Both of these cardiac disorders can be eliminated easily from consideration by appropriate studies.
In our series, percutaneous or transjugular needle liver biopsy was done preoperatively in 73 of 77 patients, and open wedge liver biopsy was done at operation in all 77 patients (see Table 77.3). All patients had intense centrilobular congestion along with centrilobular hepatocellular loss and necrosis. Of the 65 patients in our series who were referred sufficiently early to undergo surgical portal decompression, 39 (60%) had fibrosis of the liver at an early point in the illness. In three patients, the fibrosis had progressed to cirrhosis. All 12 patients who were considered candidates for liver transplantation had advanced cirrhosis (see Chapter 97A).
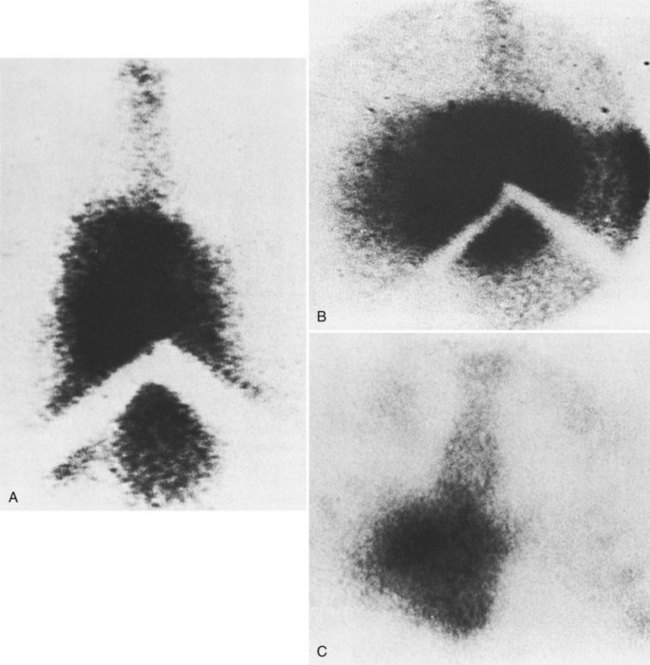
Hepatic Scintiscanning
Scintiscans of the liver (see Chapter 15) with technetium 99m-sulfur colloid or other colloidal radionuclides show the nonspecific abnormalities of decreased and nonhomogeneous hepatic uptake of radiocolloid, increased uptake of radiocolloid by the spleen and bone marrow, and hepatosplenomegaly. Central localization of radiocolloid in the liver, the so-called central hot spot, has been reported to be of diagnostic importance (Fig. 77.5; Meindok & Langer, 1976; Tavill et al, 1975). It is seen when the thrombotic process spares the small hepatic veins that drain the caudate lobe and central areas of the right and left lobes directly into the IVC, so that these areas of the liver remain healthy and even hypertrophy. The central hot spot was observed in only 19 (25%) of the 77 patients with BCS in our series.
Computed Tomography
Computed tomography (CT; see Chapter 16) of the liver after injection of contrast agents has been shown to be of diagnostic value in BCS (Baert et al, 1983; Mathieu et al, 1987; Lupescu, 2008; Erdon, 2007; Kamath, 2006; Vogelzang et al, 1987). The findings include 1) absence of opacification of the hepatic veins, which in normal subjects usually can be shown clearly; 2) nonhomogeneous and delayed uptake of contrast material in the liver; 3) delayed emptying of contrast material from venous structures at the periphery of the liver; and 4) occlusion of the IVC in patients with IVC thrombosis. CT also shows ascites and hepatomegaly. We performed CT in 64 of our 77 patients and observed these abnormalities in all of them.
Ultrasonography
Real-time and Doppler duplex ultrasonography (US) of the liver have been shown to be of diagnostic value in BCS (Baert et al, 1983; Becker et al, 1986; Bolondi et al, 1991; Chaubal et al, 2006; Grant et al, 1989; Gupta et al, 1987; Hosoki et al, 1989; Powell-Jackson et al, 1986; Rossi et al, 1981; see Chapter 13). Findings include 1) absence of normal hepatic veins draining into the IVC with flat or reversed flow, 2) an abnormal intrahepatic network of comma-shaped venous structures, 3) thrombus in IVC and flat or reversed flow in patients with IVC thrombosis, and 4) enlargement of the caudate lobe. Ascites has been shown regularly. We observed these abnormalities in all 61 patients in whom US was performed in our series. We regularly used Doppler US as an initial screening tool, but we depended on angiography for a definitive diagnosis and guide to treatment.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI; see Chapter 17) is capable of showing patency or obstruction of the hepatic veins and is particularly effective in visualizing the entire length of the IVC. It has been reported to be useful in differentiating the acute form of BCS from the subacute and chronic forms (Noone et al, 2000; Kamath, 2006; Erdon, 2007; Lupescu et al, 2008). Because of the high cost of MRI, it is not a first-line diagnostic procedure in our program; none of our 66 patients underwent MRI.
Abnormal Liver Function Tests
Results of liver function tests are usually abnormal in BCS, although the type of abnormality varies and is nondiagnostic (see Table 77.3). All 24 patients in our series who had studies of injected bromosulfophthalein or indocyanine green dye clearance had marked retention of dye, ranging from 23% to 90% (normal <6%). All 77 patients had elevation of serum alkaline phosphatase, ranging from 138 to 458 IU (normal 25 to 85 IU). Of 77 patients, 74 had prolonged prothrombin time and partial thromboplastin time, and 96% had a marked decrease in serum albumin concentration (range, 2.1 to 2.8 g/dL); 69 patients (90%) had elevations of aspartate aminotransferase (range, 76 to 540 IU), and 39 (51%) had mild to moderate elevations of serum bilirubin (range, 3.8 to 6.8 mg/dL). These biochemical abnormalities are not specific to BCS, but they indicate significant hepatic dysfunction and are an important part of the diagnostic workup.
Ascitic Fluid Analysis
Analysis of ascitic fluid in patients with BCS is of little diagnostic value, because the results vary considerably (Mitchell et al, 1982; Tavill et al, 1975). Protein concentration has been reported to range from 0.5 to 4.9 g/dL.
Diagnostic Studies in Venoocclusive Disease
The diagnosis of VOD is strongly suspected when, in the proper setting—such as following bone marrow transplantation—patients develop ascites, jaundice, hepatomegaly, and right upper quadrant abdominal pain (Senzolo et al, 2007). The most important diagnostic study in VOD is needle liver biopsy, which shows the specific abnormality of extensive occlusion of the small hepatic veins within the liver (Brooks et al, 1970; Gore et al, 1961; Shulman et al, 1995; Stuart & Bras, 1957). In the acute stage of VOD, an additional biopsy finding is centrilobular hemorrhagic necrosis of the hepatic parenchyma. In the chronic stage of VOD, the biopsy specimen shows diffuse fibrosis or cirrhosis of the liver.
Angiographic studies are not as helpful in VOD as they are in BCS (see Chapter 19). The major hepatic veins and IVC are normal on venography, but WHVP is invariably elevated, a finding that supports the diagnosis of VOD. Findings on hepatic arteriography and indirect portography are similar to the findings in BCS. Because of the significant risk of bleeding after bone marrow transplantation, it is safest in such patients to perform liver biopsy by the percutaneous transjugular route. WHVP can be measured at the same time.
Liver function test results are invariably abnormal in VOD and do not differ from the results seen in BCS; in other words, the abnormalities reflect serious hepatic dysfunction but are not specific for VOD. After bone marrow transplantation, elevated plasma levels of plasminogen activator inhibitor (PAI)-1 have been reported to be a useful marker in distinguishing VOD from several other causes of posttransplant hepatic dysfunction, such as graft-versus-host disease, drug-induced hepatotoxicity, sepsis, and viral hepatitis (Salat et al, 1997a). PAI-1 has been implicated in the pathology of VOD.
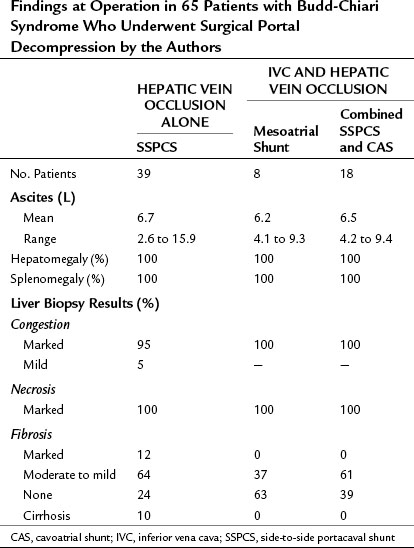
Findings at Operation
The findings at operation in the 65 patients who underwent surgical portal decompression in our series are summarized in Tables 77.4 and 77.5, and they typify what can be expected in patients with BCS. All patients had marked ascites ranging in volume from 2.6 to 15.9 L, congestive hepatomegaly, splenomegaly, and extensive portosystemic collateral veins. All of the patients had portal hypertension. In the group of 39 patients with thrombosis confined to the hepatic veins, the mean PV-IVC pressure gradient was 244 mm saline. In all 39 of these patients, portal pressure was substantially higher than IVC pressure so that a direct side-to-side portacaval shunt (SSPCS) was feasible. In contrast, in the group of 26 patients with IVC occlusion and hepatic vein occlusion, the mean PV-IVC pressure gradient was only 11 mm saline, because the high pressure in the obstructed IVC was similar to the high portal pressure. All 26 patients had a large pressure gradient between the PV and right atrium that averaged 202 mm saline. A wedge liver biopsy specimen obtained at operation showed intense centrilobular congestion and marked centrilobular cell loss and necrosis in all but one patient (see Fig. 77.1), and 35 (54%) of the 65 patients had hepatic fibrosis; this was mild or moderate in 30 patients and severe in 5 patients, 4 of whom had cirrhosis. In patients with the chronic forms of BCS, and in most patients with VOD, the operative findings are typical of cirrhosis of the liver by the time the patient receives treatment.
Nonoperative Therapy
Decompression of the liver by nonoperative measures is often not possible. Thrombolytic therapy with urokinase or streptokinase has been used in many patients in an attempt to dissolve the thrombi and restore hepatic venous outflow (Cassel & Morely, 1974; Gooneratne et al, 1979; Greenwood et al, 1983; Hodkinson et al, 1978; Hoestra et al, 2008; Malt et al, 1978; Mitchell et al, 1982; Powell-Jackson et al, 1982; Thijs et al, 1978; Warren et al, 1972; Plessier & Valla, 2008; DeLeve et al, 2009; Barrault et al, 2004; Sharma et al, 2004; Murad et al, 2009; Zimmerman et al, 2006; Menon et al, 2004). The experience with thrombolytic therapy has been recorded in sketchy anecdotal reports involving short periods of follow-up. Approximately one third of patients were believed to have had a clinical response to treatment for periods of 2 months to 1 year. Half of the patients died as a result of BCS during the brief periods of observation. In the experience of Powell-Jackson and colleagues (1982), all four patients who received thrombolytic treatment in the acute phase of BCS died without evidence of a response.
Urokinase and streptokinase have been administered by systemic intravenous (IV) infusion and by infusion directly into the IVC. In the use of thrombolytic drugs to treat deep venous thrombosis (DVT) of the lower extremities, peripheral IV infusion has been as effective as perfusing the agents directly into the clot. The recommended regimen (Greenwood et al, 1983) involves an IV loading dose (urokinase, 4400 IU/kg; streptokinase, 250,000 IU) followed by a constant IV infusion of urokinase at 4400 IU/kg/h or streptokinase at 100,000 IU/h for 2 to 7 days. Pulmonary embolization developed in two patients during thrombolytic therapy and is a potentially serious complication of this type of treatment.
Anticoagulant therapy has been used widely in BCS to prevent propagation of the thrombi (Ecker & McKittrick, 1966; Hartmann et al, 1980; Khuroo & Datta, 1980; Langer et al, 1975; Lewis et al, 1983; Liebowitz & Hartmann, 1981; Mitchell et al, 1982; Peytremann et al, 1972; Powell-Jackson et al, 1982; Thijs et al, 1978; Plessier et al, 2008; DeLeve et al, 2009; Zimmerman et al, 2006; Valla, 2009). Most reports of the effectiveness of this form of treatment have been anecdotal and lack long-term follow-up. There is no evidence that the use of either heparin or warfarin (Coumadin) brings about dissolution of established thrombosis.
In BCS caused by hematologic disorders, such as polycythemia rubra vera and paroxysmal nocturnal hemoglobinuria, some dramatic responses to IV heparin therapy have been reported, although relapses have been common (Hartmann et al, 1980; Liebowitz & Hartmann, 1981; Peytremann et al, 1972). Long-term anticoagulation with oral warfarin has been recommended to follow the initial IV use of heparin during the acute phase of BCS, although initiation or enhancement of bleeding is a potential complication of anticoagulant therapy, particularly in patients who develop cirrhosis and esophageal varices.
Control of ascites is feasible in some patients with BCS by use of the usual diuretic regimens, although ascites is resistant to therapy in many patients (see Chapter 74). Therapeutic measures include stringent sodium restriction, administration of diuretic drugs, and repeated IV infusion of salt-poor albumin or ascitic fluid. Renal function should be monitored closely during diuretic therapy to avoid precipitating the hepatorenal syndrome. There is no evidence that control of ascites influences the long-term outcome.
The prognosis of BCS with nonoperative therapy is poor (Mitchell et al, 1982; Parker, 1959; Powell-Jackson et al, 1982; Tavill et al, 1975). In his large collective review in 1959, Parker wrote, “The majority of cases have proved fatal, and there is little evidence to suggest that recovery from occlusion of the major hepatic veins occurs once symptoms have been produced.” Similarly, in 1975, Tavill and associates wrote, “The prognosis of the Budd-Chiari syndrome is almost uniformly bad….” In 1982, Mitchell and colleagues stated in their collective review, “Spontaneous resolution of hepatic vein occlusion rarely, if ever, occurs. Conventional medical therapy does little to reverse the pathophysiology in patients with hepatic vein occlusion.” Many patients with BCS that is treated nonoperatively die within a few months of the onset of symptoms. Patients who survive the acute phase usually go on to develop cirrhosis of the liver and die within a few years from hepatic failure, bleeding esophageal varices, or other complications of chronic liver disease. In the 5 years since preparation of the previous edition of this book, despite publication of numerous reviews, expert panels, and case summaries, no data have emerged to indicate that nonoperative therapy of BCS is effective.
Treatment of Hepatic Vein Occlusion by Side-to-Side Portacaval Shunt
Experimental Studies
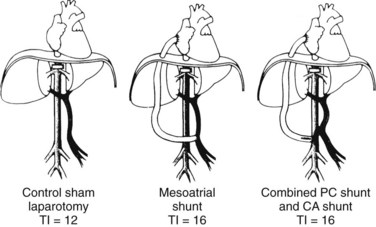
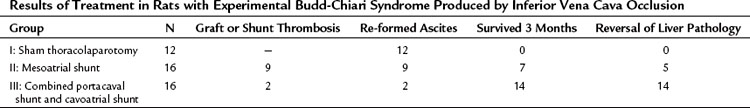
The theoretical basis for the use of SSPCS to relieve BCS resulting from occlusion of the hepatic veins is provided by substantial evidence indicating that the valveless PV can be converted into an outflow tract by an in-continuity anastomosis with the systemic venous system to decompress the obstructed hepatic vascular bed (Britton & Shirey, 1962; Britton et al, 1967; Burchell et al, 1976; Long et al, 1960; Longmire et al, 1958; Moreno et al, 1967; Mulder & Murray, 1960; Murray & Mulder, 1961; Orloff et al, 1997; Orloff & Snyder, 1961; Tamaki et al, 1968; Warren & Muller, 1959). To test this hypothesis, we conducted an experimental evaluation of SSPCS in dogs with BCS (Orloff & Johansen, 1978). Our method of producing BCS in dogs consisted of ligation and division of all hepatic veins except the large left hepatic vein, which was loosely surrounded by an ameroid constrictor (Fig. 77.6; Orloff et al, 1963, 1965; Sweat et al, 1966). The lumen in the ameroid constrictor gradually narrowed as the hygroscopic casein plastic swelled centrally so that the remaining left hepatic vein was completely occluded within several weeks, and severe hepatic outflow block, portal hypertension, and massive, intractable ascites resulted. BCS was produced in 64 dogs, which were put into three treatment groups when ascites was massive: group I underwent sham laparotomy, group II underwent end-to-side PCS, and group III underwent SSPCS.
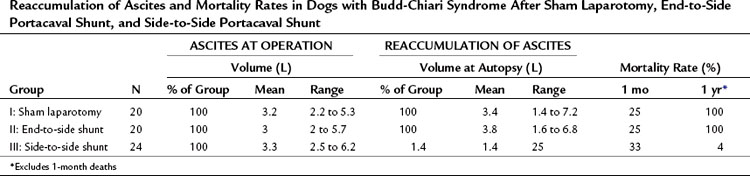
Table 77.6 summarizes the results of our experimental study. All dogs rapidly re-formed ascites after either sham laparotomy or end-to-side PCS and died with massive ascites within 6 months. In striking contrast, only 1 of the 24 dogs in group III reaccumulated ascites after SSPCS, and 67% of these dogs survived in good health without ascites until the time of sacrifice 1 year postoperatively. Before hepatic vein ligation, the liver was normal in all dogs on gross and microscopic examinations. At the time of sham laparotomy or shunt, when BCS was well established, all livers were enlarged two to four times normal size and appeared congested and friable. Liver biopsy specimens at this time showed intense centrilobular congestion, necrosis of cells around the central veins, dilated lymphatics, and subcapsular edema (Fig. 77.7). In the dogs in group I, these gross and microscopic abnormalities were unchanged at the time of death, and substantial fibrosis was found in many of the liver biopsy specimens. Similarly, all of the dogs in group II had hepatomegaly at the time of death, and severe congestion, necrosis, and fibrosis were found in all liver biopsy specimens; although, in 38% of the animals, the microscopic picture was less severe than at the time of the end-to-side shunt operation. Only one dog with SSPCS in group III, the single animal with ascites, had an enlarged liver and microscopic findings of congestion and necrosis at the time of death. The other 23 dogs in group III had a liver that was normal in size and gross appearance at autopsy; liver biopsy specimens were normal in 35% of these dogs and showed minimal fibrosis in the remaining 65%.
Table 77.6 Reaccumulation of Ascites and Mortality Rates in Dogs with Budd-Chiari Syndrome After Sham Laparotomy, End-to-Side Portacaval Shunt, and Side-to-Side Portacaval Shunt
Clinical Results of the Authors
On the basis of the results of our experimental studies, which showed SSPCS was effective in relieving BCS and reversing liver damage, we performed SSPCS in 39 patients with BCS caused by thrombosis of the hepatic veins without involvement of the IVC (Orloff & Girard, 1989; Orloff & Johansen, 1978; Orloff et al, 1992b, 2000); subjects were 21 women and 18 men, and ages ranged from 19 to 45 years. Of the 39 patients, 14 had polycythemia rubra vera, 11 had been taking oral contraceptives, 5 had Behçet disease, and 1 had protein C deficiency. In the remaining 8 patients, no condition predisposing to hepatic vein thrombosis was identified. Patients operated on before 1996 did not undergo the extensive hematologic workup shown in Box 77.2 that is currently performed. The symptoms, signs, results of diagnostic studies, and findings at operation are shown in Tables 77.2 to 77.5.
Tables 77.5 and 77.8 summarize the long-term results of SSPCS in our series. One patient underwent emergency operation, when the thrombosis of his hepatic veins suddenly extended into and occluded his previously patent IVC. An attempt to reverse a rapid downhill course by IVC thrombectomy and PCS was unsuccessful, and the patient died 6 days postoperatively with multiorgan failure and recurrence of IVC thrombosis. The remaining 38 patients (97%) recovered from the shunt operation and were long-term survivors. A striking feature of the early recovery period was a rapid gain in lean body weight that averaged 28 pounds in 3 months.
Table 77.7 Long-Term Results of Portal Decompression Operations in 65 Patients with Budd-Chiari Syndrome Treated by the Authors
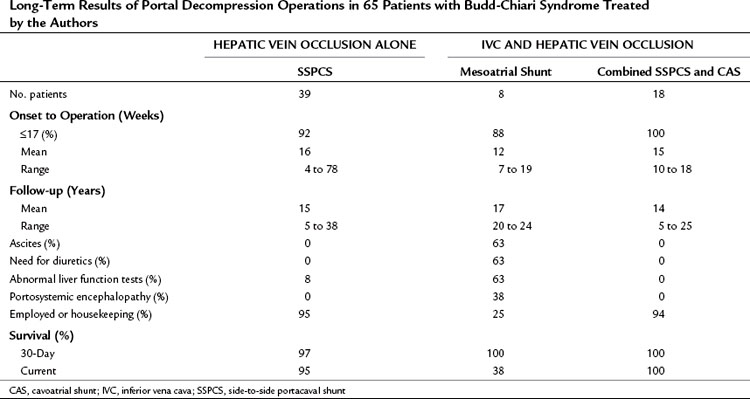
Table 77.8 Results of Follow-up Liver Biopsies Every 1 to 2 Years, Angiography, Ultrasonography, and Pressure Measurements in 64 Surviving Patients with Budd-Chiari Syndrome Who Underwent Surgical Portal Decompression by the Authors
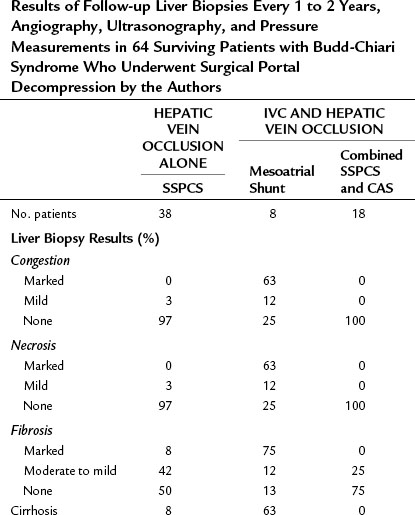
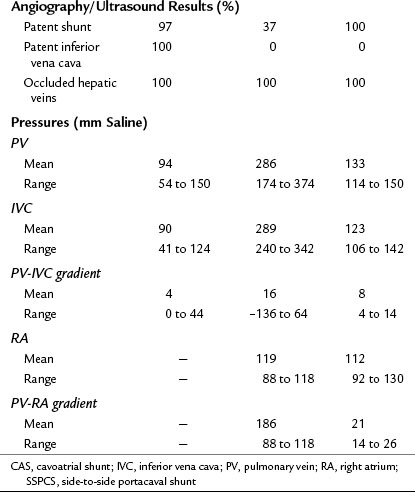
Liver biopsies and angiographic or US studies were performed periodically for 37 years after shunt in the 38 survivors, and the results are summarized in Table 77.8. Cirrhosis persisted in the three patients who already had cirrhosis by the time of the shunt operation, including the patient with Behçet disease. In 97% of the survivors, there was no longer any evidence of hepatic congestion or necrosis in the follow-up biopsy specimens, as shown in Figure 77.8. Mild to moderate fibrosis was found in 42% of the patients, but 50% had normal liver biopsy specimens. Angiography or US showed patency of the PCS and IVC, and pressure measurements showed a wide-open anastomosis with a gradient that ranged from 0 to 44 mm saline and averaged only 4 mm across the shunt. Two patients with polycythemia rubra vera developed thrombosis of the PCS 1 week and 3 months postoperatively. Both patients were operated on immediately, the shunt was taken down, fresh thrombus was removed from the PV, and the portacaval anastomosis was reconstructed with an H-graft of autologous internal jugular vein. Subsequently, both patients were maintained on anticoagulant therapy with warfarin. One patient has remained well for 28 years since revision of the shunt. The other patient developed recurrent shunt thrombosis and required liver transplantation (LT), but he is well 19 years after transplantation. It is now our policy to maintain all patients with a known coagulopathy on permanent anticoagulant therapy.
Our series is the single largest clinical experience reported to date involving the use of SSPCS in BCS confined to the hepatic veins. Our clinical experience confirmed our experimental observations regarding the efficacy of SSPCS in relieving hepatic venous outflow obstruction. All 39 of our patients in whom thrombosis remained localized to the hepatic veins survived the operation and remained free of ascites for years without requiring diuretic therapy, and the shunt remained permanently patent in all but one patient. All but three of the survivors were operated on early in the course of the disease, before liver damage was irreversible; in these patients hepatosplenomegaly disappeared, and liver function rapidly returned to normal. The results of serial liver biopsies performed for 5 to 37 years postoperatively are particularly encouraging, because they showed that the shunt had brought about substantial and long-term reversal of the striking pathologic lesions of BCS (see Fig. 77.8). SSPCS seems to be the most effective treatment of BCS confined to the hepatic veins. The long-term survival rate of the patients in our series has not been equaled by any other form of therapy, medical or surgical.
The single postoperative death in our series taught us a valuable lesson. When this 28-year-old man with polycythemia rubra vera was admitted to our hospital, clear clinical and angiographic evidence showed the occlusive process confined to the hepatic veins. During the 8-day course of his diagnostic workup, however, the thrombosis extended into the IVC, and his condition deteriorated dramatically. An emergency attempt at IVC thrombectomy and PCS failed. Extension of the thrombotic process from the hepatic veins into the IVC is a well-known event in BCS (Parker, 1959), and the potential for this devastating complication exists in every patient; therefore when the diagnosis of hepatic vein occlusion has been made, it seems unwise to delay operation.
SSPCS is indicated only in patients with BCS who have a patent IVC and an IVC pressure that is substantially lower than WHVP or portal pressure. Obstruction or occlusion of the IVC, manifested clinically by edema of the lower extremities and lower trunk and shown by angiography and IVC pressure measurements, is a contraindication to PCS. It is not unusual for patients with BCS caused by hepatic vein occlusion to have an elevated pressure in the IVC as a result of caval compression by an enlarged, congested liver or massive ascites or both. The absolute level of IVC pressure is not crucial to the effectiveness of SSPCS, as long as the portal pressure is substantially higher than caval pressure, and the IVC is shown to be patent. Others have reported relief of BCS by an in-continuity mesocaval shunt in patients with high IVC pressure but substantially higher portal pressure, showing that it is possible to decompress the liver as long as the differential is substantial between pressures in the PV and IVC (Vons et al, 1986). In our long-term follow-up studies after SSPCS, we have observed that compression of the IVC disappears with relief of hepatic congestion, and caval pressure returns to normal.
The importance of performing SSPCS early in the course of BCS cannot be overemphasized. Hepatic venous outflow obstruction produces widespread destruction of the hepatic parenchyma by pressure necrosis and ischemia, and the liver damage becomes irreversible in a surprisingly short time: some patients have developed cirrhosis within 3 or 4 months of the onset of symptoms. Relieving ascites is far less important than decompressing the liver. Recently, groups of physicians that did not include surgeons have recommended a stepwise approach to treatment of BCS, beginning with a trial of anticoagulants, then angioplasty or thrombolysis and stenting, then transjugular intrahepatic portosystemic shunt (TIPS), and finally liver transplantation (Plessier & Valla, 2009; Janssen et al, 2003; de Franchis, 2005; Hoekstra & Janssen, 2008; DeLeve et al, 2009). The stepwise plan carries with it the danger of permitting liver damage to become advanced and irreversible.
Clinical Results of Other Surgeons
Treatment by direct SSPCS or its hemodynamic equivalents—the portacaval interposition graft, mesocaval interposition graft, or splenorenal shunt—of BCS caused by occlusion of the hepatic veins has been reported in more than 300 patients by other surgeons (Ahn et al, 1987; Auvert & Farge, 1963; Bachet et al, 2007; Bismuth & Sherlock, 1991; Cameron et al, 1983; Dong et al, 2005; Eisenmenger & Nickel, 1960; Erlik et al, 1962; Fisher et al, 1999; Gentil-Kocher et al, 1988; Gibson, 1960; Hemming et al, 1996; Henderson et al, 1990; Hoyumpa et al, 1971; Huguet et al, 1979; Klein et al, 1990; Langer et al, 1975; Ludwick et al, 1967; Malt et al, 1978; McCarthy et al, 1985; Millikan et al, 1985; Montano-Loza et al, 2009; Murad et al, 2004; Noble, 1976; Panis et al, 1994; Pezzuoli et al, 1985; Powell-Jackson et al, 1982; Prandi et al, 1975; Schramek et al, 1974; Singhal et al, 2006; Slakey et al, 2001; Vons et al, 1986; Wang, 1989; Wu et al, 1990; Zeitoun et al, 1999). Many of the reports have involved descriptions of single cases. Some more recent retrospective multiinstitutional studies involving reviews of medical records have been based on analyses, as a single entity, of patients with widely different characteristics, such as the stage and type of BCS, time lapsed from diagnosis to treatment, site of hepatic venous outflow occlusion, and type of portosystemic shunt. Portacaval, mesocaval, splenorenal, mesoatrial, mesoinnominate, cavoatrial, portoatrial, and TIPS have been considered as a single entity termed a portosystemic shunt (Murad et al, 2004; Zeitoun et al, 1999). It is not possible to compare the results of these studies with our results, which were based on a prospective study of patients with BCS caused by hepatic vein thrombosis alone, all of whom underwent a direct SSPCS early in the course of their disease by a single surgeon at a single institution.
At institutions that have reported series of cases, success rates of portal decompression in BCS have been approximately 85% after direct SSPCS (Ahn et al, 1987; Bismuth & Sherlock, 1991; Hemming et al, 1996; McCarthy et al, 1985; Panis et al, 1994; Pezzuoli et al, 1985) and 67% after splenorenal shunt (Ahn et al, 1987; McCarthy et al, 1985; Millikan et al, 1985; Wang, 1989). Mesocaval or portacaval interposition grafts with autologous internal jugular vein have been reported in about 50 patients with BCS, most of them in France, with a success rate of 89% (Bismuth & Sherlock, 1991; Gentil-Kocher et al, 1988; McCarthy et al, 1985; Panis et al, 1994; Pezzuoli et al, 1985; Vons et al, 1986). Mesocaval or portacaval interposition grafts using synthetic materials, such as Dacron or polytetrafluoroethylene (Gore-Tex), have been successful in approximately 52% of 39 patients with BCS, which is the lowest success rate of the various in-continuity portal decompressive procedures (Ahn et al, 1987; Hemming et al, 1996; Henderson et al, 1990; Klein et al, 1990; McCarthy et al, 1985; Millikan et al, 1985; Pezzuoli et al, 1985; Wang, 1989). In our opinion, use of synthetic interposition portacaval or mesocaval grafts and the splenorenal shunt are inferior to direct SSPCS in the treatment of BCS, although all three shunts are hemodynamically similar. Occlusion of the shunt is a serious complication that may result in death: the splenorenal shunt and the synthetic interposition grafts have a substantial incidence of thrombosis, not only in BCS but also in other diseases that cause portal hypertension (Dowling, 1979; Hemming et al, 1996; Orloff, 1977, 1998; Orloff & Orloff, 1992); in contrast, the incidence of shunt occlusion in our series of more than 2500 direct SSPCS procedures—most of which have been followed up repeatedly by catheterization, angiography, or US—is 0.5%.
Four patients underwent end-to-side PCS for treatment of BCS. One patient had a favorable response during 1 year of follow-up (Marchal et al, 1974), but the other three died (Alpert, 1976; Brink & Botha, 1955; Langer et al, 1975). Because the hepatic side of the transected PV is ligated in an end-to-side PCS, the PV cannot function as an outflow tract to decompress the liver. It has been well established that in the presence of hepatic outflow block, the side-to-side anastomosis is substantially more effective than the end-to-side shunt in overcoming intrahepatic hypertension (Reynolds et al, 1962; Taylor & Myers, 1956; Warren & Muller, 1959).
Since our chapter on BCS was being prepared for the previous edition of this book 6 years ago, a number of reviews by nonsurgeons have been published (Janssen et al, 2003; Bachet et al, 2007; Plessier & Valla, 2008; Hoekstra & Janssen, 2008; Amarapurkar et al, 2008; DeLeve et al, 2009). These reviews, authored by physicians who had no personal experience with surgical treatment of BCS, were based on retrospective data collected from the literature, and they contained negative evaluations of the portosystemic shunt that were contrary to the results of our prospective studies. Examples of these negative evaluations that we believe are erroneous are: 1) “Overall perioperative mortality has been high, averaging 25%” (our perioperative mortality rate is 0%); 2) “The rate of shunt dysfunction due to early or late thrombosis or to late stenosis has reached 30% in series with long-term follow-up” (only 2 [5%] of our 38 patients developed thrombosis of the PCS 1 week and 3 months postoperatively, and this was permanently corrected by reoperation); 3) “Surgical portosystemic shunting … failed to show any impact on survival after adjustment for independent prognostic factors” (the survival rate in our series 5 years to 38 years postoperatively is 95%).
It is noteworthy that recent reviews, authored mainly by surgeons who have had personal experience with portosystemic shunts, have been supportive of the use and effectiveness of this important modality of the treatment in BCS (Menon et al, 2004; Klein, 2006; Zimmerman et al, 2006; Singhal et al, 2006; Montano-Loza et al, 2009).
Authors’ Technique of Side-to-Side Portacaval Shunt
We have performed more than 2500 SSPCS operations over 50 years. From this experience, we have concluded that it is possible, with the proper technique, to perform a satisfactory SSPCS in almost every patient who has a patent PV. Contrary to some statements in the literature, we have been able to perform SSPCS without difficulty in every patient with BCS on whom we have operated. We have performed shunt catheterization and angiography or US every 1 to 2 years in almost all of our patients since the 1970s, and we have observed a 99.5% long-term shunt patency rate. Our technique of SSPCS (Orloff, 1983; Orloff & Orloff 1992, 1994; Orloff et al, 2007) is illustrated in Figures 77.9 to 77.18. Important technical features of the operation are as follows:
1 The position of the patient on the operating table is crucial and can make the difference between an easy and a difficult operation.
2 A long right subcostal incision is associated with fewer postoperative complications than a thoracoabdominal incision and is much preferred.
3 Use of electrocautery throughout the operation substantially reduces both operating time and blood loss.
4 Bleeding from the many portosystemic collateral vessels is best managed by pressure with gauze sponge packs, particularly because most of the bleeding stops as soon as the portacaval anastomosis is completed, and the portal hypertension is relieved. Attempts to control each of the bleeding collaterals with ligatures and sutures prolong the operation and increase blood loss: the objective is to decompress the portal system as rapidly as possible.
5 Circumferential mobilization of the IVC between the entrance of the renal veins and the liver is essential for the side-to-side anastomosis and is neither hazardous nor difficult to perform. Apposition of the two vessels is greatly facilitated by elevation of the IVC toward the PV.
6 Essential for the side-to-side anastomosis is mobilization of a long segment of PV, which includes division of the tough fibrofatty tissue that binds the PV to the pancreas and sometimes includes division of a bit of the head of the pancreas.
7 Beware of the replaced hepatic artery crossing the PV behind the head of the pancreas! Ligation of the hepatic artery may be lethal. Palpate it with the index finger in the tunnel between the head of the pancreas and PV.
8 Resection of an enlarged caudate lobe of the liver to facilitate apposition of the two vessels is hazardous and unnecessary.
9 Pressures in the IVC and PV always should be measured after completion of the PCS. A pressure gradient greater than 50 mm saline is unacceptable and requires revision of the anastomosis.
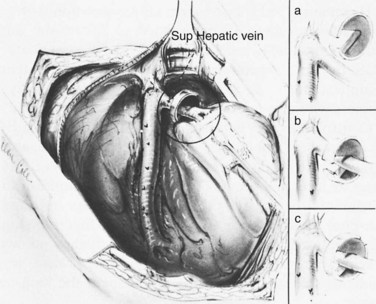
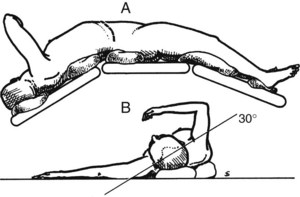
Figure 77.9 shows the position of the patient on the operating table, viewed from the patient’s right side. The patient is positioned with the right side elevated at an angle of 30 degrees to the table, and the table is “broken” at the level of the costal margin and at the knees to widen the space between the right costal margin and right iliac crest.
Figure 77.10 shows the incision. A long right subcostal incision extending from the xiphoid to well into the flank is made two fingerbreadths below the costal margin; we have used this incision in every operation over the course of 50 years. The skin is incised superficially with the scalpel, and the other layers are incised with electrocautery, which greatly reduces blood loss and shortens the operating time. When electrocautery is used, it is usually unnecessary to clamp any blood vessels with hemostats. The right rectus abdominis, external oblique, and transverses abdominis muscles are completely divided, and the medial 3 to 4 cm of the latissimus dorsi muscle is often incised. The peritoneum often contains many collateral blood vessels and is incised with the electrocautery to obtain immediate hemostasis.
Figure 77.11 shows the exposure of the operative field. The viscera are retracted by three Deaver retractors positioned at right angles to each other: the inferior retractor retracts the hepatic flexure of the colon toward the feet, the medial retractor displaces the duodenum medially, and the superior retractor retracts the liver and gallbladder toward the head. The posterior peritoneum overlying the IVC is incised with electrocautery by an extended Kocher maneuver just lateral to the descending duodenum, and the retractors are repositioned to retract the head of the pancreas medially and the right kidney caudally. Alternatively, a self-retaining retractor may be used in place of the three handheld Deaver retractors.
Figure 77.12 shows the isolation of the IVC: the anterior surface is cleared of fibroareolar tissue, and the IVC is isolated around its entire circumference, from the entrance of the right and left renal veins below to the point where it disappears behind the liver above. The IVC is surrounded with an umbilical tape. To accomplish the isolation, several tributaries may have to be ligated in continuity with fine silk ligatures and then divided. These include the right adrenal vein, one or two pairs of lumbar veins that enter on the posterior surface, and the caudal pair of small hepatic veins that enter on the anterior surface of the IVC directly from the liver. When the IVC has been mobilized completely, it can be lifted up toward the PV. Failure to isolate the IVC circumferentially is one major reason for the erroneous claim that SSPCS often cannot be performed, because the PV and IVC are too widely separated.
Figure 77.13 shows the exposure of the PV. The superior retractor is repositioned medially so that it retracts the liver and gallbladder at the point of entrance of the portal triad. The PV is located in the posterolateral aspect of the portal triad and is approached from behind. The fibrofatty tissue on the posterolateral aspect of the portal triad contains nerves, lymphatics, and lymph nodes and is divided by blunt and sharp dissection; this is a safe maneuver, because there are no portal venous tributaries on this aspect of the portal triad.
Figure 77.14 shows the mobilization of the PV behind the pancreas. Using the umbilical tape to pull the PV out of its bed, it is cleared to the point where it disappears behind the pancreas. The tough fibrofatty tissue that binds the PV to the pancreas must be divided. Several tributaries that enter the medial aspect of the PV and one tributary that enters the posterolateral aspect are divided, but it is usually unnecessary to divide the splenic vein. Wide mobilization of the PV is essential for performance of a side-to-side portacaval anastomosis. Failure to mobilize the PV behind the pancreas is a second major reason for difficulty in accomplishing an SSPCS. In some patients, it is necessary to divide a bit of the head of the pancreas to obtain adequate mobilization of the PV. Bleeding from the edges of the divided pancreas is controlled with suture ligatures. Division of a small amount of the pancreas is a helpful maneuver, and we have never observed postoperative complications, such as pancreatitis, from its performance.
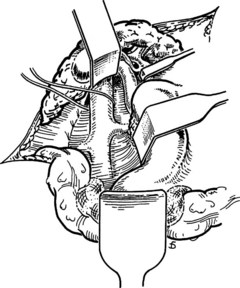
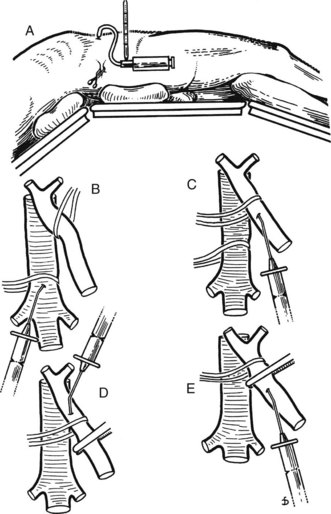
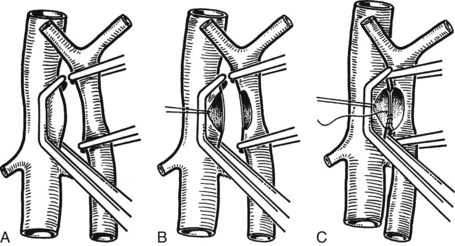
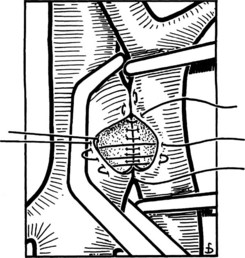
Figure 77.15 shows the measurement of pressures in the IVC and PV before performing the portacaval anastomosis. The performance of an SSPCS is illustrated in Figures 77.16 to 77.18. After it is determined that the IVC and PV can be brought together without excessive tension, a Satinsky clamp is placed obliquely across a 5-cm segment of the anteromedial wall of the IVC in a direction that is parallel to the course of the overlying PV, and the IVC is elevated toward the PV. One 5-cm segment of the PV is isolated between two angled vascular clamps, and the PV is depressed toward the IVC, bringing the two vessels into apposition (see Fig. 77.16). One 2.5-cm long, thin strip of the IVC and one 2.5-cm strip of the PV are excised with the scissors (see Fig. 77.16B). It is important to excise a thin longitudinal segment of the wall of each vessel rather than simply to make an incision in each vessel. A retraction suture of 5-0 vascular suture material is placed in the lateral wall of the vena caval opening and is weighted by attachment to a hemostat to keep the vena caval orifice open. The clamps on the PV are momentarily released to flush out any clots, then the openings in both vessels are irrigated with heparinized saline.
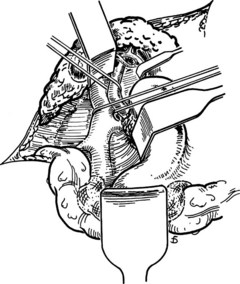
The anastomosis is started with a posterior, continuous, over-and-over suture of 5-0 vascular suture material (see Fig. 77.16C). The posterior continuous suture is tied at each end of the anastomosis. The anterior row of sutures consists of an everting, continuous, horizontal mattress stitch of 5-0 vascular material started at each end of the anastomosis (see Fig. 77.17). The suture started at the superior end of the anastomosis is discontinued after three or four throws and is deliberately left loose so that the interior surface of the vessels can be visualized as the anastomosis is completed. In this way, inadvertent inclusion of the posterior wall in the anterior row of sutures is avoided. The suture started at the inferior end of the anastomosis is inserted with continuous tension until it meets the superior suture, at which point the superior suture is drawn tight, and the two sutures are tied to each other. Before drawing the superior suture tight, the clamps on the PV are momentarily released to flush out any clots, and the anastomosis is thoroughly irrigated with heparinized saline.
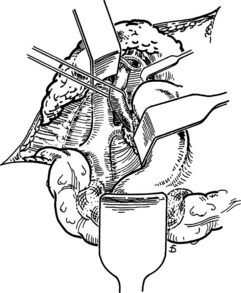
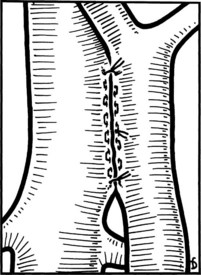
Figure 77.18 shows the finished anastomosis, upon completion of which a single interrupted tension suture is placed just beyond each end of the anastomosis to take tension off the anastomotic suture line. The clamp on the IVC is removed first, the clamp on the hepatic side of the PV is removed next, and finally the clamp on the intestinal side of the PV is removed. Bleeding from the anastomosis occurs infrequently, but it can be controlled by one or two well-placed interrupted sutures of 5-0 vascular suture material.
Treatment of Inferior Vena Cava Occlusion by Portal Decompression
Mesoatrial Shunt
Our results of mesoatrial shunting are shown in Tables 77.5, 77.7, and 77.8. A mesoatrial shunt reduced the mean gradient between the PV and right atrium from 211 to 78 mm saline. All eight patients survived the operation and left the hospital alive, although five (63%) of the eight patients subsequently developed thrombosis of the graft and died from liver failure. Three of the deaths occurred during the first postoperative year, one occurred in the second year, and one occurred in the fifth year. All five patients developed recurrence of ascites, and three developed portosystemic encephalopathy as their hepatic function deteriorated. The causes of BCS in the five patients whose mesoatrial shunt failed were oral contraceptives in one, polycythemia rubra vera in one, antithrombin III deficiency in one, and a postpartum state in one; the cause of the other one was unknown. These causes were no different from those found in the long-term survivors of portal decompression in our series. The patients whose mesoatrial shunt failed were not a higher risk group; they died because their mesoatrial shunt thrombosed 1 to 5 years after insertion, not because they were poorer risk patients to start with.
Several series of mesoatrial shunting that involve 5 or more patients have been reported in the literature, although the length of follow-up generally has been relatively short. Stringer and colleagues (1989) of London reported excellent results in 5 patients, but the follow-up period of 9 to 16 months was too short to warrant conclusions. Wang and colleagues (1989, 2005) of Beijing reported a survival rate of 61% and 50% at 5 and 10 years, respectively, in 70 patients, but his reports contain insufficient details to determine how many of the patients had patent and functioning grafts, how well the patients were followed, or the exact nature of the BCS that was being treated. Emre and colleagues (2000) of Istanbul reported an 85% survival rate of 13 patients during follow-up of 1 to 76 months, with one known thrombosed shunt. Behera and associates (2002) of Chandigarh, India, described 100% graft patency during 6 to 71 months of follow-up of 10 patients, with a survival rate of 90%. Khanna and colleagues (1991) of Chandigarh, India, reported a 62% survival rate of 13 patients during follow-up of 2 months to 7.5 years.
The results of Henderson and colleagues’ (1990) study of nine patients, of Klein and colleagues (Klein et al, 1990; Slakey et al, 2001) study of 15 patients, and of our own investigations of eight patients are representative of the experience with mesoatrial shunting in the United States. Survival rates ranged from 38% to 67%, and the percentage of patients with grafts that were patent and decompressing was only 33% to 60% during relatively short follow-up periods. Cameron and colleagues (1984; Klein et al, 1990) reported improved results with the use of an 8-cm external silicone rubber sleeve around a 16-mm ring-reinforced Gore-Tex prosthesis to prevent compression of the graft by the sternum. This innovation may reduce the frequency of graft thrombosis, but the results of mesoatrial shunting generally have been disappointing.
The high thrombosis rate of the mesoatrial shunt may be due to the fact that it is a relatively low-flow synthetic graft placed within the venous system. It does not decompress the obstructed IVC. To overcome these shortcomings, we worked in the experimental laboratory to devise a new operation for this form of BCS consisting of a combined SSPCS and cavoatrial shunt (CAS) through a Gore-Tex graft (Orloff et al, 1992a). This combined shunt was aimed at decompressing the hypertensive IVC and shunting the entire systemic venous flow from the lower two thirds of the body and the entire portal venous flow through the graft.
Experimental Studies
BCS was produced in rats by gradual occlusion of the suprahepatic IVC with an ameroid constrictor. As the hygroscopic casein plastic took up fluid and swelled centrally, the lumen in the ameroid gradually narrowed until, after 25 to 30 days, it completely occluded the IVC and produced severe hepatic outflow block, portal hypertension, and massive intractable ascites typical of BCS. One month after insertion of the ameroid constrictor around the IVC, when all rats had massive ascites, hepatomegaly, and portal hypertension, they were randomly divided into three groups (Fig. 77.19). Group I was composed of 16 control rats that underwent a sham thoracolaparotomy when BCS was well established. Group II consisted of 22 rats subjected to a mesoatrial shunt from the superior mesenteric vein to the right atrium with a 3-mm Gore-Tex graft inserted by microsurgical technique. Group III comprised 22 rats that underwent SSPCS combined with CAS from the IVC to the right atrium with a 3-mm Gore-Tex graft. Forty-four rats survived the operations and were studied.
Table 77.9 summarizes the results in the three groups of rats. All control rats that underwent sham thoracolaparotomy in group I rapidly reaccumulated ascites and died within 2 months. Nine of 16 rats with mesoatrial shunts in group II re-formed ascites and died within 2 months. All of these animals were found at autopsy to have thrombosis of the mesoatrial graft. In contrast, only 2 of 16 rats treated by combined SSPCS and CAS in group III developed CAS-graft thrombosis, re-formed ascites, and died. The remaining 14 rats in group III were found to have patent grafts and shunts when they were sacrificed after 3 months. Liver biopsy specimens in all rats were normal before IVC constriction, and after BCS was well established, they showed intense centrilobular congestion and moderate central necrosis. Severe pathologic changes persisted until death or sacrifice in all control rats in group I and in 11 of 16 rats with mesoatrial shunts in group II; however, combined SSPCS and CAS in group III reversed the liver pathology in 14 of 16 rats.
Our Clinical Results with Combined Side-to-Side Portacaval Shunt and Cavoatrial Shunt
On the basis of the encouraging results of our experimental studies, we have treated 18 seriously ill patients with BCS caused by IVC thrombosis with combined SSPCS and CAS using a 20-mm ring-reinforced Gore-Tex graft. The characteristics of the patients are summarized in Tables 77.4 and 77.5. The study included 12 men and 6 women, ranging in age from 25 to 37 years. All 18 patients were in good health before the onset of symptoms and signs, which consisted of marked ascites, edema of the lower extremities, hepatosplenomegaly, abdominal pain, weakness, and muscle wasting. The combined operation is depicted in Figure 77.20.
The procedure was performed through a long right subcostal incision for the PCS and then a medium sternotomy for the CAS. After completion of the SSPCS, a 20-mm diameter, ring-reinforced Gore-Tex graft was anastomosed to the side of the IVC at the level of the PCS. The graft was channeled anterior to the liver through an opening made in the right leaf of the diaphragm and was anastomosed to the side of the right atrium. Combined SSPCS and CAS reduced the mean gradient between the PV and right atrium from 196 mm saline to 23 mm saline. All 18 patients survived the combined shunt operation and are alive 5 to 25 years postoperatively (see Tables 77.7 and 77.8). Mean follow-up is 12 years. All patients are free of ascites, and none requires diuretics; hepatomegaly and splenomegaly are gone; and liver function tests results are normal in all survivors, with no instance of portosystemic encephalopathy. All survivors are gainfully employed or keeping house and leading productive lives of good quality.
Serial percutaneous liver biopsy specimens have shown normal architecture in 14 patients and mild fibrosis in four. Serial angiographic studies with pressure measurements and Doppler duplex US have shown patency and good function of SSPCS and CAS in all patients (Fig. 77.21). Recanalization of the IVC or hepatic veins has not occurred. Permanent anticoagulation with warfarin and low-dose acetylsalicylic acid (aspirin) has been maintained in all patients. In our program, combined SSPCS and CAS has replaced mesoatrial shunt as the preferred treatment for BCS caused by IVC obstruction.
Other Types of Surgical and Radiologic Therapy
Transjugular Intrahepatic Portosystemic Shunt
TIPS (see Chapter 76E) is SSPCS inserted percutaneously under radiographic control. As such, it is based on the same rationale for relieving hepatic venous outflow obstruction and intrahepatic portal hypertension as the surgical SSPCS. The attractiveness of TIPS lies in the fact that it can be done without a major surgical abdominal operation in patients who have liver disease, who are often quite ill. Its major disadvantage is a substantial incidence of occlusion of the TIPS that may require repeated revisions and hospitalizations and often involves recurrence of symptoms. In some patients who have complete occlusion of the hepatic veins or complete occlusion of the IVC, an additional disadvantage is technical inability to insert the TIPS. Insertion of the TIPS by direct puncture of the retrohepatic IVC, which sometimes is the only way that the shunt can be created, may be associated with a substantial incidence of complications (Rössle et al, 2004).
Between 1995 and 2004, there were many reports of TIPS treatment of BCS, each involving a few patients (Blum et al, 1995; Cejna et al, 2002; Ganger et al, 1999; Huber et al, 1997; Mancuso et al, 2003; Michl et al, 1999; Perello et al, 2002; Rogopoulos et al, 1995; Rössle et al, 1998; Uhl et al, 1996). The largest and longest reported experience with TIPS in BCS during that period was that of Rössle and colleagues (2004) at the University Hospital of Freiburg, Germany, who described their experience with 35 patients—11 acute, 13 subacute, and 11 chronic—who were followed up for a mean of 37 (± 29) months. Shunt failure occurred in 7 patients (20%): 2 were technical failures, 2 required LT (1 died), and 3 died after TIPS. Excluding the 2 patients who required LT but including the 2 who could not have TIPS for technical reasons, the 5-year survival rate was 74%. TIPS occlusion requiring revision occurred in 19 (58%) of 33 patients. One patient required 10 revisions during a follow-up period of 53 months.
Results reported by others were not as good as those of the Freiburg group. Mancuso and associates (2003) described a series of 15 patients, with 5 deaths and 1 technical failure—a 40% negative outcome. Cejna and colleagues (2002) reported a series of 8 patients; 2 (25%) died 2 weeks after TIPS, 1 developed TIPS occlusion requiring LT, and 3 others required two to seven revisions for TIPS stenosis. Only 2 (25%) of 8 of the initial series of patients had revision-free TIPS patency. Perello and colleagues (2002) reported a series of 13 patients in which there were three shunt failures (23%). The failures included 1 death, 1 TIPS thrombosis that necessitated a surgical shunt, and 1 patient that required LT. Of the remaining 10 patients, 7 (70%) developed TIPS occlusion. In 5 of these, TIPS dysfunction had not been corrected.
Since 2004, use of TIPS in BCS has increased substantially, mainly in Europe, in part as a result of the availability of polytetrafluoroethylene (PTFE)-covered stents. Most of the reports have involved retrospective reviews of small numbers of cases followed up for short periods of time. In 2006, Rössle and colleagues reported results of TIPS with PTFE-covered stents in 112 patients, 17 of whom had BCS. Of these, 12 patients were lost to follow-up, and 16 developed TIPS failure. The 1-year TIPS failure rate was 10%, 22 patients died, and 3 underwent LT. The mortality rate without and with the patients lost to follow-up was 20% and 30%, respectively. The authors concluded that the TIPS procedure was improved by the PTFE-covered stent.
Other recent reports of TIPS treatment of BCS include a 124 patients (Murad et al, 2008 [16 patients]; Garcia-Pagan et al, 2008; Eapen et al, 2006 [30 patients]; Plessier et al, 2006 [20 patients]; Gandini et al, 2006 [13 patients]; Hernandez-Guera et al, 2004 [25 patients]; Attwell et al, 2004 [17 patients]). Garcia-Pagan and colleagues (2008) conducted a retrospective review of 124 BCS patients treated with TIPS in six European centers between 1993 and 2006. It is noteworthy that 147 of 221 patients with BCS were eligible for TIPS, but 14 were excluded for technical contraindications, and attempts at TIPS failed in an additional nine patients. Thus, in a population of patients with BCS, only 60% actually underwent TIPS. Twenty-two patients had complications associated with the TIPS procedure, and 2 died as a result. Sixty-one (41%) of the 124 patients had TIPS dysfunction during follow-up that required restenting in 35, angioplasty in 20, and thrombolysis in 6. Portosystemic encephalopathy developed in 21% of patients within 1 year. During follow-up, 16 patients died (13%), and 8 required LT (6.5%). Actuarial LT-free survival rates at 1, 5, and 10 years were 88%, 78%, and 69%, respectively.
The survival rate of surgical portosystemic shunt in our series is shown in Table 77.7. In patients with acute and subacute BCS resulting from occlusion of the hepatic veins, operative mortality after SSPCS has been 3%, and 5-year survival has been 97%. In patients with BCS secondary to IVC occlusion treated by combined SSPCS and CAS, the 5-year survival has been 100%. All of the patients have been relieved of the symptoms of BCS, and readmission to the hospital has been infrequently necessary. These results are superior to the best reported results of TIPS in comparable patients. Without doubt, the long-term health care costs of TIPS are substantially higher than the costs of surgical therapy.
Liver Transplantation
LT (see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ) is a radical, drastic treatment for BCS, but it is effective when a patient’s life is at stake, and no other form of therapy would work. LT is not in competition with SSPCS or any other type of treatment. It is useful in far advanced, decompensated liver disease, when it is too late to accomplish survival with portal decompression procedures.
1 Cirrhosis with progressive liver failure that has reached the point of permitting a reasonable prediction that the patient will die within 1 year—the most common indication for LT and, incidentally, it is the same one used widely in other liver diseases (Scharschmidt, 1984; Schenker, 1984)
2 Failure of a portosystemic shunt or TIPS, usually because of thrombosis, with persistence or recurrence of symptoms and signs of BCS
3 BCS with unshuntable portal hypertension secondary to thrombosis of the PV, splenic vein, and much of the superior mesenteric vein—a rare indication that applies if patent blood vessels are available to vascularize the liver allograft
4 Acute fulminant hepatic failure—a rare indication and one that we have not encountered
Using these indications, 12 of our patients have been approved and listed for LT. Their characteristics are shown in Table 77.10. In 10 of the 12 patients, an underlying thrombogenic hematologic disorder was identified. The time lapse from clinical onset of BCS to LT was much longer than in our patients who were treated by SSPCS, ranging from 11 to 21 months and averaging 15.9 months. These patients had a chronic form of BCS. The indication for LT was cirrhosis with progressive liver failure in 11 patients whose severe hepatic decompensation precluded consideration of SSPCS, and thrombosis of a PCS with recurrence of clinical manifestations of BCS was the indication in one patient. In 11 of our patients, thrombosis was confined to the hepatic veins, and in one patient, the hepatic veins and suprahepatic IVC were occluded. All but one patient, the one with an occluded PCS, had a patent PV.
Table 77.10 Characteristics of 12 Patients Approved and Listed for Liver Transplantation for Budd-Chiari Syndrome
| Age (Years) | |
| Mean | 31 |
| Range | 25 to 37 |
| Female/male | 7/5 |
| Predisposing Condition | |
| Polycythemia rubra vera | 6 |
| Oral contraceptives | 2 |
| Protein C deficiency | 1 |
| Antithrombin III deficiency | 1 |
| Unknown | 2 |
| Onset of BCS to Liver Transplant (Months) | |
| Mean | 15.9 |
| Range | 11 to 21 |
| Indication for Liver Transplant | |
| Cirrhosis with liver failure | 11 |
| Failed portacaval shunt | 1 |
| Thrombosed hepatic veins | 12 |
| Patent inferior vena cava | 11 |
| Patent portal vein | 11 |
BCS, Budd-Chiari syndrome
Table 77.11 shows the clinical findings in our 12 patients. All patients had massive ascites, jaundice, severe muscle wasting, hepatomegaly, splenomegaly, and dilated collateral veins on the abdominal wall. Encephalopathy had occurred in 50%, and one or more episodes of bleeding varices had occurred in 58%. All patients had cirrhosis on preoperative liver biopsy and in the recipient liver removed at LT. This was a group with advanced, decompensated liver disease. All of them were Child-Turcotte-Pugh (CTP) class C (Child & Turcotte, 1964) determined by the quantitative scoring method of Campbell and colleagues (1973).
Table 77.11 Preoperative Clinical Findings in 12 Patients Approved and Listed for Liver Transplantation for Budd-Chiari Syndrome
| Finding | % |
|---|---|
| Ascites (3.8 to 7.2 L) | 100 |
| Jaundice | 100 |
| Severe wasting | 100 |
| Encephalopathy | 50 |
| Bleeding varices | 58 |
| Hepatomegaly | 100 |
| Splenomegaly | 100 |
| Dilated abdominal veins | 100 |
| Cirrhosis on biopsy | 100 |
| Child-Turcotte-Pugh class C | 100 |
Table 77.12 shows the results of preoperative liver function tests. Serum bilirubin was substantially elevated, and serum albumin was markedly depressed in every patient. Prothrombin activity was invariably low, resulting in a mean international normalized ratio of 2.0. Aspartate aminotransferase and serum alkaline phosphatase were significantly elevated. Liver function test results were highly abnormal and reflected serious hepatic failure.
Table 77.12 Preoperative Liver Function Tests in 12 Patients Approved and Listed for Liver Transplantation for Budd-Chiari Syndrome
| Liver Function Test | Mean | Range |
|---|---|---|
| Serum total bilirubin (mg/dL) | 6.0 | 5.1-6.8 |
| Serum albumin (g/dL) | 2.1 | 1.8-2.8 |
| International normalized ratio | 2.0 | 1.7-2.3 |
| Aspartate aminostransferase (IU) | 150 | 122-185 |
| Serum alkaline phosphatase (IU) | 233 | 198-282 |
Table 77.13 shows the survival rate of the 12 patients who were approved and listed for LT. Three patients died of liver failure while awaiting LT during periods of 4 to 10 months. Two patients died during the immediate period after LT, one from relentless graft rejection and one from sepsis with multisystem organ failure. A third patient died 9 months postoperatively of liver failure, but no patients were lost after the first year. The 1- and 5-year survival rates were 50%. If the three patients who died before LT could be accomplished are excluded, the actual 1-year and 5-year survival rates of the patients who underwent LT are 67%. The 5-year survival rate is comparable to the reported survival rate of LT in CTP class C patients who do not have BCS.
Table 77.13 Long-Term Results of Liver Transplantation for Budd-Chiari Syndrome in 12 Patients Approved and Listed for Orthotopic Liver Transplantation
| Survival | % |
|---|---|
| Operative mortality rate (n = 2/9) | 22 |
| One-year mortality rate (n = 6/12) | 50 |
| Five-year survival (n = 6/12) | 50 |
| Five-year survival of patients transplanted (n = 6/9) | 67 |
| Causes of Six Deaths | |
| Rejection (25 days; n = 1) | |
| Sepsis (30 days; n = 1) | |
| Liver failure (9 months; n = 1) | |
| Liver failure awaiting OLT (4 to 10 mo, n = 3) | |
| Quality of Life in Six Survivors of OLT | |
| Ascites (n = 2) | 33 |
| Need for diuretics (n = 2) | 33 |
| Normal liver function tests (n = 5) | 83 |
| Encephalopathy (n = 2) | 33 |
| Working full time (n = 5) | 83 |
| Recurrent BCS | 0 |
| Receiving anticoagulants (n = 6) | 100 |
BCS, Budd-Chiari syndrome; OLT, orthotopic liver transplantation
The six long-term survivors of LT have been followed up for more than 5 years and have had a life of good quality (see Table 77.13). Five are working full time, and BCS has not recurred in any. All survivors were placed on anticoagulant therapy postoperatively and are receiving lifelong maintenance warfarin and aspirin treatment.
Experience with Liver Transplantation in Budd-Chiari Syndrome Reported in the Literature
To date, the English literature contains reports of more than 1000 patients with BCS who have undergone LT, and Table 77.14 summarizes these reports. The largest number of case reports have come from reviews of 248 patients registered in the European Liver Transplantation Registry and 510 patients registered in the United Network for Organ Sharing (UNOS) Liver Standard Transplant Analysis and Research files. All of the reports were based on retrospective reviews of medical records. No prospective studies of LT in BCS have been done.
Table 77.14 Indications for Liver Transplantation in 15 Retrospective Series of Budd-Chiari Syndrome Reported in the Literature
| Reference | N | Indications for OLT |
|---|---|---|
| Srinivasan et al, 2002 | 19 | Failed shunt, 5 |
| Acute liver failure, 6 | ||
| Chronic liver failure, 8 | ||
| Melear et al, 2002 | 17 | Not stated |
| Hemming et al, 1996 | 10 | Failed shunt, 3 |
| Advanced cirrhosis, 4 | ||
| IVC obstruction, 2 | ||
| Ringe et al, 1995 | 43 | Unclear, 39 |
| Failed shunt, 4 | ||
| Knoop et al, 1994a, 1994b | 8 | Child class C, 5 |
| Child class B, 3 | ||
| Galati et al, 1993 | 32 | Failed shunt, 2 |
| Cirrhosis–? | ||
| Hepatic necrosis–? | ||
| Shaked et al, 1992 | 14 | Failed shunt, 2 |
| Advanced cirrhosis, 4 | ||
| Poor synthetic function, 8 | ||
| Halff et al, 1990; Iwatsuki et al, 1991 | 32 | Failed shunt, 5 |
| Intractable ascites, 17 | ||
| Recurrent variceal bleeding, 14 | ||
| Encephalopathy, 15 | ||
| Sakai & Wall, 1994 | 11 | Chronic BCS, 5 |
| Acute fulminant BCS, 3 | ||
| Failed shunt, 3 | ||
| Jamieson et al, 1991 | 26 | Failed shunt, 2 |
| Unclear, 24 | ||
| Ulrich et al, 2008 | 42 | Cirrhosis, 11 |
| Portal vein occlusion, 10 | ||
| Hepatic necrosis, 12 | ||
| Severe acute liver failure, 8 | ||
| Hepatocellular carcinoma, 3 | ||
| Hepatic artery occlusion, 2 | ||
| Cruz et al, 2005 | 11 | Failed TIPS, 5 |
| Failed PSS, 1 | ||
| Intractable ascites, 1 | ||
| Portal-systemic encephalopathy, 3 | ||
| Plessier et al, 2006 | 11 | Failed therapy with anti-coagulation and TIPS, 11 |
| Mehta et al, 2006 | 248 | Fulminant hepatic failure, 47 |
| European Liver Transplantation Registry, 1988-1999 | Renal failure, 40 Portal vein thrombosis, 47 | |
| Failed PSS, 49 | ||
| Failed TIPS, 11 | ||
| Failed percutaneous angioplasty, 18 | ||
| Segev et al, 2007 (UNOS Registry, 1987-2006) | 510 | Not reported |
BCS, Budd-Chiari syndrome; IVC, inferior vena cava; OLT, orthotopic liver transplantation; PSS, portosystemic shunt; TIPS, transjugular intrahepatic portosystemic shunting; UNOS, United Network for Organ Sharing.
As shown in Table 77.14, the indications for LT varied greatly, and it cannot be said that LT was done in patients who had 1 year or less to live because of cirrhosis with progressive liver failure. In several series (Jamieson et al, 1991; Melear et al, 2002; Ringe et al, 1995; Segev et al, 2007), the indications were not clearly stated. Ulrich and colleagues (2008) reported 12% of the 39 patients were CTP class A, and 57% were class B, obviously not in liver failure. The report of Halff and colleagues (1990) stated, “The decrease in synthetic function was not as severe as in many other causes of end-stage liver disease.” Every series had some patients who underwent LT because of a failed portosystemic shunt. In the registry review of Mentha and associates (2006), 49 of the 248 patients had failure of a portosystemic shunt (Campbell et al, 1988; Krom et al, 1984).
Table 77.15 shows the survival statistics in the reported series. All of the studies suffer from the fact that follow-up of many of the patients was short, sometimes less than 1 year. A substantial variation in patient selection was apparent from one series to the next, particularly with regard to severity of liver disease, so that they cannot be compared with each other. The early mortality rate in series that had 10 or more patients ranged from 5% to 36% and averaged 15%. The 5-year survival rate, when it was reported, ranged from 45% to 95% and averaged 71%. Segev and colleagues (2007) in their UNOS registry review reported that since introduction in 2002 of the Model for End-Stage Liver Disease (MELD) scoring system for organ allocation by disease severity, 3-year patient survival has increased from 72.6% to 84.9%, and 3-year graft survival has increased from 64.5% to 80.6%.
Table 77.15 Survival After Liver Transplantation for Budd-Chiari Syndrome Reported in the Literature
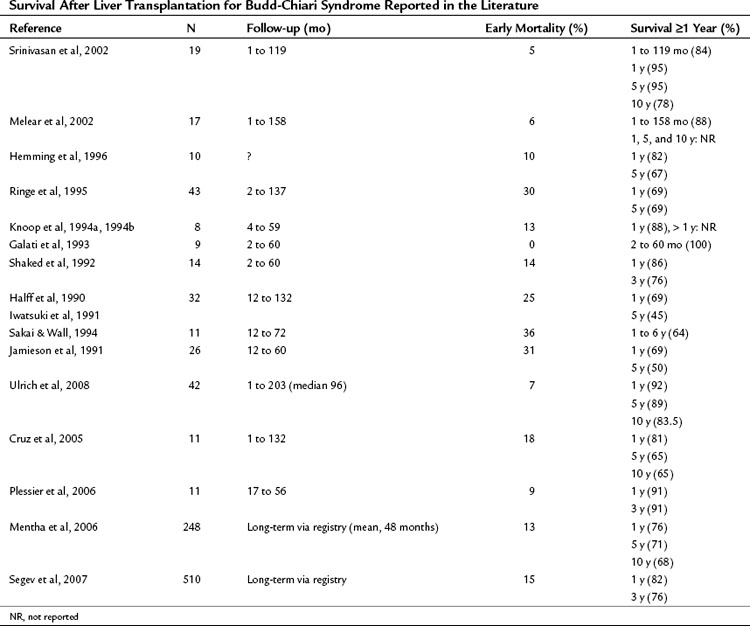
Most of the series did not report the results of pathologic examination of the liver removed from the patient with BCS. The reports of Ringe and colleagues (1995) on 43 patients, Halff and colleagues (1990) on 32 patients, Sakai and Wall (1991, 1994) on 11 patients, and Srinivasan and associates (2002) on 19 patients described the findings in the host liver, however, and confirmed the clinical impression that many patients underwent LT without having decompensated liver disease. Only 17 of the 43 patients of Ringe and colleagues (1995) had cirrhosis; the others had congestion alone (14 patients) and fibrosis alone, lesions that are found in the early stages of BCS. Similarly, in the report of Halff and colleagues (1990), none of the 32 patients had cirrhosis, and Sakai and Wall (1994) reported that 3 of 11 of their patients had central congestion and necrosis but no fibrosis or cirrhosis.
An important concern about LT in BCS is recurrence of BCS in the transplanted liver (Bahr et al, 2003; Goldstein et al, 1991). Table 77.16 shows the incidence of recurrent BCS in the liver allograft. Some investigators reported no recurrence of BCS, whereas others (Knoop et al, 1994a, 1994b; Halff et al, 1990; Cruz et al, 2005) reported substantial recurrence rates of 13% to 27% despite treatment with anticoagulants. Most series have shown a substantial incidence of posttransplant thrombosis in other splanchnic blood vessels, particularly in the PV (range, 9% to 40%), often with disastrous consequences. The frequent development of thrombosis makes it mandatory that patients receive early and lifelong anticoagulation therapy after LT. At the same time, it should be recognized that anticoagulation sometimes fails to prevent thrombosis.
Table 77.16 Reported Rates of Recurrence of Budd-Chiari Syndrome and Other Thrombosis After Liver Transplantation for Budd-Chiari Syndrome
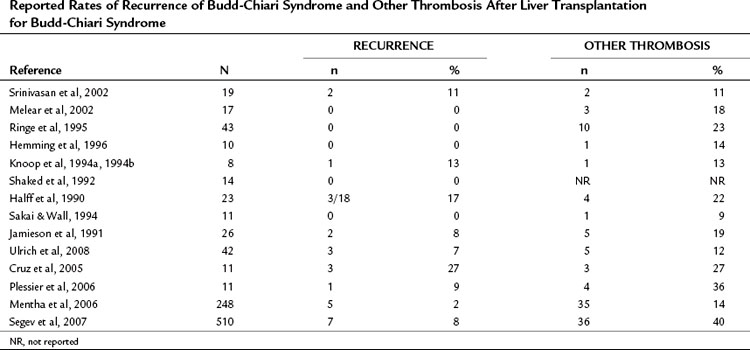
One of the potential benefits of LT in certain thrombogenic disorders, such as antithrombin III deficiency and protein-C deficiency, is correction of the underlying defect by transplantation of a liver from a normal donor. It has been suggested that LT is preferable to SSPCS in the treatment of BCS, because if SSPCS fails, the risk of performing subsequent LT is increased. It has been proposed that mesocaval shunt is preferable to SSPCS, because it is advantageous to avoid dissection of the hepatic hilum, should subsequent LT be required (McMaster, 1994; Bismuth & Sherlock, 1991; Slakey et al, 2001). In our experience, patients with BCS treated by SSPCS infrequently require LT. Of 57 patients with BCS whom we treated by SSPCS, only 1 (2%) subsequently required LT during long-term follow-up. In our experience with more than 2500 PCS procedures in patients with cirrhosis, the long-term shunt patency rate has been 99.5%.
Additionally, at least 10 more recent studies have shown that previous portosystemic shunt, regardless of type, had no influence on the outcome of LT (Aboujaoude et al, 1991; Bismuth et al, 1990; Boillot et al, 1991; Iwatsuki et al, 1988; Langnas et al, 1992; Mazzaferro et al, 1990; Minegaux et al, 1994; Turrion et al, 1991). Aboujaoude and colleagues (1991) emphasized that thrombosis of a portosystemic shunt may seriously compromise performance of LT; long-term shunt patency should therefore be the main factor that determines the type of shunt selected for the potential LT candidate, a point that is all the more true in patients with BCS. Direct PCS has had a thrombosis rate of 0.5% or less in all of our studies, compared with occlusion rates of 24% to 53% reported in the literature for mesocaval interposition shunts using synthetic grafts (Fletcher et al, 1981; Hemming et al, 1996; Smith et al, 1980; Terpstra et al, 1987). Mesocaval interposition shunts using autologous internal jugular vein grafts, which have been used widely in France, have shown patency rates comparable to direct SSPCS (Bismuth & Sherlock, 1991; Gentil-Kocher et al, 1988; Vons et al, 1986).
The third and fourth indications for orthotopic liver transplantation (OLT) in BCS are rare, and we have not encountered such cases, although they have been reported without many details in the literature. These are unshuntable patients with portal hypertension resulting from thrombosis of the portal venous system, provided that there are patent blood vessels available to vascularize the liver allograft, and acute fulminant hepatic failure (Sakai & Wall, 1994).
Surgical Removal of Venous Obstruction
Senning (1981, 1983) reported a direct method of removing obstruction of the IVC and hepatic veins in patients with chronic BCS. The operation is performed with cardiopulmonary bypass and involves opening the suprahepatic IVC and right atrium, removing any vena caval thrombus, resecting the dorsocranial part of the liver containing the confluence of the occluded major hepatic veins, and reconstructing the hepatic outflow tract by suturing the right atrium to the liver capsule. Since his initial description, Senning (1987) and others (Pasic et al, 1993) have reported the results of the operation in 17 patients, 2 of whom (12%) died within 2 weeks of operation and 4 of whom died later. Actuarial 1-year and 3-year survival rates were 76% and 57%, LT was required for 2 patients, and 3 developed recurrent thrombosis. During follow-up of 7 months to 11 years, 10 (67%) of the 15 early survivors had prolonged relief of BCS.
Kawashima and colleagues (1991) reported success of the Senning operation in 5 of 7 patients during follow-up of 2 months to 5 years, and Nakao and colleagues (1988) described success in 2 patients followed up for 1 to 2 months. In a modification of the operation, Koja and associates (Koja et al, 1996; Kuniyoshi et al, 1998) described direct removal of the IVC obstruction under partial cardiopulmonary bypass in 32 patients with chronic BCS resulting from IVC obstruction of 1 to 42 years’ duration. Koja and associates (Koja et al, 1996; Kuniyoshi et al, 1998) reopened the IVC and occluded hepatic veins under direct vision without resecting the liver and reconstructed the hepatic outflow tract with a patch graft of autologous pericardium. Many of the patients had cirrhosis or severe fibrosis of the liver, and 29 of the 32 had esophageal varices. No operative deaths were reported. During 1.5 to 17 years of follow-up, there were four late deaths, and seven patients developed hepatocellular carcinoma. Survival rates at 5 and 10 years were 93.6% and 81%. In a follow-up to their earlier report, in 2009 Kuniyoshi and colleagues (Inafuku et al, 2009) reported their results from 1979 to 2008 in 53 patients. Overall mortality rate was 32%, and 14% of survivors developed total obstruction or severe stenosis of the reconstructed IVC, therefore it does not appear that radical open-end venectomy is the first-choice operation for IVC occlusion.
Percutaneous Transluminal Angioplasty
Use of percutaneous transluminal angioplasty has been reported in more than 300 patients with BCS (Anonymous, 1982; Baijal et al, 1996; Furui et al, 1990; Griffith et al, 1996; Jeans et al, 1983; Martin et al, 1990; Sato et al, 1990; Tyagi et al, 1996; Wu et al, 2002; Xu et al, 1996; Yamada et al, 1983, 1991; Yang et al, 1996; Li et al, 2009). Most of the patients had chronic liver disease of long duration, and many of them had obstruction of the IVC of the membranous or segmental types. The obstructed vein was dilated with one or more balloon catheters. In all cases, the pressure gradient that existed before balloon dilation was substantially reduced or eliminated at the time of dilation. Stenosis often recurred, however, and required repeated balloon dilations. Most of the patients have not had long-term follow-up, so it is difficult to evaluate the ultimate effectiveness of percutaneous transluminal angioplasty. Of the few patients who have been observed for several years, the long-term success rate is less than 50% (Griffith et al, 1996; Martin et al, 1990; Sato et al, 1990; Yamada et al, 1991), although Wu and associates (2002) reported restenosis in only one of 41 patients during follow-up of 32 (± 12) months.
The use of expandable metallic stents has been added to balloon angioplasty in an attempt to maintain prolonged patency of the IVC (Baijal et al, 1996; Furui et al, 1990; Sawada et al, 1991; Xu et al, 1996, 1999; Li et al, 2009). The follow-up period of the patients who have IVC stents is too short to evaluate the efficacy of this procedure. In our opinion, based on the available evidence, percutaneous transluminal angioplasty and placement of expandable metallic stents warrant consideration only in patients with chronic BCS resulting from stenosis of the IVC. Patients treated by transluminal angioplasty should have careful follow-up that includes venography and pressure measurements every few months to detect recurrent stenosis of the IVC.
Thoracic Transposition of the Spleen
Transposition of the spleen into the left pleural cavity to create collateral venous connections between portal and pulmonary systems has been used sporadically in the treatment of portal hypertension and, in addition, has been advocated for treatment of BCS secondary to occlusion of the IVC (Akita & Sakoda, 1980; Khuroo & Datta, 1980; Schrieber & Gonzalez, 1967; Strauch, 1970). The procedure involves displacing the spleen into an opening in the left diaphragm and suturing the left lower lobe of the lung to the spleen after removing the splenic capsule and abrading the surface of the lung. It is impossible to evaluate the effectiveness of this procedure because of the paucity of information in the few case reports. Akita and Sakoda (1980) of Japan reported the use of “splenopneumopexy” in 15 patients who had diseases that they called BCS. Although few details were given, it seems that most or all of the Japanese patients had a chronic disorder that consisted of cirrhosis and esophageal varices in addition to IVC obstruction. It is possible that many of the patients had the typical MOVC that accounts for most of the cases of BCS in Japan. Portal hypertension, determined by splenic pulp pressure measurements, persisted after splenopneumopexy in all patients. It is doubtful that thoracic transposition of the spleen can decompress the liver and portal system sufficiently to prevent or reverse liver damage in the crucial early stages of BCS caused by occlusion of the IVC.
Peritoneovenous Shunt
Peritoneovenous shunt is a device that relieves ascites by transferring fluid through a one-way valve from the peritoneal cavity into the superior vena cava. It has been advocated as primary treatment for BCS by LeVeen and colleagues (LeVeen et al, 1976). The peritoneovenous shunt may be useful as a palliative measure in patients in whom portal decompression is not feasible or has been tried and failed. The peritoneovenous shunt is not indicated as the primary treatment of most patients with BCS, however, because it does not decompress the obstructed hepatic vascular bed, and it has no effect on the ongoing liver damage. Use of this device early in the course of BCS may squander the opportunity to reverse the liver damage by therapy that effectively decompresses the liver. Long-term studies of peritoneovenous shunting in patients with BCS have not been reported, probably because most patients in whom this device has been used have died from their underlying disease. There is predictable evidence, however, that the liver disease progresses to cirrhosis in the face of a functioning peritoneovenous shunt (Cameron et al, 1983).