15. Budd-Chiari Syndrome
Definition
Budd-Chiari syndrome is an uncommon condition of obstruction of intrahepatic veins. It is induced by thrombotic or nonthrombotic obstruction of hepatic venous outflow, leading to congestive hepatopathy. The hepatopathy results from large and small vein obstruction of venous outflow, producing hepatocellular injury from microvascular ischemia. It eventually results in portal hypertension and then liver insufficiency and failure.
Incidence
Exact incidence of Budd-Chiari syndrome is not known; it is classified as a rare entity both in the United States and internationally.
Etiology
Budd-Chiari syndrome frequently occurs in patients who already have some manner of thrombotic diathesis, including myeloproliferative disorders, pregnancy, tumors, chronic inflammatory diseases, clotting disorders, and infections.
Causes of Budd-Chiari Syndrome
Chronic Infections
• Amoebic abscess
• Aspergillosis
• Hydatid cysts
• Syphilis
• Tuberculosis
Chronic Inflammatory Diseases
Hematologic Disorders
• Antiphospholipid antibody syndrome
• Essential thrombocytosis
• Paroxysmal nocturnal hemoglobinuria
• Polycythemia rubra vera
• Unspecified myeloproliferative disorder
Inherited Thrombotic Diathesis
• Antithrombin III deficiency
• Factor V Leiden deficiency
• Membranous web
• Oral contraceptives
• Pregnancy/postpartum
• Protein C deficiency
• Protein S deficiency
Tumors
• Hepatocellular carcinoma
• Leiomyosarcoma
• Renal cell carcinoma
• Right atrial myoma
• Wilms‘ tumor
Miscellaneous
• α 1-Antitrypsin deficiency
• Dacarbazine
• Idiopathic causes
• Trauma
• Urethane exposure
Signs and Symptoms
• Abdominal distention
• Acute right upper quadrant pain
• Age of onset: 40 to 50 years
• Ascites
• Engorgement of vessels of chest and abdominal walls
• Polycythemia vera
• Prolonged prothrombin time
• Splenomegaly
• Tender hepatomegaly
Medical Management
Most patients present with a classic symptom triad: abdominal pain, ascites, and hepatomegaly. However, this triad is nonspecific and making the diagnosis of this disease requires that one have a high index of suspicion when the patient exhibits this symptom triad. There are four clinical variants: acute liver disease, subacute liver disease, fulminate liver disease, and liver failure. The most common variant is subacute liver failure with portal hypertension and liver decompensation.
Medical therapy can be instituted for short-term, symptomatic relief. The mortality rate associated with symptomatic treatment is 80% to 85%. Treatment includes anticoagulation, antithrombolytic therapy, and angioplasty. Ascites can be managed with sodium restriction and diuretics, with approximately 95% effectiveness. In the event of hypotension, water restriction may be incorporated. Paracentesis may be used for some patients, but it is typically reserved for the patient with tense ascites in whom rapid symptomatic relief is needed. Invasive/surgical intervention can be undertaken for decompression of the hepatic vascular congestion if portal hypertension is the underlying cause of symptoms. Peritoneovenous shunt placement is an older, almost obsolete, therapeutic measure for symptomatic relief of medically intractable ascites. The placement of this shunt can result in increased cardiac output, renal blood flow, glomerular filtration rate, urinary volume, sodium excretion, and decreased plasma renin activity and plasma aldosterone concentration. There is no evidence of improved patient survival. Currently, the emerging standard treatment is the transjugular intrahepatic portosystemic shunt (TIPS) for alleviation of the hepatic venous congestion. Prognosis for this disease can be good, based on several factors.
Complications
• Electrolyte imbalance
• Hepatic decompensation
• Hepatic encephalopathy
• Hepatorenal syndrome
• Hypercoagulation state
• Intravascular volume shifts (for large volume paracentesis)
• Portal hypertension
• Spontaneous bacterial peritonitis
• Variceal hemorrhage
Anesthesia Implications
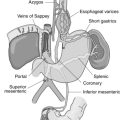
The liver receives 25% to 30% of the cardiac output when at rest. Approximately 75% of the hepatic blood flow is delivered via the portal vein. The portal system delivers 35% to 50% of the oxygen the liver receives; the remaining 50% to 65% is delivered by the hepatic artery. Portal hypertension associated with Budd-Chiari syndrome is post-sinusoidal intrahepatic in nature. The circumvention of the metabolic processing provided by the hepatocytes may produce a prolongation and exaggeration of the effects of a large number of anesthesia-related drugs, particularly those with high hepatic extraction rates.
Preoperative electrolyte balance and coagulation parameters should be ascertained. Ascites formation and its removal can result in larger shifts in the electrolytes levels. The patient with Budd-Chiari syndrome may be treated with anticoagulants and/or antithrombolytic agents. As a result, the level of treatment must be assessed and adjustments made before any elective procedure. Obviously, liver function testing is an essential preoperative evaluation. Reduced albumin levels can result in increased bioavailability of a large number of drugs.
Cardiopulmonary function should be thoroughly assessed preoperatively. Over time, liver function with ascites formation can produce transudative effusions, usually on the right side initially. Communication between the pleural and peritoneal spaces can allow ascites fluid to accumulate in the pleural cavity, causing decreased pulmonary compliance, reduced lung volumes, and elevated pleural pressures. As a result, moderate hypoxemia may ensue.