Chapter 199 Brucella
Human brucellosis, caused by organisms of the genus Brucella, continues to be a major public health problem worldwide. Humans are accidental hosts and acquire this zoonotic disease from direct contact with an infected animal or consumption of products of an infected animal. Although brucellosis is widely recognized as an occupational risk among adults working with livestock, much of the brucellosis in children is food-borne and is associated with consumption of unpasteurized milk products. It is also a potential agent of bioterrorism (Chapter 704).
Treatment
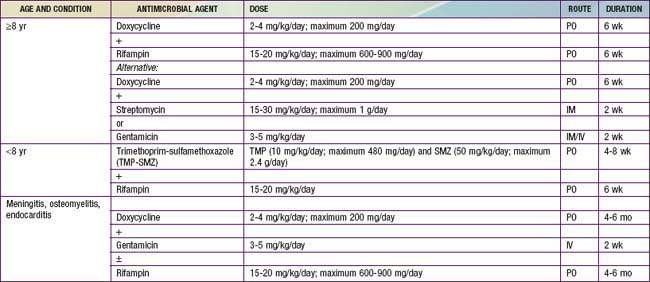
Many antimicrobial agents are active in vitro against the Brucella species, but the clinical effectiveness does not always correlate with these results. Doxycycline is the most useful antimicrobial agent and, when combined with an aminoglycoside, is associated with the fewest relapses (Table 199-1). Treatment failures with β-lactam antimicrobial agents, including the 3rd generation cephalosporins, may be due to the intracellular nature of the organism. Agents that provide intracellular killing are required for eradication of this infection. Similarly, it is apparent that prolonged treatment is the key to preventing disease relapse. Relapse is confirmed by isolation of Brucella within weeks to months after therapy has ended and is usually not associated with antimicrobial resistance.
Prognosis
Before the use of antimicrobial agents, the course of brucellosis was often prolonged and may have led to death. Since the institution of specific therapy, most deaths are due to specific organ system involvement (e.g., endocarditis) in complicated cases. The prognosis after specific therapy is excellent if patients are compliant with the prolonged therapy (see Table 199-1).
Franco MP, Mulder M, Gilman RH, et al. Human brucellosis. Lancet Infect Dis. 2007;7:775-786.
Pappas G, Akritidis N, Bosilkovski M, et al. Brucellosis. N Engl J Med. 2005;352:2325-2336.
Ramin B, MacPherson P. Human brucellosis. BMJ. 2010;341:884-885.
Shen MW. Diagnostic and therapeutic challenges of childhood brucellosis in a nonendemic country. Pediatrics. 2008;121:e1178-e1183.
Skalsky K, Yahav D, Bishara J, et al. Treatment of human brucellosis: systematic review and meta-analysis of randomizes controlled trials. BMJ. 2008;336:701-704.
Solera J, Geijo P, Largo J, et al. A randomized, double-blind study to assess the optimal duration of doxycycline treatment for human brucellosis. Clin Infect Dis. 2004;39:1776-1782.
Troy SB, Rickman LS, Davis CE. Brucellosis in San Diego: epidemiology and species-related differences in acute clinical presentations. Medicine. 2005;84:174-187.