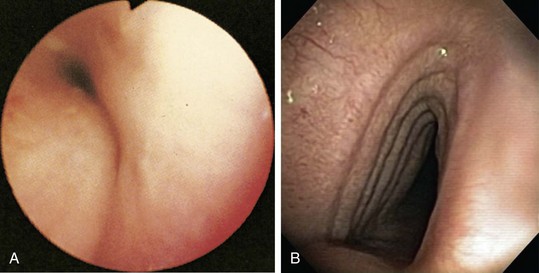
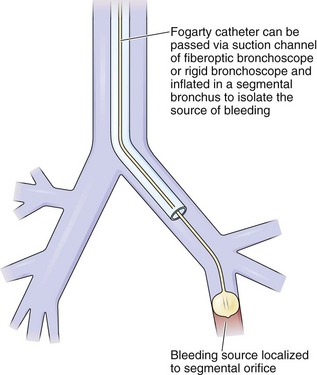
Chapter 11 Bronchoscopy
Types of Bronchoscopy and General Instrumentation
Rigid Bronchoscopy
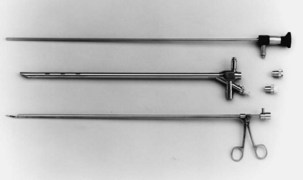
Both rigid and flexible modern systems are equipped with optic capabilities for airway observation alone. With the rigid scope, various types of telescopic rods, equipped with circumferential illumination, permit direct and magnified visualization (Figure 11-1). Specially designed telescopes allow viewing not only directly forward but also at oblique and lateral angles. Various diagnostic and therapeutic instruments can be inserted through the rigid scope while the patient remains ventilated. Rigid bronchoscopy allows a number of therapies such as laser photoresection, endobronchial stents, balloon dilation, electrocautery, argon beam coagulation, and cryotherapy to be performed safely and effectively. Perhaps most important, a rigid scope can be used to “core out” large bulky airway tumors and to dilate central airway strictures and areas of stenosis very effectively and efficiently. In addition, the rigid bronchoscope also can be used for the passage of a flexible scope, which may be necessary for dealing with tortuous airways or distal lesions.
Flexible Bronchoscopy
The flexible scope is used in most bronchoscopic procedures. Although initial flexible bronchoscopes used fiberoptic systems, most such instruments now use a charge-coupled device (CCD) camera at the tip that allows transmission of digital images to a monitor. The main advantages of flexible scopes include their ease of manipulation and greater flexibility, allowing a more complete tracheobronchial tree evaluation than with rigid bronchoscopy, and a less challenging path to expertise, permitting more rapid acquisition of skills (favorable learning curve), for use of these devices (Figure 11-2).
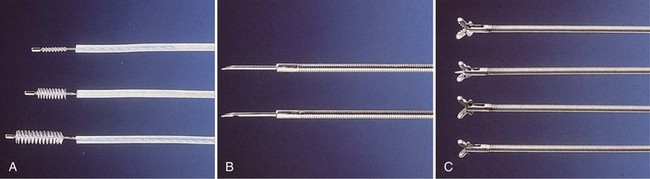
Flexible scopes vary in size, ranging from ultrathin devices allowing for endoscopy in infants and neonates to larger, adult-sized therapeutic scopes. The working channel of the bronchoscope can be used for aspiration of secretions and to accommodate various diagnostic or therapeutic accessories. Four main diagnostic tools have been developed for use during bronchoscopy in order to obtain diagnostic material: bronchoalveolar lavage (BAL), brushings, forceps biopsy, and needle aspiration (Figure 11-3). Since the inception of bronchoscopy more than 100 years ago, these diagnostic modalities have been hampered by limited ability to ensure direct localization of pulmonary nodules, masses, infiltrates, or lymph nodes. However, recent technologic developments in navigational technology and endoscopic ultrasound imaging have improved the ability to localize these lesions, to obtain diagnostic tissue, and to prevent unnecessary surgical intervention. The use of endobronchial ultrasound probes is discussed in Chapter 12.
Patient Preparation and Monitoring during Bronchoscopy
All patients undergoing bronchoscopy should undergo a complete prebronchoscopy evaluation, including a medical history, physical examination, and chest imaging (Box 11-1). Although routine laboratory tests are not required, each evaluation should be individualized on the basis of patients’ underlying conditions and the diagnostic and therapeutic procedures planned.
Box 11-1
Prebronchoscopy Checklist
1. Is there an appropriate indication for bronchoscopy?
2. Has there been a previous bronchoscopy?
3. If the answer to the preceding question is yes, were there any problems or complications?
4. Does the patient (and close relative[s] if patient is unable to communicate) fully understand the goals, risks, and complications of bronchoscopy?
5. Does the patient’s medical history (allergy to medications or topical anesthesia) and present clinical condition pose special problems or predispose to complications?
6. Are all the appropriate tests completed and the results available?
7. Are the premedications appropriate and the dosages correct?
8. Does the patient require special consideration before bronchoscopy (e.g., corticosteroids for asthma, insulin for diabetes mellitus, or prophylaxis against endocarditis) or during bronchoscopy (e.g., supplemental oxygen, extra sedation)?
9. Is the plan for postbronchoscopy care appropriate?
10. Are all the appropriate instruments and personnel available to assist during the procedure and to handle the potential complications?
Modified from Prakash UBS, Cortese DA, Stubbs SE: Technical solutions to common problems in bronchoscopy. In Prakash UBS, editor: Bronchoscopy, New York, Raven, 1994.
Technique
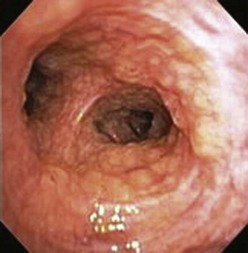
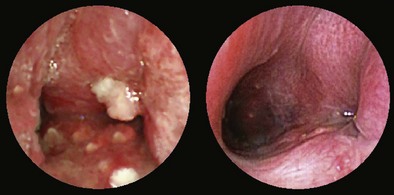
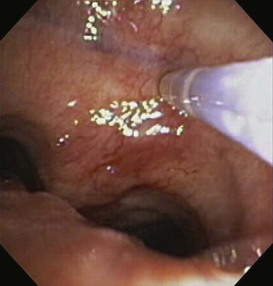
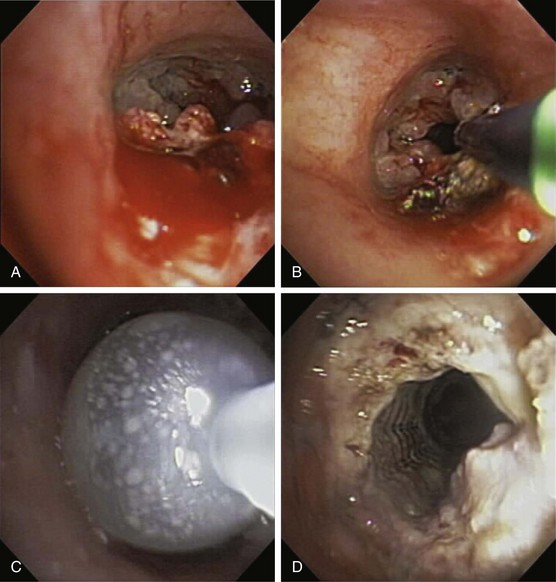
A thorough evaluation of the mucosal surface is an important part of the bronchoscopic examination. The most common abnormality is a change in mucosal coloration, with prominent hypervascular areas seen in patients with chronic bronchitis. The presence of granulation tissue can be due to reaction to a foreign body. Inflammatory mucosal reactions, although not very characteristic, should raise the possibility of mycobacterial infection, nonspecific viral and nonviral infections, and other granulomatous diseases, such as sarcoidosis (Figure 11-4). Mucosal ulcerations are more characteristic of Wegener granulomatosis or malignancy. Loss of the usual mucosal luster and presence of a roughened surface may be early signs of an infiltrative or neoplastic process.
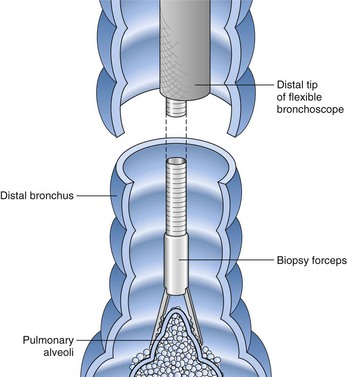
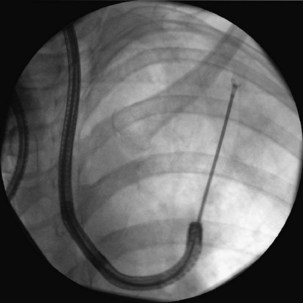
After the bronchoscopic inspection of the airways and surrounding structures has been performed, appropriate samplings should be obtained from the abnormalities identified. Aspirated secretions can be sent for microbiology cultures to determine the offending organism in cases of infection or suspected infection. Endobronchial lesions can be sampled with cytology brushes, biopsy forceps, or needles. Bronchoscopic lung biopsy can be performed for either focal abnormalities or diffuse lung diseases (Figure 11-5). For small or focal lesions, fluoroscopy helps guide peripheral forceps placement and improves the diagnostic yield of biopsies for focal lesions. The use of fluoroscopy also may obviate the need for routine chest radiography after transbronchoscopic lung biopsy. In the case of diffuse lung disease, such as sarcoidosis, use of fluoroscopy has not been demonstrated to improve the diagnostic yield of transbronchial biopsies. Fluoroscopy is useful, however, in providing information regarding the proximity of the forceps to the pleura and in more rapidly establishing the diagnosis of complications (e.g., pneumothorax). Transbronchoscopic needle aspiration (TBNA) and biopsy (TBNB) permit sampling of peribronchial lymph nodes. These transbronchial approaches provide cost-effective diagnostic modalities with less risk and a lower complication rate than with mediastinoscopy (see Chapter 17).
Bronchoalveolar lavage (BAL) is a useful and generally well-tolerated bronchoscopic sampling technique (Figure 11-6). BAL is safe, even in critically ill patients, when biopsy or brushing methods are not recommended because of bleeding risk. Normal saline solution, devoid of any bacteriostatic material, is instilled into distal air spaces through the “wedged” flexible scope and then aspirated through the instrument’s suction channel or from a sterile trap. The fluid collected can be analyzed for gross appearance to detect possible alveolar hemorrhage. The fluid also may be subjected to a variety of tests, depending on the clinical circumstances: microbiologic testing, specific cytologic analysis and cell count, immunologic parameters, presence of various biochemical mediators related to pathologic processes, tissue markers, polymerase chain reaction (PCR) assay, electron microscopy, flow cytometry, and DNA probes. The diagnostic yield of BAL very much depends on specific patient characteristics, the underlying pathologic process, and technical factors.
Indications for Diagnostic Bronchoscopy
Many potential indications have been recognized for both diagnostic and therapeutic bronchoscopy, many of which are listed in Boxes 11-2 and 11-3. The most common reason for bronchoscopy remains the evaluation of a lung mass or nodule. Other major indications include investigation of pulmonary infiltrates, evaluation of opportunistic infections in immunocompromised persons, investigation of hemoptysis, assessment for suspected foreign body, and treatment of airway complications related to neoplasms in the tracheobronchial tree. Some of these indications are discussed next.
Box 11-2
Indications for Diagnostic Bronchoscopy
Box 11-3
Indications for Therapeutic Bronchoscopy
Hemoptysis
Hemoptysis is a common clinical sign and one of the most frequent indications for bronchoscopic evaluation. The most common causes of scant hemoptysis include chronic bronchitis, tuberculosis, and bronchiectasis, whereas massive hemoptysis, usually defined as bleeding greater than 200 mL in a 24-hour period, most often is due to tuberculous cavities, lung cancer, mycetomas, or lung abscess (see Chapter 24). Bronchoscopy can be of help in localizing the site and cause of bleeding. Although the timing of the procedure should be dictated by clinical circumstances, studies have shown that early bronchoscopy (within 48 hours) is more likely to demonstrate active bleeding and allow for the determination of the bleeding site. Chest imaging, with either chest radiograph or computed tomography (CT) scan, can assist in bleeding site localization and, in stable patients without massive hemoptysis, should precede bronchoscopy. In patients with a normal appearance on the chest film, the prevalence of malignancy is approximately 5%, which in most cases is visible by CT scan. The yield of bronchoscopy in patients with normal findings on CT scan is extremely low, and a conservative approach consisting of observation and serial imaging should be considered. Beyond its role as a diagnostic tool, bronchoscopy often can be used to perform various therapeutic procedures in patients experiencing hemoptysis (see further on).
Pulmonary Infections
Bronchoscopy is a useful technique in the diagnosis of pulmonary infections, allowing for the collection of respiratory samples for evaluation with special stains and culture. Respiratory samples can be collected by one or more techniques, including bronchial washing, BAL, protected specimen brushing (PSB), bronchoscopic lung biopsy, and TBNA (Table 11-1).
Table 11-1 Bronchoscopic Techniques and Applications in Respiratory Infections
| Technique | Clinical Applications |
|---|---|
| Bronchoscopy—visualization | Assessment of mucosal, intraluminal, and extraluminal pathology |
| Evaluation of endobronchial tuberculosis, mycoses, viral vesicles (in AIDS) | |
| Evaluation of invasive tracheobronchial aspergillosis, candidiasis, and other conditions | |
| Follow-up evaluation of endobronchial disease (e.g., tuberculosis) | |
| Bronchial washing | Culture for identification of mycobacteria, fungi, and viruses and Pneumocystis smears |
| Bronchoalveolar lavage | Culture for identification of all organisms, especially mycobacteria, fungi, cytomegalovirus and other viruses and Pneumocystis smears |
| Protected specimen brushing | Culture for aerobic and anaerobic bacteria |
| Nonprotected bronchial brushing | Stains and culture for identification of mycobacteria, fungi, Pneumocystis, and viruses |
| Endobronchial biopsy | Mucosal lesions caused by mycobacteria, fungi, protozoa |
| Removal of obstructing lesions responsible for infection (e.g., tumor, foreign body) | |
| Drainage of lung abscess; piecemeal removal of mycetomas (aspergillomas, other fungus balls) | |
| Bronchoscopic needle aspiration | Stains and culture of extrabronchial lymph node specimens for identification of mycobacteria and fungi |
| Drainage of bronchogenic cyst and instillation of sclerosing agent | |
| Bronchoscopic lung biopsy | Stains and culture for identification of all organisms, especially Pneumocystis jiroveci, mycobacteria, and fungi |
| Detection of parasitic lung infections | |
| Rigid or flexible bronchoscopy—therapeutic intervention | Insertion of tracheobronchial prosthesis (stent) to overcome airway obstruction caused by intrinsic stenosis (posttuberculosis or fungal) or extrinsic compression caused by mediastinal fibrosis due to histoplasmosis |
Mycobacterial Infections
In cases in which pulmonary tuberculosis is suspected, the initial diagnostic evaluation should consist of serial examination of sputum for the presence of acid-fast bacilli (AFB) in stained smears. Ideally, induced sputum samples should be obtained. If sputum study results are negative and tuberculosis is still suspected, bronchoscopy with BAL and biopsy should be performed. Both induced sputum collection and bronchoscopy should be performed with appropriate infection control precautions to minimize the risk of nosocomial transmission. A bronchoscopy may cause the patient to produce sputum for several days afterwards; these specimens also should be collected and analyzed, if possible. The utility of bronchoscopy varies widely in the literature, with reported diagnostic yields of 50% to 95%. The yield in patients with miliary tuberculosis, in whom sputum smears frequently are negative, is approximately 70%. Bronchoscopy also is useful in tuberculosis manifesting as an endobronchial lesion or with mediastinal and hilar adenopathy, in which case diagnostic tissue can be obtained with TBNA (Figure 11-7). The yield of diagnostic procedures, including bronchoscopy, can be expected to improve as newer interferon release assays and nucleic acid amplification techniques are incorporated into everyday practice (see Chapter 31).
Bronchogenic Carcinoma
Diagnosis
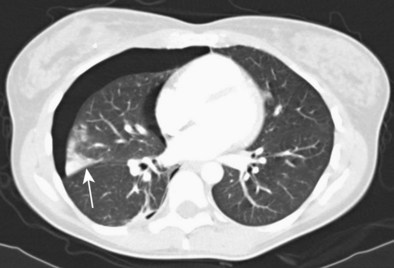
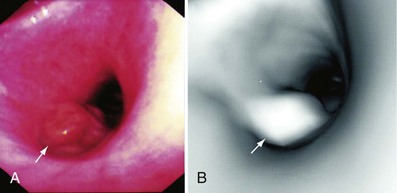
Peripheral lesions usually are sampled with a combination of bronchial wash, brushes, transbronchial biopsy, and TBNA. The diagnostic yield of bronchoscopy for peripheral lesions depends on a number of factors, including lesion size, the distance of the lesion from the central airways, and the relationship between the lesion and bronchus. The yield of bronchoscopy for lesions smaller than 3 cm varies, ranging from 14% to 50%, compared with a diagnostic yield of 46% to 80% when the lesion is larger than 3 cm. The presence of a bronchus sign on chest CT predicts a much higher yield of bronchoscopy for peripheral lung lesions. In these cases, fluoroscopic guidance should be used to ensure proper positioning of the diagnostic accessory (Figure 11-8).
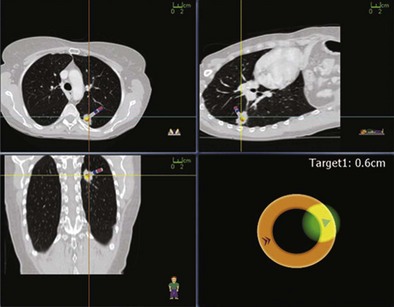
Several newer methods have been developed for the evaluation of peripheral lung lesions, including endobronchial ultrasound (EBUS) imaging (see Chapter 12) and navigational bronchoscopy. The first U.S. Food and Drug Administration (FDA)-approved navigational system, the Electromagnetic Navigation Bronchoscopy (EMN) system (InReach System, SuperDimension, Inc., Minneapolis, Minnesota), uses an electromagnetic board to generate a magnetic field around the patient, a magnetic sensor probe, an extended working channel, and three-dimensional integration of CT scan reconstruction and flexible bronchoscope position (Figure 11-9). In essence, this navigational system works on the same triangulation principle as for a global positioning system and allows the bronchoscopist to direct the flexible scope through the airways to the target. Several studies have demonstrated EMN diagnostic sensitivity to range between 67% and 74%, independent of lesion size. Studies also show improved diagnostic yield with the combination of EMN with mini-ultrasound probes for sampling of small peripheral lesions. Beyond standard diagnostic utilization, novel navigational applications have been demonstrated in targeted therapeutic delivery, such as EMN-guided stereotactic radiosurgery fiducial placement or implantation of radiotherapy monitoring devices.
Staging
TBNA has emerged as a valuable tool for the investigation of enlarged or metabolically active mediastinal lymph nodes (Figure 11-10). The procedure is particularly useful for evaluation of patients who are marginal or poor surgical candidates; in these patients, more invasive approaches, such as mediastinoscopy or mediastinotomy, may be obviated. TBNA has proved particularly useful with the use of rapid onsite evaluation (ROSE), whereby a cytopathologist working in or near the bronchoscopy suite can evaluate obtained specimens in real time (see Chapter 14).
Diffuse Lung Diseases
Certain findings on BAL can be suggestive or virtually diagnostic of a number of interstitial lung diseases (Table 11-2). It is important that the BAL findings be correlated with the clinical and HRCT findings. For example, specific characteristics of the freshly retrieved lavage fluid can support the diagnosis of alveolar hemorrhage, pulmonary alveolar proteinosis, microlithiasis, or lipid aspiration. In patients with suspected eosinophilic pneumonia, a high eosinophil count is diagnostic, and in cases of pulmonary Langerhans cell histiocytosis, BAL flow cytometry should be performed to evaluate for CD1at cells.
Table 11-2 Bronchoalveolar Lavage (BAL) in Diffuse Interstitial Disease
| Disorder | BAL Fluid Findings |
|---|---|
| Pulmonary hemorrhage | Progressive increase in RBCs with sequential aliquots; hemosiderin-laden macrophages |
| Pulmonary alveolar proteinosis (PAP) | Grossly cloudy, milky appearance; positive PAS stain |
| Eosinophilic pneumonia | Eosinophilia >25% |
| Sarcoidosis | CD4+/CD8+ ratio >3.5 |
| Pulmonary Langerhans cell histiocytosis | CD1at cells >5% |
| Hypersensitivity pneumonitis | Lymphocytosis; decreased CD4+/CD8+ ratio |
| Lipid pneumonia | Oily material that layers above aqueous phase |
| RBILD/DIP | Brown macrophages |
DIP, diffuse interstitial pneumonia; PAS, periodic acid–Schiff; RBCs, red blood cells; RBILD, respiratory bronchiolitis–associated interstitial lung disease.
In a number of disorders, BAL findings may be suggestive, but additional diagnostic procedures probably will be required. Such diseases include sarcoidosis, hypersensitivity pneumonitis, and organizing pneumonia. Bronchoscopic lung biopsy should be considered in situations in which the diagnosis has not been established by HRCT and BAL. In many situations, bronchoscopic lung biopsy can establish the diagnosis and avoid the need for surgical lung biopsy (Box 11-4). For example, with pulmonary sarcoidosis, the diagnosis usually is established by a combination of BAL and biopsy findings. The BAL can be used to exclude the presence of tuberculosis and fungal infections and can demonstrate the characteristic high CD4+/CD8+ ratio seen in sarcoidosis, whereas bronchoscopic biopsy specimens may demonstrate the classic finding of noncaseating granulomas. In general, bronchoscopic biopsy should be performed in several affected areas, and at least five or six specimens should be taken. The sensitivity for diagnosis of sarcoidosis is only approximately 60% to 70%, and many patients require further invasive testing, such as surgical lung biopsy. Recently, the use of EBUS-TBNA has been extended to the diagnosis of sarcoidosis, especially in patients with mediastinal and hilar adenopathy. The addition of TBNA to transbronchial biopsy can provide the diagnosis in more than 85% of sarcoidosis cases.
Complications of Bronchoscopy
Pneumothorax
Pneumothorax after transbronchial biopsy occurs in approximately 4% of cases, even when the procedure is done under fluoroscopic guidance (Figure 11-11). The impact of fluoroscopy on the incidence of pneumothorax remains controversial. Uncontrolled studies have not found a difference in the incidence of pneumothorax after transbronchial biopsy when performed with and without fluoroscopy.
Special Bronchoscopic Techniques
Virtual Bronchoscopy
Virtual bronchoscopy (VBS) is a novel radiographic reconstruction technique that exploits the versatility of helical (spiral) CT by transforming axial CT data into simulated three-dimensional intraluminal views of the airways. This form of perspective rendering has benefited enormously from continued advances in computing technology and currently is capable of providing images that in many ways mimic those obtained during conventional bronchoscopy (Figure 11-12). Although VBS has yet to find its place in routine clinical practice, it has nonetheless proved useful in the evaluation and management of a wide range of pulmonary diseases and conditions involving the tracheobronchial tree, including bronchogenic carcinoma, benign airway stenoses, tracheobronchomalacia, lung transplantation, and bronchiectasis. With this technique, which provides the bronchoscopist with a “virtual camera” inside the patient’s tracheobronchial tree, images and perspectives can be obtained and procedures can be planned before conventional bronchoscopy is undertaken. Specific advantages include examination of airways distal to a completely occluded bronchus, retroflexion of the bronchoscope, and en face views. Current limitations of VBS include its inability to adequately characterize mucosal abnormalities, identify subtle submucosal disease, or visualize small endobronchial lesions. Obviously, however, VBS can never completely replace conventional bronchoscopy because it does not allow biopsy or therapeutic intervention.
Optical Coherence Tomography
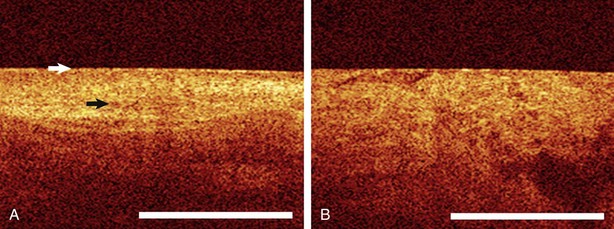
Optical coherence tomography (OCT) is the optical ultrasound analogue whereby near-infrared light transit time and reflection are used rather than sound waves and provides a macroscopic optical cross-sectional view of hollow organs. By using light instead of sound waves, OCT overcomes two major ultrasound limitations in the lung: (1) the inability to image through air and (2) poor spatial resolution. OCT resolution is between 4 and 20 nm, which is approximately 25 times higher than that of other modalities. In the airway, dysplastic, invasive cancer, or inflammatory changes appear to have unique OCT image patterns (Figure 11-13). The ability of OCT to provide an “optical biopsy” with information about microinvasive airway lesion evolution, airway wall remodeling in obstructive lung diseases, or interstitium alterations in idiopathic interstitial pneumonia (IIP) without the risk of tissue biopsy could provide a valuable bronchoscopic tool. Such data may provide diagnostic information, but more important, longitudinal evaluation in an individual patient may allow therapeutic tailoring in the future.
Therapeutic Bronchoscopy Techniques
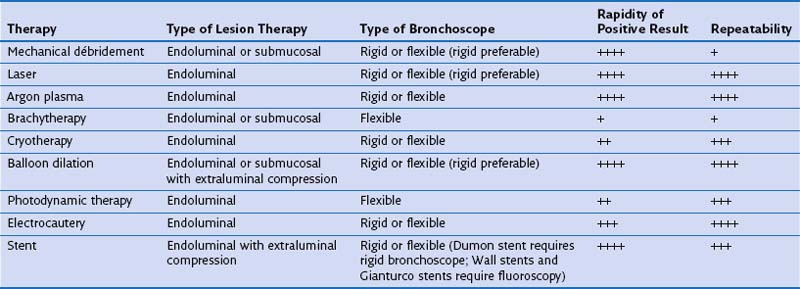
Rigid Bronchoscopic Debulking or Balloon Dilatation
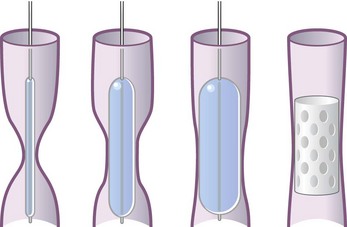
Balloon dilation has become an attractive alternative to dissection with a blunt rigid bronchoscope in less urgent cases of obstruction caused by malignant tumors and benign strictures. High-pressure balloons of various lengths and diameters commonly were used in the past. There are now balloons designed specifically for tracheobronchial use that are expandable to specific diameters by application of defined positive pressure. These are inserted through the bronchoscope working channel under direct vision or fluoroscopic guidance. The balloon, filled with saline or radiopaque contrast media, is inflated at the site of interest until the desired diameter is attained. This technique often is used in combination with bronchoscopic thermal treatments (laser, argon plasma coagulation, electrocautery) and tracheobronchial stent placement for the treatment of airway stenosis (Figure 11-14).
Tracheobronchial Stenting
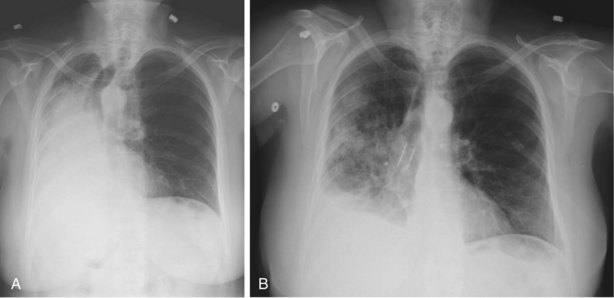
Endobronchial stents have a critical role in multimodality endoscopic approaches to both benign and malignant airway obstruction. Airway obstruction caused by locally advanced bronchogenic carcinoma can be treated with a combination of thermal tumor ablation and stent implantation to regain and to preserve airway lumen diameter by preventing tumor ingrowth (Figure 11-15). Stent placement also can be used to maintain airway patency after endobronchial brachytherapy or can be combined with laser therapy and balloon dilation in the endoscopic management of benign fibrotic strictures. Most large studies of endobronchial stent placement have demonstrated impressive efficacy. Dumon and colleagues reported excellent clinical outcomes and few complications with silicone stent use in patients with malignant airway obstruction but a lower success rate among patients with tracheal stenosis caused by other disorders. Success rates, with “success” broadly defined as symptomatic relief, have ranged in limited studies between 78% and 98%, although none of the early trials used objective measures such as the Lung Cancer Symptom Score (LCSS) to determine efficacy. In two small studies in patients who were intubated because of respiratory failure secondary to unresectable tracheobronchial and mediastinal disease, stent placement facilitated extubation in most patients.
Indications for Therapeutic Bronchoscopy
Therapeutic bronchoscopy most commonly is performed for aspiration of retained secretions and mucous plugs and for the treatment of airway obstruction. The indications for therapeutic bronchoscopy are listed in Box 11-3; many of these are discussed next.
Endoluminal Airway Obstruction
The bronchoscopic approach to management of malignant airway obstruction depends on the lesion location, the presence or absence of associated extrinsic compression, and the degree of clinical urgency (Table 11-3). Rigid bronchoscopic debulking with adjunctive thermal ablation is recommended when airway recanalization must be performed on an emergency basis. If endobronchial obstruction is accompanied by marked extrinsic compression, stent placement may be beneficial (Figure 11-16).
Tracheobronchomalacia
Tracheobronchomalacia is best diagnosed on the basis of findings on flexible bronchoscopy, performed with the patient breathing spontaneously (Figure 11-17, A), although dynamic CT scanning, with images obtained on inspiration and expiration, often is helpful. Tracheobronchomalacia should be distinguished from a saber sheath trachea, which is characterized by a fixed reduction of the transverse diameter of the intrathoracic portion of the trachea, in the presence of accentuation of the sagittal diameter (see Figure 11-17, B).
Control of Hemoptysis
In cases of hemoptysis, bronchoscopy may be of value not only for diagnosis but frequently for emergency management of endobronchial bleeding (Box 11-5). Because of difficulties with visualization, instruments with large and maximally effective suction channels should be used. Rigid bronchoscopy generally is preferred with massive bleeding and when the need to remove large clots is anticipated.
Box 11-5
Measures and Agents for Bronchoscopic Control of Hemoptysis
When continuous suctioning of blood fails to clear the airways, other means can be used. An iced saline solution can be instilled along with vasoactive drugs, such as epinephrine, to induce vasoconstriction. The bronchoscope itself can occlude the lumen of the bronchus from which the bleeding originates. The same effect, perhaps with better local control, can be achieved with bronchoscopic balloon catheters (Figure 11-18). Specially designed catheters have been developed for introduction through the working channel of the flexible bronchoscope, several permitting subsequent removal of the scope while the tamponading balloon remains in place, as well as the potential for suctioning beyond the balloon for clearance of blood from distal airways. Another effective method for control of visible sources of bleeding, particularly from endobronchial neoplasms, is Nd:YAG laser photocoagulation.
Bronchoscopic Treatments for Common “benign” Lung Diseases
Beamis JFJr, Mathur PN, Mehta AC. Interventional pulmonary medicine. New York: Marcel Dekker; 2004.
Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J. 2002;19:356–373.
Cox G, Thomson NC, Rubin AS, et al. AIR Trial Study Group: Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327–1337.
Ernst A, Silvestri GA, Johnstone D. ACCP Interventional/Diagnostic Procedures Network Steering Committee: Interventional pulmonary procedures. Guidelines from the American College of Chest Physicians. Chest. 2003;123:1693–1717.
Mehta AC, Prakash US, Garland R, et al. 2005 American College of Chest Physicians and American Association for Bronchology Consensus Statement: prevention of flexible bronchoscopy-associated infection. Chest. 2005;128:1742–1755.
Seijo LM, Sterman DH. Medical progress: interventional pulmonology. N Engl J Med. 2001;344:740–749.
Simoff MJ, Sterman DH, Ernst A. Thoracic endoscopy: advances in interventional pulmonology. Malden, Mass: Blackwell, 2006.
Wang KP, Mehta AC, Turner JFJr. Flexible bronchoscopy. Malden, Mass: Blackwell, 2004.