Chapter 3 Bronchoscopic Treatment of Silicone Stent–Related Granulation Tissue
Case Description
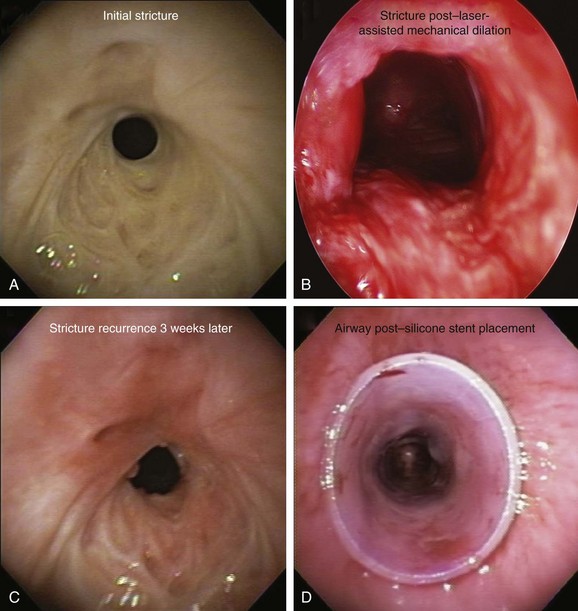
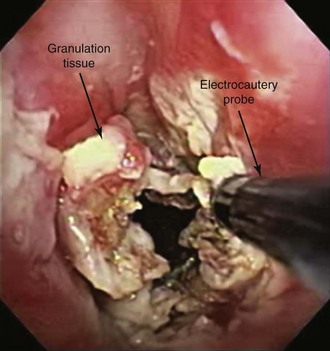
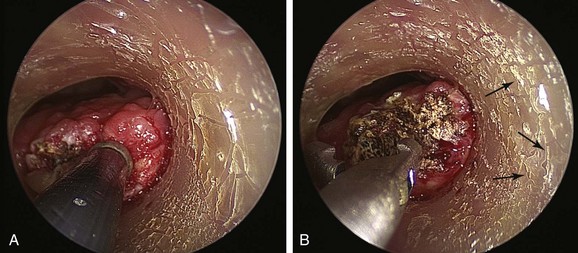
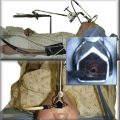
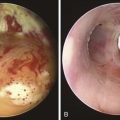
The patient was a 75-year-old man with post intubation tracheal stenosis. He had chronic obstructive pulmonary disease (COPD) (forced expiratory volume in 1 second [FEV1] 40% of predicted) requiring home oxygen supplementation at 3 L/nasal cannula. He also suffered from coronary artery disease requiring coronary artery bypass graft (CABG) surgery 5 years previously, at which time he was intubated for 3 days. He had congestive heart failure (left ventricular ejection fraction [LVEF] 40%) and required placement of a pacemaker. Three months before our encounter, he began to complain of progressive dyspnea leading to stridor. Bronchoscopy revealed a severe (4 mm), complex (three cartilaginous rings), and multilevel “hourglass” tracheal stenosis (2.5 cm in extent), suggesting a post intubation origin (Figure 3-1). His stricture rapidly recurred after rigid bronchoscopy with dilation, prompting insertion of a 12 × 40 mm straight silicone stent in the patient’s trachea 3 weeks later. The proximal aspect of the stent was located 4 cm below the vocal cords. Two months after stent placement, the patient developed progressive cough and dyspnea. On auscultation, he had rhonchi over the trachea, and stridor was heard with forced inspiration and expiration but not with tidal breathing. Bronchoscopy revealed a large amount of granulation tissue nearly completely obstructing the proximal aspect of the stent. Removal of the tissue was initiated using a flexible electrocautery probe during flexible bronchoscopy under moderate sedation (Figure 3-2).
Discussion Points
1. Describe three indications for granulation tissue removal.
2. Describe the principles of cutting and coagulation when endobronchial electrocautery and argon plasma coagulation are used.
3. Describe two potential complications resulting from electrosurgery in this patient.
4. List and explain three mechanisms of pathogenesis for granulation tissue formation.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
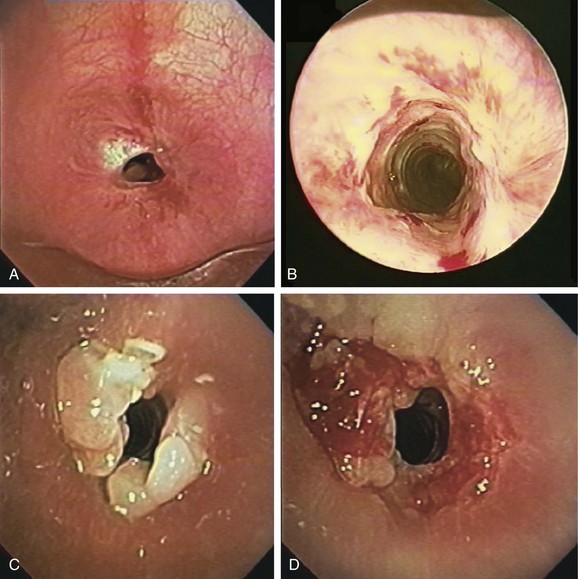
This patient presented with new exophytic endoluminal tracheal obstruction after stent placement, most likely consistent with hyperplastic granulation tissue formation, a benign form of central airway obstruction.1 The main differential diagnosis of granulation tissue in patients with indwelling airway stents is tumor overgrowth, although mucus plugging and bacterial colonization can also cause firm, necrotic-appearing obstructive lesions. The exact prevalence of stent obstruction by granulation tissue versus tumor overgrowth is somewhat confounded by the fact that studies tend to report these events together rather than separately. With this caveat, the estimated frequency of recurrent obstruction from granulation tissue or tumor is 9% to 67% in patients with metal stents, and 6% to 15% in patients with silicone stents.2 Tumor overgrowth tends to occur when only the tumor area is covered and no cancer therapy is offered; it is most often seen in patients with partially covered indwelling metal stents because malignant tissue grows through exposed wire mesh, causing obstruction (Figure 3-3). Our patient, however, had no history of cancer; his stent was placed for a benign post intubation stricture, and the rapid onset of exophytic tissue growth was not consistent with a malignant tumor growth rate.
Granulation tissue formation is less predictable. It seems that patients with known keloids or chronic airway infection are at higher risk.3 Oversizing the stent has been suspected as a risk factor, especially when stents are placed in the upper trachea or subglottis (see video on ExpertConsult.com) (Video I.3.1![]() ). For silicone, as well as metal, friction between the sharp edges of the stent and the airway mucosa may cause granulation tissue formation. In addition, when electrocautery is used, the direct (aka galvanic) currents generated* around the metal wires may be cofactors in granulation tissue formation.4 Shearing forces at the stent-mucosa interface created by differential motion of the stent relative to the airway contribute to constant stimulation of airway mucosa, further leading to reactive granulation tissue formation. Hyperplastic granulation tissue formation can be seen at the site of a lung transplant surgical anastomosis†5,6 or at the anastomosis site after tracheal sleeve resection for tracheal stenosis (Figure 3-4). One study showed that 31% of patients with a lung transplant or benign disease developed granulation tissue after placement of a self-expandable metallic stent.5
). For silicone, as well as metal, friction between the sharp edges of the stent and the airway mucosa may cause granulation tissue formation. In addition, when electrocautery is used, the direct (aka galvanic) currents generated* around the metal wires may be cofactors in granulation tissue formation.4 Shearing forces at the stent-mucosa interface created by differential motion of the stent relative to the airway contribute to constant stimulation of airway mucosa, further leading to reactive granulation tissue formation. Hyperplastic granulation tissue formation can be seen at the site of a lung transplant surgical anastomosis†5,6 or at the anastomosis site after tracheal sleeve resection for tracheal stenosis (Figure 3-4). One study showed that 31% of patients with a lung transplant or benign disease developed granulation tissue after placement of a self-expandable metallic stent.5
Silicone stent insertion performed using rigid bronchoscopy under general anesthesia was considered an acceptable alternative to surgery for our inoperable patient with complex stenosis.* In fact, silicone stents provide long-term airway patency in nonsurgical candidates with a variety of central airway obstructive lesions.†7–9 Stent-related complications, however, are not uncommon and in one series included migration (17.5%), obstruction from secretions (6.3%), and significant granulation tissue formation at the proximal or distal extremities of the stent (6.3%).10 This latter complication may promote the development of secondary stenosis.5 It is likely that other complications of stent insertion, such as kinking, fracture, or compression of mucosal vasculature due to excessive centrifugal force exerted by expanding or self-expanding stents, also contribute to granulation tissue formation. As in our patient, diagnostic flexible bronchoscopy should always be performed when a stent-related adverse event is expected. This will confirm or rule out problems such as mucus plugging or stent migration and will allow an accurate reassessment of the stenosis, the degree of mucosal inflammation, associated cartilaginous collapse, and the relative amount and location of hypertrophic fibrotic tissue.11 Bronchoscopy is the current standard for the detection and treatment of stent-related complications; in nonurgent situations, it usually involves a two-step procedure. Initially, diagnostic flexible bronchoscopy is performed to detect and characterize a stent complication; if a treatable complication is detected, rigid bronchoscopy may be required for therapeutic intervention. In our case, a large amount of obstructing granulation tissue was found proximal to the stent during flexible bronchoscopy* (see Figure 3-1).
This patient had developed significant granulation 2 months after stent placement, probably as a result of abnormal wound healing—a process that eventually led to hypertrophic fibrotic tissue formation and circumferential stenosis. The exact duration of indwelling stent placement necessary to cause airway injury and granulation is not known, and the exact molecular mechanisms responsible for this are only partially understood.† Some of the better studied mechanisms include overexpression of profibrotic transforming growth factor (TGF)-β1 in the extracellular matrix12 and the presence of high levels of vascular endothelial growth factor (VEGF) expression in the submucosal layers.13 Wound healing also depends on local and systemic factors, such as infection, pressure, tissue necrosis, age, and comorbidities.14 In this regard, patients with malignant disease develop less stent-related granulation tissue formation (≈4%)—a phenomenon that can be explained in part by the use of radiotherapy and chemotherapy, leading to a less pronounced inflammatory response to the presence of the stent.5
Comorbidities
This patient suffered from several cardiovascular comorbidities. An assessment of risk for myocardial infarction or death and methods to reduce or eliminate these risks should be addressed before surgery is performed on such patients under general anesthesia.15 Perioperative myocardial infarction causes substantial morbidity and prolonged hospitalization; mortality rates as high as 25% to 40% are associated. Noninvasive stress testing is widely used to help predict risk of perioperative complications, but the poor predictive power of these tests limits their usefulness. No data suggest benefits of percutaneous coronary intervention or CABG in reducing noncardiac surgical risk. In addition, angioplasty with stenting and its need for anticoagulation can expose patients to increased risk of perioperative bleeding. In general, stable patients who have previously undergone coronary revascularization may safely undergo surgery, especially low cardiac risk procedures such as bronchoscopic interventions. In one study of patients who had undergone high-risk noncardiac surgeries, the revascularized patients experienced significantly fewer cardiac complications perioperatively when compared with patients without previous CABG.16 It is currently recommended that asymptomatic patients who have undergone CABG in the previous 5 years should proceed directly to noncardiac surgery without further preoperative evaluation.17 From a purely cardiac standpoint, our patient had no contraindication to rigid bronchoscopy under general anesthesia, making it a possible alternative for granulation tissue removal.
Postoperative pulmonary complications are at least equally prevalent and contribute similarly or more to morbidity, mortality, and length of stay in patients undergoing noncardiac surgery.18 With regard to perioperative pulmonary risk stratification, patient-related risk factors for postoperative pulmonary complications* include advanced age,† American Society of Anesthesiologists class 2 or higher, functional dependence, COPD, and congestive heart failure (all were seen in our patient). Abnormal findings on chest examination (defined as decreased breath sounds, prolonged expiration, rales, wheezes, or rhonchi) were the strongest predictors of postoperative pulmonary complication rates (odds ratio, 5.8).18 Evidence supports procedure-related risk factors for postoperative pulmonary complications, including general anesthesia and prolonged surgery (2.5 to 4 hours). The major procedure-related risk factors (vascular, abdominal, or thoracic surgery) confer higher risk for pulmonary complications than is associated with patient-related risk factors.18 From a pulmonary standpoint, our patient was not an optimal candidate for general anesthesia, and granulation tissue was therefore removed using the flexible bronchoscope and moderate sedation.
Support System
This patient with advanced lung disease and his partner had to cope with a life limited by constant dyspnea. Although dyspnea may vary in severity from day to day, it invariably affects how patients and their partners see themselves and their place in society; people develop a feeling of isolation, helplessness, and fear.19 Indeed, results of studies show that family caregivers voiced their feelings of helplessness and their sense of relief and security once they had decided to seek help (e.g., when the patient was admitted for acute care).19 Interventions that target the family setting in which chronic disease management takes place have thus emerged as an alternative to traditional strategies that focus only on individual patients, or that consider family only as a peripheral source of positive or negative social support. When this approach is used, the educational, relational, and personal needs of all family members are emphasized with the aim of improving the quality of relationships among family members with respect to the disease.20
Procedural Strategies
Contraindications
This patient had no absolute contraindications to flexible bronchoscopy. In fact, the only contraindication to elective bronchoscopy is refractory hypoxemia.* Although hypoxemia is associated with cardiac arrhythmias in 11% to 40% of patients who undergo fiberoptic bronchoscopy, cardiac rhythm disturbances are rarely clinically important. The American Thoracic Society recommends avoiding bronchoscopy and bronchoalveolar lavage in patients with hypoxemia that cannot be corrected to at least a partial pressure of arterial oxygen (PaO2) of 75 mm Hg or to a saturated oxygen level in hemoglobin (SaO2) greater than 90% with supplemental oxygen. In case of use of electrosurgery, any high amount of supplemental fraction of inspired oxygen (FiO2) would pose a risk for airway fire and stent ignition. Our patient was on 3 L of oxygen at baseline, which is the equivalent of an FiO2 of 0.32.*
Expected Results
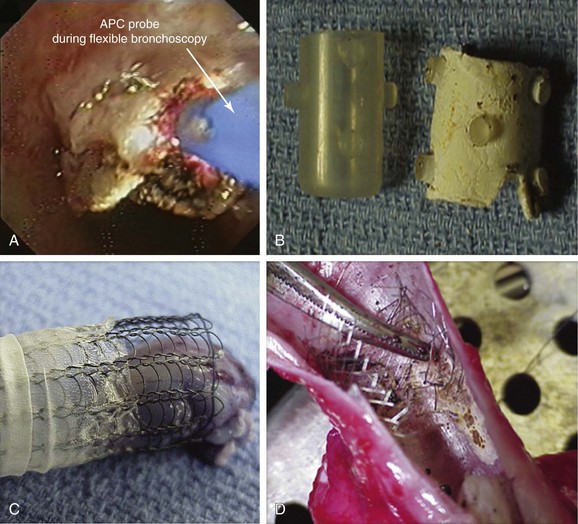
Electrocautery uses high-frequency current, which leads to thermal tissue destruction. This bronchoscopic modality has been used successfully to remove granulation tissue 21 and can be performed using flexible or rigid bronchoscopy; when the rigid scope is used, the electrode tip must not be in contact with the rigid tube or other instruments or devices (e.g., forceps, stent) to avoid formation of an electrical circuit with the equipment or the operator (Figure 3-5). The main advantage of electrocautery over other techniques (photodynamic therapy, brachytherapy, or cryotherapy) is its rapid results. Care should be taken to avoid damaging normal airway wall structures or the indwelling airway stent. Necrosis caused by electrocautery depends on the voltage difference between the probe and the tissue, the surface area of contact, the duration energy is applied,† and the presence of blood or mucus.22 For instance, in one study, superficial tissue damage was caused despite short duration of bronchoscopic electrocautery using 30 W power, use of a flexible electrocautery probe (2 mm2 surface area) for less than 2 seconds. A longer duration of coagulation (3 or 5 seconds) caused damage to the underlying cartilage.22
Therapeutic Alternatives to Granulation Tissue Removal
1. Medical therapy: Because wound healing is the source of the problem, researchers have tried to modulate and suppress this process.13 Several agents have been tested for controlling the wound-healing process in airway stenosis: (1) inflammation phase: antibiotics, steroids, and hyperbaric oxygen (HBO)*; (2) proliferative phase: antibiotics, steroids, mitomycin,† 5-fluorouracil (5-FU)‡/triamcinolone, HBO; (3) maturation phase: halofuginone,§ beta-aminopropionitrile , colchicine, penicillamine,‖ and N-acetyl-l-cysteine (NAC).¶ Most of these agents were investigated in animal models. Three modalities were more thoroughly investigated: steroids and antibiotics, mitomycin, and antireflux medications.13 Treatment with steroids and antibiotics did not have consistent results in different animal and human studies. Most studies demonstrated the superiority of mitomycin when compared with placebo if used immediately after tissue injury (i.e., on fresh, inflamed scar, containing mostly granulation tissue). Most of these studies show a tendency toward a favorable effect of mitomycin, yet results from the only prospective double-blind, randomized human study performed as of this writing did not demonstrate improvement when topical mitomycin was applied.13 Reflux prevention includes education and behavioral changes, along with drugs such as proton pump inhibitors, H2-receptor antagonists, and prokinetic agents.13 Because of limited data supporting its efficacy and the severe nature of symptoms and the degree of obstruction, simple medical therapy was not offered to our patient.
2. Neodymium-doped yttrium aluminum garnet (Nd:YAG) laser: Effect is based on thermal tissue destruction resulting from light-tissue interaction. The distribution of energy depends on the optical properties of the material and the wavelength characteristic of the laser light. As absorbed energy is converted into heat, a rise in the temperature of the target tissue or material occurs; the temperature will rise above threshold levels only if the absorbed power density exceeds the capacity of the material to conduct heat away from the impact site. For example, at low power densities, the poor absorption of the Nd:YAG laser and its pronounced scattering result in slow homogeneous heating of a large volume of tissue without serious mechanical damage to the tissue surface.23 At high power densities, however, the temperature 2 to 3 mm below the tissue surface rises rapidly, prompting vaporization of water content and a pocket of steam with pressure high enough to rupture overlying tissues.24 Certain quantities of laser energy, defined by power density, would cause local heating without the extreme temperature elevation required to disrupt stent integrity or prompt stent ignition.2 In these circumstances, laser-induced stent damage* may cause substantial morbidity from airway burn injury in case of stent ignition, or airway wall and vascular perforation in case of metal stent rupture. To identify margins of safety within which bronchoscopic Nd:YAG laser resection can be performed without damaging indwelling airway stents, an experimental in vitro study simulating a patient-care environment was conducted† using Nd:YAG laser performed at FiO2 of 0.4 using fiber-to-target distances of 10 mm and 20 mm, and noncontact, continuous-mode, 1 second pulses at power settings of 10 W, 30 W, and 40 W. Results of this study showed that uncovered Wallstent and silicone stents were not damaged when Nd:YAG laser energy was delivered using power densities less than 172 W/cm2 (10 W, 10 mm), but were damaged at power densities greater than 225 W/cm2 (30 W, 20 mm); uncovered Wallstents, covered Wallstents, and silicone stents all were damaged at power densities greater than 225 W/cm2 and at power settings greater than 30 W; covered Wallstents, however, had a high likelihood of ignition at all power densities studied (75 W/cm2, 172 W/cm2, 225 W/cm2, 300 W/cm2, 518 W/cm2, and 690 W/cm2).‡2,25 In fact, in another experimental study, investigators found that metal stents were destroyed after just one 25 W pulse delivered 4 mm from the target, prompting authors to conclude that the Nd:YAG laser should not be used in patients with indwelling Wallstent, Strecker, and Palmaz metal stents26 for fear of stent fracture. The importance of coexisting mucus or blood in the setting of indwelling airway stents was also well demonstrated in an in vitro study, in which investigators were able to ignite blood- or soot-covered silicone stents using multiple laser power settings. Clean silicone stents could not be ignited regardless of power density or oxygen concentration.27
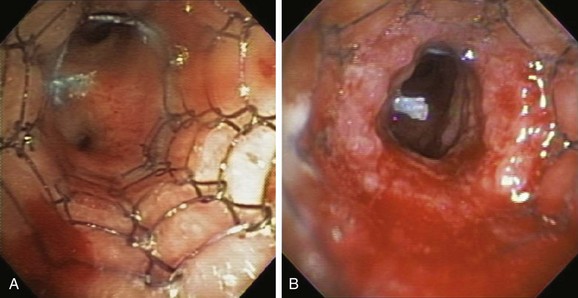
3. Argon plasma coagulation (APC): This procedure is based on argon gas ionization by current, which will lead to thermal tissue destruction. Typically indicated for hemoptysis and malignant exophytic endoluminal obstruction,28 APC has been used successfully for treating granulation tissue.29 Because argon plasma is electrically conductive, an electrical spark jumps from the tip of the electrode to the target tissue, creating a thermal effect. The probe should not touch the tissue surface at any time (Figure 3-6); the distance between the probe’s distal tip and conductive biologic tissue must be approximately 5 mm or less, and the target tissue must be conductive. Tissue that is dehydrated, carbonized, or denatured will resist the flow of electrical current. In a large prospective study of 364 patients treated over a 4-year period, authors showed that APC is effective and safe in treating a variety of central airway disorders, including stent obstruction by tumor or granulation tissue (overall 67% success rate) with a complication rate of 3.7%.30 In a small case series of three patients who developed strictures and stent-related granulation tissue after solid organ transplantation, the success rate of APC was 100% with no complications.31 When this technique is used, however, a concern is that gas forced into the airway wall may collect within a blood vessel, causing gas embolism. Argon gas is heavy, inert, and 17 times less soluble in the body than carbon dioxide, and it may pass into the systemic circulation. Both cerebral gas embolism and cardiac arrest have been reported after airway applications of APC.32,33
Use of APC in the setting of indwelling airway stents was addressed in an experimental in vitro study simulating a patient-care environment where uncovered and covered nitinol (Ultraflex) stents, uncovered and covered Wallstents, and studded silicone stents were deployed in the tracheobronchial tree of a ventilated and oxygenated heart-lung block from an expired pig. APC was performed at power settings of 40 and 80 W using FiO2 of 0.21, 0.40, and 1.00, and an argon gas flow rate of 0.8 L/min through the flexible bronchoscope. The primary outcome was the time taken for the APC to cause stent damage, defined as discoloration, ignition, or rupture. Airway fires involving all five types of stents consistently occurred in the presence of 100% oxygen at powers of 40 W and 80 W. At lower FiO2 (0.21 and 0.40), silicone stents were not damaged at 40 W and 80 W. Uncovered Ultraflex stents were undamaged using 40 W at either FiO2 (0.21 or 0.40), but could be damaged using both FiO2 levels when the power was increased to 80 W. Covered Ultraflex and both uncovered and covered Wallstents were damaged at both power settings (40 W and 80 W) and FiO2 (0.21 and 0.40) levels, with a trend toward earlier damage when higher FiO2 and power were used (see Figure 3-6). When used within the parameters identified in this study (power 40 W, FiO2 0.21, APC flow rate 0.8 L/min), APC could be a safe method for tissue destruction and could avoid the risk of airway stent ignition, especially if short bursts of APC are employed. The safety limits identified using FiO2 of 0.4, however, are also important, because some patients undergoing APC may require supplemental oxygen therapy. Nitinol and stainless steel are metals that have high electrical conductivity in comparison with polyurethane and silicone, which are nonconductive plastic materials. These differences in stent properties also explain the easy combustibility of covered metal stents compared with uncovered metal and silicone stents.34
4. Photodynamic therapy (PDT): With this modality, tissues with the retained photosensitive agent are exposed to laser light. This results in nonthermal tissue destruction. PDT for nonmalignant airway obstruction is not well described, but in animal models of trauma-induced granulation tissue, rabbits that received PDT showed patchy granulation tissue that was only 20% to 30% of the volume of that seen in untreated animals. Although PDT may be a therapeutic alternative for airway stenosis originating from granulation tissue,35 therapeutic effect is delayed, and multiple procedures may be necessary to remove necrotic debris. Furthermore, in a study comparing PDT versus electrocautery in the treatment of patients with early lung cancer, greater airway stenosis and increased subepithelial fibrosis were seen after treatment with PDT (and Nd:YAG laser).36
5. Cryotherapy: This treatment can be used for granulation tissue removal provided in a contact mode with cryoprobes. These are placed on target tissues in a succession of adjacent areas in such a way that the freeze zone margins overlap; repeated (usually three) freeze-thaw cycles, each lasting 30 seconds, will lead to tissue destruction. Tissues are subsequently removed with the use of forceps. In the search for an alternative to contact mode cryotherapy, a study of the human airway was performed using surgically resected specimens and noncontact spray cryotherapy to assess safety and histologic effects. Considerable cellular injury to the treated tissue was noted, but the supporting connective matrix was left intact. Long-term pathology findings (>100 days post-treatment) revealed a complete lack of scarring or stricture.37 The technique consists of applying medical-grade liquid nitrogen (−196° C) directly to the tissue via a low-pressure, disposable 7-French cryocatheter introduced through the working channel of a therapeutic flexible bronchoscope.38
6. Brachytherapy: The rationale for the use of ionizing radiation in the setting of granulation tissue is based on its successful use in other benign diseases such as keloids, coronary artery restenosis, peripheral vascular restenosis, and heterotopic ossification.6 Brachytherapy has been shown to successfully reduce the degree of nonmalignant airway obstruction.39,40 In one study, 80% (15/19) of patients41 with metal stent–induced granulation tissue responded favorably (a dose of 1000 cGy was applied in each treatment). In another study, investigators used one to two fractions of Ir-192 prescribed to 7.1 Gy at a radius of 1 cm in eight patients. However, the effect of brachytherapy is delayed, and repeat interventions may be necessary.6
7. Stent removal and replacement with self-expanding metallic stents (SEMS): In their uncovered form, these stents maintain epithelialization and mucociliary clearance but provide a major disadvantage in that they cause significant granulation tissue formation. Clinical reports have estimated that granulation tissue formation occurs in at least 25% to 30% of expanding metallic airway stents.5,42 This tissue ingrowth can make removal difficult and can result in substantial airway wall trauma (see video on ExpertConsult.com) (Video I.3.2![]() ).43 For our patient, we did not offer this therapy.
).43 For our patient, we did not offer this therapy.
Cost-Effectiveness
The most cost-effective management of granulation tissue has not been identified. No direct comparative studies have investigated electrocautery versus any of the other techniques mentioned previously for this patient population, or for patients with malignant obstruction,44 even though for malignancy, the effects of electrocautery seem to be equivalent to those of the Nd:YAG laser.45
Techniques and Results
Anesthesia and Perioperative Care
Electromagnetic interference generated during electrosurgery has several effects on pacemakers and automatic implantable cardioverter-defibrillators (AICDs). Electrocautery can inhibit pacing of stimuli or trigger ventricular pacing. The presence of these devices warrants precautions because their deprogramming has been reported following electrocautery. If the patient’s rhythm is not paced, the pacemaker can be inhibited* for the time of the procedure by applying a magnet over the pacemaker on the patient’s chest. The magnet inhibits sensing of cardiac or noncardiac electrical events by the pacemaker, which reverts back to the programmed routine upon removal of the magnet. If, however, the patient has a paced rhythm, a technician with instruments required to reprogram the unit should be contacted before electrocautery is used. It is advisable to have the pacemaker’s clinical support line number available, to maintain 15 cm between the active electrode and any electrocardiograph (ECG) electrode, and to have resuscitation equipment ready. Although most of these devices have a magnet response, some devices can be programmed to not respond to magnet application, and thus will need a device programmer to change the parameters. It is advisable to contact the manufacturing company to clarify these issues before beginning the procedure. For AICDs, the magnet can temporarily turn off defibrillation therapy without inhibiting pacing.† In addition to procedures requiring electrosurgery, indications for AICD deactivation include ongoing cardiac resuscitation, end-of-life care, inappropriate shocks, and transcutaneous pacing.46 This patient’s rythm was not paced, so a magnet was placed on his chest over the pacemaker site.
We performed the procedure with moderate sedation using 5 mg of midazolam intravenously. Careful management of supplemental oxygen is essential to avoid inadvertent airway injury due to ignition of gases by an electrical spark from a high-frequency generator during bronchoscopy. FiO2 is maintained below 0.4 (translating to approximately 3 liters/minute or less with use of a nasal cannula) to avoid airway fire.‡
Techniques and Instrumentation
The power setting for electrosurgery is determined by the operator. The usual power is 15 to 30 W, but power may vary according to the physician’s technique and the desired effect. For example, for deep coagulation, a 20 W power setting is selected with longer activation times. Increasing power to 40 W leads to higher energy levels at the tip of the probe, resulting in rapid but shallower effects (superficial coagulation). In our patient, we used coagulation mode at a 20 W power setting. The tissue effects of electrocautery depend not only on the settings, but also on patient-related factors. Patients have different tissue impedance according to their age and body mass, and depending on the specific disease process. To provide safe thermal effects without damage to tissue, most electrosurgical generators have an autostop function (it generates an audible tone when active),* which automatically ends current flow when tissue resistance is increased to a preset level, thus avoiding tissue charring and burning. Charred tissues have a tendency to adhere to the probe and interfere with further treatment.
Before the bronchoscope is inserted into the patient’s airway, a grounding pad is placed on the patient (on the arm or hip closest to the treatment site). This ensures that a large area of the skin† is covered by the pad to avoid burns as the current exits the body. The grounding pad serves as a conductive plate to complete the electrical circuit between the electrosurgical unit and the active electrode. The surface area must be adequate to disperse the current, so that current density cannot collect in a single spot, causing a burn. Most units have alarms for when a current leak occurs or when the grounding pad is improperly positioned.
Results and Procedure-Related Complications
After induction of moderate sedation, with the patient receiving oxygen via face mask, the flexible bronchoscope (2.8 mm working channel) was introduced through the patient’s right nostril. After local laryngeal analgesia was achieved with 300 mg of lidocaine, the scope was advanced through the cords in the patient’s subglottis and upper trachea just proximal to the granulation tissue. The electrocautery probe was then inserted, oxygen flow was reduced to 3 L/min and coagulation of granulation tissue was initiated (see video on ExpertConsult.com) (Video I.3.3![]() ). The optimal activation time for electrocautery (including APC), if possible, is during the patient’s exhalation phase or during apnea, when oxygen concentration is lowest. Electrocautery of short duration is applied because the probe is constantly moved along the tumor during the procedure and is mostly tangential to the mucosal surface. Repeat cleaning of the probe may be necessary during the procedure. Also, repeat cycles of coagulation and tissue removal with forceps are necessary when this technique is used. Withholding supplemental oxygen during actual activation avoids any increase in exposure of the electrical energy to high levels of oxygen. A patient with unstable oxygen saturation therefore may require significant time to treat. In our case, the procedure lasted 40 minutes. No procedure-related complications occurred, nor was evidence of airway wall perforation, pneumothorax, or airway fire noted.
). The optimal activation time for electrocautery (including APC), if possible, is during the patient’s exhalation phase or during apnea, when oxygen concentration is lowest. Electrocautery of short duration is applied because the probe is constantly moved along the tumor during the procedure and is mostly tangential to the mucosal surface. Repeat cleaning of the probe may be necessary during the procedure. Also, repeat cycles of coagulation and tissue removal with forceps are necessary when this technique is used. Withholding supplemental oxygen during actual activation avoids any increase in exposure of the electrical energy to high levels of oxygen. A patient with unstable oxygen saturation therefore may require significant time to treat. In our case, the procedure lasted 40 minutes. No procedure-related complications occurred, nor was evidence of airway wall perforation, pneumothorax, or airway fire noted.
Long-Term Management
Follow-up Tests and Procedures
Some operators perform surveillance flexible bronchoscopy every 3 to 6 months as part of routine evaluation of stent patency.41 The value of this practice is questionable, and evidence indicates that routine follow-up bronchoscopy with lack of symptoms is not warranted in all patients with indwelling airway stents.47 Clinical judgment has to be applied, however, on a case-by-case basis. In this patient, who had developed significant obstructing granulation tissue with stridor, we proceeded with surveillance flexible bronchoscopy after 8 weeks. At that time, the stent was still patent.
Quality Improvement
We did not objectively quantify the amount of granulation tissue. Some investigators grade the amount of granulation tissue on a 4-point scale: 0, no granulation tissue; 1, mild (less than 25%) obstruction; 2, moderate (25% to 50%) obstruction; and 3, severe (above 50%) obstruction.41 Nor did we perform a multidetector computed tomography (MDCT) scan. MDCT might be a noninvasive imaging alternative to diagnostic bronchoscopy for the detection of stent complications. One study showed that MDCT accurately detected 29 of 30 (97%) complications diagnosed by bronchoscopy in 21 patients who underwent stent placement (11 of the 21 stents were metallic). In one false-negative case, MDCT failed to detect a stent fracture. No false-positive diagnoses of stent complications were reported. However, this study examined granulation tissue and secretions, which together were responsible for 13 of 30 (43%) detected complications.48 We propose that distinguishing between the two before bronchoscopy is important because procedural preparation may be different. For instance, if granulation tissue is the cause of obstruction, rigid bronchoscopy may be planned, or, alternatively, a therapeutic scope should be available to perform APC, cryotherapy, or electrocautery. On the other hand, if mucus plugging is the culprit for obstruction seen on MDCT, flexible bronchoscopy with therapeutic aspiration of secretions may suffice. MDCT may be useful if the stent needs to be replaced because a custom stent can be designed on the basis of measurements obtained by MDCT.48
Discussion Points
1. Describe three indications for granulation tissue removal.
2. Describe the principles of cutting and coagulation when endobronchial electrocautery and argon plasma coagulation are used.
3. Describe two potential complications resulting from electrosurgery in this patient.
4. List and explain three mechanisms of pathogenesis for granulation tissue formation.
In granulation tissue formation, the balance between synthesis and breakdown of the matrix is lost in a sequence of events divided into three overlapping phases14:
Expert Commentary
An alternative approach worth considering is the use of argon plasma diathermy, also known as argon plasma coagulation (APC), at a power setting of 30 to 40 W delivered in short bursts. The electrical current, which is conducted in the argon plasma, arcs from the electrode to the nearest grounded conductive material, which in this case would be tissue. The silicone stent material is not a good conductor, and the current therefore preferentially goes to tissue, which becomes denatured. Nevertheless, it is possible for the stent to catch fire if tissue adjacent to it is flammable or reaches a high temperature.34 The potential advantage of APC diathermy is that it may not penetrate so deeply as electrocautery* and therefore may be less likely to induce additional local fibrosis and inflammatory reactions. The risk of argon emboli is lessened if short bursts are used in a space that is not too confined so that excessive pressures are avoided. This should be possible in treating granulation tissue at the proximal end of the stent.
Another alternative is cryotherapy-assisted tissue removal. Rather than using the freeze-thaw technique generally employed for debulking endobronchial tumor—a technique with delayed action—a short freeze time of 3 to 5 seconds or so might allow granulation tissue to be pulled away from the stent orifice. Clearly no risk of thermal damage to the stent is present, and underlying cartilaginous structures within the trachea are protected. A little local bleeding may occur, although usually this is easily controlled.*The authors used a flexible bronchoscope with moderate sedation, partly in accord with the patient’s preference to avoid general anesthesia (GA), which clearly needs to be respected. The patient had a background history of COPD, as well as coronary artery disease and coronary artery bypass graft surgery. Bronchoscopy should be avoided if possible within 6 weeks of a myocardial infarction,50 but this was not an issue for this patient. The patient had no history of ischemic symptoms following his cardiac surgery; it was therefore reasonable to proceed to bronchoscopy without further cardiac evaluation.17 The patient’s medication use would be checked to determine whether he was receiving anticoagulation, aspirin, or clopidogrel. Oral anticoagulation should be stopped 3 to 5 days before the procedure or reversed by vitamin K if the situation is more urgent and tissue removal is anticipated. Heparin can be given in the interim if necessary and stopped just before the procedure is performed. Procedures usually can be undertaken in the presence of aspirin therapy, but clopidogrel confers significant risks of bleeding51 and should be ceased 5 to 7 days beforehand. Other common causes of prolonged bleeding include thrombocytopenia, which requires platelet transfusion if the platelet count is less than 50,000, and uremia. Apart from the use of electrocautery or APC diathermy, bleeding usually can be controlled with tamponade and topical application of 1 : 10,000 adrenaline (epinephrine) solution or cold saline.
1. Better ventilation (down a hollow tube rather than around a solid one).
2. Better control of bleeding (by tamponading of the bleeding area with the rigid scope; by aspiration of blood and clots with large suction catheters; and by maintenance of better vision with the ability to quickly clean the telescope or flexible scope).
3. Better removal of obstructing tissue with large forceps and suckers.
The authors mention the use of medical therapies in the treatment of granulation tissue. Such interventions are given with the intent of modulating the development of granulation tissue and fibrosis, rather than as a means of removal. As stated in the text, no conclusive evidence indicates that these therapies are of benefit, although mitomycin-C and intralesional steroids are frequently advocated, and of these, mitomycin-C has the most supportive evidence. Rojas-Solano and Becker52 provide a good description of mitomycin-C applied by soaking a cotton swab in a solution of 0.4 mg/mL and using forceps to hold the swab for 5 minutes against the region to be treated. I have used this technique on occasion, as well as an alternative technique of local injections of 40 mg of Solu-Medrol, dissolved in 2 mL of saline, into the inflamed and treated area. This may be accomplished using a transbronchial aspiration needle without a side-hole and preferably short in length (i.e., 13 mm or less). The solution of methylprednisolone can be injected into the submucosa of the airway in small aliquots of 0.1 to 0.2 mL. The direction of the needle is coaxial with the airway, and a small mucosal bleb can often be seen, giving further reassurance that the needle is not perforating the wall of the airway (see video on ExpertConsult.com) (Video I.3.4![]() ). It is difficult to judge the potential benefits of such treatments on the basis of anecdotal experience, but I sometimes employ them when inflammation post dilation is significant, or when recurrence of stenosis occurs frequently.
). It is difficult to judge the potential benefits of such treatments on the basis of anecdotal experience, but I sometimes employ them when inflammation post dilation is significant, or when recurrence of stenosis occurs frequently.
* The depth of coagulation necrosis with electrocautery is 0.2 mm for 1 second bursts, but 1.9 mm with 5 second bursts. Tissue damage underneath the necrosis measures about 0.7 mm at 5 seconds. This results in overall histologic changes of ≈2.6 mm,22 which is similar to APC (up to 3 mm). The pressure by which the electrocautery probe is pushed against the airway wall may affect the extent of tissue damage and, in practice, bronchoscopists use “soft palpation,” a subjective parameter that is not an issue with APC, a noncontact modality.
* By local application of cold saline, epinephrine, and tamponade with the bronchoscope.
1. Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278-1297.
2. Dalupang JJ, Shanks TG, Colt HG. Nd-YAG laser damage to metal and silicone endobronchial stents: delineation of margins of safety using an in vitro experimental model. Chest. 2001;120:934-940.
3. Matt BH, Myer CM3rd, Harrison CJ, et al. Tracheal granulation tissue: a study of bacteriology. Arch Otolaryngol Head Neck Surg. 1991;117:538-541.
4. Freitag L. Airway stents. In: Strausz J, Bolliger CT. Interventional Pulmonology. Lausanne, Switzerland: European Respiratory Society; 2010:190-217.
5. Saad CP, Murthy S, Krizmanich K, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124:1993-1999.
6. Tendulkar RD, Fleming PA, Reddy CA, et al. High-dose-rate endobronchial brachytherapy for recurrent airway obstruction from hyperplastic granulation tissue. Int J Radiat Oncol Biol Phys. 2008;70:701-706.
7. Brichet A, Verkindre C, Dupont J, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J. 1999;13:888-893.
8. Patelli M, Gasparini S. Post-intubation tracheal stenoses: what is the curative yield of the interventional pulmonology procedures? Monaldi Arch Chest Dis. 2007;67:71-72.
9. Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med. 2008;8:18.
10. Martinez-Ballarin JI, Diaz-Jimenez JP, Castro MJ, et al. Silicone stents in the management of benign tracheobronchial stenoses: tolerance and early results in 63 patients. Chest. 1996;109:626-629.
11. Colt HG. Functional evaluation before and after interventional bronchoscopy. In: Bolliger CT, Mathur PN. Interventional Bronchoscopy. Basel, Switzerland: S. Karger; 2000:55-64.
12. Karagiannidis C, Velehorschi V, Obertrifter B, et al. High-level expression of matrix-associated transforming growth factor-beta1 in benign airway stenosis. Chest. 2006;129:1298-1304.
13. Lee YC, Hung MH, Liu LY, et al. The roles of transforming growth factor-β1 and vascular endothelial growth factor in the tracheal granulation formation. Pulm Pharmacol Ther. 2011;24:23-31.
14. Hirshoren N, Eliashar R. Wound-healing modulation in upper airway stenosis—myths and facts. Head Neck. 2009;31:111-126.
15. Maddox TM. Preoperative cardiovascular evaluation for noncardiac surgery. Mt Sinai J Med. 2005;72:185-192.
16. Eagle KA, Rihal CS, Mickel MC, et al. Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study. Circulation. 1997;96:1882-1887.
17. Eagle KA, Berger PB, Calkins H, et al. American College of Cardiology, American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 2002;39:542-553.
18. Smetana GW, Lawrence VA, Cornell JE, American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
19. Harris S. COPD and coping with breathlessness at home: a review of the literature. Br J Community Nurs. 2007;12:411-415.
20. Fisher L, Weihs KL. Can addressing family relationships improve outcomes in chronic disease? Report of the National Working Group on Family-Based Interventions in Chronic Disease. J Fam Pract. 2000;49:561-566.
21. Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest. 2000;118:516-521.
22. van Boxem TJ, Westerga J, Venmans BJ, et al. Tissue effects of bronchoscopic electrocautery: bronchoscopic appearance and histologic changes of bronchial wall after electrocautery. Chest. 2000;117:887-891.
23. Staehler G, Halldorsson T, Langerholc J, et al. Dosimetry for neodymium:YAG laser applications in urology. Lasers Surg Med. 1980;1:191-197.
24. Halldorsson T, Langerholc J, Senatori L. Thermodynamic analysis of laser irradiation of biological tissue. Appl Optics. 1978;17:3984-3985.
25. Fisher JC. The power density of a surgical laser beam: its meaning and measurement. Lasers Surg Med. 1983;2:301-315.
26. Witt C, Schmidt B, Liebetruth J, et al. Nd: YAG laser and tracheobronchial metallic stents: an experimental in vitro study. Lasers Surg Med. 1997;20:51-55.
27. Scherer TA. Nd:YAG laser ignition of silicone endobronchial stents. Chest. 2000;117:1449-1454.
28. Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119:781-787.
29. Colt HG. Bronchoscopic resection of Wallstent-associated granulation tissue using argon plasma coagulation. J Bronchol. 1998;5:209-212.
30. Reichle G, Freitag L, Kullmann HJ, et al. Argon plasma coagulation in bronchology: a new method—alternative or complementary? Pneumologie. 2000;54:508-516.
31. Keller CA, Hinerman R, Singh A, et al. The use of endoscopic argon plasma coagulation in airway complications after solid organ transplantation. Chest. 2001;119:1968-1975.
32. Kono M, Yahagi N, Kitahara M, et al. Cardiac arrest associated with use of an argon beam coagulator during laparoscopic cholecystectomy. Br J Anaesth. 2001;87:644-646.
33. Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest. 2008;134:1066-1069.
34. Colt HG, Crawford SW. In vitro study of the safety limits of bronchoscopic argon plasma coagulation in the presence of airway stents. Respirology. 2006;11:643-647.
35. Nakagishi Y, Morimoto Y, Fujita M, et al. Photodynamic therapy for airway stenosis in rabbit models. Chest. 2008;133:123-130.
36. van Boxem AJ, Westerga J, Venmans BJ, et al. Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Cancer. 2001;31:31-36.
37. Krimsky WS, Broussard JN, Sarkar SA, Harley DP. Bronchoscopic spray cryotherapy: assessment of safety and depth of airway injury. J Thorac Cardiovasc Surg. 2010;139:781-782.
38. Krimsky WS, Rodrigues MP, Malayaman N, et al. Spray cryotherapy for the treatment of glottic and subglottic stenosis. Laryngoscope. 2010;120:473-477.
39. Kramer MR, Katz A, Yarmolovsky A, et al. Successful use of high dose rate brachytherapy for nonmalignant bronchial obstruction. Thorax. 2001;56:415-416.
40. Kennedy AS, Sonett JR, Orens JB, et al. High dose rate brachytherapy to prevent recurrent benign hyperplasia in lung transplant bronchi: theoretical and clinical considerations. J Heart Lung Transplant. 2000;19:155-159.
41. Shlomi D, Peled N, Shitrit D, et al. Protective effect of immunosuppression on granulation tissue formation in metallic airway stents. Laryngoscope. 2008;118:1383-1388.
42. Burningham AR, Wax MK, Andersen PE, et al. Metallic tracheal stents: complications associated with long-term use in the upper airway. Ann Otol Rhinol Laryngol. 2002;111:285-290.
43. Alazemi S, Lunn W, Majid A, et al. Outcomes, health-care resources use, and costs of endoscopic removal of metallic airway stents. Chest. 2010;138:350-356.
44. Tremblay A, Marquette CH. Endobronchial electrocautery and argon plasma coagulation: a practical approach. Can Respir J. 2004;11:305-310.
45. Boxem T, Muller M, Venmans B, et al. Nd-YAG laser vs bronchoscopic electrocautery for palliation of symptomatic airway obstruction: a cost-effectiveness study. Chest. 1999;116:1108-1112.
46. Saraon TS. Pacemakers and implantable cardioverter defibrillators. http://emedicine.medscape.com/article/780825-overview#MagnetInhibition, Accessed March 6, 2011.
47. Matsuo T, Colt HG. Evidence against routine scheduling of surveillance bronchoscopy after stent insertion. Chest. 2000;118:1455-1459.
48. Dialani V, Ernst A, Sun M, et al. MDCT detection of airway stent complications: comparison with bronchoscopy. AJR Am J Roentgenol. 2008;191:1576-1580.
49. Verkindre C, Brichet A, Maurage CA, et al. Morphological changes induced by extensive endobronchial electrocautery. Eur Respir J. 1999;14:796-799.
50. British Thoracic Society. Guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(Suppl 1):i1-i21.
51. Ernst A, Eberhardt R, Wahidi M, et al. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest. 2006;129:734-737.
52. Rojas-Solano J, Becker HD. Bronchoscopic application of mitomycin-C as adjuvant treatment for benign airway stenosis. J Bronchol Intervent Pulmonol. 2011;18:53-56.
* An electrical current in which electron flow moves in only one direction; galvanic currents cause fibroblast proliferation and a resultant increase in collagen synthesis—a property used for wound healing and implicated in keloid formation.
† Benign hyperplastic granulation tissue can cause airway obstruction in up to 20% of patients after lung transplantation, typically occurring at the site of bronchial anastomosis a few months after surgery.
* Most studies define complex stenoses as extensive scar ≥1 cm in vertical length, circumferential hourglass-like contraction scarring, or the presence of associated malacia.
† Coexistent diseases such as coronary heart disease, severe cardiac or respiratory insufficiency, or a poor general condition.
* Of course, granulation tissue and other stent-related complications such as obstruction by tenuous secretions can be treated at the time of the initial diagnostic flexible bronchoscopy. One should be ready for procedure-related complications such as increasing hypoxemia or bleeding. These may be caused by the stent-related complication itself or during the course of treatment. For example, while tissues or secretions are removed with forceps, the loop sutures of a metal stent might be accidentally grasped, thereby mobilizing the stent and causing complete airway obstruction. On other occasions, tissue causing complete stent obstruction might not be removable. The bronchoscopist should be able to punch a hole through the tissue or secretions during the entire length of the stent, to create a passageway for ventilation, while other means of removing tissues, such as electrocautery or cryotherapy, are prepared.
† Normally, wound healing lasts up to 2 years, but malnutrition, hemodynamic status, tissue hypoxia, and metabolic factors (e.g., acidosis, diabetes mellitus) can cause a delay in the healing process.
* Atelectasis, pneumonia, respiratory failure, and exacerbation of underlying chronic lung disease.
† Advanced age (>65 years) is an important independent predictor of postoperative pulmonary complications even after adjustment for comorbidities.
* By refractory, we mean persistent hypoxemia (<90%) despite administration of supplemental oxygen or noninvasive positive-pressure ventilation.
* The first liter is 0.03 FiO2; thereafter it is 0.04 to each liter flow of oxygen. Therefore 3 L is the equivalent of approximately 0.32 FiO2. Room air is 0.21 FiO2.
† 1 second coagulation resulted in a depth of necrosis of 0.2 ± 0.1 mm, and 0.2 ± 0.3 mm of tissue damage underneath; after 5 seconds, the depth of the crater with necrosis was 1.9 ± 0.8 mm, with clear damage to the underlying cartilage.
* HBO increases the oxygen flow and the arterial partial oxygen pressure in tissues, thus improving healing and re-epithelialization.
† Acts as an alkylating agent that inhibits DNA synthesis by cross-linking DNA; mitomycin also suppresses RNA and protein synthesis.
‡ 5-FU is a well-known antimetabolic drug that has an antiproliferative effect on human fibroblasts.
§ Halofuginone is also an antifibrotic agent owing to a number of properties; it inhibits reversibly collagen1α synthesis.
‖ It completely blocks cross-linkage of newly formed collagen.
¶ NAC interferes with disulfide bond formation.
* Stent damage was defined as visualization of blackish discoloration, blister formation, perforation, actual ignition, or rupture of the integrity of the stent. Rupture of the integrity of the metal stent was defined as a spontaneous break in the continuity of the metal mesh during laser application.
† In this study, however, the laser beam was focused on each stent, rather than on surrounding tissues, as is the case in clinical practice during removal of granulation tissue. Because laser energy is easily scattered and reflected, however, collateral absorption is frequent.
‡ The average power density delivered for each setting was computed based on the formula: Paw = 0.8635 × power (watts)/πr2, where πr2 is the spot size, and Paw is average power density. Because the laser beam diverges 11 degrees around the fiber core, the spot size at a distance was calculated using the following equation: Spot size = π ([fiber radius in centimeters] + [distance in centimeters] × tan 5.5 degrees).2 When fiber-to-target distances of 10 mm and 20 mm were used, therefore, spot sizes were 0.05 cm2 and 0.155 cm2 (0.6 mm diameter coaxial fiber).
* Placing a magnet over a permanent pacemaker does not turn the pacemaker off but temporarily “reprograms” the pacer into asynchronous mode.
† With some devices, application of a magnet produces a soft beep for each QRS complex. If the magnet is left on for approximately 30 seconds, the implantable cardioverter-defibrillator (ICD) is disabled and a continuous tone is generated. To reactivate the device, the magnet must be lifted off the area of the generator. After 30 seconds, the beep returns for each QRS complex.
‡ Higher FiO2 (up to 1.0) may be safely used in soft coagulation mode because no electrical arc formation occurs in this mode; therefore no risk of ignition is present.
* Without this function, coagulation is stopped by the operator as soon as vapors are seen, and the probe is cleaned of debris and charred tissue.
† Position the patient pad as close as possible to the operative site on a well-vascularized muscular area; one area is on the flank, over the kidney on the latissimus dorsi muscle, with the pad lateral and wrapping around the side; the pad should not be placed over bony prominences, tattoos, or scars or on hairy surfaces.
* APC should be avoided in an oxygen-rich environment; APC in the presence of foreign materials such as stents should be performed using short 1 to 2 second bursts and a lower 40 W power setting.
† Good technique with APC includes brief tissue rest time between activations. This cooling and shrinkage of tissue gives the physician a more accurate assessment of the depth of penetration from previous activations. Constant movement helps to assure that energy is not overly concentrated in one area.
‡ Users may try manipulating the probe rather than the scope/probe continuum. The scope optical chip will set the correct viewing distance, allowing the physician consistent depth perception if the probe remains stationary. The physician may want to touch the tissue then pull back 1 to 3 mm before firing to set his visual cues for proximity to the tissue.
§ Argon gas itself is not combustible, nor does it promote the combustion of combustible materials. Ignition is possible only if a combustion-promoting gas such as oxygen is also present or is mixed with the argon and applied to the combustible materials.
* These mediators are produced mainly by macrophages, which also accelerate nitric oxide synthesis by producing IL-1 and TNF-α.
† Enhanced TGF-β1–mediated proliferation of human lung fibroblasts occurs in a dose-dependent manner in the presence of mitomycin-C. The local milieu with high-level, matrix-associated expression of TGF-β1 in stenoses could therefore be one reason for the limited treatment effect of mitomycin-C.