Chapter 9 Bronchoscopic Treatment of Post Tracheostomy Tracheal Stenosis with Chondritis
Case Description
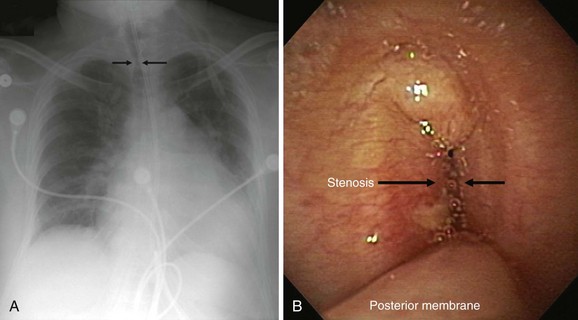
This patient is an 86-year-old woman with a history of ischemic stroke and coma requiring 4 weeks of endotracheal intubation, followed by percutaneous dilational tracheostomy (PDT). She was decannulated 3 months later in the nursing home where she lived because of required assistance for daily living activities. She had been hospitalized previously for respiratory failure due to decompensated congestive heart failure (CHF). She was extubated after CHF was treated, but within minutes of extubation she developed biphasic stridor and had to be emergently reintubated. Three days later, after administration of Solu-Medrol 60 mg intravenously every 12 hours, she was successfully extubated and transferred to the medicine ward. A few days later, however, the intern on call was called to evaluate her for recurrent stridor and increased work of breathing. The chest radiograph (CXR) shown in Figure 9-1 revealed narrowing of the upper trachea, as well as cardiomegaly and pulmonary vascular congestion. Bronchoscopy confirmed radiographic findings and showed a 1.5 cm long triangular stomal stricture 4 cm below the vocal cords. During inspiration, this narrowed the lumen by 80%, but during expiration, 100% airway lumen obstruction occurred as the result of lateral cartilaginous collapse of two tracheal rings (see Figure 9-1). The rest of the airway examination was unremarkable. No evidence of mucus plugging or secretions was found distal to the stenosis. In addition to severe CHF, her medical history included hypothyroidism, hypertension, anemia, Alzheimer’s dementia, and right hemiplegia from her ischemic stroke. Physical examination revealed an elderly woman in mild respiratory distress at rest who followed some simple commands and had biphasic stridor over the anterior neck auscultation. Oxygen saturation was 95% on room air. Karnofsky performance score before the episode of respiratory failure was 50, and American Society of Anesthesiologists (ASA) score at the time of the diagnostic bronchoscopy was 3. Laboratory data were normal. A two-dimensional echocardiogram performed during hospitalization revealed normal left ventricular function, but a moderately enlarged left atrium and diastolic dysfunction were evident. The patient has one daughter, who rarely visits her in the nursing home but has been visiting her during her most recent hospitalization. According to the staff at the nursing home, the patient enjoys watching television and seems to enjoy eating a modified dysphagia diet. At baseline, she follows some simple commands but is aphasic and does not speak.
Discussion Points
1. Describe three qualitative features of tracheal stenosis.
2. Describe three diagnostic modalities other than bronchoscopy that can be performed to assess the severity, extent, and morphology of this patient’s tracheal stenosis.
3. List four therapeutic alternatives for post tracheostomy stenosis with chondritis.
4. Identify three airway stricture characteristics that determine the need for and type of treatment.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
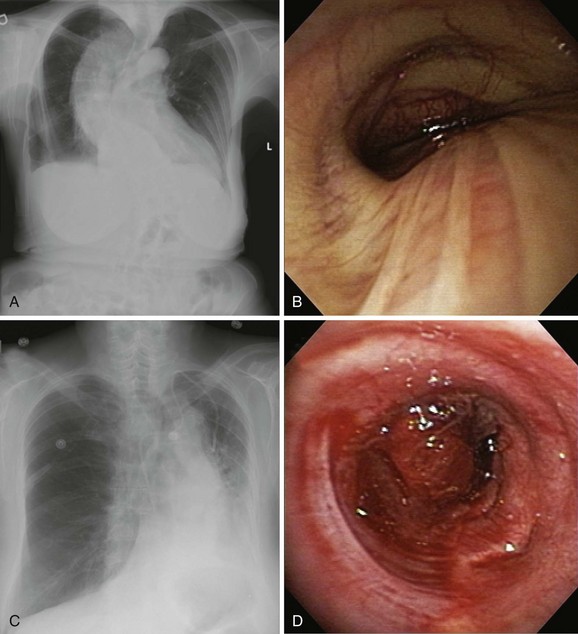
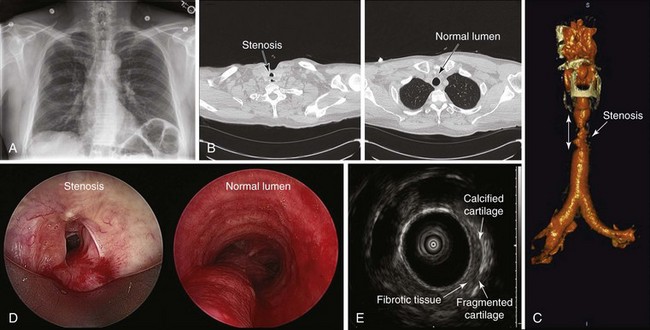
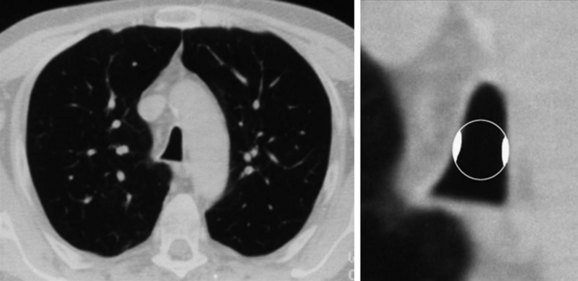
The initial diagnosis of tracheal stenosis occurred after a careful examination of the chest radiograph obtained post extubation. Chest radiographs are rarely diagnostic of central airway obstruction yet are often obtained as initial radiologic tests. Obvious pathology such as tracheal deviation from masses or severe tracheal stenosis similar to that seen in this case may be identified; CXR can also reveal changes that may alter the normal airway-vasculature relationship, such as skeletal deformities or mediastinal shift (Figure 9-2). In cases of post tracheostomy stenosis (PTS), the tracheal air column is easily overlooked by radiologists and clinicians alike; thus careful inspection is warranted in a patient who is symptomatic post tracheostomy.1 The CXR does not allow accurate determination of morphology, extent, degree of narrowing, and associated findings such as chondritis (involvement of the cartilage and resulting collapse). From this perspective, standard computed tomography (CT) scans provide much more information and the ability to document airway collapse when performed during both inspiration and expiration. Multiplanar and three-dimensional reconstruction with internal (virtual bronchoscopy) and external rendering (virtual bronchography), with excellent image quality, is achievable with the use of low-dose techniques (Figure 9-3). Analysis using these newer imaging protocols better characterizes whether the lesion is intraluminal, extrinsic, or mixed, and whether the airway distal to the obstruction is patent.2,3 In addition, the length and diameter of the lesions and their relationship to adjacent vascular structures are assessed with a higher degree of accuracy. These features may assist physicians in determining appropriate therapy.4
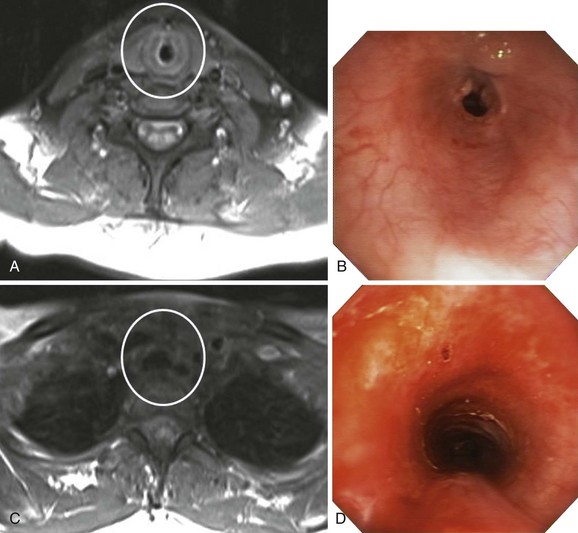
Magnetic resonance imaging (MRI) has been used in small case series of tracheal stenosis.5–7 Results of these studies show that MRI can be used to identify the relationship of the trachea to adjacent vascular structures, and to determine the degree and length of tracheal stenosis, without the use of ionizing radiation or intravenous contrast medium (Figure 9-4). Following percutaneous dilational tracheostomy, MRI provides an excellent noninvasive method of assessing the integrity of the tracheal lumen.8 Neither MRI nor CT provides information about mucosal changes, and neither can reliably image the integrity of the cartilaginous framework of the airway. Although investigators are exploring the use of high-resolution endobronchial ultrasonography (see Figure 9-3), it appears as of this writing that optical imaging using flexible bronchoscopy remains a procedure of choice to diagnose and identify the type, location, and severity of an airway stricture before therapeutic interventions are proposed.9,10
This patient had developed a postsurgical tracheo-stomy–related stricture 90 days after an indwelling size 8 cuffed tracheostomy tube with 11 mm outside diameter was inserted. The true incidence of PTS is difficult to determine accurately from the published literature because of inconsistent follow-up, but it is estimated to be 1% to 2%.11 The mean onset of strictures seems to be earlier after PDT than after open surgical tracheostomy: 5.0 weeks versus 28.5 weeks (P = .009).12
PTS appears in three locations:
1. Suprastomal stenosis: When defined as involving more than 50% of the lumen, this type of stricture was noted in 23.8% of PDT patients and in 7.3% of surgical tracheostomies in one study.13 The superior level (proximal) of the stenosis was located at a mean distance of 1.6 cm from the vocal cords in PDT patients, and at 3.4 cm from the cords in surgical tracheostomy patients (P = .04). This might be secondary to the use of incorrect needle puncture sites during PDT.
2. Stenoses at the level of the tracheostomy tube cuff: These are caused by ischemic mucosal damage when cuff-to-tracheal wall tension exceeds the mucosa capillary perfusion pressure, usually 20 to 30 mm Hg; subsequent inflammatory histologic changes may occur as soon as within 24 to 48 hours. The incidence of these lesions has been reduced 10-fold after transition to high-volume–low-pressure cuffs. It is therefore warranted that when patients have indwelling endotracheal or tracheostomy tubes, peak inspiratory and expiratory cuff pressures should be kept below 15 mm Hg, and definitely below 25 mm Hg.14
3. Stomal strictures: This is the type of stenosis described in our patient. These stenoses account for more than 85% of cases of PTS.10 They may be secondary to inadequate tracheal incisions, ongoing stomal infection, or a rigid tube-connecting system that generates excess tube motion within the trachea. In one review paper, stomal wound infection was a causative factor in 42% of stomal stenoses following open tracheostomy.15 Strictures may result from abnormal wound healing and excess granulation tissue formation around the tracheal stoma site; excess granulation tissue can also develop over cartilage fractured during tracheostomy.16 Cartilage damage can result from mechanical leverage of the tracheal tube at the stoma site from the unsupported weight of ventilator attachments. This can cause pressure necrosis of tracheal mucosa and the underlying cartilaginous frame. Because risk is high if the tracheostomy tube is too large for the airway, recommendations are to consider a size 8 tube with an 11 mm outer diameter as an upper limit in men, and a size 7 tube with a 10 mm outer diameter in women.14 We presume that the 11 mm external diameter tube used in our patient was probably too large and may have contributed to the development of her PTS.
Symptoms (at rest) in tracheal stenosis usually are not present until a 70% reduction in lumen diameter occurs, but stridor, as seen in our patient, occurs when the tracheal lumen is less than 5 mm in diameter.10 The presence of stridor on neck auscultation is consistent with a bronchoscopic classification of severe airway narrowing signaling greater than 70% airway lumen narrowing.9,17,18 Indeed, based on the Myer-Cotton classification system* for laryngotracheal stenosis, this patient has a grade III lesion because her stenotic index† was 80%.19
In addition to an accurate assessment of airway lumen, the functional status of the tracheal stenosis patient should be part of a multidimensional evaluation. On this note, the Medical Research Council (MRC) dyspnea scale was found to be highly sensitive to the presence of varying degrees of tracheal stenosis. Strong correlation was noted between the severity of the stenosis and the MRC grade. The MRC scale furthermore was found to be responsive to changes in a patient’s effort tolerance resulting from treating the obstructive lesion.20
Comorbidities
This patient had significant comorbidities, including CHF and a history of ischemic stroke. When surgical or bronchoscopic interventions are provided under general anesthesia, comorbidities could significantly increase the risks for perioperative adverse events such as decompensated CHF or new cerebral ischemia.21 Preoperative decompensated CHF, as seen in our patient, has been identified as a risk factor for other cardiac complications after surgery and often requires postponing elective surgery for a week after resolution of symptoms.22 Although a therapeutic bronchoscopic intervention in this patient is not considered a high–cardiac risk procedure (contrary to vascular, intraperitoneal, or intrathoracic procedures), her age greater than 70 years and her previous history of CHF and cerebrovascular disease are independent risk factors for major cardiac complications that need to be seriously considered.23 The presence of cerebrovascular disease is a particularly important finding in elderly patients undergoing general anesthesia. In general, at least 2 weeks should elapse after a stroke before elective surgery is attempted. Furthermore, if recurrent transient ischemic attacks (TIAs) have occurred, an evaluation for carotid artery disease is warranted. Our patient had her stroke several years before her tracheal stricture. No signs of recurrent TIAs were noted, and her CHF had been stabilized for a week before our evaluation.
Support System
The patient was a resident of a nursing home and had poor family support. The quality of social relationships for nursing home residents was shown to have a significant effect on older people’s self-perceived quality of life, life satisfaction, and well-being. Residents of nursing homes consistently report that they experience limited meaningful interaction with others and may feel socially isolated, despite the busyness of a long-term care home.24 Social interaction in these institutions can be difficult and is not always well supported by the environment or the staff. In addition, as noted in our patient, individual factors such as restricted mobility and diminished cognitive ability can further limit interaction with other people.24
Patient Preferences and Expectations
Because of her dementia and post stroke aphasia, the patient was unable to clearly express a desire for treatment. Her daughter, as the next of kin, had durable power of attorney and was the designated health care decision maker. She was informed about available therapeutic options for her mother’s tracheal stenosis, including dilation with airway stent insertion, surgery, and even repeated tracheostomy. Because it was believed that tracheostomy would further compromise her quality of life, this was considered a less optimal alternative. The likelihood of complications resulting from tracheal resection was felt to outweigh the benefits in this particular case because of her cardiac and neurologic comorbidities; in addition, from a surgical standpoint, the risk for anastomotic complications was increased in view of a previous tracheostomy and the high likelihood of peritracheal fibrosis, which limits tracheal mobility and increases tension, potentially leading to catastrophic failure of the anastomosis.25 Thus rigid bronchoscopy under general anesthesia with stent placement was offered to this patient as the therapeutic alternative of choice.
Procedural Strategies
Indications
This was a symptomatic PTS that resulted in stridor and respiratory distress at rest. A bronchoscopic procedure or open surgery may be offered to improve dyspnea, restore satisfactory airway lumen patency, and improve symptoms.9,26 Symptoms in tracheal stenosis are related mainly to the degree of airway narrowing and flow velocity through the stenosis, and to a lesser degree to the extent or morphology of the stricture. The drop in airway pressure along the stenotic area increases significantly at rest when more than 70% of the tracheal lumen is obliterated. Our patient’s inactive lifestyle caused routinely low-flow velocity through her stenotic airway. This explains why she developed symptoms only when a severe degree of airway narrowing was present. Improving airway patency to a lesser (mild) degree of narrowing (<50%) would partially or completely alleviate this patient’s shortness of breath.27 Palliation of her airway narrowing was therefore warranted.
The stricture was 1.5 cm in extent. Although this length is considered of moderate degree and may impact surgical decisions or choice of stent type and length, long stenoses show a modest difference in pressure profile with a slightly smaller magnitude of total pressure drop than the simple shorter and less than 1 cm stenosis of comparable diameter.27 It appears that in terms of flow dynamics and symptoms, the degree of airway narrowing is more important than the extent of stenosis.
Tracheal stricture morphology in this case was triangular. Triangular stenoses have been described as lambdoid, pseudoglottic, or A-shaped strictures. Experimental flow dynamic studies show that this morphologic type of stenosis results in slightly less pressure drop than an elliptical morphology of similar degree of airway narrowing,28 suggesting that an accurate description of the morphologic type (i.e., triangular, circumferential, or elliptical) may impact symptoms and eventually decisions about treatment. To our knowledge, however, no common language or nomenclature has been universally accepted for tracheal strictures.
Contraindications
No absolute contraindications to rigid bronchoscopy were known in our patient. However, the risk for perioperative cardiac complications is almost doubled when clinical signs of CHF are present preoperatively.29 Our patient had no clinical signs of CHF at the time of our evaluation. She was therefore continued on her medicines, which included angiotensin-converting enzyme (ACE) inhibitors, diuretics, and beta blockers. Decompensated CHF (New York Heart Association [NYHA] class IV), if present, should be treated to the extent possible before surgery, and postponement of surgery is often appropriate.30 Regarding her history of cerebrovascular disease, perioperative stroke is an infrequent but serious complication, occurring at a rate of 0.3% to 3.5%, with most cases occurring during the postoperative period.31
Expected Results
In patients with PTS, therapeutic success of rigid bronchoscopic interventions is variable. Factors influencing the success rate include the presence or absence of chondritis resulting in cartilaginous collapse (malacia), a characteristic of complex stenosis. This triangular stenosis, due to loss of varying amounts of anterior and lateral cartilaginous wall, will accept various sizes of dilating instruments, such as rigid bronchoscopes, but generally will not respond more than transiently with an increase in airway cross-sectional area.1 For instance, in one study of patients with complex stenosis, including those with PTS, during a follow-up period of 28 to 72 months, only 22% of patients (n = 13) were treated successfully with laser-assisted mechanical dilation, whereas 47 patients (78%) required stent placement; 22 had their stent removed after 1 year and did not require further therapy. Thirteen inoperable patients required permanent stent insertion, and 12 others were referred to surgery after failure despite numerous repeated endoscopic treatments.32 In another study, all patients with complex stenosis including PTS had undergone tracheal stent placement during an initial rigid bronchoscopy. After 6 months of follow-up, of those patients who continued to be considered inoperable (n = 10), most (n = 9) eventually required permanent bronchoscopic airway stent insertion.9
Team Experience
Experience and expediency might result in reduced complications in this patient with cardiac and neurologic comorbidities. Team experience and an available multidisciplinary airway disease program, including thoracic and ear, nose, and throat surgeons, anesthesiologists, and pulmonologists, can result in the elaboration of specific therapeutic algorithms for patients with this disease. Failure rates, mortality, and morbidity of operative and nonoperative techniques in the published literature and in the operators’ experience should be taken into account to establish a specific therapeutic approach.9
Risk-Benefit Analysis
The risks of postoperative decompensated CHF and postoperative stroke were outweighed by the benefit of improving airway patency to relieve this patient’s respiratory distress and avoid recurrent respiratory failure caused by severe tracheal stenosis. Although tracheal sleeve resection is considered the definitive treatment for this type of lesion, this patient’s poor general health, previous tracheostomy, and cardiac and cerebrovascular comorbidities would significantly increase the risk for postoperative complications after open surgical interventions.33 Therefore, other techniques have been proposed in the literature as alternatives to open surgery.
Therapeutic Alternatives for Restoring Airway Patency
• Dilation using balloon, bougies, or dilators (e.g., Jackson dilators): These tools are usually used if the stenosis shows stenotic hypertrophic fibrotic tissue in a circumferential pattern, which was not seen in our patient. Simple dilation would not be appropriate because cartilage would collapse again once the dilating instruments were removed from the stricture.34
• Laser resection using neodymium-doped yttrium aluminum garnet (Nd:YAG) lasers35: This approach can be successfully used in conjunction with dilation to release the tension of the stricture by performing radial incisions onto the hypertrophic stenotic tissues; because these were not present in our patient, this modality was not a consideration.
• Carbon dioxide (CO2) laser resection of the collapsed tracheal cartilage, with sparing of the posterior membrane to prevent circumferential restenosis (endoscopic tracheoplasty)36: This procedure is done under general anesthesia, often with muscle relaxants, high-frequency supraglottic jet ventilation, and suspension microlaryngotracheoscopy. In one study, this recently proposed technique achieved a successful outcome (decreased MRC dyspnea scale) and lack of need for repeated intervention after 6 months of symptom-free follow-up, thus avoiding the operative risks, prolonged hospitalization, and morbidity associated with open surgical tracheal resection. More experience, reproducible evidence, and longer follow-up are necessary before this treatment modality can be recommended routinely.
• Covered and uncovered metal stent insertion using flexible bronchoscopy: These techniques have been reported to be successful in series of tracheal stenoses including complex PTS.37,38 However, recurrent obstruction from granulation can occur in up to 36% of patients as the result of tissue growth through the spaces in a stent’s wire mesh. Metal stents are associated with significant complications, especially when placed in the subglottic region, prompting statements that this location should ideally be avoided,38 especially in cases of histologically proven benign airway disorders.39 A study published in the surgical literature suggests that all patients with tracheal and subglottic stenosis who had undergone covered or uncovered metallic stent placement developed new strictures or granulation tissue.40 Injuries were severe, occurred after a short duration of stent insertion, and either precluded definitive surgical treatment or required more extensive tracheal resection. Metal stents, therefore, should not be used in patients who are potential candidates for resection because these are highly likely to cause additional airway injury and to make a potentially resectable patient inoperable.41
• Self-expandable silicone stents: Unlike metal stents, silicone stents have the advantage of being easily removable. They are placed under rigid bronchoscopy or suspension laryngoscopy, and therefore require expertise in these techniques. Some silicone stents have been studied in benign airway obstruction, including tracheal stenosis and malacia.42 Although immediate symptom palliation was established in most cases, the incidence of complications was high (75%), with stent migration occurring in 69% of cases.42,43
• Silicone stent insertion is an acceptable alternative to surgery for many inoperable patients with complex stenosis. It is reported to maintain airway patency over the long term with minimal complications.9 However, the success rate of bronchoscopic treatment after stent removal (after 6 months) in cases of complex stenosis, as in patients with chondritis (as in our case), is reportedly low (17.6%). This low definitive curative yield of bronchoscopic procedures in cases of complex stenosis and the reported risk that a stent might lengthen the tracheal segment to be resected suggest that a tracheal sleeve resection should be the first option in cases of complex stenosis with chondritis if the patient is suitable for surgery.44 In fact, national societies such as the Interventional Pulmonology Study Group of the Italian Association of Pulmonologists (AIPO) recommend that when one or more cartilaginous rings are involved, endoscopic treatment is usually contraindicated unless surgery is not feasible (Level of Recommendation B).44 Other investigators have noted a high rate of airway stability and good results following stent insertion, with definitive and curative success described in 22 of 47 patients (46.8 %) after stent removal.32 In this study, airway stents had remained in place for a mean of 11.6 months, but most patients (12/22) had their stent placed for longer than 12 months. A more recent study showed that of 42 patients with complex stenoses, only 9 were surgical candidates and 33 were treated with silicone stent insertion, with a success rate of 69%.45 It is noteworthy, however, that studies evaluating complex stenoses do not specifically report separate results for post intubation and post tracheostomy subgroups. The pathogenesis and treatment approach for these stenoses, therefore, may indeed differ significantly.10
• Montgomery T-tube: This tube can be used when tracheal repair and reconstruction or dilation techniques are not available or have failed, or occasionally as an initial therapy or as a solution for patients who had silicone stent placement complicated by frequent migrations.46,47 For most patients who do not require mechanical ventilatory support, a silicone T-tube provides greater functional capability and comfort.1 In a large case series consisting of 53 patients with complex tracheal stenoses (24 post tracheostomy), silicone T-tube insertion was effective in 70% of patients with limited complications. Concurrent cardiopulmonary disease and intractable infection were the two major causes of failure after T-tube placement.48 Granulation tissue formation at the proximal end of the T-tube has also been noted. Chronic airway irritation incites infection and promotes or aggravates granulation tissue formation. The sharper edge of the proximal aspect of the T-tube (when it has to be cut) and its placement within 0.5 cm of the vocal cords are known risk factors for granulation tissue development.48 If the T-tube is not properly capped, allowing the patient to breathe through the mouth, airway secretions become dry and may cause obstruction. Patients, families, and referring health care providers probably benefit from diligent instruction on how to care for and monitor T-tubes. Frequent bronchoscopies may be necessary to remove extremely thick and sticky mucous plaques; some investigators perform three to four biweekly bronchoscopies, followed by procedures once every 4 weeks, once good stent patency has been documented.48
• Open surgical interventions such as tracheal sleeve resection or airway reconstruction are preferred initial therapies and are considered for patients who become operable candidates after short-term successful bronchoscopic intervention, as well as for operable candidates who have failed bronchoscopic treatment. Surgical tracheal sleeve resection has a failure rate ranging from 5% to 15% and a postoperative mortality ranging from 1.8% to 5%.49,50 More recently, single-stage laryngotracheal resection was performed successfully with what has been considered acceptable morbidity in specialized centers, with 3 patients (8.1%) developing major complications (2 fistulas and 1 early stenosis) that required a second surgical procedure. Long-term results were excellent to satisfactory in 36 patients (97.3%) and unsatisfactory in 1 (2.7%).51 Proponents of open surgery as the treatment of choice believe that the unique tracheal vascular anatomy and the mechanism of injury underscore the transmural nature of airway lesions, which also explains the limited success of purely endoluminal therapies (e.g., laser treatments) in the management of tracheal strictures.52
• Permanent tracheostomy is warranted in the few patients with critical stenoses who neither are candidates for surgery or stent insertion nor develop recurrence after such interventions9 and, of course, in regions where airway expertise may not be available. Referral to tertiary care centers in these instances can be beneficial for patients and caregivers.
Informed Consent
The purposes of informed consent are to promote autonomy, to protect a patient from undesired treatment, and to help patients make appropriate medical care decisions that correlate with their personal values. Elderly patients are particularly vulnerable in the informed consent process. Not only are they more likely to suffer from medical conditions that can impair cognition by virtue of age or disease morbidity, they may also suffer from physical disabilities (such as hearing loss or aphasia) that impair communication even when cognition is intact. Obtaining an informed consent supports the principle of autonomy by providing information in a context that allows voluntary choice to potential participants who are competent to decide, to affirm their understanding, and to agree to participate. Because of their cognitive impairment, persons with dementia (similar to our patient) not only are vulnerable but often are incapable (to varying degrees) of protecting their own interests during the informed consent process.53 The National Bioethics Advisory Commission specifically identified Alzheimer’s dementia as a disorder that can affect decision-making capacity. The American Geriatrics Society, however, took the position that “a diagnosis of dementia does not automatically confer decisional incapacity on affected individuals,” and that because decision making is task specific, some persons with cognitive impairment will be able to provide a valid informed consent. Establishing capacity for decision making, therefore, was an important aspect of the consent process for our patient.
The capacity to consent is usually determined by assessing specific decision-making abilities; this involves asking patients narrative questions that provide information about their understanding, appreciation, reasoning, and ability to express a choice. One must also be able to understand the consequences of one’s decision to undergo or refuse a therapeutic course of action. Expressing a choice is described as having the ability to communicate a desire to participate or not participate in a certain intervention and is considered a threshold ability: If the patient is unable to express a choice, assessment of other abilities is not necessary. Establishing decision-making capacity does not require specific subspecialty consultation. When a patient lacks decision-making capacity, of course, the clinician must document the finding, state the cause, and search for a reliable surrogate to make decisions considered to be in the best interests of the patient and/or according to what would have been the patient’s desires had he or she been able to make the decision. If the patient has an advance directive that identifies a surrogate, this choice should be respected.54 Fortunately, our patient had an advance directive that delegated her daughter as the health care decision maker. Advance directives are legally and ethically binding tools by which patients can express their own decisions regarding medical care before they actually lose the capacity to do so.
Techniques and Results
Anesthesia and Perioperative Care
With regard to her comorbidities, in general, patients with a history of CHF who are asymptomatic at the time of surgery should continue their current medical regimen. ACE inhibitors are considered safe during the perioperative period, despite concern for postoperative hypotension. It is noteworthy that hypotension was a frequently noted adverse event during rigid bronchoscopic procedures in one series of octogenarians.55 Diuretics are safe as long as attention is paid to fluid status and electrolytes. Patients with CHF already receiving beta blockers should be continued on these medications perioperatively.56 With regard to the need for invasive intraoperative monitoring, randomized controlled studies do not support the use of pulmonary artery catheters over standard care in the elderly, high-risk surgical patient.57 If postoperative pulmonary edema develops, however, the patient should be evaluated for new myocardial ischemia. Physicians should also pay close attention to volume infusion perioperatively, examining records from both the operating room and the immediate postoperative period, because excessive fluid administration is a common cause of postoperative pulmonary edema.
Results and Procedure-Related Complications
This patient was atraumatically intubated with the rigid bronchoscope, and the stricture was reassessed in terms of precise location, extent, and associated mucosal changes. The stricture indeed extended for 1.5 cm, starting at 4 cm below the vocal cords, with its distal aspect located 7 cm above the main carina. Following this evaluation, the 13 mm rigid bronchoscope was gently advanced, and the stricture was dilated. A 16 mm wide by 40 mm long straight studded silicone stent was inserted into the stricture. The stent had to be grasped using forceps, twisted, and pulled proximally so that the proximal aspect of the stent sat 1 cm above the stricture. This resulted in satisfactory positioning, with the stent’s proximal aspect located 2.5 cm below the cords, specifically, 0.5 cm below the cricoids (see video on ExpertConsult.com) (Video II.9.1![]() ). Airway patency was restored and the stenotic index was estimated at less than 50%, which was later confirmed by morphometric bronchoscopic analysis.18 The case lasted 20 minutes. The patient tolerated the procedure well, and extubation was uneventful. The patient was transferred to the postanesthesia care unit for 24 hours, during which no complications were noted. She was discharged to her nursing home 2 days later.
). Airway patency was restored and the stenotic index was estimated at less than 50%, which was later confirmed by morphometric bronchoscopic analysis.18 The case lasted 20 minutes. The patient tolerated the procedure well, and extubation was uneventful. The patient was transferred to the postanesthesia care unit for 24 hours, during which no complications were noted. She was discharged to her nursing home 2 days later.
Long-Term Management
Outcome Assessment
Improving this patient’s stenosis so that stenosis involves less than 50% of the airway lumen should enhance a patient’s exercise capacity. Because of limited mobility caused by her neurologic deficits, we were unable to objectively document improvement using the MRC dyspnea scale validated for laryngotracheal stenosis,58 but airway patency palliation resulted in resolution of stridor and respiratory distress.
Referral
In this case, multidisciplinary assessment involved thoracic and ear nose and throat surgeons, respiratory physicians, and radiologists. It was decided that given her comorbidities, a surgical intervention would not be feasible, and that if she failed stent insertion, Montgomery T-tube placement or tracheostomy would be recommended after the goals of care were discussed with the patient’s daughter. Because provision of high-quality, yet fiscally responsible, care is necessary, a palliative care consultation was warranted in this patient. Because the literature shows that providing such a service can result in reduced daily costs and length of stay, a referral to palliative care medicine was requested.59
Follow-up Tests and Procedures
In our patient, elective outpatient flexible bronchoscopy was scheduled at 30 days after the procedure to reassess stent patency and consider the need for additional therapies in case of stent-related complications. Although definitive protocols have not been universally agreed upon or studied regarding the need for or frequency of follow-up bronchoscopy, some investigators routinely perform a follow-up bronchoscopy every 4 to 6 weeks during the first 6 months after intervention.10 Others recommend bronchoscopy only in cases of new symptom development.60 Information provided by spiral CT scans with multiplanar reconstruction and virtual bronchoscopy may allow them to be considered a substitute for direct bronchoscopic examination.61
With regard to potential future interventions in case the patient does not tolerate the stent, the literature shows that most patients with complex stenoses who are considered inoperable at the initial encounter remain inoperable at 6-month follow-up.9 According to these data, it is unlikely that our patient would become a surgical candidate; however, a Montgomery T-tube and a tracheostomy could be feasible and safe alternatives to stent insertion in cases of stent failure.
Quality Improvement
A team meeting took place after the procedure. For this patient, quality of care was considered satisfactory because airway patency had been safely restored and the patient had been discharged back to her nursing home within 48 hours. We discussed whether the patient should have undergone CT or MRI after her tracheostomy tube had been decannulated, with the intention to detect earlier the development of stenosis. One study using CT scanning, for instance, in 48 patients followed for 30 months after PDT found that only 1 patient (2%) developed severe tracheal stenosis, and mild to moderate stenosis was detected in 14 patients (29.3%).62 We also discussed whether the patient should be prescribed antireflux medications, because a history of gastroesophageal reflux is often present in patients with PTS.10 We decided to prescribe proton pump inhibitors twice daily, in addition to saline nebulization, which is necessary for stent humidification.
Discussion Points
1. Describe three qualitative features of tracheal stenosis.
2. Describe three diagnostic modalities other than bronchoscopy that can be performed to assess the severity, extent, and morphology of this patient’s tracheal stenosis.
3. List four therapeutic alternatives for PTS with chondritis.
4. Identify three airway stricture characteristics that determine the need for and type of treatment.
Expert Commentary
Most acquired benign stenoses result from a mucosal trauma such as tracheostomy or long-term intubation. After a symptom-free period of several weeks, patients usually notice increasing shortness of breath on exertion. Eventually, they present with stridor or impaired cough clearance. In the text, the authors discuss the three typical levels of tracheal narrowing. One aspect that I will elaborate on is stricture shape, also known as morphology. Tracheas are not round in the first place. The horseshoe type is most common but changes with age. Sakai has shown that aging results in increased tracheal area and in distortion of the roundness.67 Traditionally, all commercially available tracheal cannulas and tubes, however, are round. This mismatch between the shape of the cannula and the shape of the trachea, especially when devices are slightly oversized, as discussed in the text, can lead to critically high localized pressures on the tracheal mucosa. In combination with impaired blood supply and hypoxia, this can easily result in permanent tissue damage. Modern low-pressure, high-volume cuffs have lowered the incidence of stenosis in the cuff region, but stomal stenoses remain a problem. In addition, as a result of irreversible damage to airway cartilage, the airway morphology may become tent shaped (also known as the A-type, or the triangular form, of benign tracheal stenosis). Now, we are forced to deal with an even worse mismatch between the actual shape of the stricture and the devices available in our therapeutic toolbox.
The following estimations are based on experimental results obtained from my laboratory.68 To compress a typical silicone stent such as a 50 mm long, 16 mm diameter, studded silicone Dumon-type stent, or a Polyflex stent down to half its diameter, a force of roughly 5 Newtons is required (weight, 500 g). Pressure is force divided by contact area. The outer surface area (2r × π × length) of this stent is approximately 25 cm2. If the expansion force of this stent would be distributed equally over its complete outer surface, this would result in a contact pressure of 2 kPa. However, if the stent wall would touch only a fifth of the inner tracheal wall, as illustrated in Figure 9-5, local pressure at that contact zone would be 10 kPa. Because the normal tracheal blood vessel pressure is in the range of 4 to 5 kPa, such high-contact pressure would result in considerable impairment of mucosal blood flow, promoting further tissue damage.
Another biomechanical aspect relates to the special location of the stenosis and the malacic component. The authors discuss the often noticed mismatch between the measurable degree of narrowing and patients’ symptoms. We have developed a technique to superimpose signals of wall pressure, pressure drops, or flows in the airway over the endoscopic image in real time. This is accomplished by obtaining physiologic data with the use of slightly modified standard instruments such as a pneumotachograph, by feeding the output signal into a computer, and by mixing the curve with the video signal from the endoscope. Using this endospirometry technique, we can study dynamic changes that occur, for example, during quiet breathing and rapid forced breathing maneuvers. Especially interesting are patients with malacic segments at the level of the thoracic inlet, such as the patient described in this chapter. Figure 9-6 shows the different cross-sectional areas of such a malacic segment in one of my own patients. During bronchoscopy, this patient was breathing through a flow transducer (pneumotachograph with mouthpiece). Superimposed over the bronchoscopic image is the airflow curve. In the upper panel of Figure 9-6, it is clearly visible that the collapse occurs during expiration, but the trachea is almost normal during inspiration. The images in the lower panel of the figure were taken from the same patient. The conditions are just the opposite—open during expiration, collapsed during inspiration. All I did was ask the patient to extend his neck. Stretching and extending his neck let the choke point migrate proximally, above the thoracic inlet, where the pressure vectors are reversed. In a certain position, this patient was almost asymptomatic, but he became stridorous when flexing his head.
1. Wain JCJr. Postintubation tracheal stenosis. Semin Thorac Cardiovasc Surg. 2009;21:284-289.
2. Remy-Jardin M, Remy J, Artaud D, et al. Volume rendering of the tracheobronchial tree: clinical evaluation of bronchographic images. Radiology. 1998;208:761-770.
3. Shitrit D, Valdsislav P, Grubstein A, et al. Accuracy of virtual bronchoscopy for grading tracheobronchial stenosis: correlation with pulmonary function test and fiberoptic bronchoscopy. Chest. 2005;128:3545-3550.
4. Boiselle PM, Feller-Kopman D, Ashiku S, et al. Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am. 2003;41:627-636.
5. Hofmann U, Hofmann D, Vogl T, et al. Magnetic resonance imaging as a new diagnostic criterion in paediatric airway obstruction. Prog Pediatr Surg. 1991;27:221-230.
6. Vogl TJ, Diebold T, Bergman C, et al. MRI in pre- and postoperative assessment of tracheal stenosis due to pulmonary artery sling. J Comput Assist Tomogr. 1993;17:878-886.
7. Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with Wegener’s granulomatosis. Laryngoscope. 1992;102:1341-1345.
8. Carney AS. The use of magnetic resonance imaging to assess tracheal stenosis following percutaneous dilatational tracheostomy. J Laryngol Otol. 1998;112:599.
9. Brichet A, Verkindre C, Dupont J, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J. 1999;13:888-893.
10. Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med. 2008;8:18.
11. De Leyn P, Bedert L, Delcroix M, et al. Belgian Association of Pneumology and Belgian Association of Cardiothoracic Surgery. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32:412-421.
12. Raghuraman G, Rajan S, Marzouk JK, et al. Is tracheal stenosis caused by percutaneous tracheostomy different from that by surgical tracheostomy? Chest. 2005;127:879-885.
13. Koitschev A, Simon C, Blumenstock G, et al. Suprastomal tracheal stenosis after dilational and surgical tracheostomy in critically ill patients. Anaesthesia. 2006;61:832-837.
14. Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 2. Complications. Chest. 1986;90:430-436.
15. Sarper A, Ayten A, Eser I, et al. Tracheal stenosis after tracheostomy or intubation: review with special regard to cause and management. Tex Heart Inst J. 2005;32:154-158.
16. Pearson FG, Andrews MJ. Detection and management of tracheal stenosis following cuffed tube tracheostomy. Ann Thorac Surg. 1971;12:359-374.
17. Hollingsworth HM. Wheezing and stridor. Clin Chest Med. 1987;8:231-240.
18. Murgu S, Colt HG. Morphometric bronchoscopy in adults with central airway obstruction: case illustrations and review of the literature. Laryngoscope. 2009;119:1318-1324.
19. Myer CM, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994;103:319-323.
20. Nouraei SA, Nouraei SM, Randhawa PS, et al. Sensitivity and responsiveness of the Medical Research Council dyspnoea scale to the presence and treatment of adult laryngotracheal stenosis. Clin Otolaryngol. 2008;33:575-580.
21. Mittnacht AJ, Fanshawe M, Konstadt S. Anesthetic considerations in the patient with valvular heart disease undergoing noncardiac surgery. Semin Cardiothorac Vasc Anesth. 2008;12:33-59.
22. Detsky AS, Abrams HB, Forbath N, et al. Cardiac assessment for patients undergoing noncardiac surgery: a multifactorial clinical risk index. Arch Intern Med. 1986;146:2131-2134.
23. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
24. Berry L. Pledges on dementia care. Nurs Older People. 2010;22:3.
25. Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg. 2004;128:731-739.
26. Simpson GT, Strong MS, Healy GB, et al. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol. 1982;91:384-388.
27. Brouns M, Jayaraju ST, Lacor C, et al. Tracheal stenosis: a flow dynamics study. J Appl Physiol. 2007;102:1178-1184.
28. Brouns M, Verbanck S, Lacor C. Influence of glottic aperture on the tracheal flow. J Biomech. 2007;40:165-172.
29. Detsky AS, Abrams HB, Forbath N, et al. Cardiac assessment for patients undergoing noncardiac surgery: a multifactorial clinical risk index. Arch Intern Med. 1986;146:2131-2134.
30. Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169-e276.
31. Hart R, Hindman B. Mechanisms of perioperative cerebral infarction. Stroke. 1982;13:766-773.
32. Cavaliere S, Bezzi M, Toninelli C, et al. Management of post-intubation tracheal stenoses using the endoscopic approach. Monaldi Arch Chest Dis. 2007;67:73-80.
33. Bisson A, Bonnette P, Ben EL, et al. Tracheal sleeve resection for iatrogenic stenoses (subglottic laryngeal and tracheal). J Thorac Cardiovasc Surg. 1992;104:882-887.
34. Rahman NA, Fruchter O, Shitrit D, et al. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg. 2010;5:2.
35. Mehta AC, Lee FY, Cordasco EM, et al. Concentric tracheal and subglottic stenosis: management using the Nd-YAG laser for mucosal sparing followed by gentle dilatation. Chest. 1993;104:673-677.
36. Nouraei SA, Kapoor KV, Nouraei SM, et al. Results of endoscopic tracheoplasty for treating tracheostomy-related airway stenosis. Clin Otolaryngol. 2007;32:471-475.
37. Dasgupta A, Dolmatch BL, Abi-Saleh WJ, et al. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: preliminary results. Chest. 1998;114:106-109.
38. Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124:1993-1999.
39. U.S. Food and Drug Administration. Metallic tracheal stents in patients with benign airway disorders. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm153009.htm, Accessed July 20, 2011.
40. Gaissert HA, Grillo HC, Wright CD, et al. Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg. 2003;126:744-747.
41. Gaissert HA, Grillo HC, Mathisen DJ, et al. Temporary and permanent restoration of airway continuity with the tracheal T-tube. J Thorac Cardiovasc Surg. 1994;107:600-606.
42. Gildea TR, Murthy SC, Sahoo D, et al. Performance of a self-expanding silicone stent in palliation of benign airway conditions. Chest. 2006;130:1419-1423.
43. Jog M, Anderson DE, McGarry GW. Polyflex stent: is it radiopaque enough? J Laryngol Otol. 2003;117:83-84.
44. Patelli M, Gasparini S. Post-intubation tracheal stenoses: what is the curative yield of the interventional pulmonology procedures? Monaldi Arch Chest Dis. 2007;67:71-72.
45. Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg. 2009;35:429-433. discussion 933-934
46. Martinez-Ballarin JI, Diaz-Jimenez JP, Castro MJ, et al. Silicone stents in the management of benign tracheobronchial stenoses: tolerance and early results in 63 patients. Chest. 1996;109:626-629.
47. Liu HC, Lee KS, Huang CJ, et al. Silicone T-tube for complex laryngotracheal problems. Eur J Cardiothorac Surg. 2002;21:326-330.
48. Liu HC, Lee KS, Huang CJ, et al. Silicone T-tube for complex laryngotracheal problems. Eur J Cardiothorac Surg. 2002;21:326-330.
49. Rea F, Callegaro D, Loy M, et al. Benign tracheal and laryngotracheal stenosis: surgical treatment and results. Eur J Cardiothorac Surg. 2002;22:352-356.
50. Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis: treatment and results. J Thorac Cardiovasc Surg. 1995;109:486-492. discussion 492-493
51. Marulli G, Rizzardi G, Bortolotti L, et al. Single-staged laryngotracheal resection and reconstruction for benign strictures in adults. Interact Cardiovasc Thorac Surg. 2008;7:227-230.
52. Grillo HC. Stents and sense. Ann Thorac Surg. 2000;70:1139.
53. Mayo AM, Wallhagen MI. Considerations of informed consent and decision-making competence in older adults with cognitive impairment. Res Gerontol Nurs. 2009;2:103-111.
54. American College of Physicians. Ethics Manual, 4th ed, Ann Intern Med, 1998;128:576-594
55. Davoudi M, Shakkottai S, Colt HG. Safety of therapeutic rigid bronchoscopy in people aged 80 and older: a retrospective cohort analysis. J Am Geriatr Soc. 2008;56:943-944.
56. Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169-e276.
57. Sandham JD, Hull RD, Brant RF, et al. Canadian Critical Care Clinical Trials Group. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5-14.
58. Nouraei SA, Nouraei SM, Randhawa PS, et al. Sensitivity and responsiveness of the Medical Research Council dyspnoea scale to the presence and treatment of adult laryngotracheal stenosis. Clin Otolaryngol. 2008;33:575-580.
59. Ciemins EL, Blum L, Nunley M, et al. The economic and clinical impact of an inpatient palliative care consultation service: a multifaceted approach. J Palliat Med. 2007;10:1347-1355.
60. Matsuo T, Colt HG. Evidence against routine scheduling of surveillance bronchoscopy after stent insertion. Chest. 2000;118:1455-1459.
61. Bauer TL, Steiner KV. Virtual bronchoscopy: clinical applications and limitations. Surg Oncol Clin N Am. 2007;16:323-328.
62. Norwood S, Vallina VL, Short K, et al. Incidence of tracheal stenosis and other late complications after percutaneous tracheostomy. Ann Surg. 2000;232:233-241.
63. Murgu SD, Colt HG. Interventional bronchoscopy from bench to bedside: new techniques for central and peripheral airway obstruction. Clin Chest Med. 2010;31:101-115.
64. Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J. 2007;30:7-12.
65. Dialani V, Ernst A, Sun M, et al. MDCT detection of airway stent complications: comparison with bronchoscopy. AJR Am J Roentgenol. 2008;191:1576-1580.
66. Boiselle PM, Ernst A. Recent advances in central airway imaging. Chest. 2002;121:1651-1660.
67. Sakai H, Nakano Y, Muro S, et al. Age-related changes in the trachea in healthy adults. Adv Exp Med Biol. 2010;662:115-120.
68. Freitag L. Airway stents. In: Strausz J, Bolliger CT. Interventional Pulomonology. Sheffield, UK: European Respiratory Society; 2010:190-217.
* A widely used staging system for classifying tracheal stenosis based on the degree of airway narrowing.
† The stenotic index (SI) represents the cross-sectional area (CSA) of the obstructed area relative to that of a normal airway lumen proximal or distal to the stenosis. SI = (CSAnormal − CSAabnormal)/(CSAnormal × 100%); the greater the SI, the greater the degree of airway narrowing and the more severe the obstruction.