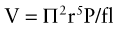Chapter 29 Bronchoscopic Treatment of a Large Right Mainstem Bronchial Stump Fistula
Case Description
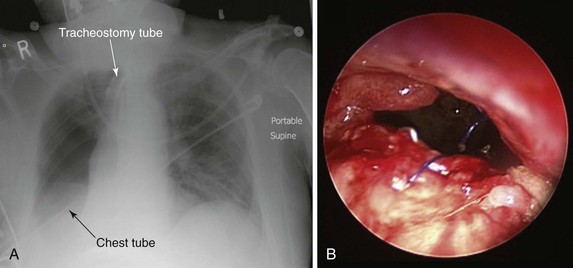
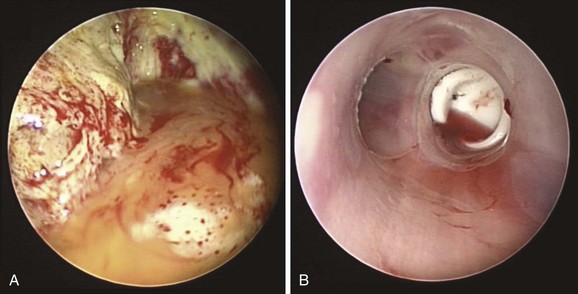
The patient was a 79-year-old male referred for treatment of a large right pneumonectomy stump fistula. He had multiple comorbidities, including severe chronic obstructive lung disease (COPD), systemic hypertension, coronary artery disease, abdominal aortic aneurysm, and carotid artery stenosis, and a remote history of thyroid carcinoma. He had been diagnosed with squamous cell carcinoma 6 months earlier at another institution, where he had undergone right upper lobectomy and radical mediastinal lymph node dissection. Positive surgical margins prompted completion of pneumonectomy several days later. Postoperative hemorrhage required repeat thoracotomy within hours after the pneumonectomy had been performed. The postoperative course was complicated by prolonged mechanical ventilation for respiratory failure, pulmonary embolism, residual left femoral deep vein thrombosis warranting anticoagulation, and placement of an inferior vena cava filter. A tracheotomy was performed on postoperative day 7. Three weeks after surgery, the patient had developed increasing-right sided pleural effusion, copious secretions, and fever. Flexible bronchoscopy revealed a post pneumonectomy stump fistula. A 32-French chest tube was placed into the right hemithorax, and this revealed the presence of a large air leak. Sputum cultures were positive for Pseudomonas aeruginosa, and the patient was started odn intravenous imipenem and amikacin. He was considered inoperable and was referred to our institution for possible bronchoscopic treatment of his right post pneumonectomy stump fistula. Chest radiograph at admission showed an air-fluid level in the right hemithorax consistent with bronchopleural fistula (BPF). Bronchoscopy showed a large BPF due to dehiscence of the right mainstem bronchial stump (Figure 29-1).
Discussion Points
1. List four risk factors associated with the development of bronchial stump fistula.
2. List three bronchoscopic treatment modalities for patients with large bronchial stump fistulas.
3. Describe and justify the use of three ventilator management strategies in patients with stump fistula and respiratory failure.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient had a central bronchopleural fistula (BPF).* Bronchial stump dehiscence (failure) represents BPF when disruption of a bronchial closure occurs after anatomic pulmonary resection. Early failure (within a few days to a few weeks following surgery) is usually the result of a technical problem such as a stapler misfiring, loose or broken sutures, or excessive tension with stump closure, causing poor apposition of tissues. Following pneumonectomy, bronchial stump dehiscence within the first few days to few weeks typically presents with a large air leak and coughing of copious quantities of secretions. Respiratory distress may occur secondary to spillage of pleural fluid through the bronchial stump into the contralateral lung or by loss of air into the empty pleural space, leading in some cases to a tension pneumothorax. Late stump failure (occurring later than 2 to 4 weeks after surgery but usually within 90 days of operation) is typically secondary to weak bronchial tissue and infection (bronchitis, empyema), which lead to loss of integrity of the bronchial stump.1 Patients present with cough and expectoration of frothy, blood-tinged secretions. Fever is often present, and patients may have sweats and chills secondary to infection within the pleural space.
BPFs after pneumonectomy, as seen in this case, are not uncommon2,3 and are associated with high mortality rates, usually resulting from empyema.4 The estimated incidence of post pneumonectomy BPF ranges between 3% and 28%. Although the perioperative mortality of pneumonectomy overall is now less than 7%, this rises to 25% when complicated by empyema, and it may be as high as 50% when associated with fistula.*3,5,6 In addition to empyema, the main complication of BPF is aspiration pneumonia caused by spillage of contaminated secretions into the contralateral healthy lung, potentially leading to adult respiratory distress syndrome (ARDS). The resulting impaired respiratory mechanics, contralateral lung contamination, and chronic pleural sepsis contribute to poor outcome.7
The bronchial stump after pneumonectomy or lobectomy could be fragile in cases of bronchial stump ischemia, extensive peribronchial dissection, inflammation at the suture line, or residual tumor at the bronchial stump margins. Several other risk factors also predispose to fistula formation and bronchial stump dehiscence, including preoperative chemotherapy or radiation therapy, right pneumonectomy, male gender, a large and long stump, postoperative mechanical ventilation, tracheostomy, and excessive dissection and tension at the bronchial suture line. Right-sided pneumonectomy, as performed in our case, is considered a major risk factor for stump disruption, in part because of the larger size and greater tendency of the right main bronchus to spring open, especially in the setting of positive-pressure ventilation, and because surgery-related alterations in the right bronchial microvasculature may lead to stump necrosis.3 BPF after right pneumonectomy occurs with significantly increased frequency compared with left pneumonectomy (13.2% vs. 5.0%).8 Pathophysiologically, this may be explained by at least two factors: First, the most common vascular supply to the right mainstem bronchus consists of a single bronchial artery, whereas the left mainstem bronchus is most commonly supplied by two bronchial arteries. Second, the left mainstem bronchus is protected beneath the aortic arch and surrounding vascularized mediastinal tissue. Another possible risk factor, present in our patient, is the use of sutures rather than staples for stump closure (Figure 29-1). In this regard, in a series of 713 pneumonectomies, Deschamps and colleagues observed an increased frequency of post pneumonectomy BPF following suture closure versus stapled closure of the bronchus (incidence of 12.5% vs. 3.8%, respectively).4
As part of the initial evaluation, chest computed tomography (CT) may reveal a direct sign of BPF: A fistulous tract is noted between the bronchus or lung and the pleural space. Indirect signs include air bubbles beneath the bronchial stump and “suspected fistula” defined as a suspicious but not definite communication between the pleural space and the airway or lung parenchyma.9 CT is useful not only for visualization and localization of BPF but also for identification of the cause, number, and size of the BPF and of underlying lung lesions (e.g., post lung resection, necrotizing pneumonia).9
Diagnosis on bronchoscopy is made by observing the direct signs of BPF. These are defined by the presence of a fistula opening at the bronchial stump or by the appearance of dye, previously injected into the airways during bronchoscopy, in the chest tube collection device. Air bubbling during the respiratory cycle (see video on ExpertConsult.com) (![]() Video VI.29.1) and purulent secretions from the segmental airways are indirect signs of peripheral BPF. As part of an initial evaluation, even asymptomatic patients with clinically or radiographically suspected BPF should undergo flexible bronchoscopy for evaluation of the stump.* A fistula will often be visible, and a small amount of air bubbling may be observed with careful inspection of the stump (see video on ExpertConsult.com) (
Video VI.29.1) and purulent secretions from the segmental airways are indirect signs of peripheral BPF. As part of an initial evaluation, even asymptomatic patients with clinically or radiographically suspected BPF should undergo flexible bronchoscopy for evaluation of the stump.* A fistula will often be visible, and a small amount of air bubbling may be observed with careful inspection of the stump (see video on ExpertConsult.com) (![]() Video VI.29.1).† In our patient, bronchoscopy revealed a large BPF due to complete stump dehiscence (see Figure 29-1).
Video VI.29.1).† In our patient, bronchoscopy revealed a large BPF due to complete stump dehiscence (see Figure 29-1).
Patient Preferences and Expectations
Because the patient’s tumor was now completely resected, the wife expressed her wish to be aggressive and “do everything possible” to restore her husband’s health. We did not consider her requests unrealistic. In today’s health care environment, physicians’ relationships with both patients and society have undergone important changes. For instance, patients are increasingly viewed as active “consumers” able to demand and are often encouraged to command and expect enhanced services, including extended hours and rapid access. In opposition to some examples in the managed care environment, where patients and health care providers might feel they are the victims of decreased access or have difficulty obtaining second opinions or referrals to specialists outside of their plans, the easy availability of health information coupled with a sense of entitlement is causing a power shift in the physician-patient relationship whereby patients might, on the basis of unrealistic expectations, impose their desire for costly tests and procedures in their attempt to do everything possible to reverse the effects of their illness, even if such tests or procedures are not believed to be medically indicated.10
Procedural Strategies
Indications
There was little doubt that without treatment of the BPF, this patient would have continued to deteriorate and eventually would have died from empyema and pneumonia in the contralateral lung. Given the reasonable reluctance to re-intervene surgically, a bronchoscopic attempt at closure of the fistula was warranted. Management strategies for BPF depend on a number of factors, including underlying cause, size, time of onset of the fistula post surgery, and health status of the patient. Surgery is the treatment of choice for this condition, but bronchoscopic techniques have been advocated as an option when surgery is not possible or has to be postponed.7 Management of patients who have developed BPF and require mechanical ventilation is even more complex. Surgical repair is not a good option for these patients because postoperative mechanical ventilation is associated with a high failure rate owing to persistent barotrauma on the repaired stump.7
In general, nonsurgical, bronchoscopic strategies are categorized as follows: (1) occlusion with indwelling airway stents, (2) occlusion with glue or other materials, and (3) procedures that induce scar tissue formation at the fistula site. The goal of the stent, as chosen in this case, is to provide a tight seal in the airway to prevent spillage of infected pleural fluid into the contralateral lung. Because many types of stents with different biomechanical properties are available, selection of a stent requires consideration of the physical characteristics of the stent and potential associated short-term and long-term complications.11 Several case reports of endobronchial stent insertion for isolated fistulas have been published, but case series of more than two patients are few.12 Obviously, the effect of case selection is difficult to quantify from these publications. However, the feasibility of the technique in selected patients is encouraging. In one study, for instance, authors used specially designed covered metal stents (with a blind ending arm to fit the bronchial stump) in six patients. Patients underwent follow-up CT scan and bronchoscopy to confirm satisfactory stent placement. The mortality rate (mean follow-up, 316 days) was 0% with 67% of patients having the fistula closed; this led to resolution of the empyema.12
Contraindications
No absolute contraindications to rigid bronchoscopy were noted, but general anesthesia was obviously risky in this patient with sepsis from empyema, pulmonary embolism, and multiple cardiovascular problems. In this regard, the physical status classification system of the American Society of Anesthesiologists (ASA) is a relatively simple system that has proved effective in stratifying overall preoperative risks of morbidity and mortality for patients undergoing anesthesia and surgery.13 Complications are much more likely to occur in patients with preexisting disease states. For example, an ASA class 1 patient has no underlying conditions or limitations, but a class 5 patient is not expected to survive the next 24 hours without the proposed intervention. Risks inherent to a specific procedure are not incorporated into the ASA class. The relative risk of serious perioperative complications is 4.4 for ASA patient status 4, as was assigned to ours (i.e., a severe incapacitating disease process that is a constant threat to life), illustrating that increasing serious comorbidities reflected in the ASA classification increase perioperative morbidity.14 Our patient was considered nonoperable because of his poor clinical status and high operative risk. Surgical contraindication was determined by consensus at our multidisciplinary meeting, which included critical care physicians, the interventional pulmonologist, and the consulting thoracic surgeon. The choice of using stent insertion, rather than repeat surgical repair, was thus based on the patient’s unstable respiratory status, poor general health status, and inflamed appearance of BPF margins (see Figure 29-1).
Expected Results
Most published experience related to airway fistulas describes occlusion of tracheal or bronchoesophageal fistulas,15,16 as well as case reports, small case series, and a few review articles pertaining to postsurgical bronchial stump fistulas.17–21 In addition to their described use in patients with an inoperable or unresectable tumor involving the mainstem bronchi and lower trachea, Y stents have been used successfully in patients with trachea-broncho-esophageal fistulas, resulting in improved dyspnea and quality of life and a reduced rate of recurrent infection.22 A Y stent has been reported to successfully cover a BPF in a patient who underwent right upper lobectomy for lung cancer.23 However, the Y stent has patent distal bronchial limbs and thus cannot completely occlude a major post pneumonectomy stump dehiscence. For this purpose, modified versions of the Y stent have involved occlusion of one of the bronchial limbs to occlude or cover the airway defect in patients who are not surgical candidates. In one report, the authors shortened the right limb of a studded Dumon Y stent (Novatech, Cedex, France) and closed the right distal bronchial limb with silicone material cut out from the stent itself.17 This resulted in successful occlusion of a 2 mm bronchial stump fistula. Another modified version of the Y stent is obtained by requesting the manufacturer to customize the stent at the time of manufacture by shortening the right bronchial limb and sealing its distal aspect. Such a stent was used successfully in a patient with a major right bronchial stump fistula and respiratory failure.21 The fact that such a customized stent must be ordered and manufactured, however, may limit wider applicability and may delay scheduling of the therapeutic intervention.
Therapeutic Alternatives for Sealing Off the Fistula
Surgical treatment options include bronchial stump revision with omental or muscular flap coverage. Surgeons usually advocate immediate thoracoplasty and open window thoracostomy when a major bronchial stump dehiscence is suspected, to prevent empyema and aspiration pneumonia on the contralateral side.24,25 At the time of our evaluation, the patient was not considered to be a surgical candidate.
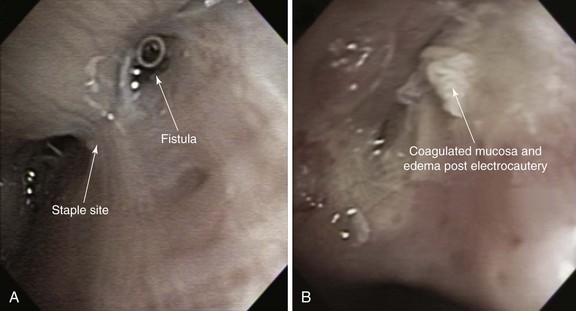
Minimally invasive techniques such as thoracoscopic and bronchoscopic procedures using different glues, coils, and sealants have been performed to attempt closure of BPFs in patients who are deemed to be poor candidates for repeat thoracotomy. Successful bronchoscopic closure of fistulas has been described with the use of ethanol, polyethylene glycol–based gel, fibrin, acrylic glue, cellulose, and gel foam, and placement of angiographic occlusion metallic coils, polidocanol, decalcified spongy calf bone, or unidirectional endobronchial valves or spigots.7 Scar-forming techniques have been reported, in which thermal energy was provided by neodymium-doped yttrium aluminum garnet (Nd:YAG) laser or electrocautery (Figure 29-2). In the absence of tumor or infection, the laser beam or electrocautery probe is directed toward the mucosa surrounding the fistula to induce tissue edema, protein denaturation, and inflammation, eventually facilitating closure of the fistula by fibrosis.26 Scar tissue and BPF closure can also be achieved by submucosal injection of the vein sclerosant polidocanol.27 According to published studies, resolution of BPF using these bronchoscopic techniques ranges from 33% to 58%. These techniques involve repeated bronchoscopic interventions in most patients.26,28,29 Furthermore, they are usually reserved for patients with small fistulas measuring less than 5 mm and for those with fistulas that occur in more distal bronchial regions. The absence of evidence supporting the use of one technique versus another justifies a multidisciplinary approach in which interventional bronchoscopists and thoracic surgeons choose a specific product or technique based on their experience and product availability.
1. Gluing techniques: Fibrin glue occlusion with spongy calf bone was used in a large series of 45 post resection fistulas (40 post pneumonectomy) up to 8 mm in diameter.30 Only 20% of cases were cured using this technique, with resolution of both fistula and empyema. Most patients had an adverse outcome, with 20% dying as inpatients, 35.6% necessitating surgery, and 15.6% left with a chronic empyema. Another series in which synthetic glue was used in 12 post pneumonectomy fistulas had a better fistula resolution rate (66.7%) and survival of 83%, but it was disappointing that only 10% of empyemas resolved.31 In another series, cyanoacrylate glue was used in three patients, with no mortality, but resolution of BPF and empyema was achieved in only one patient (33%).32
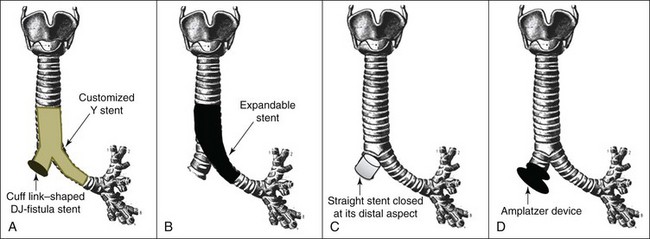
2. Amplatzer double-disk occluders: These represent a group of devices designed originally for transcatheter closure of cardiac septal defects or patent ductus arteriosus. These devices are made of Nitinol mesh with a central connector between the disks. The presence of two disks, one on either side of the lesion (Figure 29-3), leads to greater coverage and increases the likelihood of closure, as opposed to the use of glue, which is applied to the internal aspect alone. In the bronchial tree, the device induces local granulation tissue formation that potentiates its occluding properties without compromising airway patency.33 Endobronchial closure using an Amplatzer device was shown to be a safe and effective method of treating postoperative BPF in nonsurgical candidates. In one series, the authors inserted Amplatzer devices during flexible bronchoscopy under moderate sedation in 10 patients with 11 BPFs. In 9 patients, symptoms related to the BPF disappeared. Results were maintained over a median follow-up period of 9 months.33
3. Expandable metallic stent insertion: This technique has been used successfully for sealing BPFs. A large and long, straight, fully or partially covered expandable metal stent can be inserted, so that the covered portion of the main body of the stent occludes the bronchial stump fistula, while the distal aspect of the stent extends distally into the contralateral healthy bronchus and the proximal portion of the stent extends proximally into the trachea18 (see Figure 29-3). In one case series, results from seven nonoperable patients who presented with large post pneumonectomy BPF (6 to 12 mm) are reported. The authors used a dedicated customized covered conical self-expandable metallic stent.11 Cessation of the air leak and clinical improvement were achieved in all patients after stent placement. Stent-related complications (two migrations with recurrence of the leak and one stent rupture) were managed using bronchoscopic techniques in two patients and surgery in one. Mortality related to overwhelming sepsis was 57%, but definitive surgery was ultimately possible in three patients (43%).11
4. Partially covered Nitinol stent with a closed distal aspect: This device has been specifically designed and used for the treatment of bronchial stump fistula12 (see Figure 29-3).
Cost-Effectiveness
In one review of the literature, the question addressed was whether bronchoscopic approaches to the closure of BPFs were effective compared with conventional re-thoracotomy; only six case series including more than two post pneumonectomy BPF patients were identified, resulting in an overview of 85 patients with post pneumonectomy BPFs who underwent bronchoscopic repair. Success was noted in 30% of cases with use of a wide range of bronchoscopic techniques.* Mortality was 40%. Many patients required multiple bronchoscopic procedures and additional drainage procedures of their empyemas. As of this writing, it seems that bronchoscopic treatments are offered most often to patients who are too unwell to undergo re-thoracotomy.28
Techniques and Results
Anesthesia and Perioperative Care
As part of their supportive management, patients with BPF and empyema require chest tubes to drain the air accumulating in the pleural space and the empyema cavity; this leads to a continuous leak of air from the airway to the external environment, resulting in loss of airway pressure, decreased tidal volume, and potentially progressive alveolar collapse.† In some BPF patients requiring mechanical ventilation, leaks can exceed 500 mL per breath.34 For patients receiving volume-controlled ventilation, the volume of the leak is estimated as the difference between exhaled and set minute volume, assuming an intact circuit.‡ The length of the chest tube and its radius are important determinants of flow as governed by the Fanning equation, which describes turbulent flow of moist gases through a tube:
where V = flow, P = pressure, f = friction factor, l = length of the tube, and r = radius of the tube. The smallest internal diameter of a tube that would allow a maximal flow of 15 L/min at −10 cm H2O suction is 6 mm.35 Increasing negative airway pressure to compensate for lost volume while the patient is on mechanical ventilation only worsens the magnitude of the fistula. Also, negative pressures applied to a chest tube to assist drainage of the pleural space may increase flow through the fistula by increasing transpulmonary pressures and inducing auto-triggering of the ventilator. If not considered, auto-triggering may lead to inappropriate use of sedatives and paralytic agents to suppress hyperventilation. This can be avoided by decreasing sensitivity and minimizing airway pressure.36 Alternatively, separation of the lungs by means of independent lung ventilation allows appropriate ventilation and minimizes air passing through the BPF.37
Instrumentation
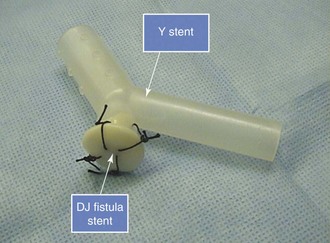
The Y stent we used had a large, 16 mm wide tracheal limb and two 13 mm wide bronchial limbs. The stent was cut, then was sculpted in such a way that the tracheal limb was 3 cm long, the right bronchial limb only 1 cm long, and the left bronchial limb 3 cm long. The right bronchial limb was cut so that its distal aspect barely entered the right pneumonectomy stump lumen. We added a cufflink-shaped silicone prosthesis known as a tracheo-esophageal DJ fistula stent, which was developed specifically to palliate small tracheo-esophageal fistulas.38 This stent was sutured onto the distal right bronchial limb of a studded Y-shaped stent to occlude the large right pneumonectomy stump fistula (Figure 29-4). The cufflink-shaped prosthesis is made of three portions: a 20 mm mushroom-shaped top, a 4 mm central limb, and a 10 mm elliptical distal aspect. The mushroom-shaped limb was pushed into the distal portion of the right bronchial limb of the Y stent, after which four 0 silk sutures were placed to tie the mushroom-shaped aspect to the Y stent, securing the cufflink-shaped prosthesis to the distal aspect of the right bronchial limb of the Y stent (see Figure 29-4). This combined studded Y-shaped and DJ fistula silicone stent was then inserted using a rigid bronchoscope and large forceps.
Anatomic Dangers and Other Risks
Our patient had already contaminated his left lung. This was noted on the admission chest radiograph (see Figure 29-1). Once contamination of the contralateral (residual) lung occurs in a septic patient, acute respiratory failure can occur from ARDS and worsening lung compliance. In our case, during rigid bronchoscopic intervention, care was taken to prevent further spillage of pus from the empyema cavity into the left bronchial tree. One initial maneuver in the management of patients with BPF is urgent positioning in the reverse Trendelenburg position (head up). Another is to place the patient in a slightly lateral decubitus position, pneumonectomy side down, to prevent spillage of pleural fluid from the post pneumonectomy space into the airway of the remaining lung.
Results and Procedure-Related Complications
Bronchoscopy revealed a large pneumonectomy stump dehiscence. The flexible bronchoscope and a 12 mm EFER ventilating rigid bronchoscope (Bryan Corp., Woburn, Mass) could be moved easily through the fistulous tract into the right hemithorax, where a large volume of purulent fluid was noted (see video on ExpertConsult.com) (![]() Video VI.29.2) (Figure 29-5). The fluid was removed by aspiration. The stump itself was severely inflamed, explaining its dehiscence. Necrotic debris was removed using forceps. The stump opening was at least 10 mm in diameter (see video on ExpertConsult.com) (
Video VI.29.2) (Figure 29-5). The fluid was removed by aspiration. The stump itself was severely inflamed, explaining its dehiscence. Necrotic debris was removed using forceps. The stump opening was at least 10 mm in diameter (see video on ExpertConsult.com) (![]() Video VI.29.3). The rigid bronchoscope, which had been inserted through the patient’s tracheostomy, was removed, and with rigid forceps, the stent was folded and inserted through the open tracheostomy and was positioned by feel onto the carina, such that the left bronchial limb could be guided down into the left main bronchus, and the right bronchial limb could be guided into the open right pneumonectomy stump. The patient’s tracheostomy allowed us to insert a stent that was large enough to completely occlude the large stump dehiscence.* The rigid bronchoscope was once more inserted into the airways, and the right bronchial limb–cufflink-shaped stent was gently pushed into position within the stump with the use of rigid forceps, completely occluding the fistulous tract (see video on ExpertConsult.com) (
Video VI.29.3). The rigid bronchoscope, which had been inserted through the patient’s tracheostomy, was removed, and with rigid forceps, the stent was folded and inserted through the open tracheostomy and was positioned by feel onto the carina, such that the left bronchial limb could be guided down into the left main bronchus, and the right bronchial limb could be guided into the open right pneumonectomy stump. The patient’s tracheostomy allowed us to insert a stent that was large enough to completely occlude the large stump dehiscence.* The rigid bronchoscope was once more inserted into the airways, and the right bronchial limb–cufflink-shaped stent was gently pushed into position within the stump with the use of rigid forceps, completely occluding the fistulous tract (see video on ExpertConsult.com) (![]() Video VI.29.4). Fibrin sealant was administered to prevent micro leaks around the stent itself. The patient then was extubated, and a No. 8 Shiley tracheotomy tube was inserted. The distal aspect of the tube was positioned such that its distal aspect was 2 cm above the proximal aspect of the indwelling Y stent. The patient was connected to the ventilator and was moved to the medical intensive care unit.
Video VI.29.4). Fibrin sealant was administered to prevent micro leaks around the stent itself. The patient then was extubated, and a No. 8 Shiley tracheotomy tube was inserted. The distal aspect of the tube was positioned such that its distal aspect was 2 cm above the proximal aspect of the indwelling Y stent. The patient was connected to the ventilator and was moved to the medical intensive care unit.
Long-Term Management
Outcome Assessment
Closure of the stump fistula was performed safely and without procedure-related complications. Immediately postoperatively, the patient’s large air leak ceased. Broad-spectrum antibiotics were continued,† and the patient was transferred back to the referring hospital in stable condition, where he was successfully weaned from mechanical ventilation. Referring physicians were asked to perform occasional flexible bronchoscopy for secretion management.
Discussion Points
1. List four risk factors associated with development of bronchial stump fistula.
Predisposition to bronchial stump dehiscence can occur as the result of preoperative (related to patient-specific characteristics), intraoperative, and postoperative risk factors.1 These include the following:
2. List three bronchoscopic treatment modalities for patients with large bronchial stump fistulas.
3. Describe and justify the use of three ventilator management strategies in patients with stump fistula and respiratory failure.
Expert Commentary
An aggressive open surgical intervention can serve as an optimal method of management for patients with post pneumonectomy empyema secondary to an associated bronchopleural fistula.42 One of these surgical interventions consists of the addition of a muscle flap to cover the fistula. For this procedure, the right thoracic cavity must first be sterilized as much as possible. In my opinion, using a chest tube alone is not enough. Not only is pleural lavage via the chest tube needed, but also or instead, an open window thoracostomy43,44 placed in the posterior aspect of the chest wall should be considered and might be more effective. To do this under general anesthesia, a double-lumen endotracheal tube should be inserted into the left main bronchus with the proximal cuff inflated in the lower trachea to establish unilateral ventilation.
Surgeons look to the chest radiograph with great interest when evaluating the stability of the mediastinum* (tendency to shift away from the midline after pneumonectomy) and whenever BPF is encountered in the early postoperative period, regardless of whether it is associated with empyema. This patient had an already stabilized mediastinum more than 3 weeks after his initial surgery. This means that his right hemithorax could be opened to atmospheric pressure.† After central venous hyperalimentation for several days with the patient under anesthesia with intercostal nerve block, a window may be created in a short time. Open drainage and changing sponges for several days could bring the thoracic empyema under control. Then a muscle flap using the latissimus dorsi or the pectoralis major could be placed over the fistula, with or without limited thoracoplasty. With thoracoplasty avoided, Clagett’s procedure* may be considered after the pleural cavity becomes healthy and granulated.44 An omental flap can also be used and is often ideal, but it is probably not indicated in this case because the patient has an aortic aneurysm.
Although outside the scope of this discussion, I wonder why this patient had not undergone a bronchoplastic procedure to save the middle and lower lobes when the upper lobe stump had turned out to be pathologically positive. From a surgical perspective, I also wonder whether the right main bronchial stump had been reinforced with some vital tissue. A pedicled flap45 of the latissimus dorsi muscle, a pericardium46 for example, or at least a good intercostal muscle flap is usually available. Such preventive methods are very important, although they are not done routinely.
* After lung removal, the mediastinum is not supported on one side and may shift. The residual pleural space should fill after surgery through a combination of mediastinal shift, diaphragm elevation, coagulation of serous drainage, and development of fibrotic tissue. If mediastinal shift is excessive, than decreased cardiac output may occur and may require mediastinal stabilization.
† Most surgeons attempt to prevent acute shift of the mediastinum by avoiding chest tubes after pneumonectomy. If they are used, tubes are clamped or are left to the water seal drainage only.
* It is a two-stage surgical procedure for treatment of empyema; the first stage involves creating an open window thoracostomy by resecting parts of ribs to create an opening into the thorax to allow drainage and antiseptic irrigation of the space. During the second stage, when the pleural space is clean and sterile, the space is filled with antibiotic solution and is closed surgically.
1. Liberman M, Cassivi SD. Bronchial stump dehiscence: update on prevention and management. Semin Thorac Cardiovasc Surg. 2007;9:366-373.
2. Wright CD, Wain JC, Mathisen DJ, et al. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: incidence, risk factors, and management. J Thorac Cardiovasc Surg. 1996;112:1367-1371.
3. Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg. 2001;7:330-336.
4. Deschamps C, Bernard A, Nichols FC3rd, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg. 2001;72:243-247.
5. Wong PS, Goldstraw P. Post-pneumonectomy empyema. Eur J Cardiothorac Surg. 1994;8:345-350.
6. Sonobe M, Nakagawa M, Ichinose M, et al. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg. 2000;18:519-523.
7. Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with a special focus on endoscopic management. Chest. 2005;128:3955-3965.
8. Darling GE, Abdurahman A, Yi Q-L, et al. Risk of right pneumonectomy: role of bronchopleural fistula. Ann Thorac Surg. 2005;79:433-437.
9. Seo H, Kim TJ, Jin KN, et al. Multi-detector row computed tomographic evaluation of bronchopleural fistula: correlation with clinical, bronchoscopic, and surgical findings. J Comput Assist Tomogr. 2010;34:13-18.
10. Edwards N, Kornacki MJ, Silversin J. Unhappy doctors: what are the causes and what can be done? Br Med J. 2002;324:835-838.
11. Dutau H, Breen DP, Gomez C, et al. The integrated place of tracheobronchial stents in the multidisciplinary management of large post-pneumonectomy fistulas: our experience using a novel customised conical self-expandable metallic stent. Eur J Cardiothorac Surg. 2011;39:185-189.
12. Han X, Wu G, Li Y, et al. A novel approach: treatment of bronchial stump fistula with a plugged, bullet-shaped, angled stent. Ann Thorac Surg. 2006;81:1867-1871.
13. Cohen MM, Duncan PG, Tate RB. Does anesthesia contribute to operative mortality? JAMA. 1988;260:2859.
14. Wolters U, Wolf T, Stutzer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217-222.
15. Colt HG, Meric B, Dumon JF. Double stents for carcinoma of the esophagus invading the tracheo-bronchial tree. Gastrointest Endosc. 1992;38:485-489.
16. Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest. 1996;110:1155-1160.
17. Ferraroli GM, Testori A, Cioffi U, et al. Healing of a bronchopleural fistula using a modified Dumon stent: a case report. J Cardiovasc Surg. 2006;1:16.
18. Garcia Franco CE, Aldeyturriaga JF, Gavira JZ. Ultraflex expandable metallic stent for the treatment of a bronchopleural fistula after pneumonectomy. Ann Thorac Surg. 2005;79:386.
19. Tayama K, Eriguchi N, Futamata Y, et al. Modified Dumon stent for the treatment of a bronchopleural fistula after pneumonectomy. Ann Thorac Surg. 2003;75:290-292.
20. Madden BP, Sheth A, Ho TB, et al. A novel approach to the management of persistent postpneumonectomy bronchopleural fistula. Ann Thorac Surg. 2005;79:2128-2130.
21. Tuskada H, Osada H. The use of a modified Dumon stent for postoperative bronchopleural fistula. Ann Thorac Surg. 2005;80:1928-1930.
22. Shiraishi T, Kawahara K, Shirakusa T, et al. Stenting for airway obstruction in the carinal region. Ann Thorac Surg. 1998;66:1925-1929.
23. Watanabe S, Shimokawa S, Yotsumoto G, et al. The use of a Dumon stent for the treatment of bronchopleural fistula. Ann Thorac Surg. 2001;72:276-278.
24. Hankins JR, Miller JE, Attar S, et al. Bronchopleural fistula: thirteen-year experience with 77 cases. J Thorac Cardiovasc Surg. 1978;76:755-762.
25. Baldwin JC, Mark JB. Treatment of bronchopleural fistula after pneumonectomy. J Thorac Cardiovasc Surg. 1985;90:813-817.
26. Kiriyama M, Fujii Y, Yamakawa Y, et al. Endobronchial neodymium:yttrium-aluminum garnet laser for non-invasive closure of small proximal bronchopleural fistula after lung resection. Ann Thorac Surg. 2002;73:945-949.
27. Varoli F, Roviaro G, Grignani F, et al. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg. 1998;65:807-809.
28. West D, Togo A, Kirk AJ. Are bronchoscopic approaches to post-pneumonectomy bronchopleural fistula an effective alternative to repeat thoracotomy? Interact Cardiovasc Thorac Surg. 2007;6:547-550.
29. Wang KP, Schaeffer L, Heitmiller R, et al. Nd:YAG laser closure of a bronchopleural fistula. Monaldi Arch Chest Dis. 1993;48:301-303.
30. Hollaus PH, Lax F, Janakiev D, et al. Endoscopic treatment of postoperative bronchopleural fistula: experience with 45 cases. Ann Thorac Surg. 1998;66:923-927.
31. Scappaticci E, Ardissone F, Ruffini E, et al. Postoperative bronchopleural fistula: endoscopic closure in 12 patients. Ann Thorac Surg. 2000;69:1629-1630.
32. Sabanathan S, Richardson J. Management of postpneumonectomy bronchopleural fistulae: a review. J Cardiovasc Surg. 1994;35:449-457.
33. Fruchter O, Kramer MR, Dagan T, et al. Endobronchial closure of bronchopleural fistulae using Amplatzer devices: our experience and literature review. Chest. 2011;139:682-687.
34. Pierson DJ, Horton CA, Bates PW. Persistent bronchopleural air leak during mechanical ventilation: a review of 39 cases. Chest. 1986;90:321-323.
35. Powner DJ, Cline D, Rodman GH. Effect of chest tube suction on gas through a BPF. Crit Care Med. 1985;13:99-101.
36. Shekar K, Foot C, Fraser J, et al. Bronchopleural fistula: an update for intensivists. J Crit Care. 2010;25:47-55.
37. Konstantinov IE, Saxena P. Independent lung ventilation in the postoperative management of large bronchopleural fistula. J Thorac Cardiovasc Surg. 2010;139:e21-e22.
38. Diaz-Jimenez P. New cufflink-shaped silicone prosthesis for the palliation of malignant tracheobronchial-esophageal fistula. J Bronchol. 2005;12:207-209.
39. Dennis JW, Eigen H, Ballantine TV, et al. The relationship between peak inspiratory pressure and positive end expiratory pressure on the volume of air lost through bronchopleural fistula. J Pediatr Surg. 1980;15:971-976.
40. Bishop MJ, Benson MS, Sato P, et al. Comparison of high frequency jet ventilation with conventional mechanical ventilation for bronchopleural fistula. Anesth Analg. 1987;66:833-838.
41. Ha DV, Johnson D. High frequency oscillatory ventilation in the management of a high output bronchopleural fistula: a case report. Can J Anaesth. 2004;51:78-83.
42. Wain JC. Management of late postpneumonectomy empyema and bronchopleural fistula. Chest Surg Clin N Am. 1996;6:529-541.
43. Regnard JF, Alifano M, Puyo P, et al. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J Thorac Cardiovasc Surg. 2000;120:270-275.
44. Massera F, Robustellini M, Pona CD, et al. Predictors of successful closure of open window thoracostomy for postpneumonectomy empyema. Ann Thorac Surg. 2006;82:288-292.
45. Abolhoda A, Bui TD, Milliken JC, et al. Pedicled latissimus dorsi muscle flap: routine use in high-risk thoracic surgery. Tex Heart Inst J. 2009;36:298-302.
46. Taghavi S, Marta GM, Lang G, et al. Bronchial stump coverage with a pedicled pericardial flap: an effective method for prevention of postpneumonectomy bronchopleural fistula. Ann Thorac Surg. 2005;79:284-288.
* BPF can be classified into central and peripheral types. Central BPF involves segmental or larger airways and occurs most frequently after lung resection or trauma. This type of BPF is diagnosed by bronchoscopy and usually requires surgical repair. Peripheral BPF is a communication between the peripheral airway or lung parenchyma and the pleural space. Peripheral BPF has more diverse causes, including necrotizing pneumonia, trauma, lung surgery, bronchoscopic lung biopsy, and malignancy.
* The mortality rate ranges from 16% to 72%.
* Sometimes BPF can “hide” at the junction of the cartilage and the posterior membrane; a careful evaluation during both inspiration and expiration is necessary.
† If no fistula is demonstrable on bronchoscopy in a patient who is otherwise asymptomatic without leukocytosis, observation without pleural drainage may be warranted, but the presence of an occult fistula should remain highly suspected.
* Bronchoscopic techniques included cyanoacrylate or fibrin glue application, YAG laser therapy, injection of the vein sclerosant polidocanol, and tracheobronchial stenting.
† Three compartment drainage systems are used for BPFs; these systems can handle flows from 10 to 42 L/min at a suction pressure of −20 cm H2O. Increasing the suction pressure to −40 cm H2O does not improve maximal flow.
‡ Air leaks may vary from less than a liter to in excess of 16 L/min.
* Otherwise, with Rusch forceps, the stent can be grasped and promptly inserted under direct laryngoscopy using the push technique of Y-stent deployment.
† Pleural fluid microbiology results had revealed Pseudomonas aeruginosa, which was treated with piperacillin/tazobactam and levofloxacin.
* Positive-pressure ventilation is the most important risk factor for BPF. Early return to spontaneous respiration without positive-pressure mechanical assistance should be a priority after surgery to avoid inherent barotraumas and risk to the bronchial stump closure.
* They may be placed using direct laryngoscopy or fiberoptic bronchoscopy or through a tracheostomy. It should be emphasized that insertion of a DLT can be challenging, especially in hypoxic patients with or without difficult airways.