Bronchopulmonary Dysplasia
After reading this chapter, you will be able to:
• List the anatomic alterations of the lungs associated with bronchopulmonary dysplasia.
• Describe the causes of bronchopulmonary dysplasia.
• List the cardiopulmonary clinical manifestations associated with bronchopulmonary dysplasia.
• Describe the general management of bronchopulmonary dysplasia.
• Describe the clinical strategies and rationales of the SOAP presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs
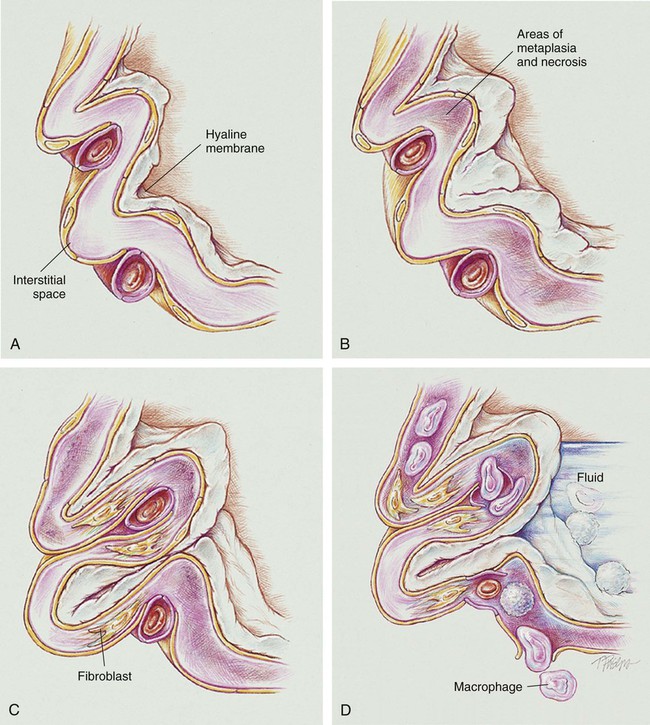
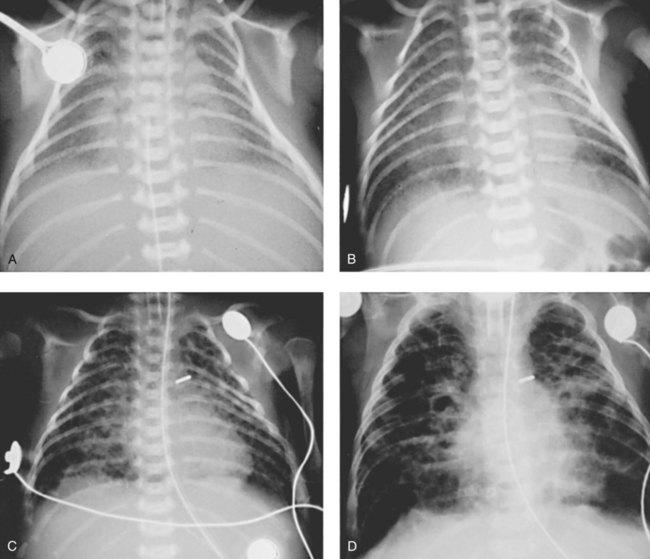
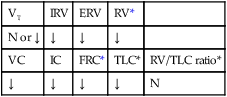
Stage I BPD was said to occur during the first 2 to 3 days of life. This stage is often indistinguishable from RDS. During this period, alveolar hyaline membranes, patches of atelectasis, and lymphatic dilation were seen. In addition, early signs of bronchial mucosal necrosis appeared during this time (see Figure 37-1, A). The chest radiographic findings revealed ground glass-like granular patterns and small lung volumes (Figure 37-2, A).
Stage II BPD was said to occur 4 to 10 days after birth. Atelectasis was more extensive during this period. In addition, metaplasia of the normal lung tissue cells caused bronchial necrosis, cellular debris, partial airway obstruction, air trapping, and alveolar hyperinflation. The pathologic findings during Stage II were commonly described as alternating areas of atelectasis and of emphysema (see Figure 37-1, B). These changes appeared on chest x-ray films as patchy opaque areas with bronchograms (areas of atelectasis) next to areas of dark translucency (areas of hyperinflation) (see Figure 37-2, B).
Stage III BPD was said to occur at 11 to 30 days of age. Pathologic findings included extensive bronchial and bronchiolar metaplasia and hyperplasia (an increased number of cells), interstitial fibrosis, and excessive bronchial airway secretions. In addition, the alveolar hyperinflation continued to form circular groups of emphysematous bullae that were surrounded by patches of atelectasis (see Figure 37-1, C). On the chest radiograph, the lungs began to show circular or cystic areas surrounded by patches of irregular density (see Figure 37-2, C).
Stage IV BPD was said to occur after 30 days of life. During this stage, massive fibrosis of the lung and destruction of the bronchial airways, alveoli, and pulmonary capillaries occured. Areas of emphysematous, or cystlike, areas continued to increase in size and number. Thin strands of atelectasis and normal alveoli were interspersed around emphysematous areas. In addition, pulmonary hypertension often developed, lymphatic and bronchial mucous gland deformation occurred, and excessive bronchial secretions continued to be a problem (see Figure 37-1, D). The chest radiographs revealed fibrosis and edema with areas of consolidation adjacent to areas of overinflation (see Figure 37-2, D). Table 37-1 provides a summary of the original BPD stages, with pathologic and radiologic correlates.
TABLE 37-1
Bronchopulmonary Dysplasia Staging (Northway)
| Stage | Days after Birth | Radiologic Findings | Pathologic Findings |
| I | 2-3 | Ground-glass granular pattern; small lung volume |
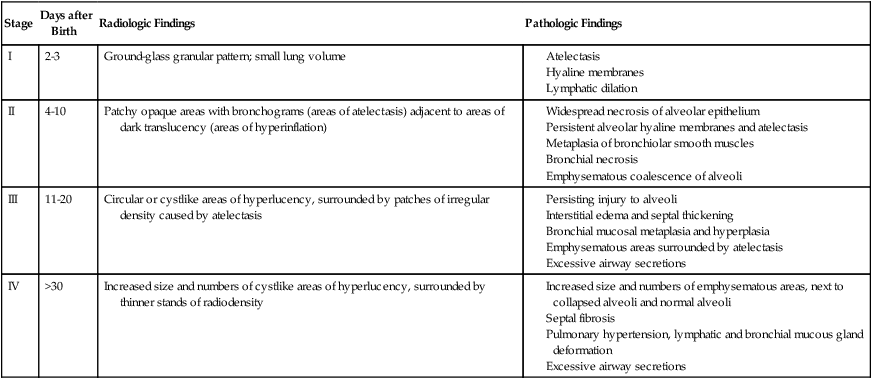
Northway WH Jr, Rosan RC, Porter DY: Pulmonary disease following respiratory therapy of hyaline-membrane disease: bronchopulmonary dysplasia, N Engl J Med 276:357-368, 1967.
The “New” Bronchopulmonary Dysplasia—Anatomic Alterations of the Lungs
In response to the awareness of the “new” BPD, the National Institutes of Health sponsored a workshop on BPD, providing a new definition of BPD.* The new definition outlines specific diagnostic criteria, including the need for oxygen, positive pressure ventilation, and/or CPAP. The definition also includes the postnatal age to better assess the severity of BPD. Table 37-2 provides an overview of the new diagnostic criteria for the “new” BPD.
TABLE 37-2
Diagnostic Criteria for the “New” Bronchopulmonary Dysplasia (BPD)
| Gestational Age | <32 Weeks | ≥32 weeks |
| Time point of assessment | 36 weeks PMA or discharge to home, whichever comes first | >28 days but <56 days postnatal age or discharge to home, whichever comes first |
| Treatment with Oxygen >21% for at Least 28 Days, PLUS | ||
| Mild BPD | Breathing room air at 36 weeks PMA or discharge, whichever comes first | Breathing room air by 56 days postnatal age or discharge, whichever comes first |
| Moderate BPD | Need* for <30% oxygen at 36 weeks PMA or discharge, whichever comes first | Need* for <30% oxygen at 56 days postnatal age or discharge, whichever comes first |
| Severe BPD | Need* for ≥30% oxygen and/or PPV or NCPAP at 36 weeks PMA or discharge, whichever comes first | Need* for ≥30% oxygen and/or PPV or NCPAP at 56 postnatal age or discharge, whichever comes first |
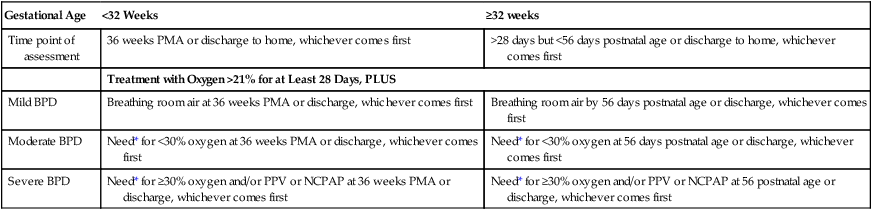
*A physiologic test confirming that the oxygen requirement at the time point of assessment has not yet been defined. This assessment may include a pulse oximetry saturation range.
Modified from Jobe AH, Bancalari E: Bronchopulmonary dysplasia, Am J Respir Crit Care Med 163:1723-1729, 2001.
Etiology and Epidemiology
BPD is the most common form of chronic lung disease in children. It is estimated that 10,000 to 12,000 infants are diagnosed with BPD in the United States annually. The current understanding of BPD indicates that there are multiple causative factors associated with BPD. Table 37-3 provides an overview of the primary causes of BPD.
TABLE 37-3
Causative Factors of Bronchopulmonary Dysplasia (BPD)
| Host susceptibility and genetic predisposition | The single most important causative factor associated with the development of BPD is prematurity. In addition, the retardation or restriction of intrauterine growth and a family history of RDS and asthma also put the infant at a higher risk for BPD. |
| Oxygen toxicity | Even in the first cases of BPD reported by Northway and colleagues in 1967, it was clear that exposure to high concentrations of oxygen was a factor in causing BPD. Subsequent reports continue to show that prolonged exposure to high levels of supplemental oxygen puts infants at risk for BPD. |
| Inflammation | A severe inflammatory response also plays a major role in the development of BPD. |
| Neonatal infection | The development of postnatal bacterial sepsis puts the infant at risk for BPD. Even airway microbial colonization without frank sepsis may increase the risk of BPD. |
| Mechanical ventilation | The development of BPD is strongly associated with mechanical ventilation. The major causative factors linked to mechanical ventilation are (1) high peak inspiratory pressures, (2) high mean airway pressures, and (3) overdistention of the lungs. Overinflation of the lungs causes stress fractures of the capillary endothelium, epithelium, and basement membranes. This mechanical injury in turn causes leakage of fluid into the alveolar spaces, with additional inflammation. |
| Pulmonary edema and patent ductus arteriosus | Abnormalities of lung fluid volume are associated with BPD. Several reports have shown that patency of the ductus arteriosus has a high correlation with the incidence of BPD. |
| Poor nutrition | All of the above causative factors are intensified by a poor nutritional status. |
General Management of Bronchopulmonary Dysplasia
Today a number of preventive methods are used to avert or treat BPD. Such measures include prenatal maternal steroid administration, the use of postnatal exogenous surfactant, gentle ventilation techniques, low oxygen concentrations, nasal CPAP, fluid restriction, vitamin A, diuretics, bronchodilator therapy, bronchial hygiene therapy, postnatal corticosteroids, and inhaled nitric oxide. In general, the management of infants who are at high risk for development of BPD or who have evolving BPD is directed at (1) minimizing the need for ventilatory support, (2) using low inspiratory pressures, (3) avoiding high mean airway pressures (MAPs), (4) minimizing the administration of high concentrations of oxygen, and (5) supporting and maintaining an adequate functional residual capacity (FRC) with PEEP or CPAP. Table 37-4 provides an overview of the therapeutic measures used to prevent or manage infants with BPD.
TABLE 37-4
Therapeutic Measures Used to Prevent or Manage Infants with Bronchopulmonary Dysplasia (BPD)
| Prenatal steroids | A single course of prenatal glucocorticoids administered to women who are at high risk for premature delivery results in a significant decrease in the mortality rate and in the morbidity associated with prematurity. |
| Gentle ventilation | In spite of the development of numerous sophisticated ventilators for the newborn, there is still no clear advantage to any one approach. The general approach is a ventilatory mode that prevents atelectasis, sustains or maintains FRC, uses a minimal tidal volume, and permits the infant to trigger his or her own ventilation as much as possible. Every effort should be made to minimize high peak inspiratory pressures, high mean airway pressures (MAPs), and overdistention of the lungs. For example, high-frequency ventilation and low tidal volumes (with the goal of maintaining the Paco2 above 55 mm Hg) are commonly used. |
| Low inspired oxygen concentrations | Every effort should be made to administer only the lowest concentration of oxygen that is necessary. |
| Nasal continuous positive airway pressure (CPAP) | The early application of nasal CPAP in high-risk respiratory distress syndrome (RDS) and BPD infants is highly recommended during postnatal care. |
| Fluid restriction | Because fluid overload is a causative factor associated with BPD, the limitation of fluids may be helpful. However, care should be taken to not be too aggressive in the limitation of fluid administration, because undernutrition is also associated with the development of BPD. |
| Vitamin A | Vitamin A is an essential nutrient for maintaining the epithelial cells of the tracheobronchial tree. |
| Diuretics | In infants with severe BPD, pulmonary edema is a major component of the disorder. There is clear evidence that either daily or alternate-day therapy with furosemide improves lung mechanics and gas exchange in infants with established BPD. |
| Bronchodilator therapy | Increased airway resistance is highly associated with BPD. Short-term therapy with inhaled or parenteral beta2-adrenergic agonists is frequently administered to infants with BPD. Inhaled albuterol has been the most widely used agent. |
| Bronchial hygiene therapy | Because of the high incidence of mucous plugging of the airways and endotracheal tubes, adequate humidification of the inspired gas is important. Postural drainage, percussion, and vigorous suctioning also are extremely beneficial. |
| Postnatal corticosteroids | The administration of postnatal corticosteroids to preterm infants has been shown to reduce lung inflammation and the incidence of BPD. Postnatal corticosteroids are also believed to increase surfactant synthesis, enhance beta-adrenergic activity, increase antioxidant production, stabilize cell and lysosomal membranes, and inhibit prostaglandin and leukotriene synthesis. |
| Inhaled nitric oxide | The administration of inhaled nitric oxide (iNO) may prevent BPD or benefit infants with evolving BPD. It is suggested that preterm infants with early BPD may have a deficiency of endogenous NO. It is hypothesized that the administration of iNO causes both pulmonary vasodilation and bronchial dilation and therefore reduces the need for oxygen and ventilatory support. |
CASE STUDY
Bronchopulmonary Dysplasia
Admitting History and Physical Examination
Respiratory Assessment and Plan
O Marginal pulmonary mechanics—decreased compliance and increased airway resistance. Coarse rhonchi and wheezes. CXR: BPD—interstitial emphysema and fibrosis. ABGs on ventilator and 40% oxygen: pH 7.36, Paco2 55, 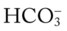 30, Pao2 50, Sao2 84%.
30, Pao2 50, Sao2 84%.
• Stiff lung with airway obstruction (pulmonary mechanics)
• Chronic ventilatory failure with moderate hypoxemia (ABGs)
• Excessive bronchial secretions (rhonchi and suctioning results)
• Possible bronchospasm (wheezes—maybe caused by bronchial secretions)
P Wean slowly per Mechanical Ventilation Protocol (decrease mandated respiratory rate slowly—decrease need for pressure and rate). Continue Oxygen Therapy Protocol (do not attempt to wean from oxygen therapy until ventilator mandated rate is down to about 5 breaths/min). Continue aggressive bronchopulmonary hygiene therapy per Bronchopulmonary Hygiene Therapy Protocol (CPT q2h and suction prn). Continue Bronchodilator Therapy Protocol (in-line neb with 0.15 mL albuterol in 2 mL normal saline q4h). Continue to monitor closely and assess frequently.
Discussion
BPD is a disorder that requires a great deal of parental education and support at the time of the baby’s discharge from the hospital. The respiratory therapist can be instrumental in working with the family both in the hospital and in the home to ensure that the parents are prepared to support the child’s respiratory care needs. For example, the parents must understand the procedures of tracheal and nasal suctioning, chest physical therapy, and aerosolized medication administration at home. Infants with BPD who have been discharged from the hospital commonly return to the hospital once or twice a year in acute respiratory distress. Therefore the importance of aggressive, long-term respiratory care in the home must be stressed to the family. For example, the value of good bronchopulmonary hygiene therapy at home to offset the clinical manifestations associated with the accumulation of Excessive Bronchial Secretions (see Figure 9-12) cannot be over emphasized.


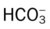

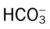

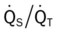
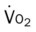
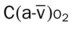
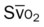

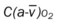 , Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 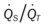 , pulmonary shunt fraction;
, pulmonary shunt fraction; 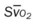 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 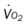 , oxygen consumption.
, oxygen consumption.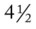 weeks, he developed pneumonia and was aggressively treated for atelectasis and excessive bronchial secretions. At that time the baby was considered to have a chronic case of bronchopulmonary dysplasia (BPD).
weeks, he developed pneumonia and was aggressively treated for atelectasis and excessive bronchial secretions. At that time the baby was considered to have a chronic case of bronchopulmonary dysplasia (BPD).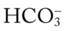 30, Pa
30, Pa