Chapter 9
Bronchoprovocation Testing
1. Describe two methods of performing bronchial challenge tests.
2. Identify a positive response to a methacholine challenge test.
3. List two indications for bronchoprovocation testing.
4. Select an appropriate protocol to test for exercise-induced asthma.
1. Describe direct versus indirect challenge mechanisms.
2. Interpret the various cut-points for determining a positive challenge test.
3. Describe the dilution process for preparation of methacholine doses.
4. Understand the physiologic determinates of exercise-induced bronchospasm.
Bronchoprovocation challenge testing
Each of these agents may trigger a bronchospasm but in slightly different ways.
Bronchoprovocation tests are classified as direct or indirect, based on their mechanism of action (Figure 9-1, Table 9-1).
Table 9-1
Agents Commonly Used in Bronchial Provocation Testing
| Direct Stimuli | Indirect Stimuli |
| Methacholine | Mannitol |
| Histamine | Adenosine (AMP) |
| Prostaglandins | Exercise |
| Leukotrienes | Eucapnic voluntary hyperventilation (EVH) |
| Propranolol (β-blockers) | |
| Hypertonic saline |
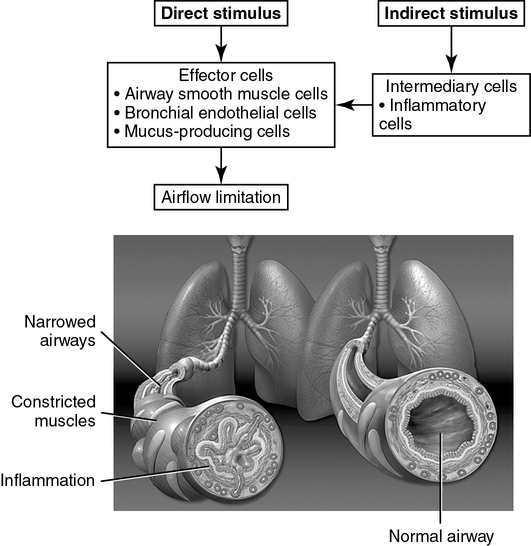
There are two categories of bronchoprovocation tests, those that act directly on smooth muscle and those that cause the airways to narrow indirectly by a release of endogenous mediators.
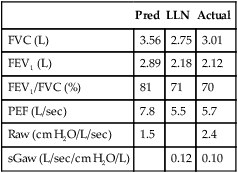
Histamine and methacholine act directly on the smooth muscle cells of the airways to cause bronchoconstriction and airway hyperresponsiveness (AHR). Indirect bronchoprovocation tests, such as mannitol and adenosine monophosphate (AMP), act through inducing the release of bronchoconstricting mediators. Hyperventilation, either at rest or during exercise, results in heat and water loss from the airway. This provokes a bronchospasm in susceptible patients. With each of these agents, pulmonary function variables are assessed before and after exposure to the challenge. FEV1 is the variable most commonly used. Other flow measurements, as well as airway resistance (Raw) and specific conductance (sGaw), may also be evaluated before and after the challenge. Additional parameters that have been used to assess response to a bronchial challenge include breath sounds, transcutaneous PO2 (tcPO2, see Chapter 11), and forced oscillation measurements of resistance and reactance.
Methacholine Challenge
Spirometry, and sometimes sGaw, is measured after each dose. Most clinicians consider the test result positive when inhalation of methacholine precipitates a 20% decrease in FEV1. The methacholine concentration at which this 20% decrease occurs is called the provocative concentration (PC20). In some references it may be termed provocative dose (PD20 ). In the doses usually used (Box 9-1), healthy subjects do not display decreases greater than 20% in FEV1. Therefore, the methacholine challenge test is highly specific for airway hyperreactivity. Many patients who have asthma experience a 20% reduction in FEV1 with doses of 8 mg/mL or less. However, bronchial hyperresponsiveness may also be seen in other pulmonary disorders such as COPD, cystic fibrosis, and bronchitis.
Patients to be tested should be asymptomatic, with no coughing or obvious wheezing. Recent upper or lower respiratory tract infections may alter airway responsiveness, so bronchial challenge testing may need to be deferred. Their baseline FEV1 should be normal or at least greater than 60%–70% of their expected value. For patients with known obstruction or restriction, FEV1 should be close to their highest previously observed value. Obvious airway obstruction (e.g., FEV1% less than the lower limit of normal) is a relative contraindication. If the patient has an FEV1 less than 1.0–1.5 L, there is a risk that a large drop in FEV1 after a methacholine challenge might leave the individual with a compromised lung function. Bronchial challenge may be indicated in obstructed patients if the clinical question is related to the degree of responsiveness. Box 9-2 lists the absolute and relative contraindications to a methacholine challenge.
If the patient has been taking bronchodilators, they should be withheld according to the schedule listed in Table 9-2. Other medications or substances can affect the validity of the challenge as well. All medications being taken at the time of testing should be recorded to assist in the evaluation of the test results.
Table 9-2
Withholding Medications Before Bronchial Challenge
| Short-acting β agonist agents (inhaled) | 8 hours |
| Long-acting β agonist agents (inhaled) | 48 hours (some may require longer) |
| Standard β agonist agents (oral) | 12 hours |
| Long-acting β agonist agents (oral) | 24 hours |
| Anticholinergic agents (ipratropium) | 24 hours |
| Standard theophylline preparations | 12–24 hours |
| Sustained-action theophylline preparations | 48 hours |
| Cromolyn sodium | 8 hours |
| Nedocromil | 48 hours |
| Antihistamines | 72–96 hours |
| Corticosteroids (inhaled or oral) | Patients challenged while taking a stable dosage* |
| Leukotriene modifiers | 24 hours |
| Caffeine-containing drinks (cola, coffee) | 6 hours |
| β-blocking agents | May increase the response |
A small-volume nebulizer is used to generate the methacholine aerosol (Figure 9-2).
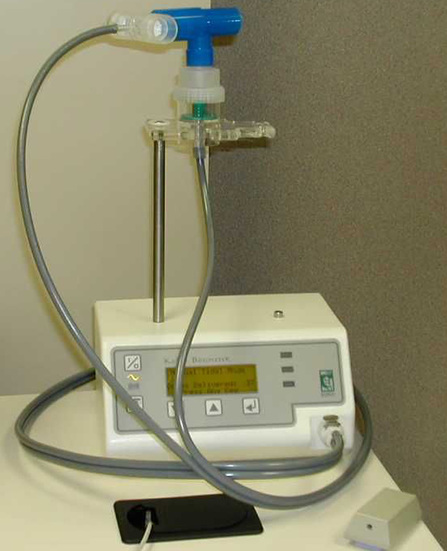
A dosimeter that controls the flow of gas to the nebulizer is also shown. The dosimeter provides a timer so that nebulization occurs for a specific time (e.g., 0.6 seconds), depending on the nebulizer used and its specific output. This dosimeter senses the patient’s inspiratory effort and triggers flow automatically; some require manual actuation. (Courtesy nSpire Health, Inc. Longmont, CO)
Two dosing routines are commonly used for a methacholine challenge (see Box 9-2). One routine uses a quadrupling (4×) increase in methacholine concentration, and the other method uses a doubling dose (2×). For each of these regimens, the highest dose is 16 mg/mL, and the dilutions can be easily prepared from a stock solution, starting with 100 mg of dry methacholine (Table 9-3). The stock solution is prepared by dissolving the powdered drug in a saline diluent. A preservative (0.4% phenol) may be added to the solution but is not required. Methacholine concentrations from 0.025–25.0 mg/mL are stable after mixing and may be kept for 5 months if refrigerated at 2°C–8°C (36-46°F). An alternate dosing scheme is provided with the FDA-approved form of methacholine (Provocholine, Methapharm, Ontario, Canada). This dosing schedule uses methacholine concentrations of 0.025, 0.25, 2.5, 10, and 25 mg/mL and is designed for use with the five-breath dosimeter method. Methacholine should be prepared by a pharmacist or individual trained in preparing drugs using a sterile technique. Appropriate precautions should be taken when handling dry powdered methacholine. Vials of methacholine should be carefully marked with labels that clearly identify the concentration.
Table 9-3
Preparation of Methacholine for Two Common Dosing Schedules*
| Methacholine | Diluent (0.9% NaCl) | Dilution |
| Doubling Dosage | ||
| 100 mg (dry powder) | 6.25 mL | 16.0 mg/mL |
| 3 mL of 16.0 mg/mL | 3 mL | 8.0 mg/mL |
| 3 mL of 8.0 mg/mL | 3 mL | 4.0 mg/mL |
| 3 mL of 4.0 mg/mL | 3 mL | 2.0 mg/mL |
| 3 mL of 2.0 mg/mL | 3 mL | 1.0 mg/mL |
| 3 mL of 1.0 mg/mL | 3 mL | 0.5 mg/mL |
| 3 mL of 0.5 mg/mL | 3 mL | 0.25 mg/mL |
| 3 mL of 0.25 mg/mL | 3 mL | 0.125 mg/mL |
| 3 mL of 0.125 mg/mL | 3 mL | 0.0625 mg/mL |
| 3 mL of 0.625 mg/mL | 3 mL | 0.031 mg/mL |
| Quadrupling Dosage | ||
| 100 mg (dry powder) | 6.25 mL | 16.0 mg/mL |
| 3 mL of 16.0 mg/mL | 9 mL | 4.0 mg/mL |
| 3 mL of 4.0 mg/mL | 9 mL | 1.0 mg/mL |
| 3 mL of 1.0 mg/mL | 9 mL | 0.25 mg/mL |
| 3 mL of 0.25 mg/mL | 9 mL | 0.0625 mg/mL |
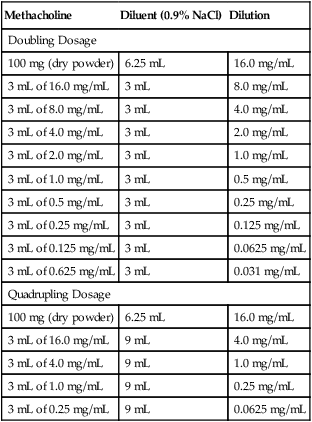
*For each schedule, 6.25 mL of saline are added to dry powdered methacholine. Subsequent dilutions then use 3 mL or 9 mL of saline added to 3 mL of the previous dilution.
Two-Minute Tidal Breathing Method
In this method, normal relaxed breathing is used as the patient inhales the aerosol. Methacholine is usually prepared in 10 doses of doubling concentrations (see Box 9-1 and Table 9-3). If the methacholine has been refrigerated, it should be allowed to come to room temperature for 30 minutes. A nebulizer capable of delivering 0.13 mL/min (±10%) driven by compressed air should be used. An accurate flowmeter allows adjustment to the flow necessary to deliver the desired volume.
< ?xml:namespace prefix = "mml" />
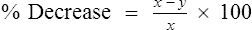
x = control FEV1 (baseline or after diluent)
y = current FEV1 after methacholine inhalation
This change is sometimes reported as a negative value (e.g., −20%) to indicate a fall in the FEV1.
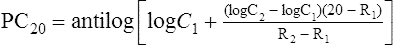
C1 = second-to-last methacholine concentration
C2 = final methacholine concentration (causing 20% or greater decrease)
R1 = percent decrease in FEV1 after C1
R2 = percent decrease in FEV1 after C2
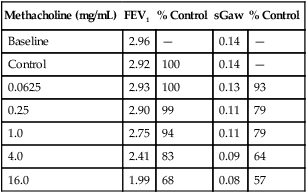
PC20 calculated this way provides a single index of bronchial responsiveness. PC20 may also be identified directly from a graph in which change in FEV1 is plotted against the log concentration of methacholine (Figure 9-3).
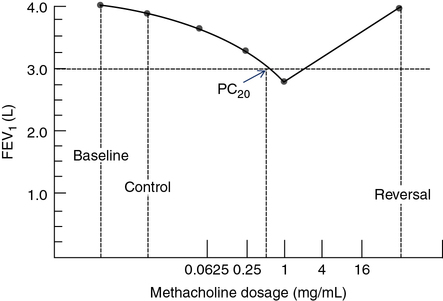
Results of data gathered during a bronchial challenge test are shown. The dosage of the challenge agent (methacholine, in this case) is plotted on a logarithmic scale on the x-axis. FEV1 (or other variable) is plotted on the y-axis. The first point represents the baseline FEV1 (4.0 L, in this example). The control value (FEV1 after inhalation of the diluent) is plotted next. FEV1 after each dose of methacholine is plotted until a 20% or greater decrease occurs. In this example, FEV1 decreased by more than 20% with a dose of 1 mg/mL. A vertical line drawn from the point at which the dose response curve crosses the 20% line defines the PC20. The patient is then given an inhaled bronchodilator to reverse the effect of the provocative agent, and the response is plotted.
See Interpretive Strategies 9-1. Airway responsiveness to methacholine can be described using the PC20. Most patients referred for bronchial challenge testing have a history or symptoms suggestive of asthma but not a definite diagnosis. For these patients, if FEV1 decreases less than 20% at the highest dose (PC20>16 mg/mL), bronchial responsiveness is probably normal and asthma is unlikely. For patients whose FEV1 decreases 20% or more at low doses of methacholine (PC20<1.0 mg/mL), the diagnosis of asthma is highly likely. For patients with PC20 values from 1–16 mg/mL, the diagnosis of asthma must be considered, based on the pre-test probability of asthma, the history of symptoms, and other possible causes for bronchial hyperreactivity. In practice, patients who have a PC20 greater than 8–16 mg/mL often do not have asthma. Patients who have a negative methacholine challenge (PC20>16 mg/mL) may have asthma that has been suppressed by anti-inflammatory medications or occupational asthma that is triggered by a specific agent. Conversely, some individuals who have PC20 values less than 8 mg/mL may not have asthma. Patients with allergic rhinitis and smokers with COPD often have bronchial hyperreactivity but not asthma.
Technical factors can also make methacholine challenge tests difficult or impossible to interpret (see Criteria for Acceptability 9-1). Changes in FEV1 after a bronchial challenge are usually not diagnostic in patients who cannot perform acceptable and reproducible baseline spirometry. Variable efforts by the patient may produce a false-positive test result (apparent reduction in FEV1 but not asthma). FEV1 values obtained at 30 and 90 seconds after each dose of methacholine should be similar. The maneuvers should meet criteria for an acceptable effort (see Chapter 2). However, because the primary endpoint is the FEV1, it may not be necessary for the patient to exhale for 6 seconds. Using a shortened exhalation requires that the patient inspires fully to TLC, and this may be difficult to determine unless a full FVC effort is performed. The usual repeatability criteria (FEV1 efforts within 150 mL) may not be met because of the effects of methacholine. Additional maneuvers may be needed at 30 and 90 seconds to verify that a real decrease has occurred. The FEV1 reported for each dose of methacholine should be the largest value obtained at that level.
The type of nebulizer and dosimeter affects the amount of agonist reaching the airways. Factors that should be controlled as much as possible include the type of nebulizer, nebulizer output, particle size, inhaled volumes, breath-hold times, and inspiratory flow. Nebulizer driving pressure or flow should be consistent throughout the test. If a single nebulizer is used, it should be thoroughly emptied between doses. If multiple nebulizers are used (one for each dose), they should be checked for similar output. Although the five-breath dosimeter and tidal breathing techniques deliver different volumes of methacholine, the sensitivity and specificity of the test are similar for both methods in adults and in children. The spirometer used should meet the minimal standards set by the ATS-ERS (see Chapter 12). It should provide spirometric tracings or F-V loops for later evaluation.
Histamine Challenge
Patient preparation for a histamine challenge is similar to that used for methacholine (see Table 9-3). Antihistamines and H1-receptor antagonists should be withheld for 48 hours before testing.
Various dosing regimens for a histamine challenge have been proposed. Box 9-3 lists one dosing protocol for a histamine challenge. These increments approximately double the concentration of drug at each level. The same criteria as those used for baseline spirometry in a methacholine challenge are observed. Diluent may be administered first to determine a control value for FEV1.
The results of a histamine challenge are reported in a manner similar to that described for methacholine. The histamine concentration that produces a 20% decrease in FEV1 is termed the PC20. Response may also be reported by graphing the percentage of change in FEV1 against the concentration (or its logarithm) of the drug. This type of plot is commonly called a dose-response graph. It permits interpolation of the precise concentration of drug that elicits the 20% decrease (see Figure 9-3).
Histamine, like methacholine, is relatively safe if testing follows the procedures described. Baseline and control values should always be established (see Criteria for Acceptability 9-1). Bronchial challenge should always begin with a low concentration of drug. The range of concentrations used should be appropriate for the patient tested. For adult patients in whom airway hyperreactivity is the suspected diagnosis, the dosing schedules previously described are recommended. Patients who have a positive response to a histamine challenge recover more quickly than those tested with methacholine. Histamine challenge can be repeated within 2 hours after the patient has returned to the baseline level of function.
Mannitol Challenge
Mannitol is a hypertonic stimulus, and inhalation increases the osmolarity of the airway that subsequently leads to the release of inflammatory mediators from mast cells and basophils. This leads to airway narrowing similar to that observed with hypertonic saline and exercise challenge tests. Mannitol is a sugar alcohol and is delivered by a special dry-powder inhaler (DPI). The DPI is trademarked as the Osmohale inhaler device and is a component of the testing kit (Aridol-Pharmaxis Ltd., Exton, PA) (Figure 9-4).
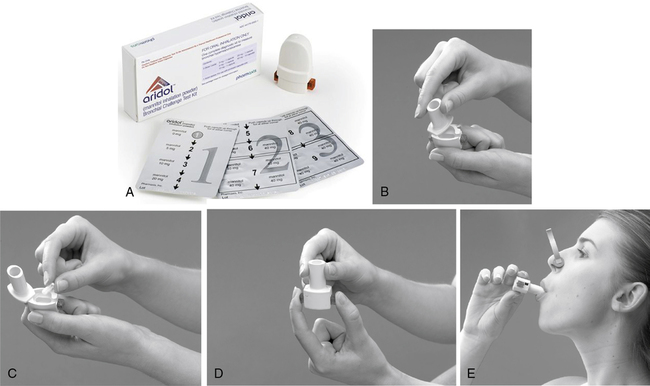
(A) Aridol kit with dry powder inhaler and capsules containing dosing scheme; (B) Twist the DPI to open the capsule chamber; (C) Insert the appropriate capsule into the holding chamber (do not use rubber gloves); (D) Puncture the capsules only once by depressing both puncture tabs; (E) Have the subject exhale, then place the mouth on the DPI with the head slightly tilted, and take a controlled deep breath, and then hold for 5 seconds. Set a timer for 60 seconds. After the 60 seconds, perform an FVC maneuver. (Pharmaxis: Product Monograph: Aridol [Aridol is a registered trademark of Pharmaxis Ltd., Frenchs Forest, Australia] [mannitol inhalation powder] Bronchial Challenge Test Kit, October 2010, Exton, PA.)
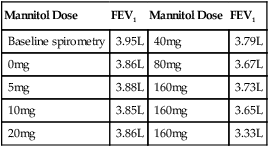
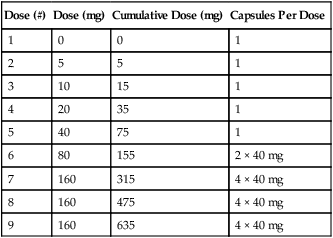
The testing kit includes the drug packaged in capsules, which contain the mannitol in the desired doses. These capsules load into the DPI device so there is not a need for additional specialized equipment or drug mixing. The dose scheme is listed in Table 9-4. Patient preparation for the mannitol challenge is similar to that used for methacholine (see Table 9-2). Mannitol is contraindicated in patients with known hypersensitivity to mannitol or to the gelatin used to make the capsules. According to the manufacturer’s package insert, it is for patients age 6 years or older who do not have apparent clinical asthma. The most common adverse reactions (rate ≥1%) were headache, pharyngolaryngeal pain, throat irritation, nausea, cough, rhinorrhea, dyspnea, chest discomfort, wheezing, retching, and dizziness. Special testing requirements are listed in Box 9-4; otherwise, the technique is similar to other challenge testing. Performance of acceptable spirometry with the largest FEV1 at least 60% of predicted is required for test performance. Following the administration of 0 mg mannitol, FEV1 is measured, and if it is not within 10% of the baseline, the test is terminated. The 0-mg dose FEV1 then becomes the baseline value and the target FEV1 is calculated:
Table 9-4
Mannitol Dose Steps for Bronchial Challenge Testing with Aridol*
| Dose (#) | Dose (mg) | Cumulative Dose (mg) | Capsules Per Dose |
| 1 | 0 | 0 | 1 |
| 2 | 5 | 5 | 1 |
| 3 | 10 | 15 | 1 |
| 4 | 20 | 35 | 1 |
| 5 | 40 | 75 | 1 |
| 6 | 80 | 155 | 2 × 40 mg |
| 7 | 160 | 315 | 4 × 40 mg |
| 8 | 160 | 475 | 4 × 40 mg |
| 9 | 160 | 635 | 4 × 40 mg |
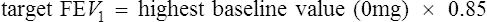
Mannitol has been shown to have the same sensitivity and specificity as methacholine testing.
Exercise Challenge
1. In patients who have shortness of breath on exertion but exhibit normal resting pulmonary function
2. In symptomatic patients in whom other bronchial provocation tests (such as methacholine challenge) produce negative or ambiguous results
3. In patients with known EIA in whom therapy is being evaluated
4. In screening patients where some risk to those with asthma might be involved (e.g., athletics, military service)
Patients referred for exercise challenge should be evaluated by means of an appropriate history and physical examination. The evaluation should include a resting electrocardiogram (ECG) to ascertain potential contraindications to exercise testing (see Chapter 7). Certain medications should be withheld as for methacholine challenge testing (see Table 9-2). Before exercise, the patient’s FEV1 should not be less than 65% of the predicted value. Patients with overt obstruction usually do not require an exercise challenge to demonstrate airway hyperreactivity. Patients should refrain from vigorous exercise for 4 hours before the test because there is a refractory period after exercise. Patients referred for EIB testing should be free from respiratory infections for 3–6 weeks before testing.
Measurement of variables such as minute ventilation (![]() E) and tidal volume (VT) may be helpful in assessing the ventilatory load imposed by the exercise. Measurement of F-V curves during exercise may be a useful adjunct in assessing the ventilatory response to increasing workloads (see Chapter 7). A spirometer that meets ATS-ERS requirements (see Chapter 11) is necessary. Resuscitation equipment, as described in Chapter 7, should be available.
E) and tidal volume (VT) may be helpful in assessing the ventilatory load imposed by the exercise. Measurement of F-V curves during exercise may be a useful adjunct in assessing the ventilatory response to increasing workloads (see Chapter 7). A spirometer that meets ATS-ERS requirements (see Chapter 11) is necessary. Resuscitation equipment, as described in Chapter 7, should be available.
In most instances, a short period of moderately heavy work is all that is required to trigger exercise-induced bronchospasm. The goal is to have the patient exercise at high intensity for 4–6 minutes, with total exercise duration of 6–8 minutes. Bronchospasm usually occurs immediately after the exercise, not during it, unless the test is extended over a longer interval (see Criteria for Acceptability 9-1). Repeated testing should be delayed for 4 hours because of a “refractory period” during which the severity of the bronchoconstriction lessens. This response is presumably caused by the release of catecholamines during exercise. An extended warm-up period before the actual exercise may also protect the airways and lessen subsequent bronchoconstriction (see section on EVH testing later in the chapter).
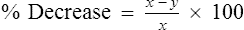
y = lowest observed post-exercise value
Patients who have VCD or other upper airway abnormalities are often referred for EIA tests. F-V curves (including maximal inspiratory flows) should be performed if the history or physical examination suggests these disorders. Measurement of tidal breathing loops during exercise (see Chapter 7) may also help define the pattern of ventilatory limitation.
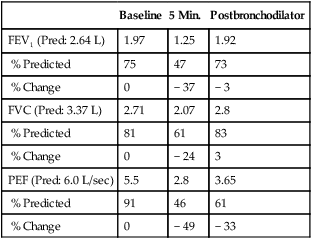
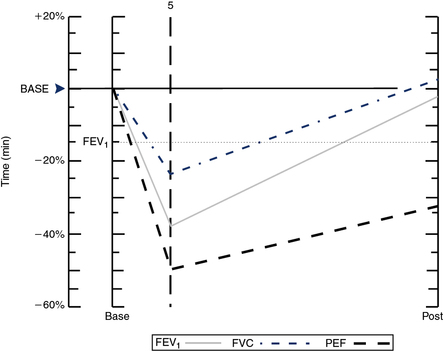
Exercise testing with cold air can also be performed using the same exercise protocols as described earlier but requires specialized equipment to refrigerate and dry inspired gas (TurboAire Challenger, VacuMed, Ventura, CA). This equipment delivers cold, dry air to the airways (relative humidity 0% and temperature −20°C) and has been shown to be slightly more sensitive and specific than testing with room-temperature gas (Figure 9-5).

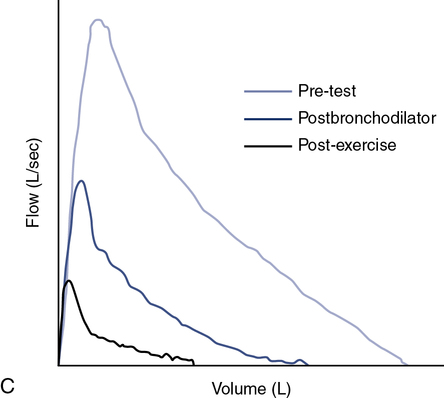
(A) Using a compressed air source via reservoir and directional valve; (B) Using a TurboAire Challenger, a device that delivers cold, dry air; (C) FVCs pre- and post-exercise challenge with a significant reduction in flow and volume following exercise and suboptimal return to baseline following the first dose of bronchodilator.
Eucapnic Voluntary Hyperventilation
Patients to be tested with eucapnic voluntary hyperventilation (EVH) should withhold bronchodilators, as suggested in Table 9-2. Baseline spirometry is performed to ascertain that airway obstruction is not present. For ventilation challenges, the baseline FEV1 is the control value with which subsequent measurements will be compared.
EVH with gas at ambient temperature also provides a stimulus for bronchospasm. In this technique, the patient breathes a mixture of 5% CO2, 21% O2, and the balance N2 at room temperature. The gas is used to fill a “target” bag or balloon of approximately 5 L (see Figure 9-6).
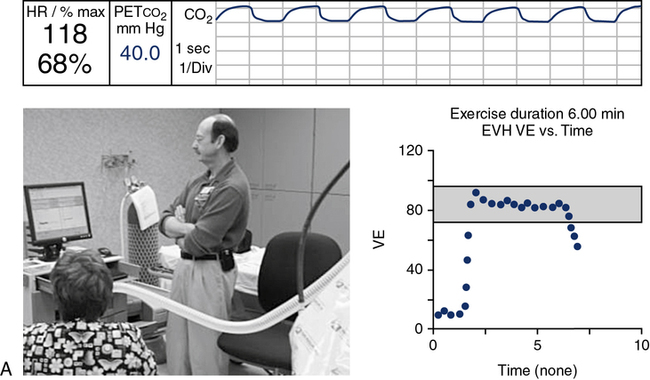
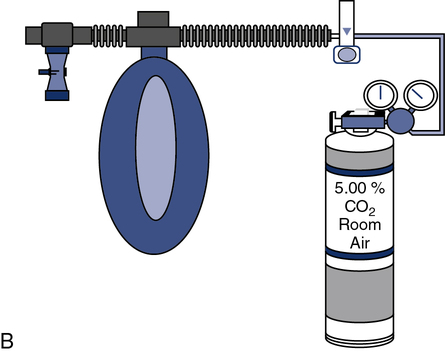
(A) A gas containing 5% CO2, 21% O2, and balance N2 is directed through a precision high-flow flowmeter to a reservoir bag. Flow is adjusted to a target ventilation level, such as 30 times the patient’s FEV1; (B) The patient is coached according to the required predetermined ventilation. The test is continued for a predetermined interval, usually 6 minutes.
The patient breathes from the bag via a nonrebreathing valve (see Chapter 11) and large-bore tubing. The patient wears a noseclip. A high-output flowmeter is used to fill the target bag. The flowmeter is adjusted to deliver gas at approximately 30 times the patient’s FEV1. The patient breathes from the bag and tries to match his or her ventilation to keep the bag partly deflated. The high level of ventilation is continued for 6 minutes. Spirometry is performed immediately after hyperventilation and then at 5-minute intervals (i.e., at 5, 10, 15, and 20 minutes). A commercial system is available, which uses a large reservoir bag for the hypercarbic gas mixture, gas analyzer, and pneumotach, which then gives feedback to the technologist and patient via the data acquisition screen on maintaining the predetermined ventilation (Figure 9-6).
For both the cold-air and room-temperature protocols, if no decrease in FEV1 occurs within 20 minutes after hyperventilation, the test may be considered negative. The percentage decrease is calculated just as for methacholine challenge testing, as described previously. A decrease of 15% is consistent with some degree of airway hyperreactivity (see Criteria for Acceptability 9-1). EVH in healthy subjects usually results in bronchodilatation. Therefore, a 15% decrease is abnormal and highly specific for increased bronchial responsiveness. Some asthmatic patients may experience significant decreases in FEV1 (20%–60%). Bronchospasm should be reversed with inhaled bronchodilators and the reversal documented with spirometry. The technologist performing the procedure should be prepared to manage severe bronchospasm if it occurs.
Summary
• Bronchial challenge tests can be done with several different agents, all of which test the airway responsiveness in slightly different ways.
• Methacholine challenge is the most commonly used and best-standardized test of airway hyperreactivity. Histamine and antigenic agents are also used.
• Mannitol is a new indirect agent to assess bronchial hyperreactivity. It is standardized and easy to use.
• Exercise testing can be specifically used to evaluate exercise-induced bronchospasm. Hyperventilation tests, with cold or room-temperature air, mimic the ventilatory load that occurs with exercise.