Chapter 15 Bronchodilators
Breathlessness and wheezing are cardinal symptoms of many respiratory diseases, and from the 19th century it was recognized that smoking cigarettes made from the leaves of the plant Atropa belladonna lessened the symptoms of asthma. This therapy was largely superseded when epinephrine (adrenaline) was purified in the early 20th century. Subsequently, synthetic analogues of epinephrine were developed, as was atropine, the first synthetic antimuscarinic agent. Both atropine and epinephrine could be added to organ baths containing airway smooth muscle, and their potency in relieving or preventing induced smooth muscle contraction could be measured. These developments led to the current understanding of the control of airway smooth muscle, as shown in Figure 15-1. Changes in airway smooth muscle length translate into changes in airway caliber—an especially important issue when airway diameter is reduced, as in chronic obstructive pulmonary disease (COPD) (Figure 15-2). In clinical practice, this aspect of pulmonary function is assessed indirectly as an increase in the forced expiratory volume in 1 second (FEV1) after drug administration. When a bronchodilator is given orally, the time to onset of action (i.e., improvement in lung function) is prolonged, reflecting the absorption and circulation of the drug; this time can be considerably shortened when treatment is administered by inhalation. Of note, not all drugs that increase FEV1 do so by changing airway smooth muscle activity; for example, antiinflammatory agents can reduce the mucosal thickening and thickening of the airway wall, thereby leading to improvements in FEV1. These processes take some time to develop, however, because agents such as corticosteroids have both nuclear and extranuclear effects on protein synthesis, which reduce the number of proinflammatory messenger molecules. Hence, for practical purposes, bronchodilators can be considered as those drugs with a proven direct effect on airway smooth muscle and a relatively rapid onset of effect (minutes to several hours) in clinical circumstances in which some measure of airway resistance is the marker for effectiveness.
Physiologic Basis for Bronchodilator Action
Under resting conditions in healthy people, the work of breathing performed by the respiratory muscles is relatively small. Inspiration is an active process, and sufficient flow has to be generated by overcoming respiratory resistive, elastive, and frictional loads to ensure adequate alveolar ventilation. Expiration is passive and ends when the expiratory recoil pressure of the lungs and the chest wall are balanced. There is little expiratory flow resistance within the bronchial tree, and expiratory flow limitation (no increase in flow despite increasing expiratory driving pressure) is detected only during the last part of the maximum forced expiration (Figure 15-3, top). Ventilation increases during exercise but not to the point at which flow limitation significantly limits performance.
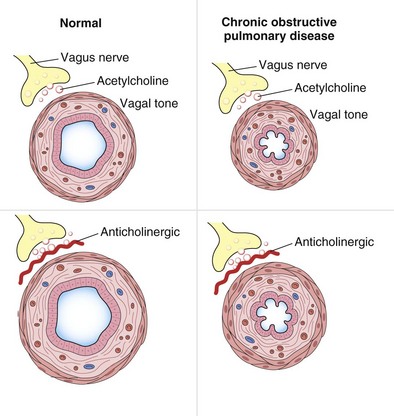
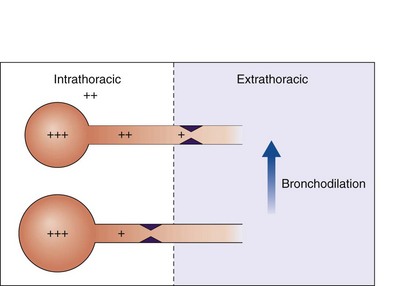
Airway resistance is influenced significantly by the caliber of the bronchial tree (see Figure 15-2). In health, most of the resistance lies in the region of the larynx, with less than 20% coming from the periphery of the lung. The evidence points to resting airway smooth muscle tone that decreases physiologically during exercise to reduce the resistive work required when ventilation has to increase. Bronchodilator drugs abolish this smooth muscle tone in both healthy persons and patients with disease. In healthy people, the increase in FEV1 after administration of a β-agonist is between 50 and 120 mL, a value similar to that in patients with COPD, whose baseline FEV1 usually is much lower. By contrast, in patients with asthma, in which airway smooth muscle bulk is greater and resting muscle tone may be increased by indirect reflex mechanisms, the response to bronchodilator drugs is more dramatic, and substantial increases in lung function occur after the acute administration of a bronchodilator. The improvement in lung emptying that results from bronchodilation has important effects on the operating lung volume and hence on the work of breathing.
In general, wheezing reflects areas of local flow limitation within the airway. Expiratory flow limitation can occur in the absence of audible wheeze and contributes to the slow emptying of the lungs and the higher lung volumes that lead to breathlessness and chest tightness in obstructive lung disease. Expiratory flow limitation may be abolished completely after a bronchodilator drug in asthma (see Figure 15-3), although asthmatic symptoms often will recur when the effects of the bronchodilator wear off (see further on). In COPD, the absolute change in lung function is much smaller than in asthma, and flow limitation often persists, particularly in severe disease, although to a less severe degree. These subtle changes in lung mechanics, however, can produce clinically relevant changes in operating lung volumes, particularly in the end-expiratory lung volume, which is significantly elevated in many patients with COPD and constitutes is a good guide to the degree of exercise impairment experienced by affected persons. A further effect of bronchodilator drugs is to increase the threshold at which symptoms are induced. This increased threshold is important in the prevention of exercise-induced asthma and also in the reduction of symptoms produced more predictably by exercise, as occurs when patients with COPD undertake certain daily activities. This ability to increase baseline lung function and reduce the impact of external stimuli on the airways is likely to be important in explaining why bronchodilator drugs are associated with fewer exacerbations of airways disease when given in effective doses over the long term.
Pharmacologic Basis of Bronchodilator Action
β-Agonists
Based on the classical work of Ahlquist in defining different subtypes of adrenoreceptors, a range of relatively specific β-agonist agents were developed. Because β2-receptors are almost the only subtype expressed on human airway smooth muscle, it makes sense to use highly selective β-agonists and there is no place for nonselective agents in clinical practice today. The chemical structures of the principal β-agonists are shown in Figure 15-4.
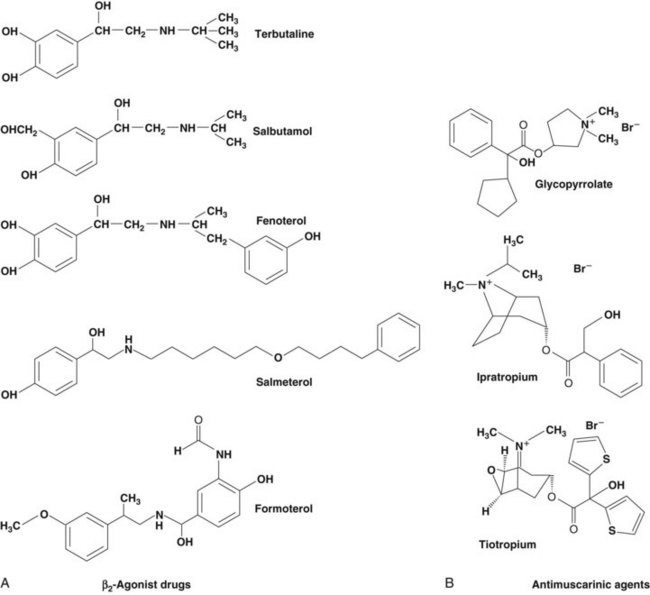
Figure 15-4 Chemical structures of commonly used bronchodilator drugs. A, β2-Agonists. B, Antimuscarinic drugs.
• A fall in the intracellular calcium ion (Ca2+) concentration by active removal of Ca2+ from the cell into intracellular stores
• Inhibition of phosphoinositide hydrolysis
• Direct inhibition of myosin light chain kinase
• Opening of large-conductance, calcium-activated potassium channels (KCa) that repolarize the smooth muscle cell and may stimulate the sequestration of Ca2+ into intracellular stores
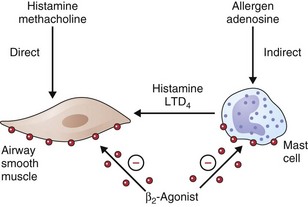
• β2-Agonists act as functional antagonists and reverse bronchoconstriction, irrespective of the contractile agent. This is an important property, because multiple bronchoconstrictor mediators (inflammatory mediators and neurotransmitters) are released in asthma.
• Inhibition of mediator release from mast cells and other inflammatory cells
• Inhibitory effects on neutrophil migration and activation
• Reduction and prevention of microvascular leakage and, consequently, development of bronchial mucosal edema after exposure to mediators such as histamine and leukotrienes
• Increased mucus secretion from submucosal glands and ion transport across airway epithelium (effects that may enhance mucociliary clearance, thereby reversing the defect in clearance found in asthma)
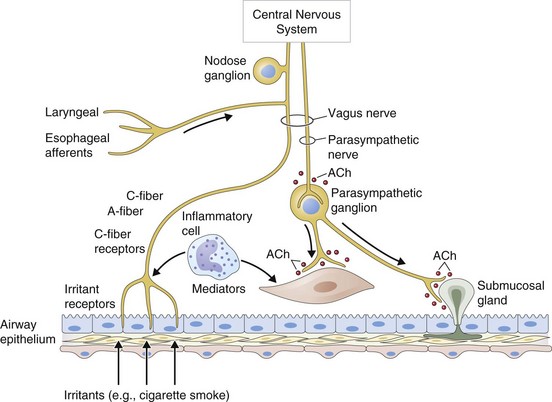
• Reduction in neurotransmitter release from airway cholinergic nerves, thus reducing cholinergic reflex bronchoconstriction
• Inhibition of the release of bronchoconstrictor and inflammatory peptides, such as substance P, from sensory nerves
How relevant any of these effects are to the observed clinical effects in disease is hard to determine. They may be more important in preventing bronchoconstriction from other stimuli in asthmatic patients than in directly influencing airway dimensions. The immediate impact on airway smooth muscle remains the most important of these various mechanisms, as illustrated in Figure 15-5. Recognized polymorphisms of the β-receptor show variable responses to agonist drugs in vitro. Translation of these observations into clinically noticeable differences in response in the clinical setting has been difficult, however, and at present these observations remain primarily of academic interest.
Antimuscarinic Agents
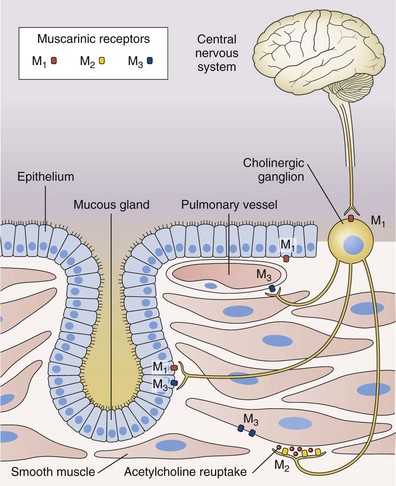
Antimuscarinic agents are specific antagonists of muscarinic receptors and inhibit cholinergic nerve–induced bronchoconstriction. Muscarinic receptors bind to acetylcholine released after stimulation of the parasympathetic nerves. These nerves innervate the bronchi and small airways of the human bronchial tree but do not extend to the respiratory bronchioles and alveoli. The receptor has seven membrane-spanning loops, the third of which shows considerable heterogeneity—explaining the existence of several different functional variants. Many of these receptors (M1 to M3) occur in different sites and with rather different functions, as shown in Figure 15-6.
β2-Agonists: Clinical Considerations
Although historically, nonselective adrenaline derivatives such as isoprenaline were used to treat airway disease, cardioselective β2-agonists are now recognized as being the safest and most effective compounds in this class. The most widely prescribed drug at present is salbutamol (albuterol in the United States), although the closely related compound terbutaline is popular in some parts of Europe. Fenoterol, a more potent β2-agonist, was widely used in the 1980s, although it was associated with an increased risk of asthma death in New Zealand. Whether this association was causal or coincidental remains a subject of heated debate, which still affects current attitudes toward drugs in this class today. Although oral β-agonists were popular for many years, their slow onset of action and significant side effect profile have led to a decline in their use, and the inhaled route is now preferred, because the effects develop more rapidly and the total dose of drug given is smaller. In general, drugs such as salbutamol and terbutaline are considered short-acting β-agonists (SABAs), to distinguish them from long-acting agents such as salmeterol and formoterol (LABAs), which also are given by inhalation. Once-daily long-acting β-agonists, such as indacaterol and vilanterol, have been developed recently and, in the case of indacaterol, are now licensed for use in certain medical conditions. Intravenous treatment with β-agonist initially was used for emergency care and is still offered to some people in intensive care units; however, this regimen has declined in popularity, and higher doses of these drugs are now given principally by nebulization. The doses, duration of action, and formulations of the most widely used agents are presented in Table 15-1.
Table 15-1 Formulations and Typical Doses of Medications for Treatment of Chronic Obstructive Pulmonary Disease*
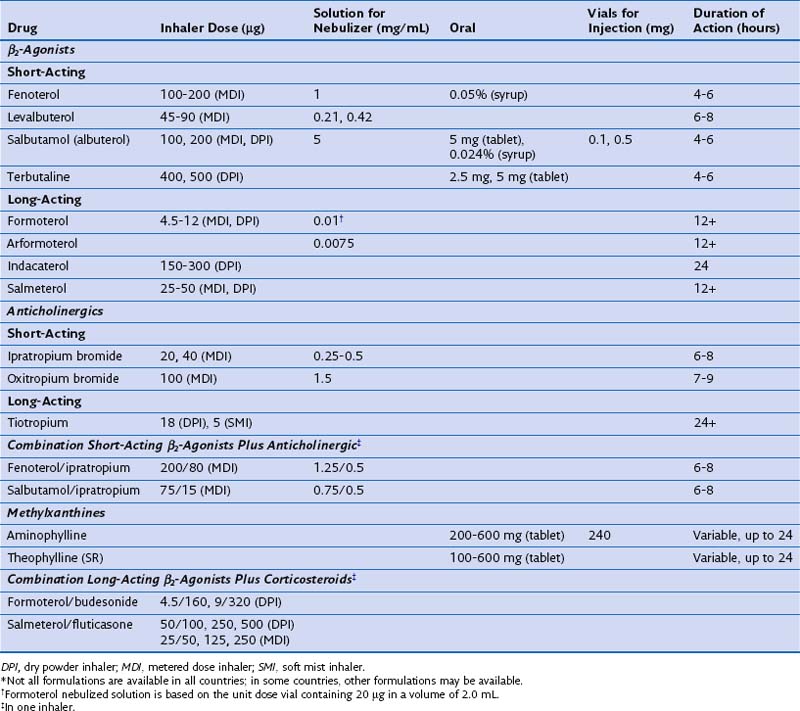
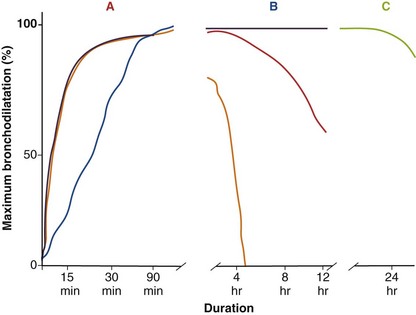
In general, SABA drugs have a duration of action (assessed by a measurable difference in FEV1 compared with that after administration of placebo) of 4 to 6 hours (Figure 15-7). Although the changes in lung function are statistically significant compared with either baseline or placebo, these effects really are very modest and often below the threshold of a clinically important difference, which for operational purposes is set at around 100 mL from baseline. Some evidence points to a much shorter period of protection against nonspecific challenges (e.g., to methacholine) than the total duration of effect measured in this way. By contrast, long-acting inhaled agents produce more substantial bronchodilation for at least 12 hours relative to their baseline value, and again, the duration of protraction against agonist challenges is much longer (see Figure 15-7). Salmeterol and formoterol are given twice-daily to maintain a bronchodilator effect over the 24-hour day. Salmeterol has a relatively slower onset of action, whereas formoterol, which is a full agonist, induces bronchodilation at a rate similar to that seen with salbutamol. This difference is important only when treatment is given on a maintenance basis, but the fast onset of action of formoterol allows it to be used as a rescue therapy; this has led to the “single inhaler” approach to asthma management. Newer data derived from patients with COPD managed with indacaterol and vilanterol show that these drugs have a rapid onset of action that lasts throughout the day after a single dose.
Side Effects
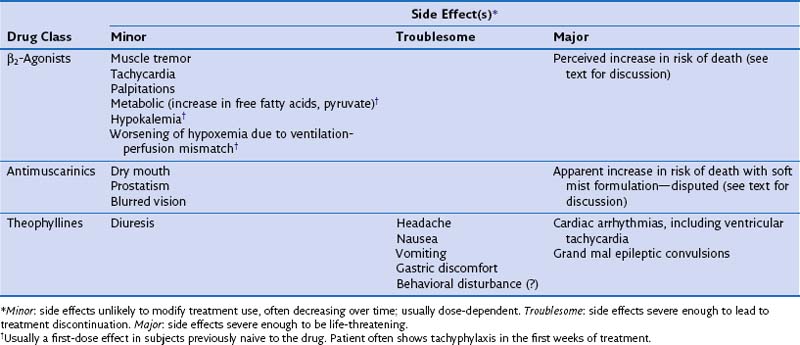
The principal side effects associated with β2-agonists are shown in Table 15-2. Although these are less evident with inhaled than oral therapy, they still occur, especially when recommended doses are exceeded, as happens in patients with poorly controlled disease with use of β2-agonist rescue therapy. The metabolic effects associated with these drugs do show tachyphylaxis, which helps explain why reports of hypokalemia after acute administration of the drugs have not been confirmed during larger clinical trials in which the drugs are given repeatedly. Also reported, however, is a variable susceptibility to tachycardia and tremor, which can be very troublesome, particularly in elderly patients. Sleep disturbance and anxiety appear to be nonspecific effects of higher doses of inhaled β-agonists, although the mechanisms leading to such problems remain unclear.
Antimuscarinic Agents: Clinical Considerations
Antimuscarinic drugs (which also are called anticholinergic agents almost interchangeably, adding an element of confusion to any consideration of this topic) have been developed exclusively for use by the inhaled route. This limited approach reflects the significant side effect profile associated with systemically available drugs such as atropine, which produce a range of pharmacologically predictable adverse events including tachycardia, dry mouth, blurred vision, constipation, and prostatism. The pharmacologic manipulation of the atropine molecule by the addition of an ammonium residue greatly reduces the ability of the drug to cross lipid membranes and hence limits the action of the agent to the airways, rather than allowing systemic absorption. By far the most widely used agent in the past has been ipratropium, which is a short-acting muscarinic antagonist (SAMA) whose duration of effects measured by FEV1 change is similar to and in general slightly greater than that for SABA drugs (see Figure 15-7). The development of tiotropium, an effective long-acting once-daily inhaled muscarinic antagonist (LAMA), has greatly reduced the use of ipratropium, and newer agents in this class also are being developed. Details of the dose, duration of action, and formulation of these drugs are presented in Table 15-1.
Theophylline
Theophyllines can be used only by the oral or intravenous route and, for reasons that remain somewhat unclear, appear to be entirely ineffective when given by inhalation. This limitation is unfortunate, because these drugs have a significant adverse event profile, as summarized in Table 15-3. The most dramatic problems are those associated with the unanticipated onset of ventricular tachycardia and of grand mal epileptic convulsions. However, a number of other troublesome side effects are typical of this group and probably are mediated by PDE4 inhibition—namely, headache, nausea, insomnia, vomiting, diarrhea, and poor appetite. These effects occur frequently with theophyllines and are dose-related. Unfortunately, the pharmacokinetics of this class of agents is rather variable, and their absorption is influenced by food and the way in which the drug is presented. Although slow-release preparations have been developed, their use increases the cost of what would otherwise be relatively inexpensive medication. It has been suggested, but not conclusively established, that introducing these drugs in low doses with gradual buildup subsequently is associated with lower incidence of unpleasant adverse events. Nonetheless, it also is a requirement that theophylline levels be monitored to ensure that the patient is not exposed to undue risks of toxicity.
Table 15-3 Factors Affecting Theophylline Metabolism in Chronic Obstructive Pulmonary Disease (COPD)*
| Increased | Decreased |
|---|---|
* Factors posing particular problems in COPD are indicated by superscript plus signs, the number depending on the likely hazards.
Drug Delivery
As noted earlier, the most effective bronchodilator drugs are delivered by the inhaled route, which minimizes the dose to the patient while increasing the directly available concentration of the drug at the desired site of action within the airways. Inhalation therapy is more complex than oral treatment, however, and in some settings and cultures, barriers to its uptake remain. As noted in Table 15-1, short-acting inhaled bronchodilators are available both as metered dose inhalers and in dry powder devices. Long-acting inhaled β-agonists also are available in both these forms, but at present tiotropium, the most widely used LAMA drug, is available only as a dry powder. No standardization of inhalation devices used to deliver these drugs has been attempted, but the general requirements for effective inhaler use have been codified and are presented in Table 15-4. An assessment of the effectiveness of the patient’s inhaler technique is one of the most important components of the clinical review on follow-up visits. The ability to use inhalers properly is not related to educational status or age and seems to be somewhat unpredictable. The use of spacer devices can improve the percentage of drug delivered and often can sidestep problems with incoordination. A variety of such devices are available for use with metered dose inhalers. Spacers can be helpful when the need to limit side effects due to inhaled corticosteroids is a primary consideration but also can increase the effectiveness of bronchodilator delivery if the drug is reaching the airway, rather than being swallowed and metabolized. Breath-activated inhalers help some patients who find it hard to coordinate inspiration and device actuation, and often a change to a different delivery system (e.g., switching to a dry powder formulation from a metered dose inhaler) can improve matters significantly. Wet nebulization formulations of both SABA and SAMA drugs are available, but their potential for greater effect reflects the much higher doses given, rather than improvement in the deposition of the drug. These systems are operator-independent and thus well suited for use by distressed patients experiencing exacerbations of symptoms. How much of this acute benefit derives from the drug and how much from the cooling effects on the face of the mist in which it is delivered remain to be quantified.
Table 15-4 Technique for Use of Metered Dose Inhalers in Obstructive Lung Disease
| Ideal Method | Difficulties Observed/Reported in Clinical Use |
|---|---|
| 1. Remove cap. | Occasionally forgotten |
| 2. Shake inhaler. | Occasionally forgotten |
| 3. Hold inhaler upright. | Often forgotten |
| 4. Tilt head back 10-55 degrees. | Often forgotten |
| 5. Hold inhaler in front of open mouth. | Confusion about this step is common |
| 6. Begin to inspire and activate inhaler. | Coordination problems |
| 7. Breathe in slowly and deeply. | Difficult if lungs are hyperinflated already |
| 8. Breath-hold for 10-15 seconds. | Breath-hold time reduced |
| 9. Breath out slowly through the nose. | Harder with high respiratory rate |
| 10. Use one puff at a time—wait 3-5 minutes between puffs. | Frequent use of multiple puffs in a single inspiration |
Use of Bronchodilator Drugs in Clinical Practice
Albert P, Calverley PM. Drugs (including oxygen) in severe COPD. Eur Respir J. 2008;31:1114–1124.
Baker WL, Baker EL, Coleman CI. Pharmacologic treatments for chronic obstructive pulmonary disease: a mixed-treatment comparison meta-analysis. Pharmacotherapy. 2009;29:891–905.
Calverley PM. COPD: what is the unmet need? Br J Pharmacol. 2008;155(4):487–493.
Cazzola M, Celli B, Dahl R, Rennard S. Therapeutic strategies in COPD. Oxford: Clinical Publishing, 2005.
Cazzola M, Matera MG. Emerging inhaled bronchodilators: an update. Eur Respir J. 2009;34:757–769.
Cazzola M, Segreti A, Matera MG. Novel bronchodilators in asthma. Curr Opin Pulm Med. 2010;16:6–12.
Global Initiative for Asthma Global strategy for asthma management and prevention (website). NHLBI/WHO Workshop Report www.ginasthma.com accessed May 2011
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD (website). www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. accessed May 2011