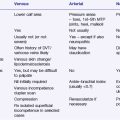
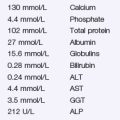
Problem 28 Breathlessness in a young woman
A 12-lead ECG showed a sinus tachycardia, normal axis, normal intervals and no ST-T wave changes.
Answers
Revision Points
Issues to Consider
Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand. http://www.heartfoundation.org.au/Professional_Information/Clinical_Practice/CHF/Pages/default.aspx, 2006. Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006
Hunt S.A., Abraham W.T., Chin M.H., et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the diagnosis and management of heart failure in adults. Journal of the American College of Cardiology, 53. 2009:e1-e90. http://content.onlinejacc.org/cgi/content/full/j.jacc.2008.11.009.
Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of CardiologyDickstein K., Cohen-Solal A., Filippatos G., et al. Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal, 29. 2008:2388-2442. http://www.escardio.org/guidelines-surveys/esc-guidelines/Pages/acute-chronic-heart-failure.aspx.